After a century since the first antimonial-based drugs were introduced to treat the disease, anti-schistosomiasis drug development is again at a bottleneck with only one drug, praziquantel, available for treatment purposes.
After a century since the first antimonial-based drugs were introduced to treat the disease, anti-schistosomiasis drug development is again at a bottleneck with only one drug, praziquantel, available for treatment purposes.
Abstract
Globally, schistosomiasis threatens more than 700 million lives, mostly children, in poor localities of tropical and sub-tropical areas with morbidity due to acute and chronic pathological manifestations of the disease. After a century since the first antimonial-based drugs were introduced to treat the disease, anti-schistosomiasis drug development is again at a bottleneck with only one drug, praziquantel, available for treatment purposes. This review focuses on promising chemotypes as potential starting points in a drug discovery effort to meet the urgent need for new schistosomicides.
1.0. Introduction
Schistosomiasis (Bilharzia) is caused by trematode worms belonging to the genus Schistosoma, of which six species are clinically relevant in humans. Prevalence is high in poor regions of tropical South America, Africa, the Arabian Peninsula, and Asia.1 In 2017, 99 million people were treated worldwide, with the Africa foci constituting 88% of the global total.2 The prevalence of the disease in the Africa region is attributable to two main species, S. mansoni and S. haematobium, which are also responsible for the occurrence of the disease in the Arabian Peninsula and South America (mainly S. mansoni). S. japonicum occurs mainly in China, the Philippines, and Indonesia. However, S. intercalatum, S. mekongi, and S. guineensis represent small foci.3 Children are more prone to high levels of infection, which is characterized by high egg and adult worm burdens, largely as a result of more frequent social and recreational activities in contaminated water bodies. Economic activities, such as fishing and farming, are known means of transmission of the infection especially in adolescents and adults.3 The disease prevalence in adolescents and adults is much lower even if they were previously infected.4
Apart from their definitive host (human), Schistosoma species require an intermediate snail host to complete their life cycle (Fig. 1). Primary infections occur after the penetration of cercariae through human skin. These infections are initially characterized by a temporal urticarial rash (cercarial dermatitis), which is very similar to the swimmer's itch. Acute schistosomiasis (Katayama fever) sets in a few weeks to months after primary infection owing to the immune system's reactions to migrating schistosomula.5S. haematobium worms reside in the pelvic venous plexus while the other five Schistosoma species harbor the mesenteric veins of the bowel.1
Fig. 1. The life cycle of human Schistosoma species (courtesy: Centers for Disease Control and Prevention).1.
The pathological manifestation of schistosomiasis is as a result of the host immune system's reaction to schistosome eggs rather than adult worms.6 Schistosome eggs secrete proteolytic enzymes that trigger immunopathological reactions resulting in the formation of defensive granulomas comprising of eosinophils, lymphocytes, neutrophils, T and B cells, and macrophages.4 Established or chronic schistosomiasis is marked by granulomatous inflammation of the host tissues, pseudopolyposis, ulceration and bleeding (in intestinal schistosomiasis). Hematuria, hydroureter, and hydronephrosis are associated with urinary schistosomiasis while intestinal schistosomiasis manifestations include abdominal pain, loss of appetite and diarrhea, with or without bleeding.6 Hepatic or hepatosplenic schistosomiasis occurs when parasite eggs are swept from the mesenteric veins to the liver and is characterized by enlargement of the spleen and the liver, especially the left lobe which, in high infections, could result in hepatosplenomegaly. Minor forms of the disease include pulmonary schistosomiasis, genital schistosomiasis, and neuroschistosomiasis.6
From a medicinal chemistry perspective, the life cycle of schistosomes and the schistosomiasis disease pathology provide opportunities for the discovery of drugs to prevent, control, and cure schistosomiasis. Schistosomicides against newly transformed schistosomula (NTS) and juvenile worms will prevent the maturation of these parasites into adult worms and hence useful for prophylaxis. Chemotherapy against adult worms will only lead to partial control of infections. However, antischistosomal agents which have activity against Schistosoma eggs, such as a reduction in egg burden, viability or development, will be necessary to prevent proliferation and transmission of parasite populations. More importantly, drugs which are capable of penetrating granulomas and specifically target Schistosoma eggs will offer both control and cure of schistosomiasis infection in the human host. In effect, medicinal chemistry efforts must, to a large extent, be geared towards drugs that are active against immature parasites and Schistosoma eggs to prevent morbidity (acute and chronic schistosomiasis) and progression of the parasite's life cycle beyond the human host.
Interventions aimed at the water stage of the Schistosoma life cycle, such as snail population control and the use of antiparasitic agents against miracidia and cercariae, will be helpful for the control and prevention of transmission of the disease. Such interventions will include the provision of safe and potable water, clean sanitation, alternative socioeconomic practices, and training on protection measures.4 Further, chemicals targeting the intermediate snail host, miracidia, and cercariae must be specific to preserve the water ecosystem, both biota and fauna. While the foregoing presents inevitable challenges, the fight against schistosomiasis, with the goal of eradication, appears more achievable via chemotherapy against the human host parasitic forms of schistosomes.7
Schistosomiasis drug discovery in the 20th century yielded a diverse class of chemical scaffolds as promising and potent schistosomicides with varying potencies against either specific or all stages of the parasite's life cycle in the human host while others, like oxamniquine (OXA) and metrifonate, were only potent against S. mansoni and S. haematobium, respectively.8 The introduction of praziquantel (PZQ) into clinical use, unfortunately, had a downturn on other promising antischistosomal agents. For over three decades of PZQ's usage as the main clinically used antischistosomal drug, coupled with a lack of any clinically relevant candidate drug in development, it has become necessary that promising but relinquished antischistosomal agents be revisited, as exemplified by the rescue of the toxic compounds lucanthone and hycanthone leading to the discovery of OXA.9
The first decade of the 21st century witnessed an increase in schistosomiasis drug discovery research necessitated by the lack of an alternative schistosomiasis drug and the over-reliance on PZQ in all endemic foci, leading to the likely emergence of widespread resistance. In this regard, medicinal chemistry approaches capable of potentially shortening the drug discovery and development timeline have been extensively explored. Notable amongst them is the repositioning and/or repurposing of established drugs.10–14 Several reviews15–20 and book chapters,21–23 which highlight progress made in the current schistosomiasis drug discovery landscape, have been reported. Notwithstanding this progress, no substitute or complement has been identified for PZQ yet, although some antimalarials are promising.24 On the other hand, new chemotypes with good antischistosomal properties, which hold the potential to populate the drug development pipeline if investigated further, have been identified. In the sections below, we focus our attention on such chemotypes (covering the period of 2009 to date) and point out their usefulness as essential starting points for medicinal chemistry structure–activity relationship (SAR) studies towards identifying potential leads. Meanwhile, we have also briefly discussed some useful chemotypes from the 20th century with special focus on their schistosomicidal properties and propose that, together with the new chemical scaffolds, medicinal chemists involved in schistosomiasis drug discovery have a plethora of arsenals to combat the disease. Finally, we discuss our perspective on the needed paradigm shift in future medicinal chemistry research to ensure the eradication of schistosomiasis.
2.0. How far have we come?
In the quest to discover the next generation of clinically useful schistosomicides, much research has gone into chemical modifications of known schistosomiasis drugs, especially PZQ and OXA, established antimalarial drugs, and approved drugs in other disease indications. For PZQ and OXA, medicinal chemistry efforts have focused on expanding their antischistosomal properties, such as identifying PZQ-based analogs with potency against both larval and juvenile schistosomes or expanding the efficacy of OXA to other Schistosoma species beside S. mansoni. New derivatives of these drugs have been identified, for example, through molecular hybridization strategies. In this regard, this section recounts new developments on old/known drugs in the schistosomiasis drug discovery venture.
PZQ (Fig. 2), an acylated quinoline–pyrazine derivative, is the mainstay of schistosomiasis treatment and the World Health Organization (WHO) recommended drug for mass drug administration programs aimed at reducing morbidity. From 2007–2018, Merck has donated more than 900 million tablets to school-age children.2 Though the drug is only effective against the mature adult worms, it is cheap, has good efficacy and safety profiles and is active against all human Schistosoma spp.8 PZQ is fast-acting despite its short half-life, arising from extensive first-pass metabolism (∼80%) in the liver.4 In the presence of PZQ, adult worms undergo tetanic contractions in their musculature, coupled with the appearance of tegumental vacuoles. The surface damage incurred causes worms to detach from the walls of veins and die.25 Although currently administered in the racemate form, the efficacy of PZQ is associated with the R-enantiomer.26,27
Fig. 2. Promising PZQ derivatives with schistosomicidal activity comparable/superior to PZQ towards juvenile and adult worms.
Initial SAR investigations leading to the discovery of PZQ revealed that the tricyclic pyrazino[2, 1-a]isoquinoline was optimal for its antihelminthic activity against S. mansoni and Hymenolepis nana. The acyl and oxo groups at positions 2 and 4, respectively, are essential for schistosomicidal activity. Similarly, the cyclohexyl ring was found to be the optimal ring size for activity, since cycloalkyl groups lower/higher in size and aryl groups resulted in less potent analogs than PZQ.28 Recent SAR studies involving the synthesis of PZQ analogs have largely focused on the phenyl ring and the acyl group. PZQ derivatives have been identified with good potency against juvenile worms in both in vitro and in vivo assays. These new analogs have provided a potentially useful avenue for further medicinal chemistry exploration.
For example, amination of the benzene ring at the C10 position (1) was more tolerated (SmIC50 = 28 μM) than other variations. Similarly, replacement of the cyclohexyl group with the 3- and 4-aminophenyl groups was also tolerated (SmIC50 = 12 μM and 18 μM, respectively) albeit these analogs were >4-fold less potent than PZQ.29 This result led to the identification of the C10 hydroxy derivative (2) which demonstrated ex vivo potency superior to PZQ (62.9% worm mortality at 50 μM) against S. japonicum adult worms with 100% worm mortality at 25 μM in 72 h. Compound 2 also showed 100% juvenile worm mortality at 15 μM in 72 h, while PZQ was inactive at >50 μM. However, in vivo, the worm burden reduction (WBR) by PZQ was 66.7% while compound 2 showed 40.2% WBR against S. japonicum adult worms after an oral dose of 200 mg kg–1. Both compounds exhibited comparable WBR (16–20%) against juvenile worms.30 The C8 bromo derivative (3) also demonstrated 100% adult worm reduction at >10 μM in 48 h in an in vitro culture of adult S. japonicum, which was comparable to PZQ. Towards juvenile worms, 3 caused <10% worm vitality reduction at 25 μM in 72 h, just as PZQ. In vivo, for an oral dose of 250 mg kg–1 given to mice harboring adult S. japonicum infection, 3 exhibited WBR of 54.3% compared to PZQ's 62.4%.31
Replacement of the phenyl ring in PZQ with thiophene, as in 4 (Fig. 2), resulted in comparable in vitro schistosomicidal activity (EC50 = 0.9 μM) to PZQ against S. mansoni adult worms.32 While maintaining the thiophene ring, Wang and colleagues undertook modifications on the acyl group at position 2 of the PZQ structure. Their work identified the chloroacetamido derivative, 5, which killed 100% of S. japonicum adult worms at 10 μM in 72 h compared to 37.5% for PZQ at the same concentration.33 In a separate study, compound 6, the ketone oxidation product of the major trans-cyclohexyl metabolite of PZQ, was strategically designed to increase the stability of PZQ towards metabolism. Although inactive in vitro, 6 demonstrated in vivo efficacy of 25% and 79% WBR against S. mansoni juvenile (PZQ, 7% WBR) and adult worms (PZQ, 96% WBR), respectively, after a single oral dose of 400 mg kg–1.34 Towards S. japonicum, in vitro, 6 caused 73.3% and 60.5% vitality reductions of adult and juvenile worms, respectively, after 50 μM of drug exposure in 24 h. In vivo, however, 6 killed more juvenile worms (60% WBR) than PZQ (19.9% WBR) after 200 mg kg–1 of drug was administered orally.35
Patra and coworkers explored organometallic PZQ analogs as potential antischistosomal agents.36–38 Most of the ferrocenyl derivatives incorporated at position 2 of PZQ were inactive at the highest concentration tested (30 μg ml–1). The most potent analog in this series was compound 7 (SmIC50 = 25.6 μM, Fig. 2).36 Two [(η6-PZQ)Cr(CO)3] derivatives (8 and 9) showed antischistosomal activity (SmIC50 ≈ 0.25 μM) against adult worms comparable to PZQ. Moreover, the compounds were non-cytotoxic (IC50 > 68 μM) against the HeLa and MRC-5 cells.37 Enantioselective schistosomicidal activity was observed for both compounds 8 and 9 with only the (R)-enantiomers showing superior activity. Metabolically, 8 produced PZQ as a major metabolite in human liver microsomes contrary to 9, which produced PZQ as one of the minor metabolites. The good in vitro activity recorded for 8 and 9 did not correlate with in vivo efficacy in a S. mansoni-mouse model, where after a single oral dose of 400 mg kg–1, only 24% and 29% WBR was recorded for 8 and 9, respectively.38
OXA (Fig. 3) is another example of a quinoline-based schistosomicide. It is cheaper and safer than PZQ, and has limited side effects. However, it is only active against S. mansoni parasites, and male worms are more susceptible to the drug than females.8 Until recently, it had been used for schistosomiasis treatment in Brazil, where S. mansoni is prevalent.39 Moreover, OXA-resistant strains have long been identified.8 OXA acts by alkylating DNA and other biomolecules, including proteins, leading to the death of the parasite. As a prodrug, it is biochemically converted to the reactive electrophilic 1,4-methineimine (11, Fig. 3) after sulfonation by S. mansoni sulfotransferase (SmSULT), followed by desulfonation. Nucleophilic attack on the reactive prodrug or its sulfonated precursor leads to parasite death. Structural differences in the active site of the sulfotransferases of S. haematobium, S. japonicum, and human hinder adequate binding and thus account for the specificity of OXA against S. mansoni.8,40,41 Although both enantiomers of OXA have schistosomicidal activity, the (S)-enantiomer is more potent than its mirror image compound due to its better binding to the active site of SmSULT.42
Fig. 3. Mechanism of action of OXA.
The SmSULT activated OXA molecule (10) has inspired the evaluation of a new series of ortho-nitrobenzyls as potential schistosomicides. These compounds, which were hypothesized to be potentially active agents against S. mansoni-resistant strains, were also assessed as inhibitors of the S. mansoni cathepsin B1 (SmCB1) enzyme involved in hemoglobin degradation in the parasite. Two compounds, 13 and 14 (Fig. 4), showed good potency against somules at 1 μM and exhibited mutagenic concentrations of at least 40 μM and 10 μM, respectively. The compounds had no effect on adult worms at 5 μM in 48 h and showed <27% inhibition of SmCB1 at 100 μM.43 Further, Rugel and coworkers explored more ortho-nitrobenzyl derivatives of OXA through a structure-based drug design approach using the active site of SmSULT. Compound 15 showed a broad-species activity of 75%, 40%, and 83% against S. mansoni, S. haematobium, and S. japonicum adult worms at 143 μM.44 This is the first report of an OXA derivative with activity against the major human Schistosoma species and hence, a good candidate for further SAR exploration.
Fig. 4. Promising OXA-based antischistosomal derivatives.
Like PZQ, organometallic complexes of OXA have been investigated as antischistosomal agents. Hess and colleagues identified the ferrocenyl (16), ruthenocenyl (17), and benzyl (18) derivatives of OXA with activity against both S. mansoni and S. haematobium. The three compounds showed comparable in vitro IC50 values of 11.4 μM, 8.7 μM, and 11.1 μM, respectively, against S. mansoni after 72 h. At a concentration of 100 μM, the compounds caused about 75% reduction in S. haematobium worm viability after 4 h and completely killed all worms after 48 h of exposure.45 Compound 16 was fast-acting on S. mansoni parasites but not on S. haematobium, to which 17 showed the quickest onset killing all males in 24 h. Similar patterns of tegumental damage were demonstrated by the three compounds against both species. Moreover, each compound showed additional distinct patterns of tegumental damage on S. mansoni worms.46In vivo, after oral administration of 100 mg kg–1 to S. mansoni-infected mice, compounds 16, 17, and 18 showed 81.0%, 93.7%, and 76.1% WBR compared to 99.2% for OXA. They also showed low toxicity with an IC50 > 73 μM against L6 rat cells after 72 h.45 According to Hess and colleagues, the schistosomicidal properties of 16, 17, and 18, namely, activity against juvenile and adult S. mansoni and S. haematobium worms in vitro, unlike OXA which lacks activity, are indicative of a possibly different mode of action for these organometallic derivatives.
In a separate study, a Mannich reaction of OXA in the presence of formaldehyde, paraformaldehyde, and acetaldehyde produced two cyclized derivatives on the alkylamine side chain and an ethyl ether derivative on the benzyl hydroxy group. Although some promising activity was observed against S. mansoni, these derivatives were highly toxic.47 Further, methacrylate and acrylamide prodrugs of OXA were explored to ensure a more prolonged pharmacological effect relative to OXA. However, in vivo, the prodrugs produced a similar biological profile to OXA.48
