Abstract
Objective
Motor vehicle collisions generate considerable transmitted forces resulting in traumatic brain injury in children presenting to emergency departments (EDs). To date, no large study has examined post‐concussive symptoms in children sustaining concussions in motor vehicle collisions. This study aimed to compare trends in acute post‐concussive symptom burden in children with concussion following motor vehicle collisions as compared to other injury mechanisms.
Methods
The study is a secondary analysis of the Predicting Persistent Post‐concussive Problems in Pediatrics study, which prospectively recruited a multicenter cohort of 3029 children 5–17 years of age presenting to the ED with concussion from 2013–2015. Post‐concussive symptom ratings were obtained at pre‐specified time points for 12 weeks post‐injury, using the validated Post‐Concussion Symptom Inventory (PCSI). Symptom severity and recovery trajectories were measured using delta scores on the PCSI (mean post‐injury symptom score minus perceived pre‐injury score). A multivariable, longitudinal model evaluated the adjusted effect of mechanism of injury (motor vehicle collisions vs other mechanisms) on mean symptom scores, compared to perceived pre‐injury reports, and the temporal change in mean scores over during recovery.
Results
Of 3029 study participants, 56 (1.8%) sustained concussion from motor vehicle collisions. Children sustaining concussion in a motor vehicle collision had lower post‐concussive symptom scores upon ED presentation, measured as differences from their perceived pre‐injury reports, as compared to other injury mechanisms (−0.36 [95% confidence interval (CI) = −0.58, −0.15]). However, the motor vehicle collisions group showed the smallest decline in symptom burden over 1 month following injury (−0.54 [95% CI = −0.81, −0.27]).
Conclusions
Children sustaining concussions in motor vehicle collisions may have lower initial symptom burdens but slower symptom recovery at 1 month compared to other mechanisms of injury and may represent a distinct population for prognostic counseling in the ED requiring further research.
Keywords: concussion, motor vehicle collision, post‐concussive symptoms, traumatic brain injury
1. INTRODUCTION
1.1. Background
Concussion, a type of mild traumatic brain injury (mTBI), is a common injury in children, resulting in >837,000 emergency department encounters annually in the United States. 1 , 2 Although concussion has been studied extensively after sports‐related injuries in children, little is known about the symptom burden and recovery trajectory of concussion after motor vehicle collisions. Motor vehicle collisions represents a unique injury mechanism because of the potential for substantial transmission of force to the brain including rotational forces and multiple impacts in a single event, in addition to injuries to the head from direct blows. 3 Given the smaller size of children, the vulnerability of their developing brains, the lack of corollary neck strength and body musculature to respond to blows, and their need for adjunct harness systems that may not always be used correctly or at all, children are at risk for significant TBI in motor vehicle collisions.
1.2. Importance
Children sustaining concussions in motor vehicle collisions may be at risk for more severe symptoms and therefore may represent a population that would benefit from early identification with prognostic counseling and coordinated medical follow‐up. 4 , 5 , 6 Additionally, other injuries sustained from motor vehicle collisions, such as orthopedic injury, cervical spine injury, or intra‐abdominal injury, present challenges in the assessment of post‐concussive symptoms in emergency settings, causing delays in or missed concussion diagnosis. 7
1.3. Goals of investigation
We hypothesized that children sustaining concussion attributable to motor vehicle collisions would have a greater total post‐concussive symptom burden on initial presentation to the ED as compared to children sustaining concussion from other mechanisms (eg, falls or blows from sports, non‐sports, or assault). We also hypothesized that children sustaining concussion in motor vehicle collisions would demonstrate longer duration of recovery.
2. MATERIALS AND METHODS
2.1. Study design
This study is an exploratory secondary analysis of data from the Predicting Persistent Post‐concussive Problems in Pediatrics (5P) study, a prospective, multicenter cohort study involving 9 pediatric EDs within the Pediatric Emergency Research Canada (PERC) network conducted from August 2013 to June 2015 to derive and validate a clinical prediction score for persistent post‐concussive symptoms in children. 8 , 9 This parent study was approved by the internal review boards at all sites.
