Abstract
BACKGROUND:
Neonatal exposure to sevoflurane induces neurobehavioral and neuroendocrine abnormalities in exposed male rats (generation F0) and neurobehavioral, but not neuroendocrine, abnormalities in their male, but not female, offspring (generation F1). These effects of sevoflurane are accompanied by a hypermethylated neuron-specific K+-2Cl− (Kcc2) Cl− exporter gene in the F0 spermatozoa and the F1 male hypothalamus, while the gene’s expression is reduced in the F0 and F1 hypothalamus. We investigated whether inhibition of DNA methyltransferases (DNMTs) prior to paternal sevoflurane exposure could alleviate the anesthetic’s F0 and F1 effects.
METHODS:
Sprague-Dawley male rats were anesthetized with 2.1% sevoflurane for 5 h on postnatal day (P) 5 and mated with control females on P90 to generate offspring. The nonselective DNMT inhibitor decitabine (0.5 mg/kg, intraperitoneally) was administered 30 min prior to sevoflurane exposure. The F0 and F1 male rats were evaluated in in vivo and in vitro tests in adulthood.
RESULTS:
Paternal exposure to sevoflurane induced impaired prepulse inhibition of the acoustic startle response and exacerbated corticosterone responses to stress in F0 males and impaired prepulse inhibition of the startle responses in F1 males. These effects were accompanied in both generations by reduced and increased expressions of hypothalamic Kcc2 and Dnmt3a/b, respectively. Decitabine deterred the effects of paternal exposure to sevoflurane in F0 and F1 males.
CONCLUSIONS:
These results suggest that similar decitabine-sensitive mechanisms regulating expression of multiple genes are involved in the mediation of neurobehavioral abnormalities in sires neonatally exposed to sevoflurane and in their future unexposed male offspring.
Keywords: Neonatal anesthesia, Intergenerational effects, Dnmt, Kcc2
INTRODUCTION
Clinical studies report learning disabilities, long-term memory impairment, and attention-deficit/hyperactivity disorder in those who had early-life procedures requiring anesthesia, especially in those who had repeated exposures.1 Recent findings in animal models show that general anesthetics may induce neurobehavioral deficiencies not only in the exposed parents, but also in their future unexposed offspring.2,3 The mechanisms mediating the adverse effects of general anesthetics in both generations remain poorly understood.1–3
We have shown that anesthetics, whose actions involve potentiation of GABA type A receptor (GABAAR) signaling, can induce impairment in developmental increases in the hippocampal, hypothalamic, and amygdala K+-2Cl− (KCC2) Cl− exporter.2–6 Other laboratories have confirmed the roles of the Cl− transporters in the neurodevelopmental effects of general anesthetics.7,8 Developmental changes in KCC2 and Na+-K+-Cl−(NKCC1) Cl− importer activities determine a proper developmental transition of GABAAR signaling from excitatory early in life to inhibitory later on.9 Importantly, delays in the GABAAR signaling transition from excitatory to inhibitory have been linked in animal models and humans to several cognitive neuropsychiatric disorders, such as schizophrenia, autism spectrum disorder, and Rett syndrome.10–16 For example, in Rett syndrome, a severe form of autism spectrum disorder, a deficiency in methyl CpG binding protein 2 (MeCP2) induced by KCC2 downregulation may play a causal role.15,16 Of particular relevance, we found that exposure of neonatal rats to sevoflurane also downregulates hippocampal MeCP2 levels.17 The effects of sevoflurane on MeCP2 may involve epigenetic mechanisms, specifically a DNA methyltransferase (DNMT)-mediated increase in DNA methylation, as a sevoflurane-induced reduction in MeCP2 and behavioral abnormalities were accompanied by increased expression of hippocampal DNMT 3A/B and hypermethylated brain-derived neurotrophic factor (Bdnf) gene. Pretreatment with the nonselective DNMT inhibitor decitabine prevented these effects of sevoflurane in the exposed rats.17
Neonatal sevoflurane-initiated DNA methylation and impaired Kcc2 expression may also be involved in the mediation of sevoflurane’s intergenerational effects.2,3 Thus, bisulfate sequencing revealed cytosine-phosphate-guanine (CpG) dinucleotide hypermethylation in the Kcc2 promoter in the paternal spermatozoa and in the hypothalamus of their male, but not female, offspring. Similar to their exposed parents, unexposed male, but not female, offspring exhibited reduced Kcc2 expression and behavioral abnormalities. Notably, in contrast to exacerbated corticosterone responses to stress induced by sevoflurane in parents, the corticosterone responses to stress in their offspring were not affected.2
To assess whether the same or similar DNA methylation-based mechanisms are involved in the initiation of the adverse effects of neonatal exposure to sevoflurane in the exposed parents and in their future unexposed male offspring, we investigated if decitabine administered prior to neonatal exposure to sevoflurane alters the previously observed intergenerational effects. To investigate homogeneous intergenerational effects of neonatal parental exposure to sevoflurane, only sires were exposed to the anesthetic because the father’s contribution to offspring development in rodents is largely limited to passing spermatozoa-containing genomic and epigenomic information. In this study, the main brain region of interest for gene expression analyses was the hypothalamus, the region with the most prominent changes in Kcc2 methylation and expression in the male offspring of the exposed sires.2
We used the prepulse inhibition (PPI) of the acoustic startle response paradigm to test the intergenerational behavioral effects of paternal neonatal treatment with decitabine prior to exposure to anesthesia with sevoflurane. We previously showed that the PPI of the startle response was impaired in sires neonatally exposed to sevoflurane and in their unexposed adult male offspring.2 The PPI of the acoustic startle response is the reduction of the startle response when the startle stimulus is preceded by a subthreshold sensory stimulus (sensorimotor gating).18 The PPI measurements in rodents, in contrast to almost all other rodent behavioral paradigms, can easily be replicated under nearly identical parameters in human studies and vice versa.19,20 Impairment in the PPI of the startle response has been demonstrated in patients with neuropsychiatric disorders associated with cognitive dysfunction, such as schizophrenia,21 obsessive-compulsive disorder,22 Asperger’s syndrome,23 22q11 syndrome,24 fragile-X syndrome,25 Tourette syndrome,26,27 panic disorder,28 and post-traumatic stress disorder,29 among others. Hence, investigation of the intergenerational PPI effects of paternal neonatal exposure to sevoflurane and related treatments may facilitate similar clinical studies in this field.
