Abstract
Purpose of Review:
This review discusses novel immunotherapeutic approaches to treat Hodgkin lymphoma (HL), specifically PD-1 inhibitors and cellular immunotherapy.
Recent findings:
PD-1 inhibitors have shown promising results in the treatment of relapsed or refractory HL, leading to FDA approval of nivolumab and pembrolizumab, although complete remissions are rare. Chimeric antigen receptor T cells directed against CD30 have been investigated with preliminary clinical trials showing minimal toxicities and some responses in heavily pre-treated patients with HL.
Summary:
HL is unique as it consists of a small percentage of malignant cells (Hodgkin Reed Sternberg cells) surrounded by an inflammatory microenvironment which promotes tumor growth and suppresses immune responses, making it an ideal target for immunotherapeutic approaches, such as PD-1 inhibitors and cellular immunotherapy. Current research is focused on overcoming barriers to efficacy via rational combinations that overcome resistance to therapy.
Keywords: Hodgkin lymphoma, checkpoint inhibitors, nivolumab, pembrolizumab, cellular immunotherapy, chimeric antigen receptor T cells
INTRODUCTION
Hodgkin Lymphoma (HL) is one of the most common malignancies in young adults and in 2017, there will be an estimated 8,260 new cases of HL in the Unites States with 1,070 deaths from the disease(1). Although the majority of patients are cured with first line therapy, about 2% of patients are refractory to therapy and about 13% will relapse after initial treatment(2). Second line therapy for HL usually involves high dose chemotherapy and autologous stem cell transplant (ASCT). For patients who relapse after ASCT, allogeneic stem cell transplant (alloSCT) offers the best chance for a sustained remission(3). The prognosis for patients with relapsed or refractory disease is poor, especially if they fail second line therapy. In addition both standard frontline therapy and salvage therapy are associated with long-term morbidity and non-relapse mortality for survivors including risk of secondary malignancies(4, 5), heart failure and cardiovascular disease(6, 7), endocrinopathies, pulmonary disease, muscle atrophy, fatigue, and infertility(7). Meanwhile, elderly patients with HL may not be able to receive aggressive frontline and salvage therapies due to comorbidities and have a substantially worse prognosis than younger patients with this disease(8, 9). Novel treatment approaches for HL are needed, both to treat patients with refractory disease who are resistant to traditional approaches and to limit long-term treatment-related complications associated with first line and salvage therapies.
Classical HL is unique because only a small fraction (<1%) of the tumor contains malignant Hodgkin and Reed Sternberg (HRS) cells, with the surrounding tumor microenvironment consisting of polyclonal T cells as well as macrophages, B cells, eosinophils, plasma cells, neutrophils, and mast cells(10-12). Numerous evidence confirms that the HL microenvironment supports and stimulates the survival of HRS cells and is inadequate in eliminating them(10, 13). HRS cells uniquely express CD30, which make this surface antigen an excellent target for therapy(14). Brentuximab vedotin (BV), the antibody-drug conjugate directed to the protein CD30, expressed by HRS cells, was approved by the FDA in 2011 for the treatment of relapsed HL and has shown excellent promise in this population(15). Although BV has changed the landscape of HL therapy by providing a therapeutic option for patients with relapsed or refractory disease, it is not usually associated with sustained remission, with less than 10% of patients achieving a durable response(16). In addition, it requires prolonged administration and is associated with toxicities such as neuropathy(15). Thus, novel therapies are still needed for patients who do not respond to or who relapse post BV. Many of the therapies currently being investigated focus on taking advantage of the unique features of HRS cells and the HL microenvironment. This review will mainly concentrate on two potential ways to tackle the altered immune system in HL: checkpoint blockade, which acts on the inhibitory signals from the tumor, and adoptive transfer of T cells to enhance cell-based immune recognition of tumor cells (antigen specific ex vivo expanded T cells and chimeric antigen receptor T cells (CAR-T)).
CHECKPOINT BLOCKADE
Rationale
Checkpoint molecules, such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death-1 (PD-1), are key players in the physiological regulation of T cell activation and expansion. Inhibition of these pathways has been extensively investigated as potential target for treatment of various malignancies.
The binding of B7-1 and B7-2 molecules (also known as CD80 and CD86) on antigen presenting cells to the CD28 molecule expressed on the surface of T cells is a critical step for lymphocyte activation(17). The CTLA-4 molecule, which is also expressed by T cells, competes with CD28 for binding of B7-1 or B7-2 but leads, in contrast, to inhibition of T cell activation(18).
Another important inhibitory axis involves PD-1/PD-L1(19). The interaction of PD-1, which is expressed on T cells, with its ligands, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2), which are expressed on antigen presenting cells, also causes inhibition of T cell receptor signaling resulting in decreased antitumor immune responses while nurturing the survival of tumor cells(20).
The PD-1 pathway appears to be an important mechanism in the HL microenvironment. PD-1 expression is increased in tumor infiltrating lymphocytes as well as peripheral T cells in HL patients and may be one mechanism that contributes to the inhibitory HL microenvironment and inability of T cells to destroy HRS cells(21). In addition, increased expression of PD-1 on tumor-infiltrating lymphocytes in HL has been associated with decreased overall survival (OS) in patients independent of disease stage(22).
HRS cells consistently express high levels of PD-L1 and PD-L2(21, 23-26), further providing a rationale for the success of PD-1 inhibition in HL. In vitro studies confirmed that blockade of the PD-1 signaling cascade with anti-PD-1 antibodies restores the function of tumor infiltrating lymphocytes, suggesting that targeting this pathway should prove beneficial(21).
Expression of PD-L1 and PD-L2 on HRS cells is induced via amplification of the chromosomal region 9p24.1, where the genes encoding both PD-L1 and PD-L2 are located(23). In addition, the 9p24.1 amplification region includes the JAK2 locus, leading to increased JAK/STAT signaling further enhancing transcription of PD-L1(23).
Some patients with classical HL have normal 9p24.1 copy number, yet they still have increased PD-L1 expression. Another etiology for increased PD-L1 expression in classical HL is attributed to Epstein Barr Virus (EBV) infection in HRS cells(24), present in about 40% of patients with HL, with results varying across different population groups(27). In the cases of EBV+ HL, the EBV-associated latent membrane protein-1 (LMP-1) mediates the activation of the JAK-STAT and activator protein-1 pathways, leading to increased PD-L1 expression(24).
