Abstract
Background & aims
The pathogenesis of chronic inflammatory bowel diseases (Crohn’s disease [CD] and ulcerative colitis) involves dysregulated TH1 and TH17 cell responses, which can be targeted therapeutically by the monoclonal antibody Ustekinumab directed against the joint p40 subunit of IL-12 and IL-23. These cytokines may also regulate the differentiation of T follicular helper (TFH) cells, which promote B cell function in germinal centers. However, the role of TFH cells in CD pathogenesis and impact of Ustekinumab therapy on TFH cell fate in patients are poorly defined.
Methods
Lymphocytes were isolated from peripheral blood (n=45) and intestinal biopsies (n=15) of CD patients or healthy controls (n=21) and analyzed by flow cytometry to assess TFH cell phenotypes and functions ex vivo. In addition, TFH cell differentiation was analyzed in the presence of Ustekinumab in vitro.
Results
TFH cell frequencies in the intestine as well as peripheral blood were associated with endoscopic as well as biochemical evidence of CD activity. CD patients with clinical response to Ustekinumab, but not those with response to anti-TNF antibodies, displayed reduced frequencies of circulating TFH cells in a concentration-dependent manner while the TFH phenotype was not affected by Ustekinumab therapy. In keeping with this notion, TFH cell differentiation was inhibited by Ustekinumab in vitro while TFH cell maintenance was not affected. Moreover, Ustekinumab therapy resulted in reduced germinal center activity in CD patients in vivo.
Conclusions
These data implicate TFH cells in the pathogenesis of CD and indicate that Ustekinumab therapy affects TFH cell differentiation, which may influence TFH-mediated immune functions in UST-treated CD patients.
Keywords: TFH, T Follicular Helper Cell, UST, Ustekinumab, Crohn’s Disease, IBD
Abbreviations used in this paper: CD, Crohn’s disease; CXCL13, C-X-C chemokine ligand 13; CXCR5, C-X-C chemokine receptor 5; HBI, Harvey-Bradshaw index; ICOS, inducible T cell costimulator; IL, interleukin; PBMC, peripheral blood mononuclear cell; PD-1, programmed cell death protein 1; TFH, follicular T helper; TGFβ, transforming growth factor β; TNF, tumor necrosis factor; UST, ustekinumab
Graphical abstract
summary.
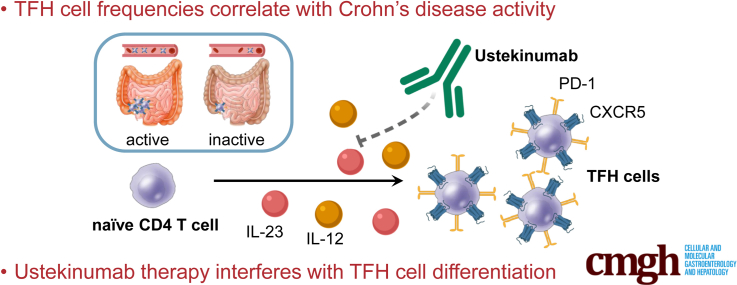
Follicular T helper (TFH) cells are essential for B cell functions. We demonstrate that TFH cell frequencies are associated with Crohn’s disease activity. Therapy with ustekinumab, an interleukin-12/interleukin-23 antibody, affects TFH cell differentiation, thus potentially affecting patients’ TFH-mediated immune responses.
The pathogenesis of Crohn’s disease (CD) is characterized by a complex interplay of intestinal barrier defects, genetic predisposition, and environmental factors, which results in an insufficiently controlled inflammatory response that is critically mediated by CD4+ T cells. Inflammation in CD patients was initially considered to involve a TH1-type response.1,2 However, more recent findings suggest that inflammation in CD patients is also mediated by interleukin (IL)-17– and interferon gamma–coproducing CD4+ T cells.3, 4, 5 While clinical development of secukinumab, a human monoclonal antibody directed against IL-17A, was discontinued in CD patients due to inefficacy and adverse events,6 ustekinumab (UST), a human monoclonal antibody directed against the shared p40 subunit of IL-12 and IL-23, is considered to inhibit the differentiation of TH1 and TH17 cells and is increasingly used to treat patients with CD.7 Besides their effects on TH1 and TH17 cell differentiation, IL-12 and IL-23 have also been implicated in the differentiation of other T cell populations, such as T follicular helper (TFH) cells.8 TFH cells provide essential T cell help to B cells allowing for germinal center formation, development of high-affinity antibodies, and memory B cells.9, 10, 11, 12 TFH cells can be characterized by their expression of the C-X-C chemokine receptor 5 (CXCR5) that mediates their migration toward germinal centers upon ligation by C-X-C chemokine ligand 13 (CXCL13),13,14 and by their high expression levels of programmed cell death protein 1 (PD-1). Activated TFH cells express the costimulatory molecules OX40 and inducible T cell costimulator (ICOS),15,16 as well as CD38.17 Owing to their functions, TFH cells are critically involved in immune responses toward pathogens as well as in vaccine-elicited immune responses and many autoantibody-mediated autoimmune diseases. However, it is still largely unclear whether TFH cells play a role in the pathogenesis of CD and whether UST therapy affects TFH cell fate in patients treated with this drug. Given the important functions of TFH cells in immune responses toward pathogens and vaccines, UST therapy may have a clinically significant impact on immune responses in CD patients. Thus, we set out to analyze the impact of UST on TFH cell differentiation in CD patients.
