 A series of N-benzothiazolyl-2-benzenesulfonamides were designed and synthesized as novel ABCA1 expression upregulators.
A series of N-benzothiazolyl-2-benzenesulfonamides were designed and synthesized as novel ABCA1 expression upregulators.
Abstract
ATP binding cassette transporter A1 (ABCA1) is a critical transporter that mediates cellular cholesterol efflux from macrophages to apolipoprotein A-I (ApoA-I). Therefore, increasing the expression level of ABCA1 is anti-atherogenic and ABCA1 expression upregulators have become novel choices for atherosclerosis treatment. In this study, a series of N-benzothiazolyl-2-benzenesulfonamides, based on the structure of WY06 discovered in our laboratory, were designed and synthesized as novel ABCA1 expression upregulators. Based on an in vitro ABCA1 upregulatory cell model, ABCA1 upregulation of target compounds was evaluated. Compounds 6c, 6d, and 6i have good upregulated ABCA1 expression activities, with EC50 values of 0.97, 0.37, and 0.41 μM, respectively. A preliminary structure–activity relationship is summarized. Replacing the methoxy group on the benzothiazole moiety of WY06 with a fluorine or chlorine atom and exchanging the ester group with a cyano group resulted in more potent ABCA1 upregulating activity. Moreover, compound 6i increased ABCA1 mRNA and protein expression and significantly promoted cholesterol efflux in RAW264.7 cells. In conclusion, N-benzothiazolyl-2-benzenesulfonamides were identified as novel ABCA1 expression upregulators.
Introduction
Coronary heart disease (CHD), stroke, and peripheral vascular disease are the leading causes of death and represent a heavy burden to healthcare systems around the world.1–4 High-density lipoprotein (HDL) is an independent protective factor against cardiovascular disease through many potential mechanisms such as inhibiting inflammation, regulating nitric oxide (NO) production, and anti-thrombotic and anti-apoptotic activities.5–8 The most established cardioprotective effect of HDL is its role in reverse cholesterol transport (RCT),8 in which HDL removes excess cholesterol from macrophages in the arterial wall and transports it back to the liver for degradation to bile acids. ATP-binding cassette transporter A1 (ABCA1) mediates the rate-controlling step in the initial stages of HDL formation and as much as 70% of the total cellular cholesterol is effluxed to apolipoprotein A-I (ApoA-I) from macrophages.9,10 Overexpression of ABCA1 in macrophages promotes HDL formation and RCT, and reduces atherosclerosis.11,12 However, humans with Tangier disease (TD), who are deficient in ABCA1, exhibit severe HDL deficiency and a higher incidence of cardiovascular disease.13 Thus, ABCA1 has been identified as a potential target for novel pharmacological agents that are designed to increase the cardiovascular protective potential of HDL.14 ABCA1 expression upregulators may be potentially used in atherosclerosis treatment.15–17 In addition, ABCA1 is also associated with the occurrence and development of other diseases such as Alzheimer's disease, inflammation, ischemic heart disease, and diabetes. Therefore, regulating ABCA1 may play an important role for the treatment of these diseases.18–21
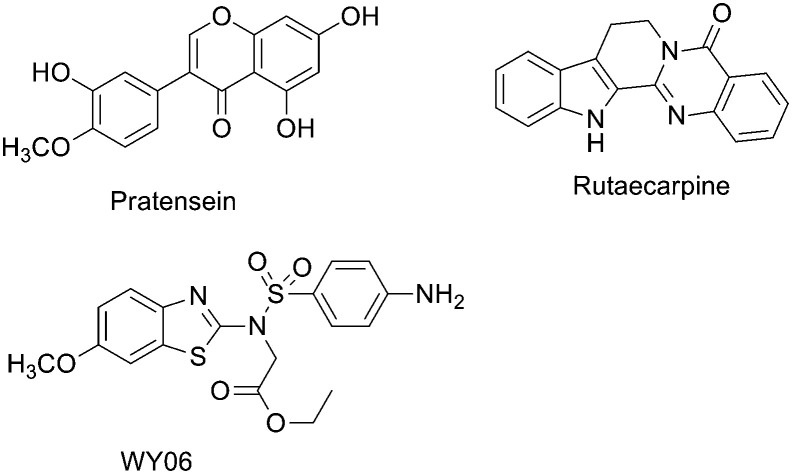
Currently, several natural or synthetic compounds such as pratensein,22 rutaecarpine (Fig. 1),23,24 and CS6253 (ref. 25) (a 26-amino acid peptide) have been reported as ABCA1 expression upregulators, but none of these has been released to the market to date. In our previous study, WY06 with a benzothiazole scaffold was found to display potent ABCA1 upregulatory activity using a high-throughput assay.22 In this study, the structure of WY06 was optimized by replacing the substituents on the benzothiazole ring and sulfonamide nitrogen atoms and by changing the position of the amine group on the benzene ring. A series of WY06 analogues were synthesized and evaluated. Our primary objective was to identify more potent ABCA1 expression upregulators. The structure and activity relationship (SAR) was summarized to facilitate further design. Moreover, the primary mechanism of action of the most potent compound was also investigated.
Fig. 1. The structure of some ABCA1 expression upregulators.
