Abstract
Background
Effectiveness and safety of pharmaceuticals is the prime concern of every osteoarthritis (OA) treatment. Chronic administration of NSAIDs, especially in case of geriatrics, through oral route tend to compromise the patient's safety, whereas topical treatments are not found to be effective owing their poor ability to deliver drug molecules.
Thus, the present study deals with a randomized, double-blind, controlled trial conducted on patients with knee osteoarthritis (OA) for comparing the performance of a novel topical gel (liposomal gel) of diclofenac with a placebo and a marketed gel.
Methods
The patients were treated and evaluated for 6 weeks as per the Western Ontario McMaster Universities (WOMAC) Index for OA. Patients were also observed for any adverse events. All the results were analyzed statistically using Kruskal-Wallis test, followed by Student's t-test at p ≤ 0.05.
Results
Patients treated with liposomal gel showed statistically significantly improvements in treatment in comparison to the other tested formulations. All the treatments were found to be well tolerated with no report of adverse event. The results unequivocally demonstrated the superiority of the diclofenac liposomal gel, in the relieving the symptoms of OA of the knee, in comparison to placebo and marketed gel.
Conclusion
From above results it was revealed that the drug in liposome have higher therapeutic potential. Thus, this can be a safe and effective option for the management of chronic OA especially for geriatric patients.
Keywords: Nanomedicine, Liposome, Topical drug delivery, Efficacy, Osteoarthritis, Patient compliance
1. Introduction
Osteoarthritis (OA) is the commonest chronic musculoskeletal disease in which the cartilages of the joints become thin. This results in the rubbing of the joint bones leading to compromised locomotion with varied degree of pain and stiffness [1]. OA is generally an age related disorder and the major risk factors for the disease include sedentary life style, genetic factors, obesity, bone mineral density, injury and gender. As per World Health Organization (WHO), around 10%–15% of the elderly patients (age above 60 years) are associated with one or other problems due to OA [2,3]. This disease mostly affects the joints of hip, knee, shoulder, hands, feet and spine. This disease severely affects the quality of life of the affected population and is regarded as the “highest-burden condition” within musculoskeletal group of diseases. Radiographic evidences of the knee give the inference that around 30% of men and women over the age of 65 are affected with OA, with almost doubles the occurrence at age group of 60. The disease has a bit of gender biasness affecting women around twice more than the men worldwide. As per the WHO reports, 80% of OA affected population has compromised locomotion, out of which, 25% cannot even perform the routine activities of the day. On economic fronts too the diseases poses a huge burden on the patients. About 82.9% of OA patients need at least one investigative test semi-annually, and around 7.9% of OA patients need to purchase various devices over the same period, as per WHO reports. As per the estimation, the approximate six-month costs arising due to OA rotates around US$ 2456, which is too huge. The economic factor is solely related to the drop outs from routine medication for OA and further severing of the disease [[1], [2], [3], [4], [5]].
Treatment options for OA are symptomatic and can be classified broadly into three categories: a) Non-pharmacological therapeutic interventions; b) Pharmacological therapeutic interventions and c) Pharmacologic operative interventions. Non-pharmacological therapeutic options refers to interventions that do not involve the use of medications to treat pain which include education programmes and social support; a host of physical treatments (aerobic exercises, muscle strengthening exercises, and patella strapping); the provision of aids and appliances through occupational therapists; and advice on weight loss [[6], [7], [8], [9]].
Operative interventions include surgical interventions like joint replacement, etc. These are applicable in case of critical and emergency cases. Much of the efforts has been spent on developing non-surgical interventions to alleviate the pain and disability in patients with OA, once the disease has become established [8,9,12,13]. Pharmacological modalities that have a place in the management of patients with osteoarthritis include simple analgesics such as paracetamol, non-steroidal anti-inflammatory agents (NSAIDs), rubifacients, and intra-articular therapy with glucocorticoids, hyaluronic acid and finally surgical interventions like knee replacements [10,11]. Though the disease is incurable, but its progression and symptoms can be minimized in early stages. Under this oral NSAIDs are frequently prescribed for chronic use, which are reported to be associated with various cardiovascular, renal and gastrointestinal (GI) adverse events [7,9,[12], [13], [14], [15]]. For minimization of risk associated with NSAID therapy, use with cautious of oral NSAIDs is recommended, especially in geriatric population [16,17].
