Abstract
Analysis and systematization of accumulated data on carbohydrate structural diversity is a subject of great interest for structural glycobiology. Despite being a challenging task, development of computational methods for efficient treatment and management of spatial (3D) structural features of carbohydrates breaks new ground in modern glycoscience. This review is dedicated to approaches of chemo- and glyco-informatics towards 3D structural data generation, deposition and processing in regard to carbohydrates and their derivatives. Databases, molecular modeling and experimental data validation services, and structure visualization facilities developed for last five years are reviewed.
Keywords: carbohydrate, spatial structure, model build, database, web-tool, glycoinformatics, structure validation, PDB glycans, structure visualization, molecular modeling
1. Introduction
Knowledge of carbohydrate spatial (3D) structure is crucial for investigation of glycoconjugate biological activity [1,2], vaccine development [3,4], estimation of ligand-receptor interaction energy [5,6,7] studies of conformational mobility of macromolecules [8], drug design [9], studies of cell wall construction aspects [10], glycosylation processes [11], and many other aspects of carbohydrate chemistry and biology. Therefore, providing information support for carbohydrate 3D structure is vital for the development of modern glycomics and glycoproteomics.
As result of growing interest to glycoprofiling, glycan microarrays, carbohydrate active enzymes (CAZy) and glycan-binding proteins (GBP) which are involved in biological processes, several major international projects (e.g., GlySpace [12], GlyCosmos [13], Glycomics@ExPASy [14], GlyGen [15], JCGGDB [16], Glytoucan [17], MIRAGE [18], CFG [19], RINGS [20], GLIC (https://glic.glycoinfo.org/), SysGlyco (https://sysglyco.org/)) were launched to integrate variety of data produced by glycobiological research. The main goal of existing glycoinformatics projects is to provide versatile resources with user-friendly access helpful for disease diagnostics [21,22], glycobioinformatics studies [23], glycosylation site prediction [24], CAZy activity prognosis [25,26] and other applications.
Appending of structural repositories with 3D structural data opens the way for computational glycobiology and modeling of carbohydrate structures at atomic resolution. Design of novel workflows and techniques to connect carbohydrate spatial structure modes and experimental data with verification, processing, analysis and deposition of associated data has gained increased popularity in glycoscience community [27]. A Carbohydrate Structure Database (CSDB, [28]) module for carbohydrate 3D structure modeling is a demonstrative example of 3D structural data integration facilities (as a database) combined with dedicated interface (as a glycoinformatics project). Further details on CSDB 3D facilities are discussed below.
The typical types of knowledge about a carbohydrate 3D structure include (Figure 1):
Primary structure (atom connectivity);
Monosaccharide ring conformation;
Rotational states of inter-residue and exocyclic linkages and their energies;
Ring puckering and transitions of glycosidic linkage conformation on a time scale;
Large-scale spatial arrangement (tertiary structure).
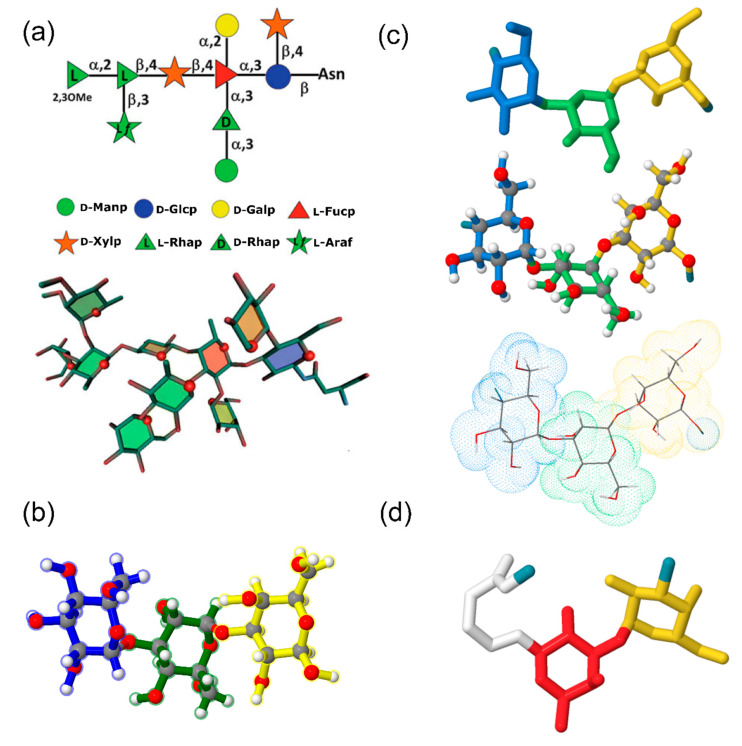
Figure 1.
