This cohort study evaluates changes in patient characteristics, wait list outcomes, and posttransplant outcomes after the heart allocation policy change in heart transplant.
Key Points
Question
What are the trends in characteristics and outcomes of adult heart transplant after implementation of the new heart allocation policy on October 18, 2018?
Findings
In this cohort study of 15 631 patients, with the heart allocation policy change, higher-risk recipients are now undergoing transplant, with higher rates of bridging with temporary mechanical circulatory support. Wait list outcomes have improved, including reductions in wait list mortality and increases in rates of transplant, and posttransplant 1-year survival has decreased from 92.1% to 87.5%.
Meaning
Although adult heart transplant wait list outcomes have improved, posttransplant survival has decreased.
Abstract
Importance
The US heart allocation policy was changed on October 18, 2018. The association of this change with recipient and donor selection and outcomes remains to be elucidated.
Objective
To evaluate changes in patient characteristics, wait list outcomes, and posttransplant outcomes after the recent allocation policy change in heart transplant.
Design, Setting, and Participants
In this cohort study, all 15 631 adults undergoing heart transplants, excluding multiorgan transplants, in the US as identified by the United Network for Organ Sharing multicenter, national registry were reviewed. Patients were stratified according to prepolicy change (October 1, 2015, to October 1, 2018) and postpolicy change (October 18, 2018 or after). Follow-up data were available through March 31, 2020.
Exposures
Heart transplants after the policy change.
Main Outcomes and Measures
Competing risk regression for wait list outcomes was performed. Posttransplant survival was compared using the Kaplan-Meier method, and risk adjustment was performed using multivariable Cox proportional hazards regression analysis.
Results
In this cohort study, of the 15 631 patients undergoing transplant, 10 671 (mean [SD] age, 53.1 [12.7] years; 7823 [73.3%] male) were wait listed before and 4960 (mean [SD] age, 52.7 [13.0] years; 3610 [72.8%] male) were wait listed after the policy change. Competing risk regression demonstrated reduced likelihood of mortality or deterioration (subhazard ratio [SHR], 0.60; 95% CI, 0.52-0.69; P < .001), increased likelihood of transplant (SHR, 1.38; 95% CI, 1.32-1.45; P < .001), and reduced likelihood of recovery (SHR, 0.54; 95% CI, 0.40-0.73; P < .001) for wait listed patients after the policy change. A total of 6078 patients underwent transplant before and 2801 after the policy change. Notable changes after the policy change included higher frequency of bridging with temporary mechanical circulatory support and lower frequency of bridging with durable left ventricular assist devices. Posttransplant survival was reduced after the policy change (1-year: 92.1% vs 87.5%; log-rank P < .001), a finding that persisted after risk adjustment (HR, 1.29; 95% CI, 1.07-1.55; P = .008).
Conclusions and Relevance
Substantial changes have occurred in adult heart transplant in the US after the policy change in October 2018. Wait list outcomes have improved, although posttransplant survival has decreased. These data confirm findings from earlier preliminary analyses and demonstrate that these trends have persisted to 1-year follow-up, underscoring the importance of continued reevaluation of the new heart allocation policy.
Introduction
Heart allocation policy for adult heart transplant in the US was changed by the Organ Procurement and Transplantation Network effective October 18, 2018.1 Namely, the existing 3-tier system was converted to a 6-tier system to provide more granular separation of wait listed patients by clinical condition and urgency of transplant. For example, patients receiving extracorporeal membrane oxygenation (ECMO) were given the highest priority, whereas stable patients with durable left ventricular assist devices (LVADs) were given a lower priority.1 The latter change was implemented because of mounting evidence of improving outcomes of durable LVAD support such that a 2-year survival of 80% could be achieved with contemporary devices.2 The intended impact of changing heart allocation policy in this manner was to reduce wait list mortality and provide broader sharing of donor organs. The aim of this study was to evaluate changes in patient characteristics, wait list outcomes, and posttransplant outcomes in adult heart transplant after the heart allocation policy change.
Methods
Study Cohort
In this cohort study, all 15 631 heart transplants performed in the US were identified using the United Network for Organ Sharing (UNOS) registry. The analysis was limited to adults 18 years or older. Multiorgan transplants, including simultaneous heart-kidney, heart-lung, heart-liver, and other combined heart and other organ transplants, were excluded from analysis. Patients were stratified into prepolicy (October 1, 2015, to October 1, 2018) and postpolicy (October 18, 2018, or after) groups. Follow-up data were available through March 31, 2020. This study was granted exempt status by the institutional review board at the University of Pittsburgh because it was based on data that are publicly available and deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Wait List Outcomes
For the wait list outcomes analysis, patients wait listed before the policy change were censored on October 17, 2018, to limit confounding that would occur in patients who were wait listed before the policy change and remained wait listed after the policy change. Baseline characteristics during the time of wait list registration were compared between eras. Competing risk regression analysis was performed, and subhazard ratios (SHRs) with 95% CIs were reported. Competing outcomes on the wait list included wait list mortality or clinical deterioration with subsequent removal from the wait list, transplant, or recovery with removal from the wait list.
Transplant Outcomes
For the analysis of transplant outcomes, patients who were wait listed and underwent transplant before the policy change were compared with patients who were wait listed and underwent transplant after the policy change. Patients who were wait listed before the policy change but underwent transplant after the policy change were therefore excluded (n = 951).
Baseline characteristics measured at the time of transplant were compared between eras. Outcomes included postoperative complications that occurred before discharge from the index hospitalization after transplant. Length of hospitalization after transplant was also compared across eras. Posttransplant longitudinal survival was compared as well.
