Abstract
Sepsis, defined as an infection with dysregulated host response leading to life‐threatening organ dysfunction, continues to carry a high potential for morbidity and mortality in children. The recognition of sepsis in children in the emergency department (ED) can be challenging, related to the high prevalence of common febrile infections, poor specificity of discriminating features, and the capacity of children to compensate until advanced stages of shock. Sepsis outcomes are strongly dependent on the timeliness of recognition and treatment, which has led to the successful implementation of quality improvement programs, increasing the reliability of sepsis treatment in many US institutions. We review clinical, laboratory, and technical modalities that can be incorporated into ED practice to facilitate the recognition, treatment, and reassessment of children with suspected sepsis. The 2020 updated pediatric sepsis guidelines are reviewed and framed in the context of ED interventions, including guidelines for antibiotic administration, fluid resuscitation, and the use of vasoactive agents. Despite a large body of literature on pediatric sepsis epidemiology in recent years, the evidence base for treatment and management components remains limited, implying an urgent need for large trials in this field. In conclusion, although the burden and impact of pediatric sepsis remains substantial, progress in our understanding of the disease and its management have led to revised guidelines and the available data emphasizes the importance of local quality improvement programs.
Keywords: antibiotic management, fluid resuscitation, pediatric, sepsis, sepsis risk factors, septic shock, severe sepsis
1. INTRODUCTION AND EPIDEMIOLOGY
Sepsis contributes to 19% of all deaths globally, with the highest age‐specific incidence in children younger than 5 years of age. 1 , 2 Pediatric sepsis resulted in 0.7% of all hospital encounters, with an incidence of 2.8% in inpatients in the United States. 3 Epidemiologic studies using clinical data have found an incidence of pediatric sepsis in up to 8% of all pediatric intensive care unit (PICU) admissions, 4 contributing to 1 in 4 deaths in PICUs. 5 Accurate estimates of pediatric sepsis are hampered by inaccuracies of diagnostic coding, resulting in gross underreporting; at the same time, increasing sepsis awareness may lead to identification earlier in the disease process, with resultant increases in apparent survival rates. 6 To address this, novel sepsis surveillance criteria derived from electronic health record data have been proposed. 7 , 8
Risk factors for sepsis and the most common sites and pathogens associated with sepsis are described in Tables 1, 9 , 10 , 11 and 2, 3 , 10 , 11 respectively. In 1 US multicenter study, the most common pathogens affecting previously healthy children were Staphylococcus aureus (9.4%), streptococcal species (7.9%), and Escherichia coli (7.1%), whereas the most common pathogens in children with chronic diseases were S. aureus (11%), Candida (9.8%), and Pseudomonas (8.1%). 12 Similar patterns have been observed in recent population‐based studies in other countries and contrast with the predominance of meningococcal infections observed in previous decades. 5 , 13 More than one‐third of children with sepsis do not have an identifiable pathogen. This may be attributed to sepsis being caused by viral etiologies or because of the limits in detection of bacterial pathogens, particularly if the volume inoculated into blood cultures is low or if the pathogens are fastidious or have specific growth requirements. Table 3 summarizes the most common pathogens by site of infection in non‐resource‐limited settings. 12
TABLE 1.
Most common comorbidities in children with sepsis in non‐resource‐limited settings
| Condition | Prevalence range (%) a |
|---|---|
| Central venous catheter | 31 |
| Congenital heart disease | 7–27 |
| Neurologic | 9–26 |
| Oncologic diagnosis | 11–17 |
| Metabolic disorder | 3–13 |
| Respiratory (including ventilator dependence) | 5–7 |
| Congenital or acquired immune deficiency | 4–7 |
| Renal | 2–6 |
| Gastrointestinal | 4–5 |
| Solid organ transplant | 4 |
| Dialysis dependence | 3 |
| Bone marrow transplantation | 3 |
Sum >100% as ranges compiled from various studies and patients may have had multiple comorbidities.
TABLE 2.
Most common sites of infection and pathogens in sepsis a
| Site | Prevalence range (%) |
|---|---|
| Respiratory | 19–57 |
| Bacteremia (primary) | 19–68 |
| Abdominal | 8 |
| Central nervous system | 4–23 |
| Genitourinary | 4–22 |
| Skin | 4–3 |
| Pathogens | Range (%) |
|---|---|
| No pathogen identified | 35–57 |
| Gram‐negative bacteria | 12–28 |
| Gram‐positive bacteria | 16–30 |
| Other bacteria | 0.4–0.7 |
| Fungal infections | 4–13 |
| Viral infections | 11–21 |
Sum >100% as ranges compiled from various studies and patients may have had multiple sites of infections or polymicrobial infections.
TABLE 3.
Most common pathogens by site of infection in children with sepsis
| Organism | Bacteremia (%) | CNS (%) | UTI (%) | SSTI (%) | Pneumonia (%) | Osteomyelitis (%) |
|---|---|---|---|---|---|---|
| S. aureus | 19 | 12 | 6 | 30 | 15 | 51 |
| S. pneumoniae | 2 | 9 | 1 | 0.2 | 4 | 1 |
| Other Gram‐positives | 28 | 25 | 9 | 11 | 6 | 16 |
| K. pneumoniae | 8 | 2 | 5 | 1 | 3 | 2 |
| E. coli | 11 | 2 | 23 | 2 | 5 | 3 |
| H. influenzae | 1 | 3 | 0.3 | 0.4 | 4 | 1 |
| Pseudomonas | 7 | 2 | 5 | 4 | 13 | 3 |
| Other Gram‐negatives | 13 | 9 | 6 | 3 | 10 | 2 |
| Candida | 9 | 9 | 5 | 7 | 7 | 5 |
| Aspergillus | 0.4 | 1 | 0.2 | 0.5 | 0.3 | 0 |
| No identifiable pathogen | N/A | 21 | 36 | 37 | 31 | 15 |
2. DEFINITIONS
Historically, the term sepsis (from Greek “sepsin,” meaning “rot, make putrid”) has been used to characterize life‐threatening infections usually caused by bacterial pathogens if untreated progress to shock and death. 14 The 2005 International Pediatric Sepsis Definition Consensus Conference classified sepsis as infection in presence of systemic inflammatory response syndrome (SIRS), severe sepsis as sepsis in the presence of organ dysfunction, and septic shock as sepsis in the presence of cardiovascular dysfunction (Table 4). 15 , 16 , 17 Although SIRS criteria have been used in many EDs to assist in the recognition of children who may have sepsis, SIRS vital sign criteria are poorly sensitive in identifying critically ill children. 18 , 19 Recently, definitions of sepsis in adults have changed, with the 2016 Sepsis‐3 consensus conference defining sepsis as infection with dysregulated host response resulting in life‐threatening organ dysfunction and septic shock as sepsis with profound circulatory, cellular, or metabolic alterations associated with substantially higher mortality. 16 There is a requirement of hypotension to meet adult shock criteria, contrary to the pediatric goal of identifying compensated shock, given that hypotension is a late‐stage finding for children. We refer to sepsis as sepsis‐associated organ dysfunction and septic shock as sepsis with cardiovascular dysfunction (including hypotension, need for treatment with vasoactive agents, or impaired perfusion) 17 in this review.
