Abstract
The role of exogenous nitric oxide (NO) application in alleviating drought stress responses by enhancing the antioxidant activities in plants is well established for several species. However, none of the studies reported its role in protecting the watermelon genotypes from drought stress. In this study, we aimed to observe the effect of NO application on the physiological and biochemical responses of the two watermelon (Citrullus lanatus var. lanatus) genotypes grown under drought stress conditions by treating the plants with 15% polyethylene glycol 6000 (PEG 6000) and 100 µM sodium nitroprusside (SNP), which is a NO donor in Hoagland solution. Among the two genotypes, one genotype, KAR 98 was drought tolerant; while another, KAR 147 was drought sensitive. Drought stress showed a decrease in the growth parameters of both the genotypes; however, as expected it was higher in the susceptible genotype, KAR 147. NO application could not prevent the reductions in the growth parameters; however, it reduced the increment in malondialdehyde (MDA) content caused by the drought stress in both watermelon genotypes. Moreover, while drought stress condition reduced the ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), and peroxidase (POX) activities in both genotypes, NO + PEG application increased the APX activity in the tolerant genotype, KAR 98. Though the obtained results does not show the direct involvement of NO in increasing drought tolerance of watermelon plants, the increase in the APX antioxidant enzyme activity on NO application under drought stress confirmed its role in protecting the watermelon genotypes from the oxidative damage caused by the drought stress. Moreover, it can be concluded that the effect of NO application on watermelons’ responses towards drought stress condition may vary according to the specific genotypes. As to date none of the studies reported the effect of NO application on the antioxidant activity of watermelon genotypes under drought stress, the present study may provide information about the mechanisms that can be focused to improve drought stress tolerance of watermelon genotypes.
Keywords: Antioxidant enzymes, Abiotic stress, NO metabolism, Physiological growth, Reactive oxygen species (ROS), Water deficit
Introduction
Drought is one of the disastrous stress conditions affecting the plants at the morphological, physiological, biochemical, and molecular levels; thus, limiting the crop yields (Abid et al. 2018). It results in an increase in the release of the highly toxic reactive oxygen species (ROS) including free radicals (such as hydroxyl radicals) and non-radicals (such as hydrogen peroxide) in plants that lead to oxidative damage causing the destruction of carbohydrates, lipids and proteins in the cells. ROS also plays an important role in stimulating the expression of a new gene at the cellular site facing the abiotic stress condition; thus, regulating the cellular growth, development and response towards a specific stress. The antioxidative defense system of plants including enzymatic and non-enzymatic molecules facilitates the scavenging of these ROS protecting the cellular structures from the possible damage (Gill and Tuteja 2010). Drought stress condition leads to the peroxidation of polyunsaturated fatty acids that leads to production of reactive aldehydes such as malondialdehyde (MDA). Thus, the changes in the MDA content during a stress condition demonstrate the extent of lipid peroxidation. NO has been identified as a potential effector and signaling molecule in plants that has been intensively studied for its role in stress regulation in plants (Simontacchi et al. 2015). It is known to act as an antioxidant molecule destroying the ROS and forming peroxynitrite that is less harmful as compared to peroxides, thus reducing the cellular destruction (Jday et al. 2016). In addition, it is known to interact with ROS and have a signaling role causing the expression of genes under abiotic stress conditions (Lamattina et al. 2003). It has also been revealed that NO leads to the accumulation of free proline in plants regulating the drought stress tolerance in plants (Farooq et al. 2009). Sodium nitroprusside (SNP) is a NO donor that leads to the alleviation in water loss from leaves via diminishing the ion leakage and transpiration rate, stimulating the closure of stomata, thus increasing the drought stress tolerance (Gan et al. 2015; Simontacchi et al. 2015; Zhang et al. 2016). Though the function of NO in alleviating drought stress responses have been studied in several plant species (Tian and Lei 2006; Gan et al. 2015; Zhang et al. 2016; Silveira et al. 2017; Santisree et al. 2015), less is known about its role in regulating drought stress in watermelon species. Watermelon (Citrullus lanatus var. lanatus) is largely grown in arid and semi-arid regions of the world that often suffer from less rainfall. In 2018, 103.9 million tonnes of watermelons were produced worldwide, with Turkey as the third major producer after China and Iran producing 4 million tonnes of watermelon (FAO, 2018). Though watermelon prefers hot and dry environment, deficient water supply during the establishment and early vegetative period (0 to approx. 40 days) lead to the development of weak plant and reduces the yield. In addition, the flowering period and fruit filling periods are sensitive to drought stress. Thus, it is crucial to understand the defense mechanism of watermelon under water deficit.
Understanding the ameliorative effect of NO on the responses of watermelon towards drought stress may facilitate the management strategies for improving its yield. Thus, the present study was aimed to explore whether the NO application has an ameliorative effect on the growth, biochemical and enzymatic activities of the two contrasting watermelon genotypes (one drought-tolerant and another drought susceptible) grown under drought stress condition. As it will be the first study to establish whether NO application have a role in modifying the antioxidant enzyme activity consequently improving the drought stress tolerance of watermelon genotypes; the obtained results can be used as a reference while establishing the management methods for watermelon to upgrade their drought tolerance in semi-arid and arid regions.
Materials and methods
Plant material and growing conditions
For this study, a drought-tolerant watermelon genotype, KAR 98 (Suyum et al. 2012; Hamurcu et al. 2015) and a drought-susceptible watermelon genotype, KAR 147 were grown in controlled hydroponic growth conditions where photoperiod, light intensity, temperature, and humidity was set to 16/8 h day/night, 14,000 lx/day, 21 ± 1 ℃, and 45–55%, respectively. In the trial, drought stress was developed in plants by polyethylene glycol (PEG) application that has been determined to stimulate drought stress in several studies, inducing hydrogen peroxide formation and lipid peroxidation, but does not have any toxic effects (Hellal et al. 2018; Emmerich and Hardegree 1990).
At first, watermelon seeds were treated with 5% sodium hypochlorite for 10 min and thoroughly rinsed 3 times with sterile deionized water (dI-H2O). After surface sterilization, seeds were imbibed in water for 2 h, put on humid filter papers in Petri plates, and then kept at 4 ℃ overnight. Further, germinated seeds were incubated on nylon net in 0.5 mM CaCl2 solution at 25 ℃ under dark. Later, germinated seedlings were transferred to 1/5 Hoagland solution (pH 6.0) in the aerated growth room. At three-leaf stage, 15% PEG 6000 and 100 µM nitric oxide was supplied to the plants within 1/5 Hoagland solution. Hoagland solution was replaced every 3 days. Samples were harvested after the 10th day of PEG and nitric oxide treatment when they started showing morphological symptoms of drought stress. Leaf samples were immediately frozen in liquid nitrogen, and stored at − 80 ℃ until enzymatic analyses.
