Abstract
Objective
To develop a validated instrument that measures knowledge about prescription opioid overdose.
Methods
Within an integrated health care system, we adapted, piloted, and tested the reliability and predictive validity of a modified Opioid Overdose Knowledge Scale (OOKS) instrument specific to prescription opioids (Rx-OOKS) with a patient population prescribed long-term opioid therapy and potentially at risk of opioid overdose. We used an interdisciplinary team approach and patient interviews to adapt the instrument. We then piloted the survey on a patient sample and assessed it using Cronbach’s alpha and logistic regression.
Results
Rx-OOKS (N = 56) resulted in a three-construct, 25-item instrument. Internal consistency was acceptable for the following constructs: “signs of an overdose” (10 items) at α = 0.851, “action to take with opioid overdose” (seven items) at α = 0.692, and “naloxone use knowledge” (eight items) at α = 0.729. One construct, “risks of an overdose” (three items), had an α of 0.365 and was subsequently eliminated from analysis due to poor performance. We conducted logistic regression to determine if any of the constructs was strongly associated with future naloxone receipt. Higher scores on “actions to take in an overdose” had nine times the odds of receiving naloxone (odds ratio [OR] = 9.00, 95% confidence interval [CI] = 1.42–57.12); higher “naloxone use knowledge” scores were 15.8 times more likely to receive naloxone than those with lower scores (OR = 15.83, 95% CI = 1.68–149.17).
Conclusions
The Rx-OOKS survey instrument can reliably measure knowledge about prescription opioid overdose recognition and naloxone use. Further, knowledge about actions to take during an opioid overdose and naloxone use were associated with future receipt of naloxone.
Keywords: Prescription Opioid, Overdose, Psychometric Measures
Introduction
Opioids are the leading substance involved in drug overdoses and drug fatalities in the United States [1, 2] and globally [3], creating a critical need for effective strategies to prevent opioid overdoses. Naloxone is an effective opioid antidote that prevents death from opioid overdose [4–6]. It was first used in hospital settings [7] and by paramedics [8] to reverse opioid-induced respiratory depression [9]. More recently, it has been used by nonmedical first responders [10]. Naloxone has also been distributed through community-based programs with people who use heroin and illicit opioids [11, 12]. Such programs—which include education—have been shown to be effective at preventing overdose fatalities [13–17].
Although prescription opioids contribute significantly to opioid overdose rates [18–20], programs to effectively distribute naloxone to patients with chronic pain to whom opioids have been prescribed are lacking [21]. Barriers to prescribing naloxone in primary care settings include a lack of knowledge among patients, practitioners, and pharmacists [21–23] about the availability of naloxone, negative attitudes of providers and pharmacists toward naloxone distribution [22, 24–27], and patients’ perceptions of being at low risk of an overdose [24, 28]. Intervention studies designed to overcome these barriers should be evaluated using validated survey instruments. However, available tools that measure opioid overdose and naloxone knowledge have been designed largely to assess illicit opioid overdose risk factors [29–32]. One such instrument is a 45-items survey, Opioid Overdose Knowledge Scale (OOKS), which evaluates caregiver and family naloxone knowledge acquisition after a short training about take-home naloxone [30]. More recently, a three-factor, 12-item knowledge screening tool, Brief Opioid Overdose Knowledge (BOOK) [33], was developed. However, the brief screener’s focus was general opioid overdose knowledge across both illicit and prescription opioid populations and did not include questions to specifically measure naloxone knowledge.
The purpose of this study was to adapt, pilot, and test the reliability and predictive validity of a modified Opioid Overdose Knowledge Scale instrument specific to prescription opioids (Rx-OOKS) with a patient population prescribed long-term opioid therapy (LTOT) and potentially at risk of opioid overdose. Additionally, for descriptive purposes, we used longitudinal data from Rx-OOKS to show the ability to measure change in knowledge over time.
Methods
Survey Adaptation and Assessment
The Rx-OOKS survey instrument was adapted and assessed in two phases. In phase 1, the research team and expert stakeholders adapted the OOKS instrument through qualitative assessment, providing face and content validity. In phase 2, we quantitatively validated the adapted survey instrument by administering it at two time points to a sample population prescribed LTOT to describe the sample and to measure change over time, as well as to assess its internal consistency and predictive validity.
Setting
The study was conducted from November 2016 through December 2017 at Kaiser Permanente Colorado (KPCO), an integrated health care delivery system with more than 540,000 members in the metropolitan Denver area. KPCO uses an electronic health record (EHR) that captures demographic, medical encounter, and prescription data on all members. All study activities were reviewed and approved by the Kaiser Permanente Colorado Institutional Review Board.
