Abstract
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has been proposed as a novel approach to managing non‐traumatic cardiac arrest (NTCA). During cardiac arrest, cardiac output ceases and perfusion of vital organs is compromised. Traditional advanced cardiac life support (ACLS) measures and cardiopulmonary resuscitation are often unable to achieve return of spontaneous circulation (ROSC). During insertion of REBOA a balloon‐tipped catheter is placed into the femoral artery and advanced in a retrograde manner into the aorta while the patient is undergoing cardiopulmonary resuscitation (CPR). The balloon is then inflated to fully occlude the aorta. The literature surrounding the use of aortic occlusion in non‐traumatic cardiac arrest is limited to animal studies, case reports and one recent non‐controlled feasibility trial. In both human and animal studies, preliminary data show that REBOA may improve coronary and cerebral perfusion pressures and key physiologic parameters during cardiac arrest resuscitation, and animal data have demonstrated improved rates of ROSC. Multiple questions remain before REBOA can be considered as an adjunct to ACLS. If demonstrated to be effective clinically, REBOA represents a potentially cost‐effective and generalizable intervention that may improve quality of life for patients with non‐traumatic cardiac arrest.
Keywords: arrhythmias cardiac, cardiopulmonary resuscitation, endovascular procedures, heart arrest, intra‐aortic balloon pumping, resuscitation, therapeutic occlusion
1. INTRODUCTION
Cardiac arrest affects >500,000 adults each year in the United States and fewer than 10% survive to hospital discharge. 1 Despite advances in technology and management of cardiac arrest over the past decades, patient‐centered outcomes have remained frustratingly poor. 1 During non‐traumatic cardiac arrest (NTCA), traditional advanced cardiac life support (ACLS) interventions are frequently unable to achieve return of spontaneous circulation (ROSC). 2 Chest compressions provide only 20%–30% of baseline cardiac output despite proper technique and ideal conditions. 3 The limited blood flow generated during cardiopulmonary resuscitation (CPR) fails to adequately distend the aorta and sufficiently increase aortic diastolic pressure, the principal determinant of coronary perfusion pressure (CPP) and myocardial blood flow.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) was originally developed as a hemorrhage control technique to manage non‐compressible truncal bleeding. It is most frequently employed to temporize the shock state of trauma patients with intra‐abdominal hemorrhage. 4 A balloon‐tipped catheter is inserted into the femoral artery and advanced in a retrograde manner into the aorta. The balloon is then inflated to occlude the aorta to maximize perfusion to organs proximal to the balloon and minimize any distal flow to slow blood loss. 5 REBOA is a lessinvasive alternative to resuscitative thoracotomy and aortic cross‐clamping when used in traumatically injured patients, although the evidence surrounding its efficacy in trauma is mixed and lacking randomized trial data. 6
Aortic occlusion with REBOA may improve myocardial and cerebral blood flow by restricting cardiac output to the thoracic aortic vasculature. Given these advantageous physiologic changes, REBOA has been proposed as a novel intervention for treatment of NTCA. The use of REBOA in conjunction with closed chest CPR, shown in Figure 1, may improve aortic pressure, CPP, and cerebral blood flow. This would effectively increase the efficiency of CPR and would provide emergency physicians with a unique mechanism to optimize conditions for ROSC and neurologically intact survival.
FIGURE 1.
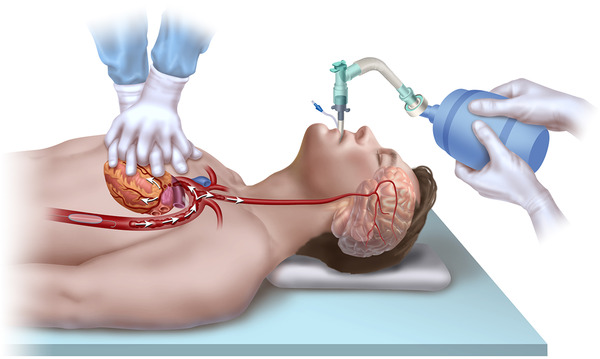
Resuscitative endovascular balloon occlusion of the aorta (REBOA) placement during non‐traumatic cardiac arrest. The REBOA balloon is inflated in Zone 1 of the aorta, causing full aortic occlusion. This redirects the limited cardiac output generated during cardiopulmonary resuscitation towards the heart and brain, generating improved aortic pressure, coronary perfusion pressure, and cerebral perfusion
This article reviews the use of REBOA during NTCA and animal and human data involving REBOA and discusses the future of REBOA for NTCA. The EMBASE and SCOPUS databases were searched for relevant articles published from 1946 to May 2020. Key words used in the search were composed of combinations of “aortic balloon occlusion,” “REBOA,” and “cardiac arrest.” Abstracts were evaluated by the last author and all relevant papers were included in this review. References for each paper identified were scrutinized for any articles involving the use of REBOA in NTCA that were not identified in the initial search. Given the growing experience of REBOA for use in trauma, it provides a relevant analogue for a discussion of NTCA and will be referenced. However, in light of the distinct differences in the epidemiology of pathophysiology of trauma and NTCA, the detailed use of REBOA for traumatically injured patients is outside the scope of this review.
