Key Points
Question
Do outcomes differ in patients with venous thromboembolism with or without concomitant moderate to severe chronic kidney disease?
Findings
In this cohort study of 8979 adult patients with venous thromboembolism, the presence of concomitant moderate to severe chronic kidney disease was associated with increases in the risk of death, recurrent venous thromboembolism, and major bleeding compared with mild to no chronic kidney disease.
Meaning
The study’s findings suggest that patients with venous thromboembolism and concomitant moderate to severe chronic kidney disease had worse prognoses, and further investigation is warranted to evaluate options for anticoagulation therapy in patients with venous thromboembolism who have advanced chronic kidney disease.
Abstract
Importance
Patients with venous thromboembolism (VTE) and concomitant chronic kidney disease (CKD) have been reported to have a higher risk of thrombosis and major bleeding complications compared with patients without concomitant CKD. The use of anticoagulation therapy is challenging, as many anticoagulant medications are excreted by the kidney. Large-scale data are needed to clarify the impact of CKD for anticoagulant treatment strategies and clinical outcomes of patients with VTE.
Objective
To compare clinical characteristics, treatment patterns, and 12-month outcomes among patients with VTE and concomitant moderate to severe CKD (stages 3-5) vs patients with VTE and mild to no CKD (stages 1-2) in a contemporary international registry.
Design, Setting, and Participants
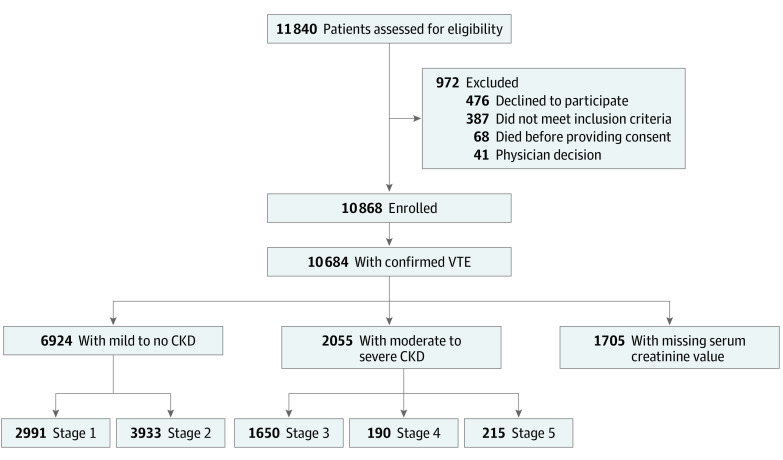
The Global Anticoagulant Registry in the Field–Venous Thromboembolism (GARFIELD-VTE) study is a prospective noninterventional investigation of real-world treatment practices. A total of 10 684 patients from 415 sites in 28 countries were enrolled in the GARFIELD-VTE between May 2014 and January 2017. This cohort study included 8979 patients (6924 patients with mild to no CKD and 2055 patients with moderate to severe CKD) who had objectively confirmed VTE within 30 days before entry in the registry. Chronic kidney disease stages were defined by estimated glomerular filtration rates. Data were extracted from the study database on December 8, 2018, and analyzed between May 1, 2019, and July 30, 2020.
Exposure
Moderate to severe CKD vs mild to no CKD.
Main Outcomes and Measures
The primary outcomes were all-cause mortality, recurrent VTE, and major bleeding. Event rates and 95% CIs were calculated and expressed per 100 person-years. Hazard ratios (HRs) were estimated with Cox proportional hazards regression models and adjusted for relevant confounding variables. All-cause mortality was considered a competing risk for other clinical outcomes in the estimation of cumulative incidences.
Results
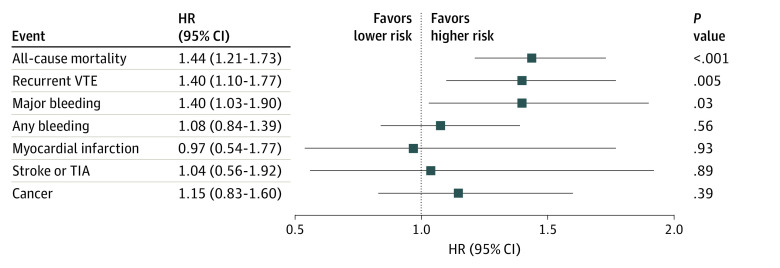
Of the 10 684 patients with objectively confirmed VTE, serum creatinine data were available for 8979 patients (84.0%). Of those, 4432 patients (49.4%) were female and 5912 patients (65.8%) were White; 6924 patients (77.1%; median age, 57 years; interquartile range [IQR], 44-69 years) were classified as having mild to no CKD, and 2055 patients (22.9%; median age, 70 years; IQR, 59-78 years) were classified as having moderate to severe CKD. Calculations using the equation from the Modification of Diet in Renal Disease study indicated that, among the 6924 patients with mild to no CKD, 2991 patients had stage 1 CKD, and 3933 patients had stage 2 CKD; among the 2055 patients with moderate to severe CKD, 1650 patients had stage 3 CKD, 190 patients had stage 4 CKD, and 215 patients had stage 5 CKD. The distribution of VTE presentation was comparable between groups. In total, 1171 patients (57.0%) with moderate to severe CKD and 4079 patients (58.9%) with mild to no CKD presented with deep vein thrombosis alone, 547 patients (26.6%) with moderate to severe CKD and 1723 patients (24.9%) with mild to no CKD presented with pulmonary embolism alone, and 337 patients (16.4%) with moderate to severe CKD and 1122 patients (16.2%) with mild to no CKD presented with both pulmonary embolism and deep vein thrombosis. Compared with patients with mild to no CKD, patients with moderate to severe CKD were more likely to be female (3259 women [47.1%] vs 1173 women [57.1%]) and older than 65 years (2313 patients [33.4%] vs 1278 patients [62.2%]). At baseline, the receipt of parenteral therapy alone was comparable between the 2 groups (355 patients [17.3%] with moderate to severe CKD vs 1253 patients [18.1%] with mild to no CKD). Patients with moderate to severe CKD compared with those with mild to no CKD were less likely to be receiving direct oral anticoagulant therapy, either alone (557 patients [27.1%] vs 2139 patients [30.9%]) or in combination with parenteral therapy (319 patients [15.5%] vs 1239 patients [17.9%]). Patients with moderate to severe CKD had a higher risk of all-cause mortality (adjusted hazard ratio [aHR], 1.44; 95% CI, 1.21-1.73), major bleeding (aHR, 1.40; 95% CI, 1.03-1.90), and recurrent VTE (aHR, 1.40; 95% CI, 1.10-1.77) than patients with mild to no CKD.
