Summary
Human embryonic kidney cells HEK293 can be used for the production of therapeutic glycoproteins requiring human post-translational modifications. High cell density perfusion processes are advantageous for such production but are challenging due to the shear sensitivity of HEK293 cells. To understand the impact of hollow filter cell separation devices, cells were cultured in bioreactors operated with tangential flow filtration (TFF) or alternating tangential flow filtration (ATF) at various flow rates. The average theoretical velocity profile in these devices showed a lower shear stress for ATF by a factor 0.637 compared to TFF. This was experimentally validated and, furthermore, transcriptomic evaluation provided insights into the underlying cellular processes. High shear caused cellular stress leading to apoptosis by three pathways, i.e. endoplasmic reticulum stress, cytoskeleton reorganization, and extrinsic signaling pathways. Positive effects of mild shear stress were observed, with increased recombinant erythropoietin production and increased gene expression associated with transcription and protein phosphorylation.
Subject Areas: Bioengineering, Biophysics, Cell Biology
Graphical Abstract
Highlights
-
•
Fluid dynamics, transcriptomics, and phenotype study to understand the perfusion impact
-
•
Mild shear stress has favorable effects on protein transcription and phosphorylation
-
•
High shear stress provokes apoptosis by three different pathways
-
•
Average shear rate in hollow filter cell separation device ATF is lower than in TFF
Bioengineering; Biophysics; Cell Biology
Introduction
Operating a bioreactor in perfusion mode allows for a continuous renewal of the culture medium, generating a stable and favorable environment in the bioreactor, which can benefit the cell metabolism and growth but even more importantly allows higher volumetric yield and product quality (Chotteau, 2015; Gomez et al., 2019). This has recently awakened an increasing interest in the biopharmaceutical field. Perfusion processes require smaller bioreactors and reduced footprint compared to batch or fed-batch processes, leading to lower capital expenditure. Numerous continuous processes for mammalian cell cultures have been reported for manufacture of products such as monoclonal antibodies, glycoproteins and baculovirus (Takamatsu et al., 1996; Wang et al., 2002; Zhu, 2012; Merten et al., 1999).
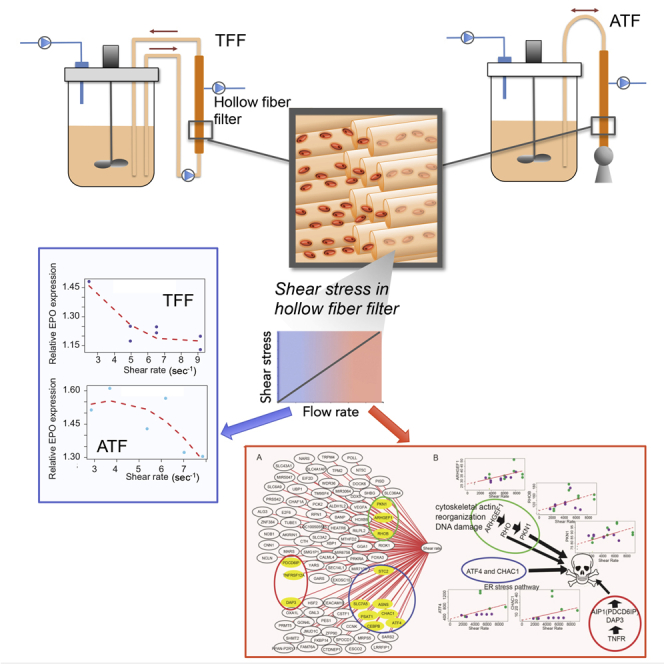
A suitable cell retention device is critical for operating a successful perfusion process. Several cell separation techniques are industrially used for perfusion operations, e.g. gravity-based cell settler, spin filters, centrifuge, tangential flow filtration (TFF) and alternating tangential flow filtration (ATF), as reviewed in previous reports (Castilho and Medronho, 2002; Voisard et al., 2003; Woodside et al., 1998). TFF can minimize the filter fouling since the particles are not pressed into the filter membrane. Among these systems, hollow fiber filter (HF)-based cell separation is efficient and has shown to support very high cell density for biopharmaceutical manufacturing (Gálvez et al., 2012; Clincke et al., 2013a, 2013b). Introduced by Shevitz (Shevitz, 2000), alternating TFF can further reduce the filter fouling by creating a back flush in the filter membrane. In the ATF, the cell suspension is pumped from the bioreactor to the HF and vice versa thanks to a diaphragm pump mounted at one end of the HF, while the cell suspension is circulated only in one direction in the TFF using a peristaltic pump.
Many mammalian cells are sensitive to shear or mechanical force, and various studies have shown that high levels of shear can affect the cell viability and growth (Garcia-Briones and Chalmers, 1994; Gregoriades et al., 2000). Ideally, to achieve high cell density while maintaining a high productivity, the cells should not be submitted to significant shear or mechanical damage. Therefore, the operation parameters should be carefully chosen such that the hydrodynamic conditions do not significantly damage the cells. In the HF-based perfusion culture setting, there are two main locations where shear cell damage occurs: the bioreactor and the cell separation device. In the bioreactor, the aeration and associated foam and bubble formations can be detrimental, as well as the agitation generated by the impeller(s) for culture homogenization. The energy dissipation rate (EDR) accounts for all potential types of fluid stress and is commonly used to characterize the fluid flow and characterize the hydrodynamic conditions that can damage the cells in bioprocesses of established cells. Chalmers and Ma (Chalmers and Ma, 2015) studied the EDR generated from different sources in the bioreactor and identified non-lethal and lethal effects on the cells. The EDR and shear stress are related in a way specific to the source of the shear damage. Concerning the cell separation device, shear is created by the passage of the cells in the hollow fiber lumens. The flow in a hollow fiber lumen can be characterized as Poiseuille flow for Newtonian fluids.
Whilst Chinese Hamster Ovary (CHO) cells are the workhorse of the biopharmaceutical industry, alternative cells of human origin such as HEK293 cells can provide recombinant glycoproteins with human post-translational modifications that are of critical importance for certain therapeutic indications. For instance, HEK293 cells have been recently adopted for the production of several recombinant factors of the blood coagulation cascade such as factor VIII but also commercially explored for the production of erythropoietin or enzymes (Kumar, 2015; Lalonde and Durocher, 2017; Llop et al., 2008; Swiech et al., 2012).
The purpose of the present study was to understand how the shear stress generated by a HF used for cell separation by TFF or ATF could impact HEK293 cells. We studied theoretically the velocity profile and shear stress for these two filtration systems. To support these results, we studied the sensitivity of a HEK293 cell line producing recombinant human erythropoietin (rhEPO) to hydrodynamic forces in parallel batch experiments using mini bioreactors system equipped with TFF or ATF at different flow rates. This phenotypic study was complemented by a systematic investigation of the global functional response of HEK293 cells to shear stress by transcriptomics. We introduced a functional map of the impact of shear stress on the HEK293 cells, manifested in cellular functions including immune and inflammatory responses, oxidative stress, cytoskeleton reorganization, endoplasmic reticulum (ER) stress, apoptosis, and cell cycle. This study provides a detailed experimental and theoretical study to understand the shear stress effects on HEK293 cells occurring in TFF and ATF systems during perfusion operation.