TABLE 4.
Approaches to defining sepsis in children
| Term | Term | Definition |
|---|---|---|
| 2005 International Pediatric Sepsis Definition Consensus conference | SIRS |
Meets ≥2 of the following criteria, 1 of which must be temperature or WBC count: Pyrexia (>38.5°C) or hypothermia (<36°C) Age‐dependent tachycardia or bradycardia Tachypnea or need for mechanical ventilation Abnormal WBC count or >10% bands |
| Sepsis |
SIRS and Suspected or confirmed infection |
|
| Severe sepsis |
Sepsis and Cardiovascular dysfunction, respiratory dysfunction, or ≥2 non‐cardiorespiratory organ system dysfunctions |
|
| Septic shock |
Sepsis and Cardiovascular dysfunction: defined as either hypotension, receipt of vasoactive medication, or impaired perfusion despite fluid resuscitation |
|
| Sepsis‐3 (adults) | Sepsis |
Suspected or confirmed infection and Presence of organ dysfunction (measured by SOFA score or qSOFA score increase in ≥2 points) |
| Septic shock |
Suspected or confirmed infection and Cardiovascular dysfunction defined as hypotension despite fluid resuscitation requiring vasoactive medication in presence of hyperlactatemia |
|
| Operationalization for the 2020 Pediatric surviving sepsis campaign | Sepsis |
Suspected or confirmed infection and Sepsis‐associated organ dysfunction or septic shock |
Note. Adapted from Goldstein et al,15 Shankar‐Hari et al,16 and Weiss et al.17
Abbreviations: qSOFA, quick SOFA; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment.
3. SEPSIS BUNDLES AND QUALITY IMPROVEMENT INITIATIVES
Failure to recognize sepsis increases the risk of morbidity and mortality, as illustrated by the case of Rory Staunton, a 12‐year‐old boy who died of initially unrecognized streptococcal sepsis. 20 A year after Rory's death, “Rory's Regulations” were passed, 21 requiring all New York hospitals to implement evidence‐based protocols to facilitate early recognition of sepsis through screening tools, identify individuals who qualified for sepsis care, and implement guidelines for sepsis management through bundled care, consisting of protocols to assist in sepsis recognition and subsequently prompt initiation of fluid resuscitation, parenteral antibiotics, and obtaining blood cultures. Multiple studies demonstrated an improvement in in‐hospital mortality rates after bundled sepsis care in both children 22 and adults. 23 In addition to legislative efforts, US children's hospitals have collaborated to enhance multicenter quality improvement activities in pediatric sepsis. The largest of these efforts is the ongoing Improving Pediatric Sepsis Outcomes Collaborative through the Children's Hospital Association and involves >50 hospitals across the United States. 24
The benefits of early recognition, treatment, and reversal of shock using bundled sepsis care extend beyond reductions in mortality. 22 , 23 , 25 Multiple pediatric studies have demonstrated decreased hospital length of stay after implementation of bundled care 26 , 27 and reductions in the rates of acute kidney injury. 28 Delays in receipt of antimicrobial therapy are associated with increased mortality, with children who experienced antibiotic delays of more than 3 hours having an almost 4‐fold risk of mortality in the PICU. 29 Other studies have found that each additional hour of persistent shock is associated with a >2‐fold increased odds of mortality. 30 Ultimately, many pediatric studies have evaluated the relationship between the timing of antibiotic administration and fluid resuscitation through protocol‐driven care and patient‐level outcomes, 22 , 25 , 26 , 27 , 28 driving ongoing revisions to institutional, national, and international guidelines.
4. DIAGNOSIS
Early diagnosis is critical to reversing shock. Barriers to recognition include age‐related variation in vital signs (resulting in failure to recognize abnormal vital signs), hypotension being a late manifestation, a relatively low prevalence of pediatric sepsis in EDs in high‐income countries, and alternative explanations for abnormal vital signs (fever or crying contributing to tachycardia or tachypnea). There are also associated challenges in identifying which children meeting SIRS criteria may be at risk for sepsis. As such, we review triage‐based and laboratory‐based tools that may optimize sepsis recognition.
4.1. Triage‐based recognition
Rapid, systematic identification of children with sepsis is paramount to initiating timely interventions. Without this critical step, delays in care may impact morbidity and mortality. In 2017, the American College of Critical Care Medicine (ACCCM) on Pediatric and Neonatal Septic Shock emphasized the importance of triggers or screening tools to rapidly identify patients with septic shock. 31 In 2020, the first Pediatric Surviving Sepsis Campaign (SSC) international guidelines extended the goal of identification to non‐cardiovascular dysfunction. 17
The clinical signs of those at risk for pediatric sepsis are largely based on the presence of suspected infection and abnormal physical examination findings such as perfusion abnormalities, altered mental status, hypotension, temperature abnormality (fever or hypothermia), and tachycardia. 31 , 32 A pragmatic screen/alert should be based on these readily identifiable and available parameters. Incorporating a sepsis screen into the triage process is efficient and identifies most patients with sepsis. 25 In 1 ED study, moving from a paper‐based to an electronic health record process significantly decreased time to recognition. 33
The significant mortality and morbidity associated with pediatric sepsis drives the inherent balance of sensitivity versus specificity of all screening models to favor sensitivity at the cost of specificity. However, this results in a low positive predictive value, and balancing measures related to false positive alerts and the potential for alert fatigue need to be considered. Design should consider institution‐specific characteristics (ie, tertiary/quaternary vs rural or community setting, acuity, healthcare workers awareness/sensitivity to sepsis) 17 , 34 and can include temperature‐corrected heart and/or respiratory rate, 35 , 36 age‐based vital sign adjustments, 33 , 36 , 37 , 38 , 39 and the inclusion of high‐risk conditions. 25 , 33 , 37
Some of the earliest adopters of sepsis screening in pediatric EDs primarily leveraged vital sign abnormalities to identify patients with possible sepsis (Table 5). 35 , 36 , 37 , 38 , 39 , 40 An ideal pediatric sepsis screening process should be efficient, initiated at first contact with the patient, incorporate reassessment/identification throughout the visit, and harness the strength of the electronic health record. The screen should incorporate pertinent vital sign and clinical parameters, recognize children with high‐risk conditions, involve a care team huddle/bedside assessment, and be monitored by a dedicated quality improvement team. Because clinician assessment can significantly improve screening performance, a 2‐step process consisting of a screening tool prompting timely medical evaluation has the potential to combine the advantages in terms of the sensitivity and specificity of both steps. 37 , 41 Performing a 2‐step screening process is well aligned with other care processes in the ED setting and is effective. 25 , 37 The first step is nurse driven, involves responding to an electronic alert determined by the presence of abnormal vital signs and/or clinical parameters, and takes into consideration the presence of high‐risk conditions. If the screen is positive, a bedside care team huddle is performed to assess the patient and determine if sepsis pathway management is appropriate.
TABLE 5.