Phase 1: Modification of the OOKS Survey Instrument
Assessing Face Validity
The OOKS survey instrument was modified for patients receiving LTOT by using our team’s knowledge from previous qualitative interviews with patients who have experienced an opioid overdose [24, 34] and feedback obtained from patients. Our interdisciplinary research team included an addiction physician, epidemiologists, qualitative expert, communication manager, behaviorists, and two of the original OOKS authors (Anna V. Williams, PhD, and John Strang, MD, of King’s College London).
After the research team’s input on the survey modifications, we conducted 11 interviews with patients receiving LTOT at KPCO from November through December 2016. Within these 11 interviews, we asked patients about the language used on the instrument (i.e., any offensive language used, understandability of questions) and the survey items (i.e., any concepts not asked that should be, adequate responses to items). The objective of the interviews was to test the wording and understanding of the instrument before field-testing on a large sample. Interviews were face-to-face and conducted by a research staff member with extensive experience in interviewing techniques and qualitative methods. Patients were consented into the interview study and received a $25 local retail gift card for their participation.
Phase 2: Implementation of the Modified Survey Tool
The 25-item Rx-OOKS survey was pilot-tested in a sample of patients prescribed LTOT from January through December 2017. The study sample consisted of a patient population aged 18 years and older who were enrolled in KPCO at the time of recruitment and had a health plan that included coverage for naloxone. LTOT was defined as three or more 30-day short- or long-acting opioid prescriptions in the prior 90 days. Tramadol was excluded as an eligible opioid prescription. Other exclusions included the following: non-English-speaking, enrolled in hospice, or with a do-not-resuscitate order.
We invited cohort members on LTOT from one clinic within our health system (N = 206) to participate in the survey study. The clinic was selected as the study team had prior experience working with them on system-wide prescription opioid initiatives. The paper-based survey was first mailed to participants via postal mail. We followed up with e-mails that contained the same information as the postal mailing plus a personalized Web link to complete their individual survey online. During the baseline recruitment period, up to two mailings and three e-mails were distributed to participants, reminding them to complete their survey. If participants completed the survey or requested to opt out of the study, no further recruitment contacts were made. For completion of each survey, participants were compensated with a $20 gift card. Four months after completing the baseline survey, participants were sent a follow-up Rx-OOKS survey. They received up to two mailings and three e-mails as reminders to complete their follow-up survey. We stopped contact at eight months after the baseline survey. Rx-OOKS survey items, demographic information, and other validated measures were collected by online survey methods using Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, TN, USA).
Outcomes
Outcome measures were Rx-OOKS survey total score, survey construct scores, and receipt of naloxone within KPCO. These outcomes were used to describe the population both at baseline and over time and to test the reliability and predictive validity of the instrument. Rx-OOKS survey item responses are categorical and converted to dichotomous responses of correct or incorrect. Responses of “don’t know” are scored as incorrect. These bivariate responses were summed for each of the four constructs, and a total score was created across the constructs. The higher the score on a construct, the greater the knowledge about risk of opioid overdose, opioid overdose signs, actions to take during an opioid overdose, or naloxone use. Pharmacy records were used to measure receipt of naloxone across the follow-up, which was 12 months from the baseline survey.
Analytic Plan
Descriptive variables were reported for baseline and follow-up survey time points. Rx-OOKS survey results were assessed using paired t tests for scale data and the chi-square test for categorical data and used the Fisher exact test when cell size was <5. We evaluated whether missing data were completely at random using Little’s Missing Completely at Random (MCAR) test [35]. The expectation-maximization algorithm was used to impute missing values. Demographic attributes were compared for those who responded to the survey and nonrespondents. Cronbach’s alpha [36] was used to assess the internal consistency of the Rx-OOKS survey constructs: “risks of an overdose,” “signs of an overdose,” “actions to take in an overdose,” and “naloxone use knowledge.” The Rx-OOKS instrument demonstrated good internal consistency and score reliability for three of the four constructs and for the overall survey. One construct, “risk of an overdose,” was removed from the survey after a poor Cronbach alpha performance below 0.60. Two constructs, “signs of an overdose” and “naloxone use knowledge,” were above α = 0.70, which is considered acceptable [37]. One construct, “actions to take with opioid overdose,” had an α of 0.692. We also used the Pearson correlational coefficient statistic [38] to determine the relationship between the constructs that were analyzed. Finally, logistic regression was used to assess the predictive validity of Rx-OOKS constructs (dependent variable) in receipt of naloxone (outcome).
The logistic regression models were run separately for each construct, given the sparse data. Odds ratios and 95% confidence intervals were estimated. The median of each survey construct and total score were used to classify high and low scores, while receipt of naloxone was classified as yes/no. Analyses were conducted using IBM SPSS, version 22.0 (IBM, Armonk, NY, USA), and SAS, version 9.4 (SAS Institute, Inc., Cary, NC, USA). We also conducted a descriptive assessment of those who received naloxone up to 12 months after completing the baseline survey and those who did not receive naloxone.
