Abstract
This study aimed to determine if lactation can be induced by exogenous hormonal treatment in non-pregnant sows. In experiment 1, pseudopregnant animals were divided into four groups and given: 1) 5 mg of estradiol dipropionate (EDP) 5 days before (n = 4), 2) 5 mg of EDP 10 days before (n = 3), 3) 10 mg of EDP 5 days before (n = 3) or 4) 10 mg of EDP 10 days (n = 3) before PGF2α treatment. Artificial lactation was induced in seven pseudopregnant sows (53.8%) by exogenous hormonal treatment. There was no significant effect of either an increased EDP dosage or interval from the EDP treatment to PGF2α treatment on the induction rate of artificial lactation. In experiment 2, milk samples were collected from artificial lactating and natural lactating sows (n = 6). IgG and IgA levels in the milk collected from both groups were significantly associated with time during the experimental period. Milk IgG levels 24 h after PGF2α treatment in artificial lactating sows were higher than those in the colostrum of lactating sows. In experiment 3, hormonal profiles in pseudopregnant sows with (n = 3) or without (n = 3) EDP treatment were determined. There was a significant difference in estradiol-17β levels on days 8, 7 and 5 before PGF2α treatment between groups. Progesterone and prolactin concentrations did not differ between groups. The present study revealed for the first time that lactation could be induced by exogenous hormonal treatment in non-pregnant sows and that the milk collected from these sows contained high immunoglobulin levels.
Keywords: Artificial lactation, Estradiol dipropionate, Pig, Pseudopregnancy
It was reported that artificial lactation could be induced using exogenous hormones in non-pregnant cows [1], ewes [2] and mares [3, 4]. In cows, milk yield of induced lactation animals was 60 to 70% in the previous natural lactation after calving [1]. There is some economic benefit in the case of cows, such that net present value for induction of lactation in non-breeding cows was greater than that for replacement cows [5]. The amount of immunoglobulins (Igs) within the harvested milk in artificial lactating mares corresponded with the amount in colostrum obtained from naturally delivered dams [3]. In particular, a non-pregnant mare in which lactation was artificially induced by hormonal treatment can be used as the nurse mare and raise a foal until the weaning age [4]. Thus, inducing lactation in nonpregnant farm animals could reduce the number of culling animals, economy losses and replacement costs derived from reproductive failure [6]. This approach could also be applied to pigs, but to the best of our knowledge, no study has investigated the induction of artificial lactation in non-pregnant pigs by hormonal treatment.
In recent years, genetic selection for prolificacy in pigs has resulted in a significant improvement in litter size at birth. Although the average number of piglets per litter has increased from 13.3 in 2006 to 15.8 pigs in 2016 [7], the average volume of colostrum is not affected by either litter size [8] or breed [9]. Colostrum provides the energy and proteins that are essential for the piglets to start suckling and begin to grow [10]. Thus, piglets should consume at least 200 g of colostrum during the first 24 h after birth to decrease their risk of mortality within 3 days after birth [11] or until weaning [12]. Indeed, neonatal piglets often fail to consume a sufficient amount of colostrum, which limits their passive immunity and increases their risk of death. Therefore, the main purpose of this study was to determine whether we can induce lactation by exogenous hormonal treatment in nonpregnant sows. Furthermore, we evaluated the hormonal profiles in lactating sows that were induced by pharmaceutical agents.
Materials and Methods
Animals
Six lactation sows and 19 pseudopregnant sows (Landrace, n = 22; crossbred of Landrace and Large white, n = 3; 222.8 ± 6.3 kg (mean ± SEM), 6.5 ± 0.3 parities) were used for this study. The condition of pseudopregnancy in sows was induced by estradiol dipropionate (EDP, Ovahormone Depot; ASKA Pharmaceutical, Tokyo, Japan) treatment, as previously reported by Noguchi et al. [13, 14]. Briefly, all animals were treated once with 30 mg of EDP intramuscularly on Day 10.3 ± 0.2 (Day 0 = ovulation) [14]. Estrus was monitored twice daily using the back pressure test with a boar pheromone spray, as previously described [15]. Pseudopregnancy was defined as the absence of estrus maintained throughout the day of PGF2α treatment (between 32 and 40 days in the estrous cycle), as described previously [14]. All protocols were approved by the Azabu University Ethics Committee of Animal Care and Experimentation (151016-3).