METHODS
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Sprague-Dawley rats were bred at the University of Florida animal care facility. The rats were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (23–24°C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched pairs for the rest of the study.
Treatment Groups
Postnatal day (P) 5 male rat pups (generation F0) were held in a temperature-controlled chamber (+37°C) with a continuous supply of 30% oxygen in air (1.5 L/min) during anesthesia, which was 6% sevoflurane for 3 min for anesthesia induction and 2.1% sevoflurane for 297 min for anesthesia maintenance (sevoflurane group) (see Figure 1 for illustration of experimental design). A rectal temperature probe was placed in some animals to monitor body temperature. Gas monitoring was performed using a calibrated Datex sidestream analyzer (Datex-Ohmeda, Helsinki, Finland), which samples from the animal chamber interior. Previously, we have shown that blood glucose and blood gas levels after 2.1% sevoflurane for 6 h were in the normal range.30 The vehicle (sevoflurane group) or decitabine (5-aza-2’-deoxycytidine, 0.5 mg/kg, intraperitoneal injection; decitabine + sevoflurane group) was given to animals 30 min prior to the onset of anesthesia with sevoflurane. Rats in the F0 control group were subjected to animal facility rearing only (control group). They remained with their dams. The primary argument against the use of a control group for maternal separation during sevoflurane anesthesia was that anesthetized pups do not experience separation stress, while 5 h of maternal separation is a stressor. All animals except those in the control group received a subcutaneous injection of saline (1 mL/100 g) at 2.5 h of anesthesia with sevoflurane to prevent dehydration.
Figure 1.
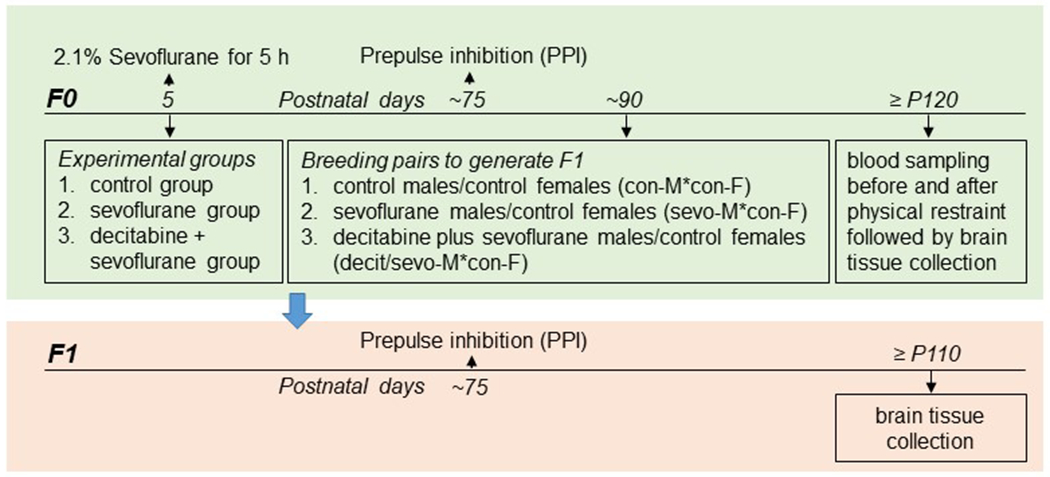
Study design.
The F0 male rats were evaluated in the PPI of the acoustic startle response on ~P75. On P90, a subset of the same F0 male rats (eight rats/group) were mated with control female rats (eight female rats/group) to generate offspring (generation F1) of: 1) control males/control females (con-M*con-F); 2) sevoflurane males/control females (sevo-M*con-F); and 3) decitabine plus sevoflurane males/control females (decit/sevo-M*con-F). The female rats were kept alone throughout the entire gestation and postpartum rearing periods. The offspring in each experimental group (15 F1 males/group) were generated by eight breeding pairs. Therefore, a given F1 male offspring experimental group included one to two rats produced by a given F0 breeding pair. On ~P120, F0 male rats were evaluated for serum corticosterone levels at rest and after physical restrain for 30 min (see Basal and Stress-Induced Levels of Corticosterone in F0 Males), followed by isolation of brain tissue samples 10 days later.