2.2. Measurements and outcomes
The Post‐Concussion Symptom Inventory (PSCI) is a validated post‐concussive symptom‐rating scale that is used to assess self‐reported post‐concussive symptom burden and allows for comparisons with perceived pre‐injury symptom reports. Unique versions of the PCSI were used depending on participant age. The PCSI consists of 13 items rated on a 3‐point scale for children 5–7 years of age, 17 items rated on a 3‐point scale for children 8–12 years of age, and 20 items rated on a 7‐point scale (0–6, 6 = maximum symptom severity) for children 13–18 years of age. 10 , 11 High total symptoms scores may reflect a combination of higher individual symptoms severity and/or higher number of symptoms reported. For each item, both a current (post‐injury) rating and a pre‐injury rating are obtained, with the difference constituting the item “delta” score. A positive delta score indicates the presence of the symptom post‐injury and the higher the delta score, the larger the difference in temporal severity. Average delta scores, reported in a single paper, may range widely from deltas of 4 through 18 in the first assessment to 0.2 through 2 at 1 month. 12 Because children of all ages and mechanisms of injury frequently omitted responses to individual items that they may not have felt were applicable, the mean of item delta scores was computed when at least 85% of the item delta scores were available (otherwise, no PCSI score was computed), to derive a uniformly comparable PCSI score for each participant. To include participants of all age groups (5–7, 8–13, and 13–18) in the same analyses, we reconciled the scaling difference between age groups by uniformly rescaling all item mean delta scores to be consistent with the 7‐point symptom‐rating scale used by the 13–18 years age‐group (ie, rescaled to a range from 0–6).
To characterize the post‐concussive symptom burden and recovery trajectory of children with concussion following motor vehicle collisions as compared to children sustaining concussion via other mechanisms (falls or blows related to: sports injury, injury occurring unrelated to sports, and assault), differences in symptom burden, compared to perceived pre‐injury reports elicited during the initial ED visit, were quantified. The differences were measured as the PSCI total delta score (post‐injury minus pre‐injury report), assessed at multiple time‐points (0, 1, 2, 4, 8, and 12 weeks post‐injury). Analyses compared PSCI total delta scores following motor vehicle collisions versus other mechanisms of injury.
2.3. Selection of participants
The 5P study included children 5–17 years of age who presented to participating EDs with an isolated acute head injury and were uniformly diagnosed with concussion in accordance with the fourth Zurich consensus statement criteria. 13 Patients were excluded for a Glasgow Coma Scale score of 13 or less, a structural abnormality on neuroimaging (if performed), neurosurgical intervention, intubation or intensive care unit admission, multisystem injury requiring hospitalization, procedural sedation, severe preexisting neurological developmental delay resulting in communication difficulties, or intoxication.
2.4. Analysis
Summary statistics (eg, median, interquartile range [IQR]) of key baseline characteristics reported in the initial ED visit were computed, with stratification of participants into the 4 mechanism of injury groups. Statistical comparisons in baseline characteristics between the 4 mechanism groups were assessed by either the Kruskal‐Wallis test (for continuous variables) or Pearson chi‐square (χ2) test (for categorical variables). To describe the symptom burden over time for each group, group means and medians with non‐parametric 95% confidence intervals (CI), as well as interquartile ranges, of the PCSI were computed at each study time point (ie, weeks 0, 1, 2, 4, 8, 12).
The Bottom Line
Concussion syndromes in children may be more heterogeneous in nature than we thought. This multi‐center study of 3029 children showed that children with concussions following motor vehicle collisions had fewer presenting symptoms but slower symptom resolution than with other mechanisms.
The primary study objective was to explore if differences existed between post‐concussive symptom burden upon ED presentation and recovery trajectory over 12 weeks in children with concussion sustained in motor vehicle collisions as compared to other mechanisms of injury. A multivariable, longitudinal model was fitted using generalized least squares with the restricted maximum likelihood estimator, where the rescaled PCSI item mean delta score (range: 0–6) was specified as the outcome variable. To account for within‐subject correlation (ie, repeated outcomes assessed for each patient), a continuous autoregressive process of order 1 correlation structure was applied to the time covariate with participant nested in site (9 levels) specified as the grouping factor to reflect the hierarchical structure of our data. In terms of model predictors, in addition to the four‐level mechanism of injury variable (sports, non‐sports, motor vehicle collisions, assault), other covariates selected a priori included: age (continuous), time (continuous, in weeks), sex (males vs females), maximum duration of previous concussion symptoms (continuous), and participant history of migraine (Yes/No), learning disability (Yes/No), ADHD (Yes/No), depression (Yes/No), anxiety (Yes/No), developmental disorders (Yes/No), and sleep disorders (Yes/No), according to parent report. These covariates were selected because they are known risk factors for prolonged recovery. 14 , 15 , 16 Before model fitting, Spearman correlations among model covariates were assessed to determine the potential for multicollinearity (largest correlation was r = 0.38 between depression and anxiety). To allow for non‐linearity of predictor effects or a non‐constant effect of injury mechanism over time, we incorporated flexible restricted cubic spline functions for all continuous predictors in the model (ie, 5 knots for age, 4 knots for maximum duration of previous concussion symptoms and week, reflecting the number of internal values) and specified an interaction term of mechanism‐of‐injury by (splined) time. 17 Leastwise deletion was performed if there were missing values (outcome or covariate). Because we adopted a longitudinal modeling strategy, individual participants could still contribute to the model even if they were missing values on the outcome at some time points. Therefore, a high proportion of our eligible sample (97%, 2953/3029) contributed to the final multivariable model (ie, they had at least one outcome value available and contributed at least 1 row of data to modeling).
