Abstract
Objective
To evaluate the effects of neuromodulation techniques in adults with phantom limb pain (PLP).
Methods
A systematic search was performed, comprising randomized controlled trials (RCTs) and quasi-experimental (QE) studies that were published from database inception to February 2019 and that measured the effects of neuromodulation in adults with PLP. Hedge’s g effect size (ES) and 95% confidence intervals were calculated, and random-effects meta-analyses were performed.
Results
Fourteen studies (nine RCTs and five QE noncontrolled studies) were included. The meta-analysis of RCTs showed significant effects for i) excitatory primary motor cortex (M1) stimulation in reducing pain after stimulation (ES = −1.36, 95% confidence interval [CI] = −2.26 to −0.45); ii) anodal M1 transcranial direct current stimulation (tDCS) in lowering pain after stimulation (ES = −1.50, 95% CI = −2.05 to 0.95), and one-week follow-up (ES = −1.04, 95% CI = −1.64 to 0.45). The meta-analysis of noncontrolled QE studies demonstrated a high rate of pain reduction after stimulation with transcutaneous electrical nerve stimulation (rate = 67%, 95% CI = 60% to 73%) and at one-year follow-up with deep brain stimulation (rate = 73%, 95% CI = 63% to 82%).
Conclusions
The evidence from RCTs suggests that excitatory M1 stimulation—specifically, anodal M1 tDCS—has a significant short-term effect in reducing pain scale scores in PLP. Various neuromodulation techniques appear to have a significant and positive impact on PLP, but due to the limited amount of data, it is not possible to draw more definite conclusions.
Keywords: Neuromodulation, Phantom Limb Pain, Meta-analysis (Source: MeSH)
Introduction
Phantom limb pain (PLP) is a chronic pain condition that is perceived in a limb that no longer exists [1]. PLP is a serious public health problem that can affect the physical, psychological, and functional health of amputees [2]. After limb amputation, 50–80% of patients experience PLP [2–6], which deteriorates their quality of life [3,7,8]. The occurrence of long-term PLP can lead to comorbidities, such as depression, sleep disturbances, and substance abuse, and affect the use of prostheses [5,9,10].
The current treatment options (pharmacological and behavioral) for PLP are not entirely effective [11]. The pathophysiology of PLP is related to cortical reorganization [11,12]. Nevertheless, no existing PLP treatment targets these maladaptive brain modifications.
Neuromodulation is a potential treatment option for chronic pain [13,14], which could alter maladaptive neuroplasticity and enhance descending inhibitory pathways [15,16]. Such methods include invasive central (deep brain stimulation [DBS], motor cortex stimulation [MCS], and spinal cord stimulation [SCS]) and peripheral (dorsal root ganglion stimulation [DRGS]) interventions and noninvasive central (transcranial magnetic stimulation [rTMS], transcranial direct current stimulation [tDCS]) and peripheral (transcutaneous electrical nerve stimulation [TENS], neuromuscular electrical stimulation [NMES], and peripheral nerve stimulation [PNS]) techniques.
Systematic reviews have attempted to evaluate these options but have encountered several limitations: They analyzed the efficacy of noninvasive brain stimulation without comparing invasive or peripheral techniques [17,18] focused on chronic pain conditions in general, including PLP patients, but failed to target PLP specifically [17]. Therefore, the efficacy of neuromodulation in PLP and the ideal option for treating PLP are unknown.
This study systematically reviewed the evidence on the efficacy and safety of neuromodulation techniques in the treatment of PLP to provide initial data on the type of neuromodulation that has significant effect sizes for further research and future clinical applications.
Methods
Study Design
We performed a systematic review. The study protocol was registered at PROSPERO, number CRD42018117998. This study follows the Cochrane handbook [19] and PRISMA guidelines [20] (Supplementary Data).
Eligibility Criteria
Our inclusion criteria were as follows: i) studies that evaluated any beneficial or adverse effect of the use of neuromodulation techniques in the treatment of adults (>18 years old) with a PLP diagnosis. We included i) invasive central nervous system (CNS) stimulation (DBS, MCS, and SCS), noninvasive CNS stimulation (rTMS and tDCS), and peripheral nervous system stimulation—TENS, PNS, and NMES; ii) randomized controlled trials (RCTs), including parallel-group and crossover designs, and quasi-experimental (QE) studies, including noncontrolled and nonrandomized studies; iii) studies with pain scales (visual analog scale [VAS], numeric rating scale [NRS], McGill Pain Questionnaire, or universal pain score [UPS]) as the primary outcome (and this information had to include mean and standard deviation before and after the intervention); iv) without language restriction. We excluded case–control studies, cohort studies, case series, review articles, conference abstracts, case reports, letters, and editorials. We excluded the studies with full text not available after trying to contact the authors. The most recent or the largest sample size publication was included when the authors published several studies using the same database.
Literature Search and Study Selection
We performed a literature search using five databases: Medline, Central Cochrane Library, Embase, Scopus, and Web of Science. The last updating search was run in February 2019. No additional filters (e.g., language or publication year) were set. The complete search strategy is available in the Supplementary Data. A manual search was also conducted to find other potential articles based on identified references in the individual articles.
Before titles and abstract screening, two reviewers (XM and KP-B) agreed on a standard approach. Two random samples of 50 search results were selected for training purposes. Reviewers screened these titles and abstracts, and inter-rater agreement and kappa estimator were computed, aiming for an inter-rater agreement of at least 90% (Supplementary Data). After this standardization process, duplicate records were removed, and two reviewers (XM and KP-B) screened all titles and abstracts following the prespecified framework and selection criteria. Discrepancies were resolved by a third reviewer independently (FF). After the title and abstract selection, full texts of selected reports were sought and analyzed; differences were resolved by consensus between all authors. These selection processes—titles and abstracts as well as full texts—were conducted using Endnote software. The complete list of excluded articles and the reasons for exclusion at this full-text stage is available in the Supplementary Data.