Statistical Analysis
Categorical variables are presented as number (percentage) and continuous variables as mean (SD). Categorical variables were compared using the χ2 test. Continuous variables were tested for normality, with normally distributed variables compared using a 2-tailed, unpaired t test and variables not normally distributed compared using the Wilcoxon rank sum test. For the wait list outcomes analysis, competing risk regression and cumulative incidence curves were analyzed using the Fine and Gray method.3 Survival was calculated using the Kaplan-Meier method and compared with the log-rank test. A multivariable Cox proportional hazards regression model was constructed using all baseline variables with biologic plausibility as well as those associated with posttransplant survival in univariate Cox proportional hazards regression analysis (exploratory 2-sided P < .05). Variables with more than 10% missing data were excluded from the multivariable model. Variables with more than 10% missing data included prior pregnancy if female, peak panel reactive antibody levels, pulmonary function tests, peak oxygen consumption, crossmatch between donor and recipient, donor inotrope use, donor left ventricular ejection fraction, and duration of donor cardiopulmonary resuscitation. Collinearity among all variables was evaluated. Missing data were handled with multiple imputation. All statistical tests were 2-sided, and P < .05 was considered to indicate statistical significance. Analyses were performed using Stata software, version 14.2 (StataCorp LLC).
Results
Baseline Characteristics of Wait Listed Patients
Of the 15 631 patients undergoing transplant, 10 671 (mean [SD] age, 53.1 [12.7] years; 7823 [73.3%] male) were wait listed before and 4960 (mean [SD] age, 52.7 [13.0] years; 3610 [72.8%] male) were wait listed after the policy change (Table 1). After the policy change, a higher percentage of Black and Hispanic patients were wait listed, and the mean body mass index at the time of wait listing was higher in the more recent era. After the policy change, a higher proportion of congenital heart disease, restrictive cardiomyopathy, and hypertrophic cardiomyopathy was found as the listed cause of heart failure (Table 1). Intra-aortic balloon pumps (IABPs) and ECMO were more commonly present at listing, and durable LVADs were less common at listing after the policy change.
Table 1. Patient Characteristics at Wait List Registration Before vs After the Change in Heart Allocation Policya.
| Characteristic | Before change (n = 10 671) | After change (n = 4960) | P value |
|---|---|---|---|
| Age, mean (SD), y | 53.1 (12.7) | 52.7 (13.0) | .06 |
| Male sex | 7823 (73.3) | 3610 (72.8) | .67 |
| Race/ethnicity | |||
| White | 6846 (64.5) | 3073 (62.2) | <.001 |
| Black | 2440 (23.0) | 1198 (24.3) | |
| Hispanic | 910 (8.6) | 483 (9.8) | |
| Asian | 345 (3.3) | 161 (3.3) | |
| Other | 70 (0.7) | 22 (0.5) | |
| BMI, mean (SD) | 27.9 (5.0) | 28.1 (5.0) | .01 |
| Blood type | |||
| A | 4050 (38.0) | 1844 (37.2) | .60 |
| AB | 504 (4.7) | 224 (4.5) | |
| B | 1501 (14.1) | 728 (14.7) | |
| O | 4615 (43.3) | 2164 (43.6) | |
| Cause of heart failure | |||
| Nonischemic dilated cardiomyopathy | 5776 (54.1) | 2587 (52.2) | <.001 |
| Ischemic cardiomyopathy | 3273 (30.7) | 1365 (27.5) | |
| Congenital heart disease | 316 (3.0) | 167 (3.4) | |
| Restrictive cardiomyopathy | 339 (3.2) | 192 (3.9) | |
| Valvular heart disease | 124 (1.2) | 51 (1.0) | |
| Failed primary heart transplant | 255 (2.4) | 105 (2.1) | |
| Hypertrophic cardiomyopathy | 299 (2.8) | 154 (3.1) | |
| Other cause | 289 (2.7) | 339 (6.8) | |
| Diabetes mellitus | 3102 (29.1) | 1330 (28.0) | .15 |
| Serum creatinine, mean (SD), mg/dL | 1.22 (0.52) | 1.22 (0.41) | .49 |
| Prelisting mechanical ventilation | 186 (1.7) | 95 (1.9) | .45 |
| Intra-aortic balloon pump | 549 (5.1) | 622 (12.5) | <.001 |
| Extracorporeal membrane oxygenation | 186 (1.7) | 138 (2.8) | <.001 |
| Ventricular assist device at listing | |||
| None | 7172 (67.3) | 3328 (69.8) | .001 |
| Left ventricular assist device | 3350 (31.4) | 1360 (28.5) | |
| Right ventricular assist device | 9 (0.1) | 7 (0.2) | |
| Total artificial heart | 39 (0.4) | 12 (0.3) | |
| Biventricular assist device | 95 (0.9) | 59 (1.2) | |
| Type of left ventricular assist device | |||
| HeartMate 2 | 1711 (16.0) | 245 (4.9) | <.001 |
| HeartWare | 992 (9.3) | 384 (7.7) | .001 |
| HeartMate 3 | 67 (0.6) | 600 (12.1) | <.001 |
| Other durable device | 627 (5.9) | 155 (3.1) | <.001 |
| Temporary device | 93 (0.9) | 98 (2.1) | <.001 |
| Status at listing | |||
| Old status | NA | ||
| 1A | 2610 (24.5) | NA | |
| 1B | 4959 (46.5) | NA | |
| 2 | 2794 (26.2) | NA | |
| New status | |||
| 1 | NA | 195 (3.9) | |
| 2 | NA | 951 (19.2) | |
| 3 | NA | 604 (12.2) | |
| 4 | NA | 1906 (38.4) | |
| 5 | NA | 2 (0.04) | |
| 6 | NA | 1156 (23.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Data are presented as number (percentage) of patients unless otherwise indicated.