Survey Development Results
Phase 1: Survey Modification Results
Team Input
Our interdisciplinary study team modified survey items to better reflect prescription opioids rather than heroin use and prioritized survey items for inclusion. We also changed the wording of the survey items from United Kingdom to American English for understandability of the survey items in the United States.
Qualitative Interviews
In this sample of 11, participants had an average age of 50 years with a range of 28 to 63 years, 64% were female, and they were prescribed LTOT for an average of 15 years, with a range from three to 36 years. Participants suggested changes to wording, requested additional clarification to the meaning of the questions, and expanded response options. For example, during an interview, one participant asked if they could administer naloxone to themselves, leading to a novel question: “Is it possible to administer naloxone (Narcan) to yourself in the event of an opioid overdose emergency?” Other novel survey items reflected knowledge we hoped to influence through our interventions. For example, “I can get naloxone directly from a pharmacy without a prescription from my doctor” assesses specific processes and policies within a health care system. We also learned from participants that we should include both the generic and brand name for the medication—naloxone and Narcan, because of the interchangeable meaning in the United States. Overall, participants helped us use patient-centered wording, include items about misconceptions about naloxone (e.g., impossibility of self-administration), and include items about the risks and signs of a prescription opioid overdose. Participants also suggested a “true, false, don’t know” option to the survey questions that originally had a checklist. This provided participants with an option to select “don’t know” rather than leave the item blank. We incorporated these suggestions into existing or new survey items to create a preliminary Rx-OOKS instrument (Supplementary Data).
Modification of Survey Items and Instrument
After incorporating suggestions from patient input into the Rx-OOKS survey instrument, the following sequential steps were taken to modify the instrument. First, we removed the original OOKS construct “risk of an overdose,” as these nine questions were about heroin overdose knowledge. We then developed three novel “risk” questions, specific to risk of prescription opioid use. Overall, there were 10 novel questions (including the three novel risk questions) explicit to the current naloxone dispensing environment, which were grouped into one of the four survey constructs: “risk of an overdose,” “naloxone use knowledge,” “actions to take during an overdose,” and “signs of an overdose.” Lastly, we removed 12 items from the constructs “naloxone use knowledge” and “actions to take during opioid overdose.” These original OOKS items were excluded because they were not specific enough to prescription opioids, were only relevant to people who used heroin, were specific to injectable naloxone only, or did not address risk factors explicit to people at risk for a prescription opioid overdose. These 12 items were assessed by the study team as to whether the items contributed meaningful measurement before removal. The modifications resulted in 28 Rx-OOKS survey items within four constructs (Supplementary Data). These modifications shortened the length of the finalized survey instrument (Supplementary Data).
Phase 2: Pilot Survey Results
Survey Cohort
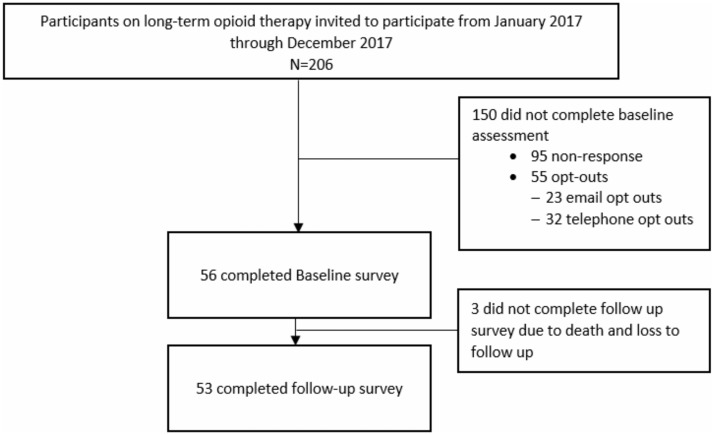
Two hundred six individuals were screened for eligibility criteria and invited to participate in the pilot survey study. Fifty-six (27%) completed the baseline assessment. The mean age of participants (SD) was 65 (10.3) years, and participants were mostly white, 63% female, and highly educated. Approximately 15% had engaged in substance use disorder treatment in the past, and ∼7% had been prescribed pharmaceutical treatment for an opioid use disorder at some point in the past. Most participants were not employed, likely due to age eligibility to retire from employment (Table 1). Fifty-three (95%) completed surveys at both baseline and follow-up (Figure 1). Of the three who did not complete the follow-up survey, the reasons included death and loss to follow up. The average number of days from baseline to survey follow-up (range) was 152 (118–229) days. We compared those who participated in the survey study with those who did not. There were no statically significant differences in age, ethnicity, race, clinical diagnoses (mental conditions, tobacco use, drug use), median household income, insurance type, or receipt of naloxone between those who participated vs those who did not participate in the survey study.
Table 1.