After model fitting, the overall contribution of individual predictors (ie, their adjusted effects) was summarized with the Wald χ2 statistic (and associated P‐value), which provides an indication of the relative importance of individual predictors. To quantify the influence of mechanism‐of‐injury on recovery in further detail, we performed 2 sets of post‐model fit contrasts to elucidate its effects on the outcome at various time points over the study periods. The first set of contrasts provided adjusted comparisons of the estimated outcome (ie, predicted PCSI values) among injury mechanisms at selected time points (weeks 0, 2, 4, and 12), whereas the second set of contrasts compared temporal changes in outcome (ie, predicted changes in PCSI) among injury mechanisms at selected time intervals (week 0 vs 1, 0 vs 2, 0 vs 4) to gain insights into potential differences in rate of recovery. For all contrasts, continuous covariates were set to their median value, while categorical covariates were set to their modal value. To account for multiplicity in our hypothesis testing, statistical significance of individual contrasts was appraised against a Holm‐Bonferroni corrected alpha of 0.05 to control Type I error for all contrasts from the same time‐point or interval (ie, each set of such contrasts represent a family of hypothesis tests). All analyses were conducted using R version 3.3.2. 18
3. RESULTS
3.1. Characteristics of study subjects
A total of 3029 participants were included in the complete analysis, with 2065 (68.2%) in the sports injury group, 866 (28.6%) in the non‐sports injury group, 56 (1.8%) in the motor vehicle collisions group, and 42 (1.4%) in the assault group. Summary statistics (eg, median, IQR, %) of key pre‐injury characteristics of the eligible sample stratified by injury mechanism is provided in Table 1.
TABLE 1.
Key pre‐injury characteristics of sample stratified by injury mechanism
| Variable | N | Sports (n = 2065) | Non‐sports (n = 866) | Motor vehicle collisions (n = 56) | Assault (n = 42) |
|---|---|---|---|---|---|
| Age, median (IQR) | 3029 | 12.7 (10.0, 14.9) | 10.1 (7.3, 12.9) | 15.0 (12.2, 16.5) | 13.8 (12.5, 15.6) |
| Sex, freq/n (%) | 3028 | ||||
| Male | 1303/2065 (63.1) | 477/865 (55.1) | 23/56 (41.1) | 30/42 (71.4) | |
| Female | 762/2065 (36.9) | 388/865 (44.9) | 33/56 (58.9) | 12/42 (28.6) | |
| Maximum duration of previous concussion(s), freq/n (%) | 3014 | ||||
| Never had concussion | 1538/2053 (74.9) | 713/863 (82.6) | 44/56 (78.6) | 33/42 (78.6) | |
| <1 wk | 224/2053 (10.9) | 67/863 (7.8) | 5/56 (8.9) | 2/42 (4.8) | |
