Abstract
Iron is important for many cellular functions, including ATP synthesis and cell proliferation. Insufficient of iron in the diet causes iron deficiency anemia (IDA), which often occurs in people living in the world. Since 50% of women with IDA show amenorrhea, the relationship of between iron deficiency and reproductive function was assessed using mice fed a low Fe diet (LFD). The estrous cycle in the LFD mice was blocked at diestrus, which impair follicle development, and fertility. Further, even LFD mice were injected with exogenous pregnant mare serum gonadotropin (PMSG), follicular development was ceased at the secondary follicle stage, and preovulatory follicles were not observed. Amount of ATP decreased in the ovary of the LFD mice, and expression of follicle development markers (Fshr, Cyp19a1, Ccnd2) and estradiol-17β (E2) was low level compared to levels mice fed a normal diet. Feeding a normal diet with sufficient iron to the LFD mice for an additional 3 weeks completely reversed absence the effects of iron insufficient on the estrous cycle and infertility. Thus, iron restriction depresses ovary functions, especially follicular development from secondary follicle to antral follicles and infertility. These effects are fully reversible by supplementation of a normal diet containing iron.
Keywords: Follicular development, Infertility, Iron
Iron is an essential dietary cofactor supplied by foods to the body. According to a systematic analysis of the global anemia burden reported by Kassebaum et al., anemia is a common sequela of malnutrition that may affect approximately 2.2 billion people (30% of the total population) [1]. Ninety-five percent of the causes of anemia is caused by iron deficiency and is termed iron deficiency anemia (IDA). IDA is the disease that is caused by the starvation that affects 30−48% of the people living in developing countries [2]. IDA is also observed in young women in developed countries who face severe dietary restriction and a biased an unbalanced diet. IDA is associated with many symptoms such as headache, paleness, fatigue, dyspnea, alopecia, and neurocognitive dysfunction [3]. IDA also affects reproduction, pregnancy, and birth [4].
Many reports about the effects of IDA on the maternal body and fetus in gestational phase and on infants have been conducted [4,5,6,7], but there is little information about the effect of IDA on follicular development and ovulation during the estrous cycle [8]. The mammalian menstrual cycle is important for producing offspring. Progression through this cycle is regulated by two major pituitary derived gonadotropins; follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH induces follicular development from the small antral stage to the preovulatory stage, as a prelude to producing and releasing mature oocytes. FSH increases the expression of Cyp19a1 mRNA-coded P450 aromatase in granulosa cells and produces estradiol-17β (E2) from the androgen produced in intrathecal cells [9, 10]. E2 increases the gene expression of follicular development markers, including Ccnd2 mRNA (a cell-cycle regulator gene) to induce further follicular development [11]. When dominant follicles reach the preovulatory stage, E2 induces a transient secretion of LH (LH surge) from the pituitary gland [12]. LH induces the expansion of the cumulus-oocytes complex (COC), the meiotic resumption of meiosis in oocytes from prophase I to metaphase II (oocyte maturation), and ovulation [13]. Kim et al. recently showed that mineral components including iron affect the menstrual cycle and hormone concentrations in humans [14]. Another investigation revealed that 50% of the study patients suffering from anemia had amenorrhea [15]. Thus, iron deficiency in the body seems to be closely associated with female reproduction, especially the menstrual cycle. However, no reports are available on the direct effects of iron deficiency on the estrous cycle, follicle development, or ovulation.
In the present study, mice were fed with normal laboratory chow (normal diet [ND]) or iron-free laboratory chow (low-Fe diet [LFD]) for 3 weeks. The effects of iron deficiency on the estrous cycle were examined by comparing the ND mice and LFD mice. Since follicular development was impaired in the LFD mice, the relative expressions of genetic markers of follicular development in the ovary were measured. The LFD rescue (LFDR) mice, i.e., the LFD mice fed the ND for an additional 3 weeks, were examined to determine whether the estrous cycle impairment was reversible.
Materials and Methods
Materials
PBS (–) was dissolved 0.8% NaCl (Fujifilm Wako Chemicals, Osaka, Japan), 0.02% KCl (Nacalai Tesque, Kyoto, Japan), 0.28% NaHPO4·12H2O (Nacalai Tesque) and 0.02% KH2PO4 (Nacalai Tesque) in ultrapure water. PBS (+) was dissolved 0.1% CaCl2 (Hayashi Pure Chemical Ind., Osaka, Japan), 0.1% MgCl2·6H2O (Katayama Chemical Industries, Osaka, Japan) and 1% glucose (Nacalai Tesque) in ultrapure water. PBS was dissolved 9% PBS (–), 10% PBS (+), 1% PVP (Sigma-Aldrich, St. Lous, MO, USA) and 0.2% penicillin streptomycin (Nacalai Tesque) in ultrapure water.