Owing to the obvious side-effects of oral NSAIDs in the chronic management of OA, patents and clinicians are exploring other options including topical administration of NSAIDs for the management of pain in the early to moderate stages of OA [18,19]. A numerous clinical trials have established the efficacy of topical NSAIDs, esp. diclofenac vis-à-vis the oral dosage forms [[20], [21], [22], [23], [24], [25], [26]]. However, there is immense need to deliver the drug to the desired site, in substantial amounts for desired duration for better therapeutic outcomes. In this regard, the novel drug delivery systems have established their potential to do so [[27], [28], [29], [30], [31]]. Henceforth, it was envisioned to explore the promises of novel topical liposomal gel (i.e., lipogel) of diclofenac in human subjects and compare the outcomes statistically to that from a market product (i.e., Emulgel®) and placebo.
2. Material and methods
2.1. Material
Diclofenac diethylamine and saturated phospholipid (soy phosphatidylcholine) were generous gifts from Biochem Pharmaceutical Industries (Mumbai, India) and Lipoid GmbH (Ludwigshafen, Germany), respectively. Triethanolamine (TEA) and sorbitan monooleate were procured from Sigma Chemicals Co. (St Louis, MO, U.S.A.). Carbopol 980 was obtained ex-gratis from Lubrizol Co. (Wickliffe, OH, U.S.A.). Diclofenac gel (Voveran® Emulgel®, Novartis India Limited, Bangalore) was procured from local pharmacy store. All other chemicals used in the study were of analytical grade.
2.2. Methods
The novel lipogel of diclofenac was optimized, developed, characterized and evaluated in-vitro and in-vivo using suitable animal model. This is based on the concept of advance drug delivery and contains nano-sized drug loaded vesicular systems composed of phospholipids [32]. For comparison a popular and highly recommended market product i.e. Voveran Emulgel was selected.
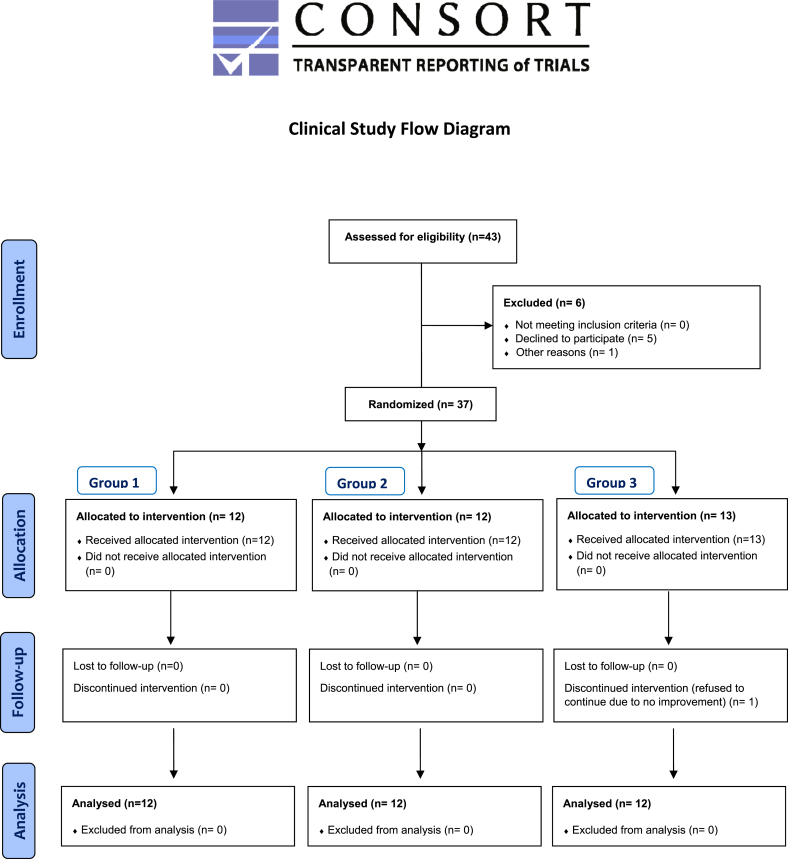
In order to test its clinical performance, the patients with signs and symptoms of OA of the knee were selected. The clinical study was initiated after obtaining the requisite approval for the study protocol by the Ethical Committee of the Post-Graduate Institute of Medical Education & Research (PGIMER), Chandigarh (Ref. No. Micro/2008/3614; NKG/545). The trial was conducted in accordance with the Indian Ethical Guidelines for Biomedical Research on Human Participants [33]. The study was carried out at PGIMER, a tertiary-level referral hospital located at Chandigarh, India. Patients were recruited from the out-patient clinics of the Department of Orthopaedic Surgery of the institute. The trial aimed to enroll a total of subjects/patients with signs and symptoms of OA. All the patients furnished their informed and written consent, after they were duly explained about the nature and details of the study. The clinical trial was registered at Clinical Trial Registry India.