Conclusions and Relevance
In this study of patients with VTE, the presence of moderate to severe CKD was associated with increases in the risk of death, VTE recurrence, and major bleeding compared with the presence of mild to no CKD.
This cohort study uses data from participants in the Global Anticoagulant Registry in the Field–Venous Thromboembolism study to examine outcomes among adult patients with venous thromboembolism with and without moderate to severe chronic kidney disease.
Introduction
Venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common factor associated with cardiovascular death.1 Patients with chronic kidney disease (CKD) have a higher risk of developing VTE than those with normal kidney function.2 In addition, decreased kidney function after PE has been found to be a short-term and long-term independent factor associated with increased mortality rates.3 The prevalence of both CKD and VTE increases with age and the accumulation of risk factors, including type 2 diabetes and hypertension.4,5 Furthermore, the choice of therapy can be more limited in patients with severe CKD owing to the clearance of direct oral anticoagulant (DOAC) medications by the kidneys.6
Randomized clinical trials support the use of DOAC therapy for the treatment of patients with VTE and mild to moderate CKD.7,8 A meta-analysis indicated that DOAC therapy has a benefit-risk profile superior to that of vitamin K antagonist (VKA) therapy in patients with early-stage CKD.9 The DOAC medications have varying degrees of kidney excretion; therefore, in one of the clinical trials, doses were adjusted for each patient,7 although this adjustment was not performed in the other clinical trial.8
Data on the efficacy and safety of DOAC therapy for the treatment of patients with advanced CKD are limited. Phase 3 randomized clinical trials investigating the receipt of DOAC therapy among patients with VTE have excluded patients with severe kidney impairment (defined as a creatinine clearance level <25-30 mL/min).7,8,10,11 Certain DOAC medications are approved for the treatment of VTE in patients with moderate to severe CKD and are licensed for use in patients with creatinine clearance levels as low as 15 mL/min.12 Large-scale data are needed to clarify the impact of CKD for anticoagulant treatment strategies and clinical outcomes of patients with VTE.
The Global Anticoagulant Registry in the Field–Venous Thromboembolism (GARFIELD-VTE) study is an ongoing worldwide prospective noninterventional registry designed to observe initial and extended therapeutic strategies and clinical outcomes in patients with VTE who are receiving treatment according to local standard practices. In the present analysis, baseline characteristics, treatment patterns, and 12-month outcomes were compared between patients with moderate to severe CKD (stages 3-5) and mild to no CKD (stages 1-2) who were enrolled in the GARFIELD-VTE.
Methods
A detailed description of the rationale and design of the GARFIELD-VTE (ClinicalTrials.gov identifier: NCT02155491) has been published previously.13 The registry is conducted in accordance with the Declaration of Helsinki,30 the International Council for Harmonization Guideline for Good Clinical Practice,31 and the International Society of Pharmacoepidemiology Guidelines for Good Pharmacoepidemiological Practice32 and adheres to all applicable national laws and regulations. Independent ethics committees for each participating country and hospital-based institutional review boards approved the design of the registry. All patients provided written informed consent to participate. The confidentiality and anonymity of patients recruited for this registry are maintained. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies.
Patient data relevant to VTE were collected through review of clinical records and patient notes. Data were captured using an electronic case report form designed by eClinicalHealth Services (Stirling, UK) and submitted electronically via a secure website to the registry coordinating center at the Thrombosis Research Institute (London, UK). The GARFIELD-VTE protocol requires that 10% of all electronic case report forms be monitored against source documentation, that there be an electronic audit trail for all data modifications, and that critical variables be subjected to additional audit. The data were extracted from the study database on December 8, 2018.
Design, Setting, and Participants
The GARFIELD-VTE enrolled patients 18 years and older who received diagnoses and treatment across a range of care settings at 415 sites in 28 countries between May 2014 and January 2017. Race and ethnicity data were collected from all participants via self-reporting. A previous study14 suggested that variation exists among different races and ethnicities regarding the risk of developing venous thrombosis, and information about race and ethnicity was necessary to clarify the association between CKD stage and clinical outcome among patients with VTE. Eligible patients were required to have an objective diagnosis of VTE (excluding superficial vein thrombosis) within 30 days before they were entered in the registry. Patients with recurrent VTE must have completed their treatment for the previous event. Patients were excluded if long-term follow-up was not planned or if they were participating in other studies that required clinical visits, diagnostic procedures, or receipt of treatments. The aim of the registry was to record local treatment practices; therefore, no specific treatments or procedures were mandated by the study protocol. Decisions to initiate, continue, or change treatment were made solely at the discretion of the treating physicians and their patients.