In addition to being expressed on HRS cells, PD-L1 can be found on tumor-infiltrating macrophages in the tumor microenvironment, further contributing to the ineffectiveness of T cells in eradicating HRS cells(26). This may be a contributing factor to previous reports describing an association between increased number of tumor associated macrophages and poor outcome in HL(12).
The anti-CTLA-4 monoclonal antibody, ipilimumab, was the first checkpoint inhibitor approved for cancer therapy, but there have been limited studies of this therapy in HL, largely due to its increased toxicity compared to PD-1 inhibitors. In addition, the high importance of the PD-1/PD-L1 axis in classical HL makes this pathway an excellent therapeutic target. Recently, two PD-1 inhibitors, nivolumab and pembrolizumab, have shown great success for treatment of relapsed or refractory HL and have been FDA approved for this indication.
Ipilimumab
There have been limited studies of ipilimumab, the CTLA-4 inhibitor, in HL. Ipilimumab was evaluated in patients with relapsed hematologic malignancies post alloSCT with the goal of increasing graft versus tumor effect. In a phase 1 study, 14 patients with relapsed HL post alloSCT were treated with a single dose of ipilimumab (with the option of redosing if progression occurred after initial response) with 2 patients showing a complete response (CR)(28). In a follow up study again looking at relapsed hematologic malignancies after alloSCT, ipilimumab was given for 4 doses with the option of maintenance therapy for patients with clinical benefit(29). In the 7 patients with HL included in this study, 1 patient had a partial response and 3 patients had stable disease lasting up to 1 year. Although the efficacy data of ipilumimab in HL is not impressive, it is important to consider that this was a particularly refractory population. Of the 28 patients treated, 4 patients experienced graft versus host disease (GVHD), which interestingly seems lower than that experienced in patients receiving PD-1 inhibitors post-alloSCT, although patients with a history of GVHD were excluded from this study. Thus, ipilimumab may be a preferable option in relapsed patients post alloSCT who are at high risk for GVHD.
Given the overall modest results with ipilimumab in HL, there has been greater interest in investigating PD-1 inhibition in this disease.
Nivolumab
Nivolumab is the first PD-1 inhibitor studied in HL(25). In a phase I study of nivolumab, 23 patients with relapsed or refractory disease received 3 mg/kg every 2 weeks until disease progression or CR or for a maximum of 2 years(25). This was a heavily pre-treated population, with 15 patients having received at least 4 lines of therapy, 18 patients having already been treated with BV, and 18 patients relapsing post ASCT. The overall response rate (ORR) was 87% with 4 CR and 16 partial responses (PR) and the progression free survival (PFS) was 86% at 6 months. Although the majority of patients did not achieve a CR, the responses were durable and in an updated report of this study, after a median follow up of 101 weeks, the median PFS had not been reached and the 1.5 year OS was 83%(32).
In general, the drug was well tolerated in this population with similar rates of adverse events as seen in solid tumor trials(25). The most common adverse events were rash (5 patients) and thrombocytopenia (4 patients). Immune-related adverse events, which have been seen in trials with checkpoint inhibitors, were observed infrequently and did not require treatment discontinuation.
In a phase II study, the ORR for 80 patients treated with nivolumab who had relapsed after BV and ASCT was 66% with 9% CR and 58% PR(33). The median PFS was 10 months with a 6 month PFS and OS of 77% and 99%, respectively. Given some responses seen after progression in solid tumor trials with nivolumab, 9 patients continued treatment after progression and, in 5 patients, there was reduction in tumor burden even after appearance of new lesions, suggesting that there may be some benefit in continuing this drug even after initial disease progression. Again, this drug was well tolerated with the most common drug related adverse events being fatigue (25%), infusion-related reactions (20%), and rash (16%).
The results of this study led to the accelerated approval of nivolumab by the FDA in May, 2016 for the treatment of classical HL which had relapsed or progressed after ASCT and BV.
Further follow up of the phase 2 study of nivolumab was reported at the American Society of Hematology meeting in 2016(34). At a median follow up of 15.4 months, 43 patients were still on therapy. Out of the 37 patients who discontinued therapy, the most common reasons were disease progression (19 patients), proceeding to alloSCT (7 patients) and adverse events (5 patients). The median duration of CR was not reached and the median duration of PR was 13.1 months. This data further supported the use of nivolumab in heavily pretreated patients and again showed that although the CR rate was not high, patients with a PR can achieve a durable response and benefit from this therapy.
Pembrolizumab
Pembrolizumab, another anti-PD1 antibody, has also demonstrated promising results in relapsed and refractory patients with classical HL. In the phase I study, 31 patients who had progressed after prior BV therapy received 10 mg/kg of pembrolizumab every 2 weeks(35). This was a heavily pretreated population in which 17 patients had received 5 or more prior lines of therapy and 22 patients had received prior ASCT. The ORR was 65% with 5 patients in CR and 15 patients in PR. After a median follow up of 24.9 months, the median PFS was 11.4 months and the median OS was not reached(36). Similarly to nivolumab, the medication was well tolerated with no grade 4 toxicities or treatment-related deaths(35).
The phase II study of pembrolizumab was divided into 3 cohorts(37). In the cohort of 69 patients with relapsed or refractory HL after ASCT who were also treated with BV, the ORR was 74% with 22% CR. In the cohort of 81 patients who were ineligible for ASCT due to chemoresistance and who failed to respond to BV, the ORR was 64% with 25% CR. In the cohort of 60 patients with relapsed/refractory HL after ASCT who were not treated with BV, the ORR was 70% with CR rate of 20%. Although the follow up for this study is too short to accurately portray duration of response, the 6 month PFS was 72.4%. Based on the results of these studies, pembolizumab was approved by the FDA in March, 2017 for treatment of patients with refractory HL or who have relapsed after 3 or more prior lines of therapy.
Biomarkers and PD-1 Inhibitors
In an effort to further investigate the mechanism of benefit of PD-1 inhibitors in HL as well as to explore features predictive of response to therapy, both PD-1 inhibitor clinical trials included biomarker assessments.
