Key Points
Question
Can coronary artery calcium score identify individuals likely to derive net benefit from aspirin therapy for primary prevention of atherosclerotic cardiovascular disease?
Findings
In this cohort study, using participants from the Dallas Heart Study cohort who were free from atherosclerotic cardiovascular disease and not taking aspirin at baseline, we found that increasing coronary artery calcium was associated with both bleeding and atherosclerotic cardiovascular disease events. Modeling the effects of aspirin for primary prevention, a high coronary artery calcium score identified individuals who would experience net benefit from aspirin from those who would not, but only in individuals at lower bleeding risk and intermediate cardiovascular risk.
Meaning
These findings support the consideration of coronary artery calcium to help select individuals for primary prevention aspirin therapy only in the setting of lower bleeding risk and estimated atherosclerotic cardiovascular disease and risk that is not low.
Abstract
Importance
Higher coronary artery calcium (CAC) identifies individuals at increased atherosclerotic cardiovascular disease (ASCVD) risk. Whether it can also identify individuals likely to derive net benefit from aspirin therapy is unclear.
Objective
To examine the association between CAC, bleeding, and ASCVD and explore the net estimated effect of aspirin at different CAC thresholds.
Design, Setting, and Participants
Prospective population-based cohort study of Dallas Heart Study participants, free from ASCVD and not taking aspirin at baseline. Data were analyzed between February 1, 2020, and July 15, 2020.
Exposures
Coronary artery calcium score in the following categories: 0, 1-99, and 100 or higher.
Main Outcomes and Measures
Major bleeding and ASCVD events were identified from International Statistical Classification of Diseases and Related Health Problems, Ninth Revision codes. Meta-analysis–derived aspirin effect estimates were applied to observed ASCVD and bleeding rates to model the net effect of aspirin at different CAC thresholds.
Results
A total of 2191 participants (mean [SD], age 44 [9.1] years, 1247 women [57%], and 1039 black individuals [47%]) had 116 major bleeding and 123 ASCVD events over a median follow-up of 12.2 years. Higher CAC categories (CAC 1-99 and ≥100 vs CAC 0) were associated with both ASCVD and bleeding events (hazard ratio [HR], 1.6; 95% CI, 1.1-2.4; HR, 2.6; 95% CI, 1.5-4.3; HR, 4.8; 95% CI, 2.8-8.2; P < .001; HR, 5.3; 95% CI, 3.6-7.9; P < .001), but the association between CAC and bleeding was attenuated after multivariable adjustment. Applying meta-analysis estimates, irrespective of CAC, aspirin use was estimated to result in net harm in individuals at low (<5%) and intermediate (5%-20%) 10-year ASCVD risk and net benefit in those at high (≥20%) ASCVD risk. Among individuals at lower bleeding risk, a CAC score of at least 100 identified individuals who would experience net benefit, but only in those at borderline or higher (≥5%) 10-year ASCVD risk. In individuals at higher bleeding risk, there would be net harm from aspirin irrespective of CAC and ASCVD risk.
Conclusions and Relevance
Higher CAC is associated with both ASCVD and bleeding events, with a stronger association with ASCVD. A high CAC score identifies individuals estimated to derive net benefit from primary prevention aspirin therapy from those who would not, but only in the setting of lower bleeding risk and estimated ASCVD risk that is not low.
This study examines the association between coronary artery calcium scores, bleeding, and atherosclerotic cardiovascular disease and explores the net estimated effect of aspirin at different coronary artery calcium thresholds.
Introduction
Aspirin has a robust evidence base for secondary prevention of cardiovascular disease. In this setting of high-baseline cardiovascular risk, aspirin lowers the absolute risk of cardiovascular events substantially, which more than offsets the increase in bleeding.1 However, in the primary prevention setting, the role of aspirin is unclear.2,3 In three 2018 primary prevention randomized clinical trials,4,5,6 aspirin, taken with a background of contemporary primary prevention pharmacologic therapies, conferred no or marginal benefit while carrying significant bleeding risk leading to a net neutral or net harmful risk-benefit profile. Based on these studies, aspirin was downgraded to a weak class IIb recommendation for primary prevention in the 2019 American College of Cardiology/American Heart Association (ACC/AHA) Primary Prevention guideline,7 compared with a more supportive recommendation in the 2016 US Preventive Task Force guidelines. The ACC/AHA Prevention Guidelines did state that aspirin may be considered in select individuals who are at higher ASCVD risk but not at increased bleeding risk.7 However, how to discern those who meet this optimal balanced profile is unclear.
Some have suggested that coronary artery calcium (CAC) scanning might be a method to identify those with the greatest net benefit from aspirin in primary prevention.8,9 However, it has been shown that bleeding risk increases with cardiovascular risk and, as such, might associate with CAC.1 Thus, although the association of increasing CAC with ASCVD events is well known, there are little published data regarding the association of CAC with bleeding to help inform clinical decision making.
In this study, we examined the association between CAC, bleeding, and ASCVD using observed bleeding and ASCVD events in the Dallas Heart Study (DHS) cohort, including participants at increased risk of bleeding. Using meta-analysis–derived aspirin effect estimates applied to observed ASCVD and bleeding rates, we then explored the net effect of aspirin at different CAC thresholds.