Wait List Outcomes
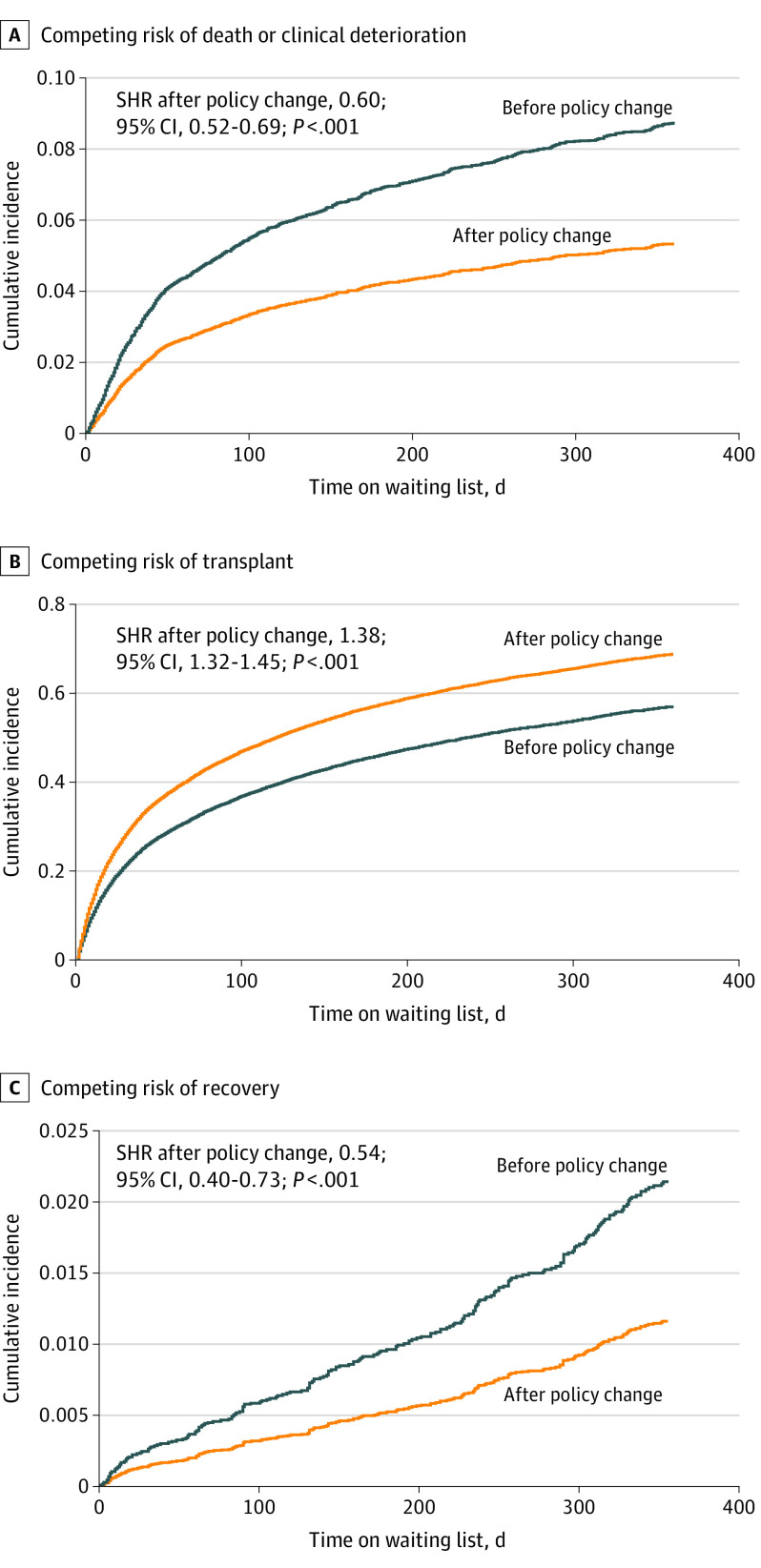
A comparison of competing wait list outcomes found significant changes after the policy change. The risk of death or clinical deterioration was reduced after the policy change (subhazard ratio [SHR], 0.60; 95% CI, 0.52-0.69; P < .001) (Figure 1A). The odds of undergoing transplant were significantly greater after the policy change (SHR, 1.38; 95% CI, 1.32-1.45; P < .001) (Figure 1B). The likelihood of wait list removal because of recovery was low in both groups but significantly decreased after the policy change (SHR, 0.54; 95% CI, 0.40-0.73; P < .001) (Figure 1C). Rates of 90-day wait list mortality (6.3% vs 5.0%; P = .02) and 1-year wait list mortality (13.3% vs 11.7%; P = .03) were lower after the policy change.
Figure 1. Competing Risk Regression Analysis .
The analysis reveals a reduced likelihood of death or clinical deterioration (A), an increased likelihood of transplant (B), and a reduced likelihood of recovery while on the waiting list after the policy change (C). SHR indicates subhazard ratio.
Baseline Characteristics at Transplant
A total of 6078 patients underwent heart transplant during the prepolicy study period, and 2801 underwent heart transplant after the policy change (Table 2).