2. CARDIAC ARREST PHYSIOLOGY
Normally, coronary blood flow occurs primarily during diastole and is largely regulated by local tissue oxygen demand and microvascular autoregulatory mechanisms over the physiologic range of aortic pressure. 7 In cardiac arrest states, profound tissue hypoxia results in complete microvascular dilatation and the principal driver of coronary blood flow is the pressure gradient across the coronary vasculature. 8 This CPP is defined as the aortic pressure minus the right atrial pressure. 9 Although an absence of myocardial contractions allows for continuous coronary blood flow, research has shown CPP is greatest during the relaxation phase of chest compressions. 9 , 10 , 11
During NTCA, cardiac output ceases, blood pools within the venous system, and peripheral arteries progressively dilate as CPP drops toward zero. The goal of traditional ACLS interventions is to increase CPP to promote ROSC. Studies have demonstrated that a sustained CPP above 15 mmHg during NTCA is highly predictive of increased rates of ROSC and survival. 9 , 12 , 13 Traditional interventions are often unable to generate a CPP >15 mmHg even under optimal conditions. 14 CPR blood flow is marginal; it can take up to 60 seconds of CPR to generate a positive CPP and 90 seconds to obtain a CPP >15 mmHg. 9 , 15 Furthermore, intravenous epinephrine, recommended to increase CPP, has not yielded improved neurologic outcomes. 16 , 17 Given these shortcomings, NTCA mortality rate has been largely unchanged for decades. 18 The dearth of effective standard therapies, combined with improvements in intravascular device technology, has driven researchers to consider novel therapies such as REBOA as adjuncts to traditional ACLS. 5
3. REBOA DEPLOYMENT DURING CPR
REBOA catheterization can be performed while the chest, head, and upper extremities remain accessible to others to perform CPR and ACLS interventions. 19 The common femoral artery is accessed using point‐of‐care ultrasonography and Seldinger technique. 5 In the hands of an experienced practitioner, REBOA can be deployed by a single physician in approximately 5 to 10 minutes. 20
The REBOA catheter is advanced retrograde into 1 of 3 zones of the aorta: Zone 1, the descending thoracic aorta between the left subclavian artery and the celiac axis; Zone 2, the paravisceral aorta from the celiac axis to the renal arteries; and Zone 3, the infrarenal aorta. 21 Zone 1 placement is currently suggested for NTCA, as shown in Figure 2. The ER‐REBOA catheter (Prytime Medical, Boerne, TX), the most common device currently in use, can be placed without fluoroscopy based upon an estimated distance using markers integrated into the catheter shaft. 22 By contrast, over‐the‐wire catheters, such as the Coda Balloon Catheter (Cook Medical, Bloomington, IN) or REBOA Balloon Kit (Reboa Medical, Norway), are inserted after the depth of a guidewire tip has been confirmed radiographically. 23 , 24 Blind placement technique using anatomic landmarks and estimated distance has been developed for the use of REBOA in trauma patients. 25 , 26 This blind technique is likely adequate for use during NTCA and is currently in use in a Phase 1 clinical trial at Yale University. 27
FIGURE 2.