Data Extraction
Two authors systematically extracted data from each included study using a standardized form. The form used for data extraction documented the most relevant items, including sample characteristics, study design, and intervention characteristics. The PLP intensity (visual analog scale), quality of life, anxiety and depression scales, and adverse events were extracted to evaluate the efficacy and safety of the use of neuromodulation techniques.
Risk of Bias Assessment
To assess the risk of bias (RoB) of RCTs, we used the Jadad Scale [21] following the standard score system from 0 to 5 (high number means low risk of bias). To assess the risk of bias of QE studies, we used the Methodological Index for Non-Randomized Studies (MINORS) tool [22]. For QE controlled studies, the MINORS tool was scored from 0 to 2; 0 for not reported information, 1 for inadequately reported information, and 2 for well-reported information. We considered a low risk of bias when the information was well reported, a high risk of bias when it was not reported, and an unclear risk of bias when it was reported inadequately [22]. The RoB evaluation was assessed by two reviewers (XM and KP-B), and discrepancies were solved by a third reviewer independently (FF).
Data Synthesis
First, we reported the articles in a narrative approach. We decided to present results separately according to study design (RCTs vs QE studies), given the differences in the quality of evidence between these two types of studies.
Interventions were categorized into one of four groups: i) excitatory noninvasive CNS stimulation, ii) inhibitory noninvasive CNS stimulation, iii) invasive CNS stimulation, iv) PNS stimulation. Pain outcome was categorized according to time, based on a previous study [17], in three groups: i) short term (end of intervention or less than one week), ii) medium term (more than one week to less than six weeks), and iv) long term (more than six weeks).
Then, with the RCT data, we performed an exploratory meta-analysis using Hedge’s g for pain intensity. Although within the treatment categories are interventions with different parameters, we decided to do an exploratory synthesis to compare across the spectrum of the available neuromodulation techniques. When possible, we used pre and post scores of the pain analog scales for each outcome to calculate the mean difference between groups. The difference was then converted to an effect size (ES). Given that Cohen’s d has a slight bias to overestimate in small sample sizes, we adjusted Cohen’s d to Hedge’s g by applying a correction factor.
With the QE data, we pooled the rate of pain reduction per individual study by implementing a proportions meta-analysis approach. Briefly, we estimated 95% confidence intervals (CIs) using the exact binomial method and incorporated the Freeman-Tukey double arcsine transformation for computation of pooled proportions [23].
We assessed heterogeneity using the I2 statistic, and we considered low heterogeneity I2 < 40% [24]. We considered it appropriate to use random-effects models due to the overall heterogeneity of the evaluation (in population and intervention) [25]. Metaregression and publication bias were not assessed, as the number of studies pooled for each meta-analysis was <10 [26]. The data were processed using Stata, version 15.0.
Management of Missing Data
When the primary outcome data (i.e., PLP visual analog scale) were missing or unclear, we contacted the authors. We used Web Plot Digitizer, version 3.11, to extract data from relevant graphs. If we could not reach the authors or obtain the data graphically, we excluded the study from the quantitative analysis.
Results
Search Results
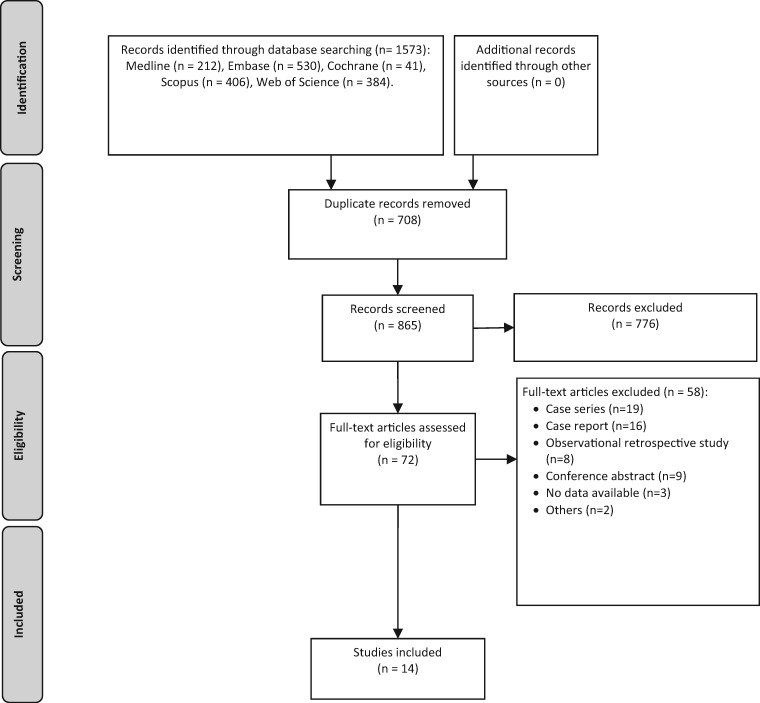
The search retrieved 1,573 results. After removing duplicates, 865 titles and abstracts were screened, and of these, 776 were excluded. Seventy-two studies were evaluated in full text, and 14 were included (Figure 1) [27–40].
Figure 1.
Prism flowchart (study selection).
Studies Characteristics
Regarding the included studies, nine were RCTs [27–31,33,35,36,38], including five crossover RCTs [28–30,33,36], and five were noncontrolled QE studies [32,34,37,39,40]. We included 236 PLP patients in this review. The number of participants ranged from eight to 54 in RCTs, and from three to 10 in QE studies.