Animals
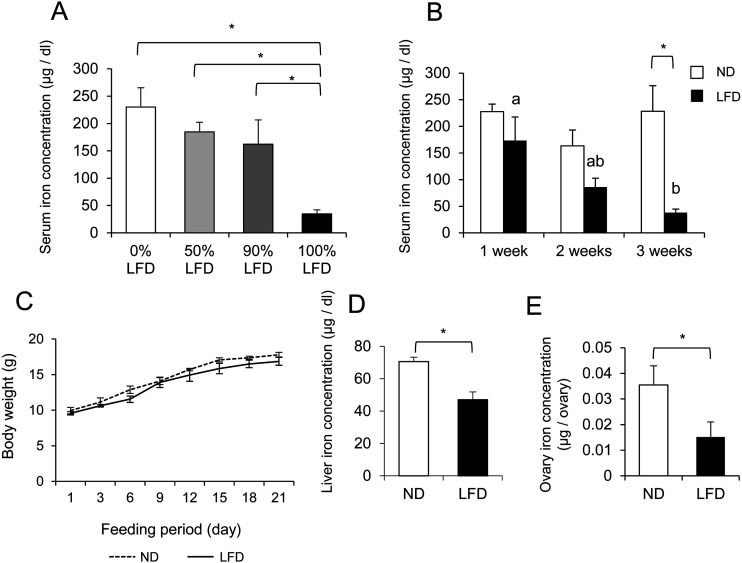
Female C57BL/6JJcl mice (3 weeks) were obtained from Clea Japan (Tokyo, Japan), housed under 12 h light/ 12 h dark cycle (12 h light: 0600–1800 h, 12 h dark: 1800–0600 h) with stable temperature condition (22 ± 1°C), relative humidity of 55 ± 10%, and provided food and water ad libitum. Previous reports showed that opening of vaginal orifice is essential for entrance of first estrous and the average timing of first vaginal orifice open was approximately 3 weeks after birth [16]. Thus, we used already vaginal orifice-opened mice in this study. The ND iron content was 37.64 mg /100 g of laboratory chow, and the LFD iron content was 5.97 mg /100 g of laboratory chow (Supplementary Table 1: online only). The LFD iron content rate was approximately 16% of the ND iron content. Three-week-old female mice were fed with various proportions of the LFD chow (0, 50, 90, 100% LFD) for 3 weeks (Fig. 1A). For other experiment groups, 3-week-old female mice were fed with the ND chow (CE-2, Clea Japan) or LFD chow (Clea Japan) for 1, 2, 3 or 6 weeks (Fig. 1B, C, D). The LFDR mice were fed by the LFD for 3 weeks, and further fed by the ND for additional 3 weeks (Fig. 1D). The three mice were bred at a plastic gauge to avoid iron contamination and CL-4161 (Clea Japan) was used for bedding. The mice were treated in accordance with National Institutes of Health Guide for the Care and the experiments were approved by the committee for Ethics on Animal Experiments in the Prefectural University of Hiroshima (Protocol Number: 16SA010). Component of normal diet chow or low Fe diet chow was shown as Supplementary Table 1.
Fig. 1.
Schematic illustrating the generation of the normal diet (ND), low-Fe diet (LFD) and LFD rescue (LFDR) mice. A) 0% LFD; 3-week-old mice were fed with the ND for 3 weeks. 50% LFD; 3-week-old mice were fed with the mixed chow of 50% ND and 50% LFD for 3 weeks. 90% LFD; 3-week-old mice were fed with the mixed chow of 10% ND and 90% LFD for 3 weeks.100% LFD; 3-week-old mice were fed with the LFD for 3 weeks. B) LFD 1 week; 3-week-old mice were fed with the LFD for 1 week. LFD 2 weeks; 3-week-old mice were fed with the LFD for 2 weeks. LFD 3 weeks; 3-week-old mice were fed with the LFD for 3 weeks. C) ND; 3-week-old mice were fed with the ND for 3 weeks. LFD; 3-week-old mice were fed with the LFD for 3 weeks. D) ND; 3-week-old mice were fed with the ND for 6 weeks. LFD; 3-week-old mice were fed with the LFD for 6 weeks. LFDR; 3-week-old mice were fed with the LFD for 3 weeks, and then further fed with the ND for an additional 3 weeks.
Measurement of body weight
To examine the effect on feeding the LFD on body weight, 3-week-old female mice were fed with the ND or LFD for additional 3 weeks. The body weight of the ND mice and LFD mice was measured by electronic scale on every morning. Three individual mice were used for this experiment.
Vaginal smear collection and observation
Vaginal mucus was continuously obtained from 6-week-old ND (n = 4) or LFD (n = 4) mice for 27 days, and 9-week-old ND (n = 3), LFD (n = 3) or LFDR (n = 3) for 14 days. Obtained vaginal mucus was smeared with slide grass and dried in room temperature. Dried vaginal smear was fixed by methanol and stained by Giemsa (Fujifilm Wako Chemicals). Stained vaginal smear was discriminated to four estrous stages, diestrus, proestrus, estrus and metestrus under an upright microscope (Olympus, CH, Tokyo, Japan) referred by Byers et al. [17].
Collection of serum, ovary, liver and COCs
The ND, LFD and LFDR mice at diestrus phase after 6-week-old were injected i. p. with 5 IU pregnant mare serum gonadotropin (PMSG) (Aska Pharmaceutical, Tokyo, Japan) followed 48 hours later with 6 IU human chorionic gonadotropin (hCG) (Aska Pharmaceutical) to induce follicular development and ovulation, respectively. Liver, ovary or ovulated COCs were collected from the mice. The mice were anesthetized with isoflurane (Fujifilm Wako Chemicals), and then euthanized by cervical dislocation. The samples were obtained from the euthanized mice. One of the ovaries was isolated by dissection to obtain COCs under microscope, and unilateral ovary weight was measured by electronic scale (n = 14). Whole blood was obtained from heart of euthanized mouse after anesthesia. Serum was obtained by centrifugation of whole blood at 2,500 rpm for 5 min at 4ºC. Serum was stored at –80ºC and used for measurement of iron and FSH concentration.