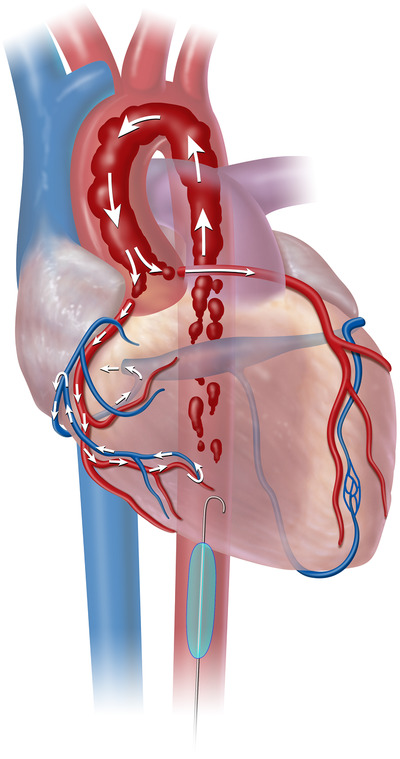
Resuscitative endovascular balloon occlusion of the aorta (REBOA) deployment in aorta Zone 1. Zone 1 aortic occlusion with REBOA allows for the cardiac output generated from cardiopulmonary resuscitation to be directed toward cardiac and cerebral vessels. As shown, higher aortic pressures improves flow to the coronary arteries and raises cerebral perfusion pressure
Once in the desired position the balloon at the tip of the REBOA catheter is inflated with a radiopaque solution, fully occluding the aorta. The radiopaque solution allows physicians to confirm REBOA placement on fluoroscopy or plain film imaging, although confirmation may be possible using ultrasound. 28 , 29 If ROSC is obtained the balloon can be deflated with the REBOA catheter remaining in place. After the catheter is removed, the arterial introducer sheath may be used for arterial pressure monitoring and cardiac catheterization or upsized for extracorporeal membrane oxygenation (ECMO). For large REBOA catheters (>8 Fr), vascular repair may be required once removed, although this is much less likely for smaller catheters. 30
4. REBOA IN ANIMAL NON‐TRAUMATIC CARDIAC ARREST
The majority of REBOA research is performed in swine models of ventricular fibrillation, as swine cardiac and aortic anatomy is similar to humans. 31 The hemodynamic benefits of REBOA in CPR were first outlined over 20 years ago. 32 , 33 Multiple studies have shown thoracic aortic occlusion improves cerebral and coronary perfusion pressures, coronary blood flow, end‐tidal carbon dioxide, and overall mortality. 32 , 34 , 35 , 36 , 37 , 38 , 39 In one notable study of adult swine, during alternating periods of intra‐aortic balloon inflation and deflation, balloon inflation increased CPP by an average of 13.7 mmHg (60%) and coronary blood flow increased by 15.5 mL/min (8.5%). 35 Of note, there is substantial variance in response to REBOA during cardiac arrest despite laboratory models attempting to control for factors that influence hemodynamics during CPR.
Traditionally animal studies have utilized Zone 1 placement of REBOA, as a more proximal level of occlusion would theoretically result in maximal hemodynamic benefit. Recent animal studies have further divided Zone 1 into 3 subzones (a‐c). Occlusion in Zone 1c (at the level of the diaphragm) augmented proximal arterial pressures and reduced arterial lactate concentrations better than occlusion in Zone 1b (at the level of the heart). 40 This may be because of impaired ventricular expansion due to the location of the balloon directly deep to the heart, although it is unclear if this finding will translate to human physiology.
Two recent studies published in 2020 have demonstrated improved proximal physiology with Zone 1 placement when compared to Zone 3 placement. 41 , 42 Although Zone 3 occlusion would benefit visceral perfusion and possibly prevent ischemic injury, any hemodynamic benefit was transient or did not translate to improved outcomes. 41 , 42
5. REBOA IN HUMAN NON‐TRAUMATIC CARDIAC ARREST
The literature surrounding the use of aortic occlusion in human patients is limited to case reports and one recent non‐controlled feasibility trial. 19 The first description of aortic occlusion, from Deakin et al in 1996, describes 2 patients who suffered cardiac arrests with an intra‐aortic balloon pump in place. Both patients underwent aortic occlusion through the continuous inflation of the intra‐aortic balloon pump and had immediate increases in systolic, diastolic, and coronary perfusion pressure. Neither patient survived. 43 The generalizability of this report is limited as one patient had infective endocarditis and the other was immediately post coronary artery bypass graft.