In the phase I trial of nivolumab, a subgroup of 10 patients with tumor samples available for analysis all had gain or amplification of PD-L1 and PD-L2 by FISH and increased expression of PDL-1 and PD-L2 on HRS cells(25). In addition, tumor cells were tested for phosphorylated STAT3 expression and all were found to be positive, signifying activation of the JAK/STAT pathway(25).
In the 45 patients with available tumor biopsy samples in the phase II trial of nivolumab, FISH analysis of HRS cells revealed alterations of the PD-L1 and PD-L2 loci in all patients(33). In addition, as in the previous study, all evaluated patients had positive staining of phosphorylated STAT3 in HRS cells, indicating active signaling of JAK/STAT. There was an association between the magnitude of 9p24.1 gain and level of PD-L1 protein expression on HRS cells. The PD-L1 “H score” was calculated by multiplying the percentage of HRS cells with positive staining by the average staining intensity and the results were divided into quartiles(38). The authors noted an association between best overall response and H score, with patients with higher PD-L1 expression on HRS cells appearing to be more responsive to therapy. Finally, patients with 9p24.1 amplification were more responsive to therapy than those with copy gain or polysomy. This is in contrast to a prior study, which found that amplification of 9p24.1 and increased PD-L1 expression in HRS cells correlated with a decreased PFS in patients treated with standard chemotherapy regimens(38). This further supports the rationale for assessing PD-L1 expression in patients with HL and prompts future studies to identify patients who would most benefit from immunotherapy approaches. As the majority of patients with 9p24.1 polysomy or PD-L1 expression in the first quartile achieved a partial response, clear cut offs for selecting candidate patients who should receive PD-1 inhibitors remain questionable.
The phase I study of pembrolizumab also evidenced a high level of PD-L expression in the tumor samples, with PD-L1 positivity in tumor cells for 15 of 16 patients and PD-L2 positivity of tumor cells in 9 of 10 patients assessed(36). There was a significant increase in the absolute number of T cells, CD4 and CD8 subsets, and NK cells, as well as significant upregulation of the IFN-γ induced signature via RNA profiling from pre-treatment to post-treatment in available samples, suggesting that PD-1 blockade stimulates the expansion of immune cells and the activation of the IFN-γ signaling pathway(36). However, these changes did not predict response. In the phase II study of pembrolizumab, clinical activity was seen even in the small group of patients with low levels of PD-L1 expression(37). Further studies are warranted to identify biomarkers in patients receiving checkpoint inhibitors and investigate factors associated with response.
Practical Questions about PD-1 Inhibitors
Thanks to the recent success and FDA approval of nivolumab and pembrolizumab in patients who had been refractory to other treatments, these medications will be more extensively used in patients with classical HL.
One remaining question is whether treatment with PD-1 inhibitors is safe for patients who relapse post alloSCT, an exclusion criteria in the above described trials. In a retrospective analysis of 20 patients with HL who relapsed after alloSCT and subsequently received nivolumab, the ORR was 95% and the median PFS was not reached at a median follow up of 370 days(39). These results are encouraging in a refractory patient population who lacks alternative treatment options. However, reactivation of GVHD remains a serious concern with PD-1 inhibitor therapy. In this study, 13 patients had previous history of acute GVHD post transplant, and GVHD occurred in 6 of these patients after nivolumab, resulting in 2 patient deaths. Given the remarkable response rates in this patient population and the limited treatment options, nivolumab should still be considered in patients post alloSCT, although we should be vigilant and closely monitor patients with a history of acute GVHD.
A second related pending problem is whether patients who proceed to alloSCT after PD-1 inhibition experience a higher rate of GVHD. Out of the 17 patients from the CheckMate nivolumab clinical trials who underwent alloSCT, 14 experienced acute GVHD(40). There were 2 patients with hyperacute GVHD occurring less than 2 weeks post transplant and 5 deaths due to GVHD. Although this is a small cohort, there are concerns that prior anti-PD-1 exposure could increase the risk of GVHD post-transplant and studies in larger patient populations would need to address this complication. Another continuing argument about PD-1 inhibitors pertains to the length of treatment. Patients on immunotherapy regimens, including checkpoint inhibitors, sometimes appear to initially experience progressive disease (tumor flare or pseudo-progression) despite attaining clinical benefits(41). Pseudo-progression is usually attributed to inflammation, edema and necrosis associated with immune cell infiltration. New response criteria have been introduced for patients on immunomodulatory therapy to aid in the dilemma of discriminating true progression from pseudoprogression, and account for possible delayed responses and/or pseudo-progression and to avoid premature discontinuation of treatment(41). However, it is unresolved if and at what point treatment can be discontinued in responding patients. In long term follow up studies of nivolumab, responses were maintained for more than 40 weeks in some patients who discontinued therapy, with one patient having a late progression but still responding and achieving a second CR after nivolumab was re-introduced(32). This argues for the potential safe discontinuation of nivolumab in some patients who respond to therapy without compromising efficacy, to allow a treatment-free period for these individuals. Regardless, only additional studies would answer the remaining queries about optimal duration of therapy in responding patients.
Future Directions
Although the introduction of PD-1 inhibitors has staged a new, relatively well tolerated treatment option for patients with refractory HL, unanswered questions leave room for improvements.
First of all, there is a proportion of patients refractory to checkpoint blockade, raising doubts about the etiology of this lack of response, as well as the potential benefits for combination therapies.
Secondly, this treatment is not curative and most patients do not achieve a sustained CR, and eventually progress. There has been interest in combination approaches that could lead to deeper and more sustained remissions. There are currently several clinical trials investigating the combination of nivolumab with various other therapies such as BV, ibrutinib, lenalidomide, and chemotherapy (Table 1). Given the promising results with the combination approach in melanoma(30), combined immune checkpoint blockade with ipilimumab and nivolumab has been explored in patients with lymphoma. In patients with relapsed or refractory HL, however, the combination of ipilimumab and nivolumab did not appear to have added benefit(31), although there is hope that other combination therapies will have a more synergistic effect. Preliminary results of the combination of BV and nivolumab in patients with relapsed or refractory HL were recently presented and this combination is well tolerated and has an excellent response rate although it is too early to comment on duration of response(42).
