Summary
Several assays have recently been developed to measure and characterize the replication-competent HIV-1 reservoir, which constitutes the barrier to cure. To date, application of these assays to studies in children and in limited-resource settings has been limited, primarily due to their expense, large required blood volumes, high costs, and labor-intensive technologies. In vertically HIV-1 infected children who initiated suppressive antiretroviral therapy (ART) regimens in infancy, HIV-1 specific antibody levels are associated with levels of viral persistence and could be used as tools to estimate the size of the residual latent reservoir on ART. This may be particularly useful for screening these children on suppressive ART for enrollment into therapeutic vaccine and other protocols aimed at achieving HIV-1 remission.
Introduction
In 2018, an estimated 37.9 million people worldwide were living with HIV-1 and among them, 1.7 million are children (<15 years old1). Most of these children currently live in sub-Saharan Africa and were vertically infected during pregnancy, childbirth or breastfeeding.2 With the introduction of antiretroviral prophylaxis and maternal antiretroviral therapy (ART), new mother-to-child HIV-1 infections among children have declined by 35% (from 270,000 in 2010 to 160,000 in 2018).1 Early ART reduces HIV-1 associated mortality in infants3; thus, current treatment guidelines4–6 recommend initiation of antiretroviral treatment as early as possible after birth regardless of clinical and immunological status. Successful implementation of testing at birth and again within the first few weeks of life could allow earlier HIV-1 diagnosis and treatment initiation, especially in resource limited settings.7
Although ART has reduced HIV-1 related diseases and mortality in children, it is not curative, due to the early establishment of a long-lived latent reservoir, consisting of replication-competent, proviral HIV-1 DNA integrated into the genomes of CD4 T cells.8–10 These cells are not cleared by ART and are a source of viral rebound if treatment is discontinued.11 The identification of biomarkers, including immune responses or measures of the latent HIV-1 reservoir, that could accurately predict the risk, rate, and amplitude of viral load rebound off ART is important for advancing clinical trials testing HIV-1 remission strategies.
An ever-increasing array of assays is available to measure and characterize the latent HIV-1 reservoir on ART. Application of many early assays to studies in children and in limited-resource settings was limited, primarily due to their expense, large required blood volumes, high costs, and labor-intensive technologies. Some newer assays are simpler, less expensive, and require smaller blood volumes, and are now being used. They are also being incorporated into clinical trials examining strategies that could further reduce the size of the latent reservoir in children, and/or that could enhance immune control of HIV-1 replication. For example, proof of concept studies exploring in African settings the ability of a therapeutic vaccine strategy (NIH 5U01AI135941) and a dual neutralizing antibody regimen (NCT03707977; Clinicaltrials.gov) to reduce the size of HIV viral reservoir in perinatally HIV-infected children (PHIV) were funded by the National Institutes of Health. Simple, inexpensive approaches that use small blood volumes to measure viral persistence in children on suppressive ART is of critical importance for advancing these and similar initiatives. This review discusses the potential utility of using HIV-1 specific antibodies to monitor response to ART and to estimate cell-associated HIV-1 reservoirs in vertically HTV1 - infected children on suppressive ART.
Search strategy and selection criteria
References for this review were identified through PubMed searches conducted from June, 2019 through January, 2020 using the following terms: “latent reservoir”, “children”, “cell associated HIV-1 DNA”, “cell associated HIV-1 RNA”, “HIV-1 antibodies”, “measures of viral reservoirs”, and “early therapy”. References were selected based on their relevance to the topics presented in this review.
Measurement and characterization of the latent HIV-1 reservoir:
Several assays (reviewed in 12, have been used to estimate the size (1 in 105-108 CD4+ T cells) and nature of the latent HIV-1 reservoir in adults and children on ART. Table 1 summarizes available assays and those used to date in pediatric studies.
Table 1: Assays used to measure and characterize the latent HIV-1 reservoir.