Emergency department sepsis screens and performance characteristics
| Components | Performance | ||||||
|---|---|---|---|---|---|---|---|
| Authors | Integrated into EHR | Vital signs, ± temperature adjustment | Clinical signs | Bedside huddle | High‐risk conditions included | Sensitivity (%) | PPV (%) |
| Cruz et al (2012)35 | + | +, internally derived | + | + | + | 81 | 4 |
| Sepanski et al (2014)36 | + | +, modified SIRS | + | − | + | 97 | 49 |
| Lane et al (2016)25 | + | −, modified PALS | + | + | + | 99 | 20 |
| Balamuth et al (2017)37 | + | +, modified PEWS | + | + | + | 86 | 25 |
| Lloyd et al (2018)33 | + | +, modified PALS | + | + | + | NR | NR |
| Powell et al (2018)40 | − | −, PEWS | + | + | + | NR | NR |
Note. −, heart rate not adjusted for pyrexia; +, heart rate adjusted for pyrexia.
Abbreviations: EHR, electronic health record; NR, not reported; PALS, pediatric advanced life support; PEWS, pediatric early warning signs; PPV, positive predictive value; SIRS, systemic inflammatory response syndrome.
Successful implementation and subsequent administration of a pediatric sepsis screening process is a large quality improvement effort requiring a dedicated and engaged workgroup. Screening pathways require adherence and performance monitoring with modifications to improve compliance and optimize sensitivity and specificity.
4.2. Laboratory‐based diagnostics
A WBC count ≥15,000 cells/μL was historically used to identify febrile young children at higher risk of occult bacteremia. 42 However, studies conducted in the post–pneumococcal conjugate vaccine era have demonstrated that WBC count has poor test characteristics for bacterial infections across age groups and that no single cut‐off value has a sufficient sensitivity or specificity for clinical utility. 43 , 44 , 45 , 46 Although elevated absolute band count >1500 cells/μL has high specificity (>90%) for bacterial infections, the sensitivity is very low (<30%). 45 , 47 Similarly, an absolute neutrophil count >10,000 cells/μL has moderate to high specificity (78%–88%) but poor sensitivity (<50%) for bacterial infection when used in isolation. 44 , 45 , 46 The absolute neutrophil count is part of the step‐by‐step approach and Pediatric Emergency Care Applied Research Network (PECARN) prediction rule to identify febrile infants ≤60 days of age at low risk of bacterial infections (Table 6). 48 , 49
TABLE 6.
Step‐by‐step approach and PECARN prediction rule for identification of well‐appearing febrile infants with bacterial infections
| Step‐by‐step approach | PECARN prediction rule | |
|---|---|---|
| Age range | ≤90 days | ≤60 days |
| History | No source of fever |
Gestational age ≥36 weeks No antibiotics in preceding 48 hours No soft‐tissue infections No chronic medical conditions Not critically ill |
| Criteria |
|
|
| Low risk | None of the above present | None of the above present |
| Test characteristics for bacterial infections |
Sensitivity: 97.8% (95% CI, 96.1–98.8) b Specificity: 58.3% (95% CI, 55.9–60.6) b |
Sensitivity: 98.2% (95% CI, 94.8–99.6) c Specificity: 58.1% (95% CI, 55.7–60.6) c |
Note. Adapted from Gomez et al48 and Kuppermann et al.49 The majority of patients with bacterial infections in these studies did not have organ dysfunction.
Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CRP, C‐reactive protein; PCT, procalcitonin; PECARN: pediatric emergency care applied research network.
Roit iunded cut‐off values.
Sensitivity and specificity calculated using data from the validation study.
Sensitivity and specificity calculated from combined derivation and validation data using rounded cut‐off values.
C‐reactive protein is often integrated into identification of febrile infants with bacterial infections. A C‐reactive protein cut‐off of 2 mg/dL has moderate sensitivity (88%) and specificity (60%) for identification of febrile children with bacterial infections, with higher levels (eg, >8 mg/dL) having higher specificity. 50 Other studies have found lower diagnostic utility for C‐reactive protein used in isolation to identify septic children. 51 , 52 Yet all of these laboratory parameters have low positive predictive value for predicting sepsis.
Procalcitonin (PCT) has the most favorable test characteristics for the identification of children with bacterial infections, particularly for invasive bacterial infection (IBI) (bacteremia and/or bacterial meningitis). 45 , 53 Among febrile infants ≤60 days of age, a PCT level of <0.5 ng/mL should be used in combination with other clinical and laboratory parameters to identify infants at low risk of IBI (Table 6). 48 , 49 For febrile older children, a PCT level of >0.5 ng/mL has low sensitivity (55%) and moderate specificity (85%) for bacterial infections, although its sensitivity is higher for IBI (82%). 53 A PCT level of >2 ng/mL has low sensitivity (61%) for IBI but high specificity (94%) and can be used to identify febrile children at higher risk of sepsis. 53 PCT use in adults with suspected sepsis has demonstrated mixed results. 54 , 55 Further data are needed on the role of PCT in children with suspected sepsis. A major limitation of currently available evidence relates to the fact that serious bacterial infection rather than sepsis, or infection with organ dysfunction, was used as the outcome in diagnostic accuracy studies.
Serum lactate >2 mmol/L (>18 mg/dL) is a component of the Sepsis‐3 definition of septic shock in adults. 16 Studies have reported that increasing lactate levels, both venous and arterial, 56 are associated with a higher risk of organ dysfunction and mortality in children with infection, in particular if >4 mmol/L (>36 mg/dL). 57 , 58 Although a normal lactate does not exclude a sepsis diagnosis in children, high levels should raise suspicion for sepsis and septic shock in the appropriate clinical context and prompt aggressive resuscitation. 31
4.3. Novel biomarkers and sepsis phenotypes
The complexity of pediatric sepsis makes it unlikely that any single biomarker in isolation will have sufficient diagnostic or prognostic capability. Consequently, most successful strategies in sepsis biomarker development have taken a multimarker approach. Because sepsis pathophysiology can affect multiple organ systems, particular interest has been paid to mechanisms spanning organ systems including immune, vascular, and bioenergetic dysfunction.
Novel biomarkers can define pediatric sepsis endotypes. Wong et al 59 identified 5 markers for the Pediatric Sepsis Biomarker Risk Model (PERSEVERE) biomarker risk model: C‐C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kDa 1B (HSPA1B), granzyme B (GZMB), and matrix metallopeptidase 8 (MMP8). These markers in combination were associated with 28‐day mortality. Addition of the platelet count (PERSEVERE II) 60 and elements of the tumor protein 53 pathway (PERSEVERE‐XP) allowed more precise prediction of 28‐day mortality. 61 Wong et al 62 identified higher pathogen burden in children with higher PERSEVERE II scores. One important caveat is that this work focused on children in the ICU with established sepsis diagnoses who were already severely ill at time of biomarker assessment. The performance of PERSEVERE or similar ICU‐based markers in a more undifferentiated population of children with possible sepsis, where the aim is to identify those about to deteriorate, remains unknown.