Table 1:
Current Checkpoint Inhibitor Trials for Classical Hodgkin Lymphoma
|
Clinicaltrials.gov Identifier |
Drug | Concept | Phase | Disease | Included Ages |
|---|---|---|---|---|---|
| Relapsed/Refractory | |||||
| NCT02603419 | Avelumab | PD-L1 antibody | Ib | cHL | Adults |
| NCT02684292 | Pembrolizumab vs BV | PD-1 antibody compared to anti-CD30 ADC | III | cHL | Adults |
| NCT02572167 | Nivolumab and BV | PD-1 antibody combined with anti-CD30 ADC | I/II | cHL | Adults |
| NCT02927769 | Nivolumab and BV (followed by BV and bendamustine in those with suboptimal response) | PD-1 antibody combined with anti-CD30 ADC after failure of first line therapy | II | cHL | Children and adults (5 years to 30 years) |
| NCT02940301 | Nivolumab and ibrutinib | PD-1 antibody combined with BTK inhibitor | II | cHL and NLPHL | Adults |
| NCT02665650 | Pembrolizumab and AFM13 | PD-1 antibody combined with bispecific anti-CD30/CD16A antibody | Ib | cHL | Adults |
| NCT03015896 | Nivolumab and lenalidomide | PD-1 antibody and immunomodulator | I/II | HL and NHL | Adults |
| NCT02875067 | Pembrolizumab and lenalidomide | PD-1 antibody and immunomodulator | I/II | HL and NHL | Adults |
| NCT03016871 | Nivolumab and ICE | PD-1 antibody and chemotherapy as second line therapy | II | cHL | Adults, children (15 years and older) |
| NCT03077828 | Pembrolizumab and ICE | PD-1 antibody and chemotherapy | II | cHL | Adults |
| NCT01896999 | Nivolumab, ipilimumab, BV | PD-1 antibody, CTLA-4 antibody and anti-CD30 ADC | I | cHL | Adults |
| Consolidation therapy after ASCT | |||||
| NCT02362997 | Pembrolizumab | PD-1 antibody | II | cHL, DLBCL, PTCL | Adults |
| NCT03057795 | Nivolumab and BV | PD-1 antibody and anti-CD30 ADC | II | cHL | Adults |
| First Line | |||||
| NCT03033914 | A(B)VD followed by nivolumab | Conventional chemotherapy followed by PD-1 antibody | I | cHL (high risk) | Adults (2nd cohort for patients 60 and older) |
| NCT03004833 | AVD and nivolumab | PD-1 antibody and conventional chemotherapy | II | cHL (early stage unfavorable) | Adults (18 to 60 years) |
| NCT02758717 | Nivolumab and BV | PD-1 antibody and anti-CD30 ADC | II | cHL (unable to receive standard chemotherapy) | Children, adults (focus on age 60 and older) |
cHL = classical Hodgkin lymphoma, BV = brentuximab vedotin, ADC = antibody drug conjugate, HL= Hodgkin lymphoma, NHL = Non-Hodgkin lymphoma, NLPHL = nodular lymphocyte predominant Hodgkin lymphoma, ICE = ifosfamide, carboplatin, etoposide, ASCT = autologous stem cell transplant, A(B)VD = doxorubicin, (bleomycin), vinblastine, dacarbazine
As PD-1 inhibitors have gained FDA approval for relapsed and refractory HL, there is interest in where these novel therapies will fit in the current treatment paradigm for HL. If the combination approaches described above show efficacy and durable responses, these treatments will likely move up in the treatment order. In addition, although the current chemotherapy approaches are successful in most patients with HL, there are groups of patients that would benefit from alternative first line options. These include elderly patients who poorly tolerate chemotherapy, have limited treatment options at diagnosis, and need alternative therapeutic strategies with a more tolerable side effect profile. Currently, a clinical trial based on the combination of nivolumab and BV in the frontline setting for elderly patients not eligible for standard first line therapy is underway. Incorporating PD-1 inhibitors in earlier lines of therapy is speculated to produce greater anti-tumor responses, the rationale being that an immune system naive to multiple lines of therapy would be healthier and more manageable to this “reinvigoration”-based strategy(19, 43). There are currently clinical trials investigating the combination of nivolumab with conventional chemotherapy in the treatment of newly diagnosed high risk HL. The results of the above studies will soon elucidate the role of PD-1 inhibitors in frontline therapy. Finally, novel agents are being developed and tested that could, in combination with checkpoint inhibitors, stimulate improved and more durable responses.
CELLULAR IMMUNOTHERAPY
Rationale
Immune-mediated elimination of cancer cells requires the coordinated effort of effector cells, which are responsible for the lysis and removal of transformed cells. T cells are key players in this army and advances in the mechanisms of tumor recognition and elimination, and our ability to ex vivo expand large quantities of these cells and improve their function by gene engineering have significantly fostered novel accomplishments in this arena. As evidenced by the response with PD-1 inhibitors, HL is undoubtedly susceptible to T-cell immune-mediated elimination. This is facilitated by endogenous T cells targeting tumor antigens, which for HL ranges from tumor specific antigens (TSA), like the EBV-associated ones, Cancer Testis Antigens (CTA), like MAGE or PRAME, to Tumor associated antigens (TAA), such as the CD30 molecule(44).
Antigen Specific T Cells
TSA, which arise in cancer cells, are often derived from oncogenic viral proteins or from non-synonymous somatic mutations, and have the unique advantage of being absent from normal cells. In HL, EBV associated antigens are an example of targetable TSA. About 40% of patients with HL harbor EBV in HRS cells(24). As a consequence, these HRS cells express EBV associated LMP 1 and 2 proteins, which, upon processing and presentation in the context of the appropriate MHC provide epitopes for targeting by cytotoxic T lymphocytes (CTL)(45). However, LMP1/2 proteins are subdominant antigens and the frequency of circulating LMP1/2 specific CTLs is negligible, which accounts for HRS cells being neglected by the immune system. To overcome the limited frequency of these circulating CTLs in patients, the adoptive transfer of ex vivo reactivated polyclonal CTLs targeting the whole arrays of EBV-antigens (EBNAs and LMPs) has been tested in relapsed EBV+ HL, resulting in CR in 5 of 14 patients as well as 1 PR and 5 patients with stable disease(46). To maximize the potential of targeting HRS cells, LMP2 and LMP1/2-CTLs were ex vivo enriched in the infused population(47). These strategies have resulted in sustained remissions for patients with HL in remission at time of T cell infusion and prolonged clinical responses in patients with disease(48). While these therapies have remarkable potential for patients with EBV+ HL, alternative targets are desirable for patients with EBV− HL.