Characteristics of survey respondents (N = 56)
| Characteristics | Baseline Survey |
|---|---|
| Participant age, M (SD), y | 65.5 (10.3) |
| Participant gender, No. (%) | |
| Female | 36 (64.3) |
| Race, No. (%) | |
| White | 47 (83.9) |
| Other race | 9 (16.1) |
| Ethnicity, No. (%) | |
| Hispanic | 3 (5.4) |
| Non-Hispanic | 52 (92.9) |
| Don’t know | 1 (1.8) |
| Annual household income, No. (%) | |
| $0–$49,000 | 22 (39.3) |
| $50,000–$75,000+ | 33 (58.9) |
| Missing | 1 (1.8) |
| Education, No. (%) | |
| Some high school/high school graduate | 4 (7.2) |
| Some college/technical school | 17 (30.4) |
| College graduate | 19 (33.9) |
| Graduate school | 16 (28.6) |
| Employment, No. (%) | |
| Currently employed | 16 (28.6) |
| Currently not employed | 36 (64.3) |
| Looking for work | 2 (3.6) |
| Employed but not currently working | 2 (3.6) |
| Living arrangements, No. (%) | |
| Own home or apartment | 51 (91.1) |
| Someone else’s home or apartment | 4 (7.1) |
| Rooming/boarding house | 1 (1.8) |
| Substance use disorder treatment, No. (%) | |
| No, never | 46 (82.1) |
| Yes, but not in past 4 mo | 9 (16.1) |
| Yes, in past 4 mo | 1 (1.8) |
| Prescribed methadone or buprenorphine for opioid use disorder, No. (%) | |
| No, never | 53 (94.6) |
| Yes, but not in past 4 mo | 3 (5.4) |
| Yes, in past 4 mo | 0 (0.0) |
Figure 1.
Participant recruitment flow diagram for Rx-OOKS pilot surveys in phase 2 of validation.
Survey Administration Results
An analysis of missingness of the data was conducted. For both baseline and follow-up Rx-OOKS data, there were <2% missing data on any one survey item. The Little’s MCAR test [35] was not statistically significant (χ2(24, N = 56) = 226.89, P = 0.618), indicating that data were missing completely at random.
Secondary Analysis—Change in Rx-OOKS Construct Scores over Time
We also conducted a secondary analysis to look at construct scores over time (the mean time between the baseline and follow-up surveys was 152 days). The total Rx-OOKS survey scores increased significantly from baseline (Mean (M) = 10.86, follow-up M = 12.04, P ≤ 0.01). For two constructs, “actions to take in an overdose” and “naloxone use knowledge,” there were significant changes between baseline and the follow-up survey, while “signs of an overdose” was not significant. “Actions to take in an overdose” scores at baseline (M = 5.09) significantly increased at follow-up (M = 5.55, P ≤ 0.01). For “naloxone use knowledge,” baseline scores (M = 1.05) differed from follow-up (M = 1.74, P ≤ 0.01). “Signs of an overdose” was not significant between baseline (M = 4.71) and follow-up (M = 4.75) (Table 2). Comparing other survey items and measures, over time, the cohort statistically increased their perceptions of risk of overdose and increased familiarity with naloxone.
Table 2.
Baseline and follow-up Prescription Opioid Overdose Knowledge (Rx-OOKS) survey items
| Survey item | Baseline (N = 56) | Follow-up (N = 53) |
|---|---|---|
| Signs of prescription opioid overdose construct, M (SD) | 4.71 (2.13) | 4.75 (1.65) |
| Actions to take in a prescription opioid overdose construct, M (SD)** | 5.09 (1.64) | 5.55 (1.22) |
| Naloxone use knowledge construct, M (SD)** | 1.05 (1.35) | 1.74 (1.61) |
| Rx-OOKS total score construct, M (SD)** | 10.86 (4.27) | 12.04 (3.63) |
| Given naloxone information by health provider,** No. (%) | ||
| Yes | 1 (1.8) | 4 (7.5) |
| No | 54 (96.4) | 48 (90.6) |
| Don’t know | 1 (1.8) | 1 (1.9) |
| Reasons for not obtaining naloxone, No. (%)† | ||
| Not familiar with naloxone** | 17 (30.4) | 11 (20.8) |
| Not offered | 42 (75.0) | 38 (71.7) |
| Cost | 3 (5.4) | 2 (3.8) |
| Not at risk of overdose** | 21 (37.5) | 18 (34.0) |
| Hard to obtain* | 2 (3.6) | 1 (1.9) |
| Will not be used | 3 (5.4) | 4 (7.5) |
| Complicated to use | 1 (1.8) | 0 (0.0) |
| No one around takes pain medication* | 6 (10.7) | 4 (7.5) |
| No one around to administer naloxone | 4 (7.1) | 3 (5.7) |
| Afraid of naloxone | 1 (1.8) | 0 (0.0) |
| Worried about naloxone side effects | 1 (1.8) | 1 (1.9) |
| Worried doctor will lower pain medications if filling naloxone | 3 (5.4) | 3 (5.7) |
| Opioid medication is taken as prescribed* | 41 (73.2) | 36 (67.9) |
| Do not want to ask pharmacist for naloxone | 2 (3.6) | 4 (7.5) |
| Do not want to ask doctor for naloxone | 3 (5.4) | 3 (5.7) |
| What level of risk for opioid pain medication overdose?,* No. (%) | ||
| No risk | 32 (57.1) | 32 (60.4) |
| Low risk | 22 (39.3) | 20 (37.7) |
| Moderate risk | 1 (1.8) | 1 (1.9) |
| High risk | 1 (1.8) | 0 (0.0) |
M = mean; SD = standard deviation; No. = number
P ≤ 0.05;
P < 0.01.