Results
TFH Cell Frequencies Are Increased in Intestinal Tissue and Peripheral Blood of CD Patients With Active Disease
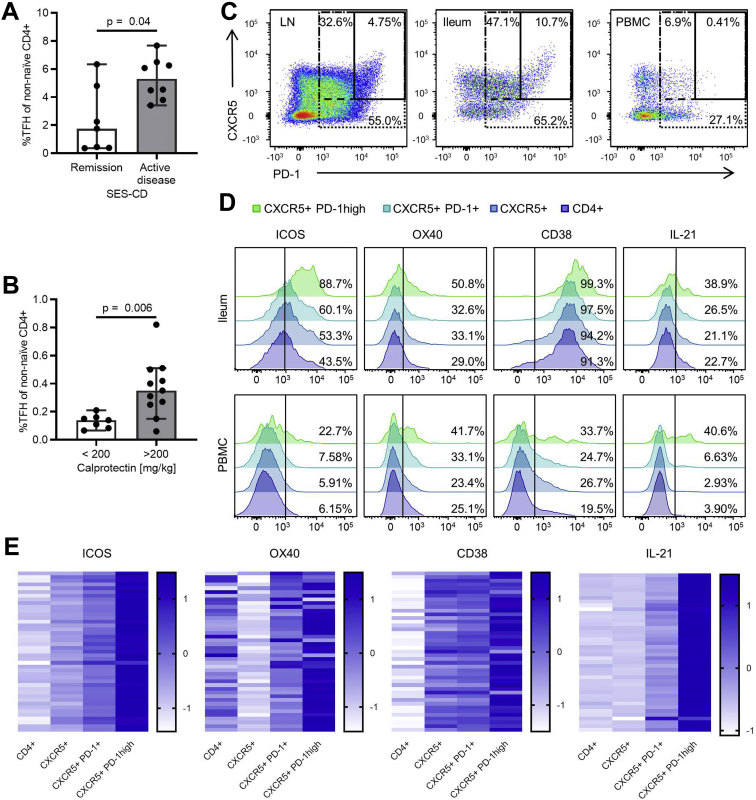
In order to determine potential associations between TFH cell frequencies and disease activity in CD patients, mononuclear cells were isolated from intestinal biopsies from the terminal ileum and peripheral blood of CD patients, and TFH cell frequencies were quantified by flow cytometry. Of note, frequencies of CXCR5+ PD-1 high TFH cells in the terminal ileum were significantly increased in patients with active disease as compared with remission as determined by the Simple Endoscopic Score for Crohn’s Disease (Figure 1A),18 the most widely used endoscopic CD activity marker in clinical trials. Importantly, TFH cell frequencies in the peripheral blood were also significantly increased in CD patients with active intestinal inflammation as determined by fecal calprotectin concentrations above 200 mg/kg, a threshold with high sensitivity and specificity to predict the presence of postoperative recurrence (Figure 1B).19
Figure 1.
TFH cell frequencies correlate with CD activity. (A) TFH cell frequencies in ileal biopsies of CD patients (n = 15) were significantly increased in patients with active inflammation (Simple Endoscopic Score for Crohn’s Disease [SES-CD] ≥3 points) as compared with patients in endoscopic remission (SES-CD ≤2 points). Mann-Whitney test was used to test for statistical significance. (B) Patients with active inflammation (stool calprotectin >200 mg/kg; n = 11) showed significantly higher TFH cell frequencies in the peripheral blood compared with patients in remission (stool calprotectin <200 mg/kg; n = 7). Mann-Whitney test was used to test for statistical significance. Note that this cohort contained patients with both ileal and colonic CD manifestations. (C) Representative flow cytometry plot of TFH cells isolated from a lymph node (LN), the ileum, and the peripheral blood of a CD patient. (D) Representative histograms of phenotypic and functional TFH markers gated on non-naïve CD4+ T cells, CXCR5+ CD4+ T cells, CXCR5+ PD-1+ CD4+ T cells, and CXCR5+ PD-1 high CD4+ T cells isolated from the peripheral blood of a CD patient. (E) CXCR5+ PD-1 high CD4+ T cells from the peripheral blood of CD patients show increased expression of TFH cell activity markers (n = 43) and increased IL-21 production (n = 42) compared with broader TFH definitions (CXCR5+ PD-1+ CD4+ T cells; CXCR5+ CD4+ T cells) and non-naïve CD4+ T cells. Expression after z-score transformation is depicted. TFH cell frequencies are shown as percentage of non-naïve CD4+ T cells.
TFH cells are predominantly localized in lymphatic tissues. The frequencies of CXCR5+ PD-1 high TFH cells were therefore lower in the peripheral blood as compared with terminal ileum or lymph node (Figure 1C). Analysis of the TFH cell activation markers ICOS, OX40, and CD38, as well as production of the master TFH cell cytokine IL-21, revealed that the population of CXCR5+ PD-1 high CD4+ T cells, although present with low frequencies, was strongly enriched for putative functional TFH cells in the intestine and peripheral blood as compared with CXCR5+ PD-1+ CD4+ T cells, CXCR5+ CD4+ T cells, and bulk CD4+ T cells (Figure 1D and E). Therefore, CXCR5+ PD-1 high expression in CD4+ cells was used to define TFH cells in our further experiments.
UST Affects TFH Cell Frequencies in CD Patients in a Concentration-Dependent Manner
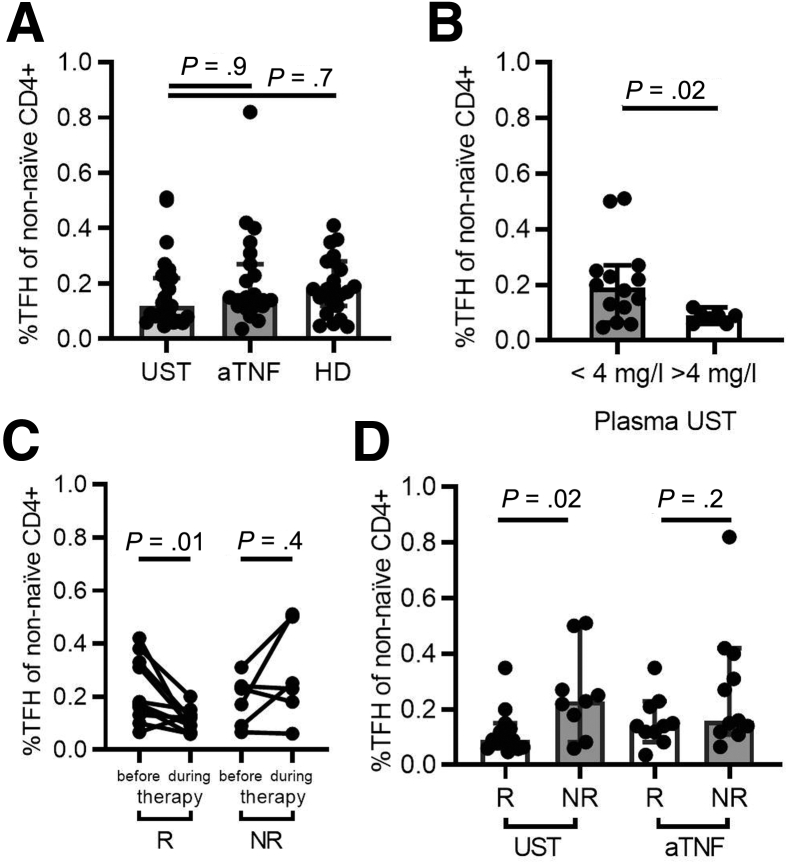
Several cytokines have been implicated in the differentiation of TFH cells from naïve CD4+ T cell precursors.20,21 In humans, both IL-12 and IL-23, in conjunction with transforming growth factor β (TGFβ), have been shown to be of central relevance in this process.8,22 To address the impact of IL-12 and IL-23 inhibition on TFH cells in vivo, we analyzed TFH cell frequencies in the peripheral blood of CD patients treated with UST (mean duration of therapy 15.6 ± 11.8 weeks) or anti-tumor necrosis factor (TNF) antibodies as well as healthy donors (Figure 2A). Disease activity in UST and anti-TNF–treated patients was comparable at baseline as determined by HBI, serum concentrations of C-reactive protein and leukocyte counts in peripheral blood (Table 1). Interestingly, no apparent differences in TFH cell frequencies were observed between these groups. However, it is likely that the effects of UST on T cell populations may be affected by patient-specific factors and differences in UST pharmacokinetics (eg, the time interval between the date of analysis and last UST injection). We therefore measured the UST concentrations in plasma samples of UST-treated CD patients by mass spectrometry and compared CD patients with UST plasma concentrations >4 mg/L, which can be considered as a therapeutic trough concentration,23 with those demonstrating plasma concentrations below 4 mg/L. Importantly, HBI and objective inflammation markers were comparable in these 2 groups (Table 2). Patients with high UST plasma concentrations had significantly reduced TFH cell frequencies compared with CD patients with UST plasma concentrations <4 mg/L (Figure 2B). In keeping with this notion, TFH cell frequencies decreased after initiation of UST therapy in CD patients with clinical response to UST (Figure 2C). Importantly, patients with clinical response to UST had significantly lower peripheral TFH cell frequencies than nonresponders, while this effect was absent in patients treated with TNF inhibitors (Figure 2D). These data suggest that the reduction of TFH cell frequencies is dependent on UST, rather than on a decrease in CD activity, and establish a plasma concentration-dependent effect of UST on TFH cell frequencies in CD patients.