2.2.1. Design of the trial
The efficacy and safety of the developed formulations was evaluated in a double blind randomized, placebo controlled, clinical trial in patients with signs and symptoms of OA of the knee [24,26,34].
2.2.2. Drug administration and treatment regimen
Patients were randomly provided with collapsible tubes (identified by a specific code number) containing DLF lipogel or Emulgel® or placebo gel, 20 g each. DLF lipogel and Emulgel® contains equivalent concentration/dose of diclofenac. Each patient was asked to apply the gel on the knees, twice a day. Treatment was continued for 6 weeks. One type of formulation was provided to the same patient throughout the study period regardless of the clinical improvement. Following completion of the treatment period and after all the definite results were obtained from all the participating patients, the formulation codes were opened [20].
Time period: 6 weeks (evaluation started 1st day [i.e. 0] and then at 1, 2, 4 & 6 weeks).
Groups & treatments: Each patient received one of the following formulations (which were assigned randomly) for the complete period of study. The scheme has been shown in consort flow chart:
Group 1: Diclofenac lipogel
Group 2: Market Product (i.e., Voveran® Emulgel®)
Group 3: Placebo lipogel (i.e., lipogel without drug)
Sample size: In this study, three different treatments are attributed to three different groups of patients. The study aims to compare/find significant difference amongst the treatments. For this purpose, statistical tests were applied for comparing the outcomes of groups (i.e., scores). The required sample size in each group was calculated by following Eqn. (1):
| Minimum sample size = [power of test] / [p X (absolute difference)2] | (1) |
At p ≤ 0.05 with absolute difference of 19 and power of the test 80%, the minimum required sample size was found to be 4 patients for each group, i.e., a total of 12 patients. A higher number of patients were enrolled for the trial following inclusion and exclusion criteria of WOMAC for Osteoarthritis. Finally, 36 patients completed the study of 6 weeks (See CONSORT flow chart).
Prior to the treatment, the detailed history of all the patients was recorded. A minimum of four weeks of wash-over period was elapsed, during which no topical or systemic anti-arthritic therapy was administered to the patients.
Outcome measures: Primary efficacy outcome measure selected was the change from baseline to end of study on the WOMAC index of pain, stiffness and physical function having scores on 5-point Likert scale as shown in Table 1. And change from baseline and mean value per visit for WOMAC index [34,35].
Table 1.