Not mutually exclusive.
Internal Reliability Testing of OOKS
The following constructs were tested using Cronbach’s alpha: “risk of an overdose” (three items) was α = 0.365; “signs of an overdose” (10 items) was α = 0.851; “action to take with opioid overdose” (seven survey items) was α = 0.692; and “naloxone use knowledge” (eight survey items) was α = 0.729. One survey item (“There is no need to call for an ambulance if I know how to manage an overdose”) was moved from the survey construct “naloxone use knowledge” to “actions to take with opioid overdose,” as it fit better statistically and conceptually with the latter construct. Since the construct “risk of an overdose” performed poorly, these 3 items were removed from the Rx-OOKs and subsequently removed from further analyses. Overall, the survey instrument (all 25 survey items) had an alpha of 0.877, which is considered very good [36]. Correlational relationships among the constructs were r ≤ 0.60, P < 0.01 (Supplementary Data), indicating that the OOKS constructs are at least moderately correlated and have some expected relationship with one another.
Predictive Validity of Survey Constructs
We conducted logistic regression to determine if any of the constructs were strongly associated with future naloxone receipt. The median value is reported for each construct as follows: for the construct “signs of an opioid overdose,” the median split was 5; for “actions to take in an opioid overdose,” the median split was 6; for “naloxone use knowledge,” the median split was 1; and overall score had a median split of 11. Six individuals from the cohort had receipt of naloxone after baseline for one year. Additional descriptive assessment of those who received naloxone and those who did not was conducted (Supplementary Data), showing higher overall means of each construct in those who received naloxone.
When compared with those with lower scores, individuals with higher scores on the construct “actions to take in an overdose” had nine times the odds of receiving naloxone (odds ratio [OR] = 9.00, 95% confidence interval [CI] = 1.42–57.12). Those with high scores on the construct “naloxone use knowledge” were 15.8 times more likely to receive naloxone than those with lower scores (OR = 15.83, 95% CI = 1.68–149.17). For the construct “signs of an overdose,” those who had higher scores were nonsignificantly less likely to receive naloxone. The odds ratio of the total score was 6.75 (95% CI = 0.69–58.50) and was not statistically significant (Table 3).
Table 3.
Logistic regression of survey constructs associated with naloxone receipt
| Construct | OR | 95% CI | P Value |
|---|---|---|---|
| Signs of opioid overdose* | |||
| High score | 0.54 | 0.09–3.23 | 0.501 |
| Low score | 1.00 | – | – |
| Actions to take in an opioid overdose† | |||
| High score | 9.00 | 1.42–57.12 | 0.020 |
| Low score | 1.00 | – | – |
| Naloxone use knowledge‡ | |||
| High score | 15.83 | 1.68–149.17 | 0.016 |
| Low score | 1.00 | – | – |
| Total score§ | |||
| High score | 6.75 | 0.69–58.50 | 0.102 |
| Low score | 1.00 | – | – |
Each construct was modeled separately.
CI = confidence interval; OR = odds ratio.
Signs: median = 5, range = 0–9.
Actions: median = 6, range = 0–7.
Naloxone use knowledge: median = 1, range = 0–7.
Total score: median = 11, range = 0–20.
Discussion
In this cohort study, we describe the process of modifying an existing, validated questionnaire intended to measure opioid knowledge about heroin use and overdose for longitudinal use in populations on long-term prescription opioid therapy. The items on the survey instrument were refined to reflect knowledge about prescription opioids (instead of illicit opioids), and novel items were added, while items irrelevant to prescription opioids were removed from the instrument. The number of items was significantly reduced from the original OOKS survey instrument, thus reducing administration and patient burden, yet retaining equivalent or improved psychometric performance. We observed good internal consistency for each of the three scales on the survey instrument and overall. Correlational analysis demonstrated that there were moderate relationships between the constructs “signs of an overdose” and “naloxone use knowledge” and between “actions to take in an opioid overdose” and “naloxone use knowledge.” This makes sense, as knowledge about naloxone is related conceptually with overdose signs and actions yet demonstrates theoretically different constructs. The relationship between “signs of opioid overdose” and “actions to take in an opioid overdose” had a higher correlation, which would be expected, as identifying signs of an overdose should lead to taking action. Two of the scales, “naloxone use knowledge” and “actions to take in an opioid overdose,” had strong predictive validity of receipt of naloxone.