The F1 male rats, which were subjected to facility rearing only, were evaluated in the PPI of the startle on ~P75, followed by isolation of brain tissue samples on ~P110 for gene expression analyses (Figure 1).
Measurements of the PPI of the Startle
The PPI of the startle tests were performed using an SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory.2–4 The % PPI for each prepulse level was calculated using the formula: % PPI = 100 × [(pulse alone) − (prepulse + pulse)] / pulse alone. Data were collected as Vmax amplitude.
Basal and Stress-Induced Levels of Corticosterone in F0 Males
Blood samples (~300 µL) were collected using the “tail clip” method at rest and at 10, 60, and 120 min after the restraint as previously described by our laboratory.2–4 Physical restraint was administered using rodent holders (Kent Scientific Corporation, Torrington, CT). Serum corticosterone was measured using commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instructions.
Tissue Collection
Rats were anesthetized with sevoflurane and decapitated. The hypothalamus was isolated by making an anterior cut at the level of the optic chiasm, a posterior coronal section anterior to the mammillary bodies, two sagittal cuts parallel to the lateral ventricles, and a dorsal horizontal cut at the level of the anterior commissure. All tissue samples were placed in vials filled with RNAlater solution (Invitrogen, Carlsbad, CA).
qRT-PCR
Levels of mRNA for Dnmt3a, Dnmt3b, Dnmt1, and Kcc2 in the hypothalamus were analyzed via qRT-PCR in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA) as previously described by our laboratory.2–6 RNA was extracted from the samples using an RNeasy Plus Kit (Qiagen, Valencia, CA), reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Hercules, CA), and analyzed via qRT-PCR. Taqman probes specific for the above genes were obtained from Applied Biosystems (Carlsbad, CA): Dnmt3a (Rn01027162), Dnmt3b (Rn01536414_g1), Dnmt1 (Rn00709664_m1), and Kcc2 (Rn00592624_m1). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA (Rn01775763_g1). Gene expression was calculated using the ΔΔCT method and data are presented as relative fold change from that of control animals.
Statistical Analysis
Statistical analyses were conducted on raw data using SigmaPlot 14.0 software (Systat Software Inc., San Jose, CA), which automatically checks if the data set meets test criteria (Shapiro-Wilk for normality test and Brown-Forsythe for equal variance test). Values are reported as mean ± SEM. A two-way repeated measures analysis of variance (ANOVA) was used to analyze the PPI data, with the treatment and prepulse intensity as independent variables. A two-way repeated measures ANOVA was used to analyze changes in the serum corticosterone levels at rest and at three time points after the restraint, with experimental groups and time as the independent variables. To assess the differences in total corticosterone concentrations, the area under the curve with respect to ground [AUCg, g – ground (level of corticosterone at rest)] was calculated and compared across experimental groups using one-way ANOVA. We used one-way ANOVA to assess the differences in the mRNA levels for Dnmt3a, Dnmt3b, Dnmt1, and Kcc2. All multiple pairwise comparisons were done using the Fisher Least Significant Difference method. P < .05 was considered significant. Statistical details are presented in the text and figure legends. The sample sizes in this study were based on previous experience with the same experimental techniques.2–6,17 According to those studies, the sample size of 15 rats/group would allow us to detect significant behavioral effects during the PPI of the acoustic startle response test. Therefore, we studied 45 F0 rats (15 rats/group × 3 treatment groups) and 45 F1 rats (15 rats/group × 3 treatment groups).
Results
Decitabine Prevented Sevoflurane-Induced Neurobehavioral Abnormalities in F0 Males
There was a significant treatment × prepulse intensity interaction in the PPI of the acoustic startle response test in adult F0 male rats (F(4,84) = 5.422; P < .001; Figure 2A). Multiple pairwise comparisons indicated that neonatal exposure to sevoflurane led to impaired PPI of the startle responses in F0 male rats at prepulse intensities of 3 dB (P < .001 vs all other groups) and 12 dB (P = .04 vs control and P = .018 vs decitabine + sevoflurane; Figure 2A). Pretreatment with decitabine significantly alleviated the sevoflurane-impaired PPI of startle at a prepulse intensity of 3 dB (P = .062 vs control) and at a prepulse intensity of 12 dB (P = .741 vs control; Figure 2A). The startle amplitudes were similar among all experimental groups of F0 male rats.
Figure 2.