Figure 2.
Response to UST therapy is associated with reduced TFH cell frequencies in CD patients in a concentration-dependent manner. (A) CD patients treated with UST (n = 23) or anti-TNF therapy (aTNF) (n = 21) as well as healthy donors (HD) (n = 22) demonstrate similar TFH cell frequencies in the peripheral blood. Patients were matched for age and gender. Kruskal-Wallis test and Dunn’s multiple comparisons test were used to test for statistical significance. (B) UST-treated CD patients with high UST plasma concentrations (n = 7) show significantly lower TFH cell frequencies compared with CD patients with lower UST plasma concentrations (n = 14). Mann-Whitney test was used to test for statistical significance. (C) Longitudinal analysis of blood samples collected before initiation of therapy and during therapy was performed. TFH cell frequencies significantly decreased in clinical responders to UST (R) (n = 10) following initiation of therapy. This effect was absent in nonresponders (NR) (n = 6). Wilcoxon matched-pairs test was used to test for statistical significance. (D) Responders to UST therapy (n = 14) show lower TFH cell frequencies in the peripheral blood compared with nonresponders (n = 9). This effect is absent in aTNF-treated patients (R: n = 10; NR: n = 11). Mann-Whitney test was used to test for statistically significant differences. TFH cell frequencies are shown as percentage of non-naïve CD4+ T cells.
Table 1.
Characteristics of Patients Recruited Into the Study
| UST (n = 23) | TNF (n = 21) | HD (n = 22) | P Value | |
|---|---|---|---|---|
| Patients included in longitudinal analysis | 17 | — | — | — |
| Age, y | 35.1 ± 10.7 | 35.38 ± 9.0 | 34.8 ± 10.9 | — |
| Female | 16 (70) | 15 (71) | 15 (68) | — |
| HBI | 6.7 ± 9.1 | 4 ± 3.9 | — | .4 |
| C-reactive protein, mg/L | 23 ± 38 | 26 ± 77 | — | .14 |
| Leukocytes, ×103/μL | 8.9 ± 2.3 | 9.3 ± 4.1 | — | .9 |
| Current therapy with prednisone | 2 (9) | 0 (0) | — | — |
| Current therapy with budesonide | 1 (4) | 1 (5) | — | — |
| Previous therapy with azathioprine | 18 (76) | 16 (76) | — | — |
| Previous therapy with MTX | 7 (30) | 2 (10) | — | — |
| Previous therapy with 6-mercaptopurine | 2 (9) | 1 (5) | — | — |
| Previous therapy with mesalamine | 12 (52) | 4 (19) | — | — |
| Previous therapy with 1 anti-TNF-AB | 9 (39) | 5 (24) | — | — |
| Previous therapy with 2 anti-TNF-AB | 12 (52) | 0 (0) | — | — |
| Previous therapy with 3 anti TNF-AB | 2 (9) | 0 (0) | — | — |
| Previous therapy with vedolizumab | 4 (17) | 1(5) | — | — |
| Stricturing disease | 13 (57) | 11 (58) | — | — |
| Penetrating disease | 10 (43) | 8 (41) | — | — |
| Previous CD-related surgery | 15 (65) | 11 (58) | — | — |
| Clinical responders | 14 (61) | 10 (48) | — | — |
Values are mean ± SD or n (%). Markers of disease activity and inflammation between UST and anti-TNF–treated patients were analyzed by Mann-Whitney test.
AB, antibody; CD, Crohn’s disease; HBI, Harvey-Bradshaw Index; HD, healthy donor; MTX, methotrexate; TNF, tumor necrosis factor; UST, ustekinumab.
Table 2.
Inflammation Markers in UST-Treated CD Patients
| UST <4 mg/L (n = 14) | UST >4 mg/L (n = 7) | P Value | |
|---|---|---|---|
| HBI | 6.5 ± 7.7 | 7 ± 12.7 | .3 |
| C-reactive protein, mg/L | 17 ± 17 | 34 ± 60 | .65 |
| Leukocytes, ×103/μL | 8.9 ± 2.7 | 8.7 ± 1.9 | >.9 |
Values are mean ± SD.
CD, Crohn’s disease; HBI, Harvey-Bradshaw Index; UST, ustekinumab.