Methods
Study Population
The Dallas Heart Study is a multiethnic probability-based population cohort study comprising 6101 Dallas County, Texas, residents aged 18 to 65 years with deliberate oversampling of African American individuals. The study protocol was approved by the University of Texas Southwestern Medical Center institutional review board, and all participants provided written informed consent. Details of the study design and characteristics of this cohort have been described previously.10 Briefly, at baseline, participants were invited to 3 sequential visits for the collection of detailed health and demographic data survey, fasting laboratory samples and various imaging studies, and electron beam computed tomography for CAC scoring. For this study, we excluded individuals with preexisting ASCVD, those taking aspirin at baseline, and those not followed up in the Dallas Fort Worth Hospital Council Data Initiative Database (DFWHC) database (eFigure 1 in the Supplement).
Covariate Measurement
Demographic data, including age, sex, race/ethnicity, smoking status, and medical history, were collected at the time of the baseline visits by self-report. Definitions for diabetes and hypertension used in the DHS have previously been described.11 Participants were asked to bring their medications to the clinic visit, where medication profiles were reviewed and medication names were directly transcribed into the electronic database.
Coronary Artery Calcium Scores
Detailed methods for electron beam computed tomography scans in the DHS cohort have previously been reported.12 Measurements were obtained at 80% of the risk ratio interval in duplicate 1 to 2 minutes apart using an Imatron 150 XP (Imatron Inc) scanner. Both scores were expressed in Agatson units, and the mean of the scores was used as the final score.12
Clinical Outcomes
Our primary outcomes were first bleeding event and first ASCVD event associated with a hospitalization or death. Hospitalization or death events were tracked using the DFWHC database and National Death Index, respectively. All patients included in this study consented to being tracked quarterly for hospital admissions using the DFWHC, which includes hospital admission data for 70 of 72 hospitals in the Dallas/Fort Worth, Texas, metroplex.13 Complete International Classification of Diseases, Ninth Revision (ICD-9) codes for all diagnoses encountered for each hospitalization were provided, inclusive of both nonfatal bleeding and ASCVD events. For nonfatal ASCVD events, participants were contacted annually to participate in detailed health surveys. Primary records were requested for all suspected ASCVD events, and these events were adjudicated separately by 2 cardiologists blinded to all study variables.
The bleeding events considered include gastrointestinal, intracranial (including hemorrhagic stroke), and other major events (ocular and respiratory). The ICD-9 codes used to identify these events have previously been applied and are listed in eTable 1 in the Supplement.14 Hospitalizations or deaths associated with a bleeding event were defined as those for which an ICD-9 code for bleeding was assigned as a diagnosis for the hospitalization or death at any coding position. The primary ASCVD outcome was a composite of nonfatal myocardial infarction, coronary heart disease death, and nonfatal and fatal stroke. Fatal ASCVD events were ascertained from the National Death Index through to 2013 using ICD-9 codes I00-I99. Deaths were considered secondary to ASCVD events if they included these codes.
Statistical Analysis
Baseline demographic and clinical characteristics were compared using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Study participants were divided by CAC score into 3 groups; 0, 1 to 99, and 100 or higher, to reflect CAC thresholds commonly used in clinical practice.15 Absolute event rates for both bleeding events and ASCVD were calculated per 1000 person-years for each group. The 95% CIs for absolute event rates were calculated using Poisson regression. Cumulative incidence curves were compared across CAC categories using the log-rank test.
The associations of CAC with incident bleeding and with incident ASCVD were tested using Cox proportional hazard models, with CAC = 0 group as the referent. Multivariable models for bleeding were adjusted for age, sex, race/ethnicity, systolic blood pressure, antihypertensive use, smoking status, diabetes mellitus, peptic ulcer disease (PUD), proton pump inhibitor (PPI)/antacid, and corticosteroid. Multivariable models for ASCVD were adjusted for age, sex, race/ethnicity, diabetes mellitus, smoking, total and high-density lipoprotein cholesterol, antihypertensive use, systolic blood pressure, and statin use. We examined for interaction between race/ethnicity and CAC and sex and CAC for incident ASCVD and bleeding.
The expected relative associations of primary prevention aspirin use with ASCVD and bleeding events were extrapolated from a 2019 aspirin meta-analysis16 of 13 randomized clinical trials that included 164 225 participants. This approach has previously been used to model the net effect of aspirin at different CAC thresholds.8,9 In the meta-analysis, aspirin use was associated with an 11% relative risk reduction in composite ASCVD events (ASCVD mortality, nonfatal myocardial infarction, and nonfatal stroke) and a 43% relative risk increase in major bleeding events. To estimate the absolute effect aspirin would have on ASCVD and bleeding events in individuals stratified by CAC, we applied these aspirin effect estimates to observed absolute ASCVD and bleeding 10-year event rates in ASCVD risk groups (10-year ASCVD risk estimate <5%, 5%-20%, and >20%) stratified by CAC score. Sensitivity analyses were conducted using effect estimates (10% relative risk reduction in composite ASCVD events and a 39% relative risk increase in major bleeding events) from trials conducted after the year 2000.16
Additional sensitivity analyses were performed using 5-year observed event rates, and with individuals stratified by age (<50 years and ≥50 years), and baseline bleeding risk. The lower bleeding risk group excluded individuals taking nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, anticoagulants, selective serotonin reuptake inhibitors (SSRIs), statins, PPI/antacids, antiplatelet agents, and those with PUD, cirrhosis, cancer, and chronic kidney disease (CKD) and uncontrolled hypertension (systolic blood pressure ≥160 mm Hg and diastolic blood pressure ≥100 mmHg).7,17 Those excluded from the lower bleeding risk group were included in the higher bleeding risk group. To account for aspirin initiation during the follow-up period, we conducted a sensitivity analysis using a subgroup that excluded participants taking aspirin (n = 279) at the time of a follow-up DHS visit 7 years later. Statistical tests were considered significant at a 2-sided P value of less than .05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc).