Results and discussion
Chemistry
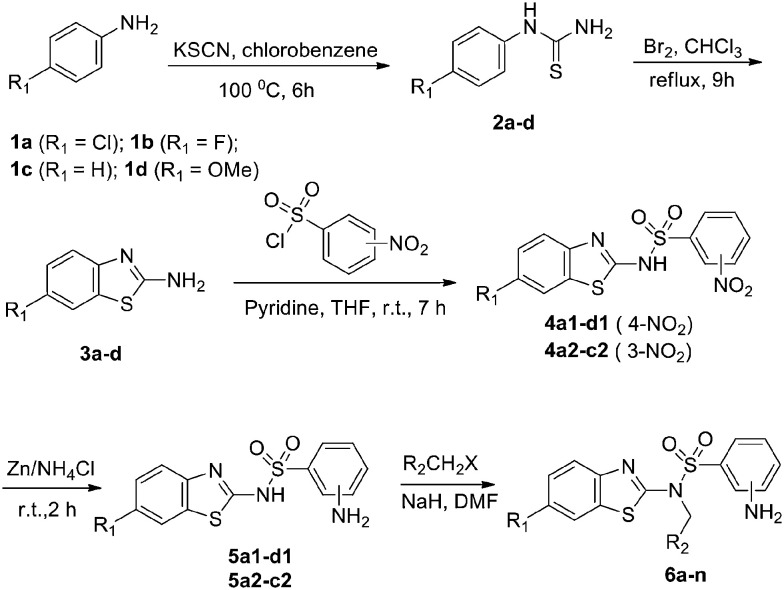
The detailed synthetic pathway to target compounds 6a–n is shown in Scheme 1. Anilines 1a–d were treated with potassium thiocyanate in chlorobenzene at 100 °C to yield thioureas 2a–d, which were cyclized in the presence of Br2 to yield benzo[d]-2-amines 3a–d. Coupling of the amines 3a–d with 3-/4-nitrobenzenesulfonyl chloride afforded benzenesulfonamides 4a–d, and then the nitro group was reduced with Zn powder and NH4Cl to yield 3-/4-aminobenzenesulfonamides 5a–d. Targets 6a–n were obtained by nucleophilic substitution of the resulting sulfonamides 5a–d with substituted haloalkanes in the presence of NaH in DMF.
Scheme 1. Synthesis of target compounds 6a–n.
Pharmacology
Table 1 presents the series of WY06 derivatives that were synthesized and evaluated for their ABCA1 upregulatory activities. We first examined the influence of substituents on the benzothiazole moiety. Removing or replacing the methoxy group on the benzothiazole moiety with a fluorine or chlorine atom, as in 6b–d, resulted in more potent activity, including increased upregulating value and decreased EC50 value. In particular, compound 6d with a chlorine atom showed a 17-fold increase in potency (EC50, 0.37 ± 0.08 μM) compared with WY06 (EC50, 6.38 ± 0.09 μM). Subsequently, the ester group at the R2 position, which might be hydrolyzed in vivo, was exchanged with a cyano group or vinyl group (6e–j). Generally, most of these analogues displayed lower potency than the corresponding ester targets. The contribution of R2 to the EC50 activity seemed to be as follows: ester > cyano > vinyl (6bvs.6evs.6f; 6cvs.6gvs.6h; 6dvs.6ivs.6j). However, compound 6i with the cyano group not only displayed comparable EC50 activity to 6d, but also exhibited enhanced upregulating potency. In addition, a series of compounds 6k–m with an amino group at the meta-position of benzene were also synthesized and evaluated, all of them exhibiting decreased potency or even lost potency (6k and 6l, EC50 > 50 μM).
Table 1. Structures and activity of target compounds 6a–n.
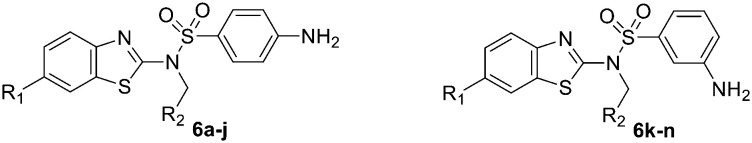
| ||||
| Compd. | R1 | R2 | Max. a (%) | EC50 b (μM) |
| 6a (WY06) | OMe | CO2Et | 138 | 6.38 ± 0.09 |
| 6b | H | CO2Et | 209 | 1.24 ± 0.33 |
| 6c | F | CO2Et | 167 | 0.97 ± 0.03 |
| 6d | Cl | CO2Et | 174 | 0.37 ± 0.08 |
| 6e | H | CN | 157 | 8.51 ± 0.12 |
| 6f | H | CH CH2 | 115 | 33.42 ± 1.21 |
| 6g | F | CN | 163 | 2.57 ± 0.15 |
| 6h | F | CH CH2 | 135 | 7.4 ± 0.32 |
| 6i | Cl | CN | 206 | 0.41 ± 0.05 |
| 6j | Cl | CH CH2 | 135 | 10.57 ± 0.16 |
| 6k | H | CH CH2 | 136 | >50 |
| 6l | H | CN | 124 | >50 |
| 6m | F | CH CH2 | 152 | 25.98 ± 0.45 |
| 6n | Cl | CN | 168 | 5.84 ± 0.57 |
aMaximum upregulating value (%).
bData represent the average of multiple determinations (n ≥ 3) ± standard error of the mean (SEM).
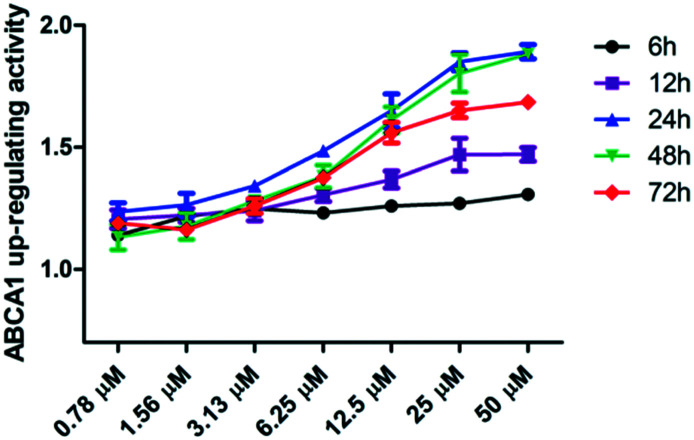
Table 1 shows that compounds 6d and 6i exhibit exceptional EC50 values, which are >15-fold higher than that of lead compound WY06, and 6i showed slightly higher ABCA1 upregulating activity than 6d. Subsequently, the luciferase assay of compound 6i at 6 h, 12 h, 24 h, 48 h, and 72 h was examined to further understand the ABCA1 upregulatory process.
The results (Fig. 2) show that compound 6i exhibited dose-dependent upregulating ABCA1 activity at different time points, and the upregulating ABCA1 activity peaked at 24 h and 48 h.
Fig. 2. The luciferase assay of compound 6i at different time points.