Typical components of a carbohydrate 3D structure exemplified on sucrose: (a) primary structure (in Symbol Nomenclature for Glycans (SNFG)); (b) superimposed conformational states and Cremer–Pople diagram; (c) conformational space of a two-torsion glycosidic linkage (Ramachandran plot); (d) transitions of glycosidic dihedrals.
Herein we focus on the important aspects of carbohydrate 3D structure availability to researchers: structural repositories; glycoinformatics tools and workflows to assist structure building, modeling and erroneous molecular geometry data detection and remediation; carbohydrate 3D structure presentation and visualization methods.
2. Structural Databases
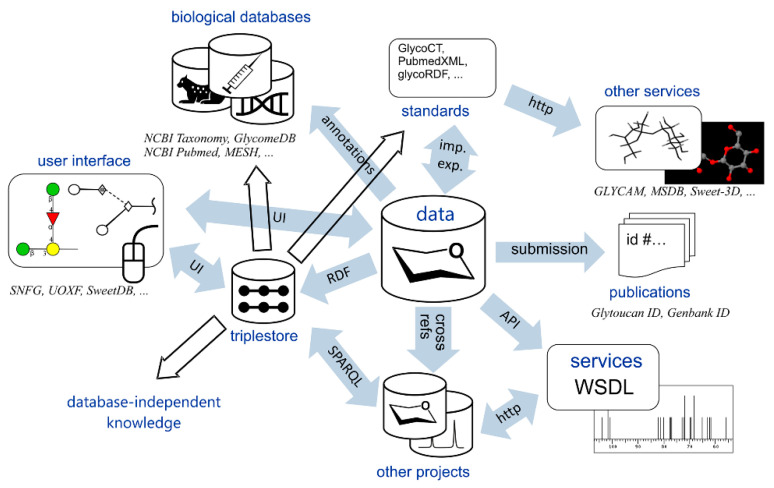
Structural databases make significant contribution to bringing information technologies to glycoscience [29]. With no focus on spatial structure, glycan databases and online tools have been recently reviewed [30,31,32]. Depositing huge number of carbohydrates with detailed data for each entry, databases are valuable sources of structural information, biological assignments, references and external links. Structural data are often accompanied by original and sometimes assigned experimental observables: NMR spectra, HPLC and MS profiles, etc. The services built on top of the databases can include 3D structure simulation, validation, and storage. A viewpoint of the authors at the ideal integration of data resources and services in glycoinformatics is summarized in Figure 2. A subject of this review is databases providing theoretical or empirical 3D structures of carbohydrates and related data-mining tools.
Figure 2.
Networking between glycoinformatics projects and related services that promotes achievement of data integration in glycomics. Reproduced with permission from [29], © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
The majority of existing repositories for carbohydrate 3D structures offer open-access data via web interface. Deposited datasets can be represented by glycoproteins, protein-carbohydrate complexes, poly- and oligosaccharides with 3D structure experimentally resolved or specified by means of NMR, X-ray crystallography, cryoEM, small angle X-ray scattering, etc. [27]. Several databases such as GLYCAM-Web, EK3D, 3DSDSCAR, GlycoMapsDB contain data from molecular dynamics simulations. We have also mentioned databases featuring information on protein structures involving carbohydrate moiety in terms of glycosylation (as post-translational modification, dbPTM), carbohydrate active enzymes (CAZy) and homology modeling (SWISS-MODEL). Table 1 displays currently active structural databases maintaining three-dimensional data on carbohydrates.