Experiment 1. Induction of artificial lactation by an additional EDP treatment in pseudopregnant sows
To determine the dosage and timing of the EDP treatment required for the induction of artificial lactation, pseudopregnant animals were given 1) 5 mg of EDP 5 days before (n = 4), 2) 5 mg of EDP 10 days before (n = 3), 3) 10 mg of EDP 5 days before (n = 3) or 4) 10 mg of EDP 10 days before (n = 3) PGF2α treatment. All animals were treated twice with 15 mg of dinoprost (a synthetic analog of PGF2α) (Panacelan Hi; Meiji Seika Pharma, Tokyo, Japan) intramuscularly in 12-h intervals on Day 36.8 ± 0.8. The gross development of mammary glands and lactation were observed from 24 to 144 h after the first PGF2α treatment. When artificial lactation was induced in sows, milk samples were collected from all teats by the hand-milking method that was performed by two people for 15 min twice daily at 0800 and 2000 h. The milk harvested from seven sows responding to treatment was passed through double gauze filters into a 50 ml tube and stored at ‒30°C after the milk yield was recorded. Successful induction of artificial lactation was defined as total volume of milk ejection of more than 20 ml/day between 24 to 120 h after PGF2α treatment.
Experiment 2. Immunoglobulin levels in milk harvested from artificial lactating sows
IgG and IgA concentrations in milk harvested from seven artificial lactating sows in experiment 1 were measured with an enzyme-linked immunosorbent assay (ELISA) kit (Pig IgG and IgA ELISA Quantitation Set; Bethyl Laboratories, Montgomery, TX, USA). Milk samples from 0, 24, 48, 72, 96 and 120 h after farrowing in six lactating sows were also collected within 10 min after oxytocin (25 IU) injection (Atonin O; Aska Animal Health, Tokyo, Japan) and measured concentrations of IgG and IgA.
Experiment 3. Hormonal profiles in pseudopregnant sows treated with additional EDP
Six pseudopregnant sows were used to determine the effect of EDP treatment before PGF2α treatment on artificial lactation by analyzing the profiles of circulating hormones before and after luteolysis. Each sow was fitted with an indwelling catheter in the auricular vein 2 or 3 days before the first PGF2α treatment [16]. Animals were either treated with 5 mg of EDP 10 days before the PGF2α treatment (treatment group, n = 3) or not treated (control group, n = 3). Blood samples were collected at least once per day throughout the study as well as every 12 h from 10 days before the first PGF2α treatment to the first PGF2α treatment and every 6 h from 0 to 7 days after the PGF2α treatment via an indwelling catheter inserted into the auricular vein. Plasma was obtained after the centrifugation of blood and stored at –30°C.
Plasma concentrations of estradiol-17β and progesterone were measured by ELISA. Estradiol-17β and progesterone were extracted from the plasma samples with diethyl ether before being used in the assays. The secondary antibody used was goat anti-rabbit IgG (AP132; Merck, Tokyo, Japan). The primary antibody used an anti-17 beta-estradiol antibody (ab215528; Abcam, Tokyo, Japan) for estradiol-17β and an anti-Progesterone-3(E)-CMO-BSA (FKA 203-E; Cosmo Bio, Tokyo, Japan) for progesterone, respectively. The horseradish peroxidase (HRP)-linked antigen used an estradiol-6-CMO-HRP (LA310; EastCoast Bio, North Berwick, ME, USA) for estradiol-17β and a progesterone-3-CMO-HRP (FKA201; Cosmo Bio) for progesterone, respectively. The intra- and inter-assay coefficient of variations (CVs) were 8.4 and 11.0% for estradiol-17β and 7.3% and 5.8% for progesterone, respectively.
Concentrations of prolactin (PRL) in the plasma of sows were determined by competitive immunoassays using europium (Eu)-labeled porcine PRL as probes. In the porcine PRL analysis, anti-porcine PRL serum (AFP084255) was used as a primary antibody, a porcine PRL antigen (AFP11609) was used for Eu-labeling and as a reference standard (Porcine PRL immunoassay kits; National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA, USA). The intra- and inter-assay CVs were 9.4 and 7.8%, respectively.
Data analyses
All statistical analyses were performed using SAS software (SAS, version 9.4; SAS Institute, Cary, NC, USA). In experiment 1, the data were collected from thirteen pseudopregnant sows. Chi-squared tests were used to assess the differences in the relative frequency of induction of artificial lactation between the dose of additional EDP and the intervals from additional EDP treatment to PGF2α treatment. In addition, repeated measures ANOVA was used to investigate the effect of time after PGF2α treatment, the dose of additional EDP and the intervals from additional EDP treatment to PGF2α treatment on the milk yield following artificial lactation. The dependent variable was the milk yield in artificial lactation. The independent variables were time after PGF2α treatment (24, 36, 48, 60, 72, 84, 96, 108, 120, 132 and 144 h), the dose of additional EDP (5 mg and 10 mg) and the intervals from additional EDP treatment to PGF2α treatment (5 days and 10 days). The repeated measure was time, and the model was tested using sow ID as the subject term.