Results
Study Participants
After applying exclusion criteria, 2191 participants were included in the main analysis (eFigure 1 in the Supplement). Of these, 1063 (49%) had a CAC score of 0, and 161 (7%) had a score of at least 100. The cohort had a mean (SD) age of 44.4 (9.1) years and included 1247 women (57%) and 1039 Black individuals (47%). Characteristics of this population stratified by incident bleeding status are displayed in Table 1. Compared with those who did not experience a bleeding event, participants with bleeding were older (49.0 vs 44.1 years; P < .001), more likely to be Black (72.5% vs 46.3%; P < .001), to smoke (current smoker, 38.5% vs 25.7%; P = .005), to have hypertension (45.9% vs 26.8%; P < .001), to have diabetes (20.2% vs 8.4%; P < .001), and to be taking corticosteroids (3.9% vs 0.7%; P = .01).
Table 1. Baseline Characteristics of the Study Population Stratified by Incident Bleeding Event.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All (N = 2191) | Bleeding event | |||
| Yes (n = 116) | No (n = 2075) | |||
| Demographics | ||||
| Age, mean (SD), y | 44.4 (9.1) | 49.0 (9.8) | 44.1 (9.0) | <.001 |
| BMI, mean (SD) | 29.8 (6.3) | 30.8 (7.2) | 29.8 (6.3) | .14 |
| Male | 944 (43.1) | 43 (39.5) | 901 (43.4) | .43 |
| Race/ethnicity | ||||
| White | 714 (32.6) | 18 (16.5) | 696 (33.5) | <.001 |
| Black | 1039 (47.4) | 79 (72.5) | 960 (46.3) | <.001 |
| Hispanic | 384 (17.5) | 10 (9.2) | 374 (18.0) | .02 |
| Other | 47 (2.1) | 2 (1.8) | 45 (2.2) | >.99 |
| ASCVD risk profile, mean (SD) | ||||
| Tc, mg/dL | 181.9 (39.4) | 185.4 (45.5) | 181.7 (39.1) | .42 |
| HDL-c, mg/dL | 50.4 (14.8) | 50.2 (14.8) | 50.4 (14.7) | .74 |
| HTN | 607 (27.7) | 50 (45.9) | 557 (26.8) | <.001 |
| Smoking status | ||||
| Current | 575 (26.2) | 42 (38.5) | 533 (25.7) | .01 |
| Former | 386 (17.6) | 23 (21.1) | 363 (17.5) | .37 |
| Never | 1220 (55.7) | 44 (40.4) | 1176 (56.8) | <.001 |
| Diabetes | 196 (8.9) | 22 (20.2) | 174 (8.4) | <.001 |
| Estimated 10-y ASCVD riska | 4.3 (6.2) | 8 (8) | 4 (6) | <.001 |
| Medical history | ||||
| PUD | 232 (10.6) | 16 (14.7) | 216 (10.4) | .15 |
| Cirrhosis | 14 (0.6) | 1 (0.9) | 13 (0.6) | .51 |
| Cancer | 87 (4.0) | 7 (6.4) | 80 (3.9) | .20 |
| CKDb | 26 (1.2) | 2 (1.7) | 24 (1.2) | .65 |
| Medications | ||||
| Antihypertensive | 361 (16.5) | 27 (23.2) | 334 (16.1) | .02 |
| NSAID | 314 (14.3) | 17 (14.7) | 297 (14.3) | .67 |
| Corticosteroid | 19 (0.9) | 4 (3.9) | 15 (0.7) | .01 |
| Anticoagulant | 3 (0.1) | 3 (0.2) | 0 | >.99 |
| SSRI | 89 (4.1) | 6 (5.8) | 83 (4.1) | .44 |
| Statin | 106 (4.8) | 5 (4.8) | 101 (4.9) | >.99 |
| PPI/antacid | 83 (3.8) | 8 (7.7) | 75 (3.7) | .06 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL-c, high-density lipoprotein cholesterol; HTN, hypertension; NSAID, nonsteroidal anti-inflammatory; PCE, pooled cohort equation; PPI, proton pump inhibitor; PUD, peptic ulcer disease; SSRI, selective serotonin reuptake inhibitor; Tc, total cholesterol.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
The ASCVD risk was calculated using the pooled cohort equation.
Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 mL per minute per 1.73.2
Incident Bleeding and ASCVD Events
A total of 116 first major bleeding events and 123 ASCVD events occurred over a mean (SD) follow-up period of 12.2 (1.9) years. Eighty-one bleeding events (70%) were gastrointestinal and 18 (16%) were intracerebral (eTable 2 in the Supplement). There were 24 fatal bleeds (21% of total).