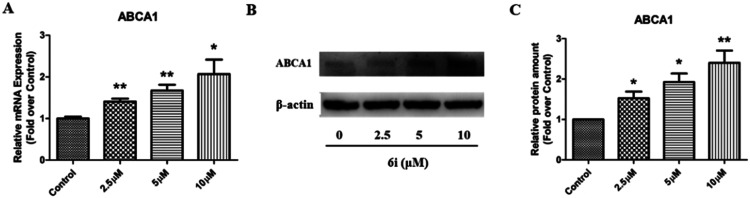
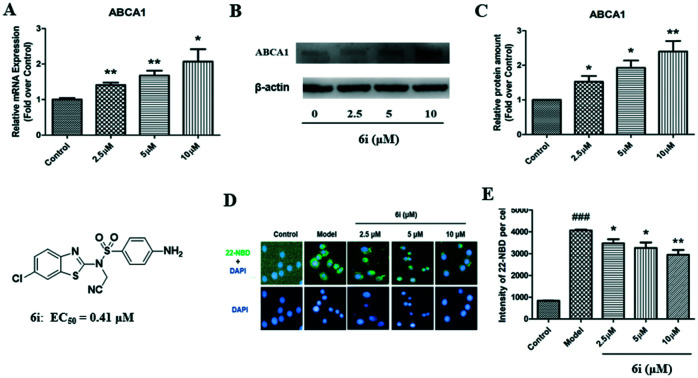
To confirm the above luciferase assay results, compound 6i was further evaluated in terms of ABCA1 mRNA and protein expression levels in RAW264.7 cells. Western blot and real time quantitative PCR (RT-qPCR) analyses were performed to investigate the effect of 6i on ABCA1 mRNA and protein expression. RT-qPCR analysis showed that 6i significantly increases ABCA1 mRNA expression, with a maximum upregulating rate of 210% (Fig. 3A). Western blot analysis revealed that ABCA1 protein levels significantly increased in a dose-dependent manner when incubated with 2.5, 5, or 10.0 μM 6i (Fig. 3B), with a maximum upregulating rate of 240% compared to the control group (Fig. 3C).
Fig. 3. Compound 6i increases ABCA1 expression in RAW264.7 cells. (A) ABCA1 mRNA expression levels in RAW264.7 cells. (B) Representative images of ABCA1 and β-actin. (C) Relative ABCA1 protein expression levels in RAW264.7 cells were normalized to β-actin. *P < 0.05, **P < 0.01 vs. control group.
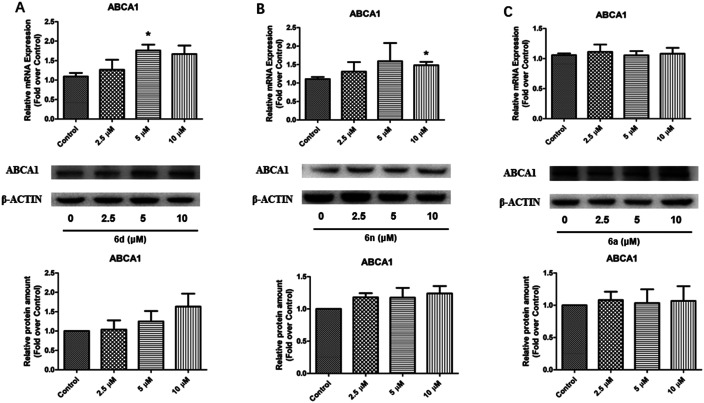
In addition, compounds 6a, 6d, and 6n with an ABCA1 upregulating EC50 gradient were selected for ABCA1 mRNA and protein analysis. Fig. 4 shows that compound 6d (EC50: 0.37 μM, maximum value: 1.74-fold higher) significantly increased ABCA1 mRNA expression in RAW264.7 cells at a concentration of 5.0 μM, and increased ABCA1 protein expression in a dose-dependent manner. Compound 6n (EC50: 5.84 μM, maximum value: 1.68-fold higher) significantly increased ABCA1 mRNA expression in RAW264.7 cells at a concentration of 5.0 μM with a maximum value of 1.5-fold, and slightly increased ABCA1 protein expression in a dose-dependent manner. Compound 6a (EC50: 6.38 μM, maximum value: 1.38-fold higher) had no obvious effect on ABCA1 mRNA and protein expression in RAW264.7 cells. These results coincided with the above SAR.
Fig. 4. The ABCA1 mRNA and protein analysis of compounds 6a, 6d, and 6n in RAW264.7 cells. RAW264.7 cells were treated with compounds 6a, 6d, or 6n [0 (control), 2.5, 5, 10 μM] as described in Experimental methods. ABCA1 mRNA and protein expression levels induced by compounds 6a (A), 6d (B), and 6n (C) were analyzed by RT-qPCR and western blotting. Representative images of ABCA1 and β-actin. Relative ABCA1 protein levels in RAW264.7 cells were normalized to β-actin. *P < 0.05 vs. control group.
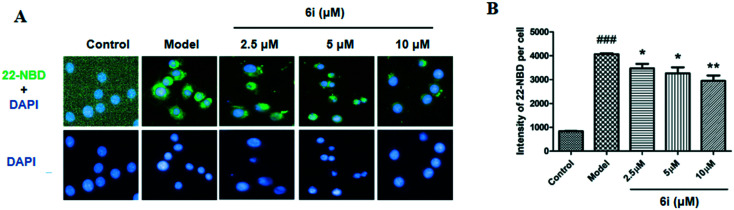
ABCA1 is one of the crucial transporter proteins that facilitate cholesterol efflux from macrophages. Considering that compound 6i could increase the expression of ABCA1 in RAW264.7 cells, a 22-NBD-cholesterol efflux assay to investigate the cholesterol efflux effect of compound 6i in RAW264.7 cells was performed. Fig. 5 shows that the 22-NBD-cholesterol levels (green color) of cells treated with compound 6i significantly decreased compared to that of the model group (Fig. 5A). Quantitative analysis results showed that compound 6i significantly decreased cellular 22-NBD-cholesterol content (Fig. 5B), which agreed with the imaging results. In summary, these results indicate that compound 6i significantly increases cholesterol efflux from RAW264.7 macrophages.
Fig. 5. Compound 6i promotes cholesterol efflux in RAW264.7 cells. (A) Representative images of the effects of 6i on cholesterol efflux in RAW264.7 cells that were treated as described in Experimental methods (400×). (B) Fluorescence intensity of 22-NBD-cholesterol per cell from three independent experiments. *P < 0.05, **P < 0.01 vs. model group.