Regarding the interventions, they consisted of invasive or noninvasive neuromodulation techniques:
Three studies used tDCS: All were RCTs and used anodal primary motor cortex (M1)/cathodal supraorbital [29,30,33]. Also, one used anodal posterior parietal cortex (PPC)/cathodal supraorbital as a part of the experimental arms [30].
Three studies used rTMS: All were RCTs and used excitatory frequencies (20 Hz, 10 Hz, 5 Hz) with intensities from 80% to 95% of the motor threshold [27,28,31]. Additionally, one used an inhibitory frequency (1 Hz) [28]. All of the studies targeted the primary motor cortex (M1).
Two studies used DBS: Both were noncontrolled QE studies. One used contralateral ventroposterior-lateral nucleus (VPL) stimulation [34], and the other used contralateral periventricular gray stimulation [39].
Four studies used TENS: Two were RCTs [36,38], and two were noncontrolled QE studies [37,40]. Two used stimulation in the PLP location on the nonamputated limb [37,38]; one study used the stump location [40], and the other used auricular TENS stimulation [36].
One QE study used PNS (femoral nerve and sciatic nerve trunk stimulation on the amputee side) [32], and one QE study used NMES (stimulation of quadriceps muscles of both legs) [35].
Intervention Characteristics
Interventions were heterogeneous in terms of stimulation parameters (area of stimulation, current intensity, pulse frequency, duration of the session, frequency, and the number of sessions) (Table 1). Regarding the control group, most of the studies used sham stimulation as a comparator, and the other few studies used, respectively, 2-Hz TMS [28], mirror therapy [38], and placebo (TENS device off) [36]. One study reported the use of phantom hand movements during brain stimulation (anodal tDCS) [33]; no other intervention combinations in the active group were reported.
Table 1.
Study characteristics of individual studies
| Author Year (Country) | Design | Population, Age, Sex, Cause of Amputation | No. of Patients | Intervention | Stimulation Location and Procedure | Control | Outcome (VAS or NRS) and P Value |
|---|---|---|---|---|---|---|---|
| Ahmed 2011 (Egypt) | RCT |
|
I: 17, C: 10 | rTMS:
|
Optimal scalp position determined from where transcranial magnetic stimulation evoked motor potentials of maximum peak-to-peak amplitude in the muscle proximal to the stump | Sham stimulation: coil elevated and angled away from the head | I = 3.4 ± 1.2, C = 7.4 ± 0.84 (P = 0.001) |
| Malavera 2016 (Colombia) | RCT |
|
I: 27, C: 27 | rTMS:
|
M1 contralateral to the amputated leg (corresponding to the first dorsal interosseous muscle of the hand contralateral to pain) | Sham stimulation (sham coil) | I = 2.28 ± 2.51, C = 3.71 ± 2.97 (P = 0.03) |
| Irlbacher 2006 (Germany) | RCT – crossover |
|
14 | rTMS:
|
M1 area corresponding to affected phantom limb. Optimal placement defined by maximal motor response | Sham stimulation: 2 Hz, identical placement of coil that looks and sounds identical and produces same scalp sensation | I (1Hz) = 4.22 ± 2.79, I (5Hz) = 4.99 ± 2.33, C (2Hz) = 4.37 ± 2.97 (P = 0.02) |
| Bolognini 2013 (Italy) | RCT – double-blind, sham-controlled crossover |
|
8 |
|
|
|
I = 0.8 (−69%), C = 2.6 (−21%, P = 0.02) |
| Bolognini 2015 (Italy) | RCT – double-blind, sham-controlled crossover |
|
8 | Anodal tDCS, 15-min session, ramping period of 10 sec at beginning and end, intensity of 1.5 mA, 5 consecutive d | M1
|
Sham stimulation (current lasted for 30 sec) | I = 3.3 (−41%), C = 4.7 (−17%, P = 0.04) |
| Kikkert 2018 (UK) | RCT – double-blind sham-controlled crossover |
|
8 | tDCS (anodal, amputation contralateral M1, cathodal, amputation ipsilateral supraorbital area) 3 sessions, active 1 session, 20 min, 1mA | M1
|
Sham:The stimulator was turned off after the impedance was stable (after ∼30 sec) | I = 1.73 ± 3.05 (−29.5%), C = 2.50 ± 2.56 (1.2%, P = 0.01) |
| Katz 1991 (Canada) | RCT – controlled crossover |
|
11/28 | TENS, 30 min (each session was divided into three consecutive 10-min periods, including an initial resting baseline [B1], bilateral ear stimulation [BES], and a final resting baseline [B2]). Throughout the 30-min session, the subject monitored changes in (painful and/or nonpainful) phantom limb intensity by turning the dial, 1 d | Outer ears (auricular, bilateral ear stimulation) | Placebo | I = 5.1 ± 1.05, C = 9.0 ± 2.16 (P < 0.01) |
| Tilak 2016 (India) | RCT |
|
26 | TENS, (contralateral leg) 20 min, one session of TENS for 4 consecutive d | Contralateral leg at the site exactly where they have PLP on the amputated leg | Mirror therapy | I = 2.46 ± 1.561, C = 2.08 ± 1.621 (P = 0.003) |
| Brede 2017 (USA) | RCT |
|
44 | MARP + NMES (15 min/session on 5 days/wk) to stimulate contractions for 12 wk | Quadriceps muscles of both legs | MARP only | I = 0.8 ± 0.7, C = 1.6 ± 0.7 (P = 0.005) |
| Mulvey 2013 (UK) | QE |
|
10 | TENS (60 min) one time | Above stump = 8, on stump = 2 | – | −1.8 ± 1.6 (P < 0.05) |
| Kawamura 1997 (Japan) | QE |
|
10 | Arteriosclerosis obliterans, malignant tumor, or trauma | The sites of the contralateral limb that exactly corresponded to the sites of the amputated limb where the patients felt | – | −1.2 ± 1.9 (P < 0.001) |
| Rauck 2014 (USA) | QE |
|
9/16 | Peripheral nerve stimulation for two weeks | Needle electrode was inserted into the trunk of a major peripheral nerve (i.e., the femoral nerve trunk and/or the sciatic nerve trunk) | – | −81% ± 28% (P < 0.002) |
| Pereira 2013 (UK) | QE |
|
5 | DBS
|
|
– | −2.8 ± 2.6 (−65.3% ± 25%, P = 0.001) |
| Bittar 2005 (Australia) | QE |
|
3 | DBS
|
DBS (contralateral) of PVG and somatosensory thalamus. Two patients: PVG only. One patient: PVG and thalamic stimulation | – | −6.17% ± 7.4% (range = 55–70%, P = 0.02) |
BKN = below knee; C = control; DBS = deep brain stimulation; EEG = electroencephalography; I = intervention; F = female; M = male; MARP = military amputee rehabilitation program; NMES = neuromuscular electrical stimulation; NR = not reported; NRS = numeric rating scale; PLP = phantom limb pain; PVG = ; QE = quasi-experimental; RCT = randomized controlled trial; rTMS = transcranial magnetic stimulation; tDCS = transcranial direct current stimulation; TENS = transcutaneous electrical nerve stimulation; VAS = visual analog scale.