COCs were collected from the ovary of the ND or LFD mice injected PMSG 48 h later. The oocytes were obtained from COCs under a stereo microscope with a pasteur pipette. The oocyte was washed by PBS and was observed under inverted phase contrast microscope. The number of ovulated COCs was counted by COC existed in the ampulla tube after 16 h later of hCG injection.
Measurement of iron concentration in serum, ovary and liver
Twenty mM Tris-HCl (pH 7.4) was dissolved 20 mM Tris (Nacalai Tesque) in ultrapure water and adjusted pH 7.4 by HCl (Fujifilm Wako Chemicals). Cell lysis buffer was dissolved 200 mM NaCl (Nacalai Tesque), 2.5 mM MgCl2 and 1% TritonX-100 in 20 mM Tris-HCl (pH 7.4).
Liver and unilateral ovary were dissolved and homogenized with cell lysis buffer. The solution was added with 6 M HCl to adjust pH (2–3), and put under room temperature for 30 min. Subsequently, the solution was centrifuged for 10 min at 10,000 rpm (Hitachi, Tokyo, Japan) and was used for measurement of iron concentration. Iron concentration in serum, ovary and liver was measured by iron assay kit (Funakoshi, Tokyo, Japan) according with manufacturer’s instructions. Three individual mice were used for this experiment.
Examination of pregnancy rate
An untreated ND male mouse (6 weeks) was mated with a 6-week-old ND or LFD female mouse, and a 9-week-old ND, LFD or LFDR female mouse for a month. Mating was started to mate at every evening (1800 h), and male mouse and female mouse were separated at next morning (0900 h). Male mice were exchanged to another one on every day. The pregnancy was identified by existence of pups, and the pregnancy rate was calculated as the rate of birthed female mice. Pups number was counted as a first litter in this experiment. Three individual mice were used for this experiment.
Morphological observation of ovary
Ovaries were fixed by 4% paraformaldehyde (Nacalai Tesque), dehydrated by staged ethanol (Nacalai Tesque) series, cleaned in xylene (Fujifilm Wako Chemicals), and embedded in paraffin (Sakura, Tokyo, Japan). Embedded ovary was cut at 5 μm using microtome (Leica RM2125RTS; Leica, Germany). The section was deparaffinized by xylene, and hydrated by ethanol series, and washed by PBS. The section was stained by hematoxylin and eosin (Sakura). Morphological analysis was observed by BZ-X700 microscope (Keyence, Tokyo, Japan). The type of follicles in ovary was subjected according to previous studies [18]. In brief, whole unilateral ovary was cut, and approximately 30–40 sections were observed per ovary at 25 μm intervals. The number of secondary, preantral, antral and preovulatory follicles existed in the whole unilateral ovary were counted (n = 3).
RNA collection and Quantitative RT-PCR
The isolation of total RNA from ovary was performed according our previous report [19]. Briefly, Total RNA was extracted from ovary using a RNeasy mini kit (Qiagen Sciences, MD, USA) according to the instruction manual and dissolved in nuclease-free water. The total RNA concentration was measured by Nanodrop One (Thermo Fisher Scientific, MA, USA). The final total RNA concentration was 5 ng/μl. Reverse transcription was performed as previously described [20]. Briefly, total RNA was reverse transcribed using 500 ng of poly-dT (Promega, Madison, WI, USA) and 0.25 U of avian myeloblastosis virus reverse transcriptase (Promega) for 75 min at 42°C and for 5 min at 95°C.
Real-time PCR analysis was performed as previously described [19, 20]. Briefly, cDNA and primers were added to the KAPA SYBER Fast Universal qPCR kit (Kapa Biosystems, MA, USA) for a total reaction volume of 15 μl. PCR reactions were then performed using the MiniOpticon Real-Time PCR Detection System (Bio-Rad, CA, USA). Conditions were set to the following parameters: 30 sec at 95°C, followed by 40 cycles each of 5 sec at 95°C, and 45 sec at 60°C. Specific primers were selected and analyzed as indicated in Supplementary Table 2 (online only). The results were first normalized to the expression levels of a housekeeping gene Rapl19. To avoid false positive signals, dissociation-curve analysis was performed at the end of amplification and the PCR products were applied to agarose gel electrophoresis to confirm the size. The data was shown by relative values of ND mice of at least 3 independent experiments (n = 3–5).
Measurement of FSH concentration in serum
Blood was collected from the ND or LFD mice, and then centrifuged at 4ºC at 2,500 rpm for 5 min. FSH concentration in serum was measured by fluorescence microscopy (ARVO X4; PerkinElmer, MA, USA) using FSH ELISA Kit (LSBio, WA, USA) according with manufacture’s instruction. Four individual mice were used for this experiment.
Quantitative determination of E2 levels
Whole cell extract buffer (WCEB) was dissolved 100 mM NaCl, 100 mM Na4P2O7 (Nacalai Tesque), 50 mM NaF (Nacalai Tesque), 0.1 mM NaVO4 (Sigma-Aldrich), 1% TritonX-100 (Nakalai Tesque) 2.5 mM HEPES (pH 7.5) (Dojindo Laboratories, Kumamoto, Japan), 5 mM EDTA (Dojindo Laboratories) and 5 mM EGTA (Nacalai Tesque) in ultrapure water.