The antischistosomal properties of antimalarials have already been reviewed elsewhere.49 Notably, the artemisinins, trioxolanes, trioxaquines, and mefloquine are promising schistosomicidal agents worthy of further SAR investigations. Here, we recapitulate recent advances made with these chemical scaffolds with respect to newly identified and hybrid derivatives. For example, four artesunate–PZQ hybrid molecules (19–22, Fig. 5) were explored for activity against juvenile and adult S. japonicum worms ex vivo and in vivo.30 Compounds 19 and 20 killed 100% of adult worms ex vivo after 72 h of exposure to 20 μM of the drugs while complete death of juvenile worms was achieved at 25 μM of the drugs (19, 20, and 22) under similar conditions. In vivo, 19, 20, and 22 exhibited comparable WBR (41–57%) against the adult worms. However, 20 caused 70.3% WBR of juvenile worms compared to 19.8% by PZQ alone. In the same study, compounds 23–26 were less potent than the artemisinin-based hybrid molecules. The 1,2,4,5-tetraoxane hybrid, 23, demonstrated ex vivo complete reduction in motility of both adult and juvenile worms at 50 μM after 72 h. Compound 23 recorded low in vivo efficacy, 38.7% WBR and 25.1% WBR, against adult and juvenile S. japonicum worms, respectively.30
Fig. 5. Antischistosomal PZQ–artemisinin-based hybrid molecules.
Trioxaquines (hybrid molecules of 1,2,4-trioxane and aminoquinoline antimalarials) have also shown good activity against both juvenile and adult worms of S. mansoni.50,51 Recently, the in vivo efficacy of Synriam™ (a combination of OZ277 and piperaquine) in an S. mansoni-infected mouse model was described.52 Synriam™ exhibited good prophylactic properties against 1 day-old schistosomula with 85.4%, 68%, and 68.5% reductions in worm, liver, and intestinal egg counts, respectively. When orally administered [dosage 240 mg kg–1 (1 : 6 ratio of OZ277 : piperaquine)] at day 21 post-infection, worm, liver, and intestinal egg counts were reduced by 88.7%, 83.8%, and 87%, respectively. The drug's activity against juvenile worms was also evident in the significant reductions observed in the number and size of hepatic granulomas. The in vivo efficacy of Synriam™ was maintained even after 42 days post-infection with 70–81% reduction in parasite populations. Morphologically, the drug caused extensive alterations in teguments and sub-tegumental tissues of worms after exposure. Further, Mossallam and colleagues52 observed that activation of Synriam™ to elicit optimum parasite death was dependent on the presence of hemin. The schistosomicidal activity of Synriam™, being active against all S. mansoni life stages in the human host, including egg granulomas, makes it a viable candidate for further development as a promising schistosomicide and opens the opportunity for investigating such antimalarial drug combinations.
A systematic SAR investigation of endoperoxides comprising bridged 1,2,4,5-tetraoxanes, alphaperoxides, and tricyclic monoperoxides was conducted by Ingram and coworkers, who identified compounds 27 and 28 (Fig. 6) as lead antischistosomal agents in their study.53 About half of the synthesized analogs exhibited IC50 < 15 μM against NTS. Generally, the bridged 1,2,4,5-tetraoxalanes were the most potent series of analogs tested. Compound 27 showed significant activity against both NTS (IC50 = 0.1 μM) and adult worms (IC50 = 0.3 μM). A single oral dose of 400 mg kg–1 to S. mansoni-infected mice led to 43.1% WBR of juvenile worms and 75.4% WBR of chronic adult infections. The tricyclic monoperoxide analog, 28, was less active in vitro, (NTS, IC50 = 14.4 μM; adult worms, IC50 = 11.7 μM) but it caused 82.8% and 18.9% WBR in adult and juvenile worm infections, respectively. In a separate study, the 1,2,6,7-tetraoxaspiro[7,11]nonadecane molecule (29) exhibited in vitro activity (EC50 = 16 nM) against schistosomula of S. mansoni.54 A WBR of 63% was observed after 14 days post-infection of mice harboring S. mansoni, after a 300 mg kg–1 oral dose of the drug. No significant reduction in worm burden was observed when 29 was administered 5 weeks after infection. Hemozoin quantities were significantly reduced after drug exposure but no apparent tegumental damage was observed in adult worms. In a related study, 30 (a congener of 29) was used to show that this series of synthetic endoperoxides accumulates in and targets acidic lysosome-like organelles and disrupts their function, leading to worm death.55
Fig. 6. Antimalarial-derived compounds with promising antischistosomal activity.
The mefloquine-related arylmethanols, 31 and 32, caused high adult worm burden reductions in S. mansoni-infected mice.56 Compound 31 showed the highest in vivo efficacy with 87% WBR (oral dose, 100 mg kg–1) and 100% WBR (oral dose, 200 mg kg–1) of adult S. mansoni and S. haematobium worms, respectively. Two congeners of 31 exhibited 75–82% WBR of adult worms in S. mansoni-infected mice after an oral dose of 100 mg kg–1. Enpiroline (32) and its threo isomers reduced worm burdens by >82% (oral dose, 200 mg kg–1). In fact, (+)-threo-enpiroline completely cleared worms at this dosage. Compounds 31 and 32, therefore merit further SAR investigations. The ferrocenyl and ruthenocenyl derivatives of mefloquine demonstrated comparable activity to mefloquine against adult S. mansoni worms in vitro.57 More importantly, both compounds demonstrated lower toxicity than mefloquine. Unfortunately, the in vitro activity was not translated to in vivo efficacy. The ferrocenyl derivative showed no activity with a single oral dose of 200 mg kg–1 while the WBR for the ruthenocenyl derivative was 27.4%.
Although amodiaquine is inactive against S. mansoni,58 a hybrid molecule with furoxan, 33, exhibited in vitro and ex vivo schistosomicidal activity comparable to the phenyl furoxan counterpart alone, causing 100% complete death of S. mansoni worms at 50 μM after 24 h in the ex vivo experiment.59 Meanwhile, ferroquine, hydroxyl ferroquine and ruthenoquine were found to be poor antischistosomal agents, showing WBR values of 0–20% after a single oral dose of 200 mg kg–1 to a 49 day-old adult S. mansoni infection. The WBR of ferroquine increased to only 35.6% after worms were exposed to a dose of 800 mg kg–1.60 The bisquinoline tetraazamacrocycle 34 (Fig. 6) and its Fe(ii) and Mn(ii) metal complexes had IC50 values of 1.6 μM, 1.3 μM, and 4.1 μM against ex vivo adult S. mansoni, respectively.61 Despite their toxicity, both Fe(ii) and Mn(ii) complexes reduced worm burdens by 75% and 88%, respectively, when administered orally at a single dose of 400 mg kg–1.
Like the antimalarials, other drug families have also been screened to identify possible leads for repurposing against schistosomiasis. These drugs (Fig. 7) are promising hits for repositioning, requiring further SAR investigations to improve their in vivo activity and broad-species efficacy. These drugs demonstrated >50% reduction in Schistosoma parasite burdens in an infected animal model or, where in vivo results are not available (for example, paroxetine62), showed significant in vitro activity. At 10 μM in vitro, the decoquinate derivative (35) killed 100% S. japonicum adult worms in 72 h but the parent drug was inactive at 100 μM.63
Fig. 7. Promising antischistosomal hits identified through screening of approved drugs.11,12,62–69 .
Ro 13-3978 (36, Fig. 8), discovered by Hoffmann-La Roche (Switzerland), is a hydantoin-based compound with high in vivo schistosomicidal activity against the major schistosomes but possesses antiandrogenic side effects.70–73 However, recent interest in 36, particularly investigations to explore its SAR, have led to compound 37 (Fig. 8) with comparable antischistosomal activity and less antiandrogenic than 36.74–76 The privileged hydantoin scaffold is core to some clinically useful drugs such as the anticonvulsants phenytoin and its prodrug (fosphenytoin) and the antiandrogenic enzalutamide (a thiohydantoin). Wang and colleagues explored the multiple positions available for substitution on the hydantoin scaffold to elucidate the SAR requirements for antischistosomal and antiandrogenic activities based on 36.76 Further SAR investigations are needed to optimize the schistosomicidal properties of 37.
Fig. 8. Optimization of Ro 13-3978. aS. mansoni-infected mice (200 mg kg–1, p.o). bS. mansoni-infected mice (100 mg kg–1, p.o). cInhibition of dihydrotestosterone-induced androgen luciferase reporter activity in the LAPC4 cell line, a cell line with a wild-type androgen receptor.
Like OXA, the identification of 37 is a classic example of the medicinal chemistry efforts needed to rescue some of the promising schistosomicides of the 20th century which were abandoned, some due to toxicity issues. Such efforts are inevitable in this period when schistosomiasis drug development is at a bottleneck. In this regard, the relinquished antischistosomal agents Ro 11-3128 (38, Fig. 9) and Ro 15-5458 (39) also need revisiting. Ro 11-3128 (meclonazepam) contains the benzodiazepine privileged scaffold. It is highly potent against schistosomula and S. mansoni male adults in a culture medium. In hamsters, it exhibited comparable activity against both S. mansoni and S. haematobium parasites and similarly cured human patients infected with either of the two species.8,77,78 The drug was, however, found to be less active against S. japonicum parasites. Meclonazepam exhibits similar effects on parasite musculature like PZQ.79 Thibaut and coworkers have reported that the ability of 38 to cause contraction of worms is not related to the benzodiazepine binding site of schistosomes.80 Ro 11-3128 was, however, withdrawn from further development as a schistosomicide due to its severe sedative effects, coupled with ataxia and muscle relaxation at doses above 0.1 mg kg–1.8 The first reported SAR study on 38 was conducted by Menezes and colleagues.81 Twelve analogs were investigated in the study. This showed that the principal pharmacophore of 38 was the unsubstituted amide moiety of the benzodiazepine scaffold. Substitution of the amide carbonyl to its thiocarbonyl isostere was tolerated. The imine, chloro, and nitro groups were also found to be necessary for activity.81 While this preliminary investigation is useful, a more extensive SAR study aimed at improving the selectivity of 38 towards antischistosomal activity is needed. Like 38, 39 (a tricyclic 9-acridanone-hydrazone derivative) is potent against all immature stages of schistosomes.77 Complete cures were reported in three separate trials in primates.82–84 It acts by reducing the levels of rRNA and mRNA thereby reducing the levels of the parasite's genes and proteins. Other promising but abandoned antischistosomal agents are shown in Fig. 9.
Fig. 9. Relinquished antischistosomal agents from the 20th century.8.
3.0. What's new?
In this section, we describe novel antischistosomal chemotypes discovered in the last decade through phenotypic whole cell screening, medium/high throughput screening (HTS), of drug-like libraries, and in silico approaches, as well as the use of natural products. Chemotypes, which have exhibited in vitro activity or enzyme inhibition activity in a biochemical assay, with accompanying preliminary SAR studies and/or in vivo efficacy results are described in detail as useful starting points for further medicinal chemistry exploration.
Ureas
The 400 compound sets of the Medicines for Malaria Venture (MMV) Malaria, Stasis, and Pathogen boxes have been very useful in the search for potential antischistosomal agents endeavor. Phenotypic screening of these compounds via an initial in vitro evaluation against schistosomula followed by in vitro and in vivo studies on adult parasites have identified potential starting points for further SAR studies (Table 1, Fig. 10 and 11).85–87
Table 1. Antischistosomal activities of some hits identified through phenotypic screening of the MMV malaria, stasis and pathogen boxes.
| Compound |
S. mansoni IC50
a
(μM) |
Adult S. mansoni b (% WBR) | Compound |
S. mansoni IC50
a
(μM) |
Adult S. mansoni b (% WBR) | Adult S. haematobium effect (%), 10 μM, 72 h | ||
| NTS | Adult | NTS | Adult | |||||
| 40 | 4.7 | 0.8 | 52.5 | 54 | 7.2 | 7.5 | 51.6 | — |
| 41 | 2.7 | 0.8 | 0 | 55 | 1.7 | 7.0 | — | — |
| 42 | 1.8 | 3.4 | 1.8 | 56 | 0.8 | 3.0 | — | 100.0 |
| 43 | 3.4 | 6.3 | 18.7 | 57 | 0.6 | 2.0 | 70.7 | 100.0 |
| 44 | — | 0.8 | 40.8 | 58 | 2.6 | 3.0 | 67.8 | 75.4 |
| 45 | 3.5 | 2.5 | 0 | 59 | 0.9 | 6.8 | — | 87.7 |
| 46 | 0.6 | 2.0 | 0 | 60 | 0.8 | 4.7 | — | 75.4 |
| 47 | 1.2 | 2.7 | 12.2 | 61 | 0.2 | 2.1 | — | 100.0 |
| 48 | 1.5 | 2.6 | 3.2 | 62 | 0.4 | 3.7 | 55.2 | 90.8 |
| 49 | 2.1 | 2.0 | 35.9 | 63 | 0.4 | 2.5 | — | 100.0 |
| 50 | 6.3 | 3.1 | 0 | 64 | 2.2 | 3.4 | — | 75.4 |
| 51 | 0.7 | 3.7 | 10.0 | 65 | 2.9 | 4.9 | 22.4 | 82.5 |
| 52 | 0.9 | 2.2 | — | 66 | 16.7 | 7.9 | 32.8 | 50.9 |
| 53 | 0.5 | 2.9 | 0 | |||||
aIC50 after 72 h.
b In vivo studies in mice: 400 mg kg–1 single oral dose for 40–44 and 200 mg kg–1 single oral dose for 45–66.
Fig. 10. Examples of antischistosomal hits identified from the MMV Malaria and Stasis boxes.85,86 .
Fig. 11. Antischistosomal hits identified from the MMV Pathogen box.87.
From the MMV Malaria box, the N,N′-diarylurea (40, Table 1, Fig. 10) and the 2,3-dianilinoquinoxaline (44) were identified as promising early leads, according to Ingram-Sieber and coworkers.85 To explore the SAR requirements for schistosomicidal activity, 46 commercially available compounds structurally related to 40 were identified by using the Tanimoto–Rogers similarity algorithm (cutoff = 0.85) and screened against S. mansoni NTS and adult worms in vitro.88 These compounds belonged to the 5 chemical classes of N,N′-diarylureas, N-aryl, N′-alkylureas, N-phenylbenzamides, N-cyclohexylbenzamides and N-arylphenylcarbamates. The in vitro screening revealed significant potency in all compound classes except the N-aryl, N′-alkylureas. From a SAR perspective, the N,N′-diarylurea series required electron-withdrawing group substituents on both aryl units, preferably in the meta or para positions, for activity. N-Phenyl benzamides with the salicylanilide moiety and at least one trifluoromethyl group at the N-phenyl moiety exhibited good activity. Even though no conclusion on SAR was possible with the carbamates, they could serve as alternatives to the ureas.88
Regardless of the promising in vitro antischistosomal activity of these compounds, only the N-phenyl benzamide (67, Fig. 12) significantly reduced worm burden in vivo (66%) after a single oral dose of 400 mg kg–1. Although the in vivo activity was better than the lead 40, more medicinal chemistry optimization is required. The low in vivo activity was explained to be due to phase II metabolism (glucuronidation) leading to fast elimination. Low aqueous solubility is another contributing factor to the low bioavailability of the N,N′-diarylurea analogs.88 To improve aqueous solubility and subsequently improve in vivo efficacy, azaheterocycle and aroyl moieties with polar substituents were explored (68, Fig. 12). Analogs with a 3-trifluoromethyl-4-pyridyl substituent displayed the highest plasma exposure but there was no direct correlation with in vivo efficacy.89 On the other hand, their 4-fluoro-3-trifluoromethylphenyl congeners showed 40% and 50% WBR in S. mansoni infected mice.
Fig. 12. Potent analogs from SAR studies based on 40. Sm (S. mansoni), Sj (S. japonium); p.o (oral administration).
In a related study, SAR explorations led to the identification of N,N′-diarylurea analogs which induced the death of juvenile S. japonicum within 72 h at 32 μM. Compounds with electron-withdrawing groups like halogen, trifluoromethyl and cyano showed excellent activity, with the meta and para positions more tolerated than the ortho position. Generally, the compounds were more potent towards juvenile worms than adult worms. Unfortunately, worm burden reductions of <35% were recorded after administration of a single oral dose of 200 mg kg–1 or 400 mg kg–1 to S. japonicum-infected mice.90 Compound 69 was the most potent analog identified in this study.
Ro 13-3978 (36) undergoes hydrolysis in an alkaline medium to produce the urea carboxylic acid 70 (Fig. 13), which shows moderate activity (66% WBR) in vivo.72,76 Rudimentary SAR exploration, which focused on modifications of the N-aryl, the urea (open or ring), the gem-dimethyl, and the terminal acyl groups, led to 71, an analog with comparable in vivo activity (70% WBR) but more liable to metabolism and more lipophilic than 70. Wu and coworkers concluded that except for the gem-dimethyl substructure and the distal nitrogen atom of the urea functional group, the rest of the structure of 70 is required for in vivo antischistosomal activity.91
Fig. 13. Chemical structures of 70 and 71.
Aminopyrazinamides
This class of promising antischistosomal agents, exemplified by 56 (Table 1, Fig. 14), was identified as one of the lead compounds from the MMV Pathogen box. It exhibited fast-killing of S. mansoni NTS parasites causing 100% death after 24 h of 10 μM drug exposure. Against adult worms, 70% worm reduction was observed after 24 h but all worms were cleared after 48 h of 10 μM of drug exposure.87 The good activity of 56 warranted the schistosomicidal evaluation of 17 congeners to acquire preliminary SAR information. Briefly, the amido group was found to be essential for activity, a replacement with the methyl ester group decreases activity while the carboxylic acid group is detrimental to activity. In fact, the activity of these aminopyrazinamides is highly dependent on the amido group. Fast-killing activity can be achieved if the substituents on the phenyl group are electron-withdrawing. Additionally, the terminal N-methylpiperazinyl group, but not the piperidinyl and the morpholino groups, complements the fast-killing activity. The propyl linker can be shortened to the ethyl linker to maintain activity. Lastly, substituting the pyrimidino unit with the 3-pyridyl group is tolerated. Analogs of 56, which caused >50% NTS worm reductions in 24 h were equally potent against adult worms. However, in vivo studies in S. mansoni-infected mice resulted in one derivative (72, Fig. 9) showing low WBR (37.6%, 200 mg kg–1, p.o). Notwithstanding the poor in vivo result, the aminopyrazinamide-based chemotype has demonstrated potential prophylactic properties in vitro and activity against adult worms and requires a more extensive SAR exploration.87
Fig. 14. Chemical structures of compounds 56 and 72.
Pyrazolopyrimidine-5-carboxamides
Compound 57 was also identified from the screening of the MMV Pathogen box. It exhibited good in vivo reduction of worm burden (70%, 200 mg kg–1, p.o) and showed good in vitro activity against S. mansoni NTS and adult worms, and S. haematobium adult schistosomes (Table 1, Fig. 15).87 To further understand the SAR around this scaffold, 39 derivatives were first tested against S. mansoni NTS worms at 10 μM and later adult worms if >50% activity against NTS was observed after 24 h. From a SAR perspective, the N-(4-piperazinyl)phenyl carboxamide on the pyrimidino unit, but not the pyrazolo core, is required for activity. The (piperazinyl)phenyl group is essential for activity but the phenyl, benzyl or cyclohexyl groups lead to loss of activity. The piperazinyl group is tolerated, however, the piperidinyl, morpholino, and the N-(tert-butylcarbamoyl)piperazinyl groups are detrimental to activity. On the pyrazolo core, substituted phenyl and pyridyl groups are tolerated but not five-membered aryl groups. Electron withdrawing and releasing groups give active analogs, but para substitution is more favored than the corresponding meta and ortho isomers. Compound 57 also displayed good pharmacokinetic properties in preliminary studies in two male Sprague Dawley rats.87
Fig. 15. Chemical structure of compound 57.