CTA are also absent on normal cells except for reproductive tissues, so are potential ideal targetable antigens. Several of these proteins are expressed by HRS cells, including MAGE, NY-ESO-1, PRAME, SSX, and survivin. By expanding the breadth of targeted antigens, multi-tumor associated antigen specific T cell lines can be generated ex vivo for adoptive transfer to make it more difficult for tumors to evade immune attack(49). This approach has been validated for HL in a study where 3 of 7 patients with active disease at time of treatment achieved CR (49). Targeting a range of tumor associated antigens appears to be safe and could be beneficial for patients at high risk of relapse or for those with relapsed or refractory disease. However, limitations remain including the ex vivo procedure itself, the potential for generating toxicities, and immune-tolerance mechanisms.
CAR-T Cells
Failure of adoptively transferred antigen specific T cells or endogenous T cells, even reinvigorated by checkpoint inhibitors, to eliminate transformed cells may be related to the deficient and/or defective antigen processing and presentation by classical MHC molecules. Deletions in the β2-microglobulin molecules are frequent in classical HL and reported to be associated with inferior outcomes independent of 9p24.1 status(50). T cells engendered with CAR appear specifically suited to overcome this issue. CAR-T cells consist of patient-generated cytotoxic T cells that are engineered to express an artificial receptor molecule created by fusing the single chain variable fragment from an antibody with the intracellular component of the T cell receptor machinery. Through this novel chimeric molecule, T cells can recognize a specific antigen expressed on the surface of tumor cells in an MHC independent fashion(51). The inclusion of a costimulatory endodomain in the CAR molecule provides improved signal and function even when tumor cells lack expression of costimulatory molecules. Clinical antitumor activity of CAR-T cells against the CD19 molecule has been validated, with remarkable efficacy in treating B-cell acute lymphoblastic leukemia and lymphomas(52, 53).
As stated above, HRS cells are an excellent target for antibody directed therapy, since the CD30 antigen is expressed on the surface of virtually all HRS cells and only in small subsets on activated lymphocytes(54), suggesting little potential for off tumor on target toxicity. BV, the antibody drug conjugate targeting CD30, has had excellent activity with a good safety profile in patients with relapsed or refractory HL(55, 56). However, although the responses in some patients are durable, the majority of patients eventually progress on therapy. CAR-T cells targeted against CD30 should provide potential advantages in terms of persistence(57, 58) and active tumor penetration(58, 59).
Two phase I clinical trials investigating CAR-T cells targeted against CD30 have been reported. In the first published trial, the antiCD30-CAR contained a 41BB costimulatory endodomain and was delivered into T cells via a lentiviral vector(60). Eighteen patients with relapsed or refractory CD30+ lymphoma (17 with HL, 1 with anaplastic large cell lymphoma) received CAR-T cells preceded by conditioning with 1 of 3 regimens, which all included cyclophosphamide. In this study, 5 patients had received BV and 9 patients had received prior ASCT. The treatment was overall well tolerated with no instances of cytokine release syndrome (CRS). Out of 18 patients treated, 7 had PR and 6 had stable disease with a median PFS of 6 months. In addition, among the 5 patients who received a second CAR-T cell infusion, 3 patients maintained a PR and 1 patient maintained stable disease, while one patient progressed after having stable disease. There was no significant difference in response based on the different conditioning regimens given and the investigators found that in general, lymph node disease appeared to respond better than extranodal disease, with an especially poor response in pulmonary lesions. CAR-T cells, which were delivered over a 3 day course, produced CAR specific signal detectable in the circulation by polymerase chain reaction (PCR) which peaked between 3 and 9 days post infusion. In addition, they found increased number of CAR transgene copies in biopsied tissue from tumor sites suggesting that CAR-T cells can traffic to tumor sites. Another dose escalation trial of CD30-CAR-T cells has been conducted in patients with relapsed CD30+ HL (7 patients) and NHL (2 with CD30+ T cell lymphoma)(61). Eight of the patients had previously received BV and ASCT(62). In contrast to the other trial, this specific CAR contained the CD28 endodomain, was delivered into T cells with a retroviral vector(62) and adoptive transfer was not preceded by lymphodepletion. There were no significant adverse events noted in this trial including no evidence of CRS. CAR-T cell expansion peaked at 1 week after infusion and was documented both by PCR and flow cytometry. Responses were observed in 3 patients including 2 CR, while 3 patients experienced stable disease and the remaining 3 progressed. Although some responses with minimal toxicities were demonstrated in patients with refractory disease who may have progressed on BV, these two studies highlight the need for more trials based on CAR in this disease There are currently several open trials investigating anti-CD30 directed CAR-T cell therapy in patients with HL and one trial using CAR.CD19 for HL, which will provide further information about safety and efficacy (Table 2).