| 1–2 wk | 114/2053 (5.6) | 32/863 (3.7) | 5/56 (8.9) | 4/42 (9.5) | |
| 3–4 wk | 78/2053 (3.8) | 18/863 (2.1) | 0/56 (0.0) | 0/42 (0.0) | |
| 5–8 wk | 39/2053 (1.9) | 9/863 (1.0) | 1/56 (1.8) | 0/42 (0.0) | |
| >8 wk | 60/2053 (2.9) | 24/863 (2.8) | 1/56 (1.8) | 3/42 (7.1) | |
| Personal history of migraines, freq/n (%) | 3016 | ||||
| No | 1794/2053 (87.4) | 753/865 (87.1) | 48/56 (85.7) | 35/42 (83.3) | |
| Yes | 259/2053 (12.6) | 112/865 (12.9) | 8/56 (14.3) | 7/42 (16.7) | |
| History of learning disability, freq/n (%) | 3017 | ||||
| No | 1907/2060 (92.6) | 791/861 (91.9) | 45/55 (81.8) | 35/41 (85.4) | |
| Yes | 153/2060 (7.4) | 70/861 (8.1) | 10/55 (18.2) | 6/41 (14.6) | |
| History of attention deficit disorder, freq/n (%) | 3014 | ||||
| No | 1874/2058 (91.1) | 794/860 (92.3) | 46/55 (83.6) | 34/41 (82.9) | |
| Yes | 184/2058 (8.9) | 66/860 (7.7) | 9/55 (16.4) | 7/41 (17.1) | |
| History of depression, freq/n (%) | 3025 | ||||
| No | 2004/2062 (97.2) | 844/865 (97.6) | 52/56 (92.9) | 38/42 (90.5) | |
| Yes | 58/2062 (2.8) | 21/865 (2.4) | 4/56 (7.1) | 4/42 (9.5) | |
| History of anxiety, freq/n (%) | 3023 | ||||
| No | 1909/2059 (92.7) | 791/866 (91.3) | 50/56 (89.3) | 37/42 (88.1) | |
| Yes | 150/2059 (7.3) | 75/866 (8.7) | 6/56 (10.7) | 5/42 (11.9) | |
| History of other developmental disorders, freq/n (%) | 3003 | ||||
| No | 1975/2047 (96.5) | 821/859 (95.6) | 52/55 (94.5) | 36/42 (85.7) | |
| Yes | 72/2047 (3.5) | 38/859 (4.4) | 3/55 (5.5) | 6/42 (14.3) | |
| History of sleep disorder, freq/n (%) | 3018 | ||||
| No | 2016/2056 (98.1) | 848/864 (98.1) | 52/56 (92.9) | 40/42 (95.2) | |
| Yes | 40/2056 (1.9) | 16/864 (1.9) | 4/56 (7.1) | 2/42 (4.8) | |
| Baseline PCSI (median item delta score, 0–6), (IQR) | 2985 | 1.8 (1.1, 2.6) | 1.6 (1.0, 2.5) | 1.4 (0.7, 2.3) | 2.1 (1.3, 2.7) |
3.2. Symptom burden over time
The individual and summarized trajectories of PCSI delta scores over the study period for each of the mechanism of injury groups are illustrated in Figure 1. In all 4 groups, clear decreases in mean and median PCSI over time were evident, with the largest declines occurring during the initial weeks (1–2 weeks after initial ED visit). In addition, all 4 groups were characterized by some heterogeneity in recovery trajectory, especially during the initial weeks. For the motor vehicle collisions group, the median delta PCSI (95% CI) at initial ED visit (week 0) was 1.35 (0.88, 2.05). At week 2, the median PCSI (95% CI) was 0.25 (0.10, 0.69). By the end of the study (week 12), the median PCSI (95% CI) had decreased to 0.05 (0.00, 0.20).
FIGURE 1.