Biaryl sulfonamides
This promising antischistosomal chemotype is exemplified by compound 58 (Table 1, Fig. 16), which demonstrated both in vitro activity against NTS and adult worms of S. mansoni, as well as adult S. haematobium worms. It reduced worm burden (67.8%) in vivo in mice harboring S. mansoni adult worms after an oral dose of 200 mg kg–1. The biological evaluation of 56 analogs related to 58 afforded rudimentary SAR analyses (Fig. 16). All analogs maintained the central biaryl unit. On the left-hand side of the molecule, the N-methyl-4-aminopiperidinylmethylene moiety is essential for activity. Shortening of the aminomethylene linker is not tolerated for activity. Replacement of the 4-aminopiperidinyl group with its acyclic 1,3-diaminopropyl group maintains activity but not the cyclohexyl or morpholino group. On the right-hand side, the sulfonyl group can be replaced with the carbonyl group to preserve activity. While the benzylamino unit is required for activity, the anilino group is also tolerated. Substitution on the phenyl ring is tolerated, in which case the chloro or trifluoromethyl group seems to be more preferred to the fluoro or methoxy group. Moreover, there is no discrimination for para or meta substitution with regards to activity. Replacement of the phenyl group with other heterocycles, such as the pyridyl, oxo-pyridyl, pyrimidinyl, and pyridazinyl groups, gave inactive compounds. Compound 58, identified from the MMV Pathogen box, is a promising starting point for further SAR exploration, which could harness the importance of the central biaryl scaffold to schistosomicidal activity.
Fig. 16. Preliminary SAR analyses of the biaryl-sulfonamide-based chemotype for fast-killing activity (>50%, 24 h, 10 μM) against S. mansoni NTS.
Hydroxamic acids
The hydroxamic acid functionality has emerged as a useful moiety for inhibition of class I Schistosoma histone deacetylases (SmHDACs). HDACs are involved in the deacetylation of lysine residues in histone. The antischistosomal properties of human HDAC inhibitors (hHDACi) suggested the potential of these enzymes as druggable targets in schistosomes.92,93 Eighteen different enzymes make the hHDACs and are classified into four categories, class I (HDAC1, -2, -3, and -8), class IIa (HDAC4, -5, -7, and -9), class IIb (HDAC6 and -10), class III [Sirtuins, (Sirt1–7)], and class IV (HDAC11).94 The schistosome ortholog of the class I type (HDAC1, -3, and -8) are expressed in all stages of the parasite's lifecycle, being predominated by SmHDAC8, which has received a lot of attention lately.95In vivo studies in infected mice have revealed the crucial importance of SmHDAC8 for the parasite's survival and maturation in its host.96
Based on a repositioning approach, from the X-ray crystal structure of the initial hit 73 (a hHDACi, Fig. 17) complexed to the zinc-dependent SmHDAC8,97 Heimburg and colleagues initially designed and synthesized a preliminary set of 3-aminobenzohydroxamate and 3-amidobenzohydroxamate derivatives as a novel series for antischistosomal agents.98 The good biochemical potency exhibited by the 3-amidobenzohydroxamate derivatives (exemplified by 74), coupled with an X-ray structure of 74 complexed to SmHDAC8 (Fig. 18), inspired further SAR studies leading to analogs 75–77 with low nanomolar potency against the SmHDAC8 and good selectivity over the hHDAC1 and -6 but not hHDAC8 (Fig. 17). In an in vitro screen against the S. mansoni schistosomula worms, the compounds exhibited dose-dependent killing of the larvae with 100% mortality at 20 μM after 48 h of drug exposure. The most lethal compound, 76, showed marked separation of adult worms, with 90% separation at 20 μM after 5 days and 80% egg-laying impairment of the worms at 20 μM dose.98
Fig. 17. Hydroxamic acid-based antischistosomal agents.
Fig. 18. Binding interactions of 74 in the active site of SmHDAC8 showing the typical bidentate chelation of the hydroxamate functionality with zinc. Red dotted lines represent hydrogen bonding. Blue dotted arcs represent hydrophobic interactions.
In a related study, Stenzel and colleagues explored alkoxyamide (78, Fig. 19) and hydrazide-based (79) isophthalic acid derivatives as SmHDAC8 inhibitors.99 Substituted phenyl, benzyl, and naphthyl groups were explored as a replacement for the alkoxy units. Apart from 79, an aniline group was also explored on the hydrazide unit. The new analogs were significantly less active against hHDAC1 (<10% inhibition at 1 μM). They, however, remained non-selective between SmHDAC8 and hHDAC8. Moreover, this new series showed minimal antischistosomal properties.
Fig. 19. Representative structures of hydroxamic acid-based antischistosomal agents.
Further, a novel series of N-(2,5-dioxopyrrolidin-3-yl)-n-alkylhydroxamate derivatives exemplified by 80, was identified through a virtual screening approach using the SmHDAC8 crystal structure.100 These hydroxamates showed weak activity against SmHDAC8 (IC50 > 4.4 μM) in a biochemical assay and poor selectivity for hHDAC1, -6, and -8. Compound 80 and its 4-bromophenyl congener induced apoptosis in S. mansoni schistosomula. Meanwhile, bicyclic and cinnamic acid hydroxamate derivatives have shown good potency against SmHDAC8 in a biochemical assay. However, as noted above, poor selectivity over hHDAC8 continued to remain a challenge. Ex vivo, the cinnamic acid ether (81) and thioether (82) derivatives caused 89% (EC50 = 6.5 μM) and 97% (EC50 = 11.8 μM) lethality on S. mansoni schistosomula at 20 μM after 48 h, respectively.101
Pyrimidopyrimidines
The hit compound 66, identified through phenotypic screening of the MMV Pathogen box (Table 1, Fig. 20),87 was also identified by Monaldi and colleagues through an in vitro screening of the GSK Kinetobox library against S. mansoni lysine deacetylase Sirtuin 2 (SmSirt2), a member of SmHDACs.102 The Kinetobox library contains 592 compounds with potent growth inhibitory activity against the kinetoplastid parasites Leishmania donovani, Trypanosoma cruzi, and T. brucei with low human cellular cytotoxicity.103 Compound 66 showed low micromolar inhibition of SmSirt2 and modest selectivity over the human ortholog (hSirt2, Fig. 20).102
Fig. 20. Pyrimidopyrimidine-based antischistosomal agents.
Initial SAR studies employed a fragment-based approach to elucidate the pharmacophoric substructure(s) of 66. In this regard, the 2,4,7-triaminopyrimidopyrimidine moiety was kept constant while introducing simple modifications such as secondary, tertiary and cyclic amines at the Nα position. Further studies focused on investigating the contribution of substituents on the Nα position, the benzylic methylene position, and the methoxy group. These 1st generation analogs were all inactive (<15% inhibition) against SmSirt2 except 83 (Fig. 20). Subsequent SAR studies led to 2nd generation analogs with improved inhibition against SmSirt2 and good selectivity over hSirt2. Briefly, 1-(4-phenoxyphenyl)-ethyl (as in 66) or 1-([1,1′-biphenyl]-4-yl)ethyl (as in 83) substituent at Nα are crucial for activity. A methyl substituent at the Nα position and a phenyl group at the Cβ position led to potent analogs. The activity against SmSirt2 is also complemented by the methoxy group, especially at the para position or 3,5-disubstituted positions. Moreover, the thiophenoxy group is tolerated for inhibition of SmSirt2, as opposed to the phenoxy group.
In ex vivo antischistosomal activity experiments, the most potent analog, 84, completely reduced the viability of schistosomula at 10 μM after 72 h of drug exposure. Similarly, 84 completely reduced adult worm pairing at 20 μM after 72 h and prevented egg-laying by >90% at 10 μM after 24 h. Incidentally, the two analogs, 85 and 86, which were inactive against SmSirt2 and toxic to HL-60 cells, were equally potent against schistosomula and adult worms like 84, thus suggesting the contributory role of other targets (off-targets) to the schistosomicidal activity of these pyrimidopyrimidine compounds.
Thiazoles and phthalyl derivatives
Thiazole and phthalimide derivatives possess a broad spectrum of pharmacological properties including analgesic, anticonvulsant, antitubercular, hypolipidemic, anxiolytic, anti-inflammatory, antimicrobial and antipsychotic properties.104–109 Santiago and coworkers explored the antischistosomal properties of 10 new thiosemicarbazone, phthalyl thiosemicarbazone, phthalyl thiazole, and phthalyl thiazolidinone pharmacophores as required core scaffolds for activity. The phthalyl thiazole series exhibited in vitro antischistosomal activity against adult S. mansoni worms with significant ultrastructural changes in worms and were noncytotoxic on splenocytes relative to praziquantel.110 The lead compound 87 (Fig. 21) caused 60% mortality of adult S. mansoni worms in 72 h but complete death of parasites only after 144 h of 100 μg ml–1 drug exposure. It displayed significant ultrastructural alterations, with tegument destruction in both male and female worms. Moreover, 87 was noncytotoxic at the highest test concentration of 100 μg ml–1 to spleen cells of BALB/c mice. In other experiments conducted on macrophages, the production of nitrite and tumor necrosis factor alpha (TNF-α), indicative of immunomodulatory activity, was demonstrated by 87.
Fig. 21. Promising phthalyl thiazole-based antischistosomal agents.
In a related study, the phthalyl thiazolidinone derivative (88, Fig. 21) and three other congeners (89–91) were evaluated for in vitro activity towards schistosomula and adult worms.111 Significant reduction in motility, oviposition inhibition, and mortality were observed in both immature and adult worms. Compounds 90 and 91 showed significant improvement in the schistosomicidal activity against adult S. mansoni worms in vitro compared to 87. Thus, 90 and 91 completely cleared adult worms at 100 μg ml–1 after 72 h and 24 h, respectively, while maintaining their noncytotoxic effects on BALB/c mice spleen cells above 138 μM, compared to <3.20 μM for PZQ. These compounds also possess promising prophylactic properties. Against schistosomula, EC50 values of 5.15 μM and 9.77 μM were observed for 90 and 91, compared to 37.5 μM and 38.7 μM towards adult worms, respectively. Evaluation of the morphology of worms after drug exposure suggested that 90 and 91 targeted the parasite's tegument causing gradual damage to its surface, inducing destruction and decomposition of the tegument in the same areas and exposition of the subtegumental tissue and the muscle tissue.111
In further studies, 92 promoted 100% mortality of larval forms up to doses of 2.5 μg ml–1 within 48 h.112 Complete mortality of adult S. mansoni worms was observed after 96 h of 100 μg ml–1 of drug exposure (EC50 = 50.6 μM). In vivo, 85% WBR was observed after a single oral dose of 400 mg kg–1 but this increased to 95% WBR at an oral dosage of 200 mg kg–1 for 5 consecutive days to S. mansoni-infected mice harboring adult worms. In addition, scanning electron microscopy revealed that 92 kills the parasites by tegumental damage and bubble generation.112 These results highlight the antischistosomal properties of molecules incorporating the thiazole and phthalimide scaffolds. However, it will be necessary to identify a more potent lead molecule while a more extensive SAR exploration is required to harness the structural requirements for activity, while noting that the thiazolo-phenyl biaryl moiety seems to be required for activity.
Imidazoles
The imidazole-based compounds 93 (Fig. 22) and 94 were identified from phenotypic screening of 265 compounds from a cyclic nucleotide phosphodiester (PDE)-focused library for antischistosomal activity by exploring the Schistosoma PDE as a potential drug target.113 The antischistosomal effects against adult S. mansoni worms were recorded as worm killing, sluggish worm movement, unpairing, absence or reduction in egg numbers, and worm hypermotility or spastic contraction. A hit rate of 64% was observed. Compound 93 demonstrated a worm killing effect of 56% at 100 μM although it was more susceptible to male worms (100%) than females (0%). On the other hand, 94 showed less male worm killing effect (33%) at 100 μM but caused complete uncoupling and reduction of eggs at 10 μM. In vivo efficacy studies in S. mansoni-infected mice harboring adult worms at an oral dosage of 20 mg kg–1 per day (5 days) of 93 resulted in 29% and 35% WBR and intestinal tissue egg burden reduction, respectively. Meanwhile, 94 was even less potent, causing <17% worm or egg burden reduction. However, when administered in combination with PZQ (10 mg kg–1 per day for 5 days; p.o), 93 and 94 significantly increased the efficacy of PZQ from 60% to 96% and 88% (WBR), respectively. Similarly, intestinal tissue egg burden reduction for PZQ was increased from 67% to 83% for both compounds.113
Fig. 22. Chemical structures of potent antischistosomal agents based on the imidazole scaffold.
Compounds containing the privileged benzimidazole scaffold have shown broad-spectrum anthelmintic properties. Compound 95 (BTP-Iso, Fig. 22), a novel benzimidazole designed based on thiabendazole and cambendazole, was evaluated for schistosomicidal activity in S. mansoni-infected mice at oral doses of 200 mg kg–1 and 300 mg kg–1.114 The antischistosomal effects, which were assessed on the basis of worm burden, oogram pattern, tissue egg load, granuloma measurement, and scanning electron microscope study of adult schistosomes showed that at 200 mg kg–1, 95 caused 66% and 44% female worm and total worm burden reductions, respectively, with no significant male worms reduction. Conversely, 95, at 300 mg kg–1, showed statistically significant higher reductions in female (76.5%), male (34.6%) and total worms (55.3%). Significant reductions in the immature ova, tissue egg load, and granuloma populations were also observed. The significant effect of 95 against female worms is relevant to the clinical pathology and transmission of schistosomiasis due to the concomitant reductions in egg populations. The morphological effects observed in treated worms included tegumental damage, especially in female worms, while the milder effects of swelling of tegumental ridges, bleb formation, and mild erosion were observed in male worms.114 These results provide proof-of-concept that the anthelminthic benzimidazoles can be repositioned as schistosomicides and thus, a rational design of analogs toward ascertaining the SAR requirements for antischistosomal activity is warranted.
Long and colleagues discovered that the S. mansoni polo-like kinase (SmPLK) 1, an ortholog of the human PLK1 (huPLK1), is a druggable target. This was achieved through RNA interference knockdown of the S. mansoni messenger RNA for SmPLK1, which resulted in disruptive changes in the morphology of immature and adult parasites.115 An initial phenotypic screening of 11 commercially available PLK1 inhibitors identified the benzimidazole thiophene compound 96 (Fig. 23), which showed significant phenotypic alterations, especially against somules, at concentrations as low as 1 μM. Further screening of 38 benzimidazole thiophene inhibitors from the GlaxoSmithKline Published Kinase Inhibitor Set (PKIS) 1 and 2 against somules and adult worms was conducted and SAR requirements for activity were delineated as follows: a 2-trifluoromethylbenzyloxy or 2-chlorobenzyloxy group and a carboxamido or acetoxy group on the thiophene unit are tolerated, and the benzimidazole thiophene biaryl moiety bearing solubilizing aliphatic amines is also needed for activity. Molecular docking of 96 in the ATP-binding sites of huPLK1 and a homology model of SmPLK1 showed similar binding poses. The four most potent compounds 97–100 demonstrated effects on adult worms at 1 or 2 μM in 5 h while effects were more adverse in somules.115 While these benzimidazole-thiophene-based inhibitors are promising as antischistosomal agents, further SAR studies aimed at identifying more selective SmPLK1 inhibitors, as well as in vivo proof-of-concept studies in an infected animal model, will be worthwhile pursuing.
Fig. 23. Benzimidazole-thiazole schistosomicides.
The pyrido[1,2-a]benzimidazole (PBI) chemotype has demonstrated antibacterial, antifungal, antiviral, antitumor, and anti-trypanosomal activities.116,117 Okombo and colleagues explored the antischistosomal properties of 57 PBI analogs (1st generation, Fig. 24). Against S. mansoni NTS parasites at 10 μM, a hit rate of 84% corresponding to >70% mortality of worms was recorded. Subsequently, 37 compounds caused >60% mortality of adult worms at 10 μM. These hits demonstrated IC50 values of 0.2–39 μM against adult worms.118In vivo studies in a 49 day-old S. mansoni-infected mice orally dosed with 400 mg kg–1 of the drug led to the most potent analog 101 (IC50 = 2.44 μM), showing 58.7% and 61.3% total and female worm burden reductions, respectively. Pharmacokinetics (PK) studies in mice following oral and intravenous doses revealed that the in vivo efficacy of 101 was related to its low exposure on oral dosing, due to solubility-limited absorption, and a relatively short half-life due to high hepatic clearance. Preliminary mechanistic studies via beta-hematin inhibition suggested that this mechanism contributes to the antischistosomal properties of the PBI analogs.
Fig. 24. SAR studies on the PBI core scaffold and potent analogs 101 and 102.
As a follow-up study, Mayoka and colleagues designed and synthesized 55 analogs, pursuing further SAR studies that incorporated N-aryl substitutions on the PBI scaffold (2nd generation, Fig. 24).119 The antischistosomal activities (IC50 = 0.2–3.3 μM) of the new analogs corroborated the initial results of Okombo and colleagues. Compound 102 (IC50 = 0.38 μM) reduced adult worm burden by 69% when S. mansoni-infected mice were dosed with 400 mg kg–1 orally. Like 101, in vivo PK studies revealed poor solubility-related effects on the in vivo efficacy results. Compound 102 attained peak plasma concentrations (0.3 μM) after 3 h post-oral administration and displayed a short half-life (0.3 h), slow clearance (0.81 mL min–1 kg), and low volume of distribution (0.18 L kg–1) with significantly low bioavailability (<1%). Though a promising scaffold, future SAR studies must aim to improve solubility, PK, and efficacy.
1,2,4-Oxadiazole-2-oxides
The antischistosomal properties of this class of compounds was identified through a quantitative HTS of 71 028 compounds comprising the Molecular Libraries Small Molecule Repository and NIH Chemical Genomics Center libraries, followed by confirmatory and target deconvolution experiments. The hit compound, furoxan (103, Fig. 25), demonstrated significant schistosomicidal activities in S. mansoni-infected mice, causing WBR of 99%, 88%, and 94%, from treatments against skin-, liver-, and adult-stage parasites, respectively, and egg-associated pathologies.120 The antischistosomal properties of the 1,2,4-oxadiazole-2-oxide derivatives have been investigated in relation to their inhibition of schistosome thioredoxin glutathione reductase (TGR) in both S. mansoni and S. japonicum.120,121 TGR is a single multifunctional selenocysteine-containing flavoenzyme, which replaces both glutathione reductase and thioredoxin reductase in the parasite. It is therefore the main enzyme responsible for the detoxification of reactive radical species in the parasite, thus essential for its survival.122
Fig. 25. Representative structures of oxadiazole-2-oxide-based antischistosomal agents.
SAR studies prompted by 103 have highlighted the significance of the N-oxide group at the 2-position for activity. Similarly, the cyano group at position 2 is optimal for activity, although the acetamido group is tolerated. On the phenyl group, compounds containing meta and para bromo groups, like 103 (Fig. 25), completely cleared parasites ex vivo after 24 h of 10 μM drug exposure. Replacement of the phenyl group with the 2-thiophenyl and 2-furyl groups led to the loss of antischistosomal activity although activity against TGR was maintained. Further, bis oxadiazole-2-oxides (for example, 104) also showed promising biochemical and schistosomicidal activities (Fig. 25).123