The scale “risk of an overdose,” which did not approach acceptable internal consistency, had three novel questions. This construct is important for measuring perceived risk of opioid overdose over time, with and without intervention. Future psychometric evaluation would benefit from expanding the number of risk questions and potentially adapting the original OOKS risk scale to incorporate content pertinent to prescription opioids.
The original OOKS has been administered to a range of populations, from law enforcement to family members of individuals at risk of opioid overdose [39–41]. However, few studies have used OOKS for patient populations using prescription opioids [33]. Studies that have used OOKS for prescription opioid–focused training and evaluation have modified the instrument by eliminating heroin-related items or creating novel items, without conducting evaluation of the modifications to the instrument and its impact on the validity and reliability of the original constructs. It has also been translated into languages other than English [42]. Modifying the OOKS for use with individuals using prescription opioids and evaluating its psychometric performance are necessary steps in developing reliable measurement scales.
Additionally, the original OOKS was conducted as a randomized controlled trial with family members and did not find an increase in perceived risks of an overdose after training [29]. Several other studies have used constructs of OOKS to measure retention of information and knowledge after training [43–45]. The Rx-OOKS can also be used in this manner, which would provide valuable information before and after intervention training, as well as a measurement of retention some time after intervention to determine effectiveness over time. Other studies have specifically modified the construct “risk of an overdose” and have found that it measures a person’s perception of risk of overdose and not the actual risk-based assessment of an overdose [40, 46] and that it lacks sensitivity as a measure of change over time [43].
In this study, patients were receiving LTOT and at potential risk for opioid overdose. It is important to develop and evaluate interventions to increase uptake of naloxone. Use of validated survey instruments is paramount to measuring the effectiveness of interventions designed to improve naloxone distribution. Evidence-based intervention outcomes facilitate the translation of research into clinical practice.
Two of the survey constructs, “actions to take in an opioid overdose” and “naloxone use knowledge,” were highly predictive of receipt of naloxone. These results suggest that education about how to recognize an overdose, the importance of acquiring naloxone, and how to administer naloxone may facilitate the uptake and accessibility of naloxone among patients prescribed LTOT.
A key strength of our study and the associated development and testing of the Rx-OOKS was its longitudinal design. By conducting it in an integrated health care system, we could identify patients at risk for overdose, administer the survey at two time points, and measure naloxone receipt using the EHR. This design helped to ensure the validity of our results.
This study has some limitations that should be addressed in future studies. The survey was conducted by administration within one health care system and therefore may not be generalizable. However, KPCO represents the larger Denver metro area demographically. Future development of the survey instrument in younger populations would be beneficial. Additionally, our study had small numbers: a cohort of 56, in which six obtained naloxone through our pharmacy system. Nevertheless, we observed very large effect sizes (ORs = 9.00, 15.83). Our small numbers also limited our ability to conduct an exploratory factor analysis. However, three of the four survey constructs demonstrated good internal consistency as measured by Cronbach’s alpha calculations. Lastly, we decided to include the commercial brand name in the instrument (Narcan), in addition to naloxone. While unorthodox to do so, our interviews with patients informed us of the interchangeable terminology for naloxone in nasal form. For future use in other countries, “Narcan” would need tailoring, as Narcan as a nasal form does not exist outside the United States. Instead, different approved nasal sprays (e.g., Nyxoid, Nalscue) could be added [16]. Further psychometric validation assessment in larger sample populations to allow factor analysis and concurrent validity with other measures is warranted.
In conclusion, our newly developed Rx-OOKS survey instrument can reliably measure knowledge about prescription opioid overdose recognition and naloxone use. With additional validation, and on larger sample sizes, further refinement will occur. As interventions to increase naloxone acceptability and use are being developed, we believe that the Rx-OOKS instrument can be used to test their effectiveness in various health care settings across the United States and internationally.
Supplementary Material
Acknowledgments
We wish to acknowledge the valuable survey data administration assistance of Ruth P. Bedoy and the contribution to the design of the methods by Komal Narwaney, PhD, Institute for Health Research, Kaiser Permanente Colorado. Ms. Bedoy and Dr. Narwaney have given permission to be mentioned here.
Contributor Information
Jo Ann Shoup, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado.
Shane R Mueller, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado.
Ingrid A Binswanger, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado; Colorado Permanente Medical Group, Denver, Colorado; Division of General Internal Medicine, Department of Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Anna V Williams, National Addiction Centre, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK.
John Strang, National Addiction Centre, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK.
Jason M Glanz, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado; Department of Epidemiology, Colorado School of Public Health, Aurora, Colorado, USA.