Table 2:
Current CAR-T Trials for Classical Hodgkin Lymphoma
|
Clinicaltrials.gov Identifier |
Target | Conditioning Regimen |
Disease | Main Inclusion Criteria |
Included Ages |
Location |
|---|---|---|---|---|---|---|
| NCT02624258 | CD19 RNA redirected CAR-T | Cy | HL | No available curative treatment options | 18-24 years | University of Pennsylvania |
| NCT02259556 | CD30.CAR-T | Cy/flu | CD30+ HL and NHL | Relapse after ASCT or refractory to 2 multidrug regimens; newly diagnosed and unable to receive standard chemotherapy | 16-80 years | Chinese PLA General Hospital |
| NCT02690545 | CD30.CAR-T | Bendamustine | CD30+ HL and NHL | Failed at least 1 salvage regimen | 18 years and older | University of North Carolina |
| NCT02917083 | CD30.CAR-T | Cy/flu | CD30+ HL and NHL | Relapsed/refractory or unable to receive standard therapy | Children and adults | Baylor College of Medicine |
| NCT03049449 | CD30.CAR-T | Cy/flu | CD30+ HL and NHL | Relapsed after 2 prior lines of therapy including ASCT or after 3 prior lines of therapy | 18-73 years | National Cancer Institute |
| NCT02274584 | CD30.CAR-T with self-withdrawal mechanism (FKBP-iCasp9) | CD30+ HL and NHL | Relapsed/refractory and not eligible for or relapsed post ASCT | 18 years and older | Peking University and University of Florida | |
| NCT02663297 | CD30.CAR-T consolidation after ASCT | ASCT | CD30+ HL and NHL | Recurrent disease eligible for ASCT considered at high risk for relapse | 3 years and older | University of North Carolina |
CAR-T = chimeric antigen receptor T-cell, Cy = cyclophosphamide, flu = fludarabine, HL = Hodgkin lymphoma, NHL = Non-Hodgkin lymphoma, ASCT = autologous stem cell transplant
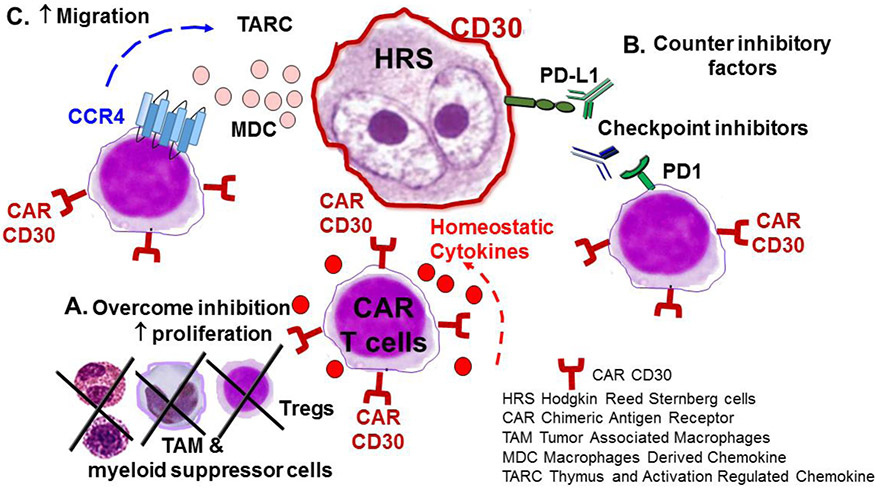
Approaches to Improve Efficacy of CD30.CAR-T Cells (Figure 1)
Figure 1: Improving Efficacy of CD30-redirected CAR-T Cells in HL.
A. Lymphodepletion leads to decreased regulatory T cells and disruption of the inhibitory tumor environment. In addition, lymphodepletion increases levels of homeostatic cytokines such as IL-15 and Il-7 which stimulates CAR-T cell expansion.
B. PD-L1, which is expressed on HRS cells, interacts with PD-1, which is found on T cells including a subset of CAR-T cells, leading to inhibition of T cell receptor signaling. Antibodies to PD-1 will counter these inhibitory factors leading to improved expansion and persistence of CAR-T cells as well as increased activity of endogenous cytotoxic T cells.
C. HRS cells produce the chemokines TARC and MDC which attract Th2 cells and regulatory T cells that express CCR4. Further engineering CAR T cells to express the CCR4 molecule will improve their migration to tumor cells.
The most logical strategy to improve the performance of T cells in HL should be sought in its microenvironment, which consists of a multitude of suppressive cell including T helper cells (Th2), regulatory T cells (Tregs), TAM and myeloid derived suppressor cells(63, 64). One method of consistently disrupting these vicious surroundings is via lymphodepletion. While cyclophosphamide and fludarabine-based regimens in part fulfil this task for CAR.CD19-based trials(65), the ideal protocol is yet to be identified for HL(60) and may require different drug combinations depending on the specific disease. ASCT seems particularly versatile, as it leads to production of high levels of homeostatic cytokines, such as IL-7 and IL-15, to sustain CAR-T cell expansion in the absence of competing endogenous lymphoid cells(66). There is currently a clinical trial investigating CD30.CAR-T cell therapy as consolidation after ASCT in high risk patients with HL as well as CD30+ NHL (Table 2).
One rational approach for HL is to combine CAR-T cells with checkpoint inhibitors since they have shown safety and some efficacy in HL as single agents. As described above, checkpoint inhibitors have impressive single agent activity although CRs are rare and responses are usually not durable. The HL microenvironment and characteristics of HRS cells, including PD-L1 expression, make them an excellent target for checkpoint inhibition. In addition, a subset of CAR-T cells retain expression of PD1(62) and/or will upregulate PD1 expression upon engagement and thus activation at the tumor site, making them sensitive to its ligand-mediated inhibition. Therefore, combination of checkpoint inhibition with CAR-T cell therapy is expected to improve expansion and persistence of CAR-T cells, as well as reinvigorate endogenous tumor infiltrating cytotoxic T cells, leading to improved anti-tumor activity. CAR-T cells could augment the efficacy of checkpoint inhibitors by leading to targeted killing of tumor cells in an MHC-unrestricted fashion. Several pre-clinical studies have demonstrated that combination with PD-1 blockade can improve CAR-T cell function(67, 68). Recently, a case report and case series have shown improved CAR-T cell expansion and persistence and clinical responses in patients who received pembrolizumab after failing to respond to CD19-directed CAR-T cells (69), suggesting that the combination of CD30-directed CAR-T cells and PD-1 inhibitors in HL could be similarly safe and feasible. Considering the safety of CAR-Ts, studying synergies in clinical trials represents a rational step forward.
Another potential improvement to CD30.CAR-T cells can be accomplished through the enhanced trafficking of these cells to the tumor site. HRS cells produce the chemokines thymus- and activation-regulated chemokine/CC chemokine ligand 17 (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22) to attract Th2 cells and Tregs that express their cognate (70)receptor CCR4(71, 72). However, CD8+ effector T cells, which do not express CCR4, do not respond to these chemokines and are indeed less commonly found within HL tumors. As a consequence, the HL microenvironment provides not only an inhibitory barrier (by recruiting cells producing inhibitory molecules, like Tregs, TAM, etc) but also a physical barricade to T cells. Forced expression of CCR4 by effector T cells increased their migration to HL cells both in in vitro and in vivo preclinical models(73). The addition of CCR4 expression to CD30.CAR-T cells thus represent the next rational manipulation to improve the efficacy of this promising approach.