Growth parameters
Root and shoot samples of watermelon genotypes were harvested on the 10th day after the PEG and NO treatment for measuring the growth parameters. Root–shoot length and fresh weight were measured immediately after the harvest. Root–shoot dry weights were measured after drying the samples at 70 ℃ for 72 h.
Photosynthesis measurements
The photosynthetic parameters including photosynthetic activity, stomatal activity, transpiration flow and internal CO2 were measured using Li-Cor LI-6400 XT instrument (Sperlich et al. 2016).
Na, K, Ca and Mg contents in plants
Na, K, Ca and Mg contents were measured employing ICP-AES machine (Inductively Coupled Plasma Atomic Emission Spectrometer) (Varian Vista AX, Australia) (Burt 2004; Pandey et al. 2016). Around half gram of dried leaf and root samples were digested with concentrated HNO3 in a microwave system CEM, Mars 5 (CEM Corp., USA). After filtering the obtained mixture, the concentration of elements in the supernatant was estimated.
Determination of malondialdehyde (MDA) content
In this study, thiobarbituric acid was used to estimate the lipid peroxidation in leaves by measuring the MDA content (Rao and Sresty 2000; Bayram et al. 2014). The MDA concentration was determined from the absorbance at 532 nm and measurements were rectified for nonspecific turbidity by subtracting the absorbance at 600 nm. The MDA concentration was estimated employing an extinction coefficient of 155/mM/cm.
Estimation of proline
Proline content was estimated by ninhydrin assay at A520 nm according to the method described by Wang et al. (2015) and Bates et al. (1973).
Hydrogen peroxide (H2O2) accumulation level
H2O2 contents were measured employing the method used by Velikova et al. (2000) and Terzi et al. (2014). Leaf tissues (0.1 g) were homogenized with 1 mL of 0.1% (w/v) trichloroacetic acid (TCA) on an ice bath. Further, the homogenate was centrifuged at 12,000 × g for 15 min. The reaction mixture consisted of supernatant (0.5 mL), 0.5 mL potassium phosphate buffer (10 mM, pH 7.0) and 1 mL 1 M potassium iodide (KI). After vortexing, the absorbance was measured at 390 nm, where 0.1% TCA was used as blank. The H2O2 content was determined from a standard curve, and the determined values were expressed as μmol/g fresh weight.
Hydroxyl (•OH) scavenging activity
Hydroxyl radical activity was estimated as per the method followed by Kim and Minamikawa (1997) and Silva et al. 2020.
Leaf osmotic potential (ΨΠ)
Leaf osmotic potential was measured employing the method followed by Santa-Cruz et al. (2002). Leaves pieces were homogenized in Eppendorf tubes and were further centrifuged at 14,000 rpm for 15 min. Readings of obtained solution were taken using Wescor Vapro 5600 osmometer as mmol/kg which were further converted to osmotic potential in MPa by multiplying them with 2.408 × 10–3.
Cell membrane permeability
Cell membrane permeability was estimated by measuring electrolyte leakage as per the method mentioned by Kaddour et al. (2013) and Dionisio-Sese and Tobita (1998). For analysis, 0.1 g leaf samples were cut into 5-mm-long discs and transferred to the test tubes containing 10 mL of deionized water. The tubes were sealed with plastic stoppers and kept in a 32 °C water bath for 2 h. The samples were autoclaved for 20 min at 121 ℃ and the electrical conductivity of the medium was measured with EC meter (EC1). Further, the samples were cooled to 25 ℃ to measure the electrical conductivity (EC2).
Electrolyte leakage (EL) was calculated according to the following formula:
Anatomical analysis
For microscopic observations, the paraffin method (Ruzin 1999; Ouk et al. 2020) was applied to specific sections of the plants, and sections were taken using a microtome.
Enzyme extractions and assays
Around half gram of the frozen leaf samples were crushed with 50 mM sodium phosphate buffer (pH 7.8) consisting of 1 mM disodium-EDTA and 2% (w/v) polyvinylpolypyrrolidone (PVPP). After centrifugation at 14,000 rpm for 40 min at 4 ℃, supernatants were used for the enzyme activity assays. Employing bovine serum albumin as standard, the total soluble protein contents of the enzyme extracts were estimated following the method of Bradford (1976). The spectrophotometric measurements were done using a Shimadzu (UV 1600) spectrophotometer.
Superoxide dismutase (SOD, EC 1.15.1.1)
The SOD activity was estimated by its capacity to prevent the photochemical reduction of nitrobluetetrazolium (NBT) at 560 nm (Beauchamp and Fridovich 1971; Gao et al. 2008). The experiments were conducted at 25 ℃ and the reaction mixture (3 mL) contained 0.033 mM NBT, 10 mM l-methionine, 0.66 mM EDTA-Na2 and 0.0033 mM riboflavin in 0.05 mM sodium phosphate buffer (pH 7.8). Followed by the addition of riboflavin, reaction mixture was incubated under 300 µmol m−2 s−1 irradiance at 25 ℃ for 10 min. The observation of maximum color in a reaction mixture with no enzyme confirms maximum reduction rate of NBT. One unit of SOD activity was interpreted as the amount of SOD necessary for 50% inhibition of NBT and the specific enzyme activity was mentioned as units per mg protein.
Catalase (CAT, EC 1.11.1.6)
CAT activity was evaluated according to Rao et al. (1997) and Sukrong et al. (2012). The initial rate of decomposition of H2O2 was estimated at 240 nm. The reaction mixture was composed of 0.05 M sodium phosphate buffer (pH 7.0) with 0.1 mM EDTA (ethylene diamine tetraacetic acid) and 3% H2O2. The decrement in the absorption was checked for 3 min and 1 µmol H2O2 decomposed per min was considered as one unit of CAT.
Glutathione reductase (GR, EC 1.6.4.2)
GR activity was evaluated according to Foyer and Halliwell (1976) and Souri et al. (2020). The assay medium was composed of 0.025 mM sodium phosphate buffer (pH 7.8), 0.5 mM GSSG (glutathione disulfide), 0.12 mM NADPH (nicotinamide adenine dinucleotide phosphate) Na4 and 0.1 mL enzyme extracted in a final assay volume of 1 mL. Further, NADPH oxidation was conducted at 340 nm. The activity was measured using the extinction coefficient of NADPH (6.2 mM−1 cm−1). A unit of GR activity was considered as µmol/ml oxidized GSSG per min. The specific enzyme activities were mentioned as units/mg protein for all the studied enzymes.
Peroxidase (POX; EC 1.11.1.7)
POX enzyme activity was measured employing Herzog and Fahimi (1973) and Faradonbeh et al. (2020) method. The absorbance of the reaction mixture was estimated at 465 nm. A unit of POX activity was considered as µmol/ml H2O2 decomposed per min.