Assays are listed in the order of minimal estimates of the latent HIV-1 reservoir (top) to maximal estimates of the latent HIV-1 reservoir (bottom). Those used in pediatric studies are noted. Abbreviations: QVOA, quantitative viral outgrowth assay; LR, latent reservoir; ms, multiply spliced; us, unspliced; ddPCR, droplet digital PCR. Modified from source: Bruner KM. et al. Trends Microbiol. 201538
| Assay | Measure | Excludes | Method | Advantages | Disadvantages |
|---|---|---|---|---|---|
| QVOA14 | Inducible replication-competent proviral genomes (“latent reservoir”) | Non-induced replication-competent provirus | Limiting dilution of CD4+ T cells, followed by potent T cell activation, addition of T cell blasts or cell lines to amplify virions, detection of HIV p24 antigen or RNA in culture supernatant | Measures replication-competent genomes induced after one or more rounds of T cell activation. | Expensive, time and labor intensive, requires large blood volumes; underestimates true size of latent reservoir as not all intact proviruses are induced after a single round of activation. Latent reservoir size often below limit of detection with long term viral suppression after early ART in children16 |
| Tat/rev Induced Limiting Dilution Assay (TILDA)34 | Inducible intracellular multiply spliced RNA | Defective provirus and those that are intact but not transcribed after T cell activation | Detection of multiply-spliced tat/rev transcripts by real-time PCR after limiting dilution and activation of CD4 T cells | Feasible for large scale clinical studies; can be completed in 1-2 days; requires fewer cells than QVOA | Does not directly measure replication-competent virus; might not stimulate all inducible virus Pediatric cells might require greater stimulation35 |
| Single copy viral load assay (SCA) | Free virus production/ongoing viral replication | Not applicable | Real-time PCR | Measures plasma HIV RNA to a single copy level | Can be derived from defective genomes |
| Cell-associated HIV-1 RNA24,27 | Intracellular HIV-1 RNA (MS and US) | Primer mismatch. Transcriptionally silent but inducible HIV-1 DNA | ddPCR, qPCR | Feasible for large scale clinical studies. Allows for quantitation of transcriptionally active proviruses | Transcripts may be defective. |
| RNA-Seq60 | Transcriptional profiling | RNA species detected dependent on assay | Uses next generation sequencing to detect and measure viral and cellular transcripts | Assays can be varied to detect and measure coding (mRNA), as well as small or noncoding (e.g., miRNA) RNA transcripts; can be performed on single cells | High cost; gene expression may change over time or under different conditions; complex analysis, requires high performance computing. |
| Matched integration site and proviral sequencing (MIP-Seq)60 | Profiles individual intact or defective proviruses and their corresponding chromosomal integration sites | Inducible or infectious virus | Multiple displacement amplification of individual proviral species, followed by near-full-length HIV-1 next-generation sequencing and corresponding chromosomal integration site analysis | Maps chromosomal positions and orientation of intact and defective proviruses | Expensive, time and labor intensive, requires large blood volumes. |
| Transposase-accessible chromatin using sequencing (ATAC-Seq)60 | Evaluates proximity of HIV-1 proviral integration sites to active transcriptional start sites and accessible chromatin regions | Inducible or infectious virus | Tn5 transposase inserts into open chromatin sites, and simultaneously cleaves and inserts sequencing adapters into genomic DNA for next gen sequencing | Can be performed on bulk or single cells | Expensive, time and labor intensive, requires large blood volumes. |
| Intact proviral DNA assay (IPDA)35,61 | Intact and defective genomes | Inducible or infectious virus | Droplet digital PCR (ddPCR) method that distinguishes most deleted and/or hypermutated proviruses from intact proviruses using two amplicons and hypermutation discrimination probes | Allows the quantitation of intact and defective genomes and overcomes many limitations of total proviral DNA | Large blood volume required. Does not provide information on the inducibility of the proviruses within the latent reservoir . |
| Nearly full-length proviral amplification and sequencing (NFL-PAS)18 | Intact vs. defective genomes and APOBEC3G | Inducible or infectious virus; 8.8 kb fragment excludes first 793 nucleotides of HIV-1 genome; | Nested PCR | Uses primers that bind conserved HIV genome regions | Difficult to measure intact provirus, high cost, complex analysis |
| HIV-1 DNA quantitation51,59 | Total HIV-1 provirus | Primer mismatch can limit amplification of HIV-1 DNA | qPCR, droplet digital PCR | Feasible for large-scale clinical studies. Does not require viable cells. | Measures all HIV-1 proviruses and does not distinguish between integrated vs. unintegrated, defective vs. intact proviruses |
The Quantitative Viral Outgrowth Assay (QVOA) was the first assay used to define and estimate the size of the latent reservoir in adults13 and children14 and is a culture-based method in which CD4 T cells are plated at limiting dilution and then treated with a T cell activator; replication-competent viruses are expanded exponentially through addition of activated, uninfected CD4 T cells, followed by measurement of viral antigen or nucleic acids in the culture supernatant. Recent studies have shown that a single round of stimulation and viral expansion underestimates the frequency of replication-competent viruses by 60-fold; multiple rounds of T cell stimulation or use of novel T cell activators have increased the assay yield.12 Use of the QVOA has been limited in pediatric populations due to the relatively large blood volumes required. Nevertheless, studies using the QVOA have demonstrated that the latent reservoir is established early and is markedly reduced by early ART initiation.15 The size of the latent reservoir continues to decline over time to the point that replication-competent virus is infrequently recovered after a decade or more of viral suppression16,17; near full-length sequencing of non-induced proviral genomes has revealed a predominance of replication-defective, non-intact genomes and a paucity of replication-competent viruses.17,18