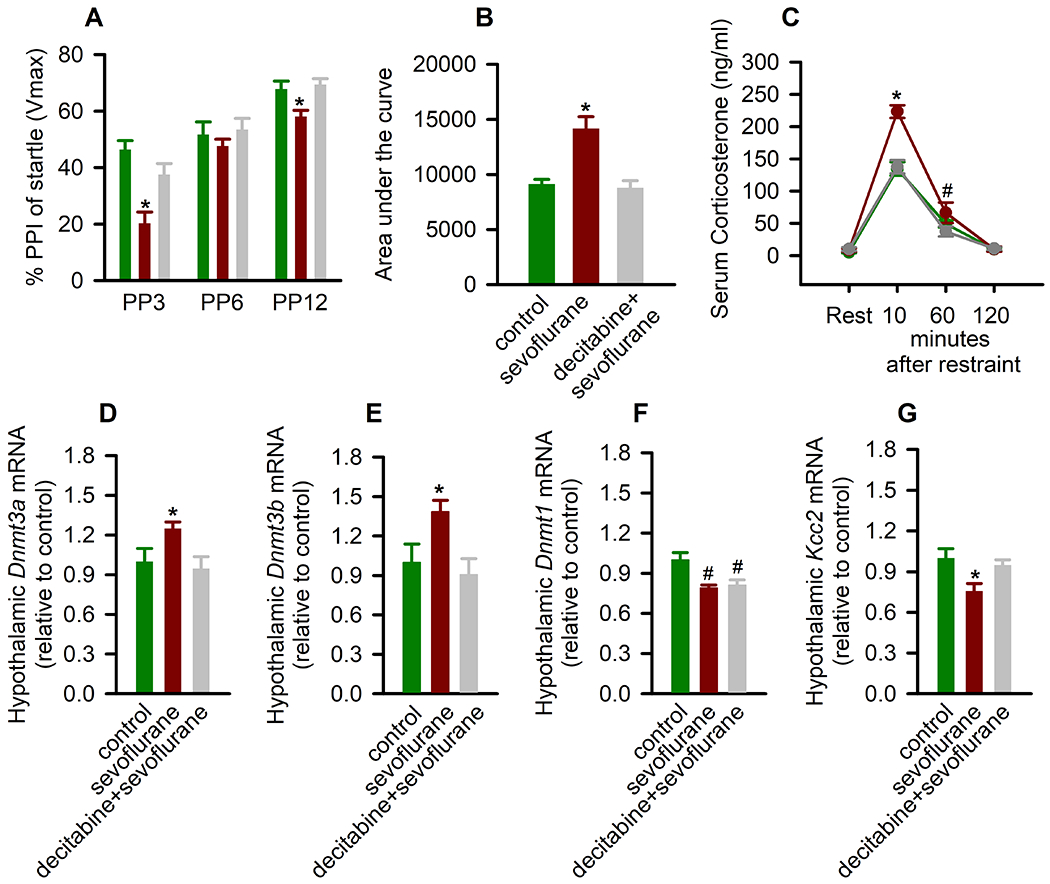
Decitabine, administered 30 min prior to anesthesia with sevoflurane on postnatal day (P) 5 reduces the ability of sevoflurane to induce impaired prepulse inhibition (PPI) of the startle responses, exacerbated corticosterone responses to physical restraint for 30 min, and changes in gene expression in the hypothalamus. (A) Histogram showing % PPI of the startle responses at prepulse intensity of 3 dB (PP3), 6 dB (PP6), and 12 dB (PP12) above background in F0 male rats. Data are means ± SEM from 15/group male rats. *P < .05 vs all other groups. (B,C) The total serum corticosterone responses (B) and the respective levels of corticosterone across each collection point (C). To assess differences in total corticosterone concentrations, area under the curve in respect to ground (AUCg; levels of corticosterone at rest were taken as a ground) was calculated. Data are means ± SEM from six animals per treatment group. *P < .05 vs all the other groups; #P = .009 vs decitabine plus sevoflurane. (D-G) The respective levels of DNA methyltransferase 3a (Dnmt3a) mRNA, Dnmt3b mRNA, Dnmt1 mRNA, and K+-2Cl− (Kcc2) Cl− exporter mRNA in the hypothalamus of F0 male rats. Data are means ± SEM from 8 rats/group (n = 9 for Kcc2 mRNA in control and sevoflurane groups). *P < .05 vs all the other groups; #P < .05 vs control. Color coding in Fig. 2B,D-G is applicable to the entire figure.
Because F0 male rats neonatally exposed to sevoflurane, but not their F1 male offspring, exhibited exacerbated corticosterone responses to stress,3 we assessed the effect of pretreatment with decitabine before sevoflurane exposure corticosterone levels in F0 males at rest and at 10, 60, and 120 min after restraint for 30 min. Similar to our previous findings, there was a significant between-subjects effect of treatment on P5 on total serum levels of corticosterone (measured as AUCg; F(2,15) = 15.629, P < .001; Figure 2B). Multiple pairwise comparisons found that compared to all the other groups, rats in the sevoflurane group had significantly higher levels of serum corticosterone (P < .001). These increases were due to higher levels of corticosterone 10 min after the restraint (P < .001 vs all the other treatment groups), as serum levels of corticosterone in the sevoflurane group were similar to those in all other groups before the restraint (P ⩾ .714) and 60 min (P = .116 vs control) and 120 min (P ⩾ .921) post-restraint (Figure 2C). The exception was that at 60 min after the restraint, the decitabine + sevoflurane group had a significantly lower level of corticosterone compared to the sevoflurane group (P = .009; Figure 2C).