Results
Theoretical Considerations - Shear Stress Characterization
Shear stress develops when a liquid is in motion due the relative movement of fluid particles with each other. The shear stress, which is the force exerted on the cells in a culture, is due to the velocity gradient and not directly to the flow rate itself. Thus the same flow rate can lead to different levels of shear stress. In a laminar flow, the shear rate is the velocity gradient and the shear stress is equal to the shear rate times the viscosity. The TFF and ATF systems were described in the Introduction and a schematic representation is given in Figure 1. In a TFF, the re-circulation fluid velocity is constant while in an ATF it has a sinusoidal profile varying between a maximum value and zero (Figure S1), due to flow in alternating directions. Consequently, for a given re-circulation flow rate, the shear rate in the HF of a TFF, γTFF, is constant while it is varying in an ATF. The maximal absolute value of the shear rate in the ATF is equal to γTFF and achieved twice per cycle but the instantaneous absolute shear rate is lower most of the time. In the range 0 to π, the average absolute shear rate in the ATF, , can be expressed as a function of its maximum absolute value (equal to γTFF), as follows:
| (Equation 1) |
where u is the velocity of the fluid (m/s), ∂U0/∂y = γTFF is the constant shear rate occurring in the TFF, ω is the frequency of the ATF cycle (s−1) calculated from the flow rate of the ATF, t is time. Detailed calculations are given in Supplemental Information - Transparent Methods - Section A.1.
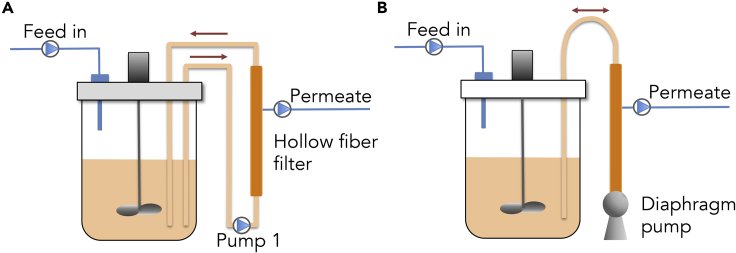
Figure 1.
Overview of a Bioreactor Connected to Hollow Fiber Filter Cell Separation for Perfusion Process by TFF or ATF
Schematic diagram of (A) tangential flow filtration, TFF, where pump 1 was an Alitea XV pump with a flow rate of up to 0.19 L/min, or two Alitea XV pumps mounted in parallel for 0.38 L/min, or the diaphragm pump of an ATF2 mounted with two one-way valves for flow rates >0.38 L/min; (B) alternating tangential flow filtration, ATF.
This result implies that the average absolute shear rate is lower in the ATF than in the TFF and is 2/π ≈ 0.637 of the shear rate in the TFF, although the instantaneous shear rate in the ATF system has a maximum equal to the constant shear rate in the TFF system. So, the average absolute shear stress in the ATF, τATF = τTFF 2/π ≈ 0.637 τTFF. For instance, for a flow rate of 1 L/min, the shear stress in the ATF and the TFF are 8.3 and 13 N/m2, respectively, (applying S.3 and S.4).
Shear Stress Effect on Cell Growth
To experimentally confirm the results obtained from the theoretical exercise of “Section Theoretical considerations - Shear stress characterization”, parallel batch cultivations were performed with cells subjected to the ATF or the TFF systems, see Figure 1. As a control in an attempt to remove the effect of the pumps and to consider only the shear stress effect from the passage in the hollow fibers of the TFF independently of the pump, a preliminary investigation was performed to characterize a system where pumping did not affect the cells. For this, the bioreactors were equipped with a system similar to the TFF perfusion culture where the HF cartridge was shortcut, i.e. removed. With this system, the effect of cell damage at a flow rate of 0.3 L/min, operated by a peristaltic pump, was studied in 5-day batch mode using two different pump tubing's of the same diameter: GORE STA-PURE and PharMed. It was observed that the PharMed pump tube led to low cell viability and poor growth after one day of culture as shown in Figure 2A. Isn contrast, the cell culture using GORE STA-PURE pump tube had a high growth rate and viability. The GORE STA-PURE has a smooth inner wall in Teflon while the PharMed has a rough inner surface deteriorating the cells during the pumping in the peristaltic pump. In all the subsequent experiments, the GORE STA-PURE tube was systematically used in the peristaltic pump of the TFF system.
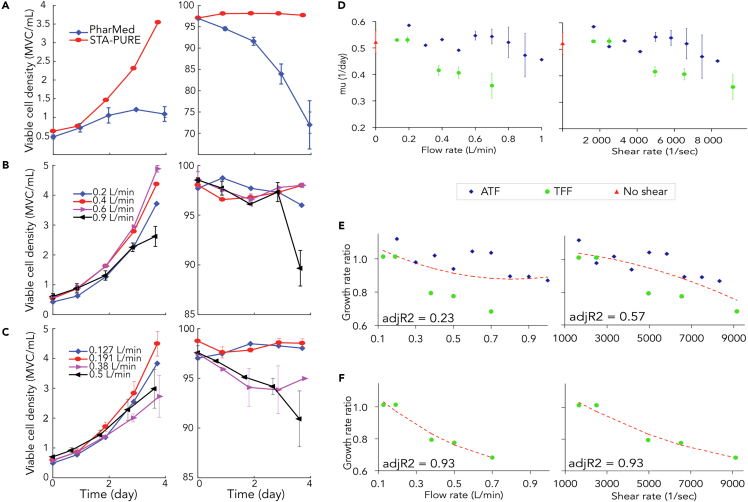
Figure 2.
Effect of the Flow Rate and the Shear Rate on the Cell Growth and Viability
Viable cell density and viability in batch cultures performed with (A) pump tubes GORE STA-PURE or PharMed, (B) ATF at different flow rates and (C) TFF at different flow rates; error bars indicate duplicate runs. Effect of flow rate and average shear rate on the cell growth in ATF and TFF systems operated at different flow rates; growth rate, average of the values at days 2 and 3, represented as function of the flow rate (D-left) and the shear rate (D-right) calculated by Equations 1 and S15 – error bars give the variation in repeated experiments listed in Material and Method. Effect of flow rate and shear rate on the average growth rate ratio (growth rate ratio = growth rate/control growth rate, where control is absence of flow 'No shear') by regression analysis (red lines) for ATF and TFF (E), and for TFF only (F) showing a strong correlation between the growth rate ratio and the flow rate or the shear rate in TFF system, as indicated by the adjusted R2 value.
The effect of the shear stress occurring in the hollow fiber cartridge in ATF and TFF systems was studied for different flow rates in 5-day batch culture as described in Transparent Methods. The operating conditions for ATF were 0.2–1 L/min (shear stress: 1.6–7.5 N/m2) and for TFF were 0.127–0.7 L/min (shear stress 1.6–9.1 N/m2), with absence of re-circulation 'No shear' as control condition.
The viable cell density and viability observed with different ATF or TFF flow rates are exemplified in Figures 2B and 2C. In both systems, there was no significant effect on the cell viability when the cells were re-circulated in these systems at the lowest flow rates, corresponding to the lowest shear stress. The cell viability decreased with time in the case of the high flow rates indicating that high flow rates and thus high shear rates led to cell death. The cell growth rate as function of increasing flow rate or shear stress using ATF and TFF systems is represented in Figure 2D-left and 2D-right. It can be seen that the growth rates using the TFF system clearly decreased with increasing flow rates for values ≥0.38 L/min. In the case of the ATF system, a similar decrease was mainly observed from flow rates ≥0.7 L/min Figure 2D-right represents the growth rate as a function of the average shear rate γ. This provides an indication of the effect of the shear rate on the cell growth or of the shear stress τ, which is directly proportional to the shear rate (Equation S3). Considering the average shear rate, which is theoretically lower by a factor 0.637 in the ATF system compared to the TFF, it was observed in Figure 2D-right that for shear rates larger than 4900 s−1 defined here as γthreshold, or shear stress larger than 5 N/m2, defined here as τthreshold, the growth rate was significantly lower, independently of the system, ATF or TFF (p value 0.0145). Polynomial regression analysis also predicted that the growth rate ratio (growth rate ratio = growth rate/control growth rate, where control is absence of flow 'No shear') is strongly associated with shear stress especially in TFF (adj R2 > 0.57, Figure 2F). It showed that high shear stress decreased the growth rate, but that a low shear stress had an enhancement effect of the growth rate.