The national coordinating investigators identified the care settings they believed most accurately represented the management of patients with VTE in their country, including vascular medicine, internal medicine, and general practice settings. Sites that agreed to participate were recruited after a qualification telephone conversation. The relevant investigator was required to complete a program that provided guidance on patient screening, enrollment, and follow-up in the registry.
Clinical Outcomes and CKD Stage
The primary clinical outcomes were all-cause mortality, recurrent VTE, and major bleeding. Major bleeding was defined as clinically overt bleeding associated with a critical site (eg, intracranial, intraspinal, or intraocular), decrease in hemoglobin of 2 g/dL or more, transfusion of 2 or more units of packed red blood cells, hemorrhagic stroke, or death. Nonmajor bleeding was defined as any overt bleeding that did not meet the criteria for major bleeding. The rates of cancer, nonhemorrhagic stroke or transient ischemic attack, and myocardial infarction were also recorded. In addition, information was collected regarding the recorded cause of death and site of bleeding. Cancer events that were diagnosed more than 30 days after the VTE diagnosis date were considered cancer end points.
The severity of CKD was centrally adjudicated according to the Kidney Disease Outcomes Quality Initiative guidelines of the National Foundation.15 The estimated glomerular filtration rate (GFR; expressed in milliliters per minute per 1.73 m squared) was calculated using the equation from the Modification of Diet in Renal Disease (MDRD) study, in which a constant of 175 is multiplied by the patient’s standardized serum creatinine level (in mg/dL) to the −1.154 power multiplied by the patient’s age (in years) to the −0.203 power; the product is then multiplied by 0.742 if the patient is female and by 1.212 if the patient is Black. Patients were classified as having mild to no CKD (stages 1-2) if their estimated GFR was 60 mL/min/1.73 m2 or higher.
Patients were classified as having moderate to severe CKD (stages 3-5) if their estimated GFR was 59 mL/min/1.73 m2 or lower (eTable 2 in the Supplement). Kidney function was confirmed by measuring creatinine clearance rate (in mL/min) using the Cockcroft Gault formula. For men, the patient’s age (in years) is subtracted from a constant of 140, then multiplied by the patient’s weight (in kg); this value is then divided by the product of the patient’s serum creatinine level (in mg/L) multiplied by a constant of 72. For women, the resulting creatinine clearance rate is multiplied by 0.85.
Statistical Analysis
Continuous variables were summarized as median and interquartile range (IQR), and categorical variables were reported as frequency and percentage. Event rates and the associated 95% CIs were estimated using Poisson regression analysis and were expressed per 100 person-years.
Time to event analyses of outcomes were performed using Cox proportional hazards models. Hazard ratios (HRs) and associated 95% CIs were calculated. All variables that were identified as potential confounders by expert clinical judgment and through literature review were included in the adjustment models (eTable 1 in the Supplement). The proportional hazards assumption was evaluated with scaled Schoenfeld residuals. Missing values were imputed using the multivariate imputation by chained equations (MICE) method.16 A sensitivity analysis was performed to assess the impact of excluded patients for the estimated HR. Cumulative incidence plots were estimated with cumulative incidence functions (CIFs) accounting for the competing risk of mortality for recurrent VTE episodes and major bleeding events.17 Spearman rank correlation coefficients were used to compare creatinine clearance and GFR.
All statistical analyses were performed using R software (R Foundation for Statistical Computing)18 and SAS software, version 9.4 (SAS Institute).19 Throughout the study, 2-sided tests were used, and the threshold for statistical significance was P = .05. Data were analyzed from May 1, 2019, to July 30, 2020.
Results
Of the 10 684 patients with objectively confirmed VTE, serum creatinine data were available for 8979 patients (84.0%). Of those, 4432 patients (49.4%) were female and 5912 patients (65.8%) were White (Table). Overall, 6924 eligible patients (77.1%) were classified as having mild to no CKD (2991 patients with stage 1 CKD and 3933 patients with stage 2 CKD), and 2055 patients (22.9%) were classified as having moderate to severe CKD (1650 patients with stage 3 CKD, 190 patients with stage 4 CKD, and 215 patients with stage 5 CKD), as calculated using the MDRD (Figure 1). The Cockcroft Gault formula revealed a similar pattern for kidney function (eTable 2 in the Supplement). Patients with moderate to severe CKD compared with those with mild to no CKD were more likely to be female (1173 women [57.1%] vs 3259 women [47.1%]) and older than 65 years (1278 patients [62.2%] vs 2313 patients [33.4%]). The Spearman correlation coefficient for estimated GFR and creatinine clearance was 0.75 (eFigure 1 in the Supplement). A full description of baseline participant characteristics is provided in the Table.
Table. Baseline Participant Characteristics .