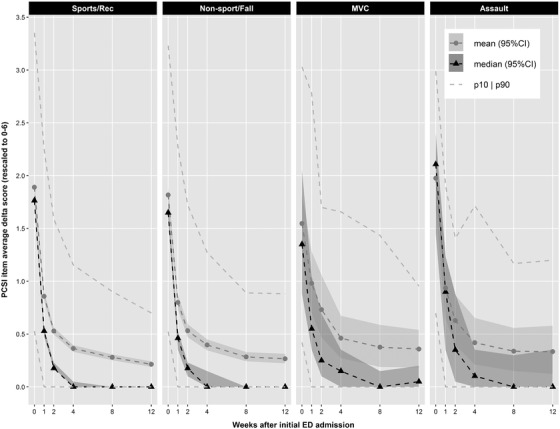
Symptom recovery trajectories stratified by injury mechanism. The individual trajectories of PCSI over the study period are presented for each of the 4 mechanisms of injury groups. In all 4 groups, clear decreases in the group‐level PCSI mean and median PCSI over time were evident, with the most dramatic changes occurring during the initial weeks after injury. In addition, all 4 groups were characterized by some heterogeneity in recovery trajectory
3.3. Influence of mechanism of injury on recovery
Using Wald χ2 for individual model predictors (see Table 2), mechanism of injury is significantly associated with post‐concussive symptom burden (χ2 for the joint test of the variable and its interaction with time = 25.82; degrees of freedom [df] = 12). Additionally, the magnitude of this association varies with time (χ2 for interaction term only = 23.95; df = 9). Among all covariates included in the model, time (week) was the strongest predictor (χ2 = 8414.56; df = 12), demonstrating a non‐linear relationship with the outcome (χ2 = 4426.24; df = 8). Non‐linear plots of the predicted values (ie, estimated PCSI delta score) over time based on the fitted model demonstrate a steep initial recovery followed by a plateauing of PCSI (Figure 2). The association of symptom burden with mechanism of injury was most prominent at the initial post‐injury assessment in the ED, occurring within 48 hours of injury, week 0 (Table 3). The estimated PCSI mean delta score associated with motor vehicle collisions was lower than sports (−0.36 [−0.58, −0.15]), non‐sports (−0.28 [−0.50, −0.06]), and assault (−0.43 [−0.75, −0.11]). However, at all other time‐points (ie, 2‐, 4‐, and 12‐weeks post‐injury), no group differences were statistically significant for any contrasts.
TABLE 2.
Multivariable model: “adjusted” effect of injury mechanism on mean PCSI delta scores
| Factor | χ2 | df |
|---|---|---|
| Age | 60.61 | 4 |
| Non‐linear | 59.06 | 3 |
| Sex | 182.16 | 1 |
| Max duration of previous concussion(s) | 43.51 | 3 |
| Non‐linear | 0.17 | 2 |
| Personal history of migraines | 14.83 | 1 |
| History of learning disability | 1.81 | 1 |
| History of attention deficit disorder | 14.28 | 1 |
| History of depression | 1.50 | 1 |
| History of anxiety | 5.10 | 1 |
| History of other developmental disorders | 0.00 | 1 |
| History of sleep disorder | 1.01 | 1 |
| Mechanism of injury (factor + higher order factors) | 25.82 | 12 |
| All interactions | 23.95 | 9 |
| Wk (factor + higher order factors) | 8414.56 | 12 |
| All Interactions | 23.95 | 9 |
| Non‐linear (factor + higher order factors) | 4426.24 | 8 |
| Mechanism of injury * wk (factor + higher order factors) | 23.95 | 9 |
| Non‐linear | 14.70 | 6 |
| Total non‐linear | 4485.61 | 13 |
| Total non‐linear + interaction | 4495.41 | 16 |
| Total | 8815.20 | 30 |
phi = 0.491, Obs = 15111, clusters = 2953
FIGURE 2.
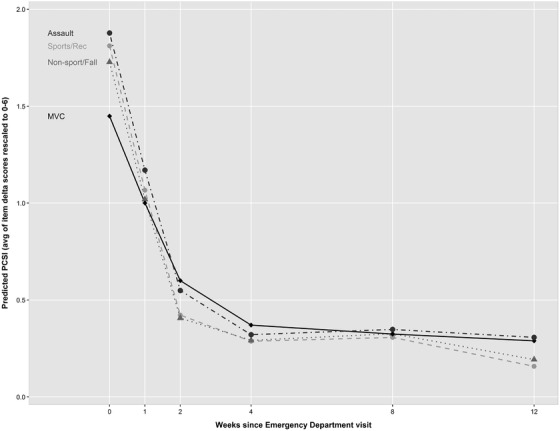
Predicted PCSI by injury mechanism. Summarized non‐linear plots of the predicted values (ie, estimated PCSI) over time based on the fitted model demonstrate a steep initial recovery followed by a plateauing of PCSI for each of the 4 mechanisms of injury groups, characterized by some heterogeneity in recovery trajectory
TABLE 3.