Funding sources: The research reported in this publication was supported by grants R34DA035952 and R01DA042059 from the National Institute on Drug Abuse of the National Institutes of Health. The National Institutes of Health did not contribute to the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit this article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Strang is based in the United Kingdom, where he is a National Institute for Health Research (NIHR) Senior Investigator, and his research is supported by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London.
Disclosure and conflicts of interest: Dr. Binswanger receives royalties for educational content on the health of incarcerated adults from UptoDate. Dr. Strang is a clinician and researcher who, through his university, has worked with pharmaceutical industry to identify new or improved treatments and from whom his employer (King’s College London) has received grants, travel costs, and/or consultancy payments; this includes investigation of new naloxone formulations and has included work (in the past three years) with Martindale, Indivior, Mundipharma (all of whom have naloxone products). His employer (King’s College London) has also registered intellectual property on a buccal naloxone formulation, naming JS and colleagues, and he was earlier named in a patent registration by a pharmaceutical company regarding concentrated nasal naloxone spray. JS has also worked as consultant for the United Nations Office on Drugs and Crime (UNODC), supporting them with a project introducing take-home naloxone to four central and western Asian countries as well as contributing to local take-home naloxone schemes. For a fuller account, see JS’s Web page at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx. No other authors declare any conflicts of interest. Jo Ann Shoup, Shane Mueller, Anna Williams, and Jason Glanz have no conflicts to report.
References
- 1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G.. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;67(5152):1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes—United States. 2018. Available at: https://www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf (accessed on June 20, 2019).
- 3. Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. Lancet 2013;382(9904):1564–74. [DOI] [PubMed] [Google Scholar]
- 4. Wermeling DP. A response to the opioid overdose epidemic: Naloxone nasal spray. Drug Deliv Transl Res 2013;3(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wermeling DP. Review of naloxone safety for opioid overdose: Practical considerations for new technology and expanded public access. Ther Adv Drug Saf 2015;6(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark AK, Wilder CM, Winstanley EL.. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 2014;8(3):153–63. [DOI] [PubMed] [Google Scholar]
- 7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER.. Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med 2014;9(2):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yealy DM, Paris PM, Kaplan RM, Heller MB, Marini SE.. The safety of prehospital naloxone administration by paramedics. Ann Emerg Med 1990;19(8):902–5. [DOI] [PubMed] [Google Scholar]
- 9. McDonald R, Campbell ND, Strang J.. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids—conception and maturation. Drug Alcohol Depend 2017;178:176–87. [DOI] [PubMed] [Google Scholar]
- 10. Strang J, Bird SM, Dietze P, Gerra G, McLellan AT. Take-Home Emergency Naloxone to Prevent Deaths from Heroin Overdose. London: British Medical Journal Publishing Group; 2014. [DOI] [PubMed]
- 11. Darke S, Hall W.. The distribution of naloxone to heroin users. Addiction 1997;92(9):1195–200. [PubMed] [Google Scholar]
- 12. Doyon S, Aks SE, Schaeffer S.. Expanding access to naloxone in the United States. J Med Toxicol 2014;10(4):431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: Interrupted time series analysis. BMJ 2013;346:f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wheeler E, Jones TS, Gilbert MK, Davidson PJ; Centers for Disease Control and Prevention. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64(23):631–5. [PMC free article] [PubMed] [Google Scholar]
- 15. Giglio RE, Li G, DiMaggio CJ.. Effectiveness of bystander naloxone administration and overdose education programs: A meta-analysis. Int J Epidemiol 2015;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strang J, McDonald R, Campbell G, et al. Take-home naloxone for the emergency interim management of opioid overdose: The public health application of an emergency medicine. Drugs 2019;79(13):1395–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald R, Strang J.. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- 19. Coe MA, Walsh SL.. Distribution of naloxone for overdose prevention to chronic pain patients. Prev Med 2015;80:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glanz JM, Narwaney KJ, Mueller SR, Gardner EM, et al. Prediction model for two-year risk of opioid overdose among patients prescribed chronic opioid therapy. J Gen Intern Med 2018;33(10):1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Binswanger IA, Koester S, Mueller SR, Gardner EM, Goddard K, Glanz JM.. Overdose education and naloxone for patients prescribed opioids in primary care: A qualitative study of primary care staff. J Gen Intern Med 2015;30(12):1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Behar E, Bagnulo R, Coffin PO.. Acceptability and feasibility of naloxone prescribing in primary care settings: A systematic review. Prev Med 2018;114:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thakur T, Frey M, Chewning B.. Pharmacist roles, training, and perceived barriers in naloxone dispensing: A systematic review. J Am Pharm Assoc 2020;60(1):178–94. [DOI] [PubMed] [Google Scholar]