| Characteristic | No. (%) | |
|---|---|---|
| Mild to no CKD | Moderate to severe CKD | |
| Total participants, No. | 6924 | 2055 |
| Female sex | 3259 (47.1) | 1173 (57.1) |
| Age, y | ||
| Median (IQR) | 57 (44-69) | 70 (59-78) |
| Category | ||
| <50 | 2482 (35.8) | 271 (13.2) |
| 50-65 | 2129 (30.7) | 506 (24.6) |
| >65-75 | 1379 (19.9) | 549 (26.7) |
| >75-85 | 774 (11.2) | 548 (26.7) |
| >85 | 160 (2.3) | 181 (8.8) |
| Race/ethnicity | ||
| White | 4485 (64.8) | 1427 (69.4) |
| Asian | 1344 (19.4) | 363 (17.7) |
| Black | 335 (4.8) | 85 (4.1) |
| Other | 389 (5.6) | 99 (4.8) |
| Missing | 371 (5.4) | 81 (3.9) |
| BMI | ||
| Median (IQR) | 27.1 (23.9-31.2) | 28.1 (24.7-32.4) |
| Category | ||
| Underweight (<18.5) | 161 (2.3) | 34 (1.7) |
| Normal (18.5-24.9) | 2001 (28.9) | 476 (23.2) |
| Overweight (25.0-29.9) | 2163 (31.2) | 674 (32.8) |
| Obese (≥30.0) | 1932 (27.9) | 702 (34.2) |
| Missing | 667 (9.6) | 169 (8.2) |
| Type of VTE | ||
| DVT alone | 4079 (58.9) | 1171 (57.0) |
| PE alone | 1723 (24.9) | 547 (26.6) |
| PE and DVT | 1122 (16.2) | 337 (16.4) |
| Site of DVT | ||
| Lower limb | 4774 (68.9) | 1423 (69.2) |
| Upper limb | 308 (4.4) | 58 (2.8) |
| Caval vein | 116 (1.7) | 24 (1.2) |
| No DVT | 1723 (24.9) | 547 (26.6) |
| Missing | 3 (0.04) | 3 (0.1) |
| Type of lower limb DVT | ||
| Proximal | 1752 (25.3) | 557 (27.1) |
| Distal | 1651 (23.8) | 419 (20.4) |
| Both | 1319 (19.0) | 428 (20.8) |
| Missing | 2202 (31.8) | 651 (31.7) |
| Care setting | ||
| Hospital | 5315 (76.8) | 1597 (77.7) |
| Outpatient setting | 1609 (23.2) | 458 (22.3) |
| Specialty | ||
| Internal medicine (hematology and intensive care) | 3311 (47.8) | 869 (42.3) |
| Vascular medicine | 2858 (41.3) | 952 (46.3) |
| Cardiology | 306 (4.4) | 100 (4.9) |
| General practitioner | 241 (3.5) | 92 (4.5) |
| Emergency medicine | 205 (3.0) | 42 (2.0) |
| Missing | 3 (0.04) | 0 |
| Region | ||
| Europe | 3856 (55.7) | 1212 (59.0) |
| Asia | 1237 (17.9) | 342 (16.6) |
| North America and Australia | 1046 (15.1) | 264 (12.8) |
| Africa and Middle East | 574 (8.3) | 165 (8.0) |
| Latin America | 211 (3.0) | 72 (3.5) |
| Provoking risk factorsa | ||
| Persistent | ||
| Active cancer | 744 (10.7) | 236 (11.5) |
| Transient | ||
| Surgery | 911 (13.2) | 219 (10.7) |
| Hospitalization | 865 (12.5) | 289 (14.1) |
| Trauma to lower limb | 548 (7.9) | 103 (5.0) |
| Oral contraception | 400 (5.8) | 49 (2.4) |
| Acute medical illness | 398 (5.7) | 145 (7.1) |
| Long traveling | 375 (5.4) | 71 (3.5) |
| Hormone replacement therapy | 98 (1.4) | 46 (2.2) |
| Predisposing risk factors | ||
| Previous VTE | 1035 (14.9) | 325 (15.8) |
| History of cancer | 901 (13.0) | 358 (17.4) |
| Family history of VTE | 459 (6.6) | 87 (4.2) |
| Chronic immobilization | 371 (5.4) | 144 (7.0) |
| Recent bleeding or anemia | 242 (3.5) | 100 (4.9) |
| Known thrombophilia | 214 (3.1) | 43 (2.1) |
| Chronic heart failure | 154 (2.2) | 138 (6.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; DVT, deep vein thrombosis; IQR, interquartile range; PE, pulmonary embolism; VTE, venous thromboembolism.
Persistent and transient provoking risk factors that occurred within 3 months before VTE diagnosis.
Figure 1. Study Population Flowchart.
CKD indicates chronic kidney disease and VTE, venous thromboembolism.
At baseline, the distribution of VTE events was comparable between groups (Table). Among patients with moderate to severe CKD, 1171 patients (57.0%) presented with DVT alone, 547 patients (26.6%) presented with PE alone, and 337 patients (16.4%) presented with PE and DVT; among patients with mild to no CKD, 4079 patients (58.9%) presented with DVT alone, 1723 patients (24.9%) presented with PE alone, and 1122 patients (16.2%) presented with PE and DVT. The most frequent site of DVT in patients with moderate to severe CKD and those with mild to no CKD was the lower limb (1423 patients [69.2%] and 4774 patients [68.9%]). The type of lower limb DVT was comparable among patients with moderate to severe CKD and those with mild to no CKD (for proximal DVT, 557 patients [27.1%] vs 1752 patients [25.3%]; for distal DVT, 419 patients [20.4%] vs 1651 [23.8%]; and for distal and proximal DVT, 428 patients [20.8%] vs 1319 patients [19.0%]). Patients with moderate to severe CKD compared with those with mild to no CKD were older (median age, 70 years [IQR, 59-78 years] vs 57 years [IQR, 44-69 years]) and more likely to be female (1173 women [57.1%] vs 3259 women [47.1%]). Other characteristics, such as race/ethnicity, body mass index (calculated as weight in kilograms divided by height in meters squared), and care setting, were similar among groups.
The prevalence of provoking risk factors, both persistent and transient, was comparable between patients with moderate to severe CKD and patients with mild to no CKD (Table). Compared with patients with mild to no CKD, patients with moderate to severe CKD were more likely to have predisposing risk factors, including chronic heart failure (154 patients [2.2%] vs 138 patients [6.7%]), chronic immobilization (371 patients [5.4%] vs 144 patients [7.0%]), and a history of cancer (901 patients [13.0%] vs 358 patients [17.4%]).