Rai and coworkers observed good selectivity of the oxadiazole-2-oxides for TGR over human glutathione reductase (hGR) and rat thioredoxin reductase (rTrxR, Fig. 25).123 It is noteworthy that some analogs active against TGR were completely inactive against schistosomes. SAR of the N-oxide moiety of furoxan by regiochemical transposition and suppression of NO donation ability led to inactive compounds against SmTGR with the compounds being ineffective ex vivo against the worms. This observation led to the conclusion that inhibition of SmTGR needs to be coupled with the ability of the compound to donate an NO radical in order to effect significant parasite death. On the contrary, Song and colleagues noted that in S. japonicum, the schistosomicidal activity does not depend on NO production or the inhibition of TGR, suggesting the presence of other functional targets of the oxadiazole-2-oxides in this schistosome species.121 Taken together, the 1,2,4-oxadiazole-2-oxide class of compound is a viable starting point for identifying useful schistosomicides against juvenile and adult worms.
Thiohydantoins
Like hydantoin-based compounds, compounds containing the thiohydantoin isostere have exhibited schistosomicidal properties.124–127 For example, compounds 105 and 106 (Fig. 26) caused 100% mortality in S. mansoni worms in vitro upon 50 μM of drug exposure after 120 h and 96 h, respectively. Treated adult worms underwent unpairing, detachment of suckers, and alterations in tegument morphology, coupled with the absence of eggs.128 Oral administration of 100 mg kg–1 of 105 in a polyethylene glycol formulation resulted in 70.5% WBR in infected adult S. mansoni mice. No distinctive patterns were observed in mature eggs compared to the immature ones, although the numbers of dead eggs increased. Further, 105 did not demonstrate immunomodulatory properties in response to the S. mansoni soluble egg antigen but downmodulated host cell response to eggs harboring the liver, thereby preventing granuloma formulation.129
Fig. 26. Chemical structures of antischistosomal imidazolidine derivatives.
In a related study, compounds 107 and 108 showed improved worm killing effects in vitro relative to 105 and 106. At 20 μM, 107 and 108 completely killed worms after 24 h and 72 h, respectively. Moreover, 107 and 108 caused similar ultrastructural alterations in worms after treatment compared to 105 and 106.130 These results highlight the promising schistosomicidal properties of the thiohydantoin series in causing morphological damage to adult schistosomes while also demonstrating in vivo efficacy.
Carbazole aminoalcohols
In a phenotypic in vitro screening against adult S. japonicum worms, the carbazole-based compounds 109 and 110 (Fig. 27) completely cleared worm populations at 10 μg ml–1. A set of 16 analogs were rationally designed and synthesized to ascertain the contribution of the carbazole core, the amine side chain and stereochemistry to antischistosomal and antiplasmodium activities.131 The new analogs (12 out of 16) maintained potency against juvenile and adult S. japonicum worms at 10 μg ml–1. From a SAR perspective, alicyclic amino side chains were required for activity against both parasite stages. In contrast, the phenyl and morpholino side chains were not tolerated. The most potent derivative 111, bearing the n-butyl amino group, completely cleared juvenile and adult S. japonicum at 5 μg ml–1 in 72 h.131 Meanwhile, activity was not compromised when either of the two enantiomerically pure isomers was tested. Inhibition of hemozoin formation as a possible mode of action of these compounds was demonstrated through molecularly docking the enantiomers of 111 with heme and confirmed by Job's plot experiments. Furthermore, 111 was evaluated for antiplasmodium activity and was found to be less active (IC50 = 0.274 μM) against the chloroquine-sensitive Plasmodium falciparum 3D7 strain than the chloroquine-resistant Dd2 strain (IC50 = 0.047 μM). The dual antiparasitic activity of 111 warrants further SAR exploration.
Fig. 27. Chemical structures of carbazole aminoalcohol hits and a potential lead (111).
Biarylalkyl carboxylic acids (BACADs)
BACADs, which are known inhibitors of the human aldose reductase (AR), have been explored as potential inhibitors of the Schistosoma orthologs of the enzyme.132–134 The Schistosoma AR is thought to play an important role in antioxidant pathways and protection of the worms from attack by its host's reactive oxygen species135 and hence makes schistosome AR an interesting target for novel antischistosomal agents. Larval and juvenile S. mansoni parasites express a separate AR ortholog (Smp_150700) from adult worms (Smp_053220).136 Eight inhibitors of the human AR were initially explored for schistosomicidal activity on the basis of observable phenotypes such as pairing stability, gut peristalsis, vitality, motility, tegumental damages, and egg production.132
The most potent inhibitor (112, Fig. 28) from the prescreen was used as a template for further SAR explorations, which focused on the phenyl ring, alkyl chain, and carboxylic acid group and led to the most potent analogs 113 and 114. In contrast to human AR, inhibitors lacking electron-withdrawing substituents on the phenyl moiety showed activity. The 3-hydroxyphenyl moiety was optimal for activity. The 5-oxopentanoic acid and pentanoic acid linkers provided the most potent compounds, while derivatization of the carboxylic acid to the carboxamide also improved activity.132 Additional SAR studies on the carboxylic acid moiety led to 82 compounds of which 6 were active against S. mansoni at concentrations up to 10 μM. The morpholine derivative 115 and the methylsulfonyl piperazine derivative 116 have emerged as good starting points for the next generation of antischistosomal BACADs.134
Fig. 28. Representative structures of BACAD antischistosomal agents.
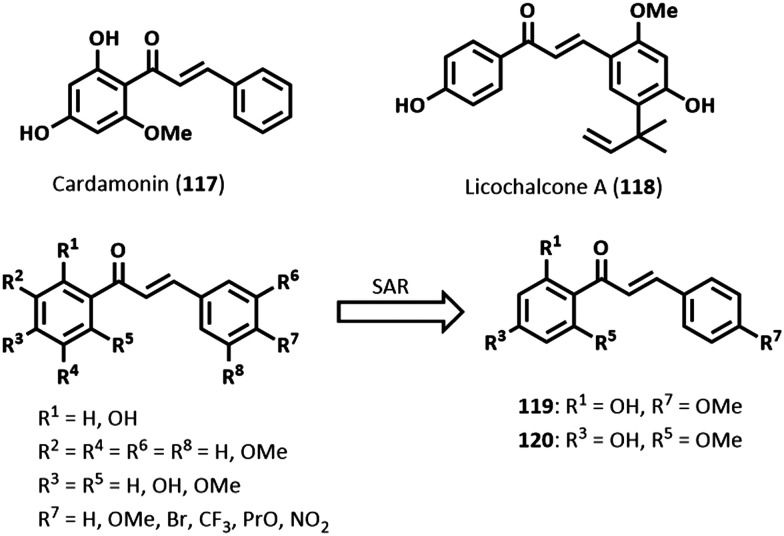
Chalcones
Chalcones are naturally occurring compounds prevalent in various plant species. They belong to the flavonoid class of secondary metabolites, being an important precursor to flavonoid compounds. Like most natural products, chalcones are characterized by diverse biological properties, including antiparasitic activities against Leishmania, Trypanosoma, and Plasmodium species.137–140 Chemically, chalcones contain the basic 1,3-diphenyl-2-propen-1-one core scaffold.
A schistosomiasis activity-guided fractionation of the dichloromethane (DCM) crude extract of the inflorescence of Piper aduncum L. led to the isolation of the chalcone cardamonin (117, Fig. 29), which demonstrated significant antischistosomal properties causing complete lethality of adult worms of S. mansoni, 100% reduction in motor activity, and extensive tegumental alterations after 24 h of 25 μM drug exposure.141 Further, 117 irreversibly inhibited oviposition. In a biochemical activity study against S. mansoni ATP diphosphohydrolase (SmATPase), 117 (40 μM) caused approximately 82% inhibition (IC50 = 23.54 μM), whereas the enzyme remained unaffected (ca. <10%) up to the highest test concentration. The SmATPases are ecto-enzymes present in the tegument and are thought to help impair host immune defenses and promote parasite survival. Molecular docking studies in a 3D-SmATPDase 1 homology model revealed good hydrophobic and hydrogen bonding interactions with the nucleotide-binding site of SmATPDase 1, which accounted for the good inhibitory activity observed.141
Fig. 29. Examples of antischistosomal chalcones.
Licochalcone A (118, Fig. 29), isolated from Glycyrrhiza inflata, exhibited LC50 (concentration lethal to 50% of exposed population) values of 9.12 μM and 9.52 μM against adult female and male worms of S. mansoni, respectively, after 24 h.142 At 12.5 μM, after 24 h, worms were completely immotile. Compound 118 reduced pairing of adult worms and decreased egg production. Further, the development of eggs was reduced in the presence of >6.25 μM of 118. Tegumental damages in male worms were marked by loss of integrity, focal lysis of the internal tissues, mitochondria swelling, and nuclear chromatin condensation after 24 h of 10 μM exposure to 118. In female worms, vacuolization of the tegument, total lysis of the internal tissue and muscular layer, and degeneration of many vitelline cells were observed. The alterations in mitochondria in the presence of 118 were rationalized to be due to an increase in superoxide anion levels and a decrease in the activity of superoxide dismutase, which could lead to oxidative stress arising from high levels of reactive oxygen species, and subsequently apoptosis. Meanwhile, 118 was noncytotoxic (IC50 = 508.3 μM) against Chinese hamster ovarian (CHO-K1) cells.142
As part of SAR studies, Pereira and colleagues synthesized a series of 15 chalcones using the Claisen–Schmidt condensation reaction.143 Compounds 119 and 120 (Fig. 29) after 24 h had completely cleared adult worms of S. mansoni at 50 μM and 25 μM, respectively. Moreover, a significant reduction in motor activity and extensive tegumental alterations were also observed. From a SAR perspective, tri-substitution of the methoxy group on either phenyl rings was not tolerated. Meanwhile, substitutions at R7 (Fig. 29) other than a methoxy group also led to loss of activity. In vivo, an oral dose of 400 mg kg–1 to S. mansoni-infected mice harboring adult worms, respectively, led to WBR of 32.8% and 31.8% by 119 and 120. When tested against SmATPDase 1, 119 demonstrated an IC50 value of 30.62 μM, while 120 was inactive. Molecular docking studies revealed a satisfactory conformation of 119 in the active site of SmATPDase 1.143
Diterpenes
Diterpenes have emerged as a useful chemotype against schistosomiasis144–146 and other major tropical diseases.147 A phytochemical investigation of the root of Lycium chinense afforded 7-keto-sempervirol (121, Fig. 30) from the DCM extract, demonstrating an LC50 value of 19.1 μM against S. mansoni schistosomula in vitro. Against adult parasites, the antischistosomal properties of 121 were evident in reductions in motility, oviposition, egg morphology, and integrity of worm surface. Moreover, 121 also affected Fasciola hepatica parasites.148
Fig. 30. SAR optimization of compound 121 to 122.
In a follow-up study, Crusco and colleagues undertook a SAR exploration on 121 to improve the anthelmintic properties and selectivity.149 The synthetic modifications focused on the core scaffold of 121 and the derivatives were classified into 6 groups I–VI (Fig. 30). Briefly, the SAR explorations assessed the importance of the rings A and B of the diterpenoid backbone, the keto group (at position 6 and/or 7 or its absence), and the effect of mono-, di-, or tri-substitution on the benzene ring. Of the 30 synthesized compounds, all 26 compounds initially screened for activity towards S. mansoni schistosomula showed a >70% effect at 50 μM. Further screens at 25 μM yielded 6 hits but screening at 10 μM led to compound 122 with improved phenotype (IC50 = 6.8 μM) and motility (IC50 = 6.3 μM) activities compared to 121 with IC50 values of 21.9 μM and 26.2 μM, respectively. No derivative belonging to class I or IV yielded a >70% effect on schistosomula at 25 μM. Against adult worms, 122 had an IC50 value of 19.9 μM, causing complete clearance of worms at 50 μM. On the other hand, no discrimination was observed between the effect of 122 on male and female motility. Moreover, egg production was significantly inhibited at concentrations as low as 6.25 μM of drug. Compound 122 was generally noncytotoxic to HepG2 human cells (IC50 = 25.6 μM) and MDBK bovine cells (IC50 = 32.5 μM).
In related work, a HTS of a small library of plant-derived diterpenoids against S. mansoni schistosomula identified labdane-type diterpenoids as promising antischistosomal agents.150 To further explore the schistosomicidal properties of this class of compounds, Crusco and coworkers conducted semi-synthetic modifications on the commercially available congener (–) sclareol (123, Fig. 31). Against schistosomula, 123 exhibited effects on phenotype (IC50 = 14.2 μM) and motility (IC50 = 12.3 μM). SAR studies explored modifications of the functional groups of the sclareol scaffold, employing reactions such as oxidation, dehydration, E1-type elimination, acetylation, and substitution of the vinyl group. Except for two analogs, phenyl derivatives at C15 (I, Fig. 31) exhibited IC50 values <10 μM against schistosomula. From a SAR perspective, the structure of sclareol seems to be optimal for activity, especially the C8- and C13-hydroxy and vinyl groups. On the phenyl moiety of the derivatives of I, halogen substituents showed improved activity while substitution at the para rather than meta position is preferred for activity. The most potent compound, 124, showed IC50 values of <2 μM against schistosomula and juvenile worms but much lower activity against adult worms (IC50 = 9.4 μM). The trisubstituted alkene derivative 125 (Fig. 31) was slightly more potent against schistosomula than 124. Adult parasites exposed to 124 experienced blebbing, swelling of the tegument, and bubble-like protrusions. Moreover, untargeted metabolomics investigation revealed that 124 affects membrane lipid homeostasis by interfering with arachidonic acid metabolism.150
Fig. 31. Sclareol-based antischistosomal agents.
Lignans
Lignans are a widespread group of phenolic natural products in plants. They contain the same phenylpropanoid monomeric core unit called lignin (126, Fig. 32) but end up as dimeric rather than polymeric compounds. The International Union of Pure and Applied Chemistry (IUPAC) identifies lignans as dimeric C6C3 coupled motifs linked at carbons 8 and 8′ (e.g.127, Fig. 32). The IUPAC identifies compounds with the coupling of the two C6C3 units at positions different from C8–C8′ as neolignans (e.g.128, Fig. 32).151 Lignans are classified into subgroups based on the cyclization pattern and the way in which oxygen is incorporated into the skeleton. The eight subgroups recognized are furofuran, furan, dibenzylbutane, dibenzylbutyrolactone, aryltetralin, arylnaphthalene, dibenzocyclooctadiene, and dibenzylbutyrolactol (Fig. 32).152 Natural and synthetic lignans are known to possess antioxidant, antifungal, antitumor and anti-inflammatory properties, amongst others.153,154
Fig. 32. Chemical structures of lignin, a furofuran lignan, and an 8,3′-neolignan.151,152 .
Purification of the ethanolic extract of the seeds of Piper cubeba afforded six lignan-based compounds (129–134, Fig. 33).155 Compounds 129–132 caused >75% reduction in the motor activity of adult S. mansoni worms at 50 μM after 24 h, although 132 maintained 100% motor activity reduction at 10 μM, while 133 and 134 had no effect at concentrations up to 100 μM. Further, at 10 μM, 132 caused >85% separation of male and female worms, followed by 129, which completely dislodged worm couples only at 100 μM but, again, 133 had no effect. Similarly, the isolated compounds, with the exception of 133, reduced the number of eggs laid during 24 h of incubation. Preliminary SAR suggested that lignans with the dibenzylbutyrolactonic skeleton (as in 129, 131, and 132) were more active than the dibenzylbutyrolactoric lignan 130, and dibenzylbutane 133 and 134. Comparison of the activities of 129 and 131 suggests that neither the methylenedioxy group nor the trimethoxy groups on ring B discriminated reduction of motor activity but not separation of worm couples. However, 129 was more potent than 131. Between 131 and 132, it is apparent that the 5-methoxy substituent (as in 132) is essential for both reduction of motor activity and separation of worm couples. Put together, and based on these analogs, the most optimal lignan is 135 with the dibenzylbutyrolactonic skeleton, methylenedioxy group at positions C3 and C4 and C3′ and C4′ of rings A and B, respectively, and a 5-methoxy group.
Fig. 33. Examples of antischistosomal lignans isolated from Piper cubeba.
Compound 136 (Fig. 34) was obtained by semi-synthesis via nitration of 129. In vitro, 136 demonstrated LC50 values of 20.4 and 102.5 μM against schistosomula and adult worms, respectively, after 72 h with much less activity (LC50 = 179.5 μM) against juvenile worms.156 Worm couples were separated after exposure to >25 μM of 136 in 24 h and egg population was also reduced by >83%, leaving <10% developed eggs in the presence of 100 μM of the drug. In in vivo efficacy studies, 136, at doses of 10 mg kg–1, 50 mg kg–1, and 100 mg kg–1, was administered through intraperitoneal injections daily for five consecutive days after day 1, day 23, and day 37 post-infection. WBR of 33–48%, 36–52%, and 39–48% were recorded after the three treatment regimens, respectively. Similarly, egg burdens were reduced by 40–48%, 52–60%, and 16–19%, respectively. Compared to the control group, spleen and liver weights reduced after the first two treatments, but not the third,.156 Using proteomics, Magalhães and colleagues suggest that 136 targets proteins involved mainly in metabolic processes, especially metabolism of carbohydrates.157
Fig. 34. Synthetic antischistosomal lignans.153,156,158 .
Furofuran lignans of natural origin possess rich structural diversity and biological activities.154 The two furofuran lignans 137 and 138 (Fig. 34) were synthesized by oxidative coupling of ferulic and sinapic acids, respectively, and evaluated for antischistosomal, antitrypanosomal, and antileishmanial activities.153 Compound 138 was more schistosomicidal (100% effect in 120 h at 25 μM) in separating worm couples than 137 (25% effect in 120 h at 25 μM). The two compounds did not cause worm mortality or tegumental alterations at up to 50 μM of drug after 120 h but 137 showed a minimal 25% reduction in motor activity after 120 h of 50 μM of test compound.
In a separate study, oxidative coupling of coumaric acid and ferulic acid methyl esters afforded the two dihydrobenzofuran neolignans 139 and 140, respectively, which were subsequently subjected to further structural modifications (hydrogenation, acetylation, methylation, or oxidation) to afford 8 new neolignan products.158 Compound 141 exhibited the most potent activity in causing worm death (100%) at 200 μM after 24 h and reducing motor activity (100%) at 100 μM after 72 h. From a SAR perspective, the Δ7,8 bond, an additional methyl group at C3′, and the absence of the Δ7′,8′ bond are detrimental to activity. Meanwhile, a C4 acetoxy group (as in 141) promotes activity.
Cryptolepines
Cryptolepis sanguinolenta (Lindi) Schitr is a plant known to Central and West African folklore medicine for its antimalarial properties, which are attributed to the alkaloid cryptolepine (142, Fig. 35) and its numerous natural congeners.159 As part of developing novel antimalarial drugs from natural products,160 El Bardicy and colleagues also explored the antischistosomal properties of synthetic derivatives of neocryptolepine (143) and norneocryptolepine (144) by incorporating a variety of aminoalkylamino substituents.161
Fig. 35. Chemical structures of cyptolepine (142) and congeners.
The neocryptolepine core was obtained from the roots of C. sanguinolenta. Using previously established chemical methods,160 a series of 16 neocryptolepine and 9 norneocryptolepine analogs were synthesized and evaluated for schistosomicidal activity against S. mansoni worms (Egyptian and Puerto Rican strains) and molluscicidal activity against adult Biomphalaria alexandrina (Ehrenberg) snails. Six neocryptolepine (145–150) and 2 norneocryptolepine analogs (151 and 152) exhibited 100% worm mortality at 5 μg ml–1 of drug after 120 h (Table 2). Against the mollusks, 7 compounds caused 100% mortality after 24 h of 5 ppm exposure to drug (Table 3). From a SAR perspective, amination at either C2 or C11, but not C3 or C9, is essential for activity. No activity was observed in the absence of an aminoalkylamino substituent. Further, activity was more pronounced by a chloro group at C2, rather than at C3 (e.g.145 and 146, Table 2). The C11 position was optimal for the aminoalkylamino group to ensure activity.
Table 2. In vitro schistosomicidal activity of neo- and norneocryptolepine derivatives on adult S. mansoni worms161.