Thirteen years later in 2009, Aslanger et al described a case that may be more representative of the majority of NTCA patients. A 74‐year‐old female was taken to the cardiac catheterization laboratory for an acute myocardial infarction and suffered from a cardiac arrest during the procedure. 44 After 25 minutes of traditional ACLS, she underwent aortic occlusion with an intra‐aortic balloon pump while in asystole, followed by sustained ROSC 1 minute later. She was discharged from the hospital eventually and made a “good recovery.” 44
In June 2019, Coniglio et al reported on the first case where a REBOA catheter was used in the emergency department for NTCA as a bridge therapy to an intra‐aortic balloon pump. 45 Following aortic balloon inflation in the ED, the patient's ETCO2 increased from 8–20 mmHg, hemodynamics stabilized, and sustained ROSC was achieved. The REBOA catheter was replaced with an intra‐aortic balloon pump. Unfortunately, the patient developed a post‐cardiac arrest coagulopathy and ultimately died. However, this report demonstrates the potential effectiveness of REBOA in NTCA to increase coronary perfusion and act as a bridge to a more definitive intra‐vascular therapy. In November 2019, a Norwegian group reported out‐of‐hospital deployment of REBOA in 10 NTCA subjects treated by helicopter emergency medical services. 19 Femoral artery cannulation for REBOA was successful on first attempt in 8 of 10 patients and on second attempt in the 2 remaining patients. The cohort consisted of 7 men and 3 women aged 50–74 years old. Mean time from dispatch to balloon occlusion was 45.6 minutes, procedural time ranged from 8–16 minutes, and ETCO2 increased a mean of 13 mmHg after 60 seconds of occlusion (P < 0.001). Similar to laboratory studies, the graph of ETCO2 measurements for individual patients showed substantial variability with some patients having large increases in ETCO2 whereas others showed little effect. This is the first report to demonstrate that REBOA is temporally associated with improvements in CPR quality as measured by ETCO2. Six of the 10 enrolled patients achieved ROSC (60%), 3 patients (30%) were admitted to the hospital, and 1 survived to hospital discharge with good neurologic outcome. In the patient who survived with a favorable neurologic outcome, investigators did not achieve aortic occlusion until 60 minutes into his asystolic NTCA. REBOA was again used as a rapidly deployable bridge therapy to a more definitive intervention and was converted to ECMO later in the patient's course.
Emergency medicine investigators at Yale University are currently enrolling subjects in the first United States Phase I clinical trial of REBOA in ou‐of‐hospital NTCA patients presenting to the ED. 27 Investigators will measure real‐time diastolic blood pressure and patients will have no‐flow times that are more typical of those seen in urban populations. Two of 5 initial subjects have been enrolled at the time of this writing, each with immediate and sustained improvements in ETCO2 and mean arterial blood pressure and transient ROSC in one. Despite this, both patients were in ventricular fibrillation that was refractory to antiarrhythmics and defibrillation and did not survive to hospital admission.
6. CHALLENGES AND UNKNOWNS
REBOA placement carries a risk of significant vascular and ischemic injury, even in the hands of experienced physicians. In a large prospective trial of REBOA for traumatically injured patients at level 1 trauma centers in the United States, outcomes were favorable. 20 Aortic occlusion was obtained in an average of 6.6 minutes, there was a low complication rate (2.2% pseudoaneurysm and 4.3% distal embolism), and REBOA had a similar survival rate to more invasive surgical techniques. 20 Several additional retrospective trials have shown improvement in mortality with REBOA with low complication rates. 6 , 46 , 47
Other large retrospective trials have shown poorer outcomes with REBOA. In 2019 Bellal et al published a large, multicentered, retrospective analysis of case‐matched outcomes involving REBOA in traumatically injured patients. 48 They found increased overall mortality (REBOA 35%, no REBOA 18.9%), increased risk of acute kidney injury (REBOA 10.7%, no REBOA 3.2%), and higher rates of lower extremity amputation (REBOA 3.6%, no REBOA 0.7%). There were no significant differences in hospital length of stay, intensive care unit length of stay, or transfusion requirements at 4 or 24 hours. 48 Furthermore, in 2 large retrospective reviews of Japanese trauma literature from 2015 and 2016, mortality was higher for the patients who received REBOA. 49 , 50 Given the vast differences between trauma patients and those with NTCA, it remains difficult to clinically contextualize the results of these studies until researchers gain more experience with this patient population.