UST Therapy Does Not Affect the TFH Cell Phenotype In Vivo
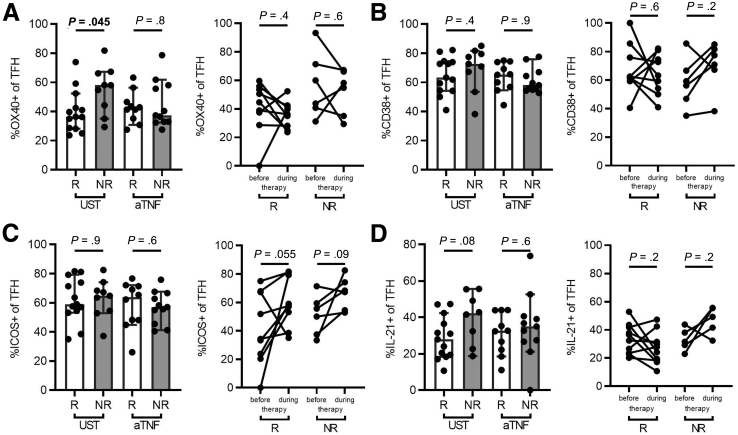
Because UST may affect both TFH cell differentiation and TFH cell phenotypes and functions, we assessed the expression of the TFH cell activation markers CD38, ICOS, and OX40 as well as the production of IL-21 by TFH cells in the peripheral blood of CD patients with response and nonresponse to UST. TFH cells in the blood of responders to UST showed a moderately but significantly reduced expression of OX40 as compared with nonresponders, which was absent in anti-TNF–treated patients (Figure 3A). In contrast, we did not observe any effects of UST therapy on the expression of CD38 or ICOS by TFH cells or on IL-21 production (Figure 3B–D). These findings suggest that UST does not profoundly alter the TFH cell phenotype and subsequent IL-21 production in vivo.
Figure 3.
UST therapy does not affect the TFH cell phenotype in vivo. (Left panels) Expression of the TFH activation markers (A) OX40, (B) CD38, and (C) ICOS, as well as (D) IL-21 production by TFHs was analyzed in responders (R) and nonresponders (NR) to UST (n = 13 and n = 8–9, respectively) or TNF inhibitors (aTNF) (n = 10 and n = 11, respectively). (Right panels) Expression of these markers was also assessed before and during therapy with UST (n = 9-10) or aTNF (n = 5–6). Wilcoxon test or Mann-Whitney tests were used as appropriate and P values are indicated.
UST Inhibits TFH Cell Differentiation In Vitro
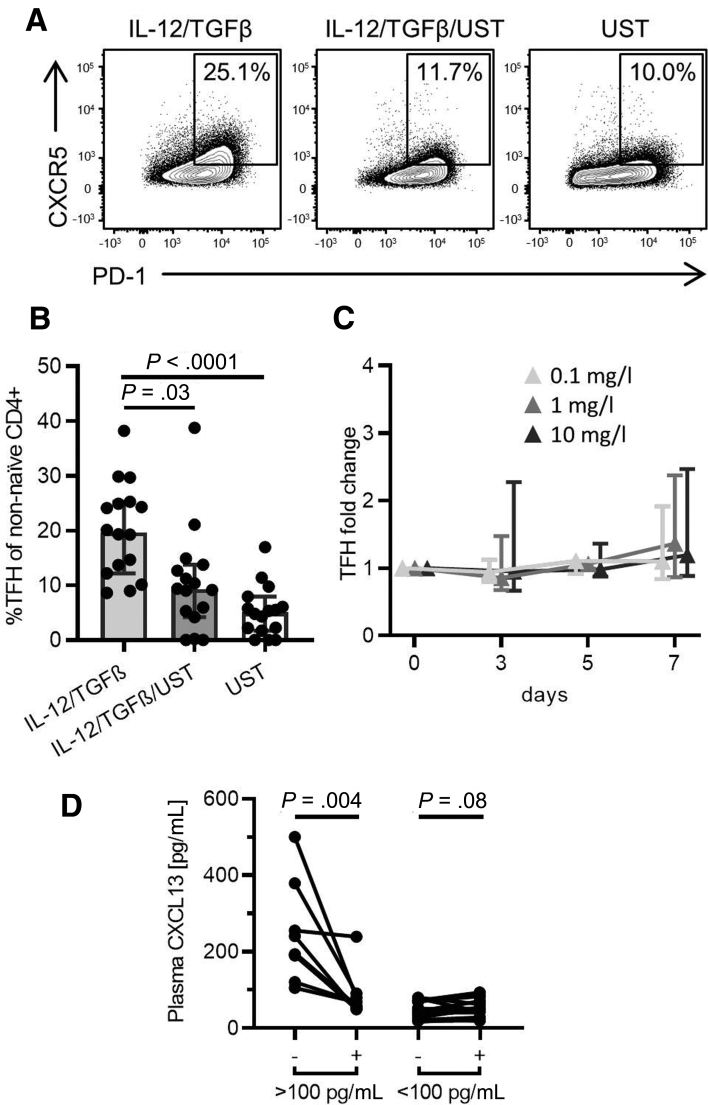
In order to address the question whether UST affects TFH cell differentiation, naïve CD4+ T cells were isolated from the peripheral blood of healthy donors and a TFH cell differentiation assay with addition of IL-12 and TGFβ was performed in the presence or absence of UST, respectively. Importantly, addition of UST significantly inhibited TFH cell differentiation in vitro (Figure 4A and B). In addition to its influence on differentiation, we sought to investigate whether UST would also affect the maintenance of TFH cells. In order to investigate this important question, we cultured peripheral blood mononuclear cells (PBMCs) in the presence of increasing concentrations of UST and analyzed TFH cell frequencies at different time points during in vitro culture. Interestingly, the presence of UST did not affect the relative frequencies of TFH cells and therefore does not appear to affect TFH cell maintenance in vitro (Figure 4C). In sum, these data demonstrate that UST has the potential to impair TFH cell differentiation from naïve CD4+ T cells but does not appear to affect the maintenance of differentiated TFH cells.
Figure 4.
UST significantly inhibits the differentiation of TFH cells in vitro and affects germinal center activity in vivo. (A, B) Representative flow cytometry plot and bar graph demonstrate inhibition of IL-12–/TGFβ-driven TFH differentiation by UST (n = 16). (C) Ustekinumab does not influence TFH cell maintenance in vitro (n = 5–6). TFH cell frequencies were normalized to untreated control patients. Friedman test and Dunn’s multiple comparisons test were used to test for statistical significance. (D) Elevated germinal center activity decreases following initiation of UST therapy. Germinal center activity was assessed by plasma CXCL13 concentrations in CD patients (>100 pg/mL: n = 9; <100 pg/mL: n = 18). Wilcoxon matched-pairs test was used to test for statistical significance. TFH cell frequencies are shown as percentage of non-naïve CD4+ T cells.
UST Therapy Affects Germinal Center Activity In Vivo
Inhibition of TFH cell differentiation may potentially have far-reaching consequences by compromising B cell function. We thus determined germinal center activity in UST-treated patients before and after 8 weeks of UST therapy by assessing plasma CXCL13 concentrations, an established blood marker of germinal center activity.24 This analysis revealed a significant reduction of plasma CXCL13 concentrations in CD patients with elevated CXCL13 concentrations at baseline (>100 pg/mL), while there were no significant alterations in patients in which CXCL13 concentrations were already low (<100 pg/mL) before initiation of UST therapy (Figure 4D). In summary, these findings provide evidence that UST therapy affects TFH cell differentiation and germinal center activity in CD patients.