Global Cellular Response to Shear Stress
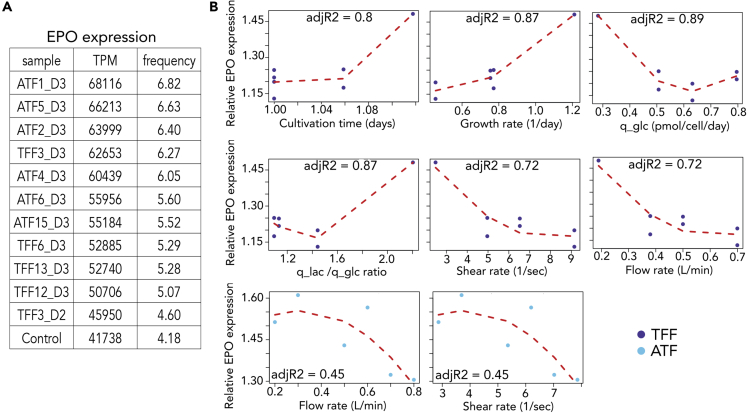
Here, we sought to characterize the global cellular response of shear stress at the transcriptome level by comparing gene expression under different conditions, i.e. filtration modes and shear rate. A list of the cultures used for the transcriptome sampling with their corresponding applied flow rate conditions is given in Table 1. Principal component analysis (PCA) showed clear differences in the overall gene expression responses to ATF, TFF, flow rate, shear stress, and cultivation time (Figures 3A and 3B). For instance, cells grown in TFF3 conditions at 0.2 L/min flow rate with two-day cultivation time (TFF3_D2) showed complete separation from those with three-day cultivation time (TFF3_D3) and ATF2_D3, all of which shared shear stress and flow rate. The TFF3_D2 samples were excluded from downstream analysis since these corresponded to a shear applied during a shorter time than three days, represented added sample heterogeneity, and showed clearly distinct responses from the remaining samples. Substantial differences were identified among all the ATF conditions in response to flow rate and shear stress, within similar cultivation time. This was the case of ATF4_D3 and ATF5_D3 at 0.5 and 0.6 L/min, which were grouped together and had similar shear stress (Table S1 - Count data and TPM data for different flow rate using ATF or TFF), and were distinct from ATF15_D3 at 0.8 L/min.
Table 1.
List of the Culture Conditions and Identifiers for Samples Subjected to Transcriptomic Analysis
| Experiment Name | Flow Rate (L/min or LPM) | Shear Rate (1/s) | Shear Stress (N/m2) | Sample Name/Culture day | Sample Name/Culture day |
|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | Control/day 2 | Control |
| TFF3 | 0.19 | 2482 | 2.48 | TFF3_D2/day 2 | TFF3_D3/day 3 |
| TFF6 | 0.38 | 4965 | 4.96 | TFF6_D2/day 2 | TFF6_D3/day 3 |
| TFF12 | 0.7 | 9146 | 9.15 | TFF12_D2/day 2 | TFF12_D3/day 3 |
| TFF13 | 0.5 | 6533 | 6.53 | TFF13_D2/day 2 | TFF13_D3/day 3 |
| ATF2 | 0.2 | 1865 | 1.86 | ATF2_D2/day 2 | ATF2_D3/day 3 |
| ATF1 | 0.3 | 2697 | 2.70 | ATF1_D2/day 2 | ATF1_D3/day 3 |
| ATF4 | 0.5 | 4361 | 4.36 | ATF4_D2/day 2 | ATF4_D3/day 3 |
| ATF5 | 0.6 | 5194 | 5.19 | ATF5_D2/day 2 | ATF5_D3/day 3 |
| ATF6 | 0.7 | 6026 | 6.03 | ATF6_D2/day 2 | ATF6_D3/day 3 |
| ATF15 | 0.8 | 6858 | 6.86 | ATF15_D2/day 2 | ATF15_D3/day 3 |
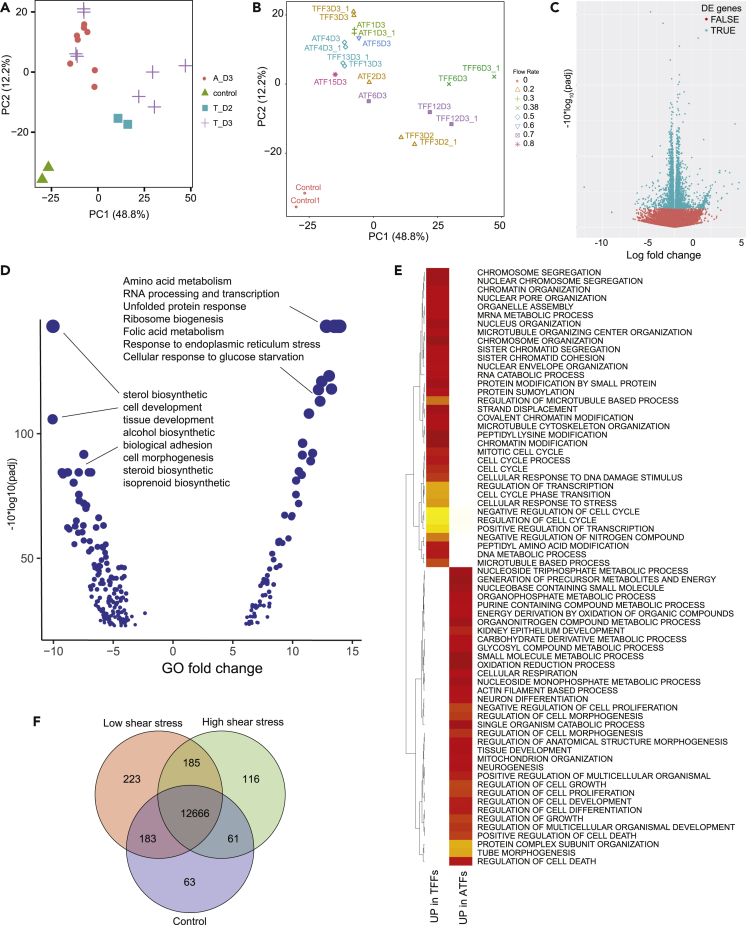
Figure 3.
Cells Display Distinct Gene Expression Responses to Shear Stress
(A) PCA of Log2-normalized gene expression in control, ATF cultivated for 3 days (ATF_D3), and TFF cultivated for 2 (TFF_D2) or 3 (TFF_D3) days.
(B) PCA with respect to different flow rates.
(C) Differentially expressed genes (cyan, FDR <0.05) and non-differentially expressed genes (red) as function of Log2-fold change differences, between ATF and TFF.
(D) Enriched biological processes (FDR <0.005) as function of biological process change, for the comparison between high and low shear stress. Dot sizes are proportional to the significance.
(E) Biological processes exclusively up-regulated (FDR <0.001) in TFF and ATF. Colors indicate up-regulation strength, from least (yellow) to most (red) enriched terms.