A recent case report from Gerard et al details the complication of REBOA balloon rupture after the initiation of CPR. 51 It is unknown if balloon rupture is caused by damage during insertion, would be worse with mechanical CPR devices, or if zone placement (1a‐1c) affects balloon rupture. The true frequency of balloon rupture during CPR is unknown but likely is low, as it is not reported elsewhere in the literature.
Currently, there is no evidence‐based guideline to inform balloon deflation. The accepted practice during use for hemorrhage control, which is based on expert opinion, is to deflate the balloon as tolerated by proximal aortic physiology. A similar approach should be rapidly followed when ROSC is obtained, in order to limit potentially deleterious increases in cardiac afterload on an already stunned myocardium. A variety of procedures have been used in animal models, involving reducing the balloon slowly over a period of 30 to 60 seconds. 52
The variability in response to REBOA during cardiac arrest, seen in both laboratory studies and the Norwegian out‐of‐hospital clinical study, does not presently have a clear explanation. REBOA may augment CPP during cardiac arrest, but the primary driver of blood flow is providing high‐quality chest compressions. Factors, such as downtime before CPR, body habitus, CPR quality, volume status, and upper body peripheral arterial vasomotor tone, may significantly influence the effect of REBOA during CPR. This variance and its effect on outcomes need further investigation.
7. THE FUTURE OF CARDIAC ARREST
REBOA is being investigated to treat NTCA along with other advanced technologies, including selective aortic arch perfusion (SAAP) and ECMO. SAAP utilizes a large‐lumen balloon catheter to occlude the thoracic aorta and provide heart and brain perfusion during cardiac arrest. 53 , 54 Although the SAAP catheter, like REBOA catheter, is inserted via a femoral artery to the thoracic aorta, SAAP is primarily an extracorporeal perfusion intervention. After aortic occlusion is obtained, an external pump infuses oxygenated perfusate into the aortic arch in to improve oxygen delivery to critical organs. Thus, SAAP has more in common with ECMO than REBOA. Aortic occlusion with SAAP is primarily to direct perfusate flow preferentially to the heart and brain to promote ROSC and favorable neurological recovery, respectively. Laboratory models of both ventricular fibrillation and hemorrhage‐induced cardiac arrest have shown high rates of ROSC. 53 SAAP is presently undergoing regulatory review for clinical trial initiation.
ECMO has been used to treat refractory cardiac arrest since the 1980s. 55 Although ECMO is primarily used in the pediatric population, its use for extracorporeal cardiopulmonary resuscitation (ECPR) has expanded in recent years. In a large retrospective trial of ECMO in NTCA, survival to hospital discharge was an impressive 29%. 56 Although these results are promising, none of the studies involving ECMO in NTCA have utilized a prospective control group, making it difficult to determine the efficacy of this intervention. ECMO is resource intensive, associated with significant cost and requires multispecialty expertise for placement and management. 57 Recent literature suggests ECMO may be cost effective, 58 but it is not yet generalizable to most community EDs where the majority of cardiac arrests are treated. REBOA, by contrast, is lower cost and requires a skillset already mastered by emergency physicians. As demonstrated by Brede and Coniglio et al, REBOA may have a role in bridging patients to more advanced interventions such as ECMO, 19 an intra‐aortic balloon pump, 45 or cardiac catheterization.
8. CONCLUSION
REBOA is a novel technique for the management of NTCA. Work with animal models has shown that REBOA placement improves CPP and cerebral perfusion pressure and leads to increased rates of ROSC. Early feasibility human trials have shown promise and Phase 1 trials for REBOA in NTCA are currently underway. Unlike more resource‐intensive interventions, REBOA is relatively low cost and requires a technical skillset that most emergency physicians have already mastered, making its widespread dissemination more feasible. REBOA for NTCA remains in its infancy and numerous questions remain about its feasibility, efficacy, and safety. If aortic occlusion with REBOA for NTCA is demonstrated to be effective in humans, the potential for change in clinical practice and improvement in quality of life is substantial.