Ovaries were collected from 6-week-old ND and LFD mice were prepared by homogenization in WCEB. Homogenized samples were centrifuged for 20 min at 5,000 rpm. The supernatants were collected in another centrifuge tube and were added 0.3 N NaOH to extract steroids into dichloromethane fraction. Extracted steroid hormones were put under vacuum, dried at 50°C to remove dichloromethane for 2 h and then dissolved with 50% methanol. Re-dissolved steroid was diluted with ELISA buffer and measured by Estrogen ELISA kit (Cayman Chemical, MI, USA) according to the manufacturer’s instruction and determined by VARIOSKAN FLASH (Thermo electron corporation, MN, USA) at 3 independent experiments (n = 3).
Measurement of ATP concentration in ovary
Unilateral ovary was homogenized with PBS, and then centrifuged at 10,000 rpm for 10 min at 4ºC. After 10 min later, the supernatants were collected. ATP amount in the ovary was measured by fluorescence microscopy using ATP measurement kit (Toyo ink, Tokyo, Japan) according with manufacture’s instruction at 4 independent experiments (n = 4).
In vitro fertilization and production of embryos
Human tubal fluid (HTF) was 101.6 mM NaCl, 4.96 mM KCl, 0.37 mM KH2PO4, 0.37 mM MgSO4·7H2O (Nacalai Tesque), 25 mM NaHCO3 (Nacalai Tesque), 2.04 mM CaCl2·2H2O (Nacalai Tesque), 2.78 mM glucose, 0.33 mM Na pyruvate (Nacalai Tesque), 21.4 mM Na lactate 60% syrup (Sigma-Aldrich), 0.4% BSA (Sigma-Aldrich), Phenol red and 1% Penicillin streptomycin in ultrapure water. Sheep oviductal monolayer with a higher K+ concentration (KSOM) medium was purchased from ARK Resource (Ark resource, Kumamoto, Japan).
HTF and KSOM medium were made two and one drop each and put at 37ºC and 5% CO2 by the day before for gas equilibrium. Sperm was collected from cauda epididymis of 3-month-old male mice and was incubated in the HTF for 90 min. After incubation, the number of sperm was counted by hemacytometer (Erma optical works, Tokyo, Japan) under the upright microscope (Olympus, CH) and was diluted to final concentration at 2 × 105 sperm/ml. Fifty COCs collected from 6-week-old ND and LFD female mice after 16 h later of hCG injection were cocultured with sperm in HTF for 6 h. Sperm and cumulus cells were removed from co-cultured oocytes. The embryos were transferred to KSOM drop and were cultured at 37ºC and 5% CO2 for 5 days. Embryos with both an embryoblast and blastocoel were defined as blastocyst-stage embryos at 3 independent experiments (n = 3).
Experimental design
Experiment 1; Effect of the LFD feeding on body weight, iron concentration in serum, liver, and ovary
First, to determine the content ratio of the low-Fe chow, 3-week-old mice were fed with 0 (ND only), 50, 90, and 100% LFD for 3 weeks (Fig. 1A). Next, to investigate the appropriate duration for serving the low-Fe chow, 3-week-old mice were fed with 100% LFD for 1, 2, or 3 weeks (Fig. 1B). After the feeding periods, the iron concentration in the serum was measured by an iron assay kit. For the assessment of the effects of the diets on the body weight and the iron concentrations in liver and ovary, a separate group of 3-week-old mice were fed with the ND or LFD for 3 weeks (Fig. 1C). Their body weights were measured every morning for 3 weeks. After the feeding periods, the iron concentrations in the liver and ovary were measured by the iron assay kit.
Experiment 2; Effect of the LFD feeding on estrous cycle and fertility
To investigate the effect of the LFD feeding on the estrous cycle, 3-week-old mice were fed with the ND or LFD for 3 weeks. For the 27 days after that 3-week feeding periods, the ND-fed mice were continuously fed with the ND, and the LFD-fed mice were fed with the LFD. Vaginal mucus was obtained from the mice every morning to determine the estrous stage. For the investigation of the pregnancy rate and litter size of the ND and LFD mice, the male mice and the ND- or LFD-fed female mice were mated for 1 month. The pregnancy rate was calculated as the rate of birthed female mice per mated female mice, and the pup number was counted.
Experiment 3; Effect of the LFD feeding on ovary
For the examination of the effect of LFD feeding on follicular development, 3-week-old mice were fed with the ND or LFD for 3 weeks, after which they were injected with PMSG. Before (Diestrus) and 48 h after the PMSG administration, ovaries and serum were obtained from the ND- and LFD-fed mice. Previous studies demonstrated that iron is an essential cofactor for enzymes in many metabolic pathways, including glycolysis, the TCA cycle, the electron transfer chain, and pentose phosphate, produce ATP, DNA, and RNA [21,22,23]. The weight of each ovary was measured, and then the ovary was used for the measurements of the E2 (PMSG 48 h) and ATP concentrations (Diestrus). The FSH concentration in serum from the ND and LFD mice was measured. Ovary was collected from the ND and LFD mice at the diestrus phase and 48 h after the PMSG injection and then used for a RT-PCR analysis of the expressions of follicular development markers. Another ovary from the ND and LFD mice injected with PMSG was fixed, and a section sample of the ovary was created. The number of secondary follicles, preantral follicles, and antral follicles were counted. In addition, PMSG-primed ND mice and LFD mice were injected with hCG. At 16 h after hCG injection, the number of ovulated COCs in the ampulla of the fallopian tube was counted. Ovulated COCs were used for in vitro fertilization for the evaluation of the developmental competence of oocytes derived from the ND- or LFD-fed mice.