(F) Venn diagram illustrating genes with TPM value > 1 for low, high, and control shear stress conditions – Data in Tables S1, S2, S3, and S4
Further reinforcing these distinct responses, several hundreds of genes were differentially expressed between ATF, TFF and shear stress levels (FDR <0.01, Table S2 – Differential gene expression between ATF, TFF, high, low or no shear). Differentially expressed genes amount to 415 between ATF vs. TFF (Figures 3C), 538 between high vs. low shear stress, among substantial differences identified between each condition and control. These differences translated into significant alterations in biological processes (FDR <0.01; Table S3 - Significant alterations in cell biological processes between ATF, TFF, high, low or no shear), and reflected shear stress-specific responses. When compared with low shear stress, high shear stress samples display up-regulated amino acid and amide metabolism, RNA processing and transcription, unfolded protein response (UPR), ribosome biogenesis and translation, folic acid metabolism, response to ER stress such as apoptosis, response to glucose starvation. On the other hand, high shear stress samples displayed down-regulated sterol, steroid, isoprenoid and alcohol biosynthesis, cell and tissue development and morphogenesis, cell-cell adhesion and signaling and lipid biosynthetic process (FDR <0.005, Figure 3D). Additionally, high and low shear rate showed up-regulation of processes associated with cell cycle proliferation and mitosis, chromosome segregation (FDR <0.005) when compared with controls (Table S3). TFF samples with high shear stress displayed up-regulated cell cycle proliferation and response to stress (Figures 3E and Table S3) in comparison with ATF samples. This corroborated the observation of Figure 2D that the growth rate significantly decreased with the shear rate in TFF samples while this trend was less marked in ATF samples. Furthermore the metabolic processes were up-regulated for these latter. This point will be discussed below with the analysis of the glucose metabolism in “Effect of the shear rate on the cell metabolism”.
Shear Stress-Related Cytoskeleton/Cell Adhesion Reorganization and Cell Death
As seen in Figure 3F, several shear stress-specific genes with TPM value > 1 could be identified. For each condition, specific on/off genes related to cytoskeleton, cell adhesion and morphogenesis were found. For instance, DCN (decorin), COL23A1, COL16A1, COL5A1, WTIP, CDH8, SBK2, NKX23, PTPRB, PSTPIP1, ESAM, CDH8, SNED1, and PCDHB11 were suppressed by high shear stress (found under normal and low shear rate), whereas SYNPO, CXCL1, ARC, PRR4, SRPX2, AOC3, CD72, ITGAX, and KLHL10 were expressed under high shear stress (Table S4 - Genes expressed at specific shear stress levels: low, high, no shear, or high and low). Furthermore, some of the genes were related to immune and inflammatory response. Besides, some of the cytoskeleton and morphogenesis genes showed expression just in the low shear stress group and included COL4A4, COL19A1, ICAM2, COL14A1, LAMB3, ITGB3, SCIN, FRY, CORO2B, MYO7A, FGD5, NUAK2, PFN4, and NTF3. Additionally, the gene set enrichment analysis showed that the expression of the genes associated with cell development and morphogenesis was down-regulated in high shear stress groups (Figure 3D).
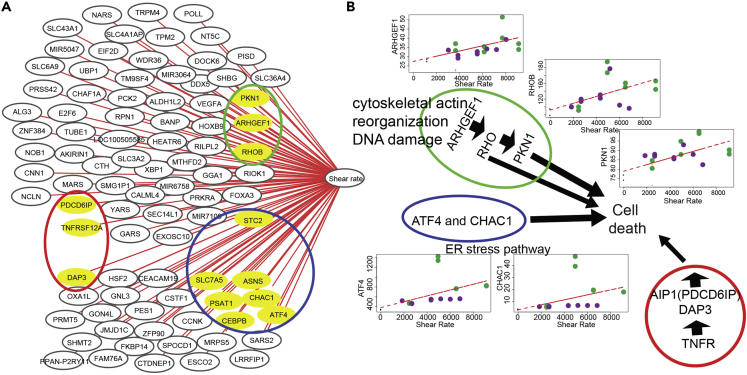
We then computed pairwise Spearman rank correlations between gene expression (TPM values) and sample metadata to identify potential relationships between metadata and co-regulated or functionally associated genes (highest Spearman's ρ 0.7, FDR <10−4). Figure 4A shows the association between gene expression and shear stress and Figure 4B illustrates the apoptosis pathways associated with shear stress. Among the significantly correlated genes, three groups were identified to be associated with apoptosis. The first group included ARHGEF1, RHOB and PKN1, which are shear stress-correlated genes and are represented in Figure 4B. These are associated with cytoskeleton reorganization and DNA damage (Table S5 – List of genes with expression associated with shear stress). They may trigger apoptosis through the activation of RhoB, a small GTPase regulating cytoskeletal reorganization and cell growth (Prendergast, 2001; Liu et al., 2001; Price and Collard, 2001; Kim et al., 2009). The second group included TNFR, DAP3, and PDCD6IP, genes associated with extrinsic signal-induced cell death (Rauert et al., 2011). The third group included genes involved in response to ER including UPR such as ATF4_gene, CHAC1, and CEBPB. UPR can be stimulated by several physiological changes including glucose shortage, hypoxia and genome instability, processes identified above through gene enrichment analysis. These genes are tightly co-regulated, for example, CEBPB controls ATF4_gene expression, which in turn regulates expression of CHAC1, a proapoptotic ER stress protein (Crawford et al., 2015) (Figure 4B). Because UPR may also be initiated in response to amino acid limitation (Su and Kilberg, 2008), we computed amino acid usage in the culture by quantifying their cell-specific consumption rate. Polynomial regression analysis showed that the consumption of all the amino acids except glutamine and alanine was weakly associated with the shear rate in ATF and had a strong correlation with the shear rate in TFF (Figure S3). Interestingly, differential expression analysis showed that the above-mentioned genes were significantly expressed in high, but not in low shear stress conditions. Overall, all observations indicated that high shear stress stimulated apoptosis associated with ER stress, cytoskeleton reorganization, and extra-cellular pathway.
Figure 4.
Association between Gene Expression and Shear Stress
(A) Genes with expression correlated with the shear stress, highlighting three groups of genes associated with apoptosis detailed in the right panel. (B) Apoptosis pathways associated with shear stress; cytoskeleton reorganization and DNA damage, ER stress pathway and extrinsic signal-induced cell death. Data in Tables S5.
Effect of the Shear Rate on the Cell Metabolism
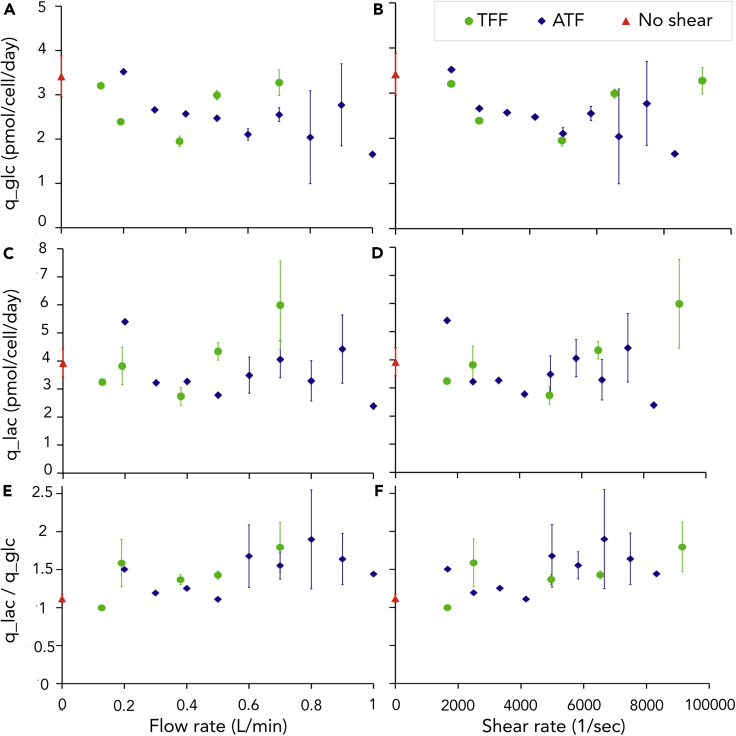
Figures 5A–5D show the cell specific rates of glucose consumption, qgluc, and lactate production, qlac, as function of increasing flow rate and shear rate in TFF and ATF systems. Both qgluc and qlac first decreased with increasing flow rates until 0.38 L/min and 0.6 L/min for the TFF and the ATF systems, respectively. It then increased with increasing flow rate. When representing qgluc and qlac as function of the shear rate, the trends for both systems were comparable with a thresholdγthreshold of 4900 s−1 (or shear stress threshold τthreshold). For shear rates < γthreshold, qgluc and qlac decreased with the shear rate while this parameter had a tendency to increase again for shear rates > γthreshold. Two regions separated by γthreshold (or) τthreshold were thus observed: a low shear region where qgluc and qlac decreased with increasing shear stress, and high shear region where qgluc and qlac tended to increase with increasing shear stress. The ratio of qlac over qgluc represented in Figures 5E and 5F indicated that qlac/qgluc was stable for shear rates < γthreshold and increasing with the flow rate and shear rate (or shear stress) for shear rates > γthreshold. This tendency was significant (p value 0.0147) according to pump had an operating range t test for qlac/qgluc separated in these two regions, i.e. shear rates < γthreshold and shear rates > γthreshold.