To exclude the effect of ApoA-I on cholesterol efflux, western blotting was performed to examine whether compound 6i affects ApoA-I protein expression in RAW264.7 cells. As shown in the ESI† (Fig. S1), compound 6i did not affect ApoA-I protein expression at the indicated concentrations [0 (control), 2.5, 5, 10 μM]. Therefore, cholesterol efflux due to compound 6i involved an increase in ABCA1 expression.
To evaluate the potential therapeutic application of the hit compounds, the cytotoxicity of six representative compounds (6a, 6b, 6c, 6d, 6i, and 6n) were evaluated in HepG2 cells using Cell Counting Kit 8 (Solarbio Life Sciences, Beijing, China). The results (ESI† Fig. S2) showed that all the tested compounds showed low cytotoxicity (CC50 > 100 μM).
Experimental methods
General
1H NMR and 13C NMR spectra were measured using a Bruker ARX-500 nuclear magnetic resonance instrument. Mass spectra were recorded with an AB Sciex 3200 LC-MS. Elemental analysis was carried out with an Elementar Vario ELIII. Commercially produced solvents and reagents were used in this study. The boiling range of petroleum ether used in column chromatography was 60–90 °C. The silica gel (300–400 mesh) for flash column chromatography was purchased from Qingdao Marine Chemical Factory in China (Qingdao, China).
Representative example: synthesis of 1-(4-chlorophenyl)thiourea (2a)
4-Chlorobenzenamine (10.0 g, 78.4 mmol) was dissolved in 70 mL of chlorobenzene. Concentrated sulfuric acid (3.0 mL, 43.1 mmol) was then added at 0 °C. The mixture was heated to 70 °C, followed by the addition of potassium thiocyanate (8.4 g, 86.2 mmol). Then, the solution was reacted at 100 °C for 6 h. After cooling to room temperature, the mixture was filtered, and the filter cake was washed with diethyl ether and water and dried in vacuo to obtain compound 2a as a white solid (yield: 63.0%). M.p.: 180.1–181.3 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 7.36 (d, J = 9.0 Hz, 2H), 7.46 (d, J = 9.0 Hz, 2H), 9.75 (s, 1H). ESI-MS, m/z: 187.2 (M + H)+. Anal. calcd for C7H7ClN2S: C 45.04, H 3.78, N 15.01; found C 45.21, H 3.84, N 15.23.
Representative example: synthesis of 6-chlorobenzo[d]thiazol-2-amine (3a)
1-(4-Chlorophenyl)thiourea (8.5 g, 45.7 mmol) was dissolved in 50 mL of chloroform, and Br2 (2.6 mL, 50.3 mmol) was added slowly at 0 °C. The mixture was heated to 70 °C for 9 h and cooled to room temperature. The reaction mixture was filtered, and the filtrate was neutralized with ammonium hydroxide, forming white solids. The precipitate was filtered, washed with water, and dried in vacuo to obtain compound 3a as a white solid (yield: 43.1%). M.p. 175.3–179.2 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 7.76 (d, J = 9.0 Hz, 2H), 7.45 (d, J = 9.0 Hz, 2H), 9.75 (s, 1H). ESI-MS, m/z: 185.2 (M + H)+. Anal. calcd for C7H5ClN2S: C 45.53, H 2.73, N 15.17; found C 45.62, H 2.84, N 15.09.
Representative example: synthesis of N-(6-chlorobenzo[d]thiazol-2-yl)-4-nitrobenzenesulfonamide (4a1)
6-Chlorobenzo[d]thiazol-2-amine (5.0 g, 27.0 mmol) was dissolved in 100 mL of THF. Pyridine (2.4 mL, 29.8 mmol) was added slowly thrice, at intervals of 4 h. After stirring for 10 min each time pyridine was added, 4-nitrobenzenesulfonyl chloride (8.9 g, 40.5 mmol) was added in three portions. About 7 h later, the reaction was completed, and the mixture was concentrated to obtain a flesh pink solid. The solid was added to 100 mL of water and stirred for 1 h and then filtered. The filter cake was washed with water and dried in vacuo, and then purified by silica gel chromatography using a solvent of petroleum ether/ethyl acetate (5 : 1) to provide the desired product 4a1 as a flesh pink solid, with a yield of 63.5%. M.p. 169.3–171.5 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 7.26 (dd, J = 2.0, 7.5 Hz, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.41 (t, J = 8.0 Hz, 1H), 7.76 (s, 1H), 7.85 (s, 1H), 8.25 (s, 1H), 13.35 (s, 1H). ESI-MS, m/z: 370.2 (M + H)+, 392.4 (M + Na)+. Anal. calcd for C13H8ClN3O4S2: C 42.22, H 2.18, N 11.36; found C 42.31, H 2.97, N 11.41.
Representative example: synthesis of 4-amino-N-(6-chlorobenzo[d]thiazol-2-yl)benzenesulfonamide (5a1)
N-(6-Chlorobenzo[d]thiazol-2-yl)-4-nitrobenzenesulfonamide (2.0 g, 5.4 mmol) was dissolved in 15 mL of water–ethanol–methanol (2 : 6 : 3) and heated to 60 °C for 20 min to obtain a clear solution. The solution was cooled to room temperature and then ammonium chloride was added. The zinc powder was added in batches. The reaction was stirred for an additional 2 h at room temperature and then filtered. The filtrate was concentrated and quenched with water, then extracted with ethyl acetate. The organic layer was washed with water and brine and then dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the obtained compound 5a1 as a yellow solid was used in the next step without any purification (yield: 52.4%). M.p. 182.5–184.6 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 5.96 (s, 2H), 7.13 (dd, J = 2.0, 7.5 Hz, 1H), 7.25 (d, J = 8.0 Hz, 1H), 7.36 (d, J = 7.5 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.68 (s, 1H), 7.98 (s, 1H), 8.23 (s, 1H), 13.26 (s, 1H). ESI-MS, m/z: 340.2 (M + H)+, 362.4 (M + Na)+. Anal. calcd for C13H10ClN3O2S2: C 45.95, H 2.97, N 12.37; found C 45.87, H 2.84, N 12.42.