Quantitative PCR-based assays (e.g., qPCR and droplet digital PCR assays, ddPCR) rapidly and directly measure HIV-1 nucleic acids, are highly sensitive, reproducible, and require small blood volumes, the latter an important consideration in studying infants and young children. Thus, PCR-based assays of peripheral blood cell-associated HIV-1 DNA have been the most widely measured approximation of the residual HIV-1 reservoir in children. However, quantitative PCR-based assays detect relatively short amplicons of conserved HIV-1 genomic regions and measure all forms of DNA, including integrated and unintegrated HIV-1 DNA. Moreover, standard quantitative HIV-1 DNA PCR assays do not differentiate between intact and defective virus19 and may overestimate the latent reservoir by up to 300-fold.20 Using quantitative PCR assays, very low levels of total and integrated HIV-1 DNA were detected in HIV-1-infected children who started ART before 6 months of age.16,21–24 These observations were confirmed in additional studies indicating that early-treated, HIV-infected infants who rapidly suppress HIV-1 replication exhibit lower levels of circulating cell-associated HIV-1 DNA compared to those who start treatment later.25–30 Pre-treatment HIV-1 DNA levels, as well as HIV-1 replication exposure over the first 12 months of ART, estimated as area-under-the-curve (AUC) of circulating plasma HIV-1 RNA levels, were significantly associated with PBMC HIV-1 DNA at one year26; PBMC HIV-1 DNA levels at 1 year and 4 years of ART correlated with age at ART initiation and age at virologic control. Similarly, Tagarro and colleagues showed that for each month delay in ART initiation, HIV-1 DNA levels (copies per 106 peripheral blood mononuclear cells, PBMCs) increased by 13%.31 In two studies of children who initiated suppressive ART at a median of two months of age, PBMC-associated HIV-1 DNA levels declined by approximately one log over the first year of ART and slowed thereafter.26,32 More recently, Veldsman et al have demonstrated much more rapid decline of PBMC-associated HIV-1 DNA levels in children who initiated suppressive ART in the first week of life compared to children who initiated suppressive ART later.33 Altogether, these data indicate that younger age at ART initiation and reduced exposure to HIV-1 replication are associated with lower PBMC HIV-1 DNA levels at initiation and after one year of age. This supports the concept that HIV-1 diagnosis and ART initiation in infants should occur as early as possible.
Several newer assays have focused on measuring the replication-competent latent HIV-1 reservoir. The Tat/rev Induced Limiting Dilution Assay (TILDA) combines in vitro stimulation with ultrasensitive detection of HIV-1 RNA to measure the frequency of cells with inducible multiply-spliced HIV-1 RNA, transcripts that are present in recently activated cells but not usually present in latently-infected cells.34 While the assay does not directly measure virus production, it is sensitive, reproducible, has a broad dynamic range, and can be completed fairly quickly (1-2 days); the relatively low cell input required makes it suitable for studying pediatric populations. Using a standard TILDA assay, Dhummakupt et al35 reported that the induced reservoir size in a cohort of perinatally infected adolescents (median age, 15.8 years) on long-term suppressive ART (median duration virologic suppression 6.7 years) was significantly lower (median 2.99 multiply-spliced RNA producing units per million CD4+ T cells; msRUPM) than in adults (median 11.92 msRUPM; p = 0.020). In addition, the proportion of induced provirus in the perinatally-infected adolescents (median, 1.62%) was significantly lower than that in adults (median = 4.03%; p = 0.030). Use of an enhanced TILDA assay with additional in vitro stimulation led to a 1.5-fold increase in the detected inducible reservoir in the perinatally-infected cohort. Total circulating HIV-1 DNA levels did not correlate with the size of the inducible reservoir as measured by either TILDA assay. Altogether, these data confirm prior studies showing that only a small fraction of circulating provirus is inducible, and that there may be a difference in HIV-1 proviral susceptibility to induction in children compared with adults. Further longitudinal studies are needed to better understand HIV-1 proviral susceptibility.
A number of factors can account for the differences in reservoir composition such as the immune landscape in which HIV-1 infection is established, including a more tolerogenic immunological environment due to higher proportions of T-regulatory cells in early infancy and childhood36; as well as differences in the distribution of memory T cell subsets at different ages37. Few pediatric studies have directly evaluated infection of CD4+ memory subsets; we have previously reported that the reservoir predominated in effector memory CD4+ T cells in perinatally infected adolescents16, highlighting possible differences in the composition of the reservoir in perinatal compared to adult infections.