Discussion
The pathogenesis of CD is linked to dysregulated CD4+ T cell functions and characterized by increased TH1 and TH17 as well as impaired regulatory T cell responses.25 In addition to these well-established CD4+ T cell populations, TFH cells have been implicated in the pathogenesis of several chronic inflammatory and autoimmune diseases.26, 27, 28, 29, 30 However, it is still unclear whether TFH cells play a role in the pathogenesis of CD, which is not dependent on the formation of autoantibodies. Here, we demonstrate that TFH cell frequencies in the terminal ileum correlate with endoscopic CD activity in patients. Moreover, frequencies of circulating TFH cells were increased in patients with active intestinal inflammation as determined by increased fecal calprotectin concentrations. These data suggest that TFH cells are indeed relevant for CD pathogenesis. Our findings are consistent with a previous report that serum concentrations of the key TFH cell cytokine IL-21 were increased in patients with inflammatory bowel disease.31 Increased expression of IL-21 was also observed in colonic tissue of patients with ulcerative colitis.32 TFH cell frequencies heavily depend on the marker definition used for their characterization. TFH cells may be defined as CXCR5+ CD4+ cells yielding TFH cell frequencies around 20% in peripheral blood (Figure 1C).33 TFH cell frequencies are considerably lower if more specific markers such as PD-1 or ICOS are used (2%–10%)26 and are well in the range of the frequencies we describe here when CXCR5+ PD-1 high cells are analyzed.34 Importantly, this subset most closely resembles lymphatic tissue–derived TFH cells.34 Moreover, analysis of putative markers of TFH cell function such as ICOS, OX40, or IL-21 revealed that the population of CXCR5+ PD-1 high CD4+ cells, although being present at rather low frequencies, contains the functionally relevant TFH cell population. However, the mechanistic functions of TFH cells in the pathogenesis of CD remain poorly defined in humans. In mice, expression of the transcription factor ATF3 protects against experimental colitis by regulating Bcl-6 transcription and subsequent TFH cell function.35 Further mechanistic analyses of circulating and intestinal TFH cells isolated from CD patients are therefore needed in order to clarify the functional relevance of TFH cells in CD.
Our present study analyzed the impact of UST therapy on TFH cell frequencies and functions in CD patients given the established role of IL-12 and IL-23 during TFH cell differentiation.20, 21, 22 UST interfered with IL-12 and TGFβ-dependent TFH cell differentiation in vitro. Moreover, UST therapy affected frequencies of circulating TFH cells in CD patients with clinical response to therapy. Importantly, by correlating TFH cell frequencies with UST plasma concentrations, we were able to take into account potential pharmacokinetic differences in UST dosage, duration of therapy, and intervals between drug injection and flow cytometric analysis. Our findings are consistent with the observation that frequencies of CXCR5+ circulating memory TFH cells in peripheral blood as well as TFH cell function in germinal centers are impaired in patients with IL-12 receptor β1 deficiency.36 In addition to these direct effects on TFH cell differentiation, UST may also exert indirect effects in that by reducing inflammation it may reduce cellular exposure to other TFH cell inducing cytokines such as IL-6. In contrast, TFH cell maintenance was not affected by co-incubation of PBMCs with UST in vitro. OX40 expression was slightly but significantly reduced in circulating TFH cells isolated from CD patients with response to UST therapy, while expression of CD38 and ICOS as well as production of IL-21 were not affected. This difference in OX40 expression is unlikely to result in biologically relevant differences in TFH phenotypes given the fact that loss of function mutations in OX40 do not affect TFH cell and antibody responses,30,37 thereby suggesting that UST indeed affects TFH cell differentiation, rather than the TFH cell phenotype.
Our study has the potential limitation that we analyzed the impact of UST therapy on circulating TFH cells, rather than intestinal TFH cells. Although the frequencies of circulating TFH cells were rather low, it was difficult to obtain repeated intestinal biopsies for ethical reasons, and it is generally accepted that circulating TFH cells are reflective of lymphoid TFH cells.38 Moreover, intestinal TFH cell analysis may be affected by sampling errors because biopsies may contain variable amounts of lymphocytes from lymphatic follicles in which TFH cells are enriched. To reduce the potential of such sampling errors, several ileal biopsies of a given patient were pooled prior to lymphocyte isolation. In this study, TFH cells were defined by CXCR5+ PD-1 high expression and were strongly enriched for putative TFH activity markers in the peripheral blood or intestine including ICOS, OX40, and CD38 as well as IL-21 production. Moreover, this definition is consistent with observations that “activated” circulating TFH cells following influenza vaccination express high levels of PD-1, ICOS, or CD38.38 The biological relevance of our findings is further validated by the observation that UST therapy of CD patients resulted in reduced plasma CXCL13 concentrations, an established marker of germinal center activity.24 These findings strongly suggest that this cell population indeed contains the functional TFH cells and that UST indeed specifically affects TFH cell frequencies.
Another potential limitation of our study is the fact that clinical response to therapy was mainly based on continuation of UST therapy, rather than on other objective disease activity markers, given a rather large heterogeneity within our CD patient cohort. Implementation of additional disease activity markers in larger and prospectively collected patient cohorts will address the question whether the observed differences in TFH cell frequencies were indeed dependent on UST or more reflective of other factors such as disease activity. However, the observation that clinical response to TNF inhibitor therapy did not affect TFH cell frequencies strongly suggests a specific interaction of UST with TFH cell fate instead of indirect effects of disease activity.
The clinical relevance of the interaction between UST therapy and TFH cell function has to be determined in future clinical studies. TFH cells are essential for B cell functions such as vaccine responses. Our observation that UST therapy results in altered CXCL-13 expression, an established marker for germinal center activity, suggests that UST indeed affects TFH cell functions in vivo. Interestingly, serological response to vaccination with pneumococcal or tetanus toxoid vaccines was not affected by concomitant UST therapy in psoriasis patients.39 However, it should be noted that considerably higher UST concentrations are used to treat CD and observations from psoriasis patients therefore do not necessarily apply to patients with CD. To address this question, vaccine responses should be carefully monitored in UST-treated CD patients in the future.
In conclusion, we demonstrate that TFH cell frequencies may play a role in CD pathogenesis and that targeting IL-12 and IL-23 by UST impairs the differentiation of TFH cells in CD patients in a plasma concentration-dependent manner, thereby potentially leading to reduced germinal center activity. These findings provide evidence that IL-12 and IL-23 are involved in the differentiation of TFH cells in this highly relevant human disease entity. The clinical implications of these findings for T cell–mediated immune responses in patients treated with this drug should be assessed in clinical studies in the future.