Risk of Bias
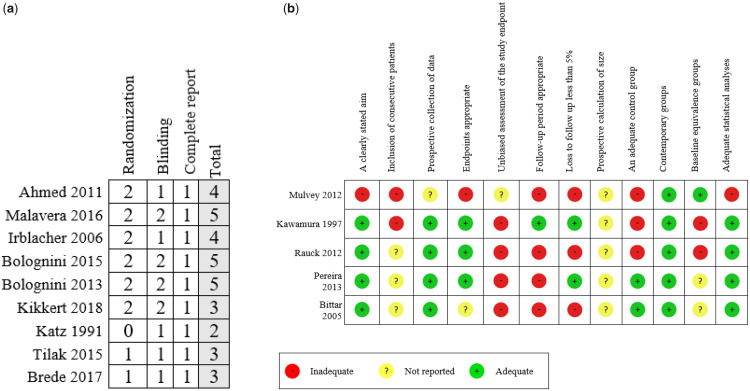
The RoB for nine RCTs was assessed using the Jadad scale. The range of points was from 2 to 5 (TMS studies had low RoB, tDCS studies had low to moderate RoB, and the other techniques had moderate to high RoB). Five studies (56%) did not report the blinding procedures or reported incomplete information about the blinded personnel. Three studies (33.3%) did not report how they generated the randomization sequence and allocation concealment, even though they specified that the allocation of the subjects was done randomly (Figure 2a). The risk of bias for the five QE studies was assessed using the MINORS tool. The studies have a moderate to high RoB. All studies did not report the sample size calculation and the procedure and personnel in charge to assess the primary outcome. Also, all the studies included consecutive patients (Figure 2b).
Figure 2.
Risk of bias assessment. a) Risk of bias of randomized controlled trials using the Jadad scale. b) Risk of bias of quasi-experimental studies using Methodological Index for Non-Randomized Studies scale.
Neuromodulation Effects on Pain
We first evaluated the pooled effect of noninvasive excitatory M1 stimulation (including high-frequency rTMS and anodal tDCS). We found a significant and large effect size for short-term pain reduction (immediately after the end of stimulation sessions—six RCTs, two parallel and four crossover studies, N = 123, ES = −1.36, 95% CI = −2.26 to −0.45) (Figure 3.1a). We also found a significant medium-term pain reduction after one week to one month of stimulation (four RCTs, two parallel and two crossover studies, N = 106, ES = −1.24, 95% CI = −2.18 to −0.31) (Figure 3.2b).
Figure 3.
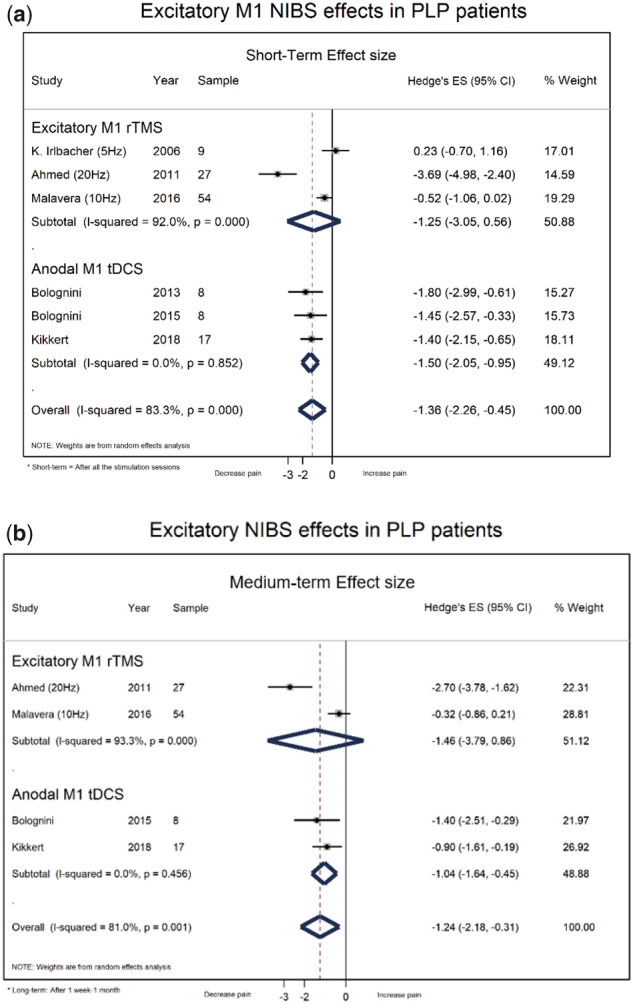
Forest plots on excitatory M1 noninvasive brain stimulation effects in phantom limb pain patients. a) Short-terms effects. b) Medium-term effects.