AUTHOR CONTRIBUTIONS
Study concept and design (Craig D. Nowadly, M. Austin Johnson, James I. Daley); drafting of the manuscript (Craig D. Nowadly, M. Austin Johnson, Guillaume L. Hoareau, James E Manning,James I. Daley); critical revision of the manuscript (Craig D. Nowadly, M. Austin Johnson, Guillaume L. Hoareau, James E Manning, James I. Daley); and approval of final manuscript (Craig D. Nowadly, M. Austin Johnson, Guillaume L. Hoareau, James E Manning, James I. Daley).
CONFLICTS OF INTEREST
Craig D. Nowadly. reports no conflict of interest. M. Austin Johnson. is a founder and stockholder of Certus Critical Care, Inc. Guillaume L. Hoareau. reports no conflict of interest. James E. Manning. is a co‐founder and stockholder of Resusitech, Inc. James I. Daley has received grant funding from Prytime Medical Devices Inc. and the American Heart Association.
DISCLAIMERS
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, or the Department of the Air Force.
ACKNOWLEDGMENTS
None.
Nowadly CD, Johnson MA, Hoareau GL, Manning JE, Daley JI. The use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for non‐traumatic cardiac arrest: A review. JACEP Open. 2020;1:737–743. 10.1002/emp2.12241
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Presentations: The information has not previously been presented and is not under consideration with any other journal at the time of submission.
Supervising Editor: Marna Rayl Greenberg, DO, MPH.
REFERENCES
- 1. Jentzer JC, Clements CM, Wright RS, et al. Improving survival from cardiac arrest: a review of contemporary practice and challenges. Ann Emerg Med. 2016;68(6):678‐689. [DOI] [PubMed] [Google Scholar]
- 2. Sanghavi P, Jena AB, Newhouse JP, Zaslavsky AM. Outcomes of basic versus advanced life support for out‐of‐hospital medical emergencies. Ann Intern Med. 2015;163(9):681‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang L, Zhang J. Mechanical cardiopulmonary resuscitation for patients with cardiac arrest. World J Emerg Med. 2011;2(3):165‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes CW. Use of an intra‐aortic balloon catheter tamponade for controlling intra‐abdominal hemorrhage in man. Surgery. 1954;36(1):65‐68. [PubMed] [Google Scholar]
- 5. Daley J, Morrison JJ, Sather J, et al. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non‐traumatic cardiac arrest. Am J Emerg Med. 2017;35(5):731‐736. [DOI] [PubMed] [Google Scholar]
- 6. Brenner M, Inaba K, Aiolfi A, et al. Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the American Association for the Surgery of Trauma's aortic occlusion in resuscitation for trauma and acute care surgery registry. J Am Coll Surg. 2018;226(5):730‐740. [DOI] [PubMed] [Google Scholar]
- 7. Ramanathan T, Skinner H. Coronary blood flow. Anaesth Crit Care Pa. 2005;5(2):61‐64. [Google Scholar]
- 8. Fries M, Tang W, Chang Y‐T, et al. Microvascular blood flow during cardiopulmonary resuscitation is predictive of outcome. Resuscitation. 2006;71(2):248‐253. [DOI] [PubMed] [Google Scholar]
- 9. Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106‐1113. [PubMed] [Google Scholar]
- 10. Niemann JT, Rosborough JP, Ung S, et al. Coronary perfusion pressure during experimental cardiopulmonary resuscitation. Ann Emerg Med. 1982;11(3):127‐131. [DOI] [PubMed] [Google Scholar]
- 11. Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3(1):11‐14. [DOI] [PubMed] [Google Scholar]
- 12. Naim MY, Sutton RM, Friess SH, et al. Blood pressure‐ and coronary perfusion pressure‐targeted cardiopulmonary resuscitation improves 24‐hour survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2016;44(11):e1111‐e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kern KB. Coronary perfusion pressure during cardiopulmonary resuscitation. Best Pract Res Clin Anaesthesiol. 2000;14(3):591‐609. [Google Scholar]
- 15. Steen S, Liao Q, Pierre L, Paskevicius A, Sjöberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation. 2003;58(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 16. Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out‐of‐hospital cardiac arrest. N Engl J Med. 2018;379(8):711‐721. [DOI] [PubMed] [Google Scholar]