WOMAC OA index parameters – interpretations and scores.
| WOMAC PARAMETERS | |
| PAIN (5) | |
| Walking, Stair Climbing; Nocturnal, Rest; Weight bearing | |
| STIFFNESS (2) | |
| Morning Stiffness; Stiffness occurring later in the day | |
| PHYSICAL FUNCTIONS (17) | |
| Descending stairs; Ascending stairs; Rising from sitting; Standing; Bending to floor; Walking on flat; Getting in or out of car; Going shopping; Putting on socks/stockings; Rising from the bed; Taking off socks/stockings; Lying in bed; Getting in/out of bath; Sitting; Getting on/off toilet; Heavy domestic duties; Light domestic duties | |
| Response | Points/score |
| None | 0 |
| Slight | 1 |
| Moderate | 2 |
| Severe | 3 |
| Extreme | 4 |
Safety measures selected were, adverse effect, dermal-irritation scores, changes in vital signs of the patient obtained at each visit.
Statistical analysis: For WOMAC index of pain, stiffness and function subscales, calculation was done at, changes from baseline at each visit for each subject, available data at that visit. The changes were analyzed from baseline on the WOMAC subscales using analysis of covariance models with treatment as fixed effect and corresponding baseline value as covariate. Statistical analyses were performed with SPSS statistical software. Standard statistical methods were used for descriptive statistics. Kruskal-Wallis test, followed by Student's t-test (a parametric test), was used to determine the significance of differences in mean WOMAC score between groups at different time intervals. All the statistical tests were two-tailed [[35], [36], [37]]. First, the efficacy of the Emulgel® was compared with placebo, when p ≤ 0.05, lipogel was compared with placebo and with Emulgel®. Thus, no adjustment was required for type I error. A threshold value of p ≤ 0.05 was considered as statistically significant.
3. Results and discussion
Patients included in the study was found to age range between 23 and 75 years, with 24 females and 12 males. It took around 1 year to complete the study on all selected patients.
The outcome data obtained were of ordinal and non-parametric nature. Hence, Kruskal-Wallis test was employed to find out the difference in treatments (p ≤ 0.05). Comparison was made amongst all the groups simultaneously for pain, stiffness and physical function [35,37,38]. Baseline characteristics for different treatments have been enlisted in Table 2.
Table 2.
Baseline characteristics of patients randomized to lipogel, Emulgel®, or placebo.
| WOMAC OA index parameter | Average score at different time intervals (week) | ||||