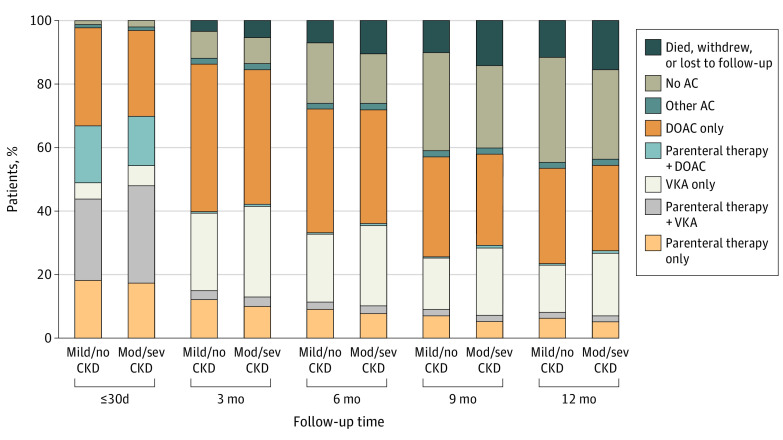
At baseline, patients with moderate to severe CKD compared with those with mild to no CKD were more likely to be receiving a VKA medication, either alone (129 patients [6.3%] vs 353 patients [5.1%]) or in combination with parenteral therapy (631 patients [30.7%] vs 1779 patients [25.7%]) and less likely to be receiving a DOAC medication, either alone (557 patients [27.1%] vs 2139 patients [30.9%]) or in combination with parenteral therapy (319 patients [15.5%] vs 1239 patients [17.9%]) (Figure 2). The receipt of parenteral therapy alone was comparable between groups (355 patients [17.3%] with moderate to severe CKD vs 1253 patients [18.1%] with mild to no CKD). Over time, a similar proportion of patients with moderate to severe CKD and mild to no CKD continued to receive anticoagulant therapy at 3 months (1778 patients [86.5%] vs 6100 patients [88.1%]), 6 months (1519 patients [73.9%] vs 5117 patients [73.9%]), 9 months (1231 patients [59.9%] vs 4085 patients [59.0%]), and 12 months (1136 patients [55.3%] vs 3829 patients [55.3%]).
Figure 2. Anticoagulant Treatment in Patients Over 12-Month Follow-Up.
Data unavailable for 13 patients with moderate to severe chronic kidney disease and 47 patients with mild to no chronic kidney disease. AC indicates anticoagulant medication; DOAC, direct oral anticoagulant medication; mild/no CKD, mild to no chronic kidney disease; mod/sev CKD, moderate to severe chronic kidney disease; and VKA, vitamin K antagonist medication.
Clinical Outcomes
The unadjusted rate of all-cause mortality at 12 months in patients with moderate to severe CKD was higher than that of patients with mild to no CKD (12.8 deaths per 100 person-years [95% CI, 11.3-14.6 deaths per 100 person-years] vs 6.7 deaths per 100 person-years [95% CI, 6.1-7.3 deaths per 100 person-years]) (eTable 3 in the Supplement). Patients with moderate to severe CKD were more likely than patients with mild to no CKD to die of cardiac-associated conditions (185 patients [9.0%] vs 339 patients [4.9%]) but less likely to die of cancer (912 patients [44.4%] vs 4141 patients [59.8%]) and VTE-associated conditions (80 patients [3.9%] vs 346 patients [5.0%]) (eTable 4 in the Supplement). The rate of recurrent VTE was higher in patients with moderate to severe CKD compared with patients with mild to no CKD (6.6 events per 100 person-years [95% CI, 5.5-7.9 events per 100 person-years] vs 5.0 events per 100 person-years [95% CI, 4.5-5.6 events per 100 person-years]). A greater proportion of PE recurrences (with or without DVT) was found in patients with moderate to severe CKD (3102 patients [44.8%]) vs patients with mild to no CKD (2285 patients [33.0%]).
Patients with moderate to severe CKD also experienced major bleeding more frequently than patients with mild to no CKD (4.6 events per 100 person-years [95% CI, 3.7-5.7 events per 100 person-years] vs 2.4 events per 100 person-years [95% CI, 2.1-2.8 events per 100 person-years]). The most frequent sites of bleeding in patients with both moderate to severe CKD and mild to no CKD were the upper gastrointestinal tract (477 patients [23.2%] and 907 patients [13.1%]) and the lower gastrointestinal tract (376 patients [18.3%] and 907 patients [13.1%]) (eTable 5 in the Supplement).