Other CAR Targets for HL
There has been interest in developing other targets for cellular therapy in HL. As described above, the immunosuppressive HL microenvironment presents a challenge for therapy. TAM are clearly playing an important role in stimulating tumor growth and inhibiting immune responses against the tumor. In a study of diagnostic biopsies in HL patients, an increased number of TAM was associated with shorter survival(12). In addition to exploring drugs that directly target TAM(74), novel therapies attempt to achieve dual targeting, ideally focusing on an antigen expressed on both HRS cells and TAM(75, 76). CD123 is one such molecule, as it is expressed on HRS in 50-60% of patients with HL as well as on several immune cells in the HL microenvironment, including TAM(77). In in vitro studies, CD123.CAR-T cells were able to recognize and kill HL cells as well as TAM(75, 76). Anti-CD123 CAR-T cells also demonstrated anti-tumor activity in a xenograft model of HL(78). As CD123.CAR-T cells can cause myelosuppression(78), this therapy would likely be explored in the setting of rescue ASCT(76).
CONCLUSIONS
Although HL has a high cure rate, the treatment of relapsed and refractory disease remains a challenge. Recently, the emphasis has been on taking advantage of the unique microenvironment and characteristics of HRS cells. PD-1 inhibitors have shown promising results, with manageable toxicities in heavily pretreated patients. Some patients remain resistant to this therapy and the majority of patients will eventually progress. To counteract resistance partially due to the multifactorial immunosuppressive tumor environment, current clinical trials are focusing on combination therapies to achieve prolonged remissions.
CAR-T cells have shown promise in other hematologic malignancies and CD30.CAR-T cells are now being investigated in HL patients. Although these therapies show some efficacy in certain patients with heavily pre-treated disease, responses are still suboptimal. Future studies with CAR-T cell therapy are warranted and should focus on overcoming barriers to efficacy, by improving migration to tumor sites, countering inhibitory factors, and disrupting the tumor microenvironment.
Footnotes
Conflict of Interest
Natalie Grover and Barbara Savoldo each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by any of the authors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Glimelius I, Ekberg S, Jerkeman M, Chang ET, Bjorkholm M, Andersson TM, et al. Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992-2009-trends in cure proportions by clinical characteristics. American journal of hematology. 2015;90(12):1128–34. [DOI] [PubMed] [Google Scholar]
- 3.Sarina B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–7. [DOI] [PubMed] [Google Scholar]
- 4.Sud A, Thomsen H, Sundquist K, Houlston RS, Hemminki K. Risk of Second Cancer in Hodgkin Lymphoma Survivors and Influence of Family History. Journal of Clinical Oncology.0(0):JCO.2016.70.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaapveld M, Aleman BM, van Eggermond AM, Janus CP, Krol AD, van der Maazen RW, et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin's Lymphoma. The New England journal of medicine. 2015;373(26):2499–511. [DOI] [PubMed] [Google Scholar]
- 6.van Nimwegen FA, Ntentas G, Darby SC, Schaapveld M, Hauptmann M, Lugtenburg PJ, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng AK, van Leeuwen FE. Hodgkin lymphoma: Late effects of treatment and guidelines for surveillance. Semin Hematol. 2016;53(3):209–15. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkholm M, Svedmyr E, Sjoberg J. How we treat elderly patients with Hodgkin lymphoma. Curr Opin Oncol. 2011;23(5):421–8. [DOI] [PubMed] [Google Scholar]
- 9.Evens AM, Helenowski I, Ramsdale E, Nabhan C, Karmali R, Hanson B, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692–5. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Sattarzadeh A, Diepstra A, Visser L, van den Berg A. The microenvironment in classical Hodgkin lymphoma: an actively shaped and essential tumor component. Seminars in cancer biology. 2014;24:15–22. [DOI] [PubMed] [Google Scholar]
- 11.Harris NL. Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol. 1999;36(3):220–32. [PubMed] [Google Scholar]
- 12.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362(10):875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glimelius I, Diepstra A. Novel treatment concepts in Hodgkin lymphoma. Journal of internal medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 14.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14. [PubMed] [Google Scholar]
- 15.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(18):2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews Immunology. 2013;13(4):227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jezersek Novakovic B. Checkpoint inhibitors in Hodgkin's lymphoma. Eur J Haematol. 2016;96(4):335–43. [DOI] [PubMed] [Google Scholar]
- 20.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8(328):328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–4. [DOI] [PubMed] [Google Scholar]
- 22.Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Human pathology. 2009;40(12):1715–22. [DOI] [PubMed] [Google Scholar]
- 23.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(6):1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372(4):311–9.** This is the phase I study of nivolumab that established the safety and efficacy of PD-1 inhibitors in HL.
- 26.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(13):3462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international data. International journal of cancer Journal international du cancer. 1997;70(4):375–82. [DOI] [PubMed] [Google Scholar]
- 28.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. The New England journal of medicine. 2016;375(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansell S, Gutierrez ME, Shipp MA, Gladstone D, Moskowitz A, Borello I, et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039). Blood. 2016;128(22):183-. [Google Scholar]
- 32.Ansell S, Armand P, Timmerman JM, Shipp MA, Bradley Garelik MB, Zhu L, et al. Nivolumab in Patients (Pts) with Relapsed or Refractory Classical Hodgkin Lymphoma (R/R cHL): Clinical Outcomes from Extended Follow-up of a Phase 1 Study (CA209-039). Blood. 2015;126(23):583-. [Google Scholar]
- 33.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94.** This is the pivotal phase 2 study of nivoulmab in patients with cHL which led to its FDA approval.
- 34.Timmerman JM, Engert A, Younes A, Santoro A, Armand P, Fanale MA, et al. Checkmate 205 update with minimum 12-month follow up: A phase 2 study of nivolumab in patients with relapsed/refractory classical Hodgkin lymphoma. Am Soc Hematology; 2016. [Google Scholar]
- 35.Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016.** This article presents results of the phase Ib study of pembrolizumab in patients with cHL who progressed on or after treatment with BV.