Materials and Methods
Study Approval
Written informed consent was obtained from all patients and controls prior to inclusion in the study and the study was conducted according to local ethics committee regulations (Albert-Ludwigs-University, Freiburg, references 407/16 and 14/17; University Ulm, reference 159/19) and the Declaration of Helsinki (1975).
Patient Cohort
CD patients were recruited in the inflammatory bowel disease outpatient clinic of the university hospitals in Freiburg and Ulm, both in Germany. Following patients’ informed consent, peripheral blood was collected before (n = 16) and during (n = 23) UST therapy. Clinical parameters including laboratory data at the respective dates of analysis were collected. CD activity was determined using the HBI.40 Peripheral blood from CD patients treated with TNF antibodies (n = 22) and from healthy donors (n = 21) was used as a control.41 UST and anti-TNF–treated patients as well as healthy control patients were matched for gender and age. Patient characteristics are detailed in Table 1. Because clinical data on disease outcome had to be collected retrospectively in rather heterogenous patient cohorts (including several patients with ileostomy), we were not able to use standard definitions of clinical response. Response was therefore defined as continuation of UST therapy for at least 1 year following patient recruitment or until last follow-up or end of data collection.
Ileal biopsies of CD patients were taken during routine endoscopy and disease activity was prospectively assessed using the Simple Endoscopic Score for Crohn’s Disease.18 To minimize sampling errors during biopsy acquisition, up to 5 ileal biopsies of each patient were pooled prior to lymphocyte isolation. Clinical chemistry and fecal calprotectin concentrations were determined by routine clinical methods (University Hospital Freiburg). Analysis of serum CXCL13 concentrations was performed in CD patients before and 8 weeks after initiation of UST therapy in the outpatient clinic of the university hospitals in Freiburg (n = 10) and Ulm (n = 17).
Lymphocyte Isolation
Isolation of mononuclear cells from the peripheral blood was performed as previously described.4,41 Processing and freezing were done at the day of sample collection. Lymphocytes were isolated from intestinal biopsies mechanically by homogenization of the biopsy through a 70-μm cell strainer. There was no further purification of lymphocytes after this mechanical isolation process. Cell populations of interest were identified using flow cytometry. Whole blood with EDTA was centrifuged for 10 minutes at 500 g at room temperature to allow for separation of plasma.
Cytokine Production and Multiparametric Flow Cytometry
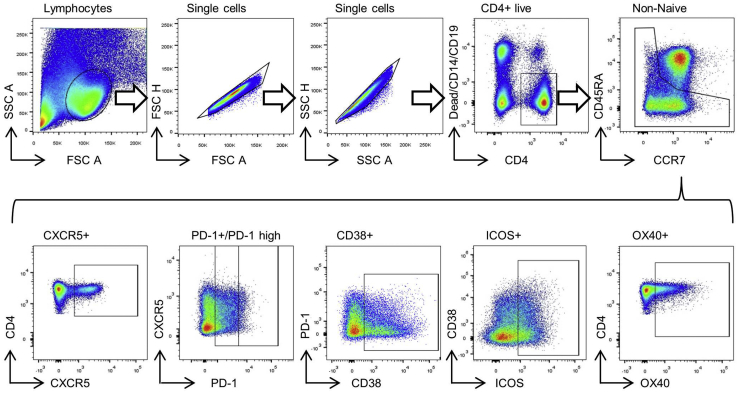
After thawing, cells were allowed to rest at 37°C for 24 hours. For intracellular cytokine staining, cells were stimulated with ionomycin (1 μL/mL; Sigma-Aldrich, Stenheim, Germany) and PMA (phorbol 12-myristate-13-acetate) (50 ng/mL; Sigma-Aldrich) in the presence of brefeldin A (GolgiPlug; 0.5 μL/mL; BD Biosciences, Heidelberg, Germany) and monensin (GolgiStop; 0.325 μL/mL, BD Biosciences) for 4 hours at 37°C. To allow for intracellular staining, cells were treated with the FoxP3 Kit (Thermo Fisher Scientific, Munich, Germany); 2% paraformaldehyde was used to fix the cells after staining. Flow cytometric analysis was performed with a LSR Fortessa (BD Biosciences). Antibodies are listed in Table 3. The applied gating strategies are shown in Figure 5.
Table 3.
Antibodies Used for Multiparametric Flow Cytometric Analysis
| Antibody (Clone) | Dilution | Manufacturer |
|---|---|---|
| Anti-CCR7-BV421 (150503) | 1:33.33 | BD Biosciences, Germany |
| Anti-CD38-BUV737 (HB7) | 1:200 | BD Biosciences, Germany |
| Anti-ICOS-BV711 (DX29) | 1:100 | BD Biosciences, Germany |
| Anti-PD-1-BV786 (EH12.1) | 1:33.33 | BD Biosciences, Germany |
| Anti-CD4-FITC (RPA-T4) | 1:33.33 | BD Biosciences, Germany |
| Anti-CD45RA-PE (HI100) | 1:200 | BD Biosciences, Germany |
| Anti-CD134(OX40)-PE-Cy7 (ACT-35) | 1:33.33 | BD Biosciences, Germany |
| Anti-CXCR5-APC (J252D4) | 1:200 | BioLegend, United Kingdom |
| Anti-fixable viability dye-eF780 | 1:5000 | eBioscience, Germany |
| Anti-CD14-eF780 (61D3) | 1:100 | eBioscience, Germany |
| Anti-CD19-eF780 (HIB19) | 1:100 | eBioscience, Germany |
| Anti-CD4-BB515 (SK3) | 1:200 | BD Biosciences, Germany |
| Anti-IL-17-PE (eBio46DEC17) | 1:50 | eBioscience, Germany |
| Anti-CD4-BV510 (SK3) | 1:10 | BD Biosciences, Germany |
| Anti-PD-1-PerCPeFlour710 (eBioJ105) | 1:20 | eBioscience, Germany |
| Anti-CXCR5-BV421(J252D4) | 1:20 | BioLegend, United Kingdom |
Figure 5.
Gating strategy for analysis of multiparametric flow cytometry staining is depicted.
CXCL13 Enzyme-Linked Immunosorbent Assay
CXCL13 plasma concentrations were determined with the Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN), following the manufacturer’s instructions. Results were analyzed with the Microplate Reader Spark (Tecan Group AG, Männedorf, Switzerland).