Association Between CAC, Incident ASCVD, and Bleeding Events
Overall, bleeding and ASCVD rates increased in a graded fashion from the lowest (CAC = 0) to the highest CAC group examined (CAC ≥100) (Figure 1). Compared with participants in the lowest CAC group, those in the highest CAC group had an unadjusted hazard ratio (HR) of 2.6 (95% CI, 1.5-4.3; P < .001) for bleeding events and 5.3 (95% CI, 3.6-7.9; P < .001) for ASCVD events (Table 2; eTable 3 in the Supplement). After multivariable adjustment for risk factors, the association between CAC and bleeding was attenuated (CAC ≥100 vs CAC 0; HR, 1.5, 95% CI, 0.8-2.6; P = .19), but the association between CAC and ASCVD remained significant in all of the CAC categories examined. These findings were replicated in the subgroup of participants without incident aspirin uptake (eTable 4 in the Supplement). There were no differences in the association of CAC and bleeding or ASCVD by age older than and younger than 50 years (HR, 1.1; 95% CI, 0.2-3.3; and HR, 1.7; 95% CI. 0.7-4.4; P interaction between age and CAC for bleeding, .70; and HR, 5.4; 95% CI, 2.0-14.4 and HR, 3.8; 1.4-10.7; P interaction between age and CAC for ASCVD, .19), Black and non-Black race (HR, 1.8; 95% CI, 0.8-3.9 and HR, 1.4; 95% CI, 0.1-2.0; P interaction for bleeding, .27; HR, 2.6; 95% CI, 1.2-5.6 and HR, 9.3; 95% CI, 2.4-36.2; P interaction for ASCVD, .09) and male vs female sex (HR, 0.6; 95% CI, 0.2-1.8 and HR, 2.2; 95% CI, 0.9-5.1; P interaction for bleeding, .12; HR, 6.0; 95% CI, 2.1-16.9 and HR, 3.5; 95% CI, 1.0-6.4, P interaction for ASCVD, .96).
Figure 1. Cumulative Incidence of Bleeding Events and Atherosclerotic Cardiovascular Disease (ASCVD) Events Stratified by Coronary Artery Calcium (CAC).
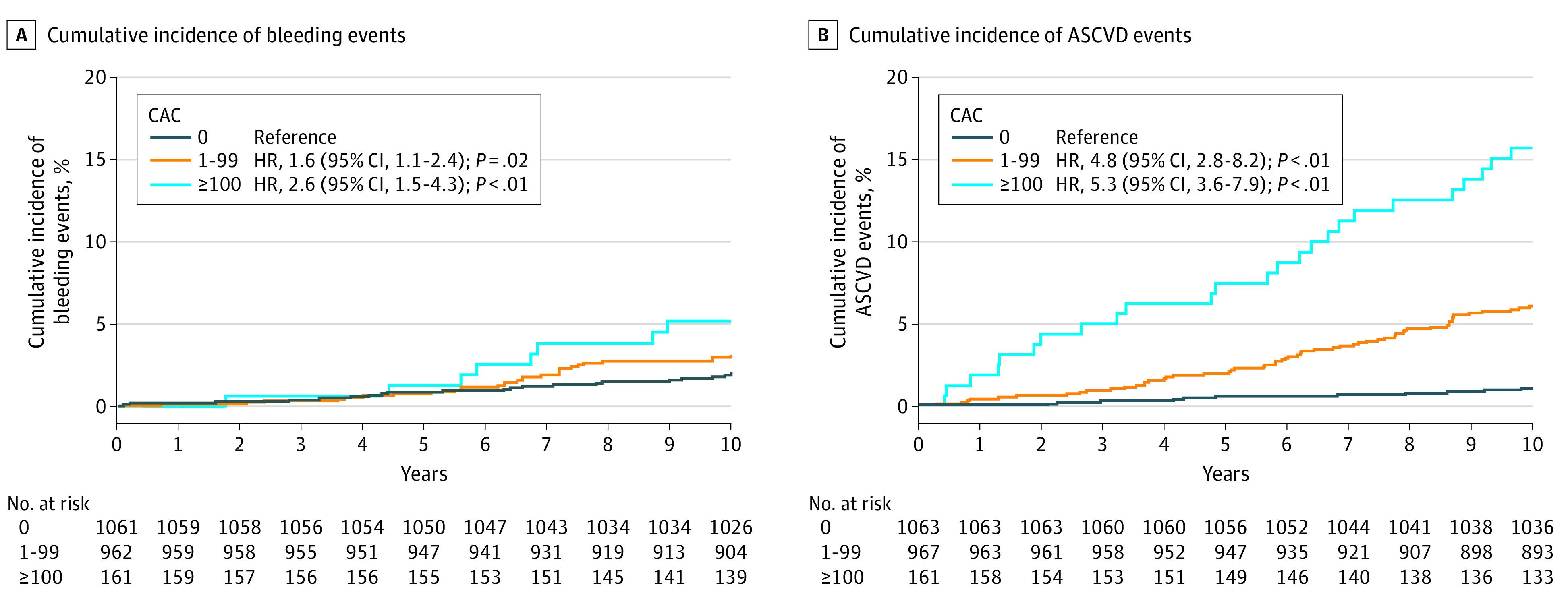
Table 2. Bleeding Event Rate and Hazard Ratios Stratified by CAC.
| CAC | No. | Bleeding events, No. | Bleeding event rate, 1000 person-years (95% CI) | Unadjusted bleeding, HR (95% CI) | P value | Adjusted bleeding HR (95% CI)a | P value |
|---|---|---|---|---|---|---|---|
| All | 2191 | 116 | 3.4 (3.4-3.5) | NA | NA | NA | NA |
| 0 | 1063 | 40 | 2.3 (2.2-2.3) | 1 [Reference] | NA | 1 [Reference] | NA |
| 1-99 | 967 | 59 | 3.8 (3.7-3.8) | 1.6 (1.1-2.4) | .02 | 0.9 (0.6-1.4) | .62 |
| >100 | 161 | 17 | 7.0 (6.9-7.1) | 2.6 (1.5-4.3) | <.001 | 1.5 (0.8-2.6) | .19 |
Abbreviations: CAC, coronary artery calcium; HR, hazard ratio; NA, not applicable.