There was a significant between-subjects effect of treatment on the hypothalamic mRNA levels of Dnmt3a (F(2,21) = 3.911, P = .036; Figure 2D), Dnmt3b (F(2,21) = 4.638, P = .021; Figure 2E), and Dnmt1 (F(2,21) = 7.641, P = .003; Figure 2F). F0 males neonatally exposed to sevoflurane had elevated levels of Dnmt3a mRNA (P = .042 vs control and P = .016 vs decitabine + sevoflurane) and Dnmt3b mRNA (P = .031 vs control and P = .009 vs decitabine + sevoflurane). Surprisingly, in contrast to our previous findings that exposure of Sprague-Dawley rats to 3% sevoflurane for 2 h daily from P7 to P9 did not affect the expression of hippocampal Dnmt1,20 in this study we found that anesthesia with 2.1% sevoflurane for 5 h on P5 induced a reduction in the hypothalamic Dnmt1 mRNA levels (P = .002 vs control). This effect of sevoflurane was not sensitive to pretreatment with decitabine (P = .723 vs decitabine + sevoflurane; Figure 2F). Consistent with our previous findings,2 there was a significant between-subjects effect of treatment on the hypothalamic mRNA levels of Kcc2 (F(2,23) = 5.323, P = .013; Figure 2G). Multiple pairwise comparisons found that the sevoflurane group had significantly lower levels of Kcc2 mRNA compared to the control group (P = .005). Pretreatment with decitabine ameliorated this effect of sevoflurane (P = .540 vs control but P = .026 vs sevoflurane).
Decitabine Prevented Paternal Neonatal Anesthesia with Sevoflurane-Induced Neurobehavioral Abnormalities in F1 Males
There was a significant parental neonatal treatment × prepulse intensity interaction in the PPI of the startle in adult F1 male offspring (F(4,84) = 2.937; P = .025; Figure 3A). Multiple pairwise comparisons indicated that paternal neonatal exposure to sevoflurane led to impaired PPI of the startle responses in F1 male offspring at a prepulse intensity of 3 dB (P = .004 vs F1 males from the con-M*con-F group). The F1 male offspring of sires neonatally pretreated with decitabine prior to sevoflurane exposure had similar PPI of the startle responses as those in F1 male offspring of both control parents (P = .188). The startle amplitudes were similar among all experimental groups of F1 male rats.
Figure 3.
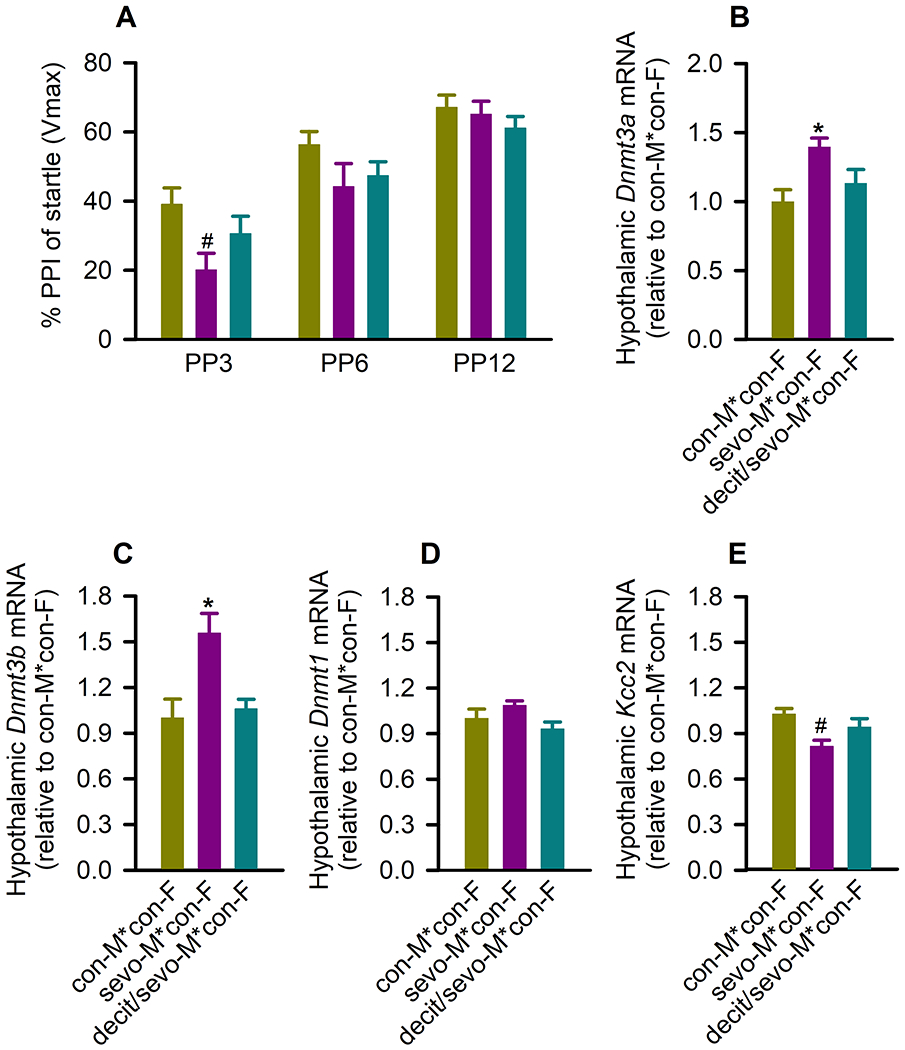
Decitabine, administered 30 min prior to paternal (F0) anesthesia with sevoflurane on postnatal day (P) 5 reduces the ability of sevoflurane to induce impaired prepulse inhibition (PPI) of the startle responses and changes in gene expression in the hypothalamus of F1 male rats. (A) Histogram showing % of PPI of the startle responses at prepulse intensity (PP) of 3 dB (PP3), 6 dB (PP6), and 12 dB (PP12) above background in F1 male rats. Data are means ± SEM from 15/group rats. #P < .05 vs F1 male from the con-M*con-F group. (B-E) The respective levels of DNA methyltransferase 3a (Dnmt3a) mRNA, Dnmt3b mRNA, Dnmt1 mRNA, K+-2Cl− (Kcc2) Cl− exporter mRNA in the hypothalamus of F1 male rats. Data are means ± SEM from eight rats/group (Dnmt3a: n = 6 from con-M*con-F group, n = 7 from sevo-M*con-F group, n = 5 from decit/sevo-M*con-F group; Dnmt1: n = 7 from sevo-M*con-F group; Kcc2: n = 9 from con-M*con-F and sevo-M*con-F groups). *P < .05 vs all other groups; #P < .05 vs F1 male from the con-M*con-F group. Color coding in Fig. 3B-D is applicable to the entire figure.