Table 2. Comparison of Recipient, Donor, and Transplant-Related Characteristics at the Time of Transplant Before and After the Change in Heart Allocation Policya.
| Characteristic | Before change (n = 6078) | After change (n = 2801) | P value |
|---|---|---|---|
| Recipients | |||
| Age, mean (SD), y | 53.9 (12.7) | 52.8 (13.1) | <.001 |
| Male sex | 4347 (71.5) | 1981 (70.7) | .44 |
| Race/ethnicity | |||
| White | 3910 (64.7) | 1754 (62.9) | .09 |
| Black | 1313 (21.7) | 629 (22.5) | |
| Hispanic | 555 (9.2) | 285 (10.2) | |
| Asian | 227 (3.8) | 113 (4.1) | |
| Other | 40 (0.7) | 9 (0.3) | |
| BMI, mean (SD) | 27.4 (4.8) | 27.3 (5.0) | .53 |
| Blood type | |||
| A | 2549 (41.9) | 1153 (41.2) | .54 |
| AB | 389 (6.4) | 173 (6.2) | |
| B | 960 (15.8) | 476 (17.0) | |
| O | 2180 (35.9) | 999 (35.7) | |
| Cause of heart failure | |||
| Nonischemic dilated cardiomyopathy | 3303 (55.8) | 1491 (56.2) | .002 |
| Ischemic cardiomyopathy | 1841 (31.1) | 743 (28.0) | |
| Congenital heart disease | 159 (2.7) | 98 (3.7) | |
| Restrictive cardiomyopathy | 241 (4.1) | 153 (5.4) | |
| Valvular heart disease | 69 (1.2) | 28 (1.1) | |
| Failed primary heart transplant | 121 (2.0) | 64 (2.4) | |
| Hypertrophic cardiomyopathy | 183 (3.1) | 85 (3.2) | |
| Diabetes | 1716 (28.2) | 727 (26.5) | .09 |
| Serum bilirubin, mean (SD), mg/dL | 0.92 (1.54) | 1.14 (2.87) | <.001 |
| Serum creatinine, mean (SD), mg/dL | 1.24 (0.76) | 1.19 (0.48) | <.001 |
| Cytomegalovirus positive | 3358 (55.3) | 1403 (50.1) | <.001 |
| Pretransplant mechanical ventilatory support | 63 (1.0) | 80 (2.9) | <.001 |
| Pretransplant intensive care unit | 1907 (31.4) | 1543 (55.1) | <.001 |
| Intra-aortic balloon pump | 534 (8.8) | 887 (31.7) | <.001 |
| Extracorporeal membrane oxygenation | 71 (1.2) | 172 (6.1) | <.001 |
| Bridge with ventricular assist device | |||
| None | 3381 (55.6) | 1823 (71.5) | <.001 |
| Left ventricular assist device | 2549 (41.9) | 651 (25.5) | |
| Right ventricular assist device | 9 (0.2) | 10 (0.4) | |
| Total artificial heart | 50 (0.8) | 14 (0.6) | |
| Biventricular assist device | 89 (1.5) | 51 (2.0) | |
| Type of left ventricular assist device | |||
| HeartMate 2 | 1168 (19.2) | 107 (3.8) | <.001 |
| HeartWare | 966 (15.9) | 203 (7.3) | <.001 |
| HeartMate 3 | 68 (1.1) | 239 (8.5) | <.001 |
| Other durable device | 399 (6.6) | 124 (4.4) | <.001 |
| Temporary device | 62 (1.0) | 105 (4.2) | <.001 |
| Donors | |||
| Age, mean (SD), y | 32.1 (10.9) | 32.4 (10.8) | .08 |
| Male sex | 4103 (67.5) | 1999 (71.4) | <.001 |
| Race/ethnicity | |||
| White | 3906 (64.3) | 1806 (64.5) | .56 |
| Black | 992 (16.3) | 430 (15.4) | |
| Hispanic | 973 (16.0) | 478 (17.1) | |
| Asian | 100 (1.7) | 42 (1.5) | |
| Other | 107 (1.8) | 45 (1.6) | |
| BMI, mean (SD) | 27.5 (6.2) | 27.7 (6.1) | .06 |
| Blood type | |||
| A | 2395 (39.4) | 1046 (37.3) | .33 |
| AB | 92 (1.5) | 43 (1.5) | |
| B | 697 (11.5) | 333 (11.9) | |
| O | 2894 (47.6) | 1379 (49.2) | |
| Mechanism of donor death | |||
| Trauma | 2684 (44.2) | 1149 (41.0) | <.001 |
| Cerebrovascular | 1023 (16.8) | 416 (14.9) | |
| Drug overdose | 1116 (18.4) | 608 (21.7) | |
| Other | 1252 (20.6) | 628 (22.4) | |
| Diabetes | 227 (3.8) | 86 (3.1) | .12 |
| Hypertension | 2047 (33.8) | 941 (35.5) | .11 |
| Hepatitis C positive | 259 (4.3) | 321 (11.5) | <.001 |
| Cytomegalovirus positive | 3696 (61.2) | 1747 (62.8) | .16 |
| Terminal serum creatinine, mean (SD), mg/dL | 1.53 (1.58) | 1.69 (1.81) | <.001 |
| Left ventricular ejection fraction <50% | 83 (1.4) | 36 (1.3) | .76 |
| Recipient-donor matching | |||
| Sex matched | 4618 (76.0) | 2175 (77.7) | .08 |
| Race/ethnicity matched | 3131 (51.5) | 1378 (49.2) | .04 |
| HLA matched (≥3 antigens) | 778 (12.8) | 354 (12.6) | .83 |
| Blood type matched | 5089 (83.7) | 2309 (82.4) | .13 |
| Cytomegalovirus status matched | 3230 (53.5) | 1441 (51.8) | .14 |
| Transplant Related | |||
| Time on wait list, mean (SD), d | 134.5 (162.6) | 55.4 (83.0) | <.001 |
| Donor hospital to transplant center distance, mean (SD), miles | 157.3 (196.8) | 279 (238.1) | <.001 |
| Cold ischemic time, mean (SD), h | 3.04 (1.05) | 3.41 (1.00) | <.001 |
| Status at transplant | |||
| Old status | NA | ||
| 1A | 4159 (68.4) | NA | |
| 1B | 1746 (28.7) | NA | |
| 2 | 173 (2.9) | NA | |
| New status | |||
| 1 | NA | 272 (9.7) | |
| 2 | NA | 1446 (51.6) | |
| 3 | NA | 544 (19.4) | |
| 4 | NA | 430 (15.4) | |
| 5 | NA | 0 | |
| 6 | NA | 108 (3.9) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
SI conversion factors: To convert bilirubin to micromoles per liter, multiply by 17.104; to convert creatinine to micromoles per liter, multiply by 88.4.