| 1st | 2nd | 3rd | 4th | 6th | |
| Group 1 | |||||
| Pain | 12.92 ± 3.5 | 12.00 ± 3.19 | 10.46 ± 3.33 | 9.31 ± 2.84 | 7.46 ± 2.73 |
| Stiffness | 6.08 ± 1.19 | 5.69 ± 1.49 | 4.92 ± 1.38 | 4.54 ± 1.26 | 3.92 ± 1.55 |
| Physical function | 50.54 ± 10.60 | 47.08 ± 11.01 | 44.46 ± 12.57 | 38.46 ± 11.36 | 32.85 ± 10.39 |
| Group 2 | |||||
| Pain | 13.75 ± 5.22 | 13.08 ± 4.83 | 11.75 ± 4.37 | 10.64 ± 3.75 | 9.73 ± 3.72 |
| Stiffness | 6.50 ± 1.31 | 6.00 ± 1.65 | 5.75 ± 1.48 | 5.45 ± 1.13 | 4.91 ± 1.14 |
| Physical function | 51.25 ± 11.04 | 49.67 ± 10.26 | 47.82 ± 10.75 | 44.64 ± 8.83 | 40.45 ± 8.60 |
| Group 3 | |||||
| Pain | 11.67 ± 4.72 | 11.67 ± 4.64 | 12.17 ± 4.88 | 12.50 ± 4.58 | 12.83 ± 4.67 |
| Stiffness | 5.67 ± 1.97 | 5.92 ± 1.93 | 6.08 ± 1.97 | 6.42 ± 1.62 | 6.5 ± 1.44 |
| Physical function | 47.25 ± 13.17 | 49.08 ± 13.63 | 49.75 ± 14.59 | 51.42 ± 14.21 | 53.67 ± 13.58 |
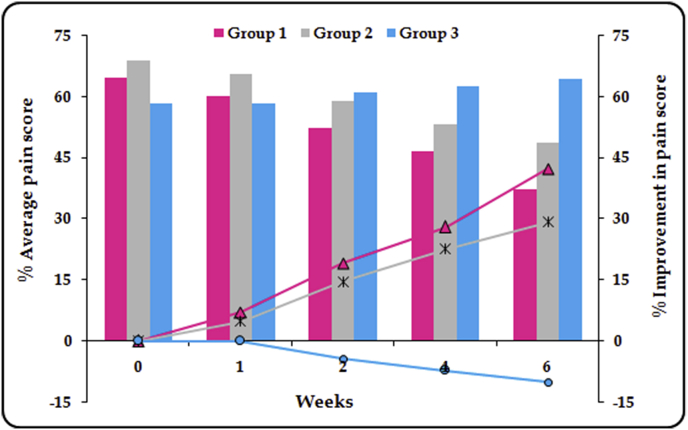
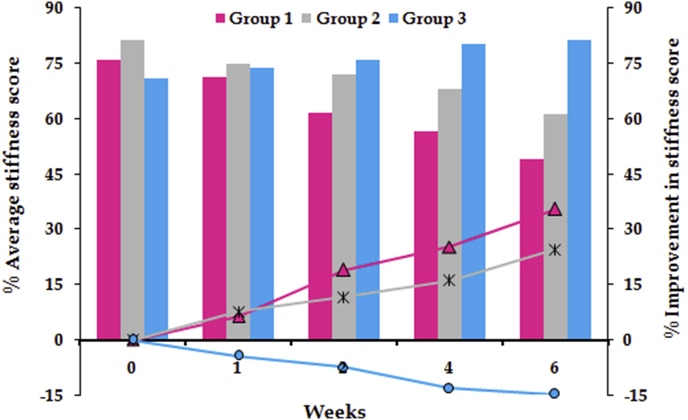
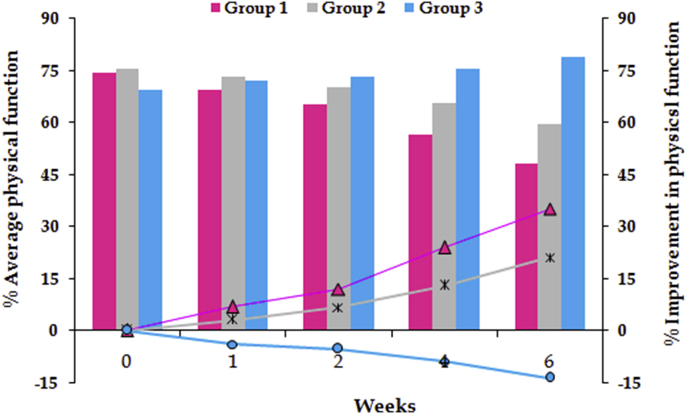
For stiffness and physical function, the significant difference between all the groups was observed after 4 weeks of treatment, while for the pain, significant difference was ascertained later on the 6th week. Followed by application of Kruskal-Wallis test, the Student's t-test for statistical comparison was performed between the two active treatments (viz. lipogel and Emulgel®) and placebo. For pain score, a significant difference (p ≤ 0.05) was observed between Group 1 and Group 3 for whole treatment period, whereas significant difference between Group 2 and Group 3 was recorded only after 4 weeks treatment (Fig. 1). For stiffness, Group 1 and Group 3 showed significant difference (p ≤ 0.01) for throughout period of treatment. On the other hand, significant difference (p ≤ 0.05) was observed between Group 2 and Group 3 only after 4 weeks of treatment (Fig. 2). Analogous results were obtained for physical function (Fig. 3). In a nutshell, it was found that both of the active treatments were superior to the placebo. Topically applied lipogel was significantly more effective than the placebo for all outcome measures i.e., pain (p = 0.002), physical function and stiffness (p = 0.01). The efficacy of the DLF lipogel was found to be significantly superior (p ≤ 0.05) to the Emulgel® for improving stiffness and physical functions of treated patients 4 weeks onwards, whereas the pain relieving action was found to be comparable. Overall change in the WOMAC index after different treatments portrayed in Fig. 4.