Model adjusted for age, sex, race/ethnicity, systolic blood pressure, antihypertensive use, smoking status, diabetes, peptic ulcer disease, protein pump inhibitor/antacid, and corticosteroid use.
Estimated Association of Aspirin With Bleeding and ASCVD Event Rates at Different CAC Thresholds
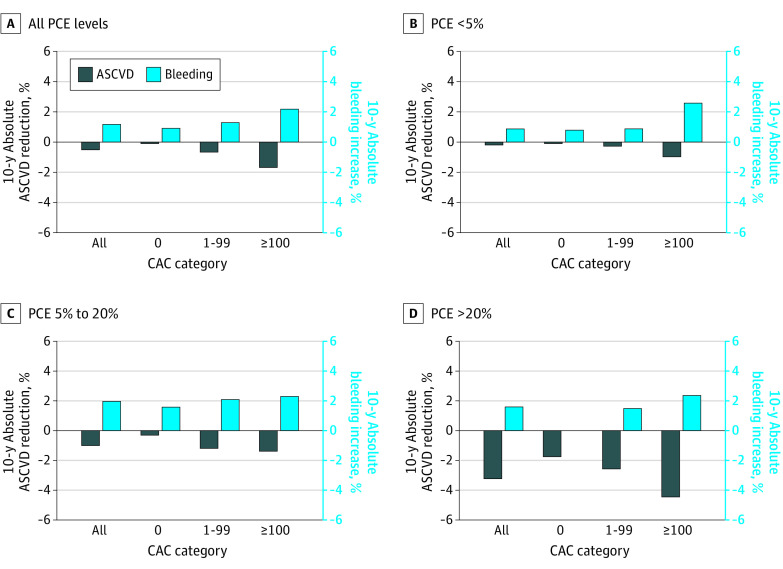
Applying effect estimates extrapolated from a 2019 primary prevention aspirin meta-analysis16 to observed 10-year bleeding and ASCVD event rates in the overall cohort, aspirin would increase bleeding more than it would reduce ASCVD events for all CAC groups (Figure 2; eTable 5 in the Supplement). This excess harm across CAC categories was consistent in those at low (<5%) and intermediate (5%-20%) 10-year ASCVD risk. However, in those at high estimated 10-year ASCVD risk (>20%), aspirin was anticipated to be net beneficial regardless of CAC value. These findings were consistent even when evaluating individuals with CAC ≥300 (eFigure 2 and eTable 5 in the Supplement). They were also replicated in sensitivity analyses in individuals without interval aspirin uptake (eFigure 3 and eTable 5 in the Supplement), in 5-year outcomes (eFigure 4 and eTable 5 in the Supplement), using aspirin effect estimates from contemporary trials (eFigure 5 and 6 and eTable 5 in the Supplement), and stratifying the cohort by age (eFigure 7 and eTable 5 in the Supplement).
Figure 2. Estimated Increase in Bleeding Event Rate vs Reduction in Atherosclerotic Cardiovascular Disease (ASCVD) Event Rate With Aspirin Use For Primary Prevention In Dallas Heart Study (DHS) Participants Stratified by ASCVD Risk and Coronary Artery Calcium (CAC).
Estimates were generated by applying an 11% relative risk reduction to 10-year ASCVD event rates and a 43% relative risk increase to 10-year bleeding event rates. The number of individuals in each subgroup were as follows: all (N = 2191), pooled cohort equation (PCE) less than 5% (n = 1063), PCE 5% to 20% (n = 967), and PCE at least 20% (n = 161).