Considering the tDCS studies only, we found also a significant and large effect size of anodal tDCS in M1 for pain reduction immediately after the end of stimulation sessions (three crossover RCTs, N = 33, ES = −1.50, 95% CI = −2.05 to 0.95) (Figure 3.2a) and a significant pain reduction at one-week follow-up (two RCTs, N = 25, ES = −1.04, 95% CI = −1.64 to 0.45) (Supplementary Data). On the other hand, we found no significant pooled effect of anodal or cathodal tDCS in PPC on pain reduction immediately after the end of the stimulation sessions (one crossover RCT, N = 8, ES = −0.80, 95% CI = −1.82 to 0.22, ES = −0.40, 95% CI = −1.39 to 0.59) (Supplementary Data). These studies did not report any adverse effects related to tDCS stimulation.
Considering the rTMS studies only, we found no significant pooled effect of rTMS in M1 on pain reduction immediately after the end of the stimulation sessions (three RCTs, one parallel and two crossover, performed excitatory rTMS, N = 90, ES = −1.25, 95% CI = −3.05 to 0.56; one RCT performed inhibitory rTMS, N = 9, ES = −0.07, 95% CI = −1.00 to 0.85) (Supplementary Data) or pain reduction at one-month follow-up (two RCTs performed excitatory rTMS, N = 81, ES = −1.46, 95% CI = −3.79 to 0.86) (Supplementary Data). These studies do not report any adverse effects related to rTMS stimulation.
For the QE studies, we calculated a pooled response rate of TENS after stimulation sessions and found a significant pooled response rate (two studies, N = 20, pooled response rate = 67%, 95% CI = 60% to 73%) (Supplementary Data) and similar results for DBS after one year of follow-up (two studies, N = 8, rate = 73%, 95% CI = 63% to 82%) (Supplementary Data).
Discussion
Our review comprised 14 studies (nine RCTs and five QE studies) that measured the effects of neuromodulation techniques (tDCS, rTMS, DBS, PNS, NMES, TENS) in patients with PLP. These studies were heterogeneous with regard to stimulation parameters and had small sample sizes and variable risk of bias (RCTs: low to moderate; QE: moderate to high). The pooled effects showed that PLP patients who underwent excitatory M1 stimulation, especially anodal tDCS, experienced a significant reduction in pain scale scores at the short-term and midterm time points. Motor cortex rTMS effected no significant difference between the active and sham groups. DBS and TENS elicited a significant decrease in PLP (noncontrolled QE studies).
Effects of tDCS and rTMS
Our exploratory meta-analysis of tDCS included three RCTs [29,30,33], demonstrating a significant decline in visual analog scale (VAS) scores for pain at the short-term and midterm end points with anodal M1 tDCS. Only cathodal tDCS to PPC decreased nonpainful phantom sensations [30]. There was no benefit for stump pain or telescoping. These results implicate a separation between the effects on painful and painless phantom sensations, likely due to the different neural circuits that are involved. None of the three trials reported moderate to severe side effects, confirming the safety of and tolerance to this technique [41].
The tDCS stimulation parameters were homogeneous; all studies targeted the motor cortex using excitatory polarization with a low current intensity (1–2 mA). Also, the session duration was 15–20 minutes. The main difference was the number of sessions [1–5]; the study with more sessions [33] reported a larger effect size, but due to the small number of included studies, we cannot suggest a potential dose response of this intervention, necessitating further studies to determine the influence of the number of sessions in PLP patients. Only one study [33] used a concomitant task (imaginary movements of the amputated limb) during tDCS stimulation, reporting a large effect on pain, indicating that the inclusion of concomitant motor tasks in the tDCS protocol may help to reactivate the deafferented motor cortex (amputated side) [42].
All of the studies on rTMS were RCTs [27,28,31]. Although Ahmed et al. [31] noted benefits of rTMS in reducing PLP, the exploratory pooled effect showed no significant difference between the active and sham groups.
One explanation of this negative result is the frequencies in these three rTMS experiments vs tDCS. Yet, all of the studies targeted the motor cortex; the forest plot results (Supplementary Data) showed a clear effect of stimulation frequency, with 1 Hz and 5 Hz showing no effect, 10 Hz having a marginal effect, and 20 Hz eliciting a significant result, suggesting that higher frequencies should be examined in future studies. This heterogeneity of the intervention makes it difficult to determine the reference stimulation parameters and might be a source of variability in the clinical effects.
This variability of parameters contrasts with the tDCS studies that had more homogeneous stimulation parameters (M1 anodal tDCS, cathoda l supraorbital, 2 mA, 20 minutes). Further, the baseline characteristics of the rTMS studies included patients who had undergone amputation less than three months earlier, which is not the best time to begin treating PLP. Conversely, more than three months had passed after amputation in most of the patients in the TDCS studies.
These trials were limited by their small sample sizes and short follow-up periods. Only well-powered trials with longer follow-ups can determine the true potential of tDCS and rTMS for PLP.
Effects of Invasive Neuromodulation Techniques
There are four reviews on DBS in patients with chronic pain, including small studies with PLP patients; three studies noted pain relief [43–45], but one showed no significant reductions in pain scores [46]. However, most of these reports were small mixed studies or case series. All available evidence has come from small trials, especially noncontrolled QE studies, and there is no consensus on how to perform DBS surgery or the parameters of stimulation [47], hampering the studies’ comparability. Although the pooled effect of the QE studies is significant in the short term and at follow-up, uncontrolled research results are inherently unreliable, and the certainty of the results should be considered low. Moreover, the risk for complications of invasive neuromodulation surgery is high; it is usually used only for severe PLP and is often chosen as a late treatment option, likely rendering it unsuitable for widespread use.