Modeled mean PCSI delta scores between injury mechanisms by time
| Contrast | Est (95% CI) |
|---|---|
| Time 0—motor vehicle collisions vs sports | −0.36 (−0.58, −0.15) |
| Time 2—motor vehicle collisions vs sports | 0.18 (−0.04, 0.39) |
| Time 4—motor vehicle collisions vs sports | 0.08 (−0.11, 0.27) |
| Time 12—motor vehicle collisions vs sports | 0.13 (−0.10, 0.36) |
| Time 0—motor vehicle collisions vs non‐sports | −0.28 (−0.50, −0.06) |
| Time 2—motor vehicle collisions vs non‐sports | 0.19 (−0.03, 0.41) |
| Time 4—motor vehicle collisions vs non‐sports | 0.08 (−0.12, 0.27) |
| Time 12—motor vehicle collisions vs non‐sports | 0.10 (−0.14, 0.34) |
| Time 0—motor vehicle collisions vs assault | −0.43 (−0.75, −0.11) |
| Time 2—motor vehicle collisions vs assault | 0.05 (−0.28, 0.39) |
| Time 4—motor vehicle collisions vs assault | 0.05 (−0.25, 0.34) |
| Time 12—motor vehicle collisions vs assault | −0.02 (−0.38, 0.35) |
Estimated temporal declines in symptom burden were smaller during recovery following motor vehicle collisions as compared to other mechanisms of injury (Table 4). For example, from week 0 to week 2, the estimated change in PCSI mean delta score for the motor vehicle collisions group was −0.54 (−0.81, 0.27), when compared to sports injury, meaning that on average, a child recovering from a sports‐related concussion would be expected to decline 0.54 points more (ie, 9% of the 0–6 PCSI range) over the first 2 weeks than a similar child with concussion arising from a motor vehicle collisions.
TABLE 4.
Modeled mean PCSI delta scores and temporal changes by injury mechanism
| Contrast | Est (95% CI) |
|---|---|
| Motor vehicle collisions (T0–T1) vs sports (T0–T1) | −0.30 (−0.45, −0.15) |
| Motor vehicle collisions (T0–T2) vs sports (T0–T2) | −0.54 (−0.81, −0.27) |
| Motor vehicle collisions (T0–T4) vs sports (T0–T4) | −0.45 (−0.72, −0.17) |
| Motor vehicle collisions (T0–T1) vs non‐sports (T0–T1) | −0.26 (−0.41, −0.11) |
| Motor vehicle collisions (T0–T2) vs non‐sports (T0–T2) | −0.47 (−0.75, −0.20) |
| Motor vehicle collisions (T0–T4) vs non‐sports (T0–T4) | −0.36 (−0.64, −0.07) |
| Motor vehicle collisions (T0–T1) vs assault (T0–T1) | −0.26 (−0.49, −0.03) |
| Motor vehicle collisions (T0–T2) vs assault (T0–T2) | −0.48 (−0.90, −0.06) |
| Motor vehicle collisions (T0–T4) vs assault (T0–T4) | −0.48 (−0.91, −0.05) |
3.3.1. Limitations
Study limitations included variability in participant characteristics in the injury mechanism groups; children in the motor vehicle collisions group were slightly older and had higher incidences of pre‐morbid learning disabilities, depression, and ADHD. Although these variables were accounted for as model predictors, their influence on injury mechanism or symptom manifestation and recovery is unclear. We acknowledge that there may be additional associated, unmeasured confounders in this population that are not accounted for in the model. Although the overall sample size was large, only 56 total subjects were in the motor vehicle collisions group, with 52 contributing to model‐fitting. This number permitted adequately powered statistical analyses, although a larger motor vehicle collision sample may have allowed for further elucidation of potential differences within this group. This study did not include a control group to compare post‐concussive symptoms ratings between children with concussion versus other injuries or medical problems. Three‐fourths of the motor vehicle collision‐related concussions were sustained in low speed collisions, which may generate forces similar to or less than those occurring in high‐speed sports. This study was unable to examine outcomes related to seatbelt use or where the child was seated in the car, as these data were not collected. Further studies including more high‐speed motor vehicle collisions‐related concussion and injury related to seat belt use are needed to understand true differences. Children who sustained concussion via mechanisms resulting in concomitant injuries may have had more severe concussive symptoms but were excluded from this study. Finally, children injured in motor vehicle collisions may exhibit post‐traumatic stress symptoms or musculoskeletal complaints, which may have significant overlap with post‐concussive symptoms, plausibly reflected by the motor vehicle collisions group's slower recovery trajectory.
4. DISCUSSION
The results of this exploratory study suggest that children who sustain concussion in motor vehicle collisions present to EDs with lower reported symptom burden immediately following injury as compared to children who sustain concussion via other mechanisms. However, they also demonstrate a slower rate of post‐concussive symptom improvement over 1 month.