RNA expression profiling also has been proposed as a tool to distinguish pathogen type in children. Blood cultures lack sensitivity (false negatives with sporadic bacteremia or low blood culture volumes) and specificity (contaminants) and are slow to result. A large multicenter study identified gene expression patterns associated with bacterial versus viral infection in febrile young infants, 63 and others have identified candidate markers in older children as well. 64 , 65 , 66
5. ED MANAGEMENT: THE 2020 PEDIATRIC SSC GUIDELINES
In February 2020, the SSC published the new guidelines (Figure 1) 17 for the management of children with sepsis. Of the 77 statements, 6 were strong recommendations and 9 were best practice statements (BPS) (Table 7). 17 For most of the guidelines, there were inadequate data to make strong recommendations in support of or against various interventions, reflecting the paucity of randomized trials in the field of pediatric sepsis. The guidelines are based on systematic literature review followed by rigorous evaluation of the evidence and discussion in the expert panel to provide guidance for most scenarios relevant to ED management.
FIGURE 1.
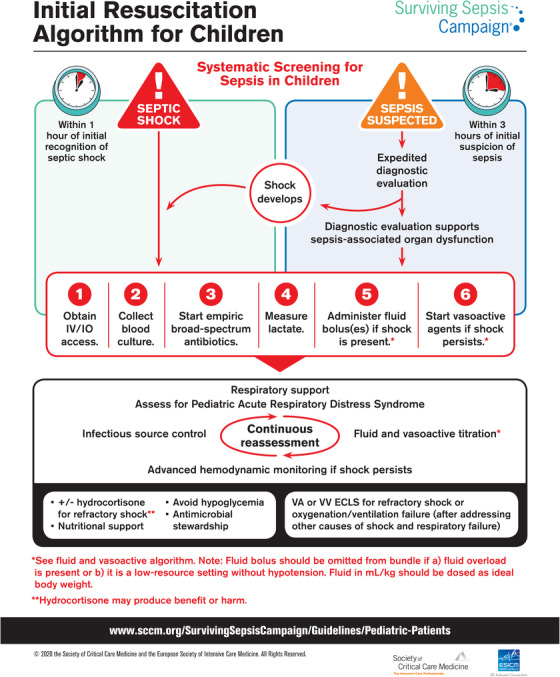
Surviving Sepsis Campaign algorithm for the initial resuscitation of children with suspected sepsis. Used with permission from the Surviving Sepsis Campaign. ECLS, extracorporeal life support; IO, intraosseous infusion; IV, intravenous; VA, veno‐arterial; VV, veno‐venous
TABLE 7.
Surviving Sepsis Campaign international guidelines for the initial management of pediatric septic shock and sepsis‐associated organ dysfunction: strong recommendations and best practice statements applicable to the emergency department setting
| Category | Recommendation |
|---|---|
| Recognition | Implement systematic screening for timely recognition of septic shock and other sepsis‐associated organ dysfunction a |
| Implement a protocol for management of sepsis‐related organ dysfunction (BPS) | |
| Obtain blood cultures before starting antimicrobial therapy if this does not delay antimicrobial administration (BPS) | |
| Antimicrobial therapy and antimicrobial stewardship | Administer antibiotics within 1 hour of recognition to children with septic shock and within 3 hours of recognition in children with sepsis‐associated organ dysfunction without shock |
| Start with empiric broad‐spectrum antibiotics to cover all likely pathogens (BPS) | |
| Narrow antimicrobial coverage after culture and susceptibility data are available (BPS) | |
| Narrow coverage or discontinue antimicrobials if no pathogen is identified, considering site of infection, clinical improvement, and patient risk factors (BPS) | |
| Optimize antimicrobial drug dosing based on pharmacokinetic data (BPS) | |
| Reassess daily for antimicrobial de‐escalation (BPS) | |
| Determine antimicrobial duration based on site of infection, etiology, clinical response, and ability to obtain source control (BPS) | |
| Source control | Remove intravascular access devices if confirmed to be source of sepsis after alternative access is obtained |
| Emergently achieve source control if possible (BPS) | |
| Fluid therapy |
If intensive care support is available, administer up to 40–60 mL/kg in bolus fluids during the first hour and monitor for signs of fluid overload If intensive care support therapies are unavailable, b administer bolus fluids only in the presence of hypotension |
| Avoid starches (hydroxyethyl starch) or gelatin in acute resuscitation | |
| Hemodynamic monitoring |
Use advanced hemodynamic monitoring, if available, in addition to bedside clinical variables to guide resuscitation Use trends in blood lactate levels to guide resuscitation |
| Respiratory support therapy |
Consider a trial of non‐invasive mechanical ventilation in children responding to resuscitation without clear indication for intubation (weak). No recommendation regarding intubation in children with fluid or catecholamine‐resistant septic shock Follow ARDS treatment recommendations including prone positioning, neuromuscular blockage, and high PEEP, do not routinely use iNO |
| Endocrine and metabolic |
Do not use IV hydrocortisone in children with septic shock responding to fluids and/or vasopressor therapy. No recommendation regarding the use of IV hydrocortisone in refractory shock Do not use insulin to target lower blood glucose levels Consider early enteral nutrition |
Note. Adapted from Weiss et al. 17
Abbreviations: ARDS, acute respiratory distress syndrome; BPS, best practice statements; iNO, inhaled nitric oxide; IV, intravenous; PEEP, positive end‐expiratory pressure.
Weak recommendation, very low‐quality evidence.
In most US settings, intensive care support therapies such as inotropes or ventilation can, at least temporarily, be administered in the emergency department and ward environments even in facilities with no on‐site ICU; this recommendation is indicated for settings where no such support can be provided.
5.1. General management
The guidelines emphasize the role of systematic screening for sepsis while acknowledging that there is no best screening/recognition tool and that implementation of screening procedures requires careful consideration of the local epidemiology and processes, with regular calibration and evaluation. Building on a body of retrospective and prospective observational studies that report on improved outcomes related to timely protocolized treatment, the new guidelines recommend the implementation of institutional protocols for the management of sepsis. Sepsis bundles should consist of the following 6 steps: obtain intravenous/intraosseous infusion access, collect blood cultures and lactate, initiate of empiric broad‐spectrum antibiotics early, administer fluid bolus if shock is present, and consider vasoactive agents if shock persists.
5.2. Antibiotic management
Empiric antibiotic therapy should be initiated as quickly as possible for children with sepsis. Empiric broad spectrum parenteral agents should be administered based on the child's age, presenting features/focus of infection, comorbidities such as immunocompromise, and local epidemiology in relation to disease prevalence and antimicrobial resistance patterns. One approach for empiric antibiotics based on the presumed site of infection and a child's comorbid medical conditions is described in Table 8. 17 , 67 , 68 , 69
TABLE 8.