Experiment 4; Feeding of normal diet to the LFD mice improve absence of estrous cycle and infertility
For determination of whether the failure of follicular development induced by the LFD in 3-week-old mice was improved by supplementation with the ND to LFD-fed mice, 3-week-old mice were fed with the LFD for 3 weeks to prevent follicular development, and then further fed with the ND for 3 weeks (LFDR mice) (Fig. 1D). As a negative and positive control, 3-week-old mice were fed with the ND or LFD for 6 weeks. After the feeding periods, the iron concentration in serum was measured by iron assay kit. Some ND, LFD, and LFDR fed mice were further fed with the ND, LFD, or ND, respectively for 14 days, and vaginal mucus was obtained from the mice every morning to determine the estrous stage.
Statistics
All results were shown means ± SEM. Statistical analyses of data from three or four replications for comparison were carried out by Student’s t-test, one-way analysis of variance (ANOVA) or two-way ANOVA followed by Student’s t-test (Statview; Abcas Concepts, Berkely, CA). P < 0.05 was showed statistically significant.
Results
Determination of the condition which the LFD feeding to mice induces iron deficiency
To determine the content ratio of the LFD, 3-week-old mice were fed with a 0, 50, 90, or 100% LFD for 3 weeks. The mice fed the 0, 50, or 90% LFD for 3 weeks showed no significant reduction in serum iron concentrations, whereas the 100% LFD significantly reduced the serum iron concentration compared to the other LFDs (Fig. 2A). For the identification of the optimal duration for serving the low-Fe chow, 3-week-old mice were fed with the 100% LFD for 1, 2, or 3 weeks. Significant differences in the iron concentration between the ND and LFD groups were not observed at 1 or 2 weeks, but the iron concentration in the mice fed with the LFD for 3 weeks (34.65 ± 7.48 μg/dl) was approximately equal to the iron concentration in another IDA mouse model reported previously [24] (Fig. 2B). The body weights over the 3 weeks after the start of the ND and LFD feeding periods were not significantly different between the ND and LFD mice (Fig. 2C). After 3 weeks of the LFD, iron deficiency was sufficiently induced in the mice. The iron concentrations in the liver (Fig. 2D) and the ovary (Fig. 2E) of the LFD mice were significantly decreased compared to those of the ND mice.
Fig. 2.
Effect of the feeding of the low-Fe diet (LFD) to mice on the iron concentration in serum, liver and ovary at diestrus phase. A) 0% LFD; 3-week-old mice were fed with the normal diet (ND) for 3 weeks. 50% LFD; 3-week-old mice were fed with the mixed chow of 50% ND and 50% LFD for 3 weeks. 90% LFD; 3-week-old mice were fed with the mixed chow of 10% ND and 90% LFD for 3 weeks.100% LFD; 3-week-old mice were fed with the LFD for 3 weeks. B) LFD 1 week; 3-week-old mice were fed with the LFD for 1 week. LFD 2 weeks; 3-week-old mice were fed with the LFD for 2 weeks. LFD 3 weeks; 3-week-old mice were fed with the LFD for 3 weeks. C) 3-week-old mice were fed with the ND or 100% LFD for 3 weeks. The body weights were measured every morning. D) 3-week-old mice were fed with the ND or 100% LFD for 3 weeks. After feeding periods, the iron concentration in the liver was measured. E) 3-week-old mice were fed with the ND or 100% LFD for 3 weeks. After feeding periods, the iron concentration in the ovary was measured. Values are mean ± SEM of 3 independent experiments (n = 3). * The significant differences were observed in between the ND and LFD mice (P < 0.05). a, b The significant differences were observed in the serum iron concentrations obtained from the mice fed for between 1 week and 3 weeks (P < 0.05).
LFD feeding for 3 weeks to mice show low iron concentrations in ovaries, impairing follicle development, and infertility in natural estrous cycle
The effect of the LFD feeding on the natural estrous cycle was evaluated using vaginal smears collected from the ND and LFD mice every morning for 27 days after 3-week feeding period (n = 4). In the ND mice, diestrus, proestrus, estrus, and metestrus were regularly observed, with cycle periods of 5.33 ± 0.88 days (Fig. 3A). The LFD mice showed only diestrus, and no LFD mice progressed to the proestrus (Fig. 3A). All of the ND-fed female mice became pregnant and the average of pup number was 8.33 ± 0.33, whereas no pregnant female mice were observed in the LFD group (n = 3) (Fig. 3B, C).
Fig. 3.
Effect of the feeding of the low-Fe diet (LFD) to mice on the estrous cycle and fertility. A) Representative chronological change of the estrous cycle in the normal diet (ND) and LFD mice. 3-week-old mice were fed with the ND or LFD for 27 days. B) Pregnancy rate of the ND or LFD mice. 6-week-old ND or LFD female mouse was mated with a sexual matured ND male mouse for 1 month. C) The number of pups in first litter of female ND or LFD mice. Values are mean ± SEM of 3 independent experiments (n = 3). N.D: Not detected.
Effect of the feeding the LFD-induced iron deficiency on follicle development
The effect of the LFD feeding on follicular development was examined herein, as was the effect of the LFD feeding on follicular development before and after an injection of PMSG. Before the PMSG injection (Diestrus), many immature follicles were observed in both the ND and LFD mice ovaries (Fig. 4A, B). The number of secondary and preantral follicles was significantly increased by the LFD, and the number of antral follicles was significantly decreased (Fig. 4E). At diestrus, ovaries from the LFD mice were significantly smaller than those of the ND mice (Fig. 4G).