Proviral inducibility may depend on several factors, including whether the proviral genome is intact, where it integrates into the host genome, and the activation or differentiation state of the host cell. Near full-length, single template next generation proviral HIV-1 sequencing of longitudinal samples from a cohort of mostly in utero infected infants who initiated ART within a few days of birth demonstrated a rapid decline in PBMC-associated intact and defective proviruses over the first 24 weeks of ART30; decay of intact proviruses was significantly faster than decay of defective proviruses. Of interest, intact proviruses appeared to be preferentially integrated in the same orientation as the host genes, which contrasts with the common integration of proviruses in the opposite direction to host genes in adults.
Another recently developed assay, the intact proviral DNA assay (IPDA) allows direct quantitation of intact vs defective (deletions or hypermutations) proviruses, and has demonstrated an excess of defective proviruses in ART-treated individuals.38 The IPDA also requires relatively small numbers of CD4 T cells and with some adaptation, may be suitable for use in pediatric populations.35
HIV-1 specific antibodies in infected children on ART:
The kinetics of plasma viral load and HIV-1 associated disease progression are strongly affected by the age at the time of infection. Primary infection in infancy is characterized by a sustained high-level plasma viral load, which decreases very slowly in the initial phases of infection39,40, while in adult infection the initial plasma virus load peak is rapidly followed by a 100- to 1,000-fold decrease in viral copies, reaching a relative stable “set point” within weeks of infection.41 This age-associated disparity in the viral kinetics of HIV-1 is thought to be due to a larger CD4 T cell compartment in infants and children42–44, combined with less robust innate or adaptive immune responses.45
Immune system differences between adults and children can also influence the ontogeny of HIV-1 specific antibodies. In HIV-1 infected adults, the initial specific antibody response is detected approximately 14 days after infection and initially targets gp4146; in the absence of treatment, HIV-1 antibody levels are stable over time and correlate with viral load.47 With the administration of antiretroviral treatment, HIV-1 specific antibody levels decline, reflecting the levels of viral persistence and providing an estimate of effective viral suppression over time.47 Infants born to HIV-1 infected women acquire maternal antibodies during the last trimester of gestation that may persist in the child’s circulation up to 18 months.48,49 In infants, de novo antibody production is generally thought to occur after 4-6 months of age against a restricted number of HIV-1 antigens; antibodies against gp160 appear first, followed by anti-gp120 and anti-gp41 antibodies.50 Infants who rapidly control HIV-1 replication after starting ART by 3 months of age clear HIV-1 antibodies of all specificities (except p17) at the same rate as HIV-1 exposed, uninfected infants51, suggesting very little de novo antibody production in the first few months of life. In one of the first early ART clinical trials, a majority (14 of 15) of infants with rapid and prolonged suppression of HIV-1 replication cleared passively-acquired (maternal) HIV-1 specific antibodies at the same rate as HIV-1 exposed, uninfected infants, and remained HIV-1 antibody seronegative45, while generating normal antibody responses to routine childhood vaccinations. Subsequent studies conducted in several different settings have demonstrated an absence of persistent HIV-1 specific antibodies in 20-60% of infants at or after 18 months of age (Figure 1) who initiated suppressive ART prior to 3 months of age.22,52,53 For example, in a study of children from South Africa, Payne and colleagues reported that −50% of children who initiated ART between 0 and 12 weeks were HIV-1 seronegative by commercial assays at nearly 2 years of age compared with only 6% of infants starting ART between 12-24 weeks, and none starting ART after 24 weeks of age.22 Age at ART initiation and cumulative early viral load were strong independent predictors of HIV-1 serostatus at 2 years of age. In addition, recent data54 suggest that in HIV-1 seronegative, infected children who initiated early ART, B- and T-cells are characterized by a reduced maturation due to shorter antigen exposure. This results in distinct qualitative differences in their memory cell compartments which can be summarized in a reduced ability of B cells to induce plasma cell differentiation and defective T/B cognate stimuli (eg, IL-21 production) in the T cell upon re-stimulation with HIV-1 antigens. Altogether, these findings are consistent with the premise that HIV-1 specific antibody production seems to be strongly affected by the age of ART start, time to viral load suppression, and duration of exposure to HIV-1 antigens; early ART rapidly removes the antigenic stimulation needed to develop and sustain an HIV-1 specific antibody response.
Figure 1. Percentage of early-treated (< 3 months) children who are HIV-1 seronegative at or after 18 months of age by age.