- 17. Long B, Koyfman A. Emergency medicine myths: epinephrine in cardiac arrest. J Emerg Med. 2017;52(6):809‐814. [DOI] [PubMed] [Google Scholar]
- 18. Chan PS, McNally B, Tang F, Kellermann A, CARES Surveillance Group . Recent trends in survival from out‐of‐hospital cardiac arrest in the United States. Circulation. 2014;130(21):1876‐1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brede JR, Lafrenz T, Klepstad P, et al. Feasibility of pre‐hospital resuscitative endovascular balloon occlusion of the aorta in non‐traumatic out‐of‐hospital cardiac arrest. J Am Heart Assoc. 2019;8(22):e014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DuBose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409‐419. [DOI] [PubMed] [Google Scholar]
- 21. Beyer CA, Hoareau GL, Tibbits EM, et al. Resuscitative endovascular balloon occlusion of the aorta induced myocardial injury is mitigated by endovascular variable aortic control. J Trauma Acute Care Surg. 2019;87(3):590. [DOI] [PubMed] [Google Scholar]
- 22. Prytime Medical. ER‐REBOA Catheter Instructions for Use. Published online January 2019. https://prytimemedical.com/wp-content/uploads/2019/02/ER-REBOA-IFU-US-and-Canada-Rev-G.pdf
- 23. Cook Medical. CODA and CODA LP Balloon Catheter Instructions for Use. https://www.cookmedical.com/data/IFU_PDF/T_CODALP_REV3.PDF
- 24. Reboa Medical. Reboa Balloon Kit. https://reboamedical.com/wp-content/uploads/2020/05/reboa_balloon_kit_brochure.png
- 25. Hilbert‐Carius P, McGreevy DT, Abu‐Zidan FM, et al. Pre‐hospital CPR and early REBOA in trauma patients — results from the ABOTrauma Registry. World J Emerg Surg. 2020;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGreevy DT, Abu‐Zidan FM, Sadeghi M, et al. Feasibility and clinical outcome of REBOA in patients with impending traumatic cardiac arrest. Shock. 2019;58(6):e831‐e832. [DOI] [PubMed] [Google Scholar]
- 27. Daley JI. The Use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct to advanced cardiac life support in non‐traumatic cardiac arrest: an early feasibility trial. clinicaltrials.gov; 2020. https://clinicaltrials.gov/ct2/show/NCT03703453 [Google Scholar]
- 28. Chaudery M, Clark J, Morrison JJ, et al. Can contrast‐enhanced ultrasonography improve Zone III REBOA placement for prehospital care?. J Trauma Acute Care Surg. 2016;80(1):89‐94. [DOI] [PubMed] [Google Scholar]
- 29. Guliani S, Amendola M, Strife B, et al. Central aortic wire confirmation for emergent endovascular procedures: as fast as surgeon‐performed ultrasound. J Trauma Acute Care Surg. 2015;79(4):549‐554. [DOI] [PubMed] [Google Scholar]
- 30. Foerster CR, Turgulov A. Prehospital endovascular occlusion of the aorta is now a technically feasible strategy for improving haemodynamics in CPR. Resuscitation. 2015;93:e25 10.1016/j.resuscitation.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 31. Crick SJ, Sheppard MN, Ho S, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193(Pt 1):105‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40(3):171‐180. [DOI] [PubMed] [Google Scholar]
- 33. Tang W, Weil MH, Noc M, et al. Augmented efficacy of external CPR by intermittent occlusion of the ascending aorta. Circulation. 1993;88(4 Pt 1):1916‐1921. [DOI] [PubMed] [Google Scholar]
- 34. Sesma J, Sara MJ, Espila JL, et al. Effect of intra‐aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20(5):453‐462. [DOI] [PubMed] [Google Scholar]
- 35. Tiba MH, McCracken BM, Cummings BC, et al. Use of resuscitative balloon occlusion of the aorta in a swine model of prolonged cardiac arrest. Resuscitation. 2019;140:106‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rubertsson S, Bircher NG, Alexander H. Effects of intra‐aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med. 1997;25(6):1003‐1009. [DOI] [PubMed] [Google Scholar]
- 37. Suzuki A, Taki K, Takeshima N. Experimental study on cardiac resuscitation—effectiveness of thoracic aorta occlusion. Masui. 1980;29(7):677‐682. [PubMed] [Google Scholar]
- 38. Spence PA, Lust RM, Chitwood WR, et al. Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res. 1990;49(3):217‐221. [DOI] [PubMed] [Google Scholar]