We could not include any study on DRG or SCS, for which there are only case reports, case series, and small retrospective cohorts (Supplementary Data). There are limited data on invasive neuromodulation (DBS, DRG, and SCS), and further research is needed.
TENS Effects
Three reviews on TENS [48–50] in mixed chronic pain populations, including few PLP patients, have been unable to judge the effectiveness of TENS for this condition. The main problem of TENS studies is the difference in stimulation site, including auricular areas [36], the contralateral leg [37,38], and above the stump [40]. All of these studies reported a significant reduction in PLP, based on the VAS, McGill Pain Questionnaire, universal pain score (UPS), and numerical rating scale (NRS). We pooled the results of the two quasi-experiments with the same stimulation parameters (contralateral to the site at which PLP was experienced on the amputated leg), and a significant reduction was found immediately after stimulation. However, the limitations of the noncontrolled QE designs prevented us from making a definitive conclusion.
Neurophysiological Hypothesis of Motor Cortex Stimulation in PLP
An important finding of this meta-analysis is that excitatory (anodal) primary motor cortex (M1) tDCS stimulation elicits a statistically and clinically significant reduction in pain intensity (by 1.5 points on the VAS) in patients with PLP [51]. The most frequently accepted hypothesis states that after amputation, PLP is associated with maladaptive reorganization of the sensory–motor cortex [52] and lower cortical inhibition [53], based on functional magnetic resonance imaging and TMS studies. Thus, PLP could be associated with decreased afference to M1, resulting in maladaptive plasticity in this area [54].
The resulting pain relief can be explained by the potential effects of tDCS on central pathophysiological mechanisms of pain that is associated with PLP [11,16]. It has been suggested that anodal M1 tDCS regulates in a top-down manner, sending signals toward the thalamocortical connections, prefrontal cortex, cingulate gyrus, and periaqueductal gray [55–57]. Based on this assumption, Lang et al. [58] reported significant activation of cortical and subcortical pain-related areas with anodal stimulation in healthy subjects. Further, one study on M1 anodal tDCS noted an increase in endogenous opioid release in the thalamus, insula, cingulate, and nucleus accumbens in a chronic pain patient [59].
Based on animal models, we hypothesized that the motor cortex not only is a passive target but also could be actively involved in the modulation of pain networks through disinhibition of the periaqueductal gray, enhancing the endogenous pain modulation system and the pain pathway of the dorsal horn in the spinal cord [60,61]. This phenomenon could result in the widespread modulation of other pain-related neural areas, such as the thalamus, thus modulating the upstream nociceptive signaling pathway. However, more mechanistic human studies are needed to truly understand the function of motor cortex stimulation as a target in the treatment of PLP patients and its influence on pain perception.
Limitations and Strengths
Due to the heterogeneity in stimulation parameters in the studies that were analyzed, it can be argued that meta-analyses do not compare similar interventions. These techniques are too heterogeneous to pool into a single unique meta-analysis; thus, we performed separate meta-analyses for similar techniques (mechanism of action and similar stimulation parameters) to reduce heterogeneity. The range in the variability of parameters was evaluated carefully to avoid a meta-analysis of opposing mechanistic effects (such as excitatory vs inhibitory stimulation, central vs peripheral intervention, and invasive vs noninvasive). For instance, the main pooled estimate in our study included noninvasive excitatory M1 stimulation. In addition, because effect estimates are needed for decision-making to guide future research and clinical applications, we found meta-analyses to be useful in providing a better overview of the results. Further, our meta-analysis recognizes the limitations in the primary studies, such as insufficient detail on the evaluation of the outcome, concomitant interventions, and the treatment that was received by the control group.
However, this study has important strengths. It followed the PRISMA guidelines and was registered in the PROSPERO database. In addition, we performed a comprehensive search strategy using multiple databases, without any language restrictions and across the articles that were cited by each of the identified studies, allowing us to retrieve all articles in previous systematic reviews [17,18] and others that were not found in these reviews. These strengths allowed us to report the state of the art in RCTs and QE controlled research on neuromodulation interventions in PLP and examine the efficacy and safety of these interventions.
Conclusions
The evidence from RCTs suggests that excitatory M1 stimulation—specifically, anodal M1 tDCS—significantly reduces pain scale scores in PLP patients after the stimulation sessions and at the midterm follow-up (one week). There are limited data on the efficacy of invasive neuromodulation techniques (DBS, DRG, and SCS). More RCTs are needed to evaluate the benefits and risks of neuromodulation techniques in patients with PLP. These studies need to be well powered, with longer follow-up (more than six weeks), and adequately reported (stimulation parameters and co-intervention description) to provide a definitive conclusion.
Supplementary Material
Acknowledgments
We thank the “Principle and Practice of Clinical Research” (PPCR) program from Harvard T.H. Chan School of Public Health for feedback on the initial version of this manuscript.
Authors’ Contributions
All authors designed the study. KP-B and XM collected the data. KP-B and XM performed statistical analyses. All authors participated in the interpretation of the results and the writing of the manuscript and approved the final version.
Contributor Information
Kevin Pacheco-Barrios, Neuromodulation Center and Center for Clinical Research Learning, Spaulding Rehabilitation Hospital and Massachusetts General Hospital, Boston, Massachusetts, USA; Universidad San Ignacio de Loyola, Vicerrectorado de Investigación, Unidad de Investigación para la Generación y Síntesis de Evidencias en Salud, Lima, Peru.