Although the U.S. National Highway Traffic Safety Administration reports that head injuries are the most common injuries sustained by children following motor vehicle collisions, the actual incidence and manifestations of motor vehicle collisions‐related concussion in children are limited. 19 , 20 , 21 This study demonstrates that among children presenting to 9 large pediatric EDs in Canada and diagnosed with concussion, only 1.8% sustained their injury in an motor vehicle collisions, as compared to 68% presenting after sports injury and 29% after non‐sports injury or assault. This represents a much smaller percentage of motor vehicle collision‐related concussion as compared to previously reported U.S. incidence rates of motor vehicle collision‐related concussion (20%) calculated from discharge codes, and may reflect differences in methods of diagnosis, diagnostic criteria, or location‐specific differences in ED utilization and triage systems. 22 , 23 , 24 , 25
This study prospectively examined a large sample of children with wide variability in injury mechanism, captured acutely at multiple centers and followed routinely to inform differences in trends of post‐concussive symptom burden in children. The follow‐up rate was good, allowing for the classification of multiple mechanisms of injury at varying time points. Methodologically, a notable aspect of our analysis was that our longitudinal modeling incorporated flexible regression splines (to uncover non‐linearity), interaction terms, and specification of a comprehensive set of post‐model fit contrasts to allow for more understanding of relationship between key predictors and outcome at different study time‐points.
Motor vehicle collisions are distressing for children and their families and often involve multiple stressful medical experiences: (1) emergency medical service transportation to the hospital; (2) c‐spine and backboard immobilization; (3) intense trauma evaluations; and (4) temporary separation of children from their families in the event that other family members also require ED care. The effect of such experiences on post‐concussive symptom self‐reports in children in the acute setting is unclear. Future research is needed to differentiate and understand associations between concussion and post‐traumatic stress disorders. Guidelines recommend that children should be triaged to “pediatric capable” trauma centers for evaluation given that forces transmitted to vehicle occupants in motor vehicle collisions can be large and result in brain injury secondary to both indirect (translated forces to the body) as well as direct injury to the head. 26 , 27 Although this study did not find an increase in post‐concussive symptom burden immediately following motor vehicle collisions as compared to other mechanisms of injury, evaluation at pediatric trauma centers is important to the management of co‐existing injury and more severe brain injury. Although assault and sport‐related injuries were associated with higher acute post‐concussive symptom reports, further research is needed to compare manifestations of injury sustained via a variety of mechanisms using objective measures of diagnosis and recovery. The results of this study suggest that concussion reassessment and prognostic counseling is important in children following motor vehicle collisions because their recovery trajectory may be slower and further research is needed to determine whether earlier subspecialty referral supports recovery.
Children sustaining concussions in motor vehicle collisions may have lower initial symptom burdens but slower symptom recovery at 1 month compared to concussions following sports injury, non‐sports injury, or assault, and may represent a distinct population for prognostic counseling in the ED requiring further research.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: ALB, KOY, and RZ. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: ALB and KT. Obtained funding: RZ. Study supervision: ALB and RZ.
FUNDING AND SUPPORT
This study was supported by operating grant 126197 from the Canadian Institutes of Health Research; grant TM1 127047 from the Canadian Institutes of Health Research‐Ontario Neurotrauma Foundation Mild Traumatic Brain Injury Team; and planning grant MRP 119829 from the Canadian Institutes of Health Research. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ACKNOWLEDGMENTS
The authors of this work would like to acknowledge the work of the 5P site investigators and their coordination and efforts in patient enrollment and data collection.
Biography
Angela Lumba‐Brown, MD, is an Assistant Professor of Emergency Medicine and Pediatrics at Stanford University, where she practices clinically in the pediatric emergency department.

Lumba‐Brown A, Tang K, Yeates KO, Zemek R, for the Pediatric Emergency Research Canada (PERC) 5P Concussion Team . Post‐concussion symptom burden in children following motor vehicle collisions. JACEP Open. 2020;1:938–946. 10.1002/emp2.12056
Trial Registration: Clinicaltrials.gov Identifier: NCT01873287.
Supervising Editor: Mike Wells, MBBCh, PhD.