In experiment 2, the data were collected from seven artificial lactating sows and six lactating sows. The profiles of immunoglobulin levels over 6 days were compared between artificial lactating and lactating sows by repeated measures ANOVA. The dependent variables were the IgG and IgA levels in artificial lactating sows and the colostrum of lactating sows. The independent variables were the sow group (artificial lactating or lactating sows) and days from PGF2α treatment in artificial lactating sows or from parturition in lactating sows. The repeated measure was days, and the model was tested using sow ID as the subject term.
In experiment 3, the data were collected from six pseudopregnant sows with or without additional EDP treatment. Plasma concentrations of estradiol, progesterone and PRL at peripartum were compared between treatments by repeated measures ANOVA. The dependent variables were estradiol, progesterone and PRL, and the independent variables were the sow group (with or without additional EDP treatment) and time at peripartum. The repeated measure was time, and the model was tested using sow ID as the subject term.
All significant effects and interactions were tested by the Tukey-Kramer multiple comparison test. A P value < 0.05 was considered significant. All data represent the mean ± the standard error of the mean.
Results
Experiment 1
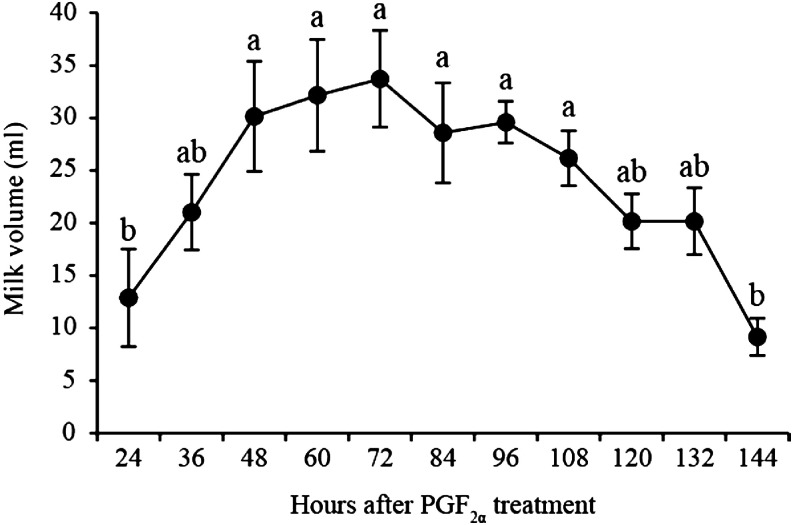
Artificial lactation was induced in seven out of 13 sows (53.8%) by additional EDP treatment. There was no significant effect of either the dose of additional EDP or the interval from the additional EDP treatment and PGF2α treatments on artificial lactation (Table 1). Figure 1 shows a change in the milk yield of artificial lactation collected from seven pseudopregnant sows after PGF2α treatment. The milk yield 48 to 108 h after PGF2α treatment was higher than at 24 h and 144 h after PGF2α treatment (P < 0.05). However, there was no effect of either the dose of additional EDP or the interval from additional EDP treatment and PGF2α treatment on the milk yield of artificial lactation. In addition to the milk yield was associated with the time after PGF2α treatment (P < 0.05), but not with the dose of additional EDP or the interval from additional EDP treatment and PGF2α treatment.
Table 1. The frequency of the induction of artificial lactation in pseudopregnant sows after additional estradiol dipropionate (EDP) treatment.
| The intervals from additional EDP treatment to PGF2α treatment |
|||
|---|---|---|---|
| 5 days | 10 days | ||
| The dose of additional EDP | 5 mg | 50.0% (2/4) | 66.7% (2/3) |
| 10 mg | 33.3% (1/3) | 66.7% (2/3) | |
Fig. 1.
Milk yield in artificial lactating sows responding to treatment (n = 7) after PGF2α treatment. Different superscripts mark significant differences among times (P < 0.05). Values are expressed as the mean ± SEM.