Table 1.
Carbohydrate databases with 3D structure support.
| Database | Years a | Description b | Data Coverage | Carbohydrate 3D Structures | References |
|---|---|---|---|---|---|
| Structure-centric | |||||
Carbohydrate Structure Database (CSDB)
|
2005– present |
|
|
|
[28,42,43,44] (http://csdb.glycoscience.ru/database) |
Glycosciences.DE
|
1997– present |
|
|
|
[45,46,47] (http://www.glycosciences.de/) |
Glyco3D
|
2015– present |
|
|
|
[48,49] (http://glyco3d.cermav.cnrs.fr/home.php) |
PolySac3DB
|
2012– present |
|
|
|
[50] (http://glyco3d.cermav.cnrs.fr/home.php) |
EK3D
|
2016– present |
|
|
|
[51] (www.iith.ac.in/EK3D/) |
3DSDSCAR
|
2010– present |
|
|
|
[52,53] (http://aliffishbay.com/Domains/3dsdscar.org/3dsdscar.html) |
MatrixDB
|
2011– present |
|
|
|
[54,55,56] (http://matrixdb.univ-lyon1.fr/) |
EPS-DB
|
2017– present |
|
|
|
[57] (http://www.epsdatabase.com) |
GlyMDB
|
2020– present |
|
|
|
[58] (http://www.glycanstructure.org/glymdb/) |
CFG Glycan Structures Database
|
2006– present |
|
|
|
[59,60] (http://www.functionalglycomics.org/glycomics/molecule/jsp/carbohydrate/carbMoleculeHome.jsp) (http://www.functionalglycomics.org/glycomics/publicdata/selectedScreens.jsp) |
| Glycoproteomic | |||||
GlycoNAVI Tcarp
|
2020– present |
|
|
|
[61] (https://glyconavi.org/TCarp/) |
GlyCosmos
|
2017– present |
|
109854 glycans glycolipids * 50113 glycoproteins 1238 lectins 20580 glycogenes |
|
[13,62,63] (https://glycosmos.org/) |
SugarBind
|
2010– present |
|
|
|
[64] (https://sugarbind.expasy.org/) |
GlyConnect
|
2019– present |
|
|
|
[65] (https://glyconnect.expasy.org/) |
ProGlycProt
|
2012– present |
|
|
|
[66,67] (http://www.proglycprot.org/) |
ProCarbDB
|
2020– present |
|
|
|
[68] (http://www.procarbdb.science/procarb/) |
Procaff
|
2019– present |
|
|
|
[69] (https://web.iitm.ac.in/bioinfo2/procaff/index.html) |
GBSDB
|
2020– present |
|
|
|
[70] (http://www.glycanstructure.org/gbs-db/pdb/) |
PROCARB
|
2010– present |
|
|
|
[71] (http://www.procarb.org/procarbdb/) |
| UniLectin3D | 2019– present |
|
|
|
[72,73] (https://www.unilectin.eu/unilectin3D/) |
Lectin Frontier
|
2015– present |
|
|
|
[74] (https://acgg.asia/lfdb2/) |
LectinDB
|
2006– present |
|
|
|
[75] (http://proline.physics.iisc.ernet.in/lectindb/) |
GlycoEpitope
|
2006– present |
|
|
|
[76,77,78] (https://www.glycoepitope.jp/epitopes) |
| GlycoCD | 2012– present |
|
|
|
[79] (http://www.glycosciences.de/glyco-cd/) |
| SACS | 2002– present |
|
|
|
[80] (http://www.bioinf.org.uk/abs/sacs/xslt.cgi?src=antibodies.xml&xsl=summary.xsl) |
SabDab
|