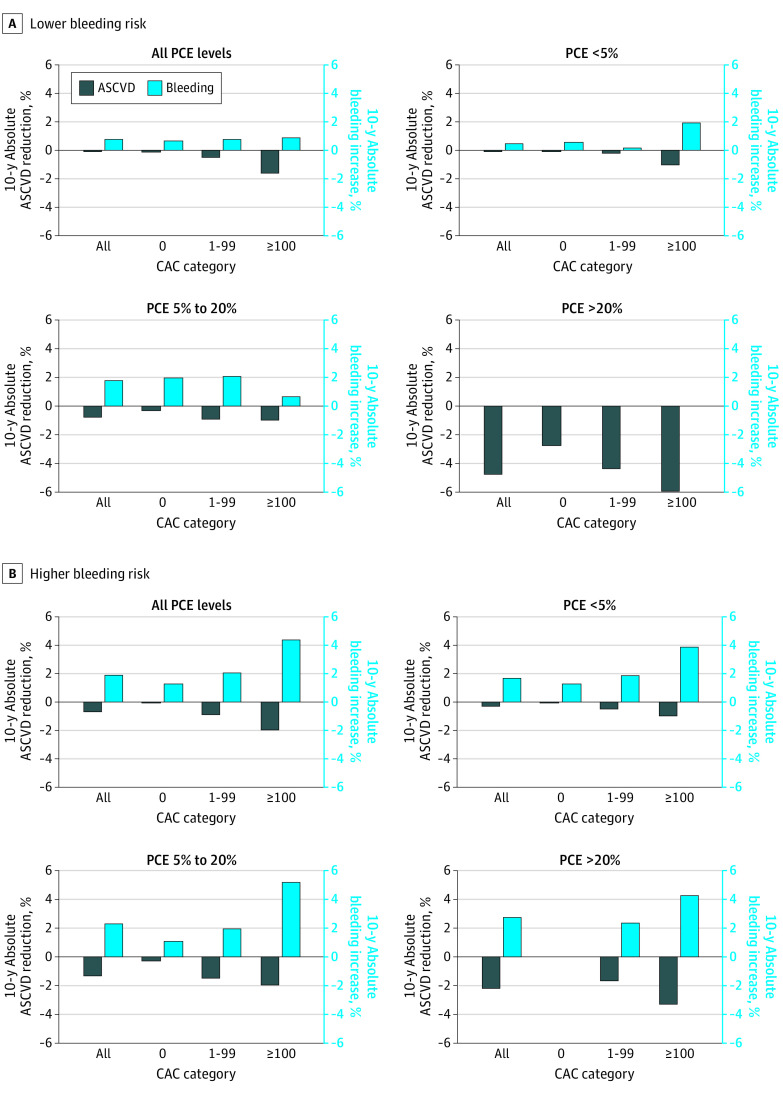
In those at lower bleeding risk, bleeding with aspirin exceeded ASCVD reduction in those with CAC of 0 and 1 to 99, but aspirin was net beneficial in those with CAC of at least 100 (Figure 3; eTable 5 in the Supplement). When stratifying by estimated 10-year ASCVD risk, those with CAC of at least 100 demonstrated potential net benefit from aspirin in the intermediate 10-year ASCVD risk (5%-20%) group but not in the low 10-year ASCVD risk (<5%) group. Similar to the overall cohort, aspirin was anticipated to be net beneficial regardless of CAC value in those at high 10-year ASCVD risk (≥20%), although no bleeding events were observed in this group. In those at higher bleeding risk, aspirin was net harmful regardless of CAC value in all ASCVD risk groups (Figure 3).
Figure 3. Estimated Increase in Bleeding Event Rate vs Reduction in Atherosclerotic Cardiovascular Disease (ASCVD) Event Rate With Aspirin Use for Primary Prevention in Dallas Heart Study (DHS) Participants at Lower and Higher Bleeding Risk Stratified by ASCVD Risk and Coronary Artery Calcium (CAC).
Estimates were generated by applying an 11% relative risk reduction to 10-year ASCVD event rates and a 43% relative risk increase to 10-year bleeding event rates. The low bleeding risk group excluded individuals that reported the use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, anticoagulants, selective serotonin reuptake inhibitors (SSRIs), statins, protein pump inhibitor (PPI)/antacids, antiplatelet agents, peptic ulcer disease (PUD), cirrhosis, cancer, chronic kidney disease (CKD), thrombocytopenia, and uncontrolled blood pressure. The number of individuals in each subgroup were as follows: lower bleeding risk: all (n = 1430), pooled cohort equation (PCE) less than 5% (n = 1050), PCE 5% to 20% (n = 294), PCE at least 20% (n = 25); higher bleeding risk: all (n = 761), pooled cohort equation (PCE) less than 5% (n = 497), PCE 5% to 20% (n = 214), and PCE at least 20% (n = 36).
Discussion
Using a large population cohort inclusive of individuals with risk factors for bleeding, with more than 10 years of follow-up and ascertainment of both bleeding and ASCVD events, we demonstrate that higher CAC scores are associated with increased bleeding events. When modeling the effects of aspirin use for primary prevention in those at lower risk of bleeding, a CAC score of at least 100 identified individuals who would experience net benefit from those that would not. After stratification by ASCVD risk, this finding held only in those at borderline or higher (>5%) 10-year ASCVD risk. Finally, in individuals at higher risk of bleeding, there would be net harm from aspirin irrespective of CAC and ASCVD risk. These findings have important implications for the use of CAC in identifying patients suitable for aspirin therapy for primary prevention of cardiovascular disease.
The recognition that CAC is associated with both bleeding and ASCVD risk is crucial to determining whether CAC can be used to select individuals for antithrombotic therapy. In our study, individuals in the highest CAC group had almost 3-fold the bleeding risk and 5-fold the ASCVD risk of those in the lowest CAC group. Our results align with those of Arps et al,18 who looked at ASCVD and major bleeding events stratified by CAC in 5196 Multiethnic Study of Atherosclerosis (MESA) participants as part of an effort to model the effects of rivaroxaban. Higher CAC was associated with both bleeding and ASCVD events, but with a greater magnitude for ASCVD. The association of CAC with both ASCVD and bleeding reflects the substantial overlap between risk factors for ASCVD and bleeding.1,7,14,19 Because both bleeding and ASCVD risk increase with CAC, both must be taken into account to accurately model the effect of antithrombotic therapy at different CAC thresholds.