Experiment 2
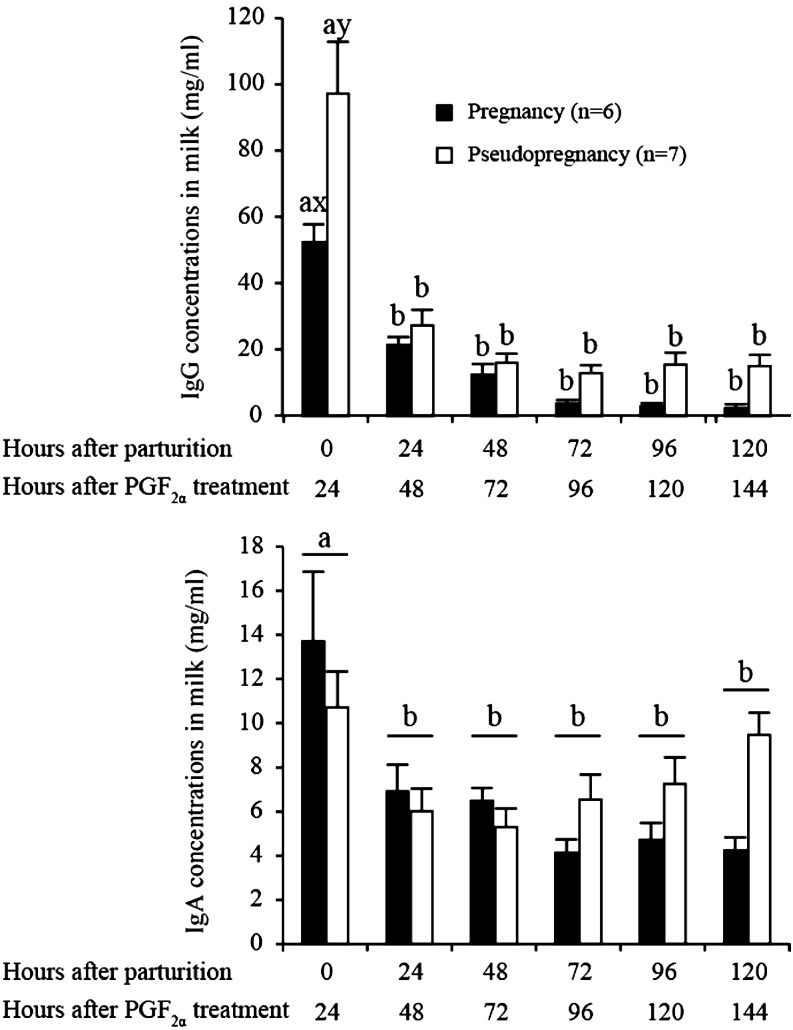
IgG and IgA concentrations on each day in artificial lactating sows and lactating sows are shown in Fig. 2. Regarding IgG, a significant interaction between sow group and days was identified (P < 0.05). The milk IgG concentration 1 day after PGF2α treatment in artificial lactating sows was higher than in the colostrum from lactating sows (97.2 ± 17.0 vs. 52.4 ± 5.4 mg/ml; P < 0.05), but there was no difference in IgG concentration between the sow groups at other timepoints. In both sow groups, the IgG concentration on the first day of correction was higher compared with other days (P < 0.05). In addition, the IgA concentration on the first day was higher than those on other days (P < 0.05), but no difference in IgA concentration was observed between sow groups.
Fig. 2.
Profiles of IgG (upper panel) and IgA (lower panel) concentrations in milk after parturition in pregnant sows (n = 6) or PGF2α treatment in pseudopregnant sows (n = 7). Different superscripts mark significant differences among times (a–b) and between groups (x–y) (P < 0.05). Values are expressed as the mean ± SEM.
Experiment 3
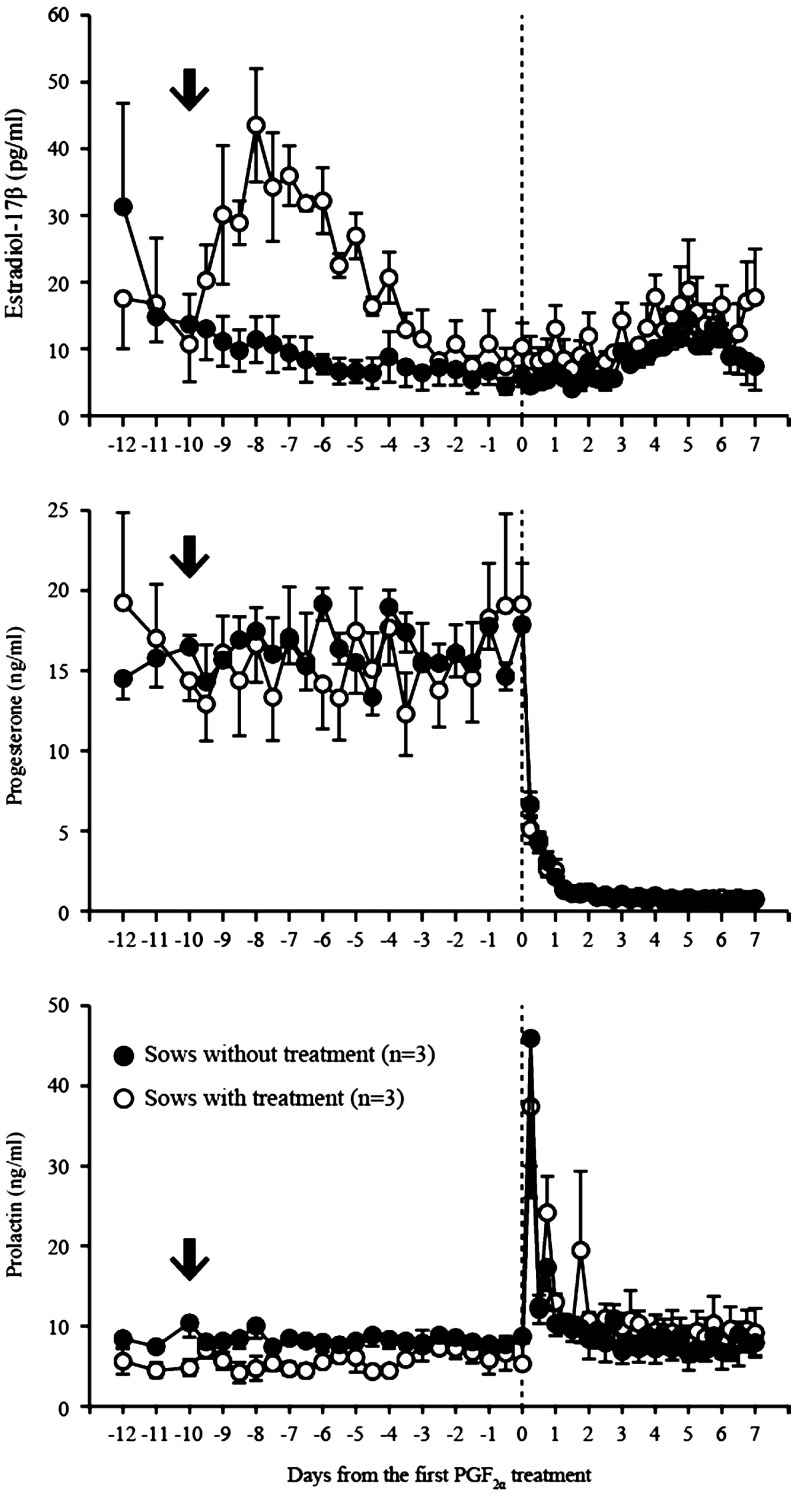
Estradiol-17β, progesterone and PRL profiles in the pseudopregnant sows with or without additional EDP treatment are shown in Fig. 3. Regarding estradiol-17β concentrations, a significant interaction between sow group and time was found (P < 0.05). In pseudopregnant sows with additional EDP treatment, there was a significant difference in estradiol-17β concentrations between different days. Specifically, the concentrations of estradiol-17β were