- 39. Wesley RCJ, Morgan DB. Effect of continuous intra‐aortic balloon inflation in canine open chest cardiopulmonary resuscitation. Crit Care Med. 1990;18(6):630‐633. [DOI] [PubMed] [Google Scholar]
- 40. Dogan EM, Beskow L, Calais F, et al. Resuscitative endovascular balloon occlusion of the aorta in experimental cardiopulmonary resuscitation: aortic occlusion level matters. Shock. 2019;52(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dogan EM, Hörer TM, Edström M, et al. Resuscitative endovascular balloon occlusion of the aorta in zone I versus zone III in a porcine model of non‐traumatic cardiac arrest and cardiopulmonary resuscitation: a randomized study. Resuscitation. 2020;151:150‐156. [DOI] [PubMed] [Google Scholar]
- 42. Nowadly CD, Hoareau GL, Grayson JK, Johnson MA. Zone 3 REBOA does not provide hemodynamic benefits during nontraumatic cardiac arrest. Am J Emerg Med. 2020;38(9):1915‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deakin CD, Barron DJ. Haemodynamic effects of descending aortic occlusion during cardiopulmonary resuscitation. Resuscitation. 1996;33(1):49‐52. [DOI] [PubMed] [Google Scholar]
- 44. Aslanger E, Golcuk E, Oflaz H, et al. Intraaortic balloon occlusion during refractory cardiac arrest. A case report. Resuscitation. 2009;80(2):281‐283. [DOI] [PubMed] [Google Scholar]
- 45. Coniglio C, Gamberini L, Lupi C, et al. Resuscitative endovascular balloon occlusion of the aorta for refractory out‐of‐hospital non‐traumatic cardiac arrest—a case report. Prehosp Disaster Med. 2019;34(5):566‐568. [DOI] [PubMed] [Google Scholar]
- 46. García AF, Manzano‐Nunez R, Orlas CP, et al. Association of resuscitative endovascular balloon occlusion of the aorta (REBOA) and mortality in penetrating trauma patients. Eur J Trauma Emerg Surg. 2020. 10.1007/s00068-020-01370-9. [DOI] [PubMed] [Google Scholar]
- 47. Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523‐530. discussion 530–532. [DOI] [PubMed] [Google Scholar]
- 48. Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154(6):500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Inoue J, Shiraishi A, Yoshiyuki A, et al. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J Trauma Acute Care Surg. 2016;80(4):559‐566. [DOI] [PubMed] [Google Scholar]
- 50. Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score‐adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721‐728. [DOI] [PubMed] [Google Scholar]
- 51. Gerard J, Skertich NJ, Wiegmann A, Bokhari F. REBOA Catheter Balloon Rupture during CPR. Am Surg. 2020;86(4):e196‐e197. [PubMed] [Google Scholar]
- 52. Davidson AJ, Russo RM, Ferencz S‐AE, et al. Incremental balloon deflation following complete resuscitative endovascular balloon occlusion of the aorta results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J Trauma Acute Care Surg. 2017;83(1):139‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manning JE, Murphy CA, Hertz CM, et al. Selective aortic arch perfusion during cardiac arrest: a new resuscitation technique. Ann Emerg Med. 1992;21(9):1058‐1065. [DOI] [PubMed] [Google Scholar]
- 54. Barnard EG, Manning JE, Smith JE, et al. A comparison of selective aortic arch perfusion and resuscitative endovascular balloon occlusion of the aorta for the management of hemorrhage‐induced traumatic cardiac arrest: a translational model in large swine. PLoS Med. 2017;14(7):e1002349 10.1371/journal.pmed.1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. del Nido PJ, Dalton HJ, Thompson AE, Siewers RD. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86(5 Suppl):II300‐304. [PubMed] [Google Scholar]
- 56. Richardson ASC, Schmidt M, Bailey M, et al. ECMO Cardio‐Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12‐years. Resuscitation. 2017;112:34‐40. [DOI] [PubMed] [Google Scholar]
- 57. Harvey MJ, Gaies MG, Prosser LA. U.S. and international in‐hospital costs of extracorporeal membrane oxygenation: a systematic review. Appl Health Econ Health Policy. 2015;13(4):341‐357. [DOI] [PubMed] [Google Scholar]
- 58. Dennis M, Zmudzki F, Burns B, et al. Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation. 2019;139:49‐56. [DOI] [PubMed] [Google Scholar]