The effects of neonatal paternal exposure to sevoflurane on expression of the hypothalamic Dnmt3a and Dnmt3b, but not on Dnmt1, were passed to F1 male offspring in a decitabine-sensitive manner. Thus, there was a significant between-subjects effect of neonatal paternal treatment on the hypothalamic mRNA levels of Dnmt3a (F(2,15) = 6.692, P = .008; Figure 3B), and Dnmt3b (F(2,21) = 7.849, P = .003; Figure 3C), but not Dnmt1 (F(2,20) = 2.458, P = .111; Figure 3D). Specifically, F1 adult male progenies of sires neonatally exposed to sevoflurane had higher hypothalamic mRNA levels of Dnmt3a (P = .003 vs the F1 males from the con-M*con-F group and P = .039 vs F1 males from the decit/sevo-M*con-F group) and Dnmt3b (P = .002 vs the F1 males from the con-M*con-F group and P = .004 vs F1 males from the decit/sevo-M*con-F group). Similar to the exposed fathers, the F1 male offspring had a reduced expression of Kcc2 in the hypothalamus (P = .003 vs the F1 males from the con-M*con-F group; Figure 3E). This reduction in expression of hypothalamic Kcc2 was not found in F1 male offspring of sires pretreated with decitabine prior to sevoflurane exposure (P = .202 vs the F1 males from the con-M*con-F group, but P = .062 vs F1 males from the decit/sevo-M*con-F group; Figure 3E).
DISCUSSION
The results of this study further confirm that paternal neonatal exposure to sevoflurane may induce long-term abnormalities not only in the exposed animals themselves, but also in their future unexposed male offspring. As previously shown, paternal neonatal anesthesia with sevoflurane induced deficiencies in sensory-motor gating function, reduced expression of hypothalamic Kcc2 in both generations, and exacerbated corticosterone responses to stress in the exposed sires.2 In addition, both the exposed sires and their F1 male offspring had a significantly upregulated expression of hypothalamic Dnmt3a and Dnmt3b. The latter suggests that the Kcc2 gene could be one of many genes involved in the mediation of the intergenerational effects of sevoflurane. Notably, decitabine, administered to sires prior to their early-life exposure to sevoflurane, prevented the effects of neonatal exposure to sevoflurane in the exposed fathers and in their distant future unexposed offspring. The alleviating intergenerational effects of paternal treatment with decitabine suggest that similar initial mechanisms are involved in the mediation of the long-term somatic (neurobehavioral) and germ cell (as evident from F1 neurobehavioral abnormalities) effects of neonatal paternal exposure to sevoflurane.