higher on days –9 to –7 compared with day –10 (day 0 indicates the day of PGF2α treatment). In contrast, there was no difference in estradiol-17β between days in pseudopregnant sows without additional EDP treatment. There was a significant difference in estradiol-17β levels on days –8, –7 and –5 between sow groups (P < 0.05). Progesterone and PRL concentrations were associated with time (P < 0.05), but not with sow group. Progesterone immediately decreased after PGF2α treatment and reached the baseline level within 2.5 days. PRL concentrations were significantly higher (P < 0.05) on day 0.25 and 0.75 than on day 0.
Fig. 3.
Plasma concentrations of estradiol-17β (upper panel), progesterone (middle panel) and prolactin (lower panel) in pseudopregnant sows treated with 5 mg of EDP (n = 3, open circle) and without treatment (n = 3, closed circle). Arrows in each panel indicate EDP treatment. Values are expressed as the mean ± SEM.
Discussion
This is the first report to investigate artificial lactation in non-pregnant sows treated with exogenous hormones. Artificial lactation induced by exogenous hormones has been reported in non-pregnant cows [1], ewes [2] and mares [3, 4], but no study has evaluated this process in sows. The present study revealed that lactation could be induced in pseudopregnant sows treated with EDP and that the milk collected from pseudopregnant sows contained Igs equivalent to colostrum. This finding may help to develop innovative technologies in pig production to address many important issues, such as the lack of teat order because of large litter sizes and the limited available treatments for weak piglets.