Figure 5.
Effect of Different Flow Rates in ATF and TFF Systems on the Cell Specific Glucose Consumption Rate, qgluc, the Cell Specific Lactate Production Rate, qlac, and the Ratio qlac/qgluc
(A) qgluc as function of the flow rate.
(B) qgluc as function of the shear rate calculated by Equations 1 and S15.
(C) qlac as function of the flow rate.
(D) qlac as function of the shear rate.
(E) qlac/qgluc as function of the flow rate.
(F) qlac/qgluc as function of the shear rate – all rates were averages of the values at days 2 and 3; the error bars give the variation in repeated experiments and control is absence of flow 'No shear'
DAVID was used to perform an enrichment analysis (Huang et al., 2009) on the KEGG metabolic pathway. It showed that amino acid biosynthesis, folate metabolism, and aminoacyl-tRNA biosynthesis were enriched in high shear stress. Importantly, most genes involved in these pathways showed strong and positive correlations with the shear stress (Figure 4). This was the case for ALDH1L2, MTHFD1L, MTHFD2, and SHMT2 in folate metabolism, YARS, GARS, NARS, MARS, and SARS2 in Aminoacyl-tRNA biosynthesis and CTH, PSAT1, and ASNS in amino acid biosynthesis. Their overexpression under high shear stress was consistent with ER stress and ATF4_gene expression, whereas folate metabolism (through MTHFD1L, MTHFD2) was associated with response to oxidative stress (Celardo et al., 2017). An overview of the main effects of high and of low shear stress on the gene expression is given in Table 2.
Table 2.
Overview of the Main Effects of High and of Low Shear Stress Observed on the Gene Expression
| Effect of High Shear Stress | |
|---|---|
| Genes increased compared to no or low shear stress | Genes decreased compared to no or low shear stress |
|
|
| Shear stress-related cytoskeleton/cell adhesion/morphogenesis and immune/inflammation | |
| Genes expressed at high shear stress compared to no or low shear stress | Genes suppressed at high shear stress compared to no or low shear stress |
|
|
| Genes of cell adhesion, cytoskeleton, cell movement, cell growth expressed only in low shear stress (not in high shear stress or in control) | |
| |
Shear Stress in HEK293 as Mammalian/Human Cell Factory Increases Erythropoietin Expression
Finally, we sought to identify the effect of shear stress and cultivation parameters on the production of rhEPO. It was observed that the cultures with low shear stress, i.e. ≤ 5 N/m2, ATF1_D3, ATF2_D3, ATF5_D3, displayed the highest EPO mRNA expression, representing >6% of the entire protein-coding gene expression pool. Additionally, in comparison with absence of shear (control), the cultures with shear stress displayed higher EPO mRNA expression (Figure 6A). It was observed as well that large growth rates promoted higher EPO mRNA expression (Figure 6B). In turn, flow rate and shear stress in both TFF and ATF systems also promoted a higher mRNA EPO expression compared to absence of shear (control), but less pronounced at higher flow or shear rates. Altogether, this indicates that shear stress has positive effects on EPO expression, especially under low shear stress levels. We also investigated the effect of shear stress on the protein secretion systems using the genes related to secretion, glycosylation and exocytosis, by collecting the genes from MSigDB (Liberzon et al., 2015). The exact Fisher test showed that perturbations of the gene expression between low and high shear stress vs. control were significantly different and significantly different between high and low shear stress. Therefore, it seems that low shear stress has a positive effect on this cell line as a human cell factory.
Figure 6.
Effect of Cultivation Parameters on EPO Production in HEK293 Cells
(A) EPO mRNA expression (TPM) and relative expression with respect to global protein-coding gene expression
(B) Relative mRNA EPO expression (with respect to control EPO expression) and relation with culture parameters.
Dark blue indicates TFF (top and middle rows), and cyan indicates ATF (bottom row)
Discussion
The relationships between the flow rate and the shear rate (S3 and S4) are well established for fluids in pipes and in particular for tangential flow filters. Here for the first time, we calculated the theoretical shear rate and the shear stress in an ATF device and showed that ATF generates a lower shear stress than TFF by a factor 0.637, owing to the difference in velocity profile between TFF and ATF. This was experimentally confirmed in a comparative study of the effect of the ATF and TFF at different flow rates in parallel batch cultures of HEK293 cells equipped with HF to mimic the shear environment that the cells experience in ATF and TFF perfusion operations. It was observed that the growth rate was lower for flow rates ≥0.7 L/min for ATF and ≥0.38 L/min for TFF, indicating that high flow rates and thus high shear rates were unfavorable for the cells. The representation of the growth rate as function of the average shear stress, using the theoretical result of Equation 1, showed a satisfying agreement of the trends obtained with ATF and with TFF. This experimentally confirmed that a lower shear stress is encountered in the HF in ATF mode compared to TFF and confirmed the theoretical relationship of the shear stress τATF = 0.637 τTFF of Equation 1.
To decouple the effect of the shear in the TFF HF from the effect of the peristaltic pumping, a preliminary study was dedicated to the selection of a pump tubing that did not harm the cells. Severe cell damage was observed using the PharMed tube, whereas the cells were not affected by pumping using a GORE STA-PURE tube. It is strongly suggested that the cell damage is attributable to the roughness of the PharMed tube inner wall surface, while the GORE STA-PURE tube has a smooth inner wall surface in PTFE. Certainly, the performance of the GORE STA-PURE tubing is preferable over that of the PharMed tubing for pumping in the TFF system. The pump effect does not occur in the ATF system since a diaphragm pump operates the cell culture re-circulation instead of a peristaltic pump as in the case of TFF.
Focusing on the cell metabolism, it was observed that the influence of the shear stress on the glucose metabolism had a biphasic behavior with two zones of low and high shear stresses separated by a shear stress threshold τthreshold. In the low shear stress region, the cell specific glucose uptake rate, qgluc, and lactate production rate, qlac, decreased with the shear stress and the flow rate, while these metabolic rates increased above the shear stress threshold τthreshold. Interestingly, the same behavior and shear stress threshold, τthreshold, were observed in the case of both ATF and TFF. This also confirmed the theoretical relationship Equation 1 of the average shear stresses occurring in these devices. This biphasic behavior can be interpreted as follows. For τ≤τthreshold the cells benefit of the shear stress, such that higher shear rate induces a more efficient glucose metabolism. However, for τ≥τthreshold the cells are adversely impacted by increasing shear stress, demonstrated by a higher metabolic rate, i.e. higher qgluc and higher qlac, and increased mRNA expression of genes associated with apoptosis.