TFH Cell Differentiation Assay
The TFH cell differentiation assay was performed with freshly isolated PBMCs from healthy donors (n = 16). Naïve CD4+ T cells were isolated by using the human Naïve CD4+ T Cell Isolation Kit II (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). A total of 500,000 naïve CD4+ T cells were cultured in complete medium (RPMI 1640 with 10% fetal calf serum, 1% penicillin/streptomycin solution and 1.5% 1M HEPES; all Thermo Fisher Scientific) in the presence of 12.5 μL/mL anti-CD3/CD28 (STEMCELL Technologies, Vancouver, Canada) in a flat-bottom 48 well plate. After 24 hours of culture, cytokines were added and cells were incubated for additional 2.5 days. A total of 5 ng/mL IL-12 + 5 ng/mL TGFβ with and without 100 ng/mL UST and 100 ng/mL UST alone were used.
TFH Maintenance Assay
Two million PBMCs isolated from healthy donors were seeded in a sterile 24-well flat-bottom plate containing complete medium (RPMI 1640 with 10% fetal calf serum, 1% penicillin/streptomycin solution, and 1.5% 1M HEPES; all Thermo Fisher Scientific). Cells were activated with 25-μL/mL anti-CD3/CD28 (STEMCELL Technologies). Ustekinumab was added in 3 different concentrations (10 mg/L, 1 mg/L, or 0.1 mg/L), and cells were cultured for 7 days. Cells were analyzed by flow cytometry on days 0, 3, 5, and 7.
Assessment of UST Plasma Concentrations
Quantification of UST plasma concentrations was performed via liquid chromatography–tandem mass spectrometry on an Acquity I-Class high-performance liquid chromatography system (Waters Corporation, Milford, MA) equipped with a TQ-XS (Waters Corporation).42
Statistics
FlowJo software version 10 (FlowJo, LLC, Ashland, OR) was used to analyze flow cytometric data. Statistical analysis was performed using Prism version 7 (GraphPad Software, San Diego, CA). Statistical tests used are indicated in the figure legends. P < .05 was considered significant. Figures show median ± 95% CI. TFH cell frequencies are shown as percentage of non-naïve CD4+ T cells.
Acknowledgments
The authors thank all patients and healthy volunteers who contributed to this study. The authors sincerely thank B. Bengsch and K. Basho for helpful discussions. Moreover, the authors are very grateful for expert technical assistance by B. Hockenjos, Ö. Sogukpinar-Beheshti, and S. Zehe. Further, the authors thank S. Hohler, T. Wehrle, and B. Starke for helping with patient recruitment.
CRediT Authorship Contributions
Anna-Maria Globig (Conceptualization: Equal; Data curation: Supporting; Formal analysis: Equal; Investigation: Equal; Supervision: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Nikola Patricia Sommer (Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Writing – original draft: Equal; Writing – review & editing: Supporting)
Katharina Wild (Formal analysis: Supporting; Investigation: Supporting)
Josefine Schardey (Investigation: Supporting)
Katharina Zoldan (Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting)
Anne Kerstin Thomann (Investigation: Supporting)
Lucas-Alexander Schulte (Investigation: Supporting)
Rupert Schreiner (Formal analysis: Supporting; Methodology: Supporting)
Wolfgang Reindl (Formal analysis: Supporting)
Jochen Klaus (Investigation: Supporting)
Christoph Mathis Schempp (Investigation: Supporting)
Maike Hofmann (Formal analysis: Supporting)
Robert Thimme (Formal analysis: Supporting; Methodology: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Tobias Boettler (Conceptualization: Equal; Formal analysis: Supporting; Methodology: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Peter Hasselblatt (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Supporting; Project administration: Lead; Supervision: Lead; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Lead)
Footnotes
Conflicts of interest These authors disclose the following: Outside of this work, Anna-Maria Globig has received travel grants from AbbVie and Janssen. Josefine Schardey has received a travel grant from AbbVie. Anne Kerstin Thomann has received travel grants from AbbVie and Takeda as well as lecture fees from MSD. Lucas-Alexander Schulte has received a travel grant from Janssen. Wolfgang Reindl has received lecture fees from AbbVie, Falk, Janssen, and Takeda. Christoph Mathis Schempp has performed clinical studies and received lecture fees from AbbVie, Janssen, and Novartis. Tobias Boettler has received lecture fees from Falk foundation and AbbVie. Peter Hasselblatt has received lecture fees from the Falk Foundation, AbbVie, Takeda, MSD, and Janssen; travel grants from AbbVie and Takeda; and fees for scientific consulting for Janssen. The remaining authors disclose no conflicts.
Funding The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and the University of Freiburg in the funding program Open Access Publishing. The study was funded by the authors' institution.
References
- 1.Neurath M.F. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 2.Strober W., Fuss I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 4.Globig A.M., Hennecke N., Martin B., Seidl M., Ruf G., Hasselblatt P., Thimme R., Bengsch B. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321–2329. doi: 10.1097/MIB.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 5.Kleinschek M.A., Boniface K., Sadekova S., Grein J., Murphy E.E., Turner S.P., Raskin L., Desai B., Faubion W.A., de Waal Malefyt R., Pierce R.H., McClanahan T., Kastelein R.A. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D., Wehkamp J., Feagan B.G., Yao M.D., Karczewski M., Karczewski J., Pezous N., Bek S., Bruin G., Mellgard B., Berger C., Londei M., Bertolino A.P., Tougas G., Travis S.P., Secukinumab in Crohn’s Disease Study Group Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feagan B.G., Sandborn W.J., Gasink C., Jacobstein D., Lang Y., Friedman J.R., Blank M.A., Johanns J., Gao L.L., Miao Y., Adedokun O.J., Sands B.E., Hanauer S.B., Vermeire S., Targan S., Ghosh S., de Villiers W.J., Colombel J.F., Tulassay Z., Seidler U., Salzberg B.A., Desreumaux P., Lee S.D., Loftus E.V., Jr., Dieleman L.A., Katz S., Rutgeerts P., UNITI-IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt N., Liu Y., Bentebibel S.E., Munagala I., Bourdery L., Venuprasad K., Banchereau J., Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 10.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good-Jacobson K.L., Szumilas C.G., Chen L., Sharpe A.H., Tomayko M.M., Shlomchik M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victora G.D., Nussenzweig M.C. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 13.Kim C.H., Rott L.S., Clark-Lewis I., Campbell D.J., Wu L., Butcher E.C. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasheed A.U., Rahn H.P., Sallusto F., Lipp M., Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 16.