Our results show that sows in which artificial lactation is induced produce good milk that contains amount of IgG and IgA equivalent to colostrum. It is well known that IgG and IgA are the main Igs in sow colostrum and milk, respectively [17,18,19]. Maternal IgG derived from colostrum provides specific systemic humoral immunity to neonates, whereas maternal IgA transferred mainly via milk until weaning provides passive mucosal immunity and local humoral immunity [20]. Given the steady increase in litter size in modern pig production, there is a need to develop a colostrum replacer or supplement [7]. Currently, there are situations where the quantity of maternal colostrum is insufficient, it is of low quality or it is not available. Piglets need to consume an adequate amount of colostrum because it provides energy and Ig for their survival and growth [10]. Colostral Ig protects piglets against infectious diseases before they develop their own adaptive immunity. The present study showed that there was no difference in either IgG or IgA levels between the milk of artificially and naturally lactating sows throughout the experimental period. Although the IgG level in milk harvested at 24 h from artificially lactating sows was higher than in the colostrum obtained from naturally lactating sows, the concentrations of IgG in both of these milk in pregnant and pseudopregnant sows in this study is the corresponded levels in colostrum as previously described [21, 22]. These findings indicate that artificial lactation collected from pseudopregnant sows can be used as a colostrum replacer or supplement for suckling piglets.
Despite good quality milk in the artificially-induced lactating sows, average milk production was low throughout 24 to 144 h after PGF2α treatment. In swine, it is well known that milk ejaculation and production are influenced by oxytocin associated with parturition and the spontaneous suckling from piglets, and thus depends on litter size and the weight of piglets [23]. During the first two weeks of lactation, suckling occurs every 30 to 70 min in sows [24, 25]. The removal of milk from the lactating mammary gland is also a major factor in maintaining milk secretion [26]. In our study, milk collection was performed only twice a day by two people for a short time. Both the frequency and technique of udder stimulation in this study may have affected milk production in the artificial lactating sows.
The present study showed that only a single additional treatment of EDP before PGF2α treatment could induce lactation in pseudopregnant sows. In bovine [1], goat [2] and horse [3], estrogen and progesterone had to be injected intramuscularly or subcutaneously into non-pregnant animals for at least 7 days to induce artificial lactation. Estrogen, progesterone and PRL are known regulators of mammary development, the initiation of lactation and colostrum production in pigs [12, 27‒29]. In particular, estrogen is an important factor for growth, morphogenesis and lactogenesis in the porcine mammary gland [27, 29, 30]. EDP (an estradiol diester with propionate in the 3- and 17-positions) works as free estradiol-17β in the body, and estradiol-17β is maintained at high levels in peripheral blood after the intramuscular administration of EDP in swine [13, 31]. High concentrations of progesterone can also be maintained in pseudopregnant pigs induced by a single EDP treatment [13, 31]. Our results and previous reports suggest that a single treatment of EDP before PGF2α treatment in pseudopregnant sows can maintain peripheral estrogen and progesterone concentrations at high levels, which is required for mammary gland development and the induction of lactation after luteolysis.
In bovine, artificial lactation was induced in 60%-69% of animals treated with an intramuscular injection of 0.1 mg/kg bw/day of estradiol-17β and 0.25 mg/kg bw/day of progesterone for 7 days [32, 33]. Estradiol-17β concentrations in pseudopregnant sows treated with additional EDP were maintained at a high level for only 3 days compared with not treated animals from 10 days before PGF2α treatment. A lack of spontaneous estrogen stimulation for mammary gland growth before PGF2α treatment may have contributed to the low induction rate of artificial lactation in this study (53.8%). Further studies are required to determine the best hormonal treatment for the induction of artificial lactation in swine.
The additional estrogen treatment before luteolysis had no effect on PRL profiles in pseudopregnant pigs. Low concentrations of PRL before farrowing stimulate mammogenesis, and then high PRL levels are needed to enhance lactogenesis after parturition in pregnant pigs [27, 28]. There was no difference in basal PRL levels with and without additional estrogen treatment in pseudopregnant pigs in this study or in previous reports [27, 34, 35]. However, the combined treatment of estrogen and PRL but not the individual treatment affected growth and morphogenesis in the mammary gland [29]. Estrogen stimulates the expression of β-casein mRNA in mammary glands, and thus lactogenesis is enhanced by exogenous estrogen in the pseudopregnant pig [30]. Lactation was not observed in pseudopregnant pigs without EDP treatment before PGF2α treatment [13, 14, 31]. From our results and previous reports, providing an estrogen supplement before luteolysis to pseudopregnant sows may not directly affect PRL secretion, but the interaction of estrogen and PRL may be important for the regulation of both mammogenesis and lactogenesis. We could not clarify the role of estrogen supplement in the induction of artificial lactation in swine in the present study. Additional studies are needed to clarify the relationships between exogenous estrogen treatment and mammogenous and/or lactogenous in the pseudoprengnant pigs.