Fig. 1.
Percent pain score and its improvement during six weeks of treatment with different formulations.
Fig. 2.
Percent stiffness and its improvement during six weeks of treatment with different formulations.
Fig. 3.
Percent score of physical function and its improvement during six weeks of treatment with different formulations.
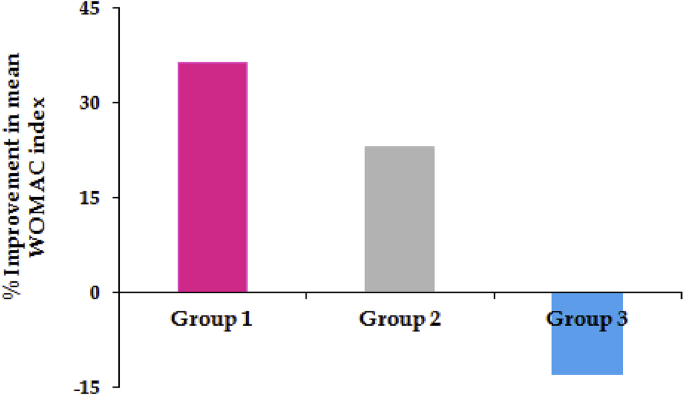
Fig. 4.
Percent improvement in mean WOMAC OA index after six weeks treatment with different active and placebo formulations.
As portrayed in Fig. 4 improvement in WOMAC index after 6 weeks was found be higher with diclofenac lipogel (i.e., 36.39% ± 4.76) as compared to Emulgel® (i.e., 22.94% ± 3.22). On the other hand it deceases with placebo gel (i.e., −13.03% ± 2.01), indicating no effect of this gel. With respect to safety evaluation, all the tested formulations were found to be safe, as no dermal as well as GI adverse event was recorded during complete period of study. The results once again affirm the potential of diclofenac lipogel, as topical drug delivery system, for the management of OA.
4. Conclusions
In conclusion, the data from this randomized trial demonstrated that the studied diclofenac lipogel offered better analgesic effect to that of the marketed product as well as placebo. Despite efficacy, the safety was substantially enhanced. As the product was better tolerated and offered significant improvement than the other test formulations, it can be concluded that the liposomal diclofenac based gel can be a better option for the treatment of symptoms of chronic osteoarthritis over the oral NASID therapy.
Registration of trial
The clinical trial was registered at Clinical Trial Registry India (reference). National Institute of Medical Statistics, Indian Council of Medical Research with reference number CTRI/2012/12/003263.
Author declaration
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Declaration of competing interest
The Author(s) declare(s) that there is no conflict of interest.
Acknowledgements
The authors acknowledge the financial support from the M/s Life Care Innovations (P) Ltd., Gurgaon, India, for the preparation of test formulation(s). The authors are grateful to M/s Biochem Pharmaceutical Industries, Mumbai, India and M/s Lipoid GmbH, Ludwigshafen, Germany, for the ex-gratis supply of diclofenac diethylamine and phospholipids, respectively.
Contributor Information
Amit Bhatia, Email: drbhatiaamit@gmail.com.
Vijay Goni, Email: vijaygoni@gmail.com.
Shruti Chopra, Email: shrutichopra0981@gmail.com.
Bhupinder Singh, Email: bsbhoop@gmail.com.
Om Prakash Katare, Email: katare@pu.ac.in.