| ||||||||
| Compound | R | R1 | R2 | Worm mortality (%) | IC50 (μM) |
IC90 (μM) |
||
| Egyptian strain | Puerto Rican strain | Egyptian strain | Puerto Rican strain | |||||
| 145 | 2-Cl | Me | NHCH(Me)(CH2)3NEt2 | 100 | 11.74 | 14.98 | 16.78 | 21.65 |
| 146 | 3-Cl | Me | NHCH(Me)(CH2)3NEt2 | 100 | 19.26 | 29.33 | 24.32 | 32.83 |
| 147 | 2-Cl | Me |

|
100 | 1.26 | 3.54 | 4.05 | 6.83 |
| 148 | 2-Cl | Me |
|
100 | 1.77 | 3.29 | 4.55 | 5.57 |
| 149 | 2-Cl | Me | NH(CH2)2NMe2 | 100 | 3.68 | 5.95 | 7.65 | 13.03 |
| 150 | 2-Cl | Me |

|
100 | 3.46 | 7.85 | 8.31 | 13.39 |
| 151 | 2-Cl | — |

|
100 | 5.41 | 6.27 | 13.68 | 17.95 |
| 152 | 2-Cl | — |

|
100 | 3.41 | 7.61 | 6.30 | 11.02 |
Table 3. Molluscicidal activity of substituted neo- and norneocryptolepines on B. alexandrina snails161.

| ||||||
| Compound | R | R1 | R2 | Snail mortality (%) | LC50 (μM) | LC90 (μM) |
| 147 | 2-Cl | Me |
|
100 | 1.3 | 2.2 |
| 148 | 2-Cl | Me |

|
100 | 3.9 | 4.8 |
| 149 | 2-Cl | Me | NH(CH2)2NMe2 | 100 | 1.47 | 2.12 |
| 150 | 2-Cl | Me |

|
100 | 2.60 | 3.50 |
| 151 | 2-Cl | — |

|
100 | 0.63 | 0.91 |
| 153 | 2-Cl | Me |

|
100 | 1.74 | 2.64 |
| 154 | 2-Cl | Me |

|
100 | 2.95 | 4.10 |
Miscellaneous compounds
Fig. 36 and 37 show examples of some promising molecules and their schistosomicidal properties are tabulated in Tables 4 and 5.
Fig. 36. Some useful antischistosomal natural products.
Fig. 37. Some antischistosomal agents identified from medicinal chemistry approaches.
Table 4. Antischistosomal properties of some promising compounds.
| Compound a | Species | Life cycle stage | In vitro b (μM) | In vivo c (% WBR) | Morphological effect (≤100 μM in ≤72 h) |
Enzyme inhibition (IC50, μM) | |||
| Worm death (%) | Tegument damage d (%) | Motor activity d (%) | Egg d (% reduction) | ||||||
| 155 162 | S. mansoni | Adult | 53.5 | 100 | 25 | 100 | |||
| 156 163,164 | S. mansoni | Juvenile | 50.2 | ||||||
| Adult | 70.0 | 100 | 100 | 100 | 80.0 | ||||
| 157 165,166 | S. mansoni | Juvenile | 58.0 | ||||||
| Adult | 60.6 | 100 | 100 | 100 | 64.1 | ||||
| 158 167 | S. mansoni | Adult | 100 | 100 | 100 | ||||
| 159 168 | S. mansoni | Larva | 88.6 | ||||||
| Juvenile | 68.3 | ||||||||
| Adult | 70.6 | ||||||||
| S. japonicum | Larva | 90.0 | |||||||
| Juvenile | 59.9 | ||||||||
| Adult | 53.2 | ||||||||
| 160 169 | S. mansoni | Adult | 100 | Y | EC50 = 8.8 μM | EC50 = 3.6 μM | SmAChE e | ||
| 161 170 | B. glabrata | Snail | 1.2 | ||||||
| 162 171 | S. mansoni | Miracidia | 99.7 | ||||||
| Cercariae | 41.0 | ||||||||
| 163 172,173 | S. mansoni | Adult | 81.2 | 100 | 20 | 100 | 54–67 | SmTGR | |
| 164 174 | S. japonicum | Larva | 12.8 | ||||||
| Adult | 15.6 | Y | |||||||
| 165 175 | S. mansoni | Adult | 83.3 | Y | Y | Y | |||
| 166 176 | S. mansoni | Larva | 2.6 | SmTGR | |||||
| Adult | 4.9 | ||||||||
| 167 177 | S. mansoni | Larva | 13.9 | ||||||
| Adult | 100 | Y | |||||||
| 168 178 | S. mansoni | rSmNACE f (2.2) | |||||||
| nSmNACE g (20) | |||||||||
| 169 179 | S. mansoni | Adult | 47.9 | Y | |||||
| 170 180 | S. mansoni | Adult | 100 | SmTGR | |||||
aReference.
bIC50/LC50.
cTotal worm burden reduction.
dSignificant effect observed.
e S. mansoni acetylcholinesterase.
fRecombinant S. mansoni NAD catabolizing enzyme.
gNative S. mansoni NAD catabolizing enzyme.
Table 5. Antischistosomal properties of some promising compounds (contd.).
| Compound a | Schistosoma species | Life cycle stage | Worm death b (%) | SmTGR (IC50, μM) |
| 171 181 | S. mansoni | Larva | 100 | 3.15 |
| Adult | 100 | |||
| 172 182 | S. mansoni | Larva | 99 | 0.33 |
| Juvenile | 39 | |||
| Adult | 62 | |||
| S. japonicum | Adult | 65 | ||
| S. haematobium | Adult | 67 | ||
| 173 182 | S. mansoni | Larva | 100 | 0.21 |
| Juvenile | 100 | |||
| Adult | 78 | |||
| S. japonicum | Adult | 50 | ||
| S. haematobium | Adult | 94 | ||
| 174 181,182 | S. mansoni | Larva | 100 | 2.84 |
| Juvenile | 100 | |||
| Adult | 100 | |||
| S. japonicum | Adult | 70 | ||
| S. haematobium | Adult | 100 | ||
| 175 181,182 | S. mansoni | Larva | 100 | 2.01 |
| Juvenile | 100 | |||
| Adult | 76 | |||
| S. japonicum | Adult | 69 | ||
| S. haematobium | Adult | 85 | ||
| 176 182 | S. mansoni | Larva | 100 | 0.06 |
| Juvenile | 57 | |||
| Adult | 67 | |||
| S. japonicum | Adult | 30 | ||
| S. haematobium | Adult | 48 | ||
| 177 182 | S. mansoni | Larva | 96 | 0.14 |
| Juvenile | 100 | |||
| Adult | 28 | |||
| S. japonicum | Adult | 100 | ||
| 178 182 | S. mansoni | Larva | 20 | 0.13 |
| Juvenile | 24 | |||
| Adult | 53 | |||
| S. japonicum | Adult | 56 |
aReference.
bPercent worm death after 24–144 h of 10–12.5 μM of test compound.
4.0. Where do we go from here?
Recent medicinal chemistry efforts towards identifying schistosomicidal agents have largely been driven by academic research groups, as evident in this review. A diverse class of molecules, exhibiting varying degrees of activity, has been reported. These schistosomicidal properties include multistage and multi-species activities, as well as activity against cercariae, miracidia, and the intermediate snail host. Phenotypic inspections on schistosomes after drug exposure have revealed, amongst others, worm mortality, uncoupling of adult worm, damage of tegument and other related tissues, reduction in motor activity, decrease in egg burdens and development, and decrease in granuloma sizes and recovery of liver and spleen weights.
Compounds that have shown activity against schistosomula are promising prophylactic agents. Worm mortality, or at least reduction in viability, especially of female worms, is of great significance to disease treatment and cure since egg production, tissue egg load, and granulomas are reduced.114 Apart from worm mortality, reduction in egg burden, viability or development can also be linked to alterations in the reproductive abilities of male and/or female worms or dislodge coupled worms.183 Thus, antischistosomal effects on adult worms, or directly on eggs, are potentially able to control the pathological manifestations of the disease and manage transmission from the human host to the environment. Furthermore, tegumental damage, evident as peeling and/or sloughing, disruption of musculature integrity, and loss of spines and sensory tissues, amongst others, after exposure to drugs lend worms to being susceptible to the hosts' immune defenses.184 These morphological defects also cause worms to detach from venal tissues thereby compromising nourishment.
Efforts to expand the schistosomicidal properties of PZQ and OXA have been worthwhile. As shown above, PZQ-hybrid molecules showing multistage activity have been identified.30 Similarly, OXA derivatives with broad species activity have been described.45,46 These results have provided a direction for further research to discover new generations of these drugs. The search for new chemotypes with novel mechanisms of action is necessary in the long term. However, expanding the potencies of already established antischistosomal drugs is important as a short to medium term chemotherapeutic intervention, for which reason more SAR studies on PZQ and OXA are worthwhile.
The antimalarials, notably the endoperoxides and mefloquine, have emerged as promising schistosomicides complementing PZQ by being efficacious against juvenile worms. However, the concurrence of malaria with schistosomiasis in most endemic areas poses a concern with regards to the extensive and broad use of the endoperoxides.49 The artemisinin-combination therapies (ACTs) have become the choice antimalarial chemotherapy of the WHO, although resistant pathogens have emerged.185 For this reason, these drugs may have limited use against schistosomiasis. The exploration of other established drugs in other disease indications may, therefore, be more advantageous. Repositioning or repurposing of such drugs, which have well-established human pharmacokinetic properties, is a low-lying fruit in schistosomiasis drug discovery. In such a venture, it can be envisaged that medicinal chemistry efforts may largely involve SAR investigations to optimize activity while maintaining or improving PK parameters necessary for anti-schistosomiasis clinical use. Unfortunately, although a handful of potential candidates for repositioning have been identified, the needed medicinal chemistry effort to optimize their activity is lagging.
Relatedly, some relinquished schistosomicides of the 20th Century lend themselves useful candidates for drug development, following an elaborate drug rescue program.24 For example, while meclonazepam's schistosomicidal properties are not related to the benzodiazepine scaffold, which is implicated in the observed toxicity, SAR investigations that involve scaffold hopping to replace the benzodiazepine scaffold will be necessary, paralleled with PK and safety studies on promising analogs. As already mentioned, Ro 15-5458, which has exhibited broad-spectrum species efficacy, coupled with significant in vivo potency in monkeys, warrants further investigation.
Moving forward, future work to identify new chemotypes must prioritize those candidates with multistage antischistosomal activity for their potential as single-dose treatments. Such candidates would possess prophylactic properties and have the ability to manage worm and egg populations, thereby offer control over pathological symptoms and morbidity and prevent further transmission. In this regard, the phenotypic evaluation of antischistosomal agents must include larval, juvenile, and adult worm assays. Further, the ability of the compound to be administered orally will be an added advantage.
Undoubtedly, the major advantage of PZQ over other schistosomicides, including OXA, is mainly because of its cross-species activity. For this reason, any new viable candidate must equally possess multi-species efficacy, at least against S. mansoni, S. haematobium, and S. japonicum. Unfortunately, as noted by Lago and colleagues, pharmacological evaluation against S. mansoni has dominated most schistosomiasis research programs (>85%).19 Furthermore, antischistosomal evaluation of promising molecules must incorporate the clinically relevant species, at least S. haematobium and S. japonicum.
The WHO-recommended dose of PZQ for adults and children over 4 years is a single 25 mg kg–1 dose for intestinal fluke infections. Complete cure rate is achieved after a dose of 25 mg kg–1 three times a day for 2 consecutive days. Meanwhile, a single dose of 40 mg kg–1 is also effective.186 The potency of a promising molecule relative to PZQ must also be considered in drug discovery efforts. Some compounds, as shown above, demonstrated comparable potency to PZQ but at lower doses. Essentially, highly potent candidates with a longer half-life than PZQ will be desirable. It will also be useful if the candidate has a novel mode of action. Furthermore, the candidate must possess a low toxicity profile. Lastly, much effort must go into ensuring that the synthesis of the molecule is cheap. It is necessary, therefore, that in efforts to identify the new anti-schistosomiasis drugs, the aforementioned factors are incorporated in the research strategy from the onset.
In summary, there is a need for a well thought through approach and a deliberate effort to populate the schistosomiasis drug development pipeline.
5.0. Conclusion
Eradication of schistosomiasis is more feasible through chemotherapy against the human host parasites. While recent research efforts in schistosomiasis drug discovery are encouraging, a more holistic approach comprising chemotherapy against human clinical schistosomes, chemotherapy against all life cycle stages of the parasite, and community involvement through maintaining good sanitation and improved living conditions, especially in endemic foci, is needed. To this end, more work is required.
Conflicts of interest
The authors have no conflict to declare.
Biographies