Ascorbate peroxidase (APX; EC 1.11.1.11)
The APX activity was estimated by Nakano and Asada (1981) and Wang et al. (2019) method. The decrease in the absorbance of the reaction mixture containing oxidized ascorbate at 290 nm was observed. A unit of APX activity was considered as µmol ml−1 oxidized ascorbate per min.
Guaiacol peroxidase (GPX; EC 1.11.1.7)
GPX activity was determined according to Quessada and Macheix (1984) and Hafsi et al. (2010).
Statistical analysis
The experiment was based on a completely randomized design. All analyses were conducted using one-way analyses of variance (ANOVA) in Minitab program and the mean differences were compared by the least significant difference (LSD) test. The data points were the mean of six replicates (n = 4), and differences between mean values with P < 0.01 were considered to be significantly different. In all the figures, the spread of values is shown as standard errors (SE) of the means.
Results and discussion
Growth parameters
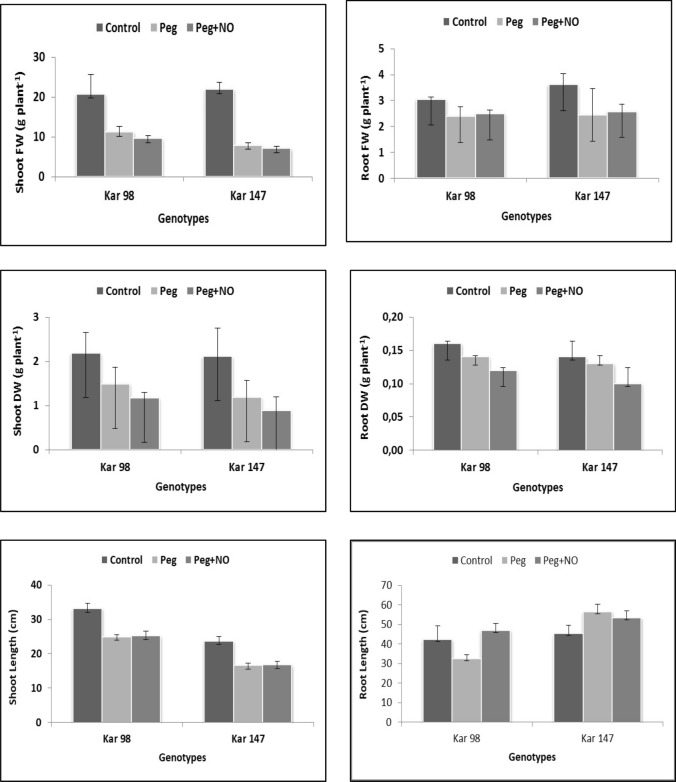
The effect of exogenous NO application on growth parameters was investigated in two different watermelon genotypes grown under drought stress. A decrease was observed in the root–shoot fresh weights, shoot dry weight, and shoot length of both the genotypes under drought stress conditions as compared to the control treatment. Moreover, this decrease was mostly greater in the case of KAR 147 genotype as compared to KAR 98 (except root dry weight). Though these results were not significant while considering the individual genotypes, the outcomes were significant when means of both the genotypes were statistically analyzed. Several studies on different plant species demonstrated a decrease in the root–shoot fresh and dry weight under drought stress conditions and the decreases were greater in sensitive genotypes (Martínez et al. 2007; Abid et al. 2018; Ayas and Demirtas 2009). Similar to our study, Kirnak and Dogan (2008) emphasized on the decrease in the dry matter production of watermelon plants (Citrullus vulgaris) under drought conditions. Turner and Begg (1981) discussed that reductions in the biomass of plants under water deficit may occur either due to a decrease in the photosynthetic activity or a reduction in the leaf growth rate. In our study, NO application under drought stress showed a statistically significant increase and decrease in the root length of the tolerant genotype, KAR 98 and susceptible genotype, KAR 147, respectively, as compared to the only drought condition (Fig. 1). This increase in the tolerant genotype showed the positive effect of NO application that may have contribution in the reduction of ROS under drought stress condition. Though NO application under drought stress decreased the shoot fresh weight and dry weight of both genotypes as compared to the only drought condition, it was not statistically significant. This decrease in the shoot fresh–dry weights of both watermelon genotypes were contrary to the results obtained by Gan et al. (2015) in barley where an increase in the fresh and dry weights were observed on NO application under drought stress as compared to the only drought condition.
Fig. 1.
The effects of NO application on the a shoot fresh weight (Shoot FW), b root fresh weight (Root FW), c shoot dry weight (Shoot DW), d root dry weight (Root DW), e hoot length and f root length of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes (tolerant, KAR 98 and susceptible, KAR 147) grown in drought stress condition. Values are mean ± S.E. (standard error) based on four replications (n = 4). Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
Photosynthesis measurements (photosynthetic activity, stomatal activity, transpiration rate, and internal CO2)
Light saturated photosynthesis (Asat) values determined at 1000 µmol/m2/s light intensity and atmospheric CO2 concentration were found to be close to each other in both genotypes in the control groups. When compared to the control group, PEG and PEG + NO applications did not significantly decreased the Asat values of KAR 98 genotype, while both applications significantly decreased the Asat values in KAR 147 genotype (Table 1). NO application under drought stress condition did not have a positive effect on the Asat values in both the genotypes as compared to the only drought treatment. These results were in accordance with the previous study conducted by Zhang et al. (2016) on Malus species where NO application under drought stress could make a positive effect on the photosynthetic rate as compared to the only drought condition.
Table 1.