REFERENCES
- 1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury‐related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Morb Mortal Wkly Rep. 2017;66:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged </ = 19 years—United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60:1337‐1342. [PubMed] [Google Scholar]
- 3. Hillary FG, Schatz P, Moelter ST, Lowry JB, Ricker JH, Chute DL. Motor vehicle collision factors influence severity and type of TBI. Brain Inj. 2002;16:729‐741. [DOI] [PubMed] [Google Scholar]
- 4. Stuart B, Mandelson B, Wilshaw R, Beckstrand RL, Heaston S. Mild traumatic brain injury: are ED providers identifying which patients are at risk? J Emerg Nurs. 2012;38:435‐442. [DOI] [PubMed] [Google Scholar]
- 5. Ganti L, Khalid H, Patel PS, Daneshvar Y, Bodhit AN, Peters KR. Who gets post‐concussion syndrome? An emergency department‐based prospective analysis. Int J Emerg Med. 2014;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruffolo CF, Friedland JF, Dawson DR, Colantonio A, Lindsay PH. Mild traumatic brain injury from motor vehicle collisions: factors associated with return to work. Arch Phys Med Rehabil. 1999;80:392‐398. [DOI] [PubMed] [Google Scholar]
- 7. Perno JF, Schunk JE, Hansen KW, Furnival RA. Significant reduction in delayed diagnosis of injury with implementation of a pediatric trauma service. Pediatr Emerg Care. 2005;21(6): 367‐371. 10.1097/01.pec.0000166726.84308.cf [DOI] [PubMed] [Google Scholar]
- 8. Zemek R, Osmond MH, Barrowman N. Predicting and preventing postconcussive problems in paediatrics (5P) study: protocol for a prospective multicentre clinical prediction rule derivation study in children with concussion. BMJ Open. 2013;3(8):e003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zemek R, Barrowman N, Freedman S, et al., for the Pediatric Emergency Research Canada (PERC) Concussion Team . Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315:1014‐1025. [DOI] [PubMed] [Google Scholar]
- 10. Gioia GA, Isquith PK, Schneider JC, Vaughan CG. New approaches to assessment and monitoring of concussion in children. Top Lang Disord. 2009;29:266‐281. [Google Scholar]
- 11. Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol. 2014;29:348‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ledoux AA, Tang K, Yeates KO, et al. Natural progression of symptom change and recovery from concussion in a pediatric population. JAMA Pediatr. 2019;173(1):e183820 10.1001/jamapediatrics.2018.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich. Br J Sports Med. 2013;47:250‐258. [DOI] [PubMed] [Google Scholar]
- 14. Lumba‐Brown A, Yeates KO, Sarmiento K, et al. Diagnosis and management of mild traumatic brain injury in children: a systematic review. JAMA Pediatr. 2018;172(11):e182847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lumba‐Brown A, Wright DW, Sarmiento K, Houry D. Emergency department implementation of the centers for disease control and prevention pediatric mild traumatic brain injury guideline recommendations. Ann Emerg Med. 2018;72:581‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lumba‐Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York: Springer; 2015. [Google Scholar]
- 18. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2016, Vienna, Austria. Available at: https://www.R-project.org/
- 19. McKay MP. National Highway Traffic Safety Administration (NHTSA) notes. Children injured in motor vehicle traffic crashes. Commentary. Ann Emerg Med. 2010;56:688‐689. [DOI] [PubMed] [Google Scholar]
- 20. Hanna R. Children injured in motor vehicle traffic crashes. (DOT HS 811 325) National Highway Traffic Safety Administration, 2010, Washington, DC. [Cited 2018 Sept 15] Available at: http://www-nrd.nhtsa.dot.gov/Pubs/811325.PDF
- 21. Viano DC, Parenteau CS, Xu L, Faul M. Head injuries (TBI) to adults and children in motor vehicle crashes. Traffic Inj Prev. 2017;18:616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meehan WP, III , Mannix R. Pediatric concussions in United States emergency departments in the years 2002–2006. J Pediatr. 2010;157:889‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zonfrillo MR, Kim KH, Arbogast KB. Emergency department visits and head computed tomography utilization for concussion patients from 2006 to 2011. Acad Emerg Med. 2015;22:872‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haarbauer‐Krupa J, Arbogast KB, Metzger KB, et al. Variations in mechanisms of injury for children with concussion. J Pediatr. 2018;197:241‐248. e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr. 2016;170:e160294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sasser SM, Hunt RC, Faul M, et al. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61:1‐20. [PubMed] [Google Scholar]
- 27. Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12:S1‐S52. [DOI] [PubMed] [Google Scholar]