In conclusion, the present study revealed for the first time that it is possible to induce lactation by exogenous hormonal treatment in non-pregnant sows and that high levels of porcine Igs are contained in the milk collected from pseudopregnant sows. These findings indicate that it would be possible to produce a colostrum supplement containing porcine Igs using pseudopregnant sows. However, this is the first step in sows, and further research should be conducted to establish the most effective way to collect artificial lactation from sows.
Acknowledgments
This study was supported by a Grant-in-Aid for Young Scientists (B) (Grant Number 16K18785 to MN) from the Japanese Society for the Promotion of Science (JSPS). We thank Dr AF Parlow, National Hormone and Peptide Program of the National Institute of Diabetes, and Digestive and Kidney Diseases, Harbor-UCLA Medical Center, Torrance, CA, USA, for providing the porcine PRL immunoassay kits.
References
- 1.Lakhani P, Thakur A, Kumar S, Singh P. Artificial induction of lactation in bovines - Scope and limitations. Int J Livest Res 2017; 7: 102–112. [Google Scholar]
- 2.Kann G. Evidence for a mammogenic role of growth hormone in ewes: effects of growth hormone-releasing factor during artificial induction of lactation. J Anim Sci 1997; 75: 2541–2549. [DOI] [PubMed] [Google Scholar]
- 3.Chavatte-Palmer P, Arnaud G, Duvaux-Ponter C, Brosse L, Bougel S, Daels P, Guillaume D, Clément F, Palmer E. Quantitative and qualitative assessment of milk production after pharmaceutical induction of lactation in the mare. J Vet Intern Med 2002; 16: 472–477. [DOI] [PubMed] [Google Scholar]
- 4.Korosue K, Murase H, Sato F, Ishimaru M, Harada T, Watanabe G, Taya K, Nambo Y. Successful induction of lactation in a barren Thoroughbred mare: growth of a foal raised on induced lactation and the corresponding maternal hormone profiles. J Vet Med Sci 2012; 74: 995–1002. [DOI] [PubMed] [Google Scholar]
- 5.Ramgattie R, Siew N, Diptee M, Stoute V, Knights M. Effect of mammary stimulation on dairy cows and heifers exposed to a lactation induction protocol. Open J Anim Sci 2014; 4: 1–12. [Google Scholar]
- 6.Inchaisri C, Jorritsma R, Vos PLAM, van der Weijden GC, Hogeveen H. Economic consequences of reproductive performance in dairy cattle. Theriogenology 2010; 74: 835–846. [DOI] [PubMed] [Google Scholar]
- 7.Kemp B, Da Silva CLA, Soede NM. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reprod Domest Anim 2018; 53(Suppl 2): 28–36. [DOI] [PubMed] [Google Scholar]
- 8.Devillers N, Farmer C, Le Dividich J, Prunier A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007; 1: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 9.Declerck I, Dewulf J, Piepers S, Decaluwé R, Maes D. Sow and litter factors influencing colostrum yield and nutritional composition. J Anim Sci 2015; 93: 1309–1317. [DOI] [PubMed] [Google Scholar]
- 10.Le Dividich J, Rooke JA, Herpin P. Nutritional and immunological importance of colostrum for the new-born pig. J Agric Sci 2005; 143: 469–485. [Google Scholar]
- 11.Devillers N, Le Dividich J, Prunier A. Influence of colostrum intake on piglet survival and immunity. Animal 2011; 5: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 12.Devillers N, van Milgen J, Prunier A, Le Dividich J. Estimation of colostrum intake in the neonatal pig. Anim Sci 2004; 78: 305–313. [Google Scholar]
- 13.Noguchi M, Yoshioka K, Suzuki C, Itoh S, Kaneko H. An efficient protocol for inducing pseudopregnancy using estradiol dipropionate and follicular development associated with changes in reproductive hormones after prostaglandin F2alpha treatment in pseudopregnant sows. Reprod Biol Endocrinol 2011; 9: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguchi M, Kashiwai S, Itoh S, Okumura H, Kure K, Suzuki C, Yoshioka K. Reproductive hormone profiles in sows on estrus synchronization using estradiol dipropionate and prostaglandin F(2α)-analogue and the reproductive performance in female pigs on commercial farms. J Vet Med Sci 2013; 75: 343–348. [DOI] [PubMed] [Google Scholar]