The effects of NO application on the light saturated photosynthesis (Asat) values of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes (tolerant, KAR 98 and susceptible, KAR 147) grown in drought stress condition
| Treatment | Asat (μmol/m2/s1) | |
|---|---|---|
| KAR 98 | KAR 147 | |
| (μmol/m2/s1) | (μmol/m2/s1) | |
| Control | 14.97 ± 2 (n = 3) | 14.7 ± 3.01 (n = 3) |
| PEG | 13.36 | 8.77 |
| PEG + NO | 12.55 | 6.27 |
Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
K, Mg, Ca, and Na concentrations
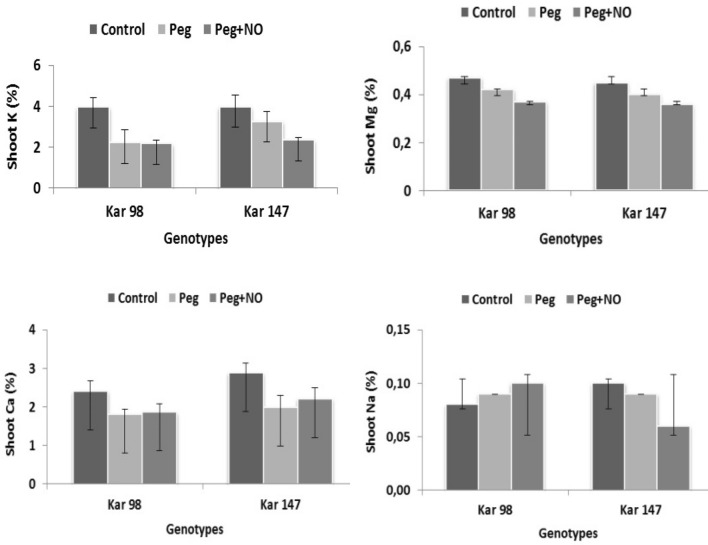
In the nutrient analysis, there were no major changes in K and Ca concentrations of KAR 98 in PEG + NO applications as compared to PEG treatment. However, in KAR 147 genotype, shoot K and Ca in PEG + NO applications decreased and increased, respectively, as compared to PEG treatment. On analyzing the mean of two genotypes, K content significantly decreased in both PEG and PEG + NO applications as compared to control. Shoot Mg concentration in both genotypes decreased in PEG and PEG + NO application as compared to control. Na concentration increased and significantly decreased in KAR 98 and KAR 147 genotype, respectively, in PEG + NO application as compared to only drought condition (Fig. 2). The increase in Ca and the decrease in K and Mg content in NO + PEG application as compared to PEG application were similar to those obtained by Bai et al. (2015) in ryegrass.
Fig. 2.
The effects of NO application on the shoot a potassium (K), b magnesium (Mg), c calcium (Ca) and d sodium (Na) content of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes (tolerant, KAR 98 and susceptible, KAR 147) grown in drought stress condition. Values are mean ± S.E. (standard error) based on four replications (n = 4). Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
Lipid peroxidation (MDA)
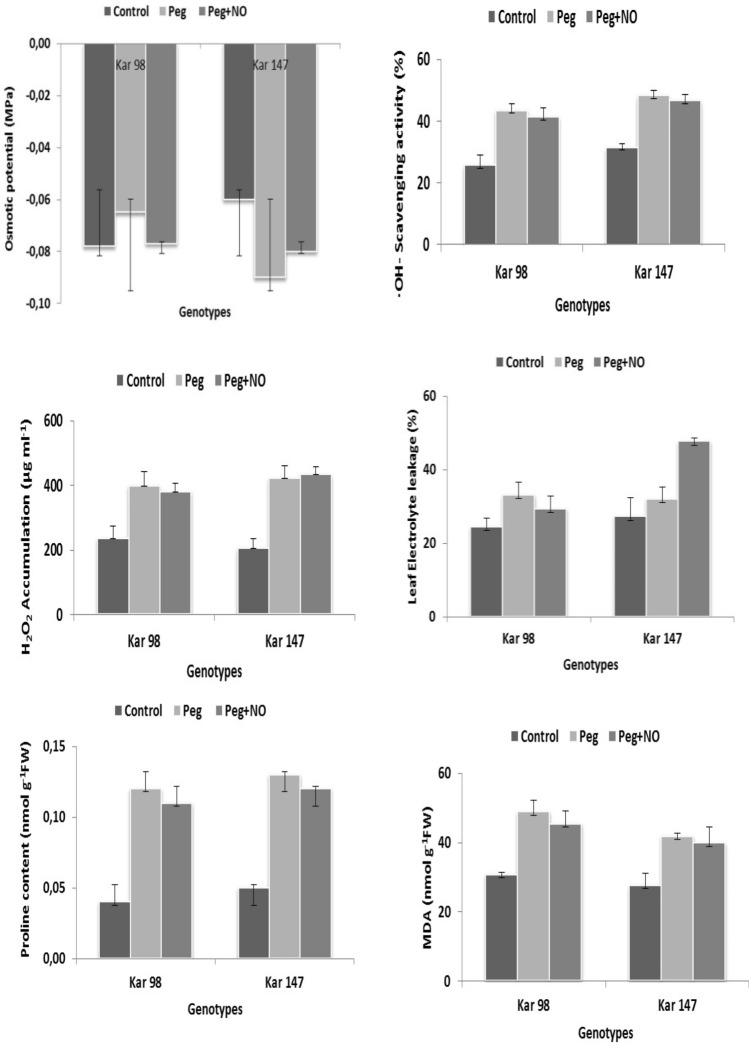
MDA content was significantly increased in both KAR 98 and KAR 147 genotype, respectively, under drought conditions as compared to control. On applying NO under drought conditions, both KAR 98 and KAR 147 genotype showed a decrease in MDA content as compared to only drought condition. These differences showed that NO application under drought stress had a positive effect on watermelon genotypes reducing the oxidative damage that decreased the MDA accumulation when compared to only PEG supply (Fig. 3). These decreases in MDA content on NO application under drought as compared to only drought treatment were in line with those obtained by Gan et al. (2015) in barley and Zhang et al. (2016) in Malus.
Fig. 3.
The effects of NO application on the a osmotic potential, b hydroxyl radical (•OH) activity, c hydrogen peroxide, d leaf electrolyte leakage, e proline and f MDA content of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes [tolerant, KAR 98 and susceptible, KAR 147] grown in drought stress condition. Values are mean ± S.E. (standard error) based on four replications (n = 4). Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
Proline
The proline accumulation increased in both KAR 98 and KAR 147 genotype in drought stress conditions as compared to control and there were decreases in the proline content in PEG + NO application as compared to drought conditions, but these changes were not significant. The percentage decrease in the osmotic potential of drought-susceptible genotype KAR 147 under PEG and PEG + NO as compared to control was higher than drought-tolerant KAR 98 (Fig. 3). The decrease in the leaf osmotic potential of watermelon genotypes in drought conditions indicates the accumulation of other osmotic compounds such as proline and glycine betaine. Similar to our results, Ahmad et al. (2018) reported that NO applications increases the synthesis of flavonoid, proline, glycine betaine and osmolites and promote the antioxidant metabolism, and metabolite accumulation, protecting the tomato plants from damage caused by salinity stress. Moreover, our results were in line with Gan et al. (2015) where NO application under drought stress increased the proline content of barley as compared to only drought condition.
Hydrogen peroxide (H2O2) content
The H2O2 content significantly increased in both genotypes under drought conditions as compared to control; however, this increase was higher in KAR 147 genotype. NO application in drought conditions increased the H2O2 content in susceptible genotype, KAR 147 while the H2O2 content of tolerant genotype, KAR 98 was decreased as compared to only drought condition, though these changes were not significant (Fig. 3). It might be possible that when NO and H2O2 pass through the peroxisome membrane, the superoxide radicals are produced on the cytosolic side of the membrane which inhibits the activity of NO along with the CAT and APX activities. This causes β-oxidation of fatty acids, increasing H2O2 concentration in susceptible genotype. In line with our study, Ozkur et al. (2009) and Qiu et al. (2008) reported an increase in H2O2 content with drought stress in Caper and wheat seedlings, respectively. The decrease in the H2O2 content of the tolerant genotype on NO supply under drought was similar to those obtained by Gan et al. (2015) in barley and Tian and Lei (2006) in wheat.