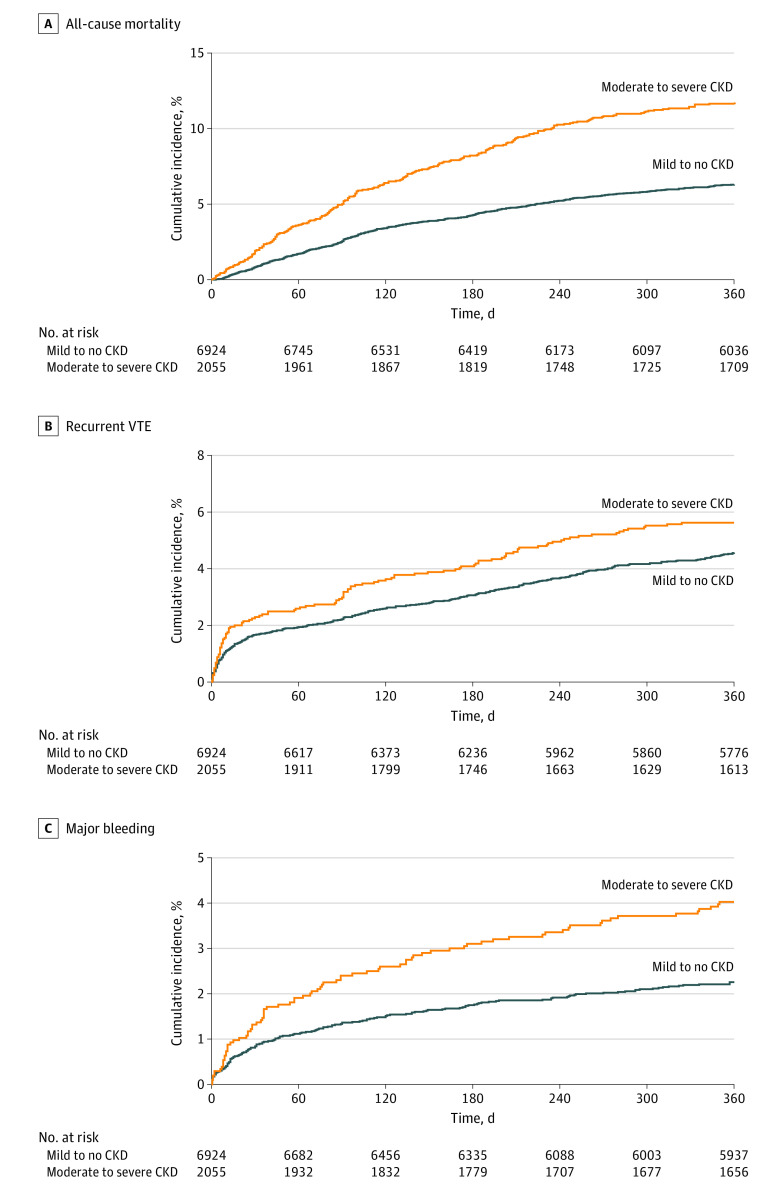
Among patients with mild to no CKD, the unadjusted rates of myocardial infarction or acute coronary syndrome (0.7 events per 100 person-years [95% CI, 0.5-0.9 events per 100 person-years]) and stroke (0.6 events per 100 person-years [95%CI, 0.4-0.8 events per 100 person-years]) did not differ from those with moderate to severe CKD (1.3 events per 100 person-years [95% CI, 0.8-1.9 events per 100 person-years] and 0.9 events per 100 person-years [95% CI, 0.6-1.5 events per 100 person-years]) (eTable 3 in the Supplement). Figure 3 shows the cumulative incidence curves for the primary end points of all-cause mortality, recurrent VTE, and major bleeding.
Figure 3. Cumulative Incidence Stratified by Stage of Chronic Kidney Disease.
Data are shown as percentages of patients experiencing event and 95% CIs. CKD indicates chronic kidney disease and VTE, venous thromboembolism. A, All-cause mortality. B, Recurrent VTE. C, Major bleeding.
After adjustment for potential confounders (eTable 1 in the Supplement), the incidence of all-cause mortality at 12 months remained higher in patients with moderate to severe CKD (adjusted HR [aHR], 1.44; 95% CI, 1.21-1.73). The incidence of recurrent VTE (aHR, 1.40; 95% CI, 1.10-1.77) and major bleeding (aHR, 1.40; 95% CI, 1.03-1.90) at 12 months was also higher in patients with moderate to severe CKD (Figure 4). The incidence of cancer did not differ between patients with mild to no CKD and moderate to severe CKD (aHR, 1.15; 95% CI, 0.83-1.60).
Figure 4. Adjusted Hazard Ratios for 12-Month Outcomes.
Reference group was patients with mild to no chronic kidney disease. eTable 1 in the Supplement lists variables used in the adjustment. HR indicates hazard ratio; TIA, transient ischemic attack; and VTE, venous thromboembolism.
A comparison of baseline characteristics between the patients included in the analysis and the 1705 patients (16.0%) omitted from the analysis owing to missing creatinine levels revealed distribution differences in VTE type (eFigure 2 in the Supplement). A sensitivity analysis estimating the HRs for the main clinical outcomes using imputed creatinine values for the excluded patients revealed comparable outcome rates.
A subanalysis of outcomes according to the type of lower limb DVT indicated that patients with proximal DVT compared with patients with distal DVT had a higher rate of all-cause mortality in those with mild to no CKD (7.9 deaths per 100 person-years [95% CI, 6.7-9.4 deaths per 100 person-years] vs 4.6 deaths per 100 person-years [95% CI, 3.6-5.8 deaths per 100 person-years]) and moderate to severe CKD (16.7 deaths per 100 person-years [95% CI, 13.4-20.7 deaths per 100 person-years] vs 10.2 deaths per 100 person-years [95% CI, 7.5-14.0 deaths per 100 person-years]). Patients with proximal DVT compared with distal DVT more frequently experienced recurrent VTE (for patients with mild to no CKD, 6.3 events per 100 person-years [95% CI, 5.2-7.7 events per 100 person-years] vs 5.3 events per 100 person-years [95% CI, 4.3-6.6 events per 100 person-years]; for patients with moderate to severe CKD, 8.9 events per 100 person-years [95% CI, 6.5-12.1 events per 100 person-years] vs 4.6 events per 100 person-years [95% CI, 2.9-7.4 events per 100 person-years]) and major bleeding (for patients with mild to no CKD, 2.6 events per 100 person-years [95% CI, 1.9-3.5 events per 100 person-years] vs 1.6 events per 100 person-years [95% CI, 1.1-2.4 events per 100 person-years]; for patients with moderate to severe CKD, 4.4 events per 100 person-years [95% CI, 2.9-6.8 events per 100 person-years] vs 3.2 events per 100 person-years [95% CI, 1.8-5.6 events per 100 person-years]) (eTable 6 in the Supplement).