Xianguo Meng, Neuromodulation Center and Center for Clinical Research Learning, Spaulding Rehabilitation Hospital and Massachusetts General Hospital, Boston, Massachusetts, USA; Shandong First Medical University & Shandong Academy of Medical Sciences, College of Sport Medicine and Rehabilitation, Jinan, Shandong Province, P.R. China.
Felipe Fregni, Neuromodulation Center and Center for Clinical Research Learning, Spaulding Rehabilitation Hospital and Massachusetts General Hospital, Boston, Massachusetts, USA.
Funding sources: This study was supported by an National Institutes of Health (NIH) RO1 grant (1R01HD082302-01A1 to Felipe Fregni).
Conflicts of interest: The authors declare that they have no compelling interests related to this article.
Protocol registration number: PROSPERO 2018 CRD42018117998.
References
- 1. Weinstein SM. Phantom limb pain and related disorders. Neurol Clin 1998;16(4):919–36. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad N, Thomas GN, Gill P, Chan C, Torella F.. Lower limb amputation in England: Prevalence, regional variation and relationship with revascularisation, deprivation and risk factors. A retrospective review of hospital data. J R Soc Med 2014;107(12):483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson C, Glenn S, Nurmikko T, Horgan M.. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain 2006;22(4):353–8. [DOI] [PubMed] [Google Scholar]
- 4. Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP.. Phantom pain and phantom sensations in upper limb amputees: An epidemiological study. Pain 2000;87(1):33–41. [DOI] [PubMed] [Google Scholar]
- 5. Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil 2000;81(8):1039–44. [DOI] [PubMed] [Google Scholar]
- 6. Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP.. Phantom pain and risk factors: A multivariate analysis. J Pain Symptom Manage 2002;24(6):578–85. [DOI] [PubMed] [Google Scholar]
- 7. Richardson C, Crawford K, Milnes K, Bouch E, Kulkarni J.. A clinical evaluation of postamputation phenomena including phantom limb pain after lower limb amputation in dysvascular patients. Pain Manag Nurs 2015;16(4):561–9. [DOI] [PubMed] [Google Scholar]
- 8. Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE.. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil 2005;86(10):1910–9. [DOI] [PubMed] [Google Scholar]
- 9. Morgan SJ, Friedly JL, Amtmann D, Salem R, Hafner BJ.. Cross-sectional assessment of factors related to pain intensity and pain interference in lower limb prosthesis users. Arch Phys Med Rehabil 2017;98(1):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padovani MT, Martins MR, Venancio A, Forni JE.. Anxiety, depression and quality of life in individuals with phantom limb pain. Acta Ortop Bras 2015;23(2):107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Investig 2018;128(6):2168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flor H. Remapping somatosensory cortex after injury. Adv Neurol 2003;93:195–204. [PubMed] [Google Scholar]
- 13. Costa B, Ferreira I, Trevizol A, Thibaut A, Fregni F.. Emerging targets and uses of neuromodulation for pain. Expert Rev Neurother 2019;19(2):109–18. [DOI] [PubMed] [Google Scholar]
- 14. Lefaucheur J-P, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 15. Naro A, Milardi D, Russo M, et al. Non-invasive brain stimulation, a tool to revert maladaptive plasticity in neuropathic pain. J Front Hum Neurosci 2016;10:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD.. Non-invasive motor cortex neuromodulation reduces secondary hyperalgesia and enhances activation of the descending pain modulatory network. J Front Neurosci 2019;13:467–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’connell NE, Marston L, Spencer S, DeSouza LH, Wand BM.. Non‐invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2018;(3):8208–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akyuz G, Giray E.. Noninvasive neuromodulation techniques for the management of phantom limb pain: A systematic review of randomized controlled trials. Int J Rehabil Res 2019;42(1):1–10. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Green S.. Cochrane Handbook for Systematic Reviews of Interventions New York: John Wiley & Sons; 2011. [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. Ann Int Med 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, Jenkinson C, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 22. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J.. Methodological Index for Non-Randomized Studies (MINORS): Development and validation of a new instrument. ANZ J Surg 2003;73(9):712–6. [DOI] [PubMed] [Google Scholar]
- 23. Nyaga VN, Arbyn M, Aerts M.. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deeks JJ, Higgins JPT, Altman DG; on behalf of the Cochrane Statistical Methods Group. Cochrane Handbook for Systematic Reviews of Interventions In: Higgins JP, Green S, ed. Analysing data and undertaking meta-analyses - Heterogeneity. New York: John Wiley & Sons; 2011:243–96. [Google Scholar]
- 25. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 26. Sterne JAC, Egger M and Moher D; on behalf of the Cochrane Bias Methods Group. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JP, Green S, ed. Detecting reporting biases. New York: John Wiley & Sons; 2011:297–334. [Google Scholar]
- 27. Malavera A, Silva FA, Fregni F, Carrillo S, Garcia RG.. Repetitive transcranial magnetic stimulation for phantom limb pain in land mine victims: A double-blinded, randomized, Sham-controlled trial. J Pain 2016;17(8):911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irlbacher K, Kuhnert J, Röricht S, Meyer BU, Brandt SA.. Central and peripheral deafferent pain: Therapy with repetitive transcranial magnetic stimulation. Nervenarzt 2006;77(10):1196–203. [DOI] [PubMed] [Google Scholar]
- 29. Bolognini N, Spandri V, Ferraro F, et al. Immediate and sustained effects of 5-day transcranial direct current stimulation of the motor cortex in phantom limb pain. J Pain 2015;16(7):657–65. [DOI] [PubMed] [Google Scholar]