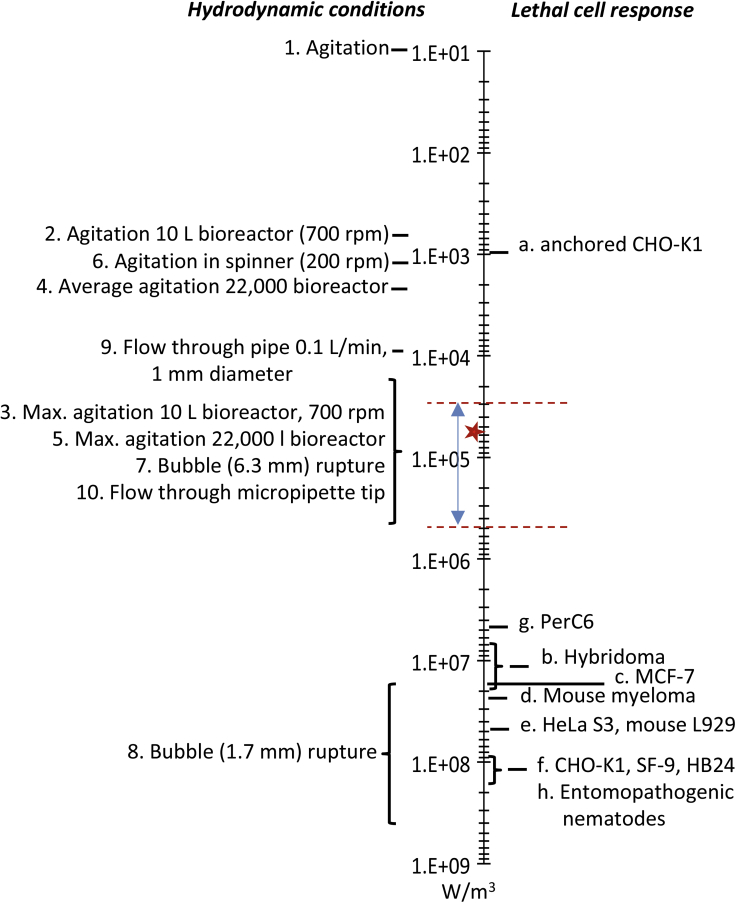
The EDR takes into account all potential types of fluid stress. This parameter is commonly used to characterize hydrodynamic conditions, in particular to evaluate the potential cell damage in bioprocesses (Chalmers, 2015). To compare the presently observed effects with the literature, the EDR in a hollow fiber for an example of 1L/min flow rate was calculated (Equation S11 Supplemental Information - Transparent Methods - Section A.2. and Figure S2), and compared to the work of Ma et al. (Ma et al., 2002) and Mollet et al. (Mollet et al., 2007) in Figure 7. For this flow rate, the EDR ranged between the dotted red lines drawn in Figure 7, in which τthreshold is indicated by a red star. These values were in the same range as previously published work, reported by Mollet et al. (Mollet et al., 2004), about a 0.1 L/min flow through a 1 mm diameter pipe and the flow through in a 200 μL micropipette tip, respectively, marked items 9 and 10 in Figure 7. Although these were performed in different conditions than reported here, these numbers were in agreement with the present findings. These EDR values were significantly lower than the lethal levels reviewed by Chalmers (Chalmers, 2015) for different established cell lines. They were however potentially in the range where non-lethal effects can occur as, for instance, observed by Keane et al. (Keane et al., 2003), who showed that increasing shear stress caused a decrease in the production of recombinant human growth hormone in CHO cells accompanied by increased glucose consumption and decreased lactate production. For high shear stress, the first two effects were observed in the present study to the contrary of the lactate production, which increased here. Notice that to our knowledge, no data of the effect of mechanical shear on HEK293 cells have been reported. Under stress conditions, it can be postulated that the cells require rapid access to energy, which is corroborated by the presently observed ratio qlac/qgluc increasing with the shear rate for τ≥τthreshold, due to a higher glycolysis and increased lactate production, i.e. Warburg effect, instead of oxidative phosphorylation (i.e. Krebs cycle). This increased Warburg effect cannot be attributed to depletion in oxygen since the higher shear rates coincide with a shorter residence time in the cell separation device. Both for TFF and ATF, higher flow rates generate a higher number of cycles per time unit, a higher shear rate and a shorter exposure time to shear per cycle compared to lower flow rates.
Figure 7.
Energy Dissipation Rate Generated by Various Hydrodynamic Effects
Energy dissipation rate generated by various hydrodynamic effects reproduced with format adaptation from Mollet et al. (2007), where the range of the energy dissipation rate for a 1 L/min flow rate (calculated for the passage through the lumen of a hollow fiber of a cartridge CFP-4-E−3MA of 13 fibers and fiber lumen of 1 mm [General Electric Healthcare]) is given between the red dotted lines; the five-pointed star indicates the threshold shear rateγthreshold identified in this study for HEK293 cells at 4900 s−1. Detailed definitions: (1) Agitation – Volume average in typical animal cell bioreactor (Varley and Birch, 1999); (2) Agitation – Volume average in a 10 L mixing vessel, RT 700 RPM (Zhou and Kresta, 1996); (3) Agitation – Maximum in a 10 L mixing vessel, RT 700 RPM (Zhou and Kresta, 1996); (4) Agitation – Volume average in a 22,000 L mixing vessel, RT 240 RPM (Wernersson and Tragarch, 1999); (5) Agitation – Maximum in the 22,000 L mixing vessel, RT 240 RPM (Wernersson and Tragarch, 1999); (6) Agitation – Maximum in spinner vessel, 200 RPM (Venkat et al., 1996); (7) Bubble rupture – Pure water bubble diameter: 6.32 mm (Garcia-Briones et al., 1994); (8) Bubble rupture – Pure water, bubble diameter: 1.7 mm (Boulton-Stone and Blake, 1993; Garcia-Briones et al., 1994); (9) Flow through pipe – Pure water, 100 mL/min, 1 mm diameter (Mollet et al., 2004); (10) Flow through a micropipette tip – Flow through a 200 μL micropipette tip in 0.2 s (Mollet et al., 2004)
Detrimental effects of high shear stress causing cell apoptosis have been previously described (Chalmers, 2015). Interestingly, we reveal here that high shear stress stimulates apoptosis associated with three pathways associated with ER stress, cytoskeleton reorganization, and extra-cellular pathway. We previously revealed oxidative stress at very high cell densities, which could be due to a higher shear stress (Zamani et al., 2018). This is consistent with the present observation of the Gene set enrichment results, where oxidative stress, folate metabolism, and UPR increased with high shear stress. Additionally, many genes involved in these pathways showed strong and positive correlations with shear stress. Folate metabolism (through MTHFD1L, MTHFD2) being associated with responses to oxidative stress is connected to ATF4_gene expression (Celardo et al., 2017). ATF4_gene expression is also involved in the UPR (Crawford et al., 2015) and mitochondrial dysfunction (Melber and Haynes, 2018). Expression of genes associated with UPR and cell stress is elevated under high shear stress conditions, while low shear stress has a positive effect on the cells with higher expression of genes associated with cellular processes, such as transcription, cell cycle, protein phosphorylation and cell division. The aforementioned biological processes steadily increased with shear stress, so that they are substantially up-regulated under high shear stress in comparison with low shear stress where the cells showed increased response to stress (Table S3).
Importantly, we revealed that low shear stress increased the production of the recombinant protein transcription, here rhEPO. To our knowledge, this favorable effect of a mild shear stress has not been reported for stable recombinant cell lines, but it can be interesting to relate this effect with known beneficial effects of mild shear on primary or stem cells. For instance, cell proliferation and protein production increased in human tendon fibroblasts subjected to stretching (Wang and Thampatty, 2006); bovine pulmonary artery endothelial cells exhibited cell proliferation and release of bFGF when subjected to hydrostatic pressure (Wang and Thampatty, 2006); in a vascularization study, low shear stress promoted the proliferation of endothelial progenitor cells and embryonic stem cells, and enhanced their ability to form new vessels (Yamamoto and Ando, 2010); finally, shockwaves are known to reduce wound size, to increase the neo- or re-vascularization, increase proliferation and reduce apoptosis (Huang et al., 2013). Although an increased cell growth was not observed in the present study, the increased rhEPO transcript at low shear rate could be mediated by a comparable mechanism to primary or stem cells.