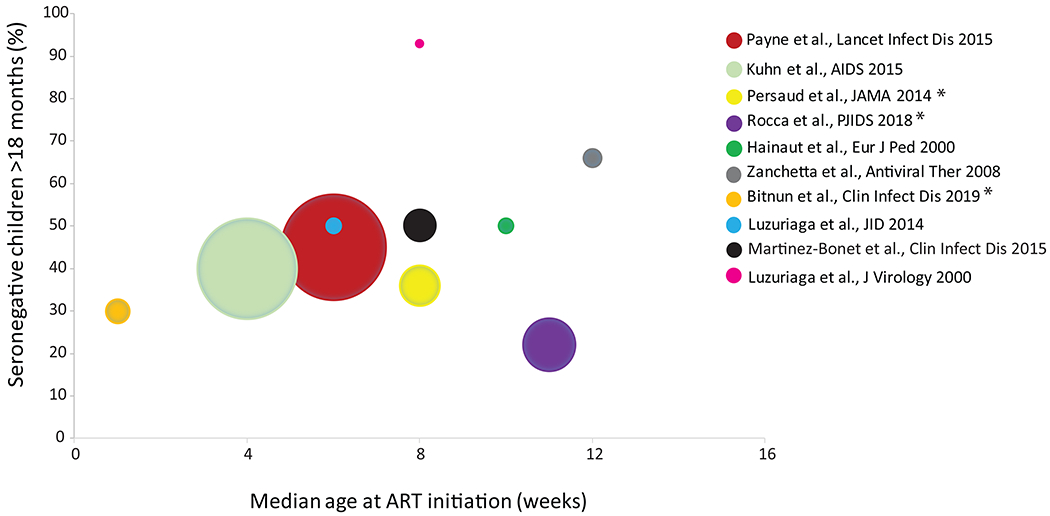
The size of individual circles reflects the size of the study cohort. The median age at ART initiation was estimated as the mid-point of the range. As described in the text, only 4-6% of children who initiate ART between 12 and 24 weeks are HIV-1 seronegative and all children who initiate ART at 6 months or older are HIV-1 seropositive. Studies that report an association between HIV-1 antibodies and PBMC-associated HIV-1 DNA are marked with an asterisk (*); these and additional studies demonstrating a relationship between HIV-1 antibodies and PBMC-associated HIV-1 DNA are reported in the text.
The magnitude of HIV-1 antibody responses predicts levels of PBMC HIV-1 DNA in virally suppressed HIV-1 infected children on ART
The quantitation and characterization of HIV-1 specific antibodies in adults55 and children29,51,56 may provide a cost-effective and highly reproducible tool to estimate viral replication and the latent reservoir in patients on long-term ART with viral suppression. The Pediatric HIV/AIDS Cohort Study (PHACS) revealed an association between early ART initiation and low PBMC-associated HIV-1 DNA levels in children; the lowest HIV-1 DNA levels were observed in children with negative or indeterminate HIV-1 serostatus determined by Western blot (Wb).23 Similarly, in the case of a child who received ART from 30 hours after birth until 18 months of age, HIV-1 specific antibodies, plasma HIV-1 RNA, and peripheral blood cell-associated DNA remained undetectable for 27 months off ART57; when virologic rebound occurred at 46 months of age, the child developed antibodies to all HIV-1 proteins.58 Research in this area is moving towards the creation of models that use HIV-1 specific antibody responses to predict levels of HIV-1 viral persistence. (Figure 2). In this context, a study conducted on 69 vertically HIV-1infected children on suppressive ART revealed that the magnitude of the HIV-1 antibody response is closely related to total PBMC HIV-1 DNA levels.59 HIV-1 antibodies were quantified by Western blot (Wb) and a score was assigned to each patient according to the different antigen positivity revealed through the assay. Lower Wb scores (range from 0 to 2·5), indicative of a reduced breadth of antibody response, were found in these early-treated children and were associated with lower levels of PBMC HIV-1 DNA (from 0 to 200 copies/106PBMCs). High Wb scores (range from 4·5 to 10, with antibody responses to many antigens, including gp160, gp120, gp41, p24, p17) were found in children who initiated ART at 1 year of age or older, and who had higher levels of circulating HIV-1 DNA.
Figure 2.