References
- 1.Haq I., Murphy E., Darce J. J. Osteoarthritis. Postgrad. Med. J. 2003;79:377–383. doi: 10.1136/pmj.79.933.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay S.I., Abajobi A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittenauer R., Smith L., Aden K. 2013. Priority Medicines for Europe and the World “A Public Health Approach to Innovation “Update on 2004 Background Paper Background Paper 6.12 Osteoarthritis World Health Organization. [Google Scholar]
- 4.Maetzel A., Li L., Pencharz J., Tomlinson G., Bombardier C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann. Rheum. Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitton R. The economic burden of osteoarthritis. Am. J. Manag. Care. 2009;15:S230–S235. [PubMed] [Google Scholar]
- 6.Gokhale C.N., Simon S.S., Hadaye R.S., Lavangare S.R. A cross-sectional study to screen community health volunteers for hip/knee-osteoarthritis and osteoporosis. J. Fam. Med. Prim. Care. 2019;8:2101–2105. doi: 10.4103/jfmpc.jfmpc_261_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman R.D., Hochberg M.C., Moskowitz R.W., Schnitzer T.J. Recommendations for the medical management of Osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Skou S.T., Roos E.M., Simonsen O., Laursen M.B., Rathleff M.S., Arendt-Nielsen L. The effects of total knee replacement and non-surgical treatment on pain sensitization and clinical pain. Eur. J. Pain. 2016;20:1612–1621. doi: 10.1002/ejp.878. [DOI] [PubMed] [Google Scholar]
- 9.Saleh K.J., Davis A. Measures for pain and function assessments for patients with osteoarthritis. J. Am. Acad. Orthop. Surg. 2016;24:e148–e162. doi: 10.5435/JAAOS-D-16-00303. [DOI] [PubMed] [Google Scholar]
- 10.Argoff C.E., Gloth F.M. Topical nonsteroidal anti-inflammatory drugs for management of osteoarthritis in long-term care patients. Therapeut. Clin. Risk Manag. 2011;7:393–399. doi: 10.2147/TCRM.S24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan K.M., Arden N.K., Doherty M., Bannwarth B., Bijlsma J.W.J., Dieppe P. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann. Rheum. Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motsko S.P., Rascati K.L., Busti A.J., Wilson J.P., Barner J.C., Lawson K.A. Temporal relationship between use of NSAIDs, including selective COX-2 inhibitors, and cardiovascular risk. Drug Saf. 2006;29:621–632. doi: 10.2165/00002018-200629070-00007. AJ. [DOI] [PubMed] [Google Scholar]
- 13.Harirforoosh S., Asghar W., Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharmaceut. Sci. 2013;16:821–847. doi: 10.18433/j3vw2f. [DOI] [PubMed] [Google Scholar]
- 14.Jung J.H., Kang K.P., Kim W., Park S.K., Lee S. Nonsteroidal antiinflammatory drug induced acute granulomatous interstitial nephritis. BMC Res. Notes. 2015;(8):793. doi: 10.1186/s13104-015-1788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungprasert P., Srivali N., Thongprayoon C. Nonsteroidal anti-inflammatory drugs and risk of incident heart failure: a systematic review and meta-analysis of observational studies. Clin. Cardiol. 2016;39:111–118. doi: 10.1002/clc.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benazzo F., Rossi S.M., Ghiara M., Zanardi A., Perticarini L., Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45:S98–S104. doi: 10.1016/j.injury.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Lanas A., Tornero J., Zamorano J.L. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann. Rheum. Dis. 2010;69:1453–1458. doi: 10.1136/ard.2009.123166. [DOI] [PubMed] [Google Scholar]
- 18.Hodge B., Skolnik D. Clinical inquiry: does topical diclofenac relieve osteoarthritis pain? J. Fam. Pract. 2015;64:124–125. [PubMed] [Google Scholar]