Adopting this approach, we found that at the extremes of ASCVD risk, CAC had no value for identifying individuals who would benefit from primary prevention aspirin therapy. Notably, aspirin would be net harmful in those at low (<5%) ASCVD risk regardless of CAC. Our results align with those from 3 contemporary randomized controlled primary prevention aspirin trials4,5,6 that showed that aspirin therapy conferred no or marginal net benefit in individuals at low observed ASCVD risk (5-year risk <10%). We also show that aspirin would result in net harm, irrespective of CAC and ASCVD risk, in individuals at higher risk of bleeding. Although the greatest risk of net harm was predicted in individuals at the lowest cardiovascular risk, those at the highest cardiovascular risk were also predicted to suffer net harm. These findings provide empirical support for the 2019 ACC/AHA guidelines7 on the primary prevention of cardiovascular disease, which recommend against the use of primary prevention aspirin in individuals at increased risk of bleeding. It is important to note that there is some subjectivity on factors associated with increased bleeding, and a “nonexhaustive list” was provided by the 2019 ACC/AHA prevention guidelines that differ somewhat from the 2016 US Preventive Services Task Force list.20 More work is needed to examine primary prevention aspirin use patterns, clearly define individuals at higher risk of bleeding, and discourage the use of aspirin for primary prevention in these individuals. In the high (≥20%) ASCVD risk group, we estimated aspirin to be beneficial in the overall cohort and net harmful in those at higher bleeding risk, regardless of CAC. However, given the small number of participants and bleeding events in this subgroup (eTable 4 in the Supplement), we believe these findings require further validation.
Individuals with CAC of 0 generally have a very low risk of ASCVD events and guidelines support de-escalating risk and deferring statin therapy in these groups owing to lack of net benefit.21 Similarly, we observed lack of net benefit of aspirin in those with CAC of 0, particularly in those at low (<5%) and intermediate (5%-20%) ASCVD risk. Although aspirin appeared to be net beneficial in those with CAC of 0 at high ASCVD risk (>20%), there were too few individuals in this group to draw any meaningful conclusions (n = 6).
Two other articles8,9 have examined the utility of CAC for identifying individuals for primary prevention aspirin therapy. In the study by Miedema et al,8 a proportional ASCVD reduction of 18% and proportional bleeding increase of 31% was applied to cardiovascular event rates in 4229 participants from the MESA cohort, free from ASCVD at baseline stratified by CAC. Here, aspirin was estimated to be net favorable in the group with CAC of at least 100.8 However, based on meta-analyses,16,22,23 the risk estimates used in this study24 overestimate the proportional reduction in ASCVD events conferred by aspirin (18% vs 10%-11%) and underestimate its effect on bleeding events (proportional increase of 31% vs 43%-50%, respectively). Furthermore, absolute bleeding rate was assumed to be constant across the CAC spectrum examined.
The second study by Cainzos-Achirica et al9 applied the relative risk estimates used in our study to observed ASCVD and bleeding rates in 3540 aspirin-naive MESA participants at low bleeding risk stratified by CAC. As in our study, they found that a CAC score of at least 100 identified individuals that would derive net benefit from aspirin. The main difference in our findings lies in the suggestion that this finding holds irrespective of baseline ASCVD risk. In the study by Cainzos-Achirica et al,9 absolute bleeding rate was assumed to be constant within each ASCVD risk stratum irrespective of CAC, while we incorporate increase in bleeding events with higher CAC. We believe this may account for the variability we observed across the ASCVD risk spectrum. Finally, differences in bleeding event rate between the studies may play a role. Our 10-year bleeding rate of 2.7% (3.4 events per 1000 person years) in the overall cohort is comparable to what is reported in other studies17,19 but higher than what is reported (1.8% 10-year bleeding rate) in the MESA study.9 This difference might be owing to more complete capture of bleeding events in the DHS, because DFWHC data collection does not depend on participant report for hospitalized events. It may also be owing to oversampling of African American individuals (approximately 50%) who have higher bleeding rates in the DHS. However, notably, we found no difference in the association between CAC and bleeding or ASCVD by race/ethnicity, which suggests that race/ethnicity did not significantly affect this association. Women were also overrepresented in our sample, and the DHS is a relatively young cohort (mean age in our sample was 44 years vs 57 years in MESA). This might have resulted in fewer ASCVD events relative to bleeding events. Here again, we found no difference by sex or age in the association between and CAC and ASCVD or bleeding.
The 2019 ACC/AHA Primary Prevention Guideline gave aspirin a cautious recommendation for use in select adults aged 40 to 70 years at higher ASCVD risk but not at increased bleeding risk.7 That guideline mentioned that CAC among other risk-enhancing factors might be considered to identify this group. Our findings support the use of CAC only in those at lower bleeding risk and borderline or higher ASCVD risk. These findings should contribute to the patient-clinician shared discussion regarding aspirin use for primary prevention.7
Limitations
Our bleeding events were identified by ICD-9 codes and were unadjudicated leading to the possibility of misclassification. However, prior studies have also relied on ICD-9 codes for bleeding,8,9 and our bleeding rate in the overall cohort was comparable with what was reported in prior studies17,19 It is possible that there is confounding by indication such that those with higher CAC scores were more likely to subsequently initiate aspirin, thus contributing to higher bleeding events with increasing CAC in our study. However, when we restricted to those without interval aspirin uptake since their baseline examination, the results were consistent with those seen in our main analysis. Given our limited event numbers, we were unable to explore more extreme thresholds of CAC (ie, >1000). Finally, our results from a US-based cohort may not be generalizable to populations outside of the United States.