- 30. Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F.. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain 2013;154(8):1274–80. [DOI] [PubMed] [Google Scholar]
- 31. Ahmed MA, Mohamed SA, Sayed D.. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res 2011;33(9):953–8. [DOI] [PubMed] [Google Scholar]
- 32. Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014;17(2):188–97. [DOI] [PubMed] [Google Scholar]
- 33. Kikkert S, Mezue M, O'Shea J, et al. The neural basis of induced phantom limb pain relief. Ann Neurol 2019;85(1):59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereira EA, Boccard SG, Linhares P, et al. Thalamic deep brain stimulation for neuropathic pain after amputation or brachial plexus avulsion. Neurosurg Focus 2013;35(3):E7–18. [DOI] [PubMed] [Google Scholar]
- 35. Brede E, Metter EJ, Talbot LA.. Neuromuscular electrical stimulation for pain management in combat-related transtibial amputees during rehabilitation and prosthetic training. J Appl Biobehav Res 2017;22(4):e12084. [Google Scholar]
- 36. Katz J, Melzack R.. Auricular transcutaneous electrical nerve stimulation (TENS) reduces phantom limb pain. J Pain Symptom Manage 1991;6(2):73–83. [DOI] [PubMed] [Google Scholar]
- 37. Kawamura H, Ito K, Yamamoto M, et al. The transcutaneous electrical nerve stimulation applied to contralateral limbs for the phantom limb pain. J Phys Ther Sci 1997;9(2):71–6. [Google Scholar]
- 38. Tilak M, Isaac SA, Fletcher J, et al. Mirror therapy and transcutaneous electrical nerve stimulation for management of phantom limb pain in amputees—a single blinded randomized controlled trial. Physiother Res Int 2016;21(2):109–15. [DOI] [PubMed] [Google Scholar]
- 39. Bittar RG, Otero S, Carter H, Aziz TZ.. Deep brain stimulation for phantom limb pain. J Clin Neurosci 2005;12(4):399–404. [DOI] [PubMed] [Google Scholar]
- 40. Mulvey MR, Radford HE, Fawkner HJ, Hirst L, Neumann V, Johnson MI.. Transcutaneous electrical nerve stimulation for phantom pain and stump pain in adult amputees. Pain Pract 2013;13(4):289–96. [DOI] [PubMed] [Google Scholar]
- 41. Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinto CB, Velez FGS, Bolognini N, Crandell D, Merabet LB, Fregni F.. Optimizing rehabilitation for phantom limb pain using mirror therapy and transcranial direct current stimulation: A randomized, double–blind clinical trial study protocol. JMIR Research Protoc 2016;5(3):e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boccard SG, Pereira EA, Aziz TZ.. Deep brain stimulation for chronic pain. J Clin Neurosci 2015;22(10):1537–43. [DOI] [PubMed] [Google Scholar]
- 44. Falowski SM. Deep brain stimulation for chronic pain. Curr Pain Headache Rep 2015;19(7):27–31. [DOI] [PubMed] [Google Scholar]
- 45. Parmar VK, Gee L, Smith H, Pilitsis JG.. Supraspinal stimulation for treatment of refractory pain. Clin Neurol Neurosurg 2014;123:155–63. [DOI] [PubMed] [Google Scholar]
- 46. Frizon LA, Yamamoto EA, Nagel SJ, Simonson MT, Hogue O, Machado AG.. Deep brain stimulation for pain in the modern era: A systematic review. Neurosurgery 2019;86(2):191–202. [DOI] [PubMed] [Google Scholar]
- 47. Honey CM, Tronnier VM, Honey CR.. Deep brain stimulation versus motor cortex stimulation for neuropathic pain: A minireview of the literature and proposal for future research. Comput Struct Biotechnol J 2016;14:234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mulvey MR, Bagnall AM, Johnson MI, Marchant PR.. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev 2010;5:7264–78. [DOI] [PubMed] [Google Scholar]
- 49. Johnson MI, Mulvey MR, Bagnall AM.. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev 2015;8:7264–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gibson W, Wand BM, Meads C, Catley MJ, O’Connell NE.. Transcutaneous electrical nerve stimulation (TENS) for chronic pain—an overview of Cochrane reviews. Cochrane Database Syst Rev 2019;2:11890–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
- 52. Raffin E, Richard N, Giraux P, Reilly K.. Primary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limb. Neuroimage 2016;130:134–44. [DOI] [PubMed] [Google Scholar]
- 53. Nardone R, Versace V, Sebastianelli L, et al. Transcranial magnetic stimulation in subjects with phantom pain and non-painful phantom sensations: A systematic review. Brain Res Bull 2019;148:1–9. [DOI] [PubMed] [Google Scholar]
- 54. Molina‐Rueda F, Navarro‐Fernández C, Cuesta‐Gómez A, Alguacil‐Diego IM, Molero‐Sánchez A, Carratalá‐Tejada M.. Neuroplasticity modifications following a lower‐limb amputation: A systematic review. P & M 2019;11(12):1326–34. [DOI] [PubMed] [Google Scholar]
- 55. Garcıa-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: A combined PET-scan and electrophysiological study. Pain 1999;83(2):259–73. [DOI] [PubMed] [Google Scholar]
- 56. Yoon EJ, Kim YK, Kim H-R, Kim SE, Lee Y, Shin HI.. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: A mechanistic PET study. Neurorehabil Neural Repair 2014;28(3):250–9. [DOI] [PubMed] [Google Scholar]
- 57. Miranda PC, Lomarev M, Hallett M.. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 2006;117(7):1623–9. [DOI] [PubMed] [Google Scholar]
- 58. Lang N, Siebner HR, Ward NS, Lee L, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 2005;22(2):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DosSantos MF, Love TM, Martikainen IK, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry 2012;3:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saavedra LC, Mendonca M, Fregni F.. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses 2014;83(3):332–6. [DOI] [PubMed] [Google Scholar]
- 61. Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR.. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: Possible pathways for antinociception. PAIN 2012;153(12):2359–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.