In conclusion, we have studied the shear stress effect in two TFF systems (ATF and TFF) for perfusion processes of HEK293 cells secreting EPO. Firstly, a theoretical relationship of the average shear stress in the ATF and the TFF systems, was established. Then, experimental investigation identified a shear stress threshold, common for ATF and TFF systems, above which reduced cell growth and altered cell metabolism were observed for HEK293 cells.
There is an increasing interest in upstream perfusion processes as part of the strategies to implement continuous manufacturing processes for economic, commercial production of therapeutic proteins (Patil and Walther, 2017). Numerous perfusion processes have already been successfully operated for CHO cells with ATF or TFF systems (Clincke et al., 2013a, 2013b), enabled by the robust nature of CHO cells. However, other more fragile cell types are emerging as important cell factories to enable the production of complex proteins that require specific post-translational modifications or are difficult-to-express in CHO cells (Tegel et al., 2020). Therefore, a better understanding of the shear stress level and its effect on cells such as HEK293 is important. Here, we have shown that a high shear stress provokes not only reduced cell growth but also a modified metabolism. However, mild shear stress can be favorable for the production of recombinant protein. Furthermore we characterized the different cell-shear interaction regions and deepened the understanding of the cells cultured in hollow fiber-based perfusion systems. We anticipate that the phenomena observed here are also likely to be occurring in other cell types and we believe these learning's should allow for improved design of new perfusion processes.
Limitations of the Study
The present study reveals a shear rate threshold, which is common for the ATF and TFF cell separation devices. It can however be observed that the detrimental effect on the cell growth of the high shear rate is more accentuated for the TFF system than for the ATF. It is unclear why this takes place since the effect of the peristaltic pump has been annihilated and, for the highest flow rate of the TFF experiments, the diaphragm pump of an ATF is used in a setting adapted for TFF. A difference, which is difficult to eliminate in this comparison, is the fact that in the ATF setting, there is only a short tube between the bioreactor and the hollow fiber cartridge, while in the TFF the connection, i.e. connection of the pump feeding the hollow fiber cartridge, is more intricate. Compared to the present small-scale system, the effect of these connections is expected to be milder in large scale due to a higher volume to wall surface ratio for the cell suspension passing the connectors and tubes.
Another aspect is that in order to generate a comparison of a large range of conditions, the present study was performed in batch mode during a time period of 5 days with cells rapidly growing in the control. A limitation is that high cell density perfusion exposes the cells to shear during a much longer time period of at least 4 weeks and most often at lower growth rate (Clincke et al., 2013b). Future investigations could address the effect of repeated shear stress and the interaction with lower growth rate in perfusion mode.
Resource Availability
Lead Contact
Veronique Chotteau (chotteau@kth.se), who should be contacted as main contact and to request further information about the protocols used, while Adil Mardinoglu (adilm@kth.se) should be contacted for bioinformatics matters.
Data and Code Availibility
The data are provided in Tables S1, S2, S3, S4, and S5; and can be found online at Mendeley Data: https://doi.org/10.1016/j.isci.2020.101653.
Materials Availability
The culture medium is commercially available. The cell line is propriety of Johan Rockberg's lab (johan.rockberg@biotech.kth.se).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work has been carried out at the Wallenberg Center for Protein Research with co-funding of the Knut and Alice Wallenberg Foundation and AstraZeneca. Thank you as well to Dr. Jeong Lee and Dr. Luigi Grassi, both from AstraZeneca, for revising the manuscript and for their insightful inputs.
Author Contributions
Conceptualization, V.C.; Methodology, C.Z., R.F., R.T., J.R., and V.C.; Investigation, C.Z., G.B., H.S., M.M., Am.M., R.F., R.T., D.H., P.V., Ad.M., J.R., and V.C.; Formal Analysis: C.Z., G.B, Ad.M. and V.C. Writing, C.Z., G.B., H.S., M.M., Am.M., R.F., R.T., D.H., P.V., Ad.M., J.R., and V.C.; Supervision, J.R., Ad.M., R.F., R.T., D.H., P.V., and V.C.
Declaration of Interests
G.B. is currently employee of AIVIVO Ltd, UK. C.S. and P.V. are currently employees of Kymab Ltd, UK.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101653.
Supplemental Information
References
- Boulton-Stone J.M., Blake J.R. Gas bubbles bursting at a free surface. J. Fluid Mech. 1993;254:437–466. [Google Scholar]
- Castilho L.R., Medronho R.A. Cell retention devices for suspended-cell perfusion cultures. In: Schügerl K., Zeng A.P., editors. Tools and Applications of Biochemical Engineering Science. Springer Berlin Heidelberg; 2002. pp. 129–169. [DOI] [PubMed] [Google Scholar]
- Celardo I., Lehmann S., Costa A.C., Loh S.H., Martins L.M. dATF4 regulation of mitochondrial folate-mediated one-carbon metabolism is neuroprotective. Cell Death Differ. 2017;24:638–648. doi: 10.1038/cdd.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J.J. Mixing, aeration and cell damage, 30+ years later: what we learned, how it affected the cell culture industry and what we would like to know more about. Curr. Opin. Chem. Eng. 2015;10:94–102. [Google Scholar]
- Chalmers J.J., Ma N. Hydrodynamic damage to animal cells. In: Al-Rubeai M., editor. Animal Cell Culture. Springer, Cham.; 2015. pp. 169–183. [Google Scholar]
- Chotteau V. Perfusion processes. animal cell culture. In: Al-Rubeai M., editor. Animal Cell Culture. Springer, Cham; 2015. pp. 407–443. [Google Scholar]
- Clincke M.F., Mölleryd C., Samani P.K., Lindskog E., Fäldt E., Walsh K., Chotteau V. Very high density of Chinese hamster ovary cells in perfusion by alternating tangential flow or tangential flow filtration in WAVE bioreactor™—part II: applications for antibody production and cryopreservation. Biotechnol. Prog. 2013;29:768–777. doi: 10.1002/btpr.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clincke M.F., Mölleryd C., Zhang Y., Lindskog E., Walsh K., Chotteau V. Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor™. Part I. Effect of the cell density on the process. Biotechnol. Prog. 2013;29:754–767. doi: 10.1002/btpr.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.R., Prescott E.T., Sylvester C.F., Higdon A.N., Shan J., Kilberg M.S., Mungrue I.N. Human CHAC1 protein degrades glutathione, and mRNA induction is regulated by the transcription factors ATF4 and ATF3 and a bipartite ATF/CRE regulatory element. J. Biol. Chem. 2015;290:15878–15891. doi: 10.1074/jbc.M114.635144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez J., Lecina M., Solà C., Cairó J.J., Gòdia F. Optimization of HEK-293S cell cultures for the production of adenoviral vectors in bioreactors using on-line OUR measurements. J. Biotechnol. 2012;157:214–222. doi: 10.1016/j.jbiotec.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Briones M.A., Chalmers J.J. Flow parameters associated with hydrodynamic cell injury. Biotechnol. Bioeng. 1994;44:1089–1098. doi: 10.1002/bit.260440910. [DOI] [PubMed] [Google Scholar]