Data are presented as number (percentage) of patients unless otherwise indicated.
After the heart allocation policy change, recipients were younger (mean [SD] age, 52.8 [13.1] years vs 53.9 [12.7] years before the policy change), had a higher total serum bilirubin level (mean [SD], 1.14 [2.87] mg/dL vs 0.92 [1.54] mg/dL before the policy change [to convert to micromoles per liter, multiply by 17.104]), and lower serum creatinine level at transplant (mean [SD], 1.19 [0.48] mg/dL vs 1.24 [0.76] mg/dL before the policy change [to convert to micromoles per liter, multiply by 88.4]) (Table 2). The cause of heart failure differed such that fewer recipients after the policy change had ischemic cardiomyopathy (1365 [27.5%] vs 3273 [30.7%] before the policy change) and more had congenital heart disease (316 [3.0%] vs 167 [3.4%] before the policy change), restrictive cardiomyopathy (339 [3.2%] vs 192 [3.9%] before the policy change), and failed primary heart transplants (255 [2.4%] vs 105 [2.1%] before the policy change).
Recipients also had higher acuity at the time of transplant after the policy change, as evidenced by higher rates of pretransplant mechanical ventilatory assistance (80 [2.9%] vs 63 [1.0%] before the policy change), intensive care unit stay (1543 [55.1%] vs 1907 [31.4%] before the policy change), IABP (887 [31.7%] vs 534 [8.8%] before the policy change), and ECMO (172 [6.1%] vs 71 [1.2%] before the policy change), with lower rates of durable LVAD support (651 [25.5%] vs 2549 [41.9%] before the policy change) (Table 2). Panel reactive antibody levels were similar before vs after the policy change (mean [SD], 11.0% [22.8%] vs 10.1% [22.0%]; P = .13), with similar proportions having levels greater than 25% (16.5% vs 15.1%; P = .15).
After the policy change, there were fewer female donors (820 [29.3%] vs 1731 [28.5%]), and terminal creatinine levels of donors were higher (1.69 [1.81] mg/dL vs 1.53 [1.58] mg/dL before the policy change) (Table 2). After the policy change, there were fewer trauma-related donor deaths (1149 [41.0%] vs 2684 [44.2%] before the policy change) and more donor deaths from drug overdose (608 [21.7%] vs 1116 [18.4%] before the policy change).
The use of hepatitis C–positive donors also increased in the more recent era (259 [4.3%] before vs 321 [11.5%] after the policy change). Race/ethnicity-matched recipients and donors were less frequent after the policy change (3131 [51.5%] before vs 1378 [49.2%] after the policy change) (Table 2). Time on the wait list was reduced (mean [SD], 134.5 [162.6] days before vs 55.4 [83.0] days after the policy change), whereas distance between donor and recipient hospital (mean [SD], 157.3 [196.8] miles before vs 279 [238.1] miles after the policy change) as well as cold ischemic time (mean [SD], 3.04 [1.05] hours before vs 3.41 [1.00] hours after the policy change) were longer after the policy change (Table 2).
Posttransplant Outcomes
Rates of postoperative new-onset dialysis (703 [11.6%] before vs 356 [14.3%] after the policy change) and stroke (171 [2.8%] before vs 98 [3.9%] after the policy change) were higher after the policy change (eTable in the Supplement). Rates of postoperative pacemaker implantation (144 [2.4%] before vs 58 [2.3%] after the policy change) as well as drug-treated short-term rejection (735 [12.1%] before vs 285 [11.5%] after the policy change) were comparable. Length of hospital stay was also similar between cohorts (mean [SD], 21.2 [21.9] days before vs 21.4 [20.5] days after the policy change) (eTable in the Supplement).