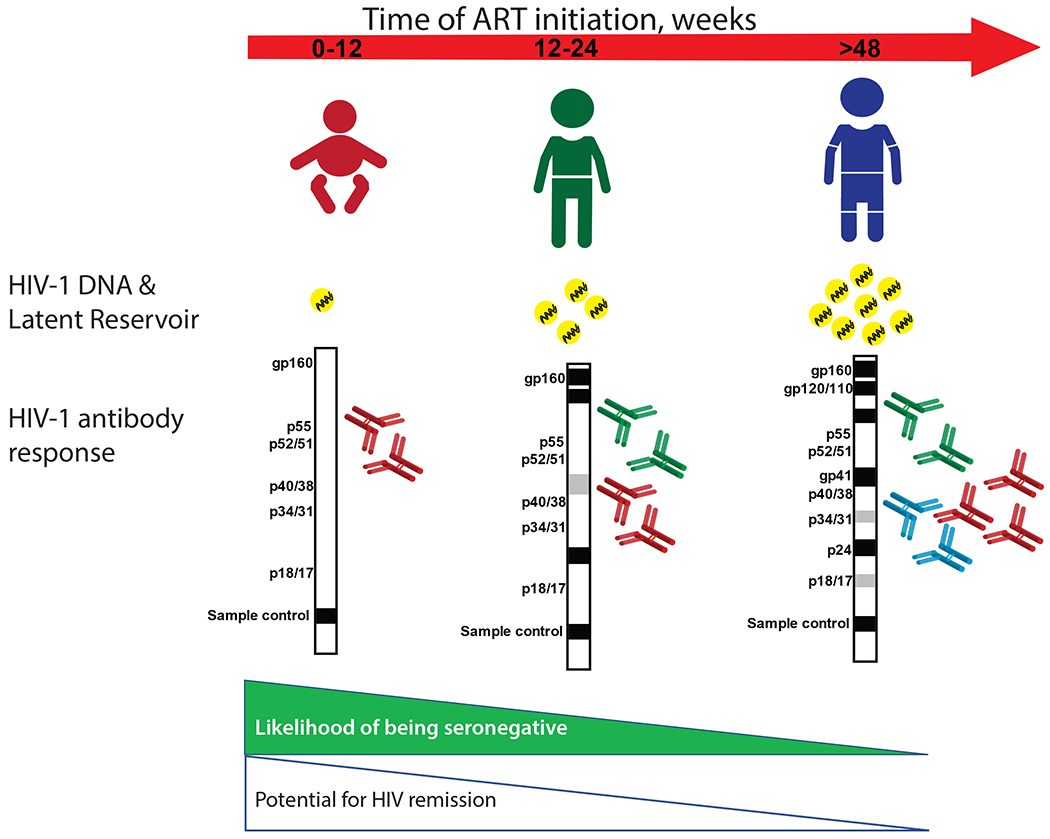
Association between timing of ART initiation, HIV-1 specific antibody responses, and HIV-1 persistence in virally suppressed perinatally HIV-infected children.
Similarly, Luzuriaga and colleagues showed that the quantitation of HIV-1 specific antibodies by ELISA assay may be useful for screening ART-treated children for control of HIV-1 replication or for PBMC HIV-1 DNA levels.51 Models were developed based on antibody clearance rates to predict the likelihood of undetectable plasma HIV-1 RNA or the level of circulating PBMC-associated HIV-1 DNA.51 In particular, gp160 and gp41-specific antibody levels were significantly associated with PBMC-associated HIV-1 DNA levels while antibodies against p31 and p17 were significantly associated with HIV-1 RNA levels. More specifically, a logistic model using gp160-specific antibody levels estimated that each unit increase in log gp160 antibody was associated with a 6-fold increase in the odds of PBMC HIV-1 DNA >1000. Bitnun and colleagues have also shown a positive correlation between HIV-1 specific antibodies measured by a routine clinical assay and peripheral blood cell-associated HIV-1 DNA levels.29
There are several potential caveats to the use of quantitative antibody levels to screen for low levels of residual latent HIV-1 reservoirs in children on suppressive ART. First, the presence of passively acquired maternal HIV-1 antibodies that can persist in a child’s blood up to 18 months of age may affect the utility of serological assays in this younger age group. Secondly, most studies have been conducted in children who have initiated early suppressive ART prior to generating a full HIV-1 specific antibody response; more work needs to be done using quantitative antibody assays to define the decay of HIV-1 antibody responses in children who initiate suppressive ART after they have developed HIV-1 specific antibody responses. Finally, while quantitative antibody levels have been shown to predict PBMC HIV-1 DNA levels, further research using new assays discussed above is necessary to understand the potential relationship between HIV-1 specific antibody levels, measures of intact and inducible HIV-1 provirus, and time to viral rebound off therapy.
Conclusions
Starting from small plasma sample volumes (less than 100 μL), serological assays allow low cost, rapid detection of HIV-1 specific antibodies to different viral components. Several studies have demonstrated that the detection and measurement of HIV-1 specific antibodies provide an effective estimate of PBMC HIV-1 DNA levels in children with durable suppression of HIV-1 replication after early ART initiation. Further work using recently developed assays is needed to determine the potential utility of quantitative HIV-1 specific antibody assays in predicting the size of the replication-competent reservoir. Simple, inexpensive approaches that use small blood volumes to measure viral persistence in children on suppressive ART may be useful for screening children for participation in clinical trials testing strategies aimed at HIV-1 remission, particularly in resource-limited settings.