Hydroxyl radical (•OH)
The •OH activities of both watermelon genotypes increased under PEG application as compared to control. However, it decreased on NO application under drought stress condition as compared to only drought condition (Fig. 3). In previous studies, it was determined that NO leads to the destruction of superoxide radical and produces peroxynitrite (ONOO−) radical that further leads to the production of highly reactive •OH radical and nitrogen oxide (NO2) on isomerization with nitrate (Singh and Shah 2014).
Leaf osmotic potential (ΨΠ)
There was an increase and decrease in the osmotic potential value of KAR 98 and KAR 147 genotype in drought conditions as compared to control. Under PEG + NO application, the osmotic potential of KAR 98 decreased; however, the osmotic potential of KAR 147 increased as compared to PEG application (Fig. 3). Similar to the results of KAR 98 in our study, Jday et al. (2016) also determined that NO application under drought stress make osmotic potential more negative as compared to the only drought treatment.
Cell membrane permeability (electrolyte leakage)
Electrolyte leakage significantly increased in both genotypes under drought conditions as compared to control. However, on applying NO with PEG applications, electrolyte leakage of tolerant genotype significantly decreased, while of susceptible genotype significantly increased as compared to only drought condition (Fig. 3). Our results in the tolerant genotype were in accordance with those obtained by Ahmad et al. (2018) and Gan et al. (2015) where exogenous application of NO decreased electrolyte leakage in salt-stressed chickpea and drought stressed barley plants, respectively. According to these results, it can be concluded that the alleviating effect of NO towards the responses of drought stress may vary according to the genotypes.
Anatomical analysis
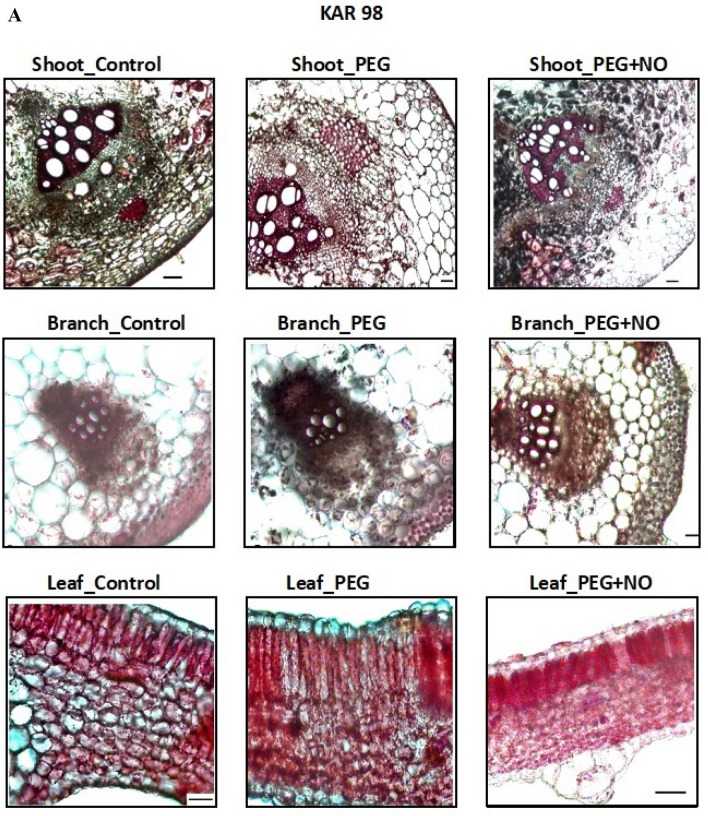
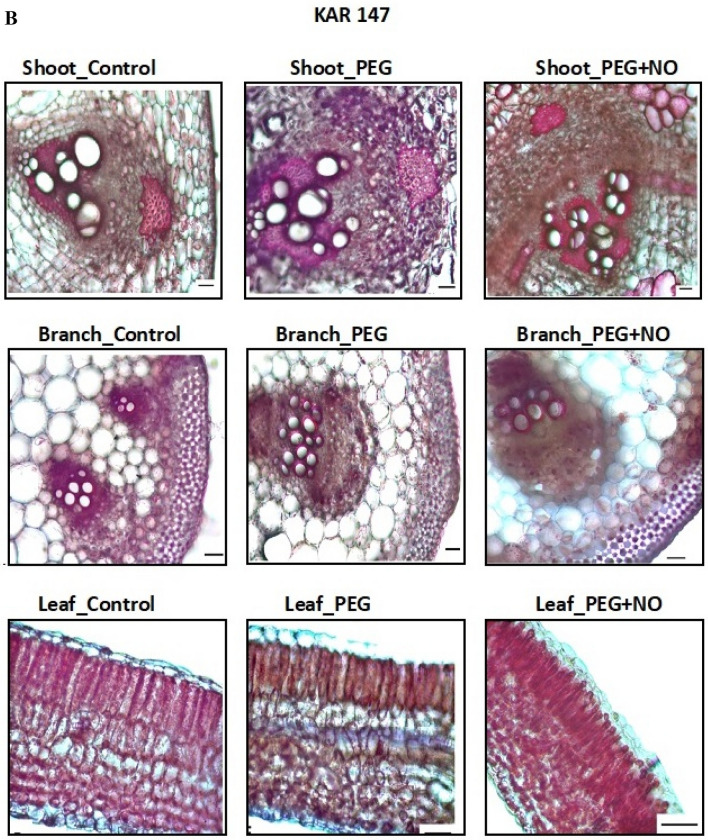
On examining the anatomical sections of shoots, an increase in the xylem counts in the vascular bundles of KAR 98 genotype depending on the applications was observed. The differences in trachea numbers were noteworthy, especially in branch sections. Some differences were observed in the number of palisade parenchyma sequences in the leaf sections of the KAR 98 genotype depending on the application (Fig. 4). Similarly, number of tracheids showed differences in the shoot sections of KAR 147 genotype. In addition, the number of cells in the sclerenchyma tissues surrounding the vascular bundles showed differences.
Fig. 4.