Empiric antimicrobial coverage in children with sepsis a
| Group | Regimen | Notes |
|---|---|---|
| Previously healthy | Third‐generation cephalosporin + vancomycin | Can use cephalosporin monotherapy in regions with minimal MRSA or resistant pneumococci; consider adding an aminoglycoside in regions with substantial ceftriaxone resistance among Gram‐negative organisms |
| Immunocompromise | Vancomycin + anti‐pseudomonal cephalosporin (eg, cefepime) or extended‐range PCN/Beta‐lactamase combination (eg, piperacillin‐tazobactam) or a broad‐spectrum carbapenem (eg, meropenem) | Vancomycin and cefepime has been associated with less AKI in adults than vancomycin combined with an extended‐range PCN/Beta‐lactamase combination |
| Central venous catheter | Vancomycin + anti‐pseudomonal cephalosporin (eg, cefepime) or extended‐range PCN/Beta‐lactamase combination (eg, piperacillin‐tazobactam) or a broad‐spectrum carbapenem (eg, meropenem) | Recognize predominance of Gram‐negative enterics in TPN‐dependent children with intestinal failure and risk of pseudomonal sepsis in febrile neutropenic children |
| Neonates | Ampicillin + third generation cephalosporin + acyclovir | Ampicillin for Listeria coverage |
| Musculoskeletal source b , c | Vancomycin | Add Gram‐negative coverage if immunocompromise or after penetrating trauma; if history of MSSA, add cefazolin or nafcillin |
| Suspected hospital‐acquired pneumonia | Vancomycin + (piperacillin/tazobactam or cefepime or ceftazidime or carbapenem) | If high risk of mortality or recent receipt of broad‐spectrum antibiotics, consider administration of 2 antibiotics with Gram‐negative coverage, trying to avoid giving 2 beta‐lactam agents together (for risk of marrow suppression, AKI) |
| Intra‐abdominal source c | Extended‐range PCN/Beta‐lactamase combination or carbapenem, or addition of metronidazole or clindamycin | Requires more robust anaerobic coverage (eg, piperacillin‐tazobactam) |
| Influenza‐like illness | Oseltamivir, peramivir, or other influenza treatment; consider Gram‐positive coverage | Biphasic illness with influenza concerning for bacterial superinfection (Staphylococcus aureus, streptococcal species) |
| Toxic shock c | Addition of clindamycin or lincomycin | Limits toxin production |
| Necrotizing fasciitis c | Vancomycin + piperacillin/tazobactam (or vancomycin + carbapenem); alternative: ceftriaxone + metronidazole | PCN + clindamycin for group A Streptococcus (Streptococcus pyogenes) |
| Travel history | Consider treatment for malaria or rickettsial diseases |
Abbreviations: AKI, acute kidney injury; MRSA, methicillin‐resistant Staphylococcus aureus; MSSA, methicillin‐susceptible Staphylococcus aureus; PCN, penicillin; TPN, total parenteral nutrition.
Variables to consider in the selection of antimicrobial agents include local epidemiology and antibiotic resistance patterns, a child's prior cultures, risk factors for specific infections, and potential drug toxicities.
Includes septic arthritis, osteomyelitis, or pyomyositis.
Role for early surgical intervention for source control and to obtain culture data to facilitate targeted antibiotic coverage.
Antibiotics should be administered as soon as possible, ideally within an hour of the recognition of septic shock. 17 This recommendation is based on the observational data demonstrating improved survival associated with early antibiotics in cohorts of children with a predominance of septic shock. 22 , 29 , 70 , 71 Antibiotic administration should follow blood culture sampling, but not be delayed while awaiting the collection or results of diagnostic testing, including lumbar puncture, in children with shock. In practice, however, many children presenting to EDs being evaluated for sepsis are not in shock, and sepsis may represent one of several diagnostic options. In children without shock, the SSC panel considered the benefits of rapid antibiotic administration with balancing measures related to the exposure of non‐septic children to potentially unnecessary antibiotics. 72 In children with sepsis without shock, the 2020 SSC recommends starting antimicrobial therapy after appropriate evaluation and within 3 hours of recognition.
5.3. Initial fluid and inotrope resuscitation, and hemodynamic monitoring
Although intravenous fluid boluses remain a cornerstone of the resuscitation of children with septic shock, an increasing number of publications have highlighted the increased morbidity and mortality associated with aggressive fluid administration. At present, the only high‐grade evidence relates to the Fluid expansion as supportive therapy (FEAST) study, 73 which observed substantially higher mortality in children in Africa with infection and organ dysfunction receiving fluid boluses. The challenge in ascertaining the relevance of these findings for high‐income settings where critical care support to deal with the side effects of fluid overload (such as respiratory failure) remains. The SSC panel therefore made a recommendation that takes the health care setting into account. In settings where intensive care interventions such as ventilation and inotropes can be provided (which is the case, at least currently, for most EDs in the United States), 40–60 mL/kg isotonic fluid boluses should be administered during the first hour in increments of 10–20 mL/kg. Fluid administration needs to be titrated to signs of perfusion and organ dysfunction and should be discontinued if signs of fluid overload develop. In contrast, in settings where intensive care is not available, fluid resuscitation should be restricted to children with hypotension using more judicious fluid amounts of 10–20 mL/kg during the first hour.
Normal saline, lactated Ringer's, and to a lesser extent PlasmaLyte are the most commonly used isotonic fluids in pediatric sepsis. The administration of normal saline results in a hyperchloremic metabolic acidosis that can exacerbate the acidosis already common in sepsis and has been associated with decreased renal perfusion and increases in morbidity. 74 , 75 , 76 Balanced fluids have been demonstrated to reduce acute and persistent kidney injury and mortality in adults with sepsis. 77 For these reasons, the new pediatric SSC recommendations suggest using balanced fluids for initial resuscitation, acknowledging that further pediatric data are needed to ascertain the optimal fluid for resuscitation of children with sepsis. 78 The group recommended against the administration of blood products for non‐bleeding children who were not anemic (hemoglobin ≥7 g/dL), as data do not support empiric administration of packed RBCs, platelets, or plasma in improving outcomes in this group.
Epinephrine or norepinephrine are recommended as first‐line vasopressors over dopamine for fluid‐refractory sepsis, with insufficient data to recommend either one specifically. Vasopressor administration initially can be through either peripheral intravenous, intraosseous, or central venous catheter (CVC). Limited data existed to suggest maximum rates to administer peripherally. Vascular access should not delay inotrope administration. The decision to place a CVC in the ED using point‐of‐care ultrasound should be made in conjunction with ICU colleagues, depending on the expertise of the ED healthcare workers in the placement of CVCs, risk of infection in a CVC placed under potentially suboptimal conditions of sterility, clinical stability of the child, and how long the child is anticipated to remain in the ED. Trending lactate levels can be used to guide resuscitation. Hydrocortisone can be considered for fluid‐refractory and vasopressor‐refractory shock, but there is no recommendation to administer intravenous hydrocortisone in the new guidelines. Finally, extracorporeal membrane oxygenation (ECMO) remains an option for children in septic shock refractory to conventional support, acknowledging that ECMO is only available at specialized centers and optimal patient selection remains challenging. 79
The previous ACCCM recommendations 31 differentiated between warm shock (bounding pulses, flash capillary refill, warm extremity temperatures, wide pulse pressure) versus for cold shock (thready pulse, slow capillary refill, cold extremities, narrow pulse pressure, mottled appearance) to select vasoconstrictors versus inotropes. However, vital signs and clinical evaluation poorly differentiate between cold and warm shock and inaccurately identify children with cardiac dysfunction. 80 Therefore, the new pediatric SSC guidelines recommended against the use of clinical parameters to categorize children as being in warm versus cold shock. Advanced hemodynamic monitoring (eg, arterial blood pressures, central venous oxygen saturations) in addition to clinical assessment may provide more reliable guidance in relation to systemic vascular resistance, filling, and cardiac output, but is operator dependent and often unavailable in the ED.