Fig. 4.
Histological analysis of the ovary obtained from the normal diet (ND) or low-Fe diet (LFD) mice. A) Representative section image in the ovary of the ND mice at diestrus phase. B) Representative section image in the ovary of the LFD mice at diestrus phase. C) Representative section image in the ovary of the ND mice at 48 h after pregnant mare serum gonadotropin (PMSG) injection. D) Representative section image in the ovary of the LFD mice at 48 h after PMSG injection. E) The number of secondary follicles, preantral follicles and antral follicles in ovary of the ND and LFD mice at the diestrus phase. F) The number of preovulatory follicles in the ovary obtained from the ND and LFD mice at 48 h after PMSG injection. G) The unilateral ovary weight of the ND and LFD mice at the diestrus phase and at 48 h after PMSG injection. Values are mean ± SEM of at least 3 independent experiments (n ≥ 3). * The significant differences were observed in between the ND and LFD mice (P < 0.05).
After the PMSG injection, in the ND ovary, follicular development to the preovulatory phase was normally induced and the number of preovulatory follicles was 24.4 ± 3.0/ovary (Fig. 4C, F). After the PMSG injection, the number of preovulatory follicles was 8.50 ± 1.66/ovary and was significantly decreased compared to the ovaries from the ND mice (n = 3) (Fig. 4D, F). The LFD ovaries were also significantly smaller than the ND ovaries at 48 h after the PMSG injection (Fig. 4G).
Iron deficiency mice decreases the amount of ATP, expression of follicular development markers, and reduces E2 production in ovary
To investigate the effect of the LFD feeding on follicular development marker, we collected ovaries from the ND and LFD mice. The expression of Fshr was significantly decreased by the LFD compared to the ND (Fig. 5A). Similarly, the expressions of Cyp19a1 and Ccnd2 mRNA were also significantly decreased compared to the ND mice (Fig. 5B, C).
Fig. 5.
Effect of the low-Fe diet (LFD) feeding to mice on the expressions of follicular development markers and estradiol-17β (E2) and ATP concentrations in the ovary, and the serum follicle-stimulating hormone (FSH) concentration. Normal diet (ND); 3-week-old mice were fed with the ND for 3 weeks. Low-Fe diet (LFD); 3-week-old mice were fed with the LFD for 3 weeks. A, B) Fshr (A) and Cyp19a1 (B) expression in the ovary at the diestrus phase. C) Ccnd2 expression in the ovary at 48 h after pregnant mare serum gonadotropin (PMSG) injection. D) FSH concentration in the serum at the diestrus phase. E) E2 concentration in the unilateral ovary at 48 h after PMSG injection. F) Amount of ATP in the unilateral ovary at the diestrus phase. Values are mean ± SEM of least 3 independent experiments (n = 3–5). * The significant differences were observed in between the ND mice and LFD mice (P < 0.05). The data were shown by relative values of the ND mice.
FSH concentration in the serum of the mice at the diestrus phase was 13.7 ± 1.2 ng/ml in the ND mice and 11.9 ± 1.0 ng/ml in the LFD mice (a nonsignificant difference) (Fig. 5D).
The E2 concentration in the ovaries of the ND mice was 1.30 ± 0.32 ng/ovary in the ND mice and 0.37 ± 0.12 ng/ovary in the LFD mice, and it was significantly decreased at 48 h after the PMSG injection compared to the ND mice (Fig. 5E).
Herein, we measured the amounts of ATP in the ovaries of the ND and LFD mice. In the ND mice, the ovarian ATP concentration was 9.31 ± 1.55 μM/ g ovary and was significantly decreased in the LFD mice at 2.22 ± 0.68 μM/ g ovary (Fig. 5F).
Effect of the feeding the LFD-induced iron deficiency on oocyte quality and blastocyst development
To evaluate the developmental competence of oocytes derived from the ND- and LFD-fed mice, we collected ovulated COCs. The initial number of ovulated COCs from the LFD mice was initially 4.5 ± 1.5, whereas in the ND mice, the number was 21.5 ± 2.5. The LFD feeding mice significantly reduced this number compared to the normal diet with iron (n = 3) (Fig. 6A). The LFD feeding also reduced the rates of fertilization and progression to blastocyst compared to the ND (Fig. 6B).
Fig. 6.
The number of the ovulated cumulus-oocyte complexes (COCs) of the normal diet (ND) and low-Fe diet (LFD) mice (A) and the evaluation of developmental competence of oocytes in the ND and LFD mice (B). Values are mean ± SEM of 3 independent experiments (n = 3). * The significant differences were observed in between the ND mice and LFD mice (P < 0.05).
Feeding of the ND to LFD mice improves progression of estrous cycle and pregnancy
For our evaluation of whether the failure of follicular development induced by the LFD fed to 3-week-old mice could be improved by the supplementation with the ND, 3-week-old mice were fed with the LFD for 3 weeks, and then further fed with the ND for 3 weeks. The serum iron concentration completely recovered in these LFDR mice, which showed iron levels comparable to these of the mice fed the ND throughout the study (Fig. 7A). In the ND mice, diestrus, proestrus, estrus, and metestrus were observed, whereas the LFD feeding for 6 weeks blocked the cycle at diestrus (Fig. 7B). The estrous cycle in the LFDR mice returned to the regular and continuous cycle (Fig. 7B), 5.3 ± 0.3 days/cycle, similar to the regular period observed in the ND mice, i.e., 4.8 ± 0.6 days/cycle. In addition, all of the LFDR mice were later successfully impregnated and gave birth to normal-sized litters (n = 3).