The effects of NO application on the anatomical shoot, branch and leaf sections of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes a KAR 98 (tolerant one) and b KAR 147 (susceptible one) (scale 50 µm). Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
Antioxidant enzyme activities
Superoxide dismutase (SOD) activity
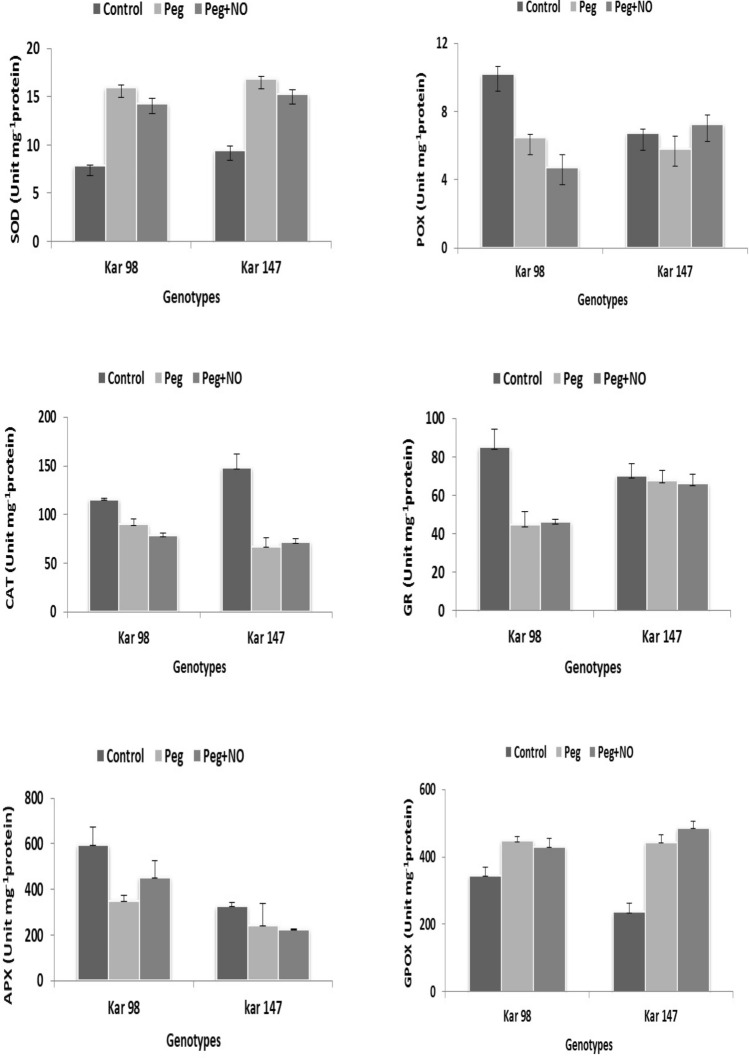
SOD activity increased in both KAR 98 and KAR 147 genotype under drought conditions and PEG + NO as compared to control (Fig. 5). While SOD enzyme activity increased under NO + PEG application as compared to control, it decreased as compared with the drought application in both watermelon genotypes. Similar to our study results, SOD enzyme activity of beans (Phaseolus vulgaris) and tepary beans (Phaseolus acutifolius) increased due to drought stress in a study conducted by Türkan et al. (2005). Although there was an increase in SOD enzyme activity due to drought stress, decreases in the enzyme activity were reported with the increasing stress concentrations (Tan et al. 2006). Moreover, on contrary with our study, NO application under drought stress increased the SOD activity in barley and Malus species, respectively, when compared to only PEG supply (Gan et al. 2015; Zhang et al. 2016). NO applications in abiotic stress conditions may have variable effects on antioxidant enzyme activity in plants (He et al. 2019; Laspina et al. 2005).
Fig. 5.
The effects of NO application on the shoot a superoxide dismutase (SOD), b peroxidase (POX), c catalase (CAT), d glutathione reductase (GR), e ascorbate peroxidase (APX), and f guaiacol peroxidase (GPX) contents of two contrasting watermelon (Citrullus lanatus var. lanatus) genotypes (tolerant, KAR 98 and susceptible, KAR 147) grown in drought stress condition. Values are mean ± S.E. (standard error) based on four replications (n = 4). Control no drought stress no SNP (NO donor), PEG drought stress treatment, PEG + NO drought stress + SNP treatment
Catalase (CAT) activity
In the drought condition, CAT enzyme activity significantly decreased in both genotypes as compared to control; however, the decrease was higher in susceptible KAR 147 genotype (55%) and lower in the tolerant KAR 98 genotype (22%) (Fig. 5). NO application under drought significantly decreased and increased the CAT activity in KAR 98 and KAR 147 genotype, respectively, as compared to the only PEG application. The increase in the CAT activity of susceptible genotype highlights the positive effect of NO application under drought stress condition. In contrary to our results of tolerant genotype, an increase in the CAT activity was observed in barley and Malus plants under PEG + NO application as compared to only PEG application (Gan et al. 2015; Zhang et al. 2016). Similar to our study, CAT activity has been reported to be inhibited by exogenous NO treatment in the studies conducted by Clarke et al. (2000) and Manai et al. (2014).
Glutathione reductase (GR) enzyme activity
Several studies reported that drought stress induces oxidative stress and causes an increase in GR activity (Gill and Tuteja 2010; Miller et al. 2010). However, in our study, GR activity significantly decreased in KAR 98 genotype under PEG treatment as compared to control. However, NO application under drought stress increased GR activity in the tolerant genotype, KAR 98 when compared to only PEG treatment. The decrease in GR activity in KAR 147 genotype under both PEG and PEG + NO application was not significant when compared to the control (Fig. 5). This showed that other than having relieving effects, NO may also have negative effects on plants when applied with some existing abiotic stress conditions. This negative effect is dependent on the nature of the genotype, type of enzymes, and types of abiotic stress.
Peroxidase (POX) activity
Under drought conditions, POX activity significantly decreased in both the genotypes; however, the decrease was greater in KAR 98 genotype. On NO application under drought, POX activity values significantly decreased and increased in KAR 98 and KAR 147 genotype, respectively, as compared to only drought application (Fig. 5). Previous studies have shown that POX enzyme activity increases with the stress conditions in plants exposed to drought stress and results of several studies were in parallel with our study (Tan et al. 2006; Türkan et al. 2005). However, contrary to our results, Wang et al. (2009) showed a higher POX activity in drought-tolerant alfalfa plants under drought stress conditions.
Ascorbate peroxidase (APX) enzyme activity
When PEG and PEG + NO applications were compared with the control group, APX activity values were observed to be significantly decreased in both watermelon genotypes. However, NO supply under drought stress had significant positive effect on the APX activity of the tolerant genotype, KAR 98 which confirmed the alleviating effect of NO application on drought stress (Fig. 5). This increase in the APX activity of the tolerant genotypes under PEG + NO application as compared to only PEG treatment was similar to the results obtained by Zhang et al. (2016) in Malus species.