5.4. Near‐infrared spectroscopy
The microcirculatory changes of sepsis precede diversion of blood from end‐organs and cannot necessarily be predicted from macro‐hemodynamic values such as vital signs. Detecting these early microcirculatory changes could lead to earlier diagnosis and improved outcomes 81 ; however, microcirculation is difficult to monitor. Microcirculatory tests such as lactic acid, acid‐base status, and central venous oxygen saturation levels are invasive, not always available, and do not offer the advantage of continuous monitoring.
Near‐infrared spectroscopy (NIRS) is a non‐invasive, real‐time, easily applied tool that continuously monitors microcirculation and regional tissue oxygen saturation (StO2) without requiring a pulsatile signal. NIRS uses the absorption of infrared light emitted from a probe that passes through skin or bone into underlying tissue, giving a venous weighted hemoglobin saturation in tissue. 82 NIRS‐derived cerebral StO2 correlates with central venous oxygen saturation. 83 , 84 , 85 , 86 Abnormal NIRS values have been shown to identify perfusion deficits earlier than lactate or base deficit. 87 A systematic review and meta‐analysis of adults showed that patients with severe sepsis or septic shock have lower levels of StO2 with survivors having higher levels of StO2 compared with non‐survivors. 88 However, the utility of StO2 in pediatric sepsis has not been well studied. The potential for StO2 as a resuscitation target in sepsis and incorporation into a risk score along with other predictor variables are possible avenues for future investigation.
5.5. Respiratory support
Many children will respond to initial sepsis therapy. If signs of respiratory distress of failure develop, a trial of non‐invasive positive‐pressure ventilation can be considered for children who lack clear indications for intubation. 17 Although the guidelines do not specifically recommend airway management, intubation should be considered for children with fluid‐refractory and catecholamine‐refractory shock. Etomidate as an induction agent should be avoided, as small studies have found significant adrenal suppression in adults with sepsis intubated with etomidate as opposed to other agents. 89 Although there are limited data on optimal induction agents for children, the use of ketamine or fentanyl (the latter potentially administered at lower doses in children with hypotension) may facilitate intubation without causing adrenal suppression. Once intubated, children with acute respiratory distress syndrome and sepsis may require higher (>10 cm H2O) positive end‐expiratory pressure to prevent alveolar collapse and optimize oxygenation, and best practices for pediatric acute respiratory distress syndrome including prone positioning and consideration for ECMO in cases of refractory respiratory failure should be followed. 79
6. OUTCOMES
Recent studies from high‐income countries indicate that ≈3%–7% of children with sepsis presenting to EDs die, with mortality rates increasing to up to 20% for those with septic shock treated in PICUs. 1 , 9 , 90 In resource‐limited settings, mortality rates as high as 50% remain a daily reality. 4 The majority of pediatric sepsis deaths occur within 48 hours of presentation, and specific risk factors for mortality have been identified (Table 9). 10 , 11 , 91 , 92 , 93 Early deaths are usually attributed to refractory shock, whereas late deaths are more often associated with multiorgan system dysfunction. 94
TABLE 9.
Risk factors for mortality in pediatric sepsis in high‐resource settings
| Category | Risk factor |
|---|---|
| Demographic | Age < 1 year |
| Comorbidities | Congenital heart disease |
| Hematology/Immunology | |
| Malignancy | |
| Immunosuppression | |
| Organ system dysfunction | AKI |
| Hypotension | |
| Cardiac arrest | |
| Ventilatory support | |
| Shock at ICU admission | |
| ECMO | |
| Laboratory or microbiologic parameter | Elevated lactate |
| Bacteremia | |
| Pneumococcal infection |
Abbreviations: AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation.
In recent years, the relevance of long‐term outcomes beyond the hospitalization of children with sepsis received increasing attention. Of concern, more than one‐third of pediatric survivors had not regained their baseline health‐related quality of life one year after an episode of community‐acquired sepsis. 95 Lower quality‐of‐life scores were associated with multiorgan dysfunction, renal replacement therapy, ECMO or cardiopulmonary resuscitation, and duration of mechanical ventilation and inotropes. 96 Similar findings were observed internationally. 11 Given that most children with sepsis are younger than 10 years of age, the long‐term impact of sepsis sequelae on children, their families, and society cannot be emphasized enough.
7. CONCLUSIONS
Sepsis remains a leading cause of death in children in the United States and globally, and its toll on short‐term and long‐term outcomes for this vulnerable patient group is substantial. Yet the relative rarity of sepsis in comparison to common febrile infections, combined with often non‐specific early manifestations, can make prompt recognition in EDs challenging. The use of clinical decision support and sepsis protocols have been shown to reduce morbidity and mortality in children with sepsis and should be implemented and audited as best practice to save lives and improve outcomes for children with sepsis.
Cruz AT, Lane RD, Balamuth F, et al. Updates on pediatric sepsis. JACEP Open 2020;1:981–993. 10.1002/emp2.12173
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Angela Lumba‐Brown, MD.
REFERENCES
- 1. Fleischman‐Struzek C, Goldfarb DM, Schlattmann P, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223‐230. [DOI] [PubMed] [Google Scholar]
- 2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease study. Lancet. 2020;395(10219):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss SL, Balamuth F, Chiutti M, et al, Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) . Identification of pediatric sepsis for epidemiologic surveillance using electronic clinical data. Pediatr Crit Care Med. 2020;21:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 6. Rudd KE, Delaney A, Finfer S. Counting sepsis, an imprecise but improving science. JAMA. 2017;318(13):1228‐1229. [DOI] [PubMed] [Google Scholar]
- 7. Hsu HE, Abanyie F, Agus MSD, et al. A national approach to pediatric sepsis surveillance. Pediatrics. 2019;144:e20191790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children's hospitals. Pediatr Crit Care Med. 2014;15(9):798‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prout AJ, Talisa VB, Carcillo JA, et al. Children with chronic disease bear the highest burden of pediatric sepsis. J Pediatr. 2018;199:194‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014;15:828‐838. [DOI] [PubMed] [Google Scholar]
- 11. Boeddha NP, Schlapbach LJ, Dreissen GJ, et al. Mortality and morbidity in community‐acquired sepsis in European pediatric intensive care units: a prospective study from the European Childhood Life‐Threatening Infectious Disease Study (EUCLIDS). Crit Care. 2018;22(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prout AJ, Talisa VB, Carcillo JA, et al. Bacterial and fungal etiology of sepsis in children in the United States: reconsidering empiric therapy. Crit Care Med. 2020;48:e192‐e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agyeman PKA, Schlapbach LJ, Giannoni E, et al. Epidemiology of blood culture‐proven bacterial sepsis in children in Switzerland: a population‐based cohort study. Lancet Child Adolesc Health. 2017;1(2):124‐133. [DOI] [PubMed] [Google Scholar]
- 14. Schlapbach LJ, Kissoon N. Defining pediatric sepsis. JAMA Pediatr. 2018;172(4):312‐314. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein B, Giroir B. Randolph A. International Consensus Conference on Pediatric Sepsis: International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2‐8. [DOI] [PubMed] [Google Scholar]
- 16. Shankar‐Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315(8):775‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis‐associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52‐e106. [DOI] [PubMed] [Google Scholar]
- 18. Scott HF, Deakyne SJ, Woods JM, et al. The prevalence and diagnostic utility of systemic inflammatory response syndrome vital signs in a pediatric emergency department. Acad Emerg Med. 2015;22(4):3810389. [DOI] [PubMed] [Google Scholar]
- 19. Schlapbach LJ, Straney L, Bellomo R, et al. Prognostic accuracy of age‐adapted SOFA, SIRS, PELOD‐2, and qSOFA for in‐hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 2018;44(2):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dwyer J. An infection, unnoticed, turns unstoppable. New York Times. https://www.nytimes.com/2012/07/12/nyregion/in-rory-stauntons-fight-for-his-life-signs-that-went-unheeded.html. Published July 11, 2012. Accessed April 13, 2020. [Google Scholar]
- 21. New York State Law: Title 10 of the New York State Codes, Rules and Regulations (Sections 405.2 and 405.4). https://www.health.ny.gov/facilities/public_health_and_health_planning_council/meetings/2013-02-07/docs/13-01.pdf. Accessed April 20, 2020.