Fig. 7.
Feeding of the normal diet to the low-Fe diet (LFD) mice improve the absence of the estrous cycle. A) The iron concentration of the normal diet (ND), LFD and LFD rescue (LFDR) mice at the diestrus phase in the serum. B) Chronological change of the estrous cycle in the ND, LFD and LFDR mice. Values are mean ± SEM of 3 independent experiments (n = 3). * The significant differences were observed (P < 0.05).
Discussion
In mammals, iron in the diet is absorbed only in the diet via small intestine, and it is then stored in the liver [25]. Stored iron in the liver is transported to various peripheral tissues, including the muscles, kidneys, and ovaries, and it functions as a vital enzyme cofactor [25]. Efforts among humans to reduce their body weight are a relatively recent phenomenon, and dietary restrictions have accompanied such effort, especially for women. IDA occurs frequently even in developed countries [2]. A study of a rat model demonstrated that iron restriction induced a disruption of the estrous cycle [26], but little information is available on the impacts of IDA on human reproductive disorders. In our present experiments, mice were fed with a 100% low-Fe diet (LFD) for 3 weeks, inducing serious iron deficiency, whereas the 50% and 90% LFDs for 3 weeks after weaning did not induce serious iron deficiency. We suspected that iron stored in the mother’s liver supplied from her milk was likely sufficient to counter the lack of dietary iron. In humans, mild IDA initially induces a reduction of the iron concentration in the liver, whereas serious IDA induces reductions of the iron concentration in both the serum and liver. In this study, since the iron concentration in both the serum and liver was significantly decreased in the LFD mice compared to the ND mice, we consider that the feeding of the 100% LFD effectively induced anemia in the mice.
In peripheral tissues, iron serves as a cofactor for the expression and activation of various metabolic enzymes involved in glycolysis [21], the TCA cycle [22], the electron transfer chain [23], and pentose phosphate pathways [21]. These pathways are essential for synthesizing ATP and protein. For example, muscle-specific Transferrin receptor 1 knockout, which inhibits the iron absorption into muscle cells, is decreased ATP production. Lack of ATP decreases cells’ ability to synthesize DNA and mRNA [27]. During follicle development in humans, the follicle size increases from 0.12 mm to 20 mm, and the number of granulosa cells increases by 100,000-fold [28]. This proliferation of granulosa cells requires FSH stimuli. The level of expression of Fshr mRNA broadly affected the mRNA expression levels of genes regulated by FSH, including Cyp19a1, Ccnd2, and Lhcgr mRNA derived from the LFD mice.
Follicle development is a stage of rapid cell proliferation and increased steroidogenesis. Both cell proliferation and steroidogenesis require ATP energy for mRNA and protein synthesis. Thus, follicle development greatly increases the demand for iron. We also observed herein that the FSH concentration in the serum of the mice was not affected by the LFD feeding for 3 weeks, suggesting that iron restriction for 3 weeks influences peripheral tissues (including the ovaries) but not the pituitary gland. In addition, since a previous study using Fshr gene target disruption mice showed reduced concentrations of LH [29] and progesterone [30] in the serum, we suspected that the LFD feeding also had an adverse effect on the ovulatory stage as was observed in the Fshr knockout mice, which may have resulted in a reduction of the oocyte’s ability to develop to the blastocyst stage in this study.
In the field of obstetrics and gynecology, superovulation is often used as hormone therapy. Our present findings suggest that even if superovulation treatment is used for patients with IDA, their fertility is not likely to improve. In humans, IDA can be treated with oral iron supplements [31]. The LFD-fed mice in this study that were later given the normal diet recovered from the effects of iron deficiency, including a return to the normal estrous cycle and fertility. Symptoms caused by iron deficiency can likely be ameliorated with iron supplementation. However, since an iron overload causes severe disease [32], the posology of iron supplementation should be fully investigated.
In conclusion, feeding low iron chow to mice for 3 weeks reduced the iron concentrations in their serum, liver and ovaries, the amounts of ATP in the ovaries, the mRNA expressions of the follicle development markers (Fshr, Cyp19a1 and Ccnd2 mRNA), and E2 production, resulting in failed follicle development and fertility. Since the significant difference was not observed in the serum FSH concentration between the ND and LFD mice, these results suggested that iron restriction in females induces comprehensive ovarian dysfunction (including metabolic pathway) at the follicular developmental phase, which resulted in decreasing fertility in mice. The infertility in the LFD mice was rescued by suppling a normal diet containing iron, and it is therefore possible that the ovarian dysfunction at the developmental phase caused by anemia in mammals can be overcome by iron supplementation in mammals.