Godwin Dziwornu
Godwin Dziwornu obtained his M.Phil. in Organic Chemistry from the University of Ghana under Prof. Dorcas Osei-Safo. He received his Ph.D. degree in Chemistry in 2019 from the University of Cape Town (UCT), South Africa, under the supervision of Profs. Kelly Chibale and Denzil R. Beukes. He is currently a postdoctoral fellow in the laboratory of Prof. Kelly Chibale at the Department of Chemistry, University of Cape Town. His research interests lie in natural products chemistry and medicinal chemistry of natural products and privileged scaffolds toward discovery of drugs against tropical diseases.

Henrietta Dede Attram
Henrietta Dede Attram is a PhD student in the Department of Chemistry at the UCT under the supervision of Prof. Kelly Chibale. She graduated from the University of Ghana in 2015 with a bachelor's degree in chemistry. Her current research work involves conceptualizing drug discovery projects to design pharmaceutically relevant and novel chemotypes for tropical and neglected tropical diseases.

Samuel Gachuhi
Samuel Gachuhi obtained his Bachelor and Master of Science in Chemistry degrees from Moi University and the University of Nairobi (Kenya) in 2009 and 2017, respectively. He is currently completing his Ph.D. work under the guidance of Prof. Kelly Chibale and Dr. Lauren Arendse at the UCT. His research interests are in drug discovery, in particular, design and development of novel drugs for treatment of tropical and neglected tropical diseases.