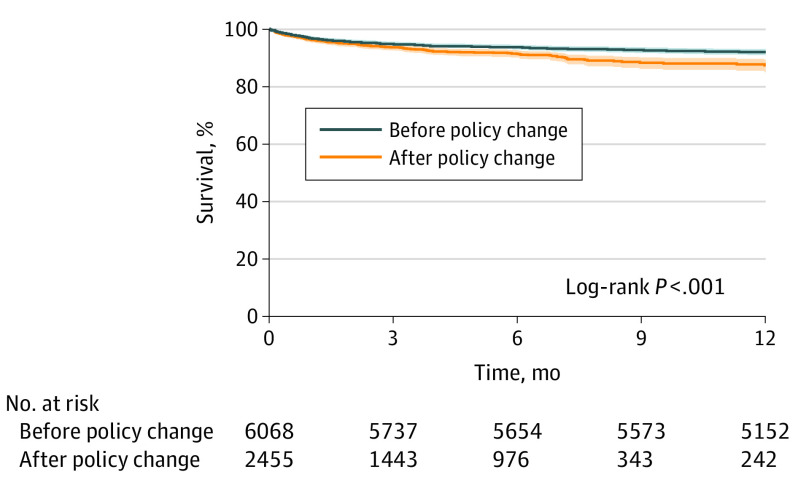
The 1-year survival was lower after the policy change (87.5%; 95% CI, 85.0%-89.5%) compared with before the policy change (92.1%; 95% CI, 91.3%-92.7%; log-rank P < .001) (Figure 2). In multivariable Cox proportional hazards regression analysis, transplant after the policy change was associated with posttransplant mortality (hazard ratio, 1.29; 95% CI, 1.07-1.55; P = .008) (Table 3). The absolute overall rate of 30-day mortality from the time of transplant was higher in the newer era (3.2% vs 4.5%; P = .01). The most pronounced differences in 1-year survival between eras occurred in UNOS region 1 (91.9% vs 79.5%; P = .02), UNOS region 5 (93.5% vs 88.9%; P = .02), and UNOS region 9 (93.4% vs 79.0%; P = .002).
Figure 2. Kaplan-Meier Curve.
The 1-year survival after heart transplant following the policy change decreased. Shaded areas indicate 95% CIs.
Table 3. Multivariable Cox Proportional Hazards Regression Model for Mortality After Heart Transplant.
| Covariate | Hazard ratio (95% CI) | P value |
|---|---|---|
| Transplant after policy change | 1.29 (1.07-1.55) | .008 |
| Age (increasing, per 1 y) | 1.01 (1.00-1.02) | .003 |
| BMI (increasing, per 1 U) | 1.02 (1.00-1.03) | .03 |
| Heart failure cause | ||
| Nonischemic dilated cardiomyopathy | 1 [Reference] | NA |
| Ischemic cardiomyopathy | 1.20 (1.03-1.40) | .02 |
| Congenital heart disease | 2.12 (1.45-3.10) | <.001 |
| Restrictive cardiomyopathy | 1.46 (1.07-1.99) | .02 |
| Valvular heart disease | 0.78 (0.38-1.62) | .51 |
| Failed primary heart transplant | 2.38 (1.67-3.41) | <.001 |
| Hypertrophic cardiomyopathy | 0.99 (0.64-1.54) | .97 |
| Diabetes | 1.18 (1.02-1.36) | .03 |
| Serum total bilirubin (increasing, per 1 mg/dL) | 1.04 (1.03-1.06) | <.001 |
| Serum creatinine (increasing, per 1 mg/dL) | 1.06 (1.01-1.11) | .02 |
| Recipient cytomegalovirus positive | 1.12 (0.98-1.29) | .09 |
| Pretransplant mechanical ventilatory support | 1.50 (0.97-2.32) | .07 |
| Extracorporeal membrane oxygenation | 1.90 (1.31-2.78) | .001 |
| Bridging with ventricular assist device | ||
| None | 1 [Reference] | NA |
| Left ventricular assist device | 1.22 (1.05-1.41) | .008 |
| Right ventricular assist device | 2.63 (1.07-6.48) | .04 |
| Total artificial heart | 2.71 (1.69-4.35) | <.001 |
| Biventricular assist device | 1.39 (0.86-2.25) | .18 |
| Donor age (increasing, per 1 y) | 1.01 (1.01-1.02) | <.001 |
| Ischemic time (increasing, per 1 h) | 1.13 (1.06-1.20) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
As a sensitivity analysis, we performed an additional analysis that included the 951 patients who were wait listed before October 18, 2018, but underwent transplant thereafter to ensure that results were not confounded by this excluded cohort. Similar findings were demonstrated in terms of changes in baseline characteristics after the policy change. Similarly, 1-year survival remained significantly lower in the more recent era when including these patients (89.4%; 95% CI, 87.9%-90.8%; log rank P = .001 compared with the prepolicy change era).
Discussion
This cohort study found multiple changes that have occurred after implementation of the revised heart allocation policy on October 18, 2018, for adult heart transplant in the US. Foremost, wait list outcomes have improved, with reduced rates of wait list mortality and higher rates of transplant. A major goal of the new policy was to reduce wait list mortality, and in this respect, the policy seems to be successful. This impetus arose from the finding that during the decade between 2006 and 2015, the demand for heart transplant increased significantly in the US, with a near doubling of active wait list candidates.4 This increase occurred while the donor supply of hearts remained relatively stagnant, leading to increases in wait list times.4
Another related goal of the heart allocation policy change was to induce broader geographic sharing of donor organs. Although both wait list mortality and posttransplant mortality were reduced with the institution of the 2006 heart allocation policy, significant regional variation was found in wait list times and dependence on mechanical circulatory support as a bridge to heart transplant.5 Again, in this regard, the present analysis suggests that the policy is successful because time on the wait list has been reduced and the mean distance between the donor hospital and recipient transplant center has increased.