General synthetic procedure for compounds 6a–6n
Amino-N-(6-chlorobenzo[d]thiazol-2-yl)benzenesulfonamide (1 mmol) was dissolved in 1 mL of DMF. NaH (3 mmol) was added at 0 °C. Chloroacetonitrile, ethyl bromoacetate, or allyl chloride (2.5 mmol) was then added. The reaction was stirred for an additional 8 h at room temperature and diluted with water (30 mL). The mixture was extracted with ethyl acetate (4 × 30 mL), washed with brine (30 mL), and dried over anhydrous sodium sulfate. The target compounds were purified by silica gel chromatography using petroleum ether and ethyl acetate as a solvent as white to light yellow solids.
Ethyl 2-(4-amino-N-(6-methoxybenzo[d]thiazol-2-yl)phenylsulfonamido)acetate (6a)
Yield 39.8%. M.p. 170.5–172.0 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 1.11 (t, J = 7.3 Hz, 3H), 3.78 (s, 3H), 4.06 (q, J = 7.0 Hz, 2H), 4.97 (s, 2H), 5.94 (s, 2H), 6.56 (d, J = 8.5 Hz, 2H), 7.02 (dd, J = 2.4, 8.5 Hz, 1H), 7.45 (d, J = 9.0 Hz, 2H), 7.48 (d, J = 9.0 Hz, 1H), 7.51 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 14.34, 46.37, 56.28, 61.84, 107.98, 112.91, 113.35, 114.69, 124.88, 127.22, 128.28, 131.32, 149.24, 153.21, 156.82, 164.67, 167.32, 175.43. ESI-MS, m/z: 422.3 (M + H)+, 444.1 (M + Na)+. Anal. calcd for C17H17N3O5S2: C 50.11, H 4.21, N 10.31; found C 50.19, H 4.30, N 10.33.
Ethyl 2-(4-amino-N-(benzo[d]thiazol-2-yl)phenylsulfonamido)acetate (6b)
Yield 41.4%. M.p. 167.5–170.2 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 1.12 (t, J = 7.0 Hz, 3H), 4.05–4.09 (m, 2H), 5.01 (s, 2H), 5.96 (s, 2H), 6.56 (d, J = 9.0 Hz, 2H), 7.30 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 8.5 Hz, 3H), 7.57 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 7.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 14.35, 46.28, 61.87, 112.62, 112.93, 123.35, 123.68, 124.50, 126.96, 127.65, 128.33, 128.46, 137.48, 153.32, 165.08, 167.32, 175.27. ESI-MS, m/z: 392.3 (M + H)+, 414.2 (M + Na)+. Anal. calcd for C16H15N3O4S2: C 50.91, H 4.01, N 11.13; found C 50.84, H 4.26, N 11.23.
Ethyl 2-(4-amino-N-(6-fluorobenzo[d]thiazol-2-yl)phenylsulfonamido)acetate (6c)
Yield 40.2%. M.p. 193.6–194.5 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 1.16 (t, J = 7.0 Hz, 3H), 4.12 (q, J = 7.0 Hz, 2H), 5.06 (s, 2H), 6.04 (s, 2H), 6.62 (d, J = 8.0 Hz, 2H), 7.38 (t, J = 7.5 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.67 (dd, J = 2.4, 8.0 Hz, 1H), 7.98 (dd, J = 2.0, 7.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 14.12, 46.72, 61.03, 109.59, 112.98. 114.03, 123.26, 125.69, 127.63, 128.10, 128.61, 136.98, 151.22, 152.42, 164.16, 167.34, 175.66. ESI-MS, m/z: 410.1 (M + H)+, 432.3 (M + Na)+. Anal. calcd for C16H14FN3O4S2: C 48.60, H 3.57, N 10.63; found C 48.72, H 3.52, N 10.73.
Ethyl 2-(4-amino-N-(6-chlorobenzo[d]thiazol-2-yl)phenylsulfonamido)acetate (6d)
Yield 42.3%. M.p. 185.0–186.7 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 1.10 (t, J = 7.0 Hz, 3H), 4.06 (q, J = 7.00 Hz, 2H), 5.00 (s, 2H), 5.98 (s, 2H), 6.57 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.5 Hz, 2H), 7.50 (dd, J = 2.4, 8.7 Hz, 1H), 7.60 (d, J = 9.0 Hz, 1H), 8.02 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 14.12, 46.72, 61.03, 109.59, 112.98, 114.03, 123.26, 125.69, 127.63, 128.10, 128.61, 136.98, 151.22, 152.42, 164.16, 167.34, 175.66. ESI-MS, m/z: 426.3 (M + H)+, 448.1 (M + Na)+. Anal. calcd for C16H14ClN3O4S2: C 46.66, H 3.43, N 10.20; found C 46.59, H 3.49, N 10.34.
N-Cyanomethyl-N-(benzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6e)
Yield 39.3%. M.p. 176.2–177.5 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 5.41 (s, 2H), 5.58 (s, 2H), 6.76 (dd, J = 2.4, 8.3 Hz, 1H), 7.00 (d, J = 7.5 Hz, 1H), 7.10 (s, 1H), 7.17 (t, J = 8.0 Hz, 1H), 7.38 (t, J = 7.7 Hz, 1H), 7.54 (t, J = 7.7 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.92 (d, J = 7.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 32.79, 110.58, 112.33, 112.77, 114.85, 117.72, 123.26, 123.40, 124.94, 127.69, 129.53, 135.55, 141.59, 149.47, 165.30. ESI-MS, m/z: 345.4 (M + H)+, 367.1 (M + Na)+. Anal. calcd for C15H12N4O2S2: C 52.31, H 3.51, N 16.27; found C 52.54, H 3.61, N 16.32.
N-Allyl-N-(benzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6f)
Yield 40.0%. M.p. 199.7–200.6 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 3.59 (d, J = 6.5 Hz, 2H), 5.03 (d, J = 10.0 Hz, 1H), 5.13 (d, J = 17.0 Hz, 1H), 5.78–5.88 (m, 1H), 7.11 (t, J = 7.5 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.25 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 7.5 Hz, 1H), 7.43 (d, J = 7.5 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 7.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 35.93, 118.07, 118.94, 121.03, 122.21, 125.40, 125.55, 125.93, 127.06, 130.07, 130.83, 133.68, 137.34, 151.72, 155.10, 173.51. ESI-MS, m/z: 346.4 (M + H)+, 368.1 (M + Na)+. Anal. calcd for C16H15N3O2S2: C 55.63, H 4.38, N 12.16; found C 55.72, H 4.28, N 12.37.