Kelly Chibale
Kelly Chibale obtained his Ph.D. in Synthetic Organic Chemistry from the University of Cambridge with Stuart Warren (1989–1992). This was followed by postdoctoral stints at the University of Liverpool as a British Ramsay Fellow with Nick Greeves (1992–1994) and at the Scripps Research Institute as a Wellcome Trust International Prize Research Fellow with K. C. Nicolaou (1994–1996). His research is in the field of global health drug discovery. He is the Neville Isdell Chair in African-centric Drug Discovery and Development, South Africa Research Chair in Drug Discovery, Director of the UCT Drug Discovery and Development Centre (H3D), and Director of the South African Medical Research Council Drug Discovery and Development Research Unit at the UCT.
References
- CDC, Parasites – Schistosomiasis, https://www.cdc.gov/parasites/schistosomiasis/biology.html.
- WHO, Neglected Tropical Diseases, https://www.who.int/neglected_diseases/news/unprecedented-treatment-coverage-bilharzia-intestinal-worms/en/.
- WHO, Schistosomiasis, https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
- McManus D. P., Dunne D. W., Sacko M., Utzinger J., Vennervald B. J., Zhou X.-N. Nat. Rev. Dis. Primers. 2018;4(1):13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- Ross A. G., Vickers D., Olds G. R., Shah S. M., McManus D. P. Lancet Infect. Dis. 2007;7(3):218–224. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- Gryseels B., Polman K., Clerinx J., Kestens L. Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Fenwick A., Webster J. P. Curr. Opin. Infect. Dis. 2006;19(6):577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Archer S. Pharmacol. Ther. 1995;68(1):35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Richards H. C., Foster R. Nature. 1969;222(5193):581–582. doi: 10.1038/222581a0. [DOI] [PubMed] [Google Scholar]
- Abdulla M.-H., Ruelas D. S., Wolff B., Snedecor J., Lim K.-C., Xu F., Renslo A. R., Williams J., McKerrow J. H., Caffrey C. R. PLoS Neglected Trop. Dis. 2009;3(7):e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N., Keiser J. Parasites Vectors. 2015;8(1):417. doi: 10.1186/s13071-015-1023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic G., Vargas M., Scandale I., Keiser J. PLoS Neglected Trop. Dis. 2015;9(7):e0003962. doi: 10.1371/journal.pntd.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves B. J., Braga R. C., Bezerra J. C. B., Cravo P. V. L., Andrade C. H. PLoS Neglected Trop. Dis. 2015;9(1):e3435. doi: 10.1371/journal.pntd.0003435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi R., Graef K. M., Dent J. Future Med. Chem. 2015;7(6):727–735. doi: 10.4155/fmc.15.26. [DOI] [PubMed] [Google Scholar]
- Bergquist R., Utzinger J., Keiser J. Infect. Dis. Poverty. 2017;6(1):74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder P., Rennar G. A., Ventura A. M. P., Grevelding C. G., Schlitzer M. ChemMedChem. 2018;13(22):2374–2389. doi: 10.1002/cmdc.201800572. [DOI] [PubMed] [Google Scholar]
- Ribeiro-dos-Santos G., Verjovski-Almeida S., Leite L. C. C. Parasitol. Res. 2006;99(5):505–521. doi: 10.1007/s00436-006-0175-2. [DOI] [PubMed] [Google Scholar]
- Thétiot-Laurent S. A.-L., Boissier J., Robert A., Meunier B. Angew. Chem., Int. Ed. 2013;52(31):7936–7956. doi: 10.1002/anie.201208390. [DOI] [PubMed] [Google Scholar]
- Lago E. M., Xavier R. P., Teixeira T. R., Silva L. M., da Silva Filho A. A., de Moraes J. Future Med. Chem. 2018;10(1):89–120. doi: 10.4155/fmc-2017-0112. [DOI] [PubMed] [Google Scholar]
- Njoroge M., Njuguna N. M., Mutai P., Ongarora D. S. B., Smith P. W., Chibale K. Chem. Rev. 2014;114(22):11138–11163. doi: 10.1021/cr500098f. [DOI] [PubMed] [Google Scholar]
- Caffrey C. R., El-Sakkary N., Mäder P., Krieg R., Becker K., Schlitzer M., Drewry D. H., Vennerstrom J. L. and Grevelding C. G., Drug Discovery and Development for Schistosomiasis, 2019, pp. 187–225. [Google Scholar]
- Tavares N. C., de Aguiar P. H. N., Gava S. G., Oliveira G. and Mourão M. M., Schistosomiasis: Setting Routes for Drug Discovery, in Special Topics in Drug Discovery, InTech, 2016. [Google Scholar]
- Gemma S., Federico S., Brogi S., Brindisi M., Butini S. and Campiani G., Dealing with Schistosomiasis: Current Drug Discovery Strategies, in Medicinal Chemistry Approaches to Malaria and Other Tropical Disease, Elsevier, 2019, pp. 107–138. [Google Scholar]
- Panic G., Keiser J. Curr. Opin. Pharmacol. 2018;42:27–33. doi: 10.1016/j.coph.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Ross A. G. P., Bartley P. B., Sleigh A. C., Olds G. R., Li Y., Williams G. M., McManus D. P. N. Engl. J. Med. 2002;346(16):1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- Meister I., Ingram-Sieber K., Cowan N., Todd M., Robertson M. N., Meli C., Patra M., Gasser G., Keiser J. Antimicrob. Agents Chemother. 2014;58(9):5466–5472. doi: 10.1128/AAC.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovač J., Vargas M., Keiser J. Parasites Vectors. 2017;10(1):365. doi: 10.1186/s13071-017-2293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P., Thomas H., Pohlke R., Seubert J. Med. Res. Rev. 1983;3(2):147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- Ronketti F., Ramana A. V., Chao-Ming X., Pica-Mattoccia L., Cioli D., Todd M. H. Bioorg. Med. Chem. Lett. 2007;17(15):4154–4157. doi: 10.1016/j.bmcl.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Duan W., Qiu S., Zhao Y., Sun H., Qiao C., Xia C. Bioorg. Med. Chem. Lett. 2012;22(4):1587–1590. doi: 10.1016/j.bmcl.2011.12.133. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen J., Qiao C. Chem. Biol. Drug Des. 2013;82(2):216–225. doi: 10.1111/cbdd.12153. [DOI] [PubMed] [Google Scholar]
- Liu H., William S., Herdtweck E., Botros S., Dömling A. Chem. Biol. Drug Des. 2012;79(4):470–477. doi: 10.1111/j.1747-0285.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-L., Song L.-J., Chen X., Yin X.-R., Fan W.-H., Wang G.-P., Yu C.-X., Feng B. Molecules. 2013;18(8):9163–9178. doi: 10.3390/molecules18089163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Chollet J., Vargas M., Mansour N. R., Bickle Q., Alnouti Y., Huang J., Keiser J., Vennerstrom J. L. Bioorg. Med. Chem. Lett. 2010;20(8):2481–2484. doi: 10.1016/j.bmcl.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Dong L., Hu C., Zhao B., Yang C., Xia C., Sun D. Bioorg. Med. Chem. Lett. 2014;24(17):4223–4226. doi: 10.1016/j.bmcl.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Patra M., Ingram K., Pierroz V., Ferrari S., Spingler B., Keiser J., Gasser G. J. Med. Chem. 2012;55(20):8790–8798. doi: 10.1021/jm301077m. [DOI] [PubMed] [Google Scholar]
- Patra M., Ingram K., Pierroz V., Ferrari S., Spingler B., Gasser R. B., Keiser J., Gasser G. Chem. – Eur. J. 2013;19(7):2232–2235. doi: 10.1002/chem.201204291. [DOI] [PubMed] [Google Scholar]
- Patra M., Ingram K., Leonidova A., Pierroz V., Ferrari S., Robertson M. N., Todd M. H., Keiser J., Gasser G. J. Med. Chem. 2013;56(22):9192–9198. doi: 10.1021/jm401287m. [DOI] [PubMed] [Google Scholar]
- da Silva V. B. R., Campos B. R. K. L., de Oliveira J. F., Decout J.-L., do Carmo Alves de Lima M. Bioorg. Med. Chem. 2017;25(13):3259–3277. doi: 10.1016/j.bmc.2017.04.031. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Carlini D., Guidi A., Cimica V., Vigorosi F., Cioli D. Mem. Inst. Oswaldo Cruz. 2006;101(suppl 1):307–312. doi: 10.1590/s0074-02762006000900048. [DOI] [PubMed] [Google Scholar]
- Valentim C. L. L., Cioli D., Chevalier F. D., Cao X., Taylor A. B., Holloway S. P., Pica-Mattoccia L., Guidi A., Basso A., Tsai I. J. Science. 2013;342(6164):1385–1389. doi: 10.1126/science.1243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. B., Pica-Mattoccia L., Polcaro C. M., Donati E., Cao X., Basso A., Guidi A., Rugel A. R., Holloway S. P., Anderson T. J. C. PLoS Neglected Trop. Dis. 2015;9(10):e0004132. doi: 10.1371/journal.pntd.0004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M. S., Suzuki B. M., Pereira G. A. do N., Probst A. C., Ferreira R. S., de Oliveira J. T., Tecchio K. B., dos Santos F. V., Caffrey C. R., de Oliveira R. B. Braz. J. Pharm. Sci. 2018;54(2):e17376. [Google Scholar]
- Rugel A., Tarpley R. S., Lopez A., Menard T., Guzman M. A., Taylor A. B., Cao X., Kovalskyy D., Chevalier F. D., Anderson T. J. C. ACS Med. Chem. Lett. 2018;9(10):967–973. doi: 10.1021/acsmedchemlett.8b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Panic G., Patra M., Mastrobuoni L., Spingler B., Roy S., Keiser J., Gasser G. ACS Infect. Dis. 2017;3(9):645–652. doi: 10.1021/acsinfecdis.7b00054. [DOI] [PubMed] [Google Scholar]
- Buchter V., Hess J., Gasser G., Keiser J. Parasites Vectors. 2018;11(1):580. doi: 10.1186/s13071-018-3132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho S. B., Gargioni C., Silva Pinto P. L., Chiodelli S. G., Gurgel Vellosa S. A., da Silva R. M., da Silveira M. A. B. Int. J. Pharm. 2002;233(1–2):35–41. doi: 10.1016/s0378-5173(01)00917-6. [DOI] [PubMed] [Google Scholar]
- Filho R. P., de Souza Menezes C. M., Pinto P. L. S., Paula G. A., Brandt C. A., da Silveira M. A. B. Bioorg. Med. Chem. 2007;15(3):1229–1236. doi: 10.1016/j.bmc.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Curr. Pharm. Des. 2012;18(24):3531–3538. [PubMed] [Google Scholar]
- Boissier J., Cosledan F., Robert A., Meunier B. Antimicrob. Agents Chemother. 2009;53(11):4903–4906. doi: 10.1128/AAC.00640-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradines V., Portela J., Boissier J., Coslédan F., Meunier B., Robert A. Antimicrob. Agents Chemother. 2011;55(5):2403–2405. doi: 10.1128/AAC.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossallam S. F., Amer E. I., El-Faham M. H. Acta Trop. 2015;143:36–46. doi: 10.1016/j.actatropica.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Ingram K., Yaremenko I. A., Krylov I. B., Hofer L., Terent'ev A. O., Keiser J. J. Med. Chem. 2012;55(20):8700–8711. doi: 10.1021/jm3009184. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kumagai T., Shimogawara R., Ichinose S., Hiramoto A., Sato A., Morita M., Nojima M., Kim H.-S., Wataya Y. Parasitol. Int. 2011;60(3):231–236. doi: 10.1016/j.parint.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Yamabe M., Kumagai T., Shimogawara R., Blay E. A., Hino A., Ichimura K., Sato A., Kim H.-S., Ohta N. Parasitol. Int. 2017;66(1):917–924. doi: 10.1016/j.parint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Ingram K., Ellis W., Keiser J. Antimicrob. Agents Chemother. 2012;56(6):3207–3215. doi: 10.1128/AAC.06177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Orchymont F., Hess J., Panic G., Jakubaszek M., Gemperle L., Keiser J., Gasser G. MedChemComm. 2018;9(11):1905–1909. doi: 10.1039/c8md00396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Chollet J., Xiao S.-H., Mei J.-Y., Jiao P.-Y., Utzinger J., Tanner M. PLoS Neglected Trop. Dis. 2009;3(1):e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott B. T., Cheng K. C.-C., Guha R., Kommer V. P., Williams D. L., Vermeire J. J., Cappello M., Maloney D. J., Rai G., Jadhav A. MedChemComm. 2012;3(12):1505. doi: 10.1039/C2MD20238G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Vargas M., Rubbiani R., Gasser G., Biot C. Parasites Vectors. 2014;7(1):424. doi: 10.1186/1756-3305-7-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. O. F., Keiser J., Amoyaw P. N. A., Hossain M. F., Vargas M., Le J. G., Simpson N. C., Roewe K. D., Freeman T. N. C., Hasley T. R. Antimicrob. Agents Chemother. 2016;60(9):5331–5336. doi: 10.1128/AAC.00778-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves B. J., Dantas R. F., Senger M. R., Valente W. C. G., Rezende-Neto J. de M., Chaves W. T., Kamentsky L., Carpenter A., Silva-Junior F. P., Andrade C. H. MedChemComm. 2016;7(6):1176–1182. [Google Scholar]
- Wang W.-L., Song L.-J., Hu B.-C., Miao L., Chen X.-Y., Fan W.-H., Yin X.-R., Shen S., Ding Z.-F., Yu C.-X. Chin. Chem. Lett. 2017;28(7):1547–1552. [Google Scholar]
- Guerra R. A., Silva M. P., Silva T. C., Salvadori M. C., Teixeira F. S., de Oliveira R. N., Rocha J. A., Pinto P. L. S., de Moraes J. Antimicrob. Agents Chemother. 2019;63(3):e01722-18. doi: 10.1128/AAC.01722-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi A., Lalli C., Perlas E., Bolasco G., Nibbio M., Monteagudo E., Bresciani A., Ruberti G. PLoS Neglected Trop. Dis. 2016;10(8):e0004928. doi: 10.1371/journal.pntd.0004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes E., Varela-M R. E., López-Abán J., Dakir E. L. H., Mollinedo F., Muro A. PLoS One. 2014;9(10):e109431. doi: 10.1371/journal.pone.0109431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago E. M., Silva M. P., Queiroz T. G., Mazloum S. F., Rodrigues V. C., Carnaúba P. U., Pinto P. L., Rocha J. A., Ferreira L. L. G., Andricopulo A. D. EBioMedicine. 2019;43:370–379. doi: 10.1016/j.ebiom.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa M. M., Mossallam S. F., Amer E. I., Younis L. K., Rashed H. A. Acta Trop. 2017;166:58–66. doi: 10.1016/j.actatropica.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Roquini D. B., Cogo R. M., Mengarda A. C., Mazloum S. F., Morais C. S., Xavier R. P., Salvadori M. C., Teixeira F. S., Ferreira L. E., Pinto P. L. Antimicrob. Agents Chemother. 2019;63(12):e01208-19. doi: 10.1128/AAC.01208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernauer K., Link H. and Stohler H., Antiandrogenic and Schistosomicidal Imidazolidine Derivatives, US Pat., 4234736, 1980.
- Forget E., Félix H., Notteghem M. J., Léger N. Bull. Soc. Pathol. Exot. Ses Fil. 1982;75:192–200. [PubMed] [Google Scholar]
- Hansen J. B., Hafliger O. J. Pharm. Sci. 1983;72(4):429–431. doi: 10.1002/jps.2600720426. [DOI] [PubMed] [Google Scholar]
- Link H., Stohler H. R. Eur. J. Med. Chem. 1984;19:261–265. [Google Scholar]
- Wang X., Dong Y., Min J., Wang C., Muniyan S., Vargas M., Vennerstrom J. L., Zhao Q., Lin M.-F., Keiser J. Am. J. Trop. Med. Hyg. 2014;90(6):1156–1158. doi: 10.4269/ajtmh.14-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Panic G., Vargas M., Wang C., Dong Y., Gautam N., Vennerstrom J. L. J. Antimicrob. Chemother. 2015;70(6):1788–1797. doi: 10.1093/jac/dkv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao Q., Vargas M., Jones J. O., White K. L., Shackleford D. M., Chen G., Saunders J., Ng A. C. F., Chiu F. C. K. J. Med. Chem. 2016;59(23):10705–10718. doi: 10.1021/acs.jmedchem.6b01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Ghani R. A., Loutfy N., Hassan A. Parasitol. Res. 2009;105(4):899–906. doi: 10.1007/s00436-009-1546-2. [DOI] [PubMed] [Google Scholar]
- Baard A. P., Sommers D. K., Honiball P. J., Fourie E. D., du Toit L. E. S. Afr. Med. J. 1979;55(16):617–618. [PubMed] [Google Scholar]
- Pax R., Bennett J. L., Fetterer R. Naunyn-Schmiedeberg's Arch. Pharmacol. 1978;304(3):309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- Thibaut J. P. B., Monteiro L. M., Leite L. C. C., Menezes C. M. S., Lima L. M., Noël F. Eur. J. Pharmacol. 2009;606(1–3):9–16. doi: 10.1016/j.ejphar.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Menezes C. M. S., Rivera G., Alves M. A., do Amaral D. N., Thibaut J. P. B., Noël F., Barreiro E. J., Lima L. M. Chem. Biol. Drug Des. 2012;79(6):943–949. doi: 10.1111/j.1747-0285.2012.01354.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. F., Bain J., Webbe G., Doenhoff M. J., Stohler H. Trans. R. Soc. Trop. Med. Hyg. 1987;81:188–192. doi: 10.1016/0035-9203(87)90210-0. [DOI] [PubMed] [Google Scholar]
- Coelho P. M. Z., Pereira L. H., Mello R. T. Rev. Soc. Bras. Med. Trop. 1995;28(3):179–183. doi: 10.1590/s0037-86821995000300003. [DOI] [PubMed] [Google Scholar]
- Pereira L. H., Coelho P. M. Z., Costa J. O., Mello R. T. Mem. Inst. Oswaldo Cruz. 1995;90(3):425–428. doi: 10.1590/s0074-02761995000300021. [DOI] [PubMed] [Google Scholar]
- Ingram-Sieber K., Cowan N., Panic G., Vargas M., Mansour N. R., Bickle Q. D., Wells T. N. C., Spangenberg T., Keiser J. PLoS Neglected Trop. Dis. 2014;8(1):e2610. doi: 10.1371/journal.pntd.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasche V., Laleu B., Keiser J. Parasites Vectors. 2018;11(1):298. doi: 10.1186/s13071-018-2855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasche V., Laleu B., Keiser J. ACS Infect. Dis. 2019;5(1):102–110. doi: 10.1021/acsinfecdis.8b00220. [DOI] [PubMed] [Google Scholar]
- Cowan N., Dätwyler P., Ernst B., Wang C., Vennerstrom J. L., Spangenberg T., Keiser J. Antimicrob. Agents Chemother. 2015;59(4):1935–1941. doi: 10.1128/AAC.04463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang C., Leas D., Vargas M., White K. L., Shackleford D. M., Chen G., Sanford A. G., Hemsley R. M., Davis P. H. Bioorg. Med. Chem. Lett. 2018;28(3):244–248. doi: 10.1016/j.bmcl.2017.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Liu F., Chen J., Li Y., Cui J., Qiao C. Bioorg. Med. Chem. Lett. 2016;26(5):1386–1390. doi: 10.1016/j.bmcl.2016.01.075. [DOI] [PubMed] [Google Scholar]
- Wu J., Wang C., Häberli C., White K. L., Shackleford D. M., Chen G., Dong Y., Charman S. A., Keiser J., Vennerstrom J. L. Bioorg. Med. Chem. Lett. 2018;28(23–24):3648–3651. doi: 10.1016/j.bmcl.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A., Cosseau C., Grunau C. Exp. Parasitol. 2009;121(3):288–291. doi: 10.1016/j.exppara.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Dubois F., Caby S., Oger F., Cosseau C., Capron M., Grunau C., Dissous C., Pierce R. J. Mol. Biochem. Parasitol. 2009;168(1):7–15. doi: 10.1016/j.molbiopara.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yang X.-J., Seto E. Nat. Rev. Mol. Cell Biol. 2008;9(3):206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oger F., Dubois F., Caby S., Noël C., Cornette J., Bertin B., Capron M., Pierce R. J. Biochem. Biophys. Res. Commun. 2008;377(4):1079–1084. doi: 10.1016/j.bbrc.2008.10.090. [DOI] [PubMed] [Google Scholar]
- Marek M., Kannan S., Hauser A.-T., Moraes Mourão M., Caby S., Cura V., Stolfa D. A., Schmidtkunz K., Lancelot J., Andrade L. PLoS Pathog. 2013;9(9):e1003645. doi: 10.1371/journal.ppat.1003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Melesina J., Hauser A.-T., Chakrabarti A., Heimburg T., Schmidtkunz K., Walter A., Marek M., Pierce R. J., Romier C. J. Chem. Inf. Model. 2014;54(10):3005–3019. doi: 10.1021/ci5004653. [DOI] [PubMed] [Google Scholar]
- Heimburg T., Chakrabarti A., Lancelot J., Marek M., Melesina J., Hauser A. T., Shaik T. B., Duclaud S., Robaa D., Erdmann F. J. Med. Chem. 2016;59(6):2423–2435. doi: 10.1021/acs.jmedchem.5b01478. [DOI] [PubMed] [Google Scholar]
- Stenzel K., Chakrabarti A., Melesina J., Hansen F. K., Lancelot J., Herkenhöhner S., Lungerich B., Marek M., Romier C., Pierce R. J. Arch. Pharm. 2017;350(8):1700096. doi: 10.1002/ardp.201700096. [DOI] [PubMed] [Google Scholar]