- 24. Mueller SR, Koester S, Glanz JM, Gardner EM, Binswanger IA.. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med 2017;32(3):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haffajee RL, French CA.. Provider perceptions of system-level opioid prescribing and addiction treatment policies. Curr Opin Psychol 2019;30:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haug NA, Bielenberg J, Linder SH, Lembke A.. Assessment of provider attitudes toward #naloxone on Twitter. Subst Abuse 2016;37(1):35–41. [DOI] [PubMed] [Google Scholar]
- 27. Bounthavong M, Suh K, Christopher ML, Veenstra DL, Basu A, Devine EB.. Providers' perceptions on barriers and facilitators to prescribing naloxone for patients at risk for opioid overdose after implementation of a national academic detailing program: A qualitative assessment. Res Social Adm Pharm 2020;16(8):1033–40. [DOI] [PubMed] [Google Scholar]
- 28. Behar E, Rowe C, Santos G-M, Murphy S, Coffin PO.. Primary care patient experience with naloxone prescription. Ann Fam Med 2016;14(5):431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams AV, Marsden J, Strang J.. Training family members to manage heroin overdose and administer naloxone: Randomized trial of effects on knowledge and attitudes. Addiction 2014;109(2):250–9. [DOI] [PubMed] [Google Scholar]
- 30. Williams AV, Strang J, Marsden J.. Development of Opioid Overdose Knowledge (OOKS) and Attitudes (OOAS) scales for take-home naloxone training evaluation. Drug Alcohol Depend 2013;132(1–2):383–6. [DOI] [PubMed] [Google Scholar]
- 31. Behar E, Santos G-M, Wheeler E, Rowe C, Coffin PO.. Brief overdose education is sufficient for naloxone distribution to opioid users. Drug Alcohol Depend 2015;148:209–12. [DOI] [PubMed] [Google Scholar]
- 32. Green TC, Heimer R, Grau LE.. Distinguishing signs of opioid overdose and indication for naloxone: An evaluation of six overdose training and naloxone distribution programs in the United States. Addiction 2008;103(6):979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunn KE, Barrett FS, Yepez-Laubach C, et al. Brief Opioid Overdose Knowledge (BOOK): A questionnaire to assess overdose knowledge in individuals who use illicit or prescribed opioids. J Addict Med 2016;10(5):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koester S, Mueller SR, Raville L, Langegger S, Binswanger IA.. Why are some people who have received overdose education and naloxone reticent to call emergency medical services in the event of overdose? Int J Drug Policy 2017;48:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Little RJ. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988;83(404):1198–202. [Google Scholar]
- 36. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16(3):297–334. [Google Scholar]
- 37. DeVellis RF. Scale Development: Theory and Applications. Thousand Oaks, CA: Sage Publications; 2016.
- 38.Pearson’s correlation coefficient. In: Kirch W, ed. Encyclopedia of Public Health. Dordrecht, the Netherlands: Springer Netherlands; 2008:1090–1.
- 39. Wagner KD, Bovet LJ, Haynes B, Joshua A, Davidson PJ.. Training law enforcement to respond to opioid overdose with naloxone: Impact on knowledge, attitudes, and interactions with community members. Drug Alcohol Depend 2016;165:22–8. [DOI] [PubMed] [Google Scholar]
- 40. Monteiro K, Dumenco L, Collins S, et al. An interprofessional education workshop to develop health professional student opioid misuse knowledge, attitudes, and skills. J Am Pharm Assoc 2017;57(2):S113–S7. [DOI] [PubMed] [Google Scholar]
- 41. Wolfson-Stofko B, Gwadz MV, Elliott L, Bennett AS, Curtis R.. “Feeling confident and equipped”: Evaluating the acceptability and efficacy of an overdose response and naloxone administration intervention to service industry employees. Drug Alcohol Depend 2018;192:362–70. in New York City. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petterson AG, Madah-Amiri D.. Overdose prevention training with naloxone distribution in a prison in Oslo, Norway: A preliminary study. Harm Reduct J 2017;14(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lott DC, Rhodes J.. Opioid overdose and naloxone education in a substance use disorder treatment program. Am J Addict 2016;25(3):221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen S, Peacock A, Lintzeris N, Bruno R, Larance B, Degenhardt L.. Knowledge of opioid overdose and attitudes to supply of take-home naloxone among people with chronic noncancer pain prescribed opioids. Pain Med 2018;19(3):533–40. [DOI] [PubMed] [Google Scholar]
- 45. Zhang X, Marchand C, Sullivan B, Klass EM, Wagner KD.. Naloxone access for emergency medical technicians: An evaluation of a training program in rural communities. Addict Behav 2018;86:79–85. [DOI] [PubMed] [Google Scholar]
- 46. Wilder CM, Miller SC, Tiffany E, Winhusen T, Winstanley EL, Stein MD.. Risk factors for opioid overdose and awareness of overdose risk among veterans prescribed chronic opioids for addiction or pain. J Addict Dis 2016;35(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.