DNA methylation, the transfer of a methyl group to the 5-position of the cytosine ring within CpG dinucleotide, is frequently associated with long-term transcriptional repression and has been linked to regulation of neurocognitive function, among many other biological effects.31 DNMT1 is selective to hemimethylated DNA and catalyzes the methylation of newly synthesized strands of DNA. DNMT3A/3B, on the other hand, are de novo DNMTs. Their DNA methylation activities are modulated by internal and external factors.31 DNMTs form covalent bond with decitabine because of decitabine’s structural similarity to cytosine. This property of decitabine underlies one of its main known effects: inhibition of DNA methylation. The covalent bond between decitabine and DNMTs precludes the DNA methylation activity of the latter.32 The alleviating effects of paternal neonatal treatment with decitabine suggest that paternal neonatal exposure to sevoflurane induces changes in DNA methylation, which may initiate mechanisms leading to neurobehavioral abnormalities in both generations. Although altered DNA methylation may contribute to neurobehavioral abnormalities in F0 and F1 rats, the decitabine-sensitive neurobehavioral abnormalities in the two generations were different. Thus, pretreatment with decitabine prevented sevoflurane-induced exacerbated corticosterone responses to stress in the exposed rats. However, such corticosterone responses to stress in the F1 male offspring of the exposed sires are normal3 and the same as those in the F1 male offspring of the unexposed parents. This suggests that altered DNA methylation is needed, but not sufficient, to mediate the adverse effects of neonatal parental exposure to sevoflurane, at least as it relates to abnormal functioning of the hypothalamic-pituitary-adrenal axis in F1 male offspring. Future studies will be needed to elucidate the relative roles of DNMT1, DNMT3A, DNMT3B, and KCC2 in the intergenerational effects of parental neonatal exposure to sevoflurane. The pretreatment with decitabine alleviated sevoflurane-induced neurobehavioral abnormalities in both generations of male rats, but did not affect anesthetic-induced changes in expression of the hypothalamic Dnmt1 in F0 males. This suggests a limited role of Dnmt1 in the intergenerational neurobehavioral effects of paternal exposure to sevoflurane.
Evidence suggests that the involvement of DNA methylation in the mediation of the developmental effects of general anesthetics is not limited to sevoflurane or hypermethylation. Wu et al. found that impaired behavior in rats neonatally exposed to isoflurane was accompanied by increased expression of DNMT1, hypermethylated Bdnf, and reduced Bdnf expression in the hippocampus.33 While Chastain-Potts et al. reported that dams neonatally exposed to sevoflurane had hypomethylated and overexpressed subiculum Arc and Junb genes, and their expression was also upregulated in the male offspring.34 Also, a study of the germ cell effects of obesity in humans found that multiple genes were either hypo- or hypermethylated in the spermatozoa of male patients just 1 week after bariatric surgery.35 Although this study was not specifically designed to investigate the epigenetic germ cell effects of anesthesia/surgery, the findings of epigenetic changes in the spermatozoa days after the surgery suggest that the anesthesia/surgery may contribute to such changes in humans. The large number of patients who require anesthesia underscore the need in specifically designed clinical studies to investigate the intergenerational effects of general anesthetics. Our findings in this study and previously published rodent studies that paternal exposure to sevoflurane can induce intergenerational impairments in PPI of the startle responses, on one hand, and a wide use of the PPI paradigms in studies in humans, on the other hand, may facilitate clinical studies of intergenerational effects of general anesthetics.
In conclusion, the findings of this study with decitabine along with our recently published findings of hypermethylated Kcc2 in paternal spermatozoa and in the brains of their male offspring suggest that early-life paternal exposure to sevoflurane-induced alterations in DNA methylation may contribute to initial mechanism(s) mediating the intergenerational effects of the anesthetic. Similar decitabine-sensitive changes in multiple genes can be involved in the mediation of neonatal sevoflurane exposure-induced neurobehavioral abnormalities in the exposed sires and in their future male offspring.
KEY POINTS.
Question: Does inhibition of DNA methyltransferases (DNMTs) prior to neonatal sevoflurane exposure in sires alleviate the anesthetic’s known adverse effects in the sires themselves and in their future offspring?
Finding: Pretreatment with the DNMT inhibitor decitabine protected sires neonatally exposed to sevoflurane and their unexposed male offspring from long-term neurobehavioral abnormalities otherwise induced by exposure to the anesthetic.
Meaning: These results suggest that sevoflurane-induced, decitabine-sensitive dysregulation of multiple genes, including Kcc2 and Dnmt genes, are involved in the mediation of the anesthetic-induced neurobehavioral abnormalities in both generations.
Acknowledgments
Financial disclosures: This work was supported by the National Institutes of Health, Bethesda, MD, USA (Nos. R01NS091542, R01NS091542-S to A.E.M.), the Escher Autism Fund (A.E.M.), the I. Heermann Anesthesia Foundation (L.S.J), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (N.G.).