2014– present |
|
|
|
[81] (http://opig.stats.ox.ac.uk/webapps/newsabdab/sabdab/) |
CAZy
|
1998– present |
|
|
|
[82,83,84] (http://www.cazy.org/) |
dbPTM
|
2006– present |
|
|
|
[85,86,87] (http://dbptm.mbc.nctu.edu.tw/) |
SWISS-MODEL Repository
|
2004– present |
|
|
|
[88,89,90] (https://swissmodel.expasy.org/repository) |
| Specialized | |||||
GlycoMaps DB
|
2004– present |
|
|
|
[91] (http://www.glycosciences.de/modeling/glycomapsdb/) |
GFDB
|
2013– present |
|
|
|
[92] (http://www.glycanstructure.org/fragment-db) |
GLYCAM-Web
|
2013– present |
|
|
|
(http://glycam.org/Pre-builtLibraries.jsp) |
a Where unknown, the year of the first publication is given. b Database is marked as curated if manual verification of data was reported in the original publication or at the database web site. c Published coverage data can be outdated; database interface provides no statistics on current coverage. * Database provides no search facilities for indicated carbohydrate 3D structural data.
For Table 1, we have selected carbohydrate and related databases using the following criteria:
Database can be freely accessed through web user interface;
Database must contain experimentally confirmed and/or predicted 3D structures (preprocessed and/or generated on-the-fly from a primary structure input) of glycans, glycoproteins, or protein-carbohydrate complexes;
Stored 3D structures must be deposited as atomic coordinates in PDB, MOL, or other format, and the structures must contain a saccharide moiety;
Databases with records linked to other large 3D data collections (e.g., RCSB PDB, PDBe, PDBj, PDBsum, UniProtKB etc.) are included in Table 1 (as long as database entries contain carbohydrate moiety, e.g., as a part of a lectin or an antibody);
Databases with derived carbohydrate 3D structural data (conformational maps, conformer energy minima, etc.) are included in Table 1 even if they provide no atomic coordinates (e.g., GlycoMapsDB and GFDB).
Despite no fit to the criteria above, assistance of large structure repositories offering only glycan primary structures (e.g., GlyToucan [17] (https://glytoucan.org/), UniCarbKB [33] (http://www.unicarbkb.org/)) can be useful for cross-referencing of existing carbohydrate resources and serve as supplementation to 3D modeling pipelines.
Some out-of-date projects, such as Complex Carbohydrate Structural Database (CCSD) [34,35], EUROCarbDB [33,36], GlycomeDB [36,37,38], Glycoconjugate Data Bank [39], GlycoSuite [40,41] are noteworthy as they had shaped the modern vision of structural glycoinformatics.