Discussion
The clinical characteristics, treatment patterns, and 12-month outcomes of patients with moderate to severe CKD (stages 3-5) and mild to no CKD (stages 1-2) were evaluated using data from the large contemporary international registry of the GARFIELD-VTE. The patient’s CKD stage was determined using the MDRD equation for estimated GFR levels, and kidney function was confirmed using the Cockcroft Gault formula for creatinine clearance measurement, indicating a Spearman correlation coefficient of 0.75 for the sample. The MDRD equation is typically used to calculate estimated GFR, which is the recommended measurement for establishing the stage and progression of CKD. The Cockcroft Gault formula is often used to analyze creatinine clearance as an indication of kidney function and as a means of calculating dosage requirements. The MDRD equation provides a greater level of accuracy for calculating kidney function than the Cockcroft Gault formula in patients with advanced CKD and in patients with impaired kidney function.20,21 Both estimations are widely used to classify the progress of the patient’s CKD stage, and the MDRD and Cockcroft Gault estimations did not differ substantially in our analysis.
Patients with moderate to severe CKD had higher rates of all-cause mortality, recurrent VTE, and major bleeding than those with mild to no CKD over a 12-month period after diagnosis with VTE, despite comparable use of anticoagulant therapy. It is of note that the cumulative incidences were diverse over time between patients with moderate to severe CKD and patients with mild to no CKD for both all-cause mortality and major bleeding. Differences in the cumulative incidence of VTE recurrence between the 2 groups were calculated at an early point after recruitment and maintained throughout the 12 months of follow-up.
Patients with moderate to severe CKD were older than those with mild to no CKD, confirming the association between older age and the prevalence of CKD5; patients with moderate to severe CKD were also more likely to be female.22 In addition, patients with moderate to severe CKD had a higher prevalence of predisposing factors, including chronic heart failure. Heart failure and CKD share many common risk factors, such as older age, hypertension, and type 2 diabetes, with more than one-half of patients with heart failure having moderate to severe CKD.23 A higher risk of mortality and bleeding in patients with VTE and concomitant moderate to severe CKD has been reported previously;24,25,26 however, unlike the GARFIELD-VTE registry, these studies did not include a substantial number of patients who were receiving DOAC therapy,24 and they were conducted over a shorter period of 3 months.24,25,26
In the contemporary international GARFIELD-VTE registry, inclusion of a substantial number of Asian patients indicated that, among those with moderate to severe CKD, mortality was primarily associated with cancer followed by cardiac conditions.27 At baseline, the choice of parenteral anticoagulation treatment alone was comparable between patients with moderate to severe CKD and those with mild to no CKD. Patients with moderate to severe CKD were more likely to receive VKA therapy (alone or in combination with parenteral therapy) and less likely to receive DOAC therapy (alone or in combination with parenteral therapy) than patients with mild to no CKD.
Chronic kidney disease is recognized as an important factor in future cardiovascular events owing to the associated hypercoagulable state.28 Even after accounting for the competing risk of death, subdistribution HRs illustrated an increased incidence of recurrent VTE and major bleeding in patients with moderate to severe CKD. A higher incidence of bleeding events in patients with moderate to severe CKD raises the question of whether the intensity of anticoagulant medication currently being used is too high. These results are consistent with those observed in the GARFIELD–Atrial Fibrillation study,29 in which patients with moderate to severe CKD had a higher incidence of mortality and bleeding complications. A comparative effectiveness analysis detailing the impact of differing anticoagulation strategies is warranted to investigate safer treatment choices for patients with VTE and concomitant moderate to severe CKD in the future.
Limitations
This study has several limitations. The absence of creatinine clearance measurements in 16.0% of patients with objectively confirmed VTE is a major limitation. Another limitation is the heterogeneous distribution of patients’ CKD stages, with only a small number of patients in the GARFIELD-VTE having advanced CKD (190 patients with stage 4 CKD and 215 patients with stage 5 CKD).
Conclusions
In this study, the presence of concomitant moderate to severe CKD among patients with VTE was associated with increases in the risk of death, recurrent VTE, and major bleeding within 12 months of VTE diagnosis compared with the presence of mild to no CKD, even after adjustment for baseline participant characteristics. Improving the quality of care for patients with VTE and concomitant moderate to severe CKD remains an important challenge. Future work within the GARFIELD-VTE will assess the impact of both the dose and the duration of anticoagulant treatment for VTE recurrence and bleeding up to 3 years after VTE diagnosis.