- 15.Langendijk P, Bouwman EG, Schams D, Soede NM, Kemp B. Effects of different sexual stimuli on oxytocin release, uterine activity and receptive behavior in estrous sows. Theriogenology 2003; 59: 849–861. [DOI] [PubMed] [Google Scholar]
- 16.Zanella AJ, Mendl MT. A fast and simple technique for jugular catheterization in adult sows. Lab Anim 1992; 26: 211–213. [DOI] [PubMed] [Google Scholar]
- 17.Markowska-Daniel I, Pomorska-Mól M, Pejsak Z. Dynamic changes of immunoglobulin concentrations in pig colostrum and serum around parturition. Pol J Vet Sci 2010; 13: 21–27. [PubMed] [Google Scholar]
- 18.Drew MD, Owen BD. The provision of passive immunity to colostrum-deprived piglets by bovine or porcine serum immunoglobulins. Can J Anim Sci 1988; 68: 1277–1284. [Google Scholar]
- 19.Porter P, Noakes DE, Allen WD. Secretory IgA and antibodies to Escherichia coli in porcine colostrum and milk and their significance in the alimentary tract of the young pig. Immunology 1970; 18: 245–257. [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol 2009; 33: 384–393. [DOI] [PubMed] [Google Scholar]
- 21.Hurley WL. Composition of sow colostrum and milk. In: Farmer C (ed.), The Gestating and Lactating Sow. Wageningen, The Netherlands: Wageningen Academic Publishers; 2015: 193–229. [Google Scholar]
- 22.Kielland C, Rootwelt V, Reksen O, Framstad T. The association between immunoglobulin G in sow colostrum and piglet plasma. J Anim Sci 2015; 93: 4453–4462. [DOI] [PubMed] [Google Scholar]
- 23.King RH. Factors that influence milk production in well-fed sows. J Anim Sci 2000; 78: 19–25. [DOI] [PubMed] [Google Scholar]
- 24.Bøe K. The process of weaning in pigs: when the sow decides. Appl Anim Behav Sci 1991; 30: 47–59. [Google Scholar]
- 25.Jensen P, Stangel G, Algers B. Nursing and suckling behaviour of semi-naturally kept pigs during the first 10 days postpartum. Appl Anim Behav Sci 1991; 31: 195–209. [Google Scholar]
- 26.Hurley WL. Mammary gland growth in the lactating sow. Livest Prod Sci 2001; 70: 149–157. [Google Scholar]
- 27.DeHoff MH, Stoner CS, Bazer FW, Collier RJ, Kraeling RR, Buonomo FC. Temporal changes in steroids, prolactin and growth hormone in pregnant and pseudopregnant gilts during mammogenesis and lactogenesis. Domest Anim Endocrinol 1986; 3: 95–105. [Google Scholar]
- 28.Farmer C, Sorensen MT, Petitclerc D. Inhibition of prolactin in the last trimester of gestation decreases mammary gland development in gilts. J Anim Sci 2000; 78: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 29.Horigan KC, Trott JF, Barndollar AS, Scudder JM, Blauwiekel RM, Hovey RC. Hormone interactions confer specific proliferative and histomorphogenic responses in the porcine mammary gland. Domest Anim Endocrinol 2009; 37: 124–138. [DOI] [PubMed] [Google Scholar]
- 30.Lee CY, Bazer FW, Simmen FA. Expression of components of the insulin-like growth factor system in pig mammary glands and serum during pregnancy and pseudopregnancy: effects of oestrogen. J Endocrinol 1993; 137: 473–483. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi M, Yoshioka K, Suzuki C, Arai S, Itoh S, Wada Y. Estrus synchronization with pseudopregnant gilts induced by a single treatment of estradiol dipropionate. J Reprod Dev 2010; 56: 421–427. [DOI] [PubMed] [Google Scholar]
- 32.Smith KL, Schanbacher FL. Hormone induced lactation in the bovine. I. Lactational performance following injections of 17 -estradiol and progesterone. J Dairy Sci 1973; 56: 738–743. [DOI] [PubMed] [Google Scholar]
- 33.Collier RJ, Bauman DE, Hays RL. Milk production and reproductive performance of cows hormonally induced into lactation. J Dairy Sci 1975; 58: 1524–1527. [Google Scholar]
- 34.Burne THJ, Murfitt PJE, Goode JA, Boulton MI, Gilbert CL. Effects of oestrogen supplementation and space restriction on PGF2α-induced nest-building in pseudopregnant gilts. Anim Reprod Sci 1999; 55: 255–267. [DOI] [PubMed] [Google Scholar]
- 35.Boulton MI, Wickens A, Brown D, Goode JA, Gilbert CL. Prostaglandin F2α-induced nest-building in pseudopregnant pigs. II. Space restriction stress does not influence secretion of oxytocin, prolactin, oestradiol or progesterone. Physiol Behav 1997; 62: 1079–1085. [DOI] [PubMed] [Google Scholar]