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haltaufderhyde K., Srikiatkhachorn A., Green S., Macareo L., Park S., Kalayanarooj S., Rothman A.L., Mathew A. Activation of peripheral T follicular helper cells during acute dengue virus infection. J Infect Dis. 2018;218:1675–1685. doi: 10.1093/infdis/jiy360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daperno M., D’Haens G., Van Assche G., Baert F., Bulois P., Maunoury V., Sostegni R., Rocca R., Pera A., Gevers A., Mary J.Y., Colombel J.F., Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 19.Tham Y.S., Yung D.E., Fay S., Yamamoto T., Ben-Horin S., Eliakim R., Koulaouzidis A., Kopylov U. Fecal calprotectin for detection of postoperative endoscopic recurrence in Crohn’s disease: systematic review and meta-analysis. Therap Adv Gastroenterol. 2018;11 doi: 10.1177/1756284818785571. 1756284818785571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H., Deng Y., Zhao M., Zhang J., Zheng M., Chen G., Li L., He Z., Lu Q. Molecular control of follicular helper T cell development and differentiation. Front Immunol. 2018;9:2470. doi: 10.3389/fimmu.2018.02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locci M., Wu J.E., Arumemi F., Mikulski Z., Dahlberg C., Miller A.T., Crotty S. Activin A programs the differentiation of human TFH cells. Nat Immunol. 2016;17:976–984. doi: 10.1038/ni.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H., He F., Tsokos G.C., Kyttaris V.C. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J Immunol. 2017;199:903–910. doi: 10.4049/jimmunol.1700418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battat R., Kopylov U., Bessissow T., Bitton A., Cohen A., Jain A., Martel M., Seidman E., Afif W. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017;15:1427–1434.e2. doi: 10.1016/j.cgh.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Havenar-Daughton C., Lindqvist M., Heit A., Wu J.E., Reiss S.M., Kendric K., Belanger S., Kasturi S.P., Landais E., Akondy R.S., McGuire H.M., Bothwell M., Vagefi P.A., Scully E., Investigators I.P.C.P., Tomaras G.D., Davis M.M., Poignard P., Ahmed R., Walker B.D., Pulendran B., McElrath M.J., Kaufmann D.E., Crotty S. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 26.Niu J., Song Z., Yang X., Zhai Z., Zhong H., Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1791–1796. doi: 10.1111/jdv.13027. [DOI] [PubMed] [Google Scholar]
- 27.Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., Lavecchio E.M., Punaro M., Pascual V., Banchereau J., Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam L., Zoldan K., Hofmann M., Schultheiss M., Bettinger D., Neumann-Haefelin C., Thimme R., Boettler T. Follicular T helper cell signatures in primary biliary cholangitis and primary sclerosing cholangitis. Hepatol Commun. 2018;2:1051–1063. doi: 10.1002/hep4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng J., Wei Y., Fonseca V.R., Graca L., Yu D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat Rev Rheumatol. 2019;15:475–490. doi: 10.1038/s41584-019-0254-2. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi S., Ueno H. Potential pathways associated with exaggerated T follicular helper response in human autoimmune diseases. Front Immunol. 2018;9:1630. doi: 10.3389/fimmu.2018.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Zhu Y., Zhang M., Hou J., Wang H., Jiang Y., Wang H., Gao P. The shifted balance between circulating follicular regulatory T cells and follicular helper T cells in patients with ulcerative colitis. Clin Sci (Lond) 2017;131:2933–2945. doi: 10.1042/CS20171258. [DOI] [PubMed] [Google Scholar]
- 32.Yu J., He S., Liu P., Hu Y., Wang L., Wang X., Han Y., Zhu X. Interleukin21 promotes the development of ulcerative colitis and regulates the proliferation and secretion of follicular T helper cells in the colitides microenvironment. Mol Med Rep. 2015;11:1049–1056. doi: 10.3892/mmr.2014.2824. [DOI] [PubMed] [Google Scholar]
- 33.Long Y., Xia C., Xu L., Liu C., Fan C., Bao H., Zhao X., Liu C. The imbalance of circulating follicular helper t cells and follicular regulatory T cells is associated with disease activity in patients with ulcerative colitis. Front Immunol. 2020;11:104. doi: 10.3389/fimmu.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindqvist M., van Lunzen J., Soghoian D.Z., Kuhl B.D., Ranasinghe S., Kranias G., Flanders M.D., Cutler S., Yudanin N., Muller M.I., Davis I., Farber D., Hartjen P., Haag F., Alter G., Schulze zur Wiesch J., Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y., Yang Q., Deng H., Tang J., Hu J., Liu H., Zhi M., Ye L., Zou B., Liu Y., Wei L., Gabrilovich D.I., Wang H., Zhou J. Transcriptional factor ATF3 protects against colitis by regulating follicular helper T cells in Peyer’s patches. Proc Natl Acad Sci U S A. 2019;116:6286–6291. doi: 10.1073/pnas.1818164116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt N., Bustamante J., Bourdery L., Bentebibel S.E., Boisson-Dupuis S., Hamlin F., Tran M.V., Blankenship D., Pascual V., Savino D.A., Banchereau J., Casanova J.L., Ueno H. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byun M., Ma C.S., Akcay A., Pedergnana V., Palendira U., Myoung J., Avery D.T., Liu Y., Abhyankar A., Lorenzo L., Schmidt M., Lim H.K., Cassar O., Migaud M., Rozenberg F., Canpolat N., Aydogan G., Fleckenstein B., Bustamante J., Picard C., Gessain A., Jouanguy E., Cesarman E., Olivier M., Gros P., Abel L., Croft M., Tangye S.G., Casanova J.L. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210:1743–1759. doi: 10.1084/jem.20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma C.S., Phan T.G. Here, there and everywhere: T follicular helper cells on the move. Immunology. 2017;152:382–387. doi: 10.1111/imm.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodmerkel C., Wadman E., Langley R.G., Papp K.A., Bourcier M., Poulin Y., Ho V., Guenther L., Kunynetz R., Nigen S., Vender R., Wasel N., Hsu M.C., Szapary P. Immune response to pneumococcus and tetanus toxoid in patients with moderate-to-severe psoriasis following long-term ustekinumab use. J Drugs Dermatol. 2013;12:1122–1129. [PubMed] [Google Scholar]
- 40.Harvey R.F., Bradshaw J.M. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 41.Schardey J., Globig A.M., Janssen C., Hofmann M., Manegold P., Thimme R., Hasselblatt P. Vitamin D inhibits pro-inflammatory T cell function in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13:1546–1557. doi: 10.1093/ecco-jcc/jjz090. [DOI] [PubMed] [Google Scholar]
- 42.Thomann A.K., Schulte L.A., Globig A.M., Hoffmann P., Klag T., Itzel T., Teufel A., Schreiner R., Scheffe N., Ebert M.P., Wehkamp J., Gauss A., Hasselblatt P., Klaus J., Reindl W. Ustekinumab serum concentrations are associated with clinical outcomes in Crohn’s disease - a regional multi-center pilot study. Z Gastroenterol. 2020;58:439–444. doi: 10.1055/a-1088-1461. [DOI] [PubMed] [Google Scholar]