N-Cyanomethyl-N-(6-fluorobenzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6g)
Yield 38.8%. M.p. 197.5–198.0 °C; 1H-NMR (DMSO-d6, 125 MHz) δ: 5.37 (s, 2H), 6.03 (s, 2H), 6.59 (d, J = 9.00 Hz, 2H), 7.41 (t, J = 9.0 Hz, 1H), 7.52 (d, J = 8.5 Hz, 2H), 7.74 (dd, J = 3.0, 9.0 Hz, 1H), 7.86 (dd, J = 8.3 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 33.29, 110.89, 111.12, 113.03, 113.75, 115.23, 115.39, 126.31, 128.58, 132.72, 153.61, 157.66, 158.47, 160.39, 164.66. ESI-MS, m/z: 363.2 (M + H)+, 385.1 (M + Na)+. Anal. calcd for C15H11FN4O2S2: C 49.71, H 3.06, N 15.46; found C 49.75, H 3.43, N 15.62.
N-Allyl-N-(6-fluorobenzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6h)
Yield 39.6%. M.p. 208.5–210.5 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 4.78 (d, J = 4.0 Hz, 2H), 4.98 (d, J = 17.0 Hz, 1H), 5.14 (d, J = 10.5 Hz, 1H), 5.78–5.86 (m, 1H), 5.97 (s, 2H), 6.57 (d, J = 8.5 Hz, 2H), 7.31 (t, J = 9.0 Hz, 1H), 7.47 (d, J = 9.0 Hz, 3H), 7.82 (dd, J = 2.4, 8.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 35.04, 108.01, 108.22, 113.37, 113.56, 117.53, 117.60, 118.83, 119.85, 119.91, 133.63, 148.80, 157.41, 159.30, 173.90. ESI-MS, m/z: 364.4 (M + H)+, 386.6 (M + Na)+. Anal. calcd for C16H14FN3O3S2: C 52.88, H 3.88, N 11.56; found C 52.91, H 3.74, N 11.62.
N-Cyanomethyl-N-(6-chlorobenzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6i)
Yield 40.4%. M.p. 183.2–185.3 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 5.35 (s, 2H), 6.03 (s, 2H), 6.59 (d, J = 8.5 Hz, 2H), 7.52 (d, J = 8.5 Hz, 2H), 7.58 (dd, J = 2.4, 8.5 Hz, 1H), 7.72 (d, J = 9.0 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 33.24, 112.95, 113.04, 113.76, 115.16, 123.40, 125.72, 126.25, 127.93, 128.40, 128.61, 129.10, 135.16, 153.62, 164.50. ESI-MS, m/z: 379.5 (M + H)+, 401.2 (M + Na)+. Anal. calcd for C15H11ClN4O2S2: C 47.55, H 2.93, N 14.79; found C 47.69, H 2.85, N 14.52.
N-Allyl-N-(6-chlorobenzo[d]thiazol-2-yl)-4-aminobenzenesulfonamide (6j)
Yield 40.3%. M.p. 180.5–182.1 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 4.77 (d, J = 5.0 Hz, 2H), 4.97 (d, J = 17.0 Hz, 1H), 5.13 (d, J = 10.5 Hz, 1H), 5.78–5.86 (m, 1H), 5.98 (s, 2H), 6.57 (d, J = 8.5 Hz, 2H), 7.47 (s, 1H), 7.48 (s, 3H), 8.01 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 15.44, 47.13, 49.05, 112.99, 114.28, 118.10, 122.97, 126.00, 127.01, 127.59, 128.35, 128.43, 130.89, 136.31, 153.30, 164.40, 173.42. ESI-MS, m/z: 380.2 (M + H)+, 402.1 (M + Na)+. Anal. calcd for C16H14ClN3O2S2: C 50.59, H 3.71, N 11.06; found C 50.70, H 3.69, N 11.32.
N-Allyl-N-(benzo[d]thiazol-2-yl)-3-aminobenzenesulfonamide (6k)
Yield 36.2%. M.p. 175.3–176.0 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 4.83 (d, J = 4.5 Hz, 2H), 5.01 (d, J = 17.5 Hz, 1H), 5.15 (d, J = 10.5 Hz, 1H), 5.58 (s, 1H), 5.81–5.89 (m, 1H), 6.73 (dd, J = 2.4, 8.0 Hz, 1H), 6.94 (d, J = 7.5 Hz, 1H), 7.05 (s, 1H), 7.13 (t, J = 8.0 Hz, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 47.14, 110.88, 113.07, 117.84, 118.25, 121.30, 123.35, 123.98, 124.71, 127.76, 129.80, 130.95, 137.11, 142.55, 149.75, 165.75. ESI-MS, m/z: 346.3 (M + H)+, 368.2 (M + Na)+. Anal. calcd for C16H15N3O2S2: C 55.63, H 4.38, N 12.16; found C 55.72, H 4.26, N 12.74.
N-Cyanomethyl-N-(benzo[d]thiazol-2-yl)-3-aminobenzenesulfonamide (6l)
Yield 35.3%. M.p. 210.0–211.5 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 5.44 (s, 2H), 5.62 (s, 2H), 6.76 (dd, J = 2.4, 7.8 Hz, 1H), 6.99 (d, J = 8.0 Hz, 1H), 7.10 (s, 1H), 7.16 (t, J = 7.8 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 7.54 (t, J = 8.0 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 32.79, 110.58, 112.33, 112.77, 114.85, 117.72, 123.26, 123.40, 124.94, 127.69, 129.53, 135.55, 141.59, 149.47, 165.30. ESI-MS, m/z: 345.1 (M + H)+, 367.3 (M + Na)+. Anal. calcd for C15H12N4O2S2: C 52.31, H 3.51, N 16.27; found C 52.52, H 3.54, N 16.34.