- 36.Armand P, Shipp MA, Ribrag V, Michot J-M, Zinzani PL, Kuruvilla J, et al. Pembrolizumab in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Long-Term Efficacy from the Phase 1b Keynote-013 Study. Am Soc Hematology; 2016. [Google Scholar]
- 37.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of Clinical Oncology. 2017;35(19):2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(23):2690–7.*This article presents data on PD-L1/PD-L2 expression in HL and association with prognosis, supporting a rationale for PD-1 inhibition.
- 39.Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin's lymphoma. Blood. 2017. [DOI] [PubMed] [Google Scholar]
- 40.Armand P, Zinzani PL, Collins GP, Cohen JB, Halwani AS, Carlo-Stella C, et al. Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after Treatment with Nivolumab for Relapsed/Refractory Hodgkin Lymphoma. Am Soc Hematology; 2016. [Google Scholar]
- 41.Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–96.*This introduces new response criteria when evaluating lymphoma patients on immunotherapy.
- 42.Diefenbach CS, Hong F, David KA, Cohen J, Robertson M, Advani R, et al. Title: A Phase I Study with an Expansion Cohort of the Combination of Ipilimumab and Nivolumab and Brentuximab Vedotin in Patients with Relapsed/Refractory Hodgkin Lymphoma: A Trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E). Blood. 2016;128(22):1106-. [Google Scholar]
- 43.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125(22):3393–400. [DOI] [PubMed] [Google Scholar]
- 44.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nature reviews Cancer. 2017;17(4):209–22.*This is an excellent review on targeting neoantigens in cancer therapy.
- 45.Chapman AL, Rickinson AB, Thomas WA, Jarrett RF, Crocker J, Lee SP. Epstein-Barr virus-specific cytotoxic T lymphocyte responses in the blood and tumor site of Hodgkin's disease patients: implications for a T-cell-based therapy. Cancer research. 2001;61(16):6219–26. [PubMed] [Google Scholar]
- 46.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. The Journal of experimental medicine. 2004;200(12):1623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):798–808.*This article presents promising data on EBV-specific CTLs in lymphoma patients.
- 49.Leen A, Tzannou I, Bilgi M, Liu H, Vera JF, Gerdemann U, et al. Immunotherapy for Lymphoma Using T Cells Targeting Multiple Tumor Associated Antigens. Blood. 2015;126(23):186-. [Google Scholar]
- 50.Roemer MG, Advani RH, Redd RA, Pinkus GS, Natkunam Y, Ligon AH, et al. Classical Hodgkin Lymphoma with Reduced beta2M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer immunology research. 2016;4(11):910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos CA, Heslop HE, Brenner MK. CAR-T Cell Therapy for Lymphoma. Annual review of medicine. 2016;67:165–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuster SJ, Svoboda J, Dwivedy Nasta S, Porter DL, Chong EA, Landsburg DJ, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood. 2015;126(23):183-. [Google Scholar]
- 53.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer research. 1998;58(6):1116–9. [PubMed] [Google Scholar]
- 55.Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(1):248–55. [DOI] [PubMed] [Google Scholar]
- 57.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–55. [PubMed] [Google Scholar]
- 59.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016.*This is one of the first published trials of CD30.CAR-T therapy.
- 61.Ramos CA, Ballard B, Liu E, Dakhova O, Mei Z, Liu H, et al. Chimeric T Cells for Therapy of CD30+ Hodgkin and Non-Hodgkin Lymphomas. Blood. 2015;126(23):185-.26024876*This is one of the first published trials of CD30.CAR-T therapy.
- 62.Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and Immunological Responses in Patients Receving T Lymphocytes Targeting the CD30 antigen. Under revision 2017.
- 63.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–62. [DOI] [PubMed] [Google Scholar]
- 64.Barath S, Aleksza M, Keresztes K, Toth J, Sipka S, Szegedi G, et al. Immunoregulatory T cells in the peripheral blood of patients with Hodgkin's lymphoma. Acta Haematologica. 2006;116(3):181–5. [DOI] [PubMed] [Google Scholar]
- 65.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(6):540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends in immunology. 2005;26(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.John LB, Devaud C, Duong CPM, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clinical Cancer Research. 2013;19(20):5636–46.*This article provides pre-clinical support for the synergistic effect of the combination of PD-1 inhibitors and CAR-T cell therapy.
- 68.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. The Journal of Clinical Investigation. 2016;126(8):3130–44.*This article provides pre-clinical support for combining PD-1 inhibitors with CAR-T cell therapy and provides insight on overcoming resistance to CAR-T therapy.
- 69.Maude SL, Hucks GE, Seif AE, Talekar MK, Teachey DT, Baniewicz D, et al. The effect of pembrolizumab in combination with CD19-targeted chimeric antigen receptor (CAR) T cells in relapsed acute lymphoblastic leukemia (ALL). American Society of Clinical Oncology; 2017. [Google Scholar]
- 70.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. The American journal of pathology. 1999;154(6):1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer research. 2006;66(11):5716–22. [DOI] [PubMed] [Google Scholar]
- 73.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, et al. An Open-Label, Multicenter, Phase I/II Study of JNJ-40346527, a CSF-1R Inhibitor, in Patients with Relapsed or Refractory Hodgkin Lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(8):1843–50. [DOI] [PubMed] [Google Scholar]
- 75.Ruella M, Kenderian SS, Shestova O, Chen T, Scholler J, Wasik MA, et al. Novel Chimeric Antigen Receptor T Cells for the Treatment of Hodgkin Lymphoma. Blood. 2014;124(21):806-. [Google Scholar]
- 76.Ruella M, Klichinsky M, Kenderian SS, Shestova O, Ziober A, Feldman MD, et al. Overcoming the Immunosuppressive Tumor Microenvironment of Hodgkin Lymphoma Using Chimeric Antigen Receptor T Cells. Am Soc Hematology; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fromm JR. Flow cytometric analysis of CD123 is useful for immunophenotyping classical Hodgkin lymphoma. Cytometry Part B, Clinical cytometry. 2011;80(2):91–9. [DOI] [PubMed] [Google Scholar]
- 78.Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, et al. Anti-CD123 Chimeric Antigen Receptor T Cells (CART-123) Provide A Novel Myeloablative Conditioning Regimen That Eradicates Human Acute Myeloid Leukemia In Preclinical Models. Blood. 2013;122(21):143-.23690447 [Google Scholar]