- 22. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York Sepsis Care Mandate and in‐hospital mortality for pediatric sepsis. JAMA. 2018;320:358‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paul R, Melendez E, Walthen B, et al. A quality improvement collaborative for pediatric sepsis: lessons learned. Pediatr Qual Saf. 2017;3(1):e051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lane RD, Funai T, Reeder R, et al. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. 2016;138(4):pii:e20154153. [DOI] [PubMed] [Google Scholar]
- 26. Paul R, Neuman MI, Monuteaux MC, et al. Adherence to PALS sepsis guidelines and hospital length of stay. Pediatrics. 2012;130:e273‐e280. [DOI] [PubMed] [Google Scholar]
- 27. Gitte YL, Mecham N, Greenberg R. An emergency department Septic Shock Protocol Care Guideline for children initiated at triage. Pediatrics. 2011;127:e1585‐e1592. [DOI] [PubMed] [Google Scholar]
- 28. Akcan Arikan A, Williams EA, Graf JM, et al. Resuscitation bundle in Pediatric Shock Decreases Acute Kidney Injury and Improves Outcomes. J Pediatr. 2015;167:1301‐1305. [DOI] [PubMed] [Google Scholar]
- 29. Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42:2409‐2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric‐neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112(4):793‐799. [DOI] [PubMed] [Google Scholar]
- 31. Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061‐1093. [DOI] [PubMed] [Google Scholar]
- 32. Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lloyd JK, Ahrens EA, Clark D, Dachenhaus T, Nuss KE. Automating a manual sepsis screening tool in a pediatric emergency department. Appl Clin Inform. 2018;9(4):803‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scott HF, Colborn KL, Sevick CJ, et al. Development and validation of a predictive model of the risk of pediatric septic shock using data known at the time of hospital arrival. J Pediatr. 2020;217:145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cruz AT, Williams EA, Graf JM, et al. Test characteristics of an automated age‐ and temperature‐adjusted tachycardia alert in pediatric septic shock. Pediatr Emerg Care. 2012;28(9):889‐894. [DOI] [PubMed] [Google Scholar]
- 36. Sepanski RJ, Godambe SA, Mangum CD, et al. Designing a pediatric severe sepsis screening tool. Front Pediatr. 2014;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balamuth F, Alpern ER, Abbadessa MK, et al. Improving recognition of pediatric severe sepsis in the emergency department: contributions of a vital sign‐based electronic alert and bedside clinician identification. Ann Emerg Med. 2017;70(6):759‐768; e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6):e1585‐e1592. [DOI] [PubMed] [Google Scholar]
- 39. Cruz AT, Perry AM, Williams EA, et al. Implementation of goal‐directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127(3):e758‐e766. [DOI] [PubMed] [Google Scholar]
- 40. Powell R, Jeavons K. Identifying paediatric sepsis: the difficulties in following recommended practice and the creation of our own pathway. Arch Dis Child. 2018;103(1):114. [DOI] [PubMed] [Google Scholar]
- 41. Balamuth F, Alpern ER, Grundmeier RW, et al. Comparison of two sepsis recognition methods in a pediatric emergency department. Acad Emerg Med. 2015;22(11):1298‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198‐1210. [DOI] [PubMed] [Google Scholar]
- 43. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a children's hospital emergency department and urgent care center. Arch Pediatr Adolesc Med. 2004;158(7):671‐675. [DOI] [PubMed] [Google Scholar]
- 44. De S, Williams GJ, Hayen A, et al. Value of white cell count in predicting serious bacterial infection in febrile children under 5 years of age. Arch Dis Child. 2014;99(6):493‐499. [DOI] [PubMed] [Google Scholar]
- 45. Mahajan P, Grzybowski M, Chen X, et al. Procalcitonin as a marker of serious bacterial infections in febrile children younger than 3 years old. Acad Emerg Med. 2014;21(2):171‐179. [DOI] [PubMed] [Google Scholar]
- 46. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramgopal S, Walker LW, Nowalk AJ, et al. Immature neutrophils in young febrile infants. Arch Dis Child. 2019;104(9):884‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomez B, Mintegi S, Bressan S, et al. Validation of the "Step‐by‐Step" approach in the management of young febrile infants. Pediatrics. 2016;138(2):pii:e20154381. [DOI] [PubMed] [Google Scholar]
- 49. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andreola B, Bressan S, Callegaro S, et al. Procalcitonin and C‐reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J. 2007;26(8):672‐677. [DOI] [PubMed] [Google Scholar]
- 51. Lamping F, Jack T, Rubsamen N, et al. Development and validation of a diagnostic model for early differentiation of sepsis and non‐infectious SIRS in critically ill children—a data‐driven approach using machine‐learning algorithms. BMC Pediatr. 2018;18(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jaye DL, Waites KB. Clinical applications of C‐reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16(8):735‐746; quiz 746‐737. [DOI] [PubMed] [Google Scholar]
- 53. Trippella G, Galli L, De Martino M, et al. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: a systematic review and meta‐analysis. Expert Rev Anti Infect Ther. 2017;15(11):1041‐1057. [DOI] [PubMed] [Google Scholar]
- 54. Andriolo BN, Andriolo RB, Salomao R, et al. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017;1:CD010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open‐label trial. Lancet Infect Dis. 2016;16(7):819‐827. [DOI] [PubMed] [Google Scholar]
- 56. Schlapbach LJ, MacLaren G, Straney L. Venous vs arterial lactate and 30‐day mortality in pediatric sepsis. JAMA Pediatr. 2017;171(8):813. [DOI] [PubMed] [Google Scholar]
- 57. Scott HF, Donoghue AJ, Gaieski DF, et al. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med. 2012;19(11):1276‐1280. [DOI] [PubMed] [Google Scholar]
- 58. Scott HF, Brou L, Deakyne SJ, et al. Association Between Early Lactate Levels and 30‐Day Mortality in Clinically Suspected Sepsis in Children. JAMA Pediatr. 2017;171(3):249‐255. [DOI] [PubMed] [Google Scholar]