N-Allyl-N-(6-fluorobenzo[d]thiazol-2-yl)-3-aminobenzenesulfonamide (6m)
Yield 39.6%. M.p. 153.3–155.0 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 3.66 (d, J = 6.5 Hz, 2H), 5.09 (d, J = 10.0 Hz, 1H), 5.25 (d, J = 16.5 Hz, 1H), 5.79–5.88 (m, 1H), 7.03 (t, J = 7.3 Hz, 1H), 7.09 (t, J = 8.0 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 7.35 (s, 1H), 7.47 (dd, J = 3.0, 8.0 Hz, 1H), 7.60 (dd, J = 2.4, 7.5 Hz, 1H), 8.05 (s, 1H), 9.28 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 35.06, 108.10, 108.56, 113.28, 113.31, 117.41, 117.79, 119.01, 119.85, 119.98, 134.21, 148.63, 157.41, 159.50, 174.20. ESI-MS, m/z: 364.1 (M + H)+, 386.2 (M + Na)+. Anal. calcd for C16H14FN3O2S2: C 52.88, H 3.88, N 11.56; found C 52.95, H 3.97, N 11.67.
N-Cyanomethyl-N-(6-chlorobenzo[d]thiazol-2-yl)-3-aminobenzenesulfonamide (6n)
Yield 39.6%. M.p. 157.0–158.6 °C; 1H-NMR (DMSO-d6, 500 MHz) δ: 5.39 (s, 1H), 5.60 (s, 2H), 6.75 (dd, J = 7.3 Hz, 1H), 6.97 (d, J = 8.0 Hz, 1H), 7.08 (s, 1H), 7.12 (dd, J = 2.4, 8.0 Hz, 1H), 7.15 (t, J = 8.5 Hz, 1H,),7.58 (s, 1H), 7.68 (d, J = 8.5 Hz, 1H). 13C-NMR (DMSO-d6, 125 MHz) δ: 33.14, 110.96, 112.88, 113.59, 115.08, 115.23, 125.28, 126.15, 128.42, 132.56, 153.45, 157.51, 158.31, 160.23, 164.50. ESI-MS, m/z: 379.1 (M + H)+, 401.2 (M + Na)+. Anal. calcd for C15H11ClN4O2S2: C 47.55, H 2.93, N 14.79; found C 47.69, H 3.84, N 14.82.
Cell culture
HepG2 cells were cultured in DMEM (Corning Inc., Corning, NY, USA) with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). RAW264.7 (ATCC, Rockville, MD, USA) cells were grown in RPMI (Corning, Inc.) with 10% FBS (medium A). Human ABCA1p-LUC HepG2 cells were grown in medium A supplemented with 500 μg mL–1 G418 (medium B). All cells were cultured at 37 °C with 5% CO2 in a cell incubator and maintained at the NHC Key Laboratory of Biotechnology of Antibiotics (CAMS & PUMC).
ABCA1 upregulating activity assay
The ABCA1p-LUC HepG2 cells were seeded into a 96-well clear bottom white plate (Costar) at a density of 5 × 105 cells per well in 100 μL medium B. The compounds were diluted to concentrations of 100, 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78125, 0.39, 0.039, 0.0039, and 0 μM with RPMI-1640 cell culture medium containing 5% FBS (medium C). After 6–8 h of incubation, the culture medium was discarded and replaced with 200 μL of cell culture medium containing the indicated concentrations of the compounds. After 24 h, the cells were washed with PBS once, and then luciferase activity was assessed using a Luciferase Assay System (Promega) and a microplate reader.22
Western blotting
RAW264.7 cells were grown in a six-well plate (Costar) and then treated with compound 6i (0, 2.5, 5, and 10 μM) for 24 h. Total proteins were extracted with RIPA buffer (Applygen Technologies, Beijing, China). The cell lysate was centrifuged at 12 000 rpm for 15 min at 4 °C. Protein concentrations of the supernatants were determined with a BCA Protein Assay kit (Thermo Fisher Scientific). Protein samples were separated using SDS-PAGE gels and then transferred onto polyvinylidene fluoride membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skim milk for 1 h and then incubated with the corresponding primary antibodies overnight at 4 °C. After washing with 1× Tris buffered saline with 0.1% Tween-20 (TBST) thrice, the membranes were incubated with the appropriate secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h at room temperature. The primary antibodies used include ABCA1 (Novus Biologicals, Littleton, CO, USA), ApoA-I (Abcam) and β-actin (Sigma-Aldrich).
Real-time quantitative PCR (RT-qPCR)
RAW264.7 cells were grown in a six-well plate and treated with compound 6i (0, 2.5, 5 and 10 μM) for 24 h. Total RNA was extracted with a QIAGEN RNeasy Mini kit (Qiagen, 40724 Hilden, Germany). Then, the total RNA was reverse transcribed using a TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China). RT-qPCR was performed using a FastStart Universal SYBR Green PCR Master (Roche Diagnostics, Lewes, UK) and special primers with an FTC-3000 real-time quantitative thermal cycler (Funglyn Biotech Inc., Richmond Hill, Canada).
Cholesterol efflux assay
RAW264.7 macrophages were plated into 96-well clear-bottom black plates (PerkinElmer, Fremont, CA, USA). After the cells had attached to the bottom of the plate, compound 6i (0, 2.5, 5, and 10 μM) was added, incubating for 18 h at 37 °C with 5% CO2 in a cell incubator. Then, the cells were treated with serum-free DMEM with 0.2% (w/v) bovine serum albumin (Sigma Aldrich) and different concentrations of compound 6i (medium A) containing 22-NBD-cholesterol (final concentration: 50 μM) (Thermo Fisher Scientific) for 6 h. Then, the cells were washed twice with phosphate-buffered saline (PBS). Then, the cells were incubated with medium A containing ApoA-I (final concentration: 10 μg mL–1) (Peking Union-Biology, Beijing, China) for another 6 h. DAPI (Invitrogen) was used as a nuclear counterstain. Fluorescence images were captured and the fluorescence was quantified using a high content analysis system (PerkinElmer, Fremont, CA, USA).
Conclusions
In this study, 14 benzothiazole derivatives were synthesized and evaluated in terms of their upregulatory effects on human ABCA1 expression. The results showed that compounds 6b, 6c, 6d, 6g, and 6i had better upregulatory activities than the lead compound 6a. Further RT-qPCR and western blot analyses showed that 6i increases both ABCA1 mRNA expression and protein expression in RAW264.7 cells. In addition, 22-NBD-cholesterol efflux assay showed that 6i significantly increases cholesterol efflux from RAW264.7 macrophages. Currently, 6i is the leading anti-atherosclerosis drug that requires further modification.