- Simoben C., Robaa D., Chakrabarti A., Schmidtkunz K., Marek M., Lancelot J., Kannan S., Melesina J., Shaik T., Pierce R. Molecules. 2018;23(3):566. doi: 10.3390/molecules23030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T., Chakrabarti A., Lancelot J., Shaik T. B., Hausmann K., Melesina J., Schmidtkunz K., Marek M., Erdmann F., Schmidt M. ChemMedChem. 2018;13(15):1517–1529. doi: 10.1002/cmdc.201800238. [DOI] [PubMed] [Google Scholar]
- Monaldi D., Rotili D., Lancelot J., Marek M., Wössner N., Lucidi A., Tomaselli D., Ramos-Morales E., Romier C., Pierce R. J. J. Med. Chem. 2019;62(19):8733–8759. doi: 10.1021/acs.jmedchem.9b00638. [DOI] [PubMed] [Google Scholar]
- Peña I., Pilar Manzano M., Cantizani J., Kessler A., Alonso-Padilla J., Bardera A. I., Alvarez E., Colmenarejo G., Cotillo I., Roquero I. Sci. Rep. 2015;5(1):8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A. M., El-Azab A. S., Al-Suwaidan I. A., ElTahir K. E. H., Asiri Y. A., Abdel-Aziz N. I., Abdel-Aziz A. A.-M. Eur. J. Med. Chem. 2015;92:115–123. doi: 10.1016/j.ejmech.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Kamiński K., Obniska J., Wiklik B., Atamanyuk D. Eur. J. Med. Chem. 2011;46(9):4634–4641. doi: 10.1016/j.ejmech.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Abdel-Aziz A. A.-M., El-Azab A. S., Attia S. M., Al-Obaid A. M., Al-Omar M. A., El-Subbagh H. I. Eur. J. Med. Chem. 2011;46(9):4324–4329. doi: 10.1016/j.ejmech.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Akgün H., Karamelekoğlu İ., Berk B., Kurnaz I., Sarıbıyık G., Öktem S., Kocagöz T. Bioorg. Med. Chem. 2012;20(13):4149–4154. doi: 10.1016/j.bmc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- Elumalai K., Ali M. A., Elumalai M., Eluri K., Srinivasan S., Sivannan S., Mohanthi S. K. Int. J. Chem. Anal. Sci. 2013;4(2):57–61. [Google Scholar]
- Williams R., Manka J. T., Rodriguez A. L., Vinson P. N., Niswender C. M., Weaver C. D., Jones C. K., Conn P. J., Lindsley C. W., Stauffer S. R. Bioorg. Med. Chem. Lett. 2011;21(5):1350–1353. doi: 10.1016/j.bmcl.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago E. de F., de Oliveira S. A., de Oliveira Filho G. B., Moreira D. R. M., Gomes P. A. T., da Silva A. L., de Barros A. F., da Silva A. C., dos Santos T. A. R., Pereira V. R. A. Antimicrob. Agents Chemother. 2014;58(1):352–363. doi: 10.1128/AAC.01900-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S. A., de Oliveira Barbosa M., Filho C. A. L. M., Oliveira A. R., de Sousa F. A., de Farias Santiago E., de Oliveira Filho G. B., de Moraes Gomes P. A. T., da Conceição J. M., Brayner F. A. Parasitol. Res. 2018;117(7):2105–2115. doi: 10.1007/s00436-018-5897-4. [DOI] [PubMed] [Google Scholar]
- de Oliveira Barbosa M., de Oliveira S. A., Filho C. A. L. M., Oliveira A. R., Fernandes C. J. B., Lucena J. P., dede SousaSousa F. A., de Barros Dias M. C. H., Brayner F. A., Alves L. C. Eur. J. Pharm. Sci. 2019;133:15–27. doi: 10.1016/j.ejps.2019.03.008. [DOI] [PubMed] [Google Scholar]
- Botros S. S., William S., Sabra A.-N. A., El-Lakkany N. M., Seif El-Din S. H., García-Rubia A., Sebastián-Pérez V., Blaazer A. R., de Heuvel E., Sijm M. Int. J. Parasitol.: Drugs Drug Resist. 2019;9:35–43. doi: 10.1016/j.ijpddr.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bialy S. A., Taman A., El-Beshbishi S. N., Mansour B., El-Malky M., Bayoumi W. A., Essa H. M. Parasitol. Res. 2013;112(12):4221–4229. doi: 10.1007/s00436-013-3614-x. [DOI] [PubMed] [Google Scholar]
- Long T., Neitz R. J., Beasley R., Kalyanaraman C., Suzuki B. M., Jacobson M. P., Dissous C., McKerrow J. H., Drewry D. H., Zuercher W. J. PLoS Neglected Trop. Dis. 2016;10(1):e0004356. doi: 10.1371/journal.pntd.0004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rida S., Soliman F. S. G., Badawey E. A. M., El-Ghazzawi E., Kader O., Kappe T. J. Heterocycl. Chem. 1988;25:1087–1093. [Google Scholar]
- Khare S., Roach S. L., Barnes S. W., Hoepfner D., Walker J. R., Chatterjee A. K., Neitz R. J., Arkin M. R., McNamara C. W., Ballard J. PLoS Pathog. 2015;11(7):e1005058. doi: 10.1371/journal.ppat.1005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okombo J., Singh K., Mayoka G., Ndubi F., Barnard L., Njogu P. M., Njoroge M., Gibhard L., Brunschwig C., Vargas M. ACS Infect. Dis. 2017;3(6):411–420. doi: 10.1021/acsinfecdis.6b00205. [DOI] [PubMed] [Google Scholar]
- Mayoka G., Keiser J., Häberli C., Chibale K. ACS Infect. Dis. 2019;5(3):418–429. doi: 10.1021/acsinfecdis.8b00313. [DOI] [PubMed] [Google Scholar]
- Sayed A. A., Simeonov A., Thomas C. J., Inglese J., Austin C. P., Williams D. L. Nat. Med. 2008;14(4):407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L. J., Luo H., Fan W. H., Wang G. P., Yin X. R., Shen S., Wang J., Jin Y., Zhang W., Gao H. Parasites Vectors. 2016;9(1):1–12. doi: 10.1186/s13071-016-1301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eweas A. F., Allam G. Mol. Biochem. Parasitol. 2018;225:94–102. doi: 10.1016/j.molbiopara.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Rai G., Sayed A. A., Lea W. A., Luecke H. F., Chakrapani H., Prast-Nielsen S., Jadhav A., Leister W., Shen M., Inglese J. J. Med. Chem. 2009;52(20):6474–6483. doi: 10.1021/jm901021k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho G. S. G., Dias R. M. P., Pavan F. R., Leite C. Q. F., Silva V. L., Diniz C. G., de Paula D. T. S., Coimbra E. S., Retailleau P., da Silva A. D. Med. Chem. 2013;9(3):351–359. doi: 10.2174/1573406411309030005. [DOI] [PubMed] [Google Scholar]
- Caterina M. C., Perillo I. A., Boiani L., Pezaroglo H., Cerecetto H., González M., Salerno A. Bioorg. Med. Chem. 2008;16(5):2226–2234. doi: 10.1016/j.bmc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- Pitta M. G., Silva A. C., AL Neves J. K., Silva P. G., Irmão J. I., Malagueño E., Santana J. V., Lima M. C., Galdino S. L., Pitta I. R. Mem. Inst. Oswaldo Cruz. 2006;101(suppl 1):313–316. doi: 10.1590/s0074-02762006000900049. [DOI] [PubMed] [Google Scholar]
- Neves J. K. de A. L., Botelho S. P. S., de Melo C. M. L., Pereira V. R. A., de Lima M. D. C. A., da Rocha Pitta I., de Azevedo Albuquerque M. C. P., Lins Galdino S. Parasitol. Res. 2010;107(3):531–538. doi: 10.1007/s00436-010-1886-y. [DOI] [PubMed] [Google Scholar]
- Neves J. K. A. L., de Lima M. D. C. A., Pereira V. R. A., de Melo C. M. L., Peixoto C. A., Pitta I. D. R., Albuquerque M. C. P. A., Galdino S. L. Exp. Parasitol. 2011;128(1):82–90. doi: 10.1016/j.exppara.2011.01.021. [DOI] [PubMed] [Google Scholar]
- da Silva A. C. A., Neves J. K. de A. L., Irmão J. I., Costa V. M. A., Souza V. M. O., de Medeiros P. L., da Silva E. C., de Lima M. de C. A., Pitta I. da R., Albuquerque M. C. P. de A. Sci. World J. 2012;2012:1–8. [Google Scholar]
- Matos-Rocha T. J., Lima M. do C. A. de, Silva A. L. da, Oliveira J. F. de, Gouveia A. L. A., Silva V. B. R. da, Almeida Júnior A. S. A. de, Brayner F. A., Cardoso P. R. G., Pitta-Galdino M. da R. Rev. Inst. Med. Trop. Sao Paulo. 2017;59:e8. doi: 10.1590/S1678-9946201759008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Q., Wei Y., Xue J., Sun X., Yu Y., Chen Z., Li S., Duan L. Int. J. Parasitol.: Drugs Drug Resist. 2017;7(2):191–199. doi: 10.1016/j.ijpddr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder P., Blohm A. S., Quack T., Lange-Grünweller K., Grünweller A., Hartmann R. K., Grevelding C. G., Schlitzer M. ChemMedChem. 2016;11(13):1459–1468. doi: 10.1002/cmdc.201600127. [DOI] [PubMed] [Google Scholar]
- Blohm A. S., Mäder P., Quack T., Lu Z., Hahnel S., Schlitzer M., Grevelding C. G. Parasitol. Res. 2016;115(10):3831–3842. doi: 10.1007/s00436-016-5146-7. [DOI] [PubMed] [Google Scholar]
- Peter Ventura A. M., Haeberlein S., Lange-Grünweller K., Grünweller A., Hartmann R. K., Grevelding C. G., Schlitzer M. ChemMedChem. 2019;14(21):1856–1862. doi: 10.1002/cmdc.201900423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger H. M., Williams D. L. Mol. Biochem. Parasitol. 2002;121(1):129–139. doi: 10.1016/s0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- Berriman M., Haas B. J., LoVerde P. T., Wilson R. A., Dillon G. P., Cerqueira G. C., Mashiyama S. T., Al-Lazikani B., Andrade L. F., Ashton P. D. Nature. 2009;460(7253):352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Wilairat P., Croft S. L., Tan A. L. C., Go M. L. Bioorganic Med. Chem. 2003;11(13):2729–2738. doi: 10.1016/s0968-0896(03)00233-5. [DOI] [PubMed] [Google Scholar]
- de Mello M. V. P., Abrahim-Vieira B. de A., Domingos T. F. S., de Jesus J. B., de Sousa A. C. C., Rodrigues C. R., Souza A. M. T. de. Eur. J. Med. Chem. 2018;150:920–929. doi: 10.1016/j.ejmech.2018.03.047. [DOI] [PubMed] [Google Scholar]
- Tajuddeen N., Isah M. B., Suleiman M. A., van Heerden F. R., Ibrahim M. A. Int. J. Antimicrob. Agents. 2018:311–318. doi: 10.1016/j.ijantimicag.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Yadav N., Dixit S. K., Bhattacharya A., Mishra L. C., Sharma M., Awasthi S. K., Bhasin V. K. Chem. Biol. Drug Des. 2012;80(2):340–347. doi: 10.1111/j.1747-0285.2012.01383.x. [DOI] [PubMed] [Google Scholar]
- de Castro C. C. B., Costa P. S., Laktin G. T., de Carvalho P. H. D., Geraldo R. B., de Moraes J., Pinto P. L. S., Couri M. R. C., Pinto P. de F., Da Silva Filho A. A. Phytomedicine. 2015;22(10):921–928. doi: 10.1016/j.phymed.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Souza R. L., Gonçalves U. O., Badoco F. R., de Souza Galvão L., Santos R. A., de Carvalho P. H. D., de Carvalho L. S. A., da Silva Filho A. A., Veneziani R. C. S., Rodrigues V. Biomed. Pharmacother. 2017;96:64–71. doi: 10.1016/j.biopha.2017.09.128. [DOI] [PubMed] [Google Scholar]
- Pereira V. R. D., Junior I. J. A., da Silveira L. S., Geraldo R. B., de F. Pinto P., Teixeira F. S., Salvadori M. C., Silva M. P., Alves L. A., Capriles P. V. S. Z. Chem. Biodiversity. 2018;15(12):e1800398. doi: 10.1002/cbdv.201800398. [DOI] [PubMed] [Google Scholar]
- Wang W.-B., Liu H.-J., Wang B.-J., Zhou X., Zhang J., Liu C.-C., Gong W., Zhu-Ge H.-X. Zhongguo Xuexichongbing Fangzhi Zazhi. 2011;23(3):268–272. [PubMed] [Google Scholar]
- Porto T. S., da Silva Filho A. A., Magalhães L. G., dos Santos R. A., Furtado N. A. J. C., Arakawa N. S., Said S., de Oliveira D. C. R., Gregório L. E., Rodrigues V. Chem. Biodiversity. 2012;9(8):1465–1474. doi: 10.1002/cbdv.201100336. [DOI] [PubMed] [Google Scholar]
- de Moraes J., de Oliveira R. N., Costa J. P., Junior A. L. G., de Sousa D. P., Freitas R. M., Allegretti S. M., Pinto P. L. S. PLoS Neglected Trop. Dis. 2014;8(1):e2617. doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros de Alencar M. V. O., de Castro E Sousa J. M., Rolim H. M. L., de Medeiros M. das G. F., Cerqueira G. S., de Castro Almeida F. R., Citó A. M. das G. L., Ferreira P. M. P., Lopes J. A. D., de Carvalho Melo-Cavalcante A. A. Phyther. Res. 2017;31(2):175–201. doi: 10.1002/ptr.5749. [DOI] [PubMed] [Google Scholar]
- Edwards J., Brown M., Peak E., Bartholomew B., Nash R. J., Hoffmann K. F. PLoS Neglected Trop. Dis. 2015;9(3):e0003604. doi: 10.1371/journal.pntd.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusco A., Bordoni C., Chakroborty A., Whatley K. C. L., Whiteland H., Westwell A. D., Hoffmann K. F. Eur. J. Med. Chem. 2018;152:87–100. doi: 10.1016/j.ejmech.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Crusco A., Whiteland H., Baptista R., Forde-Thomas J. E., Beckmann M., Mur L. A. J., Nash R. J., Westwell A. D., Hoffmann K. F. ACS Infect. Dis. 2019;5(7):1188–1199. doi: 10.1021/acsinfecdis.9b00034. [DOI] [PubMed] [Google Scholar]
- Simpson D. and Amos S., Other Plant Metabolites, in Pharmacognosy, Elsevier, 2017, pp. 267–280. [Google Scholar]
- Tsopmo A., Awah F. M. and Kuete V., Lignans and Stilbenes from African Medicinal Plants, in Medicinal Plant Research in Africa, Elsevier, 2013, pp. 435–478. [Google Scholar]
- E Silva M. L. A., Esperandim V. R., Ferreira D. da S., Magalhães L. G., Lima T. C., Cunha W. R., Nanayakkara D. N. P., Pereira A. C., Bastos J. K. Phytochemistry. 2014;107:119–125. doi: 10.1016/j.phytochem.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Xu W.-H., Zhao P., Wang M., Liang Q. Nat. Prod. Res. 2019;33(9):1357–1373. doi: 10.1080/14786419.2018.1474467. [DOI] [PubMed] [Google Scholar]
- Parreira R. L. T., Costa E. S., Heleno V. C. G., Magalhães L. G., Souza J. M., Pauletti P. M., Cunha W. R., Januário A. H., Símaro G. V., Bastos J. K. Chem. Biodiversity. 2019;16(1):e1800305. doi: 10.1002/cbdv.201800305. [DOI] [PubMed] [Google Scholar]
- Pereira A. C., Silva M. L. A. E., Souza J. M., Laurentiz R. S., Rodrigues V., Januário A. H., Pauletti P. M., Tavares D. C., Filho A. A. D. S., Cunha W. R. Acta Trop. 2015;149:195–201. doi: 10.1016/j.actatropica.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Magalhães L. G., Lima T. C., de Paula R. G., Morais E. R., Aguiar D. P., Gardinassi L. G., Garcia G. R., Laurentiz R. S., Rodrigues V., Bastos J. K. Rev. Bras. Farmacogn. 2016;26(3):334–341. [Google Scholar]
- Dias H. J., Patrocínio A. B., Pagotti M. C., Fukui M. J., Rodrigues V., Magalhães L. G., Crotti A. E. M. Chem. Biodiversity. 2018;15(7):e1800134. doi: 10.1002/cbdv.201800134. [DOI] [PubMed] [Google Scholar]
- Wright C. W. Rev. Bras. Farmacogn. 2007;59(6):899–904. doi: 10.1211/jpp.59.6.0017. [DOI] [PubMed] [Google Scholar]
- El Sayed I., Van der Veken P., Steert K., Dhooghe L., Hostyn S., Van Baelen G., Lemière G., Maes B. U. W., Cos P., Maes L. J. Med. Chem. 2009;52(9):2979–2988. doi: 10.1021/jm801490z. [DOI] [PubMed] [Google Scholar]
- El Bardicy S., El Sayed I., Yousif F., Van der Veken P., Haemers A., Augustyns K., Pieters L. Pharm. Biol. 2012;50(2):134–140. doi: 10.3109/13880209.2011.578278. [DOI] [PubMed] [Google Scholar]
- Pereira A. C., Magalhães L. G., Gonçalves U. O., Luz P. P., Moraes A. C. G., Rodrigues V., da Matta Guedes P. M., da Silva Filho A. A., Cunha W. R., Bastos J. K. Phytochemistry. 2011;72(11–12):1424–1430. doi: 10.1016/j.phytochem.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Veras L. M., Guimaraes A. M., Campelo D. Y., Vieira M. M., Nascimento C., Lima F. D., Vasconcelos L., Nakano E., Kuckelhaus S. S., Batista C. M. Curr. Med. Chem. 2012;19(13):2051–2058. doi: 10.2174/092986712800167347. [DOI] [PubMed] [Google Scholar]
- Guimarães M. A., de Oliveira R. N., Véras L. M. C., Lima D. F., Campelo Y. D. M., Campos S. A., Kuckelhaus S. A. S., Pinto P. L. S., Eaton P., Mafud A. C. PLoS Neglected Trop. Dis. 2015;9(3):e0003656. doi: 10.1371/journal.pntd.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha J. A., Andrade I. M., Véras L. M. C., Quelemes P. V., Lima D. F., Soares M. J. S., Pinto P. L. S., Mayo S. J., Ivanova G., Rangel M. Phyther. Res. 2017;31(4):624–630. doi: 10.1002/ptr.5771. [DOI] [PubMed] [Google Scholar]
- Guimarães M. A., de Oliveira R. N., de Almeida R. L., Mafud A. C., Sarkis A. L. V., Ganassin R., da Silva M. P., Roquini D. B., Veras L. M., Sawada T. C. H. PLoS One. 2018;13(5):e0196667. doi: 10.1371/journal.pone.0196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L. M. S., Carvalho L. S. A., Gazolla M. C., Silva Pinto P. L., Silva M. P. N., de Moraes J., Da Silva Filho A. A. Evid.-Based Complementary Altern. Med. 2016;2016:1–9. [Google Scholar]
- Kang N., Shen W., Gao H., Feng Y., Zhu W., Yang S., Liu Y., Xu Q., Yu D. Molecules. 2018;23(6):1431. doi: 10.3390/molecules23061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellershohn J., Thomas L., Hahnel S. R., Grünweller A., Hartmann R. K., Hardt M., Vilcinskas A., Grevelding C. G., Haeberlein S. PLoS Neglected Trop. Dis. 2019;13(3):e0007240. doi: 10.1371/journal.pntd.0007240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. R., McCue C. F., Gerwick W. H. J. Nat. Prod. 2010;73(2):217–220. doi: 10.1021/np9008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sayed E., Hamid H. A., Abu El Einin H. M. Pharm. Biol. 2014;52(6):698–705. doi: 10.3109/13880209.2013.865240. [DOI] [PubMed] [Google Scholar]
- Eweas A. F., Allam G., Abuelsaad A. S. A., ALGhamdi A. H., Maghrabi I. A. Bioorg. Chem. 2013;46:17–25. doi: 10.1016/j.bioorg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Allam G., Eweas A. F., Abuelsaad A. S. A. Parasitol. Res. 2013;112(9):3137–3149. doi: 10.1007/s00436-013-3490-4. [DOI] [PubMed] [Google Scholar]
- Zhou S., Huang G. MedChemComm. 2018;9(2):328–336. doi: 10.1039/c7md00590c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariz Gomes da Silva L. M., de Oliveira J. F., Silva W. L., da Silva A. L., de Almeida Junior A. S. A., Barbosa dos Santos V. H., Alves L. C., Brayner dos Santos F. A., Costa V. M. A., Aires A. de L. Chem.-Biol. Interact. 2018;283:20–29. doi: 10.1016/j.cbi.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Neves B. J., Dantas R. F., Senger M. R., Melo-Filho C. C., Valente W. C. G., de Almeida A. C. M., Rezende-Neto J. M., Lima E. F. C., Paveley R., Furnham N. J. Med. Chem. 2016;59(15):7075–7088. doi: 10.1021/acs.jmedchem.5b02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmelli R., Persico M., Imperatore C., Saccoccia F., Guidi A., Casertano M., Luciano P., Pietrantoni A., Bertuccini L., Paladino A. ACS Infect. Dis. 2020;6(1):124–137. doi: 10.1021/acsinfecdis.9b00252. [DOI] [PubMed] [Google Scholar]
- Muller-Steffner H., Jacques S. A., Kuhn I., Schultz M. D., Botta D., Osswald P., Maechling C., Lund F. E., Kellenberger E. ACS Chem. Biol. 2017;12(7):1787–1795. doi: 10.1021/acschembio.7b00186. [DOI] [PubMed] [Google Scholar]
- Rando D. G. G., da Costa M. O. L., Pavani T. F. A., Oliveira T., dos Santos P. F., Amorim C. R., Pinto P. L. S., de Brito M. G., Silva M. P. N., Roquini D. B. Curr. Top. Med. Chem. 2019;19(14):1241–1251. doi: 10.2174/1568026619666190620163237. [DOI] [PubMed] [Google Scholar]
- Lanfranchi D. A., Cesar-Rodo E., Bertrand B., Huang H.-H., Day L., Johann L., Elhabiri M., Becker K., Williams D. L., Davioud-Charvet E. Org. Biomol. Chem. 2012;10(31):6375. doi: 10.1039/c2ob25812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Ziniel P. D., He P., Kommer V. P., Crowther G. J., He M., Liu Q., Van Voorhis W. C., Williams D. L., Wang M.-W. Infect. Dis. Poverty. 2015;4(1):40. doi: 10.1186/s40249-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu H., Petukhov P. A., Banta P. R., Jadhav A., Lea W. A., Cheng Q., Arnér E. S. J., Simeonov A., Thatcher G. R. J., Angelucci F. ACS Infect. Dis. 2020;6(3):393–405. doi: 10.1021/acsinfecdis.9b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth L. R., Fernandes A. P. M., Rodrigues V. Rev. Inst. Med. Trop. Sao Paulo. 1996;38(6):423–426. doi: 10.1590/s0036-46651996000600006. [DOI] [PubMed] [Google Scholar]
- Van Hellemond J. J., Retra K., Brouwers J. F. H. M., van Balkom B. W. M., Yazdanbakhsh M., Shoemaker C. B., Tielens A. G. M. Int. J. Parasitol. 2006;36(6):691–699. doi: 10.1016/j.ijpara.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Tilley L., Straimer J., Gnädig N. F., Ralph S. A., Fidock D. A. Trends Parasitol. 2016;32(9):682–696. doi: 10.1016/j.pt.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, WHO Model Prescribing Information: Drugs Used in Parasitic Diseases, 2nd edn, 1995. [Google Scholar]