Conclusions
In a large, population-based cohort, we found that increasing CAC was associated with both ASCVD and bleeding events. Modeling the effects of aspirin for primary prevention, a high CAC score identifies individuals who would derive net benefit from primary prevention aspirin therapy from those who would not, but only in the setting of lower bleeding risk and borderline or higher cardiovascular risk.
eFigure 1. Participant selection
eFigure 2. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by ASCVD risk and CAC (including CAC >300 category)
eFigure 3. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants without interval aspirin uptakea
eFigure 4. Estimated increase in 5-year bleeding event rate vs reduction in adjudicated 5-year ASCVD event rate with aspirin use for primary prevention stratified by CAC
eFigure 5. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by ASCVD risk and CAC (aspirin effect estimate taken from contemporary aspirin trials)
eFigure 6. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants at lower bleeding risk stratified by ASCVD risk and CAC (aspirin effect estimate taken from contemporary aspirin trials)
eFigure 7. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by age, ASCVD risk and CAC
eTable 1. ICD codes used to identify bleeding events from the DFWHC and NDI databases
eTable 2. Bleeding event categories
eTable 3. ASCVD event rate and hazard ratios stratified by CAC
eTable 4. Bleeding event rate and hazard ratios stratified by CAC in DHS participants without interval aspirin uptake
eTable 5. ASCVD and bleeding event rates in various subgroups
References
- 1.Baigent C, Blackwell L, Collins R, et al. ; Antithrombotic Trialists’ (ATT) Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-1860. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miedema MD, Huguelet J, Virani SS. Aspirin for the primary prevention of cardiovascular disease: in need of clarity. Curr Atheroscler Rep. 2016;18(1):4. doi: 10.1007/s11883-015-0555-0 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM Should aspirin be used for primary prevention in the post-statin era? N Engl J Med. 2018;379(16):1572-1574. doi: 10.1056/NEJMe1812000 [DOI] [PubMed] [Google Scholar]
- 4.McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group . Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaziano JM, Brotons C, Coppolecchia R, et al. ; ARRIVE Executive Committee . Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi: 10.1016/S0140-6736(18)31924-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 7.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7(3):453-460. doi: 10.1161/CIRCOUTCOMES.113.000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cainzos-Achirica M, Miedema MD, McEvoy JW, et al. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019: the MESA Study (Multi-Ethnic Study of Atherosclerosis). Circulation. 2020;141(19):1541-1553. doi: 10.1161/CIRCULATIONAHA.119.045010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503-2512. doi: 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44(9):1812-1818. doi: 10.1016/j.jacc.2004.07.047 [DOI] [PubMed] [Google Scholar]
- 12.Jain T, Peshock R, McGuire DK, et al. ; Dallas Heart Study Investigators . African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44(5):1011-1017. doi: 10.1016/j.jacc.2004.05.069 [DOI] [PubMed] [Google Scholar]
- 13.Maroules CD, Khera A, Ayers C, et al. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014;16(1):33. doi: 10.1186/1532-429X-16-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selak V, Jackson R, Poppe K, et al. Predicting bleeding risk to guide aspirin use for the primary prevention of cardiovascular disease. Ann Intern Med. 2019;170(6):357-368. doi: 10.7326/M18-2808 [DOI] [PubMed] [Google Scholar]
- 15.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 16.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi: 10.1001/jama.2018.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selak V, Kerr A, Poppe K, et al. Annual risk of major bleeding among persons without cardiovascular disease not receiving antiplatelet therapy. JAMA. 2018;319(24):2507-2520. doi: 10.1001/jama.2018.8194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arps K, Rifai MA, Blaha MJ, et al. Usefulness of Coronary Artery Calcium to Identify Adults of Sufficiently High Risk for Atherothrombotic Cardiovascular Events to Consider Low-Dose Rivaroxaban Thromboprophylaxis (from MESA). Am J Cardiol. 2019;124(8):1198-1206. doi: 10.1016/j.amjcard.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307(21):2286-2294. doi: 10.1001/jama.2012.5034 [DOI] [PubMed] [Google Scholar]
- 20.Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2016;164(12):826-835. doi: 10.7326/M15-2112 [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud AN, Elgendy IY. The influence of the baseline 10-year atherosclerotic cardiovascular disease risk on cardiovascular outcomes with aspirin for primary prevention: a meta-regression analysis. Eur Hear J. 2020;6(2):175-176. doi: 10.1093/ehjqcco/qcz049 [DOI] [PubMed] [Google Scholar]
- 23.Abdelaziz HK, Saad M, Pothineni NVK, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol. 2019;73(23):2915-2929. doi: 10.1016/j.jacc.2019.03.501 [DOI] [PubMed] [Google Scholar]
- 24.Seshasai SRK, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(3):209-216. doi: 10.1001/archinternmed.2011.628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Participant selection
eFigure 2. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by ASCVD risk and CAC (including CAC >300 category)
eFigure 3. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants without interval aspirin uptakea
eFigure 4. Estimated increase in 5-year bleeding event rate vs reduction in adjudicated 5-year ASCVD event rate with aspirin use for primary prevention stratified by CAC
eFigure 5. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by ASCVD risk and CAC (aspirin effect estimate taken from contemporary aspirin trials)
eFigure 6. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants at lower bleeding risk stratified by ASCVD risk and CAC (aspirin effect estimate taken from contemporary aspirin trials)
eFigure 7. Estimated increase in bleeding event rate vs reduction in ASCVD event rate with aspirin use for primary prevention in DHS participants stratified by age, ASCVD risk and CAC
eTable 1. ICD codes used to identify bleeding events from the DFWHC and NDI databases
eTable 2. Bleeding event categories
eTable 3. ASCVD event rate and hazard ratios stratified by CAC
eTable 4. Bleeding event rate and hazard ratios stratified by CAC in DHS participants without interval aspirin uptake
eTable 5. ASCVD and bleeding event rates in various subgroups