Supplementary
Acknowledgments
We thank A Sei, S Uchihashi, M Shiroma, M Komori, M Mochizuki and H Hiramoto for technical assistance. This work was supported, in part, by PUE Research Grant Program (Advanced Research A) from the Prefectural University of Hiroshima and KAKENHI Grant Number JP22780251 and JP26450381 from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Andrade Cairo RC, Rodrigues Silva L, Carneiro Bustani N, Ferreira Marques CD. Iron deficiency anemia in adolescents; a literature review. Nutr Hosp 2014; 29: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 3.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2016; 387: 907–916. [DOI] [PubMed] [Google Scholar]
- 4.Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients 2014; 6: 4093–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambling L, Andersen HS, Czopek A, Wojciak R, Krejpcio Z, McArdle HJ. Effect of timing of iron supplementation on maternal and neonatal growth and iron status of iron-deficient pregnant rats. J Physiol 2004; 561: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006; 160: 1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993; 341: 1–4. [DOI] [PubMed] [Google Scholar]
- 8.Fujii T, Matsuki Y, Hasegawa M. Pathophysiological study of iron-deficiency anemia in adolescence. Keio J Med 1968; 17: 193–206. [DOI] [PubMed] [Google Scholar]
- 9.Silva JM, Price CA. Effect of follicle-stimulating hormone on steroid secretion and messenger ribonucleic acids encoding cytochromes P450 aromatase and cholesterol side-chain cleavage in bovine granulosa cells in vitro. Biol Reprod 2000; 62: 186–191. [DOI] [PubMed] [Google Scholar]
- 10.Silva JM, Price CA. Insulin and IGF-I are necessary for FSH-induced cytochrome P450 aromatase but not cytochrome P450 side-chain cleavage gene expression in oestrogenic bovine granulosa cells in vitro. J Endocrinol 2002; 174: 499–507. [DOI] [PubMed] [Google Scholar]
- 11.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 1998; 12: 924–940. [DOI] [PubMed] [Google Scholar]
- 12.Wesson JA, Miller KF, Ginther OJ. Response of plasma LH and FSH to gonadotropin-releasing hormone in pony foals and ovariectomized pony mares. Theriogenology 1980; 14: 113–121. [DOI] [PubMed] [Google Scholar]
- 13.Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002; 143: 2221–2232. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Wactawski-Wende J, Michels KA, Schliep KC, Plowden TC, Chaljub EN, Mumford SL. Dietary minerals, reproductive hormone levels and sporadic anovulation: associations in healthy women with regular menstrual cycles. Br J Nutr 2018; 120: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pafumi C, Leanza V, Coco L, Vizzini S, Ciotta L, Messina A, Leanza G, Zarbo G, D’Agati A, Palumbo MA, Iemmola A, Gulino FA, Teodoro MC, Attard M, Plesca AC, Soares C, Kouloubis N, Chammas M. The reproduction in women affected by cooley disease. Hematol Rep 2011; 3: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Author manuscript; available in PMC 2010. Jul 1. doi: https://doi.org/10.1002/0471142301.nsa04is48. [Google Scholar]
- 17.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One 2012; 7: e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004; 127: 569–580. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto A, Ikeda M, Kaneko A, Kishida C, Shimada M, Yamashita Y. The Novel pig in vitro maturation system to improve developmental competence of oocytes derived from atretic non-vascularized follicle. Biol Reprod 2016; 95: 7. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Okamoto M, Ikeda M, Okamoto A, Sakai M, Gunji Y, Nishimura R, Hishinuma M, Shimada M. Protein kinase C (PKC) increases TACE/ADAM17 enzyme activity in porcine ovarian somatic cells, which is essential for granulosa cell luteinization and oocyte maturation. Endocrinology 2014; 155: 1080–1090. [DOI] [PubMed] [Google Scholar]
- 21.Li YQ, Cao XX, Bai B, Zhang JN, Wang MQ, Zhang YH. Severe iron deficiency is associated with a reduced conception rate in female rats. Gynecol Obstet Invest 2014; 77: 19–23. [DOI] [PubMed] [Google Scholar]
- 22.Dhur A, Galan P, Hercberg S. Effects of different degrees of iron deficiency on cytochrome P450 complex and pentose phosphate pathway dehydrogenases in the rat. J Nutr 1989; 119: 40–47. [DOI] [PubMed] [Google Scholar]
- 23.Chitambar CR, Narasimhan J. Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology 1991; 59: 3–10. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Kato H, Hada H, Itoh-Nakadai A, Fujiwara T, Muto A, Inoguchi Y, Ichiyanagi K, Hojo W, Tomosugi N, Sasaki H, Harigae H, Igarashi K. Iron-heme-Bach1 axis is involved in erythroblast adaptation to iron deficiency. Haematologica 2017; 102: 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin DR, Matyushov DV. Electron-transfer chain in respiratory complex I. Sci Rep 2017; 7: 5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev 2010; 68: 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrientos T, Laothamatas I, Koves TR, Soderblom EJ, Bryan M, Moseley MA, Muoio DM, Andrews NC. Metabolic catastrophe in mice lacking transferrin receptor in muscle. EBioMedicine 2015; 2: 1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod 1986; 1: 81–87. [DOI] [PubMed] [Google Scholar]
- 29.Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod 2003; 69: 1281–1293. [DOI] [PubMed] [Google Scholar]
- 30.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 2000; 141: 4295–4308. [DOI] [PubMed] [Google Scholar]
- 31.Taylor S, Rampton D. Treatment of iron deficiency anemia: practical considerations. Pol Arch Med Wewn 2015; 125: 452–460. [DOI] [PubMed] [Google Scholar]
- 32.Seidman JD. The presence of mucosal iron in the fallopian tube supports the “incessant menstruation hypothesis” for ovarian carcinoma. Int J Gynecol Pathol 2013; 32: 454–458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.