- 59. Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16(5):R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong HR, Cvijanovich NZ, Anas N, et al. Pediatric sepsis biomarker risk model‐II: redefining the pediatric sepsis biomarker risk model with septic shock phenotype. Crit Care Med. 2016;44(11):2010‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wong HR, Cvijanovich NZ, Anas N, et al. Improved risk stratification in pediatric septic shock using both protein and mRNA biomarkers. PERSEVERE‐XP. Am J Respir Crit Care Med. 2017;196(4):494‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wong HR, Caldwell JT, Cvijanovich NZ, et al. Prospective clinical testing and experimental validation of the Pediatric Sepsis Biomarker Risk Model. Sci Transl Med. 2019;11(518):eaax9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahajan P, Kupperman N, Mejias A, et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Herberg JA, Kaforou M, Wright VJ, et al. Diagnostic accuracy of a 2‐transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA. 2016;316(8):835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raymond SL, Lopez MC, Baker HV, et al. Unique transcriptomic response to sepsis is observed among patients of different age groups. PLoS One. 2017;12(9):e0184159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Balamuth F, Alpern ER, Kan M, et al. Gene expression profiles in children with suspected sepsis. Ann Emerg Med. 2020;75(6):744‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Buckley MS, Hartsock NC, Berry AJ, et al. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care. 2018;48:32‐38. [DOI] [PubMed] [Google Scholar]
- 68. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10‐e52. [DOI] [PubMed] [Google Scholar]
- 69. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61‐e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lane RD, Olson J, Reeder R, et al. Antibiotic timing in pediatric septic shock. Hosp Pediatr. 2020;10(4):311‐317. [DOI] [PubMed] [Google Scholar]
- 71. Creedon JK, Vargas S, Asaro LA, et al. Timing of antibiotic administration in pediatric sepsis [published online ahead of print November 26, 2018]. Pediatr Emerg Care. 10.1097/PEC.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 72. Schlapbach LJ, Weiss SL, Wolf J. Reducing collateral damage from mandates for time to antibiotics in pediatric sepsis – primum no nocere. JAMA Pediatr. 2019;173(5):409‐410. [DOI] [PubMed] [Google Scholar]
- 73. Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483‐2495. [DOI] [PubMed] [Google Scholar]
- 74. Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS‐stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286(4):R686‐R692. [DOI] [PubMed] [Google Scholar]
- 75. Sen A, Keener CM, Sileanu FE, et al. Chloride content of fluids used for large‐volume resuscitation is associated with reduced survival. Crit Care Med. 2017;45(2):e146‐e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double‐blind crossover study on the effects of 2‐L infusions of 0.9% saline and plasmalyte®148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 77. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloid versus saline in critically ill adults. N Engl J Med. 2018;378:829‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Balamuth F, Kittick M, McBride P, et al. Pragmatic pediatric trial of balanced versus normal saline fluid in sepsis: the PRoMPT BOLUS randomized controlled trial pilot feasibility study. Acad Emerg Med. 2019;26(12):1346‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schlapbach JL, Chiletti R, Straney L, et al. Defining benefit threshold for extracorporeal membrane oxygenation in children with sepsis – a binational cohort study. Crit Care. 2019;23(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brierley J, Peters MJ. Distinct hemodynamic patterns of septic shock at presentation to pediatric intensive care. Pediatrics. 2008;122:752‐759. [DOI] [PubMed] [Google Scholar]
- 81. Creteur J. Muscle StO2 in critically ill patients. Curr Opin Crit Care. 2008;14(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 82. Hoffman GM, Ghanayem NS, Tweddell JS. Noninvasive assessment of cardiac output. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu;2005:12‐21. [DOI] [PubMed] [Google Scholar]
- 83. Abdul‐Khaliq H, Troitzsch D, Berger F, et al. [Regional transcranial oximetry with near infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygena‐tion]. Biomed Tech (Berl). 2000;45(11):328‐332. [DOI] [PubMed] [Google Scholar]
- 84. Abdul‐Khaliq H, Troitzsch D, Berger F, et al. [Regional tran‐scranial oximetry with near infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygena‐tion]. Biomedizinische Technik Biomed Eng. 2000;45(11):328‐332. [DOI] [PubMed] [Google Scholar]
- 85. Ranucci M, IsgrO G, De La Torre T, et al. Near‐infrared spectroscopy correlates with continuous superior vena cava oxygen saturation in pediatric cardiac surgery patients. Pediatr Anesthesia. 2008;18(12):1163‐1169. [DOI] [PubMed] [Google Scholar]
- 86. Ricci Z, Garisto C, Favia I, et al. Cerebral NIRS as a marker of superior vena cava oxygen saturation in neonates with congenital heart disease. Pediatr Anesthesia. 2010;20(11):1040‐1045. [DOI] [PubMed] [Google Scholar]
- 87. Putnam B, Bricker S, Fedorka P, et al. The correlation of near‐infrared spectroscopy with changes in oxygen delivery in a controlled model of altered perfusion. Am Surg. 2007;73:1017‐1022. [PubMed] [Google Scholar]
- 88. Neto AS, Pereira VG, Manetta JA, et al. Association between static and dynamic thenar near‐infrared spectroscopy and mortality in patients with sepsis: a systematic review and meta‐analysis. J Trauma Acute Care Surg. 2014;76:226‐233. [DOI] [PubMed] [Google Scholar]
- 89. Jabre P, Combes X, Lapostolle F, et al. KETASED Collaborative Study Group: etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293‐300. [DOI] [PubMed] [Google Scholar]
- 90. Ames SG, Davis BS, Angus DC, et al. Hospital variation in risk‐adjusted pediatric sepsis mortality. Pediatr Crit Care Med. 2018;19:390‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fitzgerald JC, Ross ME, Thomas NJ, et al. Risk factors and inpatient outcomes associated with acute kidney injury at pediatric severe sepsis presentation. Pediatr Nephrol. 2018;33(10):1781‐1790. [DOI] [PubMed] [Google Scholar]
- 92. Schlapbach LJ, MacLaren G, Festa M, et al. Prediction of pediatric sepsis mortality within 1 hour of intensive care unit admission. Intensive Care Med. 2017;43(8):1085‐1096. [DOI] [PubMed] [Google Scholar]
- 93. Cvetkovic M, Lutman D, Ramnarayan P, et al. Timing of death in children referred for intensive care with severe sepsis: implications for interventional studies. Pediatr Crit Care Med. 2015;16(5):410‐417. [DOI] [PubMed] [Google Scholar]
- 94. Weiss SL, Balamuth F, Hensley J, et al. The epidemiology of hospital death following pediatric severe sepsis: when, why, and how children with sepsis die. Pediatr Crit Care Med. 2017;18(9):823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zimmerman JJ, Banks R, Berg RA. Trajectory of mortality and health‐related quality of life morbidity following community‐acquired pediatric septic shock. Crit Care Med. 2020;48(3):329‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zimmerman JJ, Banks R, Berg RA, et al. Critical illness factors associated with long‐term mortality and health‐related quality of life morbidity following community‐acquired pediatric shock. Crit Care Med. 2020;48(3):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]