Conflicts of interest
The authors hereby declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of Hebei Province (grant number 19JCZDJC63900), the Drug Innovation Major Project (grant number 2018ZX09711001-003-006), the National Natural Science Foundation of China (grant number 81573482), the Health and Medical Creation Program of CAMS (grant number 2016-I2M-1-011), the Natural Science Foundation of Hebei Province (grant number H2018307055) and the Science Foundation of Hebei Normal University (grant number L2017K06). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
†Electronic supplementary information (ESI) available: NMR and MS spectra of compounds 6a–6n. See DOI: 10.1039/c9md00556k
References
- Wang E. Y., Dixson J., Schiller N. B., Whooley M. A. Am. J. Cardiol. 2017;119:27–34. doi: 10.1016/j.amjcard.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Puddu P. E., Piras P., Menotti A. Int. J. Cardiol. 2017;228:359–363. doi: 10.1016/j.ijcard.2016.11.157. [DOI] [PubMed] [Google Scholar]
- Kawada T. Am. J. Cardiol. 2014;113:571. doi: 10.1016/j.amjcard.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Snyder M. L., Love S.-A., Sorlie P. D., Rosamond W. D., Antini C., Metcalf P. A., Hardy S., Suchindran C. M., Shahar E., Heiss G. Popul. Health Metr. 2014;12:10. doi: 10.1186/1478-7954-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi S., Mehramiz M., Kelesidis T., Mobarhan M. G., Sahebkar A. H., Esmaily H., Moohebati M., Farjami Z., Ferns G. A., Mohammadpour A. H., Avan A. J. Cell. Physiol. 2019;234:1–10. doi: 10.1002/jcp.28276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapano A. L., Pirillo A., Norata G. D. Cardiovasc. Res. 2019;115:6–7. doi: 10.1093/cvr/cvy247. [DOI] [PubMed] [Google Scholar]
- Ahn N., Kim K. Integr. Med. Res. 2016;5:212–215. doi: 10.1016/j.imr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Fan B., Ma A., Shaul P. W., Zhu H. J. Lipid Res. 2015;56:986–997. doi: 10.1194/jlr.M054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L., Wang N., Tall A. R. Arterioscler., Thromb., Vasc. Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Smith J. D. BioFactors. 2014;40:547–554. doi: 10.1002/biof.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi A., Ueno T., Fukuda N., Watanabe T., Tahira K., Haketa A., Hatanaka Y., Tanaka S., Matsumoto T., Matsumoto Y., Nagase H., Soma M. J. Mol. Med. 2014;92:509–521. doi: 10.1007/s00109-013-1118-x. [DOI] [PubMed] [Google Scholar]
- Hafiane A., Bielicki J. K., Johansson J. O., Genest J. PLoS One. 2015;10:e0131997. doi: 10.1371/journal.pone.0131997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury P., Couvert P., Elbitar S., Ghateb Y., Abou-Khalil Y., Azar Y., Ayoub C., Superville A., Guerin M., Rabes J.-P., Varret M., Boileau C., Jambart S., Giral P., Carrie A., Le Goff W., Abifadel M. J. Clin. Lipidol. 2018;12:1374–1382. doi: 10.1016/j.jacl.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Gao J. H., Zeng M. Y., Yu X. H., Zeng G. F., He L. H., Zheng X. L., Zhang D. W., Ouyang X. P., Tang C. K. Biochem. Biophys. Res. Commun. 2018;500:318–324. doi: 10.1016/j.bbrc.2018.04.066. [DOI] [PubMed] [Google Scholar]
- Li Y., Jiang B., Liang P., Tong Z., Liu M., Lv Q., Liu Y., Liu X., Tang Y., Xiao X. Biochem. Biophys. Res. Commun. 2017;486:364–371. doi: 10.1016/j.bbrc.2017.03.047. [DOI] [PubMed] [Google Scholar]
- Ma X., Li S. F., Qin Z. S., Ye J., Zhao Z. L., Fang H. H., Yao Z. W., Gu M. N., Hu Y. W. Cardiovasc. Pathol. 2015;24:230–235. doi: 10.1016/j.carpath.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Hu Y. W., Wang Q., Ma X., Li X. X., Liu X. H., Xiao J., Liao D. F., Xiang J., Tang C. K. J. Atheroscler. Thromb. 2010;17:493–502. doi: 10.5551/jat.3152. [DOI] [PubMed] [Google Scholar]
- Salinas C. A., Cruz-Bautista I., Mehta R., Villarreal-Molina M. T., Pérez F. J., Tusié-Luna M. T., Canizales-Quinteros S. Curr. Diabetes Rev. 2007;3:264–267. doi: 10.2174/157339907782329979. [DOI] [PubMed] [Google Scholar]
- Yang J., Kou J., Lalonde R., Fukuchi K. Brain, Behav., Immun. 2017;65:262–273. doi: 10.1016/j.bbi.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frikke-Schmidt R. Atherosclerosis. 2010;208:305–316. doi: 10.1016/j.atherosclerosis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Liu Y., Tang C. Biochim. Biophys. Acta. 2012;1821:522–529. doi: 10.1016/j.bbalip.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Xu Y., Yang Y., Zheng Z., Jiang W., Hong B., Yan X., Si S. J. Biomol. Screening. 2008;13:648–656. doi: 10.1177/1087057108320545. [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu Q., Xu Y., Liu Y. C., Wang X., He X., Zhu N., Liu J., Wu Y., Li Y., Li N., Feng T., Lai F., Zhang M., Hong B., Jiang J., Si S. J. Lipid Res. 2014;56:1634–1647. doi: 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Feng T., Liu P., Liu C., Wang X., Li D., Li N., Chen M., Xu Y., Si S. ACS Med. Chem. Lett. 2014;5:884–888. doi: 10.1021/ml500131a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S., Bittner S., Hu J., Shen W. J., Cortez Y., Hao X., Han L., Lagerstedt J. O., Kraemer F. B., Johansson J. O. Mol. Cell. Endocrinol. 2019;480:1–11. doi: 10.1016/j.mce.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.