- 19.Goregaonkar A., Mathiazhagan K.J., Shah R.R., Kapoor P.S., Taneja P., Sharma A. Comparative assessment of the effectiveness and tolerability of lornoxicam 8 mg BID and diclofenac 50 mg TID in adult indian patients with osteoarthritis of the hip or knee: a 4-week, double-blind, randomized, comparative, multicenter study. Curr. Ther. Res. Clin. Exp. 2009;70:56–68. doi: 10.1016/j.curtheres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baer P.A., Thomas L.M., Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Muscoskel. Disord. 2005;6:44. doi: 10.1186/1471-2474-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rother M., Lavins B.J., Kneer W., Lehnhardt K., Seidel E.J., Mazgareanu S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral cefecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann. J. Rheumatol. Dis. 2007;66:1178–1183. doi: 10.1136/ard.2006.065128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revel F.B., Fayet M., Hagen M. Topical diclofenac, an efficacious treatment for osteoarthritis: a narrative review. Rheumatol. Ther. 2020;7:217–236. doi: 10.1007/s40744-020-00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niethard F.U., Gold M.S., Solomon G.S., Liu J.M., Unkauf M., Albrecht H.H. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J. Rheumatol. 2005;32:2384–2392. [PubMed] [Google Scholar]
- 24.Ueberall M.A., Mueller-Schwefe G.H., Wigand R., Essner U. Efficacy, tolerability, and safety of an oral enzyme combination vs diclofenac in osteoarthritis of the knee: results of an individual patient-level pooled reanalysis of data from six randomized controlled trials. J. Pain Res. 2016;9:941–961. doi: 10.2147/JPR.S108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller P., Roth S. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J. Pain Res. 2011;4:159–167. doi: 10.2147/JPR.S20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tugwell P.S., Wells G.A., Shainhouse J.Z. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J. Rheumatol. 2004;31:2002–2012. [PubMed] [Google Scholar]
- 27.Wagner V., Dullaart A., Bock A.-K., Zweck A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 28.Kriwet K., Müller-Goymann C.C. Diclofenac release from phospholipid drug systems and permeation through excised human stratum corneum. Int. J. Pharmaceutics. 1995;125:231–242. [Google Scholar]
- 29.Raza K., Singh B., Mahajan A., Negi P., Bhatia A., Katare O.P. Design and evaluation of flexible membrane vesicles (FMVs) for enhanced topical delivery of capsaicin. J. Drug Target. 2011;19:293–302. doi: 10.3109/1061186X.2010.499464. [DOI] [PubMed] [Google Scholar]
- 30.Cevc G., Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J. Contr. Release. 2010;14:277–299. doi: 10.1016/j.jconrel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Ethical Guidelines for Biomedical Research on Human Participants. Indian Council of Medical Research; 2006. [Google Scholar]
- 32.Bhatia A., Singh B., Katare O.P. Flexible-vesicle loaded with an anti-inflammatory agent: development, optimization, characterization and evaluation. AAPS J. 2008;10(S2):858. [Google Scholar]
- 33.Goggins J., Baker K., Felson D. What WOMAC pain score should make a patient eligible for a trial in knee osteoarthritis? J. Rheumatol. 2005;32:540–542. [PubMed] [Google Scholar]
- 34.Tubach F., Baron G., Falissard B., Logeart B.I., Dougados M., Bellamy N. Using patients' and rheumatologists' opinions to specify a short form of the WOMAC function subscale. Ann. Rheum. Dis. 2005;64:75–79. doi: 10.1136/ard.2003.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellamy WOMAC N. A 20-year experiential review of a patient-centered self- reported health status questionnaire. J. Rheumtol. 2002;29:2473–2476. [PubMed] [Google Scholar]
- 36.Escobar A., Quintana J.M., Bilbao A., Aróstegui I., Lafuente I., Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF036 after total knee replacement. Osteoarthritis Cartilage. 2007;15:273–280. doi: 10.1016/j.joca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Salaffi F., Leardini G., Canesi B., Mannoni B.A., Fioravanti A., Caporali R. Reliability and validity of the western Ontario and McMaster Universities (WOMAC) osteoarthritis index in Italian patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11:551–560. doi: 10.1016/s1063-4584(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia A., Kumar R., Katare O.P. Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J. Pharm. Pharmaceut. Sci. 2004;7:252–259. [PubMed] [Google Scholar]