Acknowledgments.
We thank Jennifer Faudella, Ilaria Pepponi, Daniel Gomezpena, Silvia Faggioni, and Inger Lindfors Rossi for administrative assistance. This work was supported by the EPIICAL Project, an investigator-led network focused on the early identification of novel therapeutic strategies for HIV infected children (https://www.epiical.org/ ). The EPIICAL project receives independent funding from ViiV Healthcare U.K through the PENTA-ID Foundation (http://penta-id.org/ ). Additional support for the work was provided by the U.S. National Institutes of Health through NCATS/NIH UL1 TR001453-03 (Luzuriaga) and U01AI135941 (Cotugno, Palma), grants from Children’s Hospital Bambino Gesú (Ricerca corrente 2018-2019), and the Associazione Volontari Bambino Gesù.
Conflict of Interest Statement:
Drs. Palma and Rossi report an independent, indirect grant from ViiV Foundation through PENTA foundation, during the conduct of the study. Ms. McManus, Dr. Rocca, and Dr. Cotugno have nothing to disclose. Dr. Luzuriaga reports grants from the U.S. NIH and consulting fees from Gilead, during the conduct of the study; consulting fees from Up To Date, outside the submitted work.
References
- 1.UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet. 2019. https://www.unaids.org/en/resources/fact-sheet2019).
- 2.Luzuriaga K, Mofenson LM. Eliminating Pediatric HIV-1 Infection. N Engl J Med 2016; 375(2): 193–4. [DOI] [PubMed] [Google Scholar]
- 3.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359(21): 2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.In: nd, ed. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva; 2016. [PubMed] [Google Scholar]
- 5.Foster C, Bamford A, Turkova A, et al. Paediatric European Network for Treatment of AIDS Treatment Guideline 2016 update: antiretroviral therapy recommended for all children living with HIV. HIV Med 2017; 18(2): 133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzon MJ, Martin-Gayo E, Pereyra F, et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol 2014; 88(17): 10056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchuenche M, Gill MM, Bollinger L, et al. Estimating the cost of diagnosing HIV at birth in Lesotho. PLoS One 2018; 13(8): e0202420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ananworanich J, McSteen B, Robb ML. Broadly neutralizing antibody and the HIV reservoir in acute HIV infection: a strategy toward HIV remission? Curr Opin HIV AIDS 2015; 10(3): 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15(8): 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley JM, Hill BJ, Ambrozak DR, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 2004; 78(3): 1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science 2009; 323(5919): 1304–7. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Simonetti FR, Siliciano RF, Laird GM. Measuring replication competent HIV-1: advances and challenges in defining the latent reservoir. Retrovirology 2018; 15(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278(5341): 1295–300. [DOI] [PubMed] [Google Scholar]
- 14.Persaud D, Pierson T, Ruff C, et al. A stable latent reservoir for HIV-1 in resting CD4(+) T lymphocytes in infected children. J Clin Invest 2000; 105(7): 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persaud D, Palumbo PE, Ziemniak C, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26(12): 1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210(10): 1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainwater-Lovett K, Ziemniak C, Watson D, et al. Paucity of Intact Non-Induced Provirus with Early, Long-Term Antiretroviral Therapy of Perinatal HIV Infection. PLoS One 2017; 12(2): e0170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katusiime MG, Halvas EK, Wright I, et al. Intact HIV Proviruses Persist in Children Seven to Nine Years after Initiation of Antiretroviral Therapy in the First Year of Life. J Virol 2020; 94(4): e01519–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharaf RR, Li JZ. The Alphabet Soup of HIV Reservoir Markers. Curr HIV/AIDS Rep 2017; 14(2): 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9(2): e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28(7): 1015–20. [DOI] [PubMed] [Google Scholar]
- 22.Payne H, Mkhize N, Otwombe K, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis 2015; 15(7): 803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persaud D, Patel K, Karalius B, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168(12): 1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J Infect Dis 2015; 212(1): 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Bonet M, Puertas MC, Fortuny C, et al. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-infected Children Initiating Very Early Antiretroviral Therapy. Clin Infect Dis 2015; 61(7): 1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McManus M, Mick E, Hudson R, et al. Early Combination Antiretroviral Therapy Limits Exposure to HIV-1 Replication and Cell-Associated HIV-1 DNA Levels in Infants. PLoS One 2016; 11(4): e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uprety P, Patel K, Karalius B, et al. Human Immunodeficiency Virus Type 1 DNA Decay Dynamics With Early, Long-term Virologic Control of Perinatal Infection. Clin Infect Dis 2017; 64(11): 1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn L, Paximadis M, Da Costa Dias B, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One 2018; 13(4): e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitnun A, Ransy DG, Brophy J, et al. Clinical correlates of HIV-1 DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Broncano P, Maddali S, Einkauf KB, et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci Transl Med 2019; 11(520): eaax7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tagarro A, Chan M, Zangari P, et al. Early and Highly Suppressive Antiretroviral Therapy Are Main Factors Associated With Low Viral Reservoir in European Perinatally HIV-Infected Children. J Acquir Immune Defic Syndr 2018; 79(2): 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uprety P, Chadwick EG, Rainwater-Lovett K, et al. Cell-Associated HIV-1 DNA and RNA Decay Dynamics During Early Combination Antiretroviral Therapy in HIV-1-Infected Infants. Clin Infect Dis 2015; 61(12): 1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldsman KA, Janse van Rensburg A, Isaacs S, et al. HIV-1 DNA decay is faster in children who initiate ART shortly after birth than later. J Int AIDS Soc 2019; 22(8): e25368–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Procopio FA, Fromentin R, Kulpa DA, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine 2015; 2(8): 874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhummakupt A, Rubens JH, Anderson T, et al. Differences in inducibility of the latent HIV reservoir in perinatal and adult infection. JCI Insight 2020; 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV infection: the potential for cure. Nat Rev Immunol 2016; 16(4): 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein N, Palma P, Luzuriaga K, et al. Early antiretroviral therapy in children perinatally infected with HIV: a unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet Infect Dis 2015; 15(9): 1108–14. [DOI] [PubMed] [Google Scholar]
- 38.Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 2015; 23(4): 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mofenson LM, Korelitz J, Meyer WA 3rd, et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1)RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis 1997; 175(5): 1029–38. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh K, Shevitz A, Zaknun D, et al. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J 1996; 15(12): 1087–91. [DOI] [PubMed] [Google Scholar]
- 41.Martinez DR, Permar SR, Fouda GG. Contrasting Adult and Infant Immune Responses to HIV Infection and Vaccination. Clin Vaccine Immunol 2016; 23(2): 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krogstad P, Uittenbogaart CH, Dickover R, Bryson YJ, Plaeger S, Garfinkel A. Primary HIV infection of infants: the effects of somatic growth on lymphocyte and virus dynamics. Clin Immunol 1999; 92(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 43.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 1997; 130(3): 388–93. [DOI] [PubMed] [Google Scholar]
- 44.Erkeller-Yuksel FM, Deneys V, Yuksel B, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr 1992; 120(2 Pt 1): 216–22. [DOI] [PubMed] [Google Scholar]
- 45.Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol 2000; 74(15): 6984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 2008; 82(24): 12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keating SM, Pilcher CD, Jain V, et al. HIV Antibody Level as a Marker of HIV Persistence and Low-Level Viral Replication. J Infect Dis 2017; 216(1): 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J 1995; 14(5): 382–7. [DOI] [PubMed] [Google Scholar]
- 49.Pahwa S Human immunodeficiency virus infection in children: nature of immunodeficiency, clinical spectrum and management. Pediatr Infect Dis J 1988; 7(5 Suppl): S61–71. [PubMed] [Google Scholar]
- 50.Pollack H, Zhan MX, Ilmet-Moore T, Ajuang-Simbiri K, Krasinski K, Borkowsky W. Ontogeny of anti-human immunodeficiency virus (HIV) antibody production in HIV-1-infected infants. Proc Natl Acad Sci U S A 1993; 90(6): 2340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McManus M, Henderson J, Gautam A, et al. Quantitative Human Immunodeficiency Virus (HIV)-1 Antibodies Correlate With Plasma HIV-1 RNA and Cell-associated DNA Levels in Children on Antiretroviral Therapy. Clin Infect Dis 2019; 68(10): 1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanchetta M, Anselmi A, Vendrame D, et al. Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther 2008; 13(1): 47–55. [PubMed] [Google Scholar]
- 53.Kuhn L, Schramm DB, Shiau S, et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS 2015; 29(9): 1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotugno N, Morrocchi E, Rinaldi S, et al. Early ART-treated perinatally HIV-infected seronegative children demonstrate distinct long-term persistence of HIV-specific T and B cell memory. AIDS (London, England) 2020: 10.1097/QAD.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burbelo PD, Bayat A, Rhodes CS, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 2014; 209(10): 1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rocca S, Zangari P, Cotugno N, et al. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J Pediatric Infect Dis Soc 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369(19): 1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372(8): 786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocca S, Zangari P, Cotugno N, et al. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J Pediatric Infect Dis Soc 2019; 8(5): 433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Einkauf KB, Lee GQ, Gao C, et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest 2019; 129(3): 988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019. 566(7742). 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