3. Carbohydrate 3D Structure Modeling
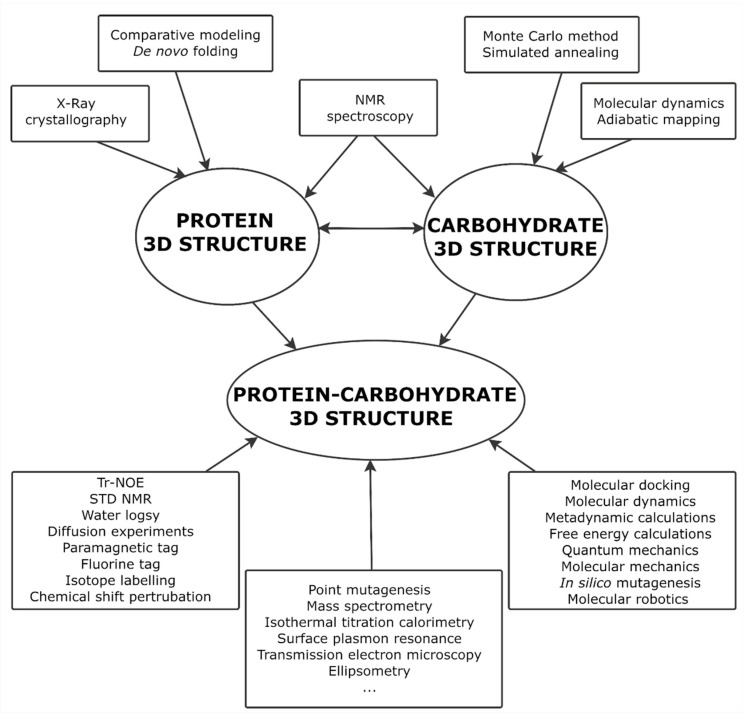
Methods to probe a 3D structure of carbohydrate-containing biomolecules has been developed for decades. NMR techniques (interatomic distances derived from NOE, and torsion angles derived from coupling constants), X-ray crystallography, and electron cryo-microscopy (the two latter being atomic models built on the basis of electron density map) are among most demanded methods for 3D strucural elucidation. These methods have been reviewed [93,94,95,96] and are beyond the scope of this review focused in information technologies. For use of instrumental methods for the validation of a simulated structure, please refer to Section 5 “Experimental data validation”.
Structural investigation of large biological systems involving protein-glycan interactions requires leveraging more resources and employing more complex experimental techniques compared to solely oligo- and polysaccharides studies. Advances in NMR methods hold great potential for direct spatial structure determination of carbohydrate-protein complexes in solution based on intermolecular NOEs which affords estimation of atomic contacts between a protein and a carbohydrate ligand [97,98]. Further extraction of NOE-derived distance restraints for a saccharide molecule results in generation of representative conformational ensembles [99,100,101].
Support of experimental data with computer simulations can significantly improve quality of 3D structures. Quantum mechanics [100,102,103,104,105,106] and molecular dynamics modeling [107,108,109,110,111] are commonly applied to conformation search and NMR signal prediction.
To date, the following theoretical models and methods are applied for in silico design of carbohydrate three-dimensional structure [112,113,114,115,116]:
Molecular mechanics (MM) and molecular dynamics (MD) calculations [117];
Ab initio simulations based on density functional theory (DFT) [124,125,126,127,128];
Hybrid QM/MM and QM/QM and ONIOM (“our own N-layered integrated molecular orbital and molecular mechanics”) approaches [129,130,131,132,133,134].
Due to computational limitations, most of publications of the recent decade have reported molecular dynamics approaches in general or dedicated force fields. With increasing computer power, other methods gain interest, however majority of applications of molecular modeling of complex carbohydrates, especially in solution, still use MM/MD methods.
Based on Scopus [135] article count we estimated the application rate for quantum mechanics (10759 publications) and molecular mechanics (14871 publications) methods applied for carbohydrate structure modeling for the recent five years (2015–2020). Search queries included abundant carbohydrate terms, typical glycan moieties, and common modeling approaches (query details are given in Supplementary Table S1). In spite of growing interest to QM approaches in carbohydrate structure simulation, the major contribution to the statistics for such resource-intensive calculations is application of QM to relatively simple model compounds. For complex bioglycans in solution predominance of MM methods is more pronounced [6,8].
Molecular Mechanics and Dynamics
Molecular dynamics methods have achieved broad scope of application in terms of reasonable computer resource consumption. They fulfill advantageous compromise between calculation accuracy and performance, when applied to glycan molecules and their structural complexity (variety of known monomeric elements, presence of ionogenic groups), high bridge flexibility and stereo-electronic effects [112,113,136,137].
In molecular mechanics simulations, Newtonian mechanics principles are applied to calculate potential energy of a system using parameter set specific for a class of compounds under study (force field). Particular features of carbohydrate moiety, e.g., ring puckering, rotational barriers, hydrogen bonds, must be taken into account to perform precise analysis of molecular behavior in vacuo or in solution [138].