eTable 1. Covariates Considered for the Adjustment of 12-Month Outcomes
eTable 2. Chronic Kidney Disease Classification Using the Modification of Diet in Renal Disease Equation and the Cockcroft Gault Calculation of Renal Function
eTable 3. Unadjusted 12-Month Clinical Outcomes
eTable 4. Cause of Death Over 12 Months of Follow-Up
eTable 5. Site of Major Bleeding in Patients With Moderate to Severe Chronic Kidney Disease and Mild to No Chronic Kidney Disease
eTable 6. Incidence Rates of Outcomes at 12 Months in Patients With Mild to No Chronic Kidney Disease and Moderate to Severe Chronic Kidney Disease According to Type of Lower Limb Deep Vein Thrombosis
eFigure 1. Spearman Correlation for Association Between Glomerular Filtration Rate and Creatinine Clearance Among Patients With Mild to No Chronic Kidney Disease and Moderate to Severe Chronic Kidney Disease
eFigure 2. Sensitivity Analysis for Standardized Differences Between Baseline Characteristics for Missing Patients and Patients Included in the Study
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3):370-372. doi: 10.1161/ATVBAHA.108.162545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19(1):135-140. doi: 10.1681/ASN.2007030308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ģībietis V, Kigitoviča D, Vītola B, Strautmane S, Skride A. Glomerular filtration rate as a prognostic factor for long-term mortality after acute pulmonary embolism. Med Princ Pract. 2019;28(3):264-272. doi: 10.1159/000497436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038-2047. doi: 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, Levey AS. Chronic kidney disease in the elderly—how to assess risk. N Engl J Med. 2005;352(20):2122-2124. doi: 10.1056/NEJMe058035 [DOI] [PubMed] [Google Scholar]
- 6.Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40. doi: 10.1093/ndt/gft209 [DOI] [PubMed] [Google Scholar]
- 7.Büller HR, Décousus H, Grosso MA, et al. ; Hokusai-VTE Investigators . Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406-1415. doi: 10.1056/NEJMoa1306638 [DOI] [PubMed] [Google Scholar]
- 8.Bauersachs R, Berkowitz SD, Brenner B, et al. ; EINSTEIN Investigators . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. doi: 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 9.Ha JT, Neuen BL, Cheng LP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181-189. doi: 10.7326/M19-0087 [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C, Kakkar AK, et al. ; RE-COVER Study Group . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352. doi: 10.1056/NEJMoa0906598 [DOI] [PubMed] [Google Scholar]
- 11.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi: 10.1056/NEJMoa1302507 [DOI] [PubMed] [Google Scholar]
- 12.Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin Cardiol. 2019;42(8):774-782. doi: 10.1002/clc.23196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weitz JI, Haas S, Ageno W, et al. Global Anticoagulant Registry in the Field - Venous Thromboembolism (GARFIELD-VTE). Rationale and design. Thromb Haemost. 2016;116(6):1172-1179. [DOI] [PubMed] [Google Scholar]
- 14.Liew NC, Alemany GV, Angchaisuksiri P, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36(1):1-20. [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2)(suppl 1):S1-S266. [PubMed] [Google Scholar]
- 16.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Soft. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 17.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2018. Accessed January 24, 2019. https://www.R-project.org/
- 19.SAS Institute . Base SAS 9.4 Procedures Guide, Seventh Edition. SAS Institute; 2017. Updated August 20, 2020. http://documentation.sas.com/api/docsets/proc/9.4/content/proc.pdf?locale=en#nameddest=titlepage
- 20.Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant. 2005;20(11):2394-2401. doi: 10.1093/ndt/gfi076 [DOI] [PubMed] [Google Scholar]
- 21.Schwandt A, Denkinger M, Fasching P, et al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complications. 2017;31(9):1376-1383. doi: 10.1016/j.jdiacomp.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 22.Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33(5):383-392. doi: 10.1159/000320389 [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4(4):387-399. doi: 10.1016/j.hfc.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falgá C, Capdevila JA, Soler S, et al. ; RIETE Investigators . Clinical outcome of patients with venous thromboembolism and renal insufficiency. Findings from the RIETE registry. Thromb Haemost. 2007;98(4):771-776. doi: 10.1160/TH07-02-0132 [DOI] [PubMed] [Google Scholar]
- 25.Spirk D, Sebastian T, Banyai M, et al. Venous thromboembolism and renal impairment: insights from the Swiss Venous Thromboembolism Registry (SWIVTER). Semin Thromb Hemost. 2019;45(8):851-858. doi: 10.1055/s-0039-1698770 [DOI] [PubMed] [Google Scholar]
- 26.Catella J, Bertoletti L, Mismetti P, et al. ; investigators of the RIETE registry . Severe renal impairment and risk of bleeding during anticoagulation for venous thromboembolism. J Thromb Haemost. 2020;18(7):1728-1737. doi: 10.1111/jth.14837 [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Li H-Y, Zhou Q, et al. Renal function and all-cause mortality risk among cancer patients. Medicine (Baltimore). 2016;95(20):e3728. doi: 10.1097/MD.0000000000003728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattazzi M, Villalta S, De Lucchi L, et al. Chronic kidney disease is associated with increased risk of venous thromboembolism recurrence. Thromb Res. 2017;160:32-37. doi: 10.1016/j.thromres.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Goto S, Angchaisuksiri P, Bassand J-P, et al. ; GARFIELD‐AF Investigators . GARFIELD-AF Investigators. Management and 1-year outcomes of patients with newly diagnosed atrial fibrillation and chronic kidney disease: results from the prospective GARFIELD-AF Registry. J Am Heart Assoc. 2019;8(3):e010510. doi: 10.1161/JAHA.118.010510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 31.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1). June 10, 1996. Accessed September 28, 2020. https://ichgcp.net/
- 32.Public Policy Committee, International Society of Pharmacoepidemiology . Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf. 2016;25(1):2-10. doi: 10.1002/pds.3891 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Covariates Considered for the Adjustment of 12-Month Outcomes
eTable 2. Chronic Kidney Disease Classification Using the Modification of Diet in Renal Disease Equation and the Cockcroft Gault Calculation of Renal Function
eTable 3. Unadjusted 12-Month Clinical Outcomes
eTable 4. Cause of Death Over 12 Months of Follow-Up
eTable 5. Site of Major Bleeding in Patients With Moderate to Severe Chronic Kidney Disease and Mild to No Chronic Kidney Disease
eTable 6. Incidence Rates of Outcomes at 12 Months in Patients With Mild to No Chronic Kidney Disease and Moderate to Severe Chronic Kidney Disease According to Type of Lower Limb Deep Vein Thrombosis
eFigure 1. Spearman Correlation for Association Between Glomerular Filtration Rate and Creatinine Clearance Among Patients With Mild to No Chronic Kidney Disease and Moderate to Severe Chronic Kidney Disease
eFigure 2. Sensitivity Analysis for Standardized Differences Between Baseline Characteristics for Missing Patients and Patients Included in the Study