A recognized limitation of the prior policy was that the most urgent status, status 1A, was too broad a category and covered a population that was too heterogeneous. Although the overall number of wait listed patients had nearly doubled during the last decade, the number of status 1A wait listed patients had increased 5-fold during the same period.6 The proportion of transplant recipients who were bridged to heart transplant with LVADs had increased from 11.1% between 1999 and 2006 to 26.6% between 2006 and 2012.5 This increase occurred in conjunction with improvements in LVAD technology and patient management, leading to improved survival such that patients with stable LVAD had lower wait list mortality risk than other status 1A patients.7,8 In addition, a multitude of exemption requests were submitted and granted for patients with an inability to tolerate inotropes, patients with congenital heart disease, and those with ventricular arrhythmias.9
By converting from a 3-tier to a 6-tier system, the recent heart allocation policy has provided a higher level of granularity and separation based on medical urgency of wait listed patients. Our analysis demonstrates notable changes in patient characteristics that are reflective of some of the intended consequences of the policy. High-acuity patients constitute a much higher percentage of heart transplants under the current system, with bridging with IABP increasing more than 3-fold and bridging with ECMO increasing more than 5-fold. During the same time, bridging with durable LVADs has decreased substantially from 41.9% to 25.5%. Because the criteria for the new status 1 to 3 groups generally corresponds to the prior status 1A, a comparison indicates that although 68.4% of prepolicy transplant patients were status 1A, 80.7% of transplants after the policy are new status 1 to 3.
The current analysis demonstrates a reduction in posttransplant survival, with an absolute decrease in 1-year survival of 4.6% after the policy change. Given the drastic shift in recipient characteristics, these findings are not unexpected. Many of the characteristics that are more prevalent in transplant recipients after the policy change are risk factors for posttransplant mortality. For example, recipients bridged with ECMO have reduced early and late survival and increased risk of primary graft dysfunction.10,11 Congenital heart disease and pretransplant mechanical ventilatory support are among the strongest factors associated with 1-year mortality after adult heart transplant, both of which were present in higher proportions in the postpolicy era.12 As broader sharing of organs has occurred, cold ischemic times have increased, as demonstrated in our analysis. Longer cold ischemic time is a known risk factor for primary graft dysfunction and for posttransplant mortality in heart transplant.10,13 The reduction in 1-year survival demonstrated in our analysis is perhaps less substantial than was anticipated based on early analyses of the impact of the allocation change in which 6-month survival was estimated at approximately 80% to 85% under the new system.14,15 This finding likely suggests that transplant centers have made appropriate adjustments as time has progressed under the new system, with perhaps more refined patient selection and more judicious use of higher-risk bridging modalities. Notably, listing policies in solid organ transplant are generally designed to reduce wait list mortality, whereas the responsibility of posttransplant mortality typically lies with individual centers.
This study also found higher rates of postoperative new-onset dialysis and stroke under the new policy. Congenital heart disease, pretransplant mechanical ventilatory support, and being in the intensive care unit before transplant, all of which were present more frequently in our analysis after the policy change, were previously identified as risk factors for new-onset dialysis after heart transplant.16 Another study17 identified preoperative IABP support, the use of which increased substantially with the policy change, as a significant factor associated with neurologic complications after heart transplant.
Prior studies14,15,18,19,20 have examined the impact of the new allocation change as well but have been limited in patient numbers, follow-up, or breadth of data compared with the current analysis, which provides a more in-depth analysis of both wait list and posttransplant trends and outcomes with follow-up to 1 year. Two prior analyses14,15 were early brief reports that demonstrated 6-month trends similar to those observed in the current analysis. However, there were notable differences; in particular, the divergence in posttransplant survival was less pronounced in the current analysis, which again lends weight to the theory of a learning curve that transplant centers have likely experienced with more refined patient selection as time has progressed under the new heart allocation policy. Another brief report18 specifically stated that ECMO bridging had increased and durable LVAD bridging had decreased under the new system. Interestingly, a 14-center study19 found that the use of temporary mechanical circulatory support for decompensated heart failure–related cardiogenic shock increased from 25.4% to 42.6% after the policy change at transplant centers but remained stable, from 24.5% to 24.1%, at nontransplant centers after the policy change. An analysis20 of trends at the time of listing found that ECMO at the time of listing had increased 1.2%, IABP at listing increased by 4.0%, and requests for exceptions increased by 12.0%, whereas bridging to heart transplant with inotrope support only decreased significantly.
The transplant community will ultimately need to weigh the advantages and disadvantages with the new allocation system as demonstrated in this analysis. One could argue that the clear improvements in wait list outcomes justify the 4.6% decrease in 1-year survival in a higher-risk patient subset. Others may argue that the substantial increase in bridging with ECMO and other temporary mechanical circulatory support modalities has resulted in worse posttransplant outcomes and adjustments to allocation should therefore be made. Although this is a complex topic that requires input from multiple stakeholders, the potential utility of developing and using a heart allocation score that balances the risk of wait list mortality with posttransplant survival should be explored, similar to what has been done in lung transplant.12,13,21
Another factor that should be considered is the improvement of survival and quality of life of patients supported with durable LVADs.2 Such durable LVADs can improve end-organ perfusion and facilitate discharge from the hospital with continued rehabilitation and optimization for heart transplant at a future time. These benefits are particularly useful in patients presenting in cardiogenic shock or decompensated heart failure because the outcomes of transplant in these settings can be suboptimal. However, the benefits of LVAD implantation must be weighed against the increased complexity of transplant when requiring LVAD explantation, including longer operative and cardiopulmonary bypass times and greater coagulopathy and blood transfusion requirements. As demonstrated in the current study, bridging with durable LVADs decreased substantially, from 42% to 26%, which suggests, along with the increase in bridging with temporary support, that many programs favor direct transplant strategies in those presenting in decompensated states.