GLOSSARY OF TERMS
- ANOVA
analysis of variance
- Bdnf
brain-derived neurotrophic factor
- CpG
cytosine-phosphate-guanine
- DNMT
DNA methyltransferase
- GABAAR
GABA type A receptor
- KCC2
K+-2Cl−
- MeCP2
methyl CpG binding protein 2
- NKCC1
Na+-K+-Cl−
- P
postnatal day
- PPI
prepulse inhibition
Footnotes
Conflicts of interest: None
Clinical trial number and registry URL: N/A
REFERENCES
- 1.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. [DOI] [PubMed] [Google Scholar]
- 2.Ju LS, Yang JJ, Morey TE, et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth. 2018;121:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju LS, Yang JJ, Xu N, et al. Intergenerational effects of sevoflurane in young adult rats. Anesthesiology. 2019;131:1092–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ju LS, Yang JJ, Gravenstein N, et al. Role of environmental stressors in determining the developmental outcome of neonatal anesthesia. Psychoneuroendocrinology. 2017;81:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Ju L, Jia M, et al. Subsequent maternal separation exacerbates neurobehavioral abnormalities in rats neonatally exposed to sevoflurane anesthesia. Neurosci Lett. 2017;661:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Ju L, Yang C, et al. Effects of combined brief etomidate anesthesia and postnatal stress on amygdala expression of Cl− cotransporters and corticotropin-releasing hormone and alcohol intake in adult rats. Neurosci Lett. 2018;685:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth. 2019;122:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera OH, Tesic V, Tat QL, Chastain S, Quillinan N, Jevtovic-Todorovic V. Sevoflurane-induced dysregulation of cation-chloride cotransporters NKCC1 and KCC2 in neonatal mouse brain. Mol Neurobiol. 2020;57:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. [DOI] [PubMed] [Google Scholar]
- 10.Merner N, Chandler M, Bourassa C, et al. Regulatory domain or CpG site variation in SLC12A5, encoding the chloride transporter KCC2, in human autism and schizophrenia. Front Cell Neurosci. 2015;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisella LI, Gaiarsa JL, Diabira D, et al. Impaired regulation of KCC2 phosphorylation leads to neuronal network dysfunction and neurodevelopmental pathology. Sci Signal. 2019;12(603). pii: eaay0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehorter N, Vinay L, Hammond C, Ben-Ari Y. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci. 2012;35:1846–1856. [DOI] [PubMed] [Google Scholar]
- 13.Miller S, Maguire J. Deficits in KCC2 and activation of the HPA axis lead to depression-like behavior following social defeat. Hormonal Studies. 2014;2:2. [Google Scholar]
- 14.Lemonnier E, Robin G, Degrez C, Tyzio R, Grandgeorge M, Ben-Ari Y. Treating Fragile X syndrome with the diuretic bumetanide: a case report. Acta Paediatr. 2013;102:e288–e290. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Kim J, Zhou L, et al. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci U S A. 2016;113:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X, Drotar J, Li K, et al. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci Transl Med. 2019;11:eaau0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju LS, Jia M, Sun J, et al. Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res. 2016;29:243–255. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher N, Azzopardi E, De Oliveira C, et al. Deciphering midbrain mechanisms underlying prepulse inhibition of startle. Prog Neurobiol. 2020;185:101734. [DOI] [PubMed] [Google Scholar]
- 19.Hantsoo L, Golden CEM, Kornfield S, Grillon C, Epperson CN. Startling differences: using the acoustic startle response to study sex differences and neurosteroids in affective disorders. Curr Psychiatry Rep. 2018;20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoji H, Miyakawa T. Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: a large-scale meta-analytic study. Mol Brain. 2018;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. [DOI] [PubMed] [Google Scholar]
- 22.Kohl S, Gruendler TO, Huys D, et al. Effects of deep brain stimulation on prepulse inhibition in obsessive-compulsive disorder. Transl Psychiatry. 2015;5:e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAlonan GM, Daly E, Kumari V, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. [DOI] [PubMed] [Google Scholar]
- 24.Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuhas J, Cordeiro L, Tassone F, et al. Brief report: sensorimotor gating in idiopathic autism and autism associated with fragile X syndrome. J Autism Dev Disord. 2011;41:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elle T, Alam M, Voigt C, Krauss JK, John N, Schwabe K. Deep brain stimulation of the thalamic centromedian-parafascicular nucleus improves behavioural and neuronal traits in a rat model of Tourette. Behav Brain Res. 2020;378:112251. [DOI] [PubMed] [Google Scholar]
- 27.Buse J, Beste C, Herrmann E, Roessner V. Neural correlates of altered sensorimotor gating in boys with Tourette Syndrome: a combined EMG/fMRI study. World J Biol Psychiatry. 2016;17:187–197. [DOI] [PubMed] [Google Scholar]
- 28.Ludewig S, Geyer MA, Ramseier M, Vollenweider FX, Rechsteiner E, Cattapan-Ludewig K. Information-processing deficits and cognitive dysfunction in panic disorder. J Psychiatry Neurosci. 2005;30:37–43. [PMC free article] [PubMed] [Google Scholar]
- 29.Pineles SL, Blumenthal TD, Curreri AJ, et al. Prepulse inhibition deficits in women with PTSD. Psychophysiology. 2016;53:1377–1385. [DOI] [PubMed] [Google Scholar]
- 30.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. [DOI] [PubMed] [Google Scholar]
- 31.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z, Li HQ, Liu F. DNA methyltransferase inhibitors and their therapeutic potential. Curr Top Med Chem. 2018;18:2448–2457. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Bie B, Naguib M. Epigenetic manipulation of brain-derived neurotrophic factor improves memory deficiency induced by neonatal anesthesia in rats. Anesthesiology. 2016;124:624–640. [DOI] [PubMed] [Google Scholar]
- 34.Chastain-Potts SE, Tesic V, Tat QL, Cabrera OH, Quillinan N, Jevtovic-Todorovic V. Sevoflurane exposure results in sex-specific transgenerational upregulation of target IEGs in the subiculum. Mol Neurobiol. 2020;57:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–378. [DOI] [PubMed] [Google Scholar]