- Garcia-Briones M.A., Brodkey R.S., Chalmers J.J. Computer Simulations of the rupture of a gas bubble at a gas-liquid interface and its implications in animal cell damage. Chem. Eng. Sci. 1994;49:2301–2320. [Google Scholar]
- Gomez N., Barkhordarian H., Lull J., Huh J., GhattyVenkataKrishna P., Zhang X. Perfusion CHO cell culture applied to lower aggregation and increase volumetric productivity for a bispecific recombinant protein. J. Biotechnol. 2019;304:70–77. doi: 10.1016/j.jbiotec.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Gregoriades N., Clay J., Ma N., Koelling K., Chalmers J.J. Cell damage of microcarrier cultures as a function of local energy dissipation created by a rapid extensional flow. Biotechnol. Bioeng. 2000;69:171–182. doi: 10.1002/(sici)1097-0290(20000720)69:2<171::aid-bit6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Holfeld J., Schaden W., Orgill D., Ogawa R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013;19:555–564. doi: 10.1016/j.molmed.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Keane J.T., Ryan D., Gray P.P. Effect of shear stress on expression of a recombinant protein by Chinese hamster ovary cells. Biotechnol. Bioeng. 2003;81:211–220. doi: 10.1002/bit.10472. [DOI] [PubMed] [Google Scholar]
- Kim D.M., Chung K.S., Choi S.J., Jung Y.J., Park S.K., Han G.H., Ha J.S., Song K.B., Choi N.S., Kim H.M. RhoB induces apoptosis via direct interaction with TNFAIP1 in HeLa cells. Int. J. Cancer. 2009;125:2520–2527. doi: 10.1002/ijc.24617. [DOI] [PubMed] [Google Scholar]
- Kumar S.R. Industrial production of clotting factors: challenges of expression and choice of host cells. Biotechnol. J. 2015;10:995–1004. doi: 10.1002/biot.201400666. [DOI] [PubMed] [Google Scholar]
- Lalonde M.E., Durocher Y. Therapeutic glycoprotein production in mammalian cells. J. Biotechnol. 2017;251:128–140. doi: 10.1016/j.jbiotec.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.X., Rane N., Liu J.P., Prendergast G.C. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop E., Gutiérrez-Gallego R., Segura J., Mallorquí J., Pascual J.A. Structural analysis of the glycosylation of gene-activated erythropoietin (epoetin delta, Dynepo) Anal. Biochem. 2008;383:243–254. doi: 10.1016/j.ab.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Ma N., Koelling K.W., Chalmers J.J. Fabrication and use of a transient contractional flow device to quantify the sensitivity of mammalian and insect cells to hydrodynamic forces. Biotechnol. Bioeng. 2002;80:428–437. doi: 10.1002/bit.10387. [DOI] [PubMed] [Google Scholar]
- Melber A., Haynes C.M. UPR(mt) regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten O.W., Manuguerra J.C., Hannoun C., Van der Werf S. Production of influenza virus in serum-free mammalian cell cultures. Dev. Biol. Stand. 1999;98:23–37. [PubMed] [Google Scholar]
- Mollet M., Godoy-Silva R., Berdugo C., Chalmers J.J. Acute hydrodynamic forces and apoptosis: a complex question. Biotechnol. Bioeng. 2007;98:772–788. doi: 10.1002/bit.21476. [DOI] [PubMed] [Google Scholar]
- Mollet M., Ma N., Zhao Y., Brodkey R., Taticek R., Chalmers J.J. Bioprocess equipment: characterization of energy dissipation rate and its potential to damage cells. Biotechnol. Prog. 2004;20:1437–1448. doi: 10.1021/bp0498488. [DOI] [PubMed] [Google Scholar]
- Patil R., Walther J. Continuous manufacturing of recombinant therapeutic proteins: upstream and downstream technologies. In: Kiss B., Gottschalk U., Pohlscheidt M., editors. New Bioprocessing Strategies: Development and Manufacturing of Recombinant Antibodies and Proteins. Springer, Cham; 2017. pp. 277–322. [Google Scholar]
- Prendergast G.C. Actin' up: RhoB in cancer and apoptosis. Nat. Rev. Cancer. 2001;1:162–168. doi: 10.1038/35101096. [DOI] [PubMed] [Google Scholar]
- Price L.S., Collard J.G. Regulation of the cytoskeleton by Rho-family GTPases: implications for tumour cell invasion. Semin. Cancer Biol. 2001;11:167–173. doi: 10.1006/scbi.2000.0367. [DOI] [PubMed] [Google Scholar]
- Rauert H., Stühmer T., Bargou R., Wajant H., Siegmund D. TNFR1 and TNFR2 regulate the extrinsic apoptotic pathway in myeloma cells by multiple mechanisms. Cell Death Dis. 2011;2:e194. doi: 10.1038/cddis.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevitz J. Fluid filtration system. US Patent. 2000;6:424. [Google Scholar]
- Su N., Kilberg M.S. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J. Biol. Chem. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech K., Picanço-Castro V., Covas D.T. Human cells: new platform for recombinant therapeutic protein production. Protein Expr. Purif. 2012;84:147–153. doi: 10.1016/j.pep.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Takamatsu H., Hamamoto K., Ishimura K., Yokoyama S., Tokashiki M. Large-scale perfusion culture process for suspended mammalian cells that uses a centrifuge with multiple settling zones. Appl. Microbiol. Biotechnol. 1996;45:454–457. doi: 10.1007/BF00578455. [DOI] [PubMed] [Google Scholar]
- Tegel H., Dannemeyer M., Kanje S., Sivertsson Å., Berling A., Svensson A.S., Hober A., Enstedt H., Volk A.L., Lundqvist M. High throughput generation of a resource of the human secretome in mammalian cells. New Biotechnol. 2020;58:45–54. doi: 10.1016/j.nbt.2020.05.002. [DOI] [PubMed] [Google Scholar]
- Varley J., Birch J. Reactor design for large scale suspension animal cell culture. Cytotechnology. 1999;29:177–205. doi: 10.1023/A:1008008021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat R.V., Stock L.R., Chalmers J.J. Study of hydrodynamics in microcarrier culture spinner vessels: a particle tracking velocimetry approach. Biotechnol. Bioeng. 1996;49:456–466. doi: 10.1002/(SICI)1097-0290(19960220)49:4<456::AID-BIT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Voisard D., Meuwly F., Ruffieux P.A., Baer G., Kadouri A. Potential of cell retention techniques for large-scale high-density perfusion culture of suspended mammalian cells. Biotechnol. Bioeng. 2003;82:751–765. doi: 10.1002/bit.10629. [DOI] [PubMed] [Google Scholar]
- Wang J.H.C., Thampatty B.P. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wang M.D., Yang M., Huzel N., Butler M. Erythropoietin production from CHO cells grown by continuous culture in a fluidized-bed bioreactor. Biotechnol. Bioeng. 2002;77:194–203. doi: 10.1002/bit.10144. [DOI] [PubMed] [Google Scholar]
- Wernersson E.S., Tragarch C. Scaleup of Rushton turbine agitated tanks. Chem. Eng. Sci. 1999;54:4245–4256. [Google Scholar]
- Woodside S.M., Bowen B.D., Piret J.M. Mammalian cell retention devices for stirred perfusion bioreactors. Cytotechnology. 1998;28:163–175. doi: 10.1023/A:1008050202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ando J.J. Differentiation of stem/progenitor cells into vascular cells in response to fluid mechanical forces. J. Biorheol. 2010;24:1. [Google Scholar]
- Zamani L., Lundqvist M., Zhang Y., Aberg M., Edfors F., Bidkhori G., Lindahl A., Mie A., Mardinoglu A., Field R. High cell density perfusion culture has a maintained exoproteome and metabolome. Biotechnol. J. 2018;13:1800036. doi: 10.1002/biot.201800036. [DOI] [PubMed] [Google Scholar]
- Zhou G., Kresta S.M. Distribution of energy dissipation between convective and turbulent flow for three frequently used impellers. Trans. Inst. Chem. Eng. 1996;74:379–389. [Google Scholar]
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012;30:1158–1170. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.