Guaiacol peroxidase (GPX) enzyme activity
GPX activity significantly increased in both the genotypes under PEG and PEG + NO applications as compared to control. However, on NO application under PEG, the GPX activity decreased and significantly increased in KAR 98 and KAR 147 genotype, respectively, as compared to only PEG application (Fig. 5). This showed that NO application under drought condition had a positive effect on the GPX activity of the tolerant KAR 98 genotype. Tian and Lei (2006) also found lower GPX activity in wheat seedlings on NO supply under drought stress as compared to only drought condition. These results were also in parallel with the other previously conducted studies such as Namdjoyan et al. (2018).
Conclusion
Based on the results, it can be concluded that the drought stress caused by PEG 6000 decreased the growth, development and caused oxidative stress in both tolerant and susceptible watermelon genotypes. The drought condition caused MDA accumulation in watermelon plants and this was associated with the changes in the enzyme activities. However, the NO applications under drought stress resulted in a decrease in MDA accumulation in the genotypes. Besides, NO application led to an increase in the root fresh weight and root length of tolerant watermelon genotype under drought stress conditions. In NO applications under drought conditions, there was a decrease in SOD activity in both genotypes when compared with the only drought treatment. Although NO applications under drought stress did not have a positive effect on the activity of all the enzymes as compared to PEG application, it did have a positive effect on APX activity of the tolerant genotype, KAR 98. In our study, NO application when applied under drought stress condition showed a positive effect on only some of the growth parameters and antioxidant enzymes of both watermelon genotypes. Thus, it can be concluded that the effect of NO application on plants grown under a specific abiotic stress condition may vary according to the type of abiotic stress, studied plant species and its specific genotypes. In future studies, the transcript levels of the specific watermelon genes for the studied parameters should be explored in detail under PEG and PEG + NO treatments and the obtained expression results can be utilized to develop drought-tolerant watermelon genotype.
Author contributions
MH, MKK and AP conceived, wrote and edited the manuscript. CO, ZZA, FE and AHO conducted the experiments. SG made intellectual contribution to the manuscript. All the authors have read and agreed to the content.
Compliance with ethical standards
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
References
- Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci Rep. 2018;8(1):4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P, AbassAhanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M. Mitigation of sodium chloride toxicity in Solanumlycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact. 2018;13(1):64–72. doi: 10.1080/17429145.2017.1420830. [DOI] [Google Scholar]
- Ayas S, Demirtas C. Deficit irrigation effects on cucumber (Cucumissativus L. Maraton) yield in unheated greenhouse condition. J Food Agric Environ. 2009;7(3):645–649. [Google Scholar]
- Bai XY, Dong YJ, Wang QH, Xu LL, Kong J, Liu S. Effects of lead and nitric oxide on photosynthesis, antioxidative ability, and mineral element content of perennial ryegrass. Biol Plant. 2015;59(1):163–170. doi: 10.1007/s10535-014-0476-8. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Bayram D, Dinler BS, Tasci E. Differential response of bean (Phaseolus vulgaris L.) roots and leaves to salinity in soil and hydroponic culture. NotulaeBotanicaeHortiAgrobotanici Cluj-Napoca. 2014;42(1):219–226. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burt R. Soil survey laboratory methods manual. Soil survey investigations report no, 42 version 4.0. Washington, DC: Natural Resources Conservation Service, US Department of Agriculture; 2004. [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J Cell MolBiol. 2000;24(5):667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135(1):1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Emmerich WE, Hardegree SP. Polyethylene glycol solution contact effects on seed germination. Agron J. 1990;82(6):1103–1107. doi: 10.2134/agronj1990.00021962008200060015x. [DOI] [Google Scholar]
- FAO (2018). FAOSTAT: Food and agriculture data,. Crop Statistics. Available at: https://www.fao.org/faostat. Accessed 19 June 2020
- Faradonbeh NH, Darbandi EI, Karimmojeni H, Nezami A. Physiological and growth responses of cucumber (Cucumissativus L.) genotypes to Egyptian broomrape (Phelipancheaegyptiaca (Pers.) Pomel) parasitism. ActaPhysiol Plant. 2020;42(8):1–15. [Google Scholar]
- Farooq M, Basra SMA, Wahid A, Rehman H. Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.) J Agron Crop Sci. 2009;195(4):254–261. doi: 10.1111/j.1439-037X.2009.00367.x. [DOI] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133(1):21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Gan L, Wu X, Zhong Y. Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Prod Sci. 2015;18(1):52–56. doi: 10.1626/pps.18.52. [DOI] [Google Scholar]
- Gao J, Xiao Q, Ding L, Chen M, Yin L, Li J, Zhou S, He G. Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroides and Oryza sativa subjected to drought stress. Plant Growth Regul. 2008;56(1):89. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Hafsi C, Romero-Puertas MC, del Río LA, Sandalio LM, Abdelly C. Differential antioxidative response in barley leaves subjected to the interactive effects of salinity and potassium deprivation. Plant Soil. 2010;334(1):449–460. doi: 10.1007/s11104-010-0395-1. [DOI] [Google Scholar]
- Hamurcu M, Demiral T, Hakki EE, Turkmen O, Gezgin S, Bell RW. Oxidative stress responses in watermelon (Citrullus lanatus) as influenced by boron toxicity and drought. Zemdirbyste-Agriculture. 2015;102(2):209–216. [Google Scholar]
- He H, Oo TL, Huang W, He L-F, Gu M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.) Sci Rep. 2019;9(1):9516. doi: 10.1038/s41598-019-46036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal FA, El-Shabrawi HM, Abd El-Hady M, Khatab IA, El-Sayed SAA, Abdelly C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J Genet EngBiotechnol. 2018;16(1):203–212. doi: 10.1016/j.jgeb.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V, Fahimi HD. A new sensitive colorimetric assay for peroxidase using 3, 3′-diaminobenzidine as hydrogen donor. Anal Biochem. 1973;55(2):554–562. doi: 10.1016/0003-2697(73)90144-9. [DOI] [PubMed] [Google Scholar]
- Jday A, Ben Rejeb K, Slama I, Saadallah K, Bordenave M, Planchais S, Savouré A, Abdelly C. Effects of exogenous nitric oxide on growth, proline accumulation and antioxidant capacity in Cakile maritima seedlings subjected to water deficit stress. Funct Plant Biol. 2016;43(10):939–948. doi: 10.1071/FP15363. [DOI] [PubMed] [Google Scholar]
- Kaddour R, Draoui E, Baâtour O, Mahmoudi H, Tarchoun I, Nasri N, Gruber M, Lachaâl M. Assessment of salt tolerance of Nasturtium officinale R. Br. using physiological and biochemical parameters. ActaPhysiol Plant. 2013;35(12):3427–3436. doi: 10.1007/s11738-013-1377-8. [DOI] [Google Scholar]