Limitations
This study has limitations. As with most multicenter registries, the UNOS registry is susceptible to errors in data entry and missing data. There may be confounders that were not available in the registry and therefore not accounted for in the analysis, such as patient adherence, granular reasons for decision making regarding individual recipient and donor selection, and practice patterns regarding selection of bridging modality. Details regarding adverse events while supported on durable LVADs and conversion from a bridge-to-transplant to destination therapy strategy because of such events could not be accounted for as well. In the UNOS registry, most data are recorded at the time of wait list registration and transplant; therefore, variables such as duration of temporary mechanical circulatory support or specific hemodynamic data at the time of instituting such support are not available. There is also the possibility that as time progresses under the new heart allocation policy, that individual centers will adjust practice and the outcome trends observed in the current analysis will change. Cost data were also not available in the registry but would be useful to better assess the broad impact of the policy change on heart transplant practice nationally.
Conclusions
This study found that significant trends have occurred in patient characteristics, wait list outcomes, and posttransplant outcomes after the heart allocation policy change for adult heart transplants in the US in October 2018. The use of temporary mechanical circulatory support as a bridge to transplant has increased substantially, whereas bridging with durable LVADs has decreased. Wait list outcomes have improved, including a reduction in wait list mortality, an increase in rate of transplants, and a reduction in wait list times. Posttransplant 1-year survival has decreased. Reassessment of these changes as time progresses under the new heart allocation policy is prudent.
eTable. Comparison of Postoperative Complications and Length of Hospital Stay Following Heart Transplantation Before Versus After the Policy Change
References
- 1.Organ Procurement and Transplantation Network Adult Heart Allocation. Accessed March 22, 2020. https://optn.transplant.hrsa.gov/learn/professional-education/adult-heart-allocation/
- 2.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device - final report. N Engl J Med. 2019;380(17):1618-1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 3.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risks in survival analysis. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 4.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant. 2018;18(suppl 1):291-362. doi: 10.1111/ajt.14561 [DOI] [PubMed] [Google Scholar]
- 5.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart Fail. 2014;2(2):166-177. doi: 10.1016/j.jchf.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1A time. J Am Coll Cardiol. 2012;60(1):36-43. doi: 10.1016/j.jacc.2012.02.031 [DOI] [PubMed] [Google Scholar]
- 7.Pinney SP Timing isn’t everything: donor heart allocation in the present LVAD era. J Am Coll Cardiol. 2012;60(1):52-53. doi: 10.1016/j.jacc.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555-564. doi: 10.1016/j.healun.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 9.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant. 2015;15(1):44-54. doi: 10.1111/ajt.13030 [DOI] [PubMed] [Google Scholar]
- 10.Russo MJ, Iribarne A, Hong KN, et al. Factors associated with primary graft failure after heart transplantation. Transplantation. 2010;90(4):444-450. doi: 10.1097/TP.0b013e3181e6f1eb [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara S, Takeda K, Kurlansky PA, Naka Y, Takayama H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg. 2018;155(4):1607-1618.e6. doi: 10.1016/j.jtcvs.2017.10.152 [DOI] [PubMed] [Google Scholar]
- 12.Kilic A, Allen JG, Weiss ES. Validation of the United States-derived Index for Mortality Prediction After Cardiac Transplantation (IMPACT) using international registry data. J Heart Lung Transplant. 2013;32(5):492-498. doi: 10.1016/j.healun.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 13.Weiss ES, Allen JG, Kilic A, et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant. 2012;31(3):266-273. doi: 10.1016/j.healun.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant. 2020;39(1):1-4. doi: 10.1016/j.healun.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Kilic A, Hickey G, Mathier MA, et al. Outcomes of the first 1300 adult heart transplants in the United States following the allocation policy change. Circulation. 2020;141(20):1662-1664. doi: 10.1161/CIRCULATIONAHA.119.045354 [DOI] [PubMed] [Google Scholar]
- 16.Kilic A, Grimm JC, Shah AS, Conte JV, Whitman GJ, Sciortino CM. An easily calculable and highly predictive risk index for postoperative renal failure after heart transplantation. J Thorac Cardiovasc Surg. 2014;148(3):1099-1104. doi: 10.1016/j.jtcvs.2014.05.065 [DOI] [PubMed] [Google Scholar]
- 17.Zierer A, Melby SJ, Voeller RK, et al. Significance of neurologic complications in the modern era of cardiac transplantation. Ann Thorac Surg. 2007;83(5):1684-1690. doi: 10.1016/j.athoracsur.2006.12.017 [DOI] [PubMed] [Google Scholar]
- 18.Hanff TC, Harhay MO, Kimmel SE, et al. Trends in mechanical support use as a bridge to adult heart transplant under new allocation rules. JAMA Cardiol. 2020;5:728-729. doi: 10.1001/jamacardio.2020.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varshney AS, Berg DD, Katz JN, et al. ; Critical Care Cardiology Trials Network Investigators . Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the United Network for Organ Sharing donor heart allocation system changes. JAMA Cardiol. 2020;5(6):1-6. doi: 10.1001/jamacardio.2020.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice changes at U.S. transplant centers after the new adult heart allocation policy. J Am Coll Cardiol. 2020;75(23):2906-2916. doi: 10.1016/j.jacc.2020.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011;92(3):914-921. doi: 10.1016/j.athoracsur.2011.04.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Comparison of Postoperative Complications and Length of Hospital Stay Following Heart Transplantation Before Versus After the Policy Change