- Kim JW, Minamikawa T. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra) Biosci Biotechnol Biochem. 1997;61(1):118–123. [Google Scholar]
- Kirnak H, Dogan E. Effect of seasonal water stress imposed on drip irrigated second crop watermelon grown in semi-arid climatic conditions. Irrig Sci. 2008;27(2):155. doi: 10.1007/s00271-008-0130-3. [DOI] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54(1):109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005;169(2):323–330. doi: 10.1016/j.plantsci.2005.02.007. [DOI] [Google Scholar]
- Manai J, Kalai T, Gouia H, Corpas FJ. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J Soil Sci Plant Nutr. 2014;14:433–446. [Google Scholar]
- Martínez JP, Silva H, Ledent JF, Pinto M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.) Eur J Agron. 2007;26(1):30–38. doi: 10.1016/j.eja.2006.08.003. [DOI] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Namdjoyan S, Kermanian H, Soorki AA, Tabatabaei SM, Elyasi N. Effects of exogenous salicylic acid and sodium nitroprusside on α-tocopherol and phytochelatin biosynthesis in zinc-stressed safflower plants. Turkish J Bot. 2018;42(3):271–279. [Google Scholar]
- Ouk R, Oi T, Taniguchi M. Three-dimensional anatomy of mesophyll cells in rice leaf tissue by serial section light microscopy. Plant Prod Sci. 2020;23(2):149–159. [Google Scholar]
- Ozkur O, Ozdemir F, Bor M, Turkan I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovataDesf to drought. Environ Exp Bot. 2009;66(3):487–492. doi: 10.1016/j.envexpbot.2009.04.003. [DOI] [Google Scholar]
- Pandey A, Khan MK, Hakki EE, Thomas G, Hamurcu M, Gezgin S, Gizlenci O, Akkaya MS. Assessment of genetic variability for grain nutrients from diverse regions: potential for wheat improvement. SpringerPlus. 2016;5(1):1912. doi: 10.1186/s40064-016-3586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z-B, Liu X, Tian X-J, Yue M. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J PhotochemPhotobiol B. 2008;90(1):17–25. doi: 10.1016/j.jphotobiol.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Quessada MP, Macheix JJ. Characterization of a peroxidase specifically implicated in lignification, in relation with graft incompatibility in the apricot tree. Physiologie Vegetale. 1984;2(5):533–540. [Google Scholar]
- Rao KM, Sresty T. Antioxidative parameters in the seedlings of pigeonpea (Cajanuscajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157(1):113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes (salicylic acid-mediated oxidative damage requires H2O2) Plant Physiol. 1997;115(1):137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Shen W, Ye M, Xu L. Protective effects of nitric oxide on salt stress-induced oxidative damage to wheat (Triticumaestivum L.) leaves. Chin Sci Bull. 2002;47(8):677. doi: 10.1360/02tb9154. [DOI] [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York: Oxford University Press; 1999. [Google Scholar]
- Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin MC. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci. 2002;162(5):825–831. doi: 10.1016/S0168-9452(02)00030-4. [DOI] [Google Scholar]
- Santisree P, Bhatnagar-Mathur P, Sharma KK. NO to drought-multifunctional role of nitric oxide in plant drought: Do we have all the answers? Plant Sci. 2015;239:44–55. doi: 10.1016/j.plantsci.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Silva AM, Martins-Gomes C, Souto EB, Schäfer J, Santos JA, Bunzel M, Nunes FM. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants. 2020;9(6):482. doi: 10.3390/antiox9060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira NM, Hancock JT, Frungillo L, Siasou E, Marcos FCC, Salgado I, Machado EC, Ribeiro RV. Evidence towards the involvement of nitric oxide in drought tolerance of sugarcane. Plant Physiol Biochem. 2017;115:354–359. doi: 10.1016/j.plaphy.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Simontacchi M, Galatro A, Ramos-Artuso F, Santa-María GE. Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Shah K. Evidences for reduced metal-uptake and membrane injury upon application of nitric oxide donor in cadmium stressed rice seedlings. Plant Physiol Biochem. 2014;83:180–184. doi: 10.1016/j.plaphy.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Souri Z, Karimi N, Farooq MA, Sandalio LM. Nitric oxide improves tolerance to arsenic stress in Isatiscappadocicadesv. Shoots by enhancing antioxidant defenses. Chemosphere. 2020;239:124523. doi: 10.1016/j.chemosphere.2019.124523. [DOI] [PubMed] [Google Scholar]
- Sperlich D, Barbeta A, Ogaya R, Sabaté S, Peñuelas J. Balance between carbon gain and loss under long-term drought: impacts on foliar respiration and photosynthesis in Quercus ilex L. J Exp Bot. 2016;67(3):821–833. doi: 10.1093/jxb/erv492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukrong S, Yun K-Y, Stadler P, Kumar C, Facciuolo T, Moffatt BA, Falcone DL. Improved growth and stress tolerance in the Arabidopsis oxt1 mutant triggered by altered adenine metabolism. Molecular Plant. 2012;5(6):1310–1332. doi: 10.1093/mp/sss065. [DOI] [PubMed] [Google Scholar]
- Suyum K, Dasgan H, Sari N, Kusvuran S, Aydoner G, Akyol M, Akhoundnejad Y, Solmaz I, Bol A. (2012) Genotypic variation in the response of watermelon genotypes to salinity and drought stresses. In: Proceedings of the Xth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae. pp 15–18
- Tan Y, Liang Z, Shao H, Du F. Effect of water deficits on the activity of anti-oxidative enzymes and osmoregulation among three different genotypes of Radix astragali at seeding stage. Colloids Surf B. 2006;49(1):60–65. doi: 10.1016/j.colsurfb.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Terzi R, Kadioglu A, Kalaycioglu E, Saglam A. Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J Plant Interact. 2014;9(1):559–565. [Google Scholar]
- Tian X, Lei Y. Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant. 2006;50(4):775–778. doi: 10.1007/s10535-006-0129-7. [DOI] [Google Scholar]
- Türkan İ, Bor M, Özdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168(1):223–231. doi: 10.1016/j.plantsci.2004.07.032. [DOI] [Google Scholar]
- Turner NC, Begg JE. Plant-water relations and adaptation to stress. Plant Soil. 1981;58(1):97–131. doi: 10.1007/BF02180051. [DOI] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. [Google Scholar]
- Wang H, Tang X, Wang H, Shao H-B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-B, Kim Y-H, Lee H-S, Kim K-Y, Deng X-P, Kwak S-S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem. 2009;47(7):570–577. doi: 10.1016/j.plaphy.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang R, Zheng J, Shen Z, Xu X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.) Ecotoxicol Environ Saf. 2019;167:10–19. doi: 10.1016/j.ecoenv.2018.08.064. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li X, Li X, Wei Z, Han M, Zhang L, Li B. Exogenous nitric oxide protects against drought-induced oxidativestress in Malus rootstocks. Turkish J Bot. 2016;40:17–27. doi: 10.3906/bot-1407-31. [DOI] [Google Scholar]