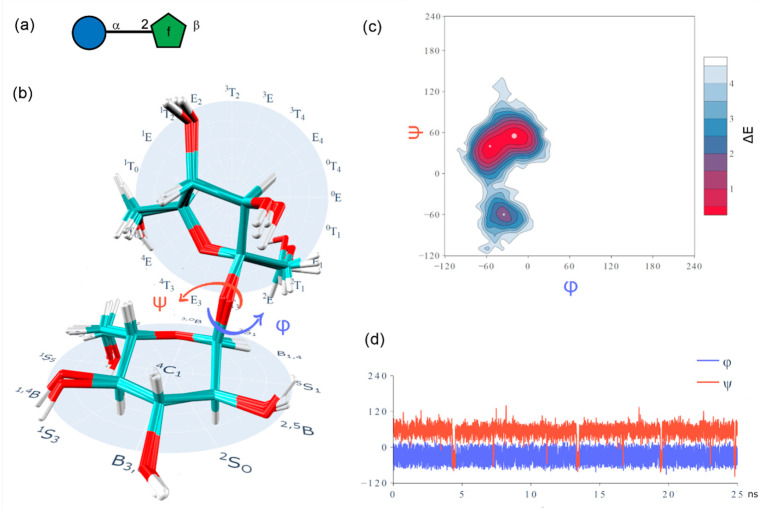
Molecular dynamics simulations consider Newtonian motion equations to observe evolution of a system during a certain timespan. Conformation ensemble generation occurs via calculation of molecular trajectory at given temperature. Accuracy of calculation depends on the employed force field and sufficient conformational sampling. MD simulations are commonly used for interpretation and analysis of the NMR and X-ray observables in the context of carbohydrate 3D structure [139]. Enhanced molecular dynamics sampling technologies, such as replica-exchange MD (REMD) [140,141], Hamiltonian replica-exchange MD (HREX) [142,143,144], multidimensional swarm-enhanced sampling MD (msesMD) [145,146], Gaussian accelerated MD (GAMD) [147,148] have been reported. Density maps or energy maps built for a set of the glycosidic torsion angles (φ, ψ, ω) are a typical way to report conformational preferences of a glycan provided by population analysis of its MD trajectory. As a representative example, conformational characteristics of highly flexible branched oligosaccharide Glc1Man9GlcNAc2 (GM9) were investigated by explicit-water REMD study and validated using paramagnetism-assisted NMR spectroscopy [149] (Figure 3a,b). Due to the structural complexity of GM9, adequate exploration of conformational space requires long-timescale simulations. Regular MD simulations of similar manno-oligosaccharides were reported to fail reproduction of experimental data [150]. Replica-exchange approach implies running periodically swapped parallel replicas of the system at different temperatures. Ensemble of GM9 conformers sampled by this method was consistent with the NMR observables. Populated areas of density maps built for glycosidic linkages of Glc1Man3 branch of GM9 (Figure 3c) were close to crystallographic conformations of a linear Glc1Man3 tetrasaccharide (a GM9 determinant recognized by lectins) from PDB.
Figure 3.
NMR-validated conformational analysis of high-mannose oligosaccharide GM9 based on replica-exchange molecular dynamics (REMD) simulation results. (a) Superimposition of 260 GM9 conformers extracted from REMD trajectory (black—GlcNAc, green—Man, blue—Glc). (b) primary structure of the GM9 oligosaccharide (SNFG representation). (c) REMD density maps for φ-ψ torsions of GM9 branch (Glc1Man3). Red dots locate glycosidic torsion angles derived from crystallographic data of Glc1Man3 tetrasaccharide ligand complexed with the lectin domain of calreticulin (PDB ID: 3O0W). Panels (a) and (c) were reproduced with permission from [149], © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Force field (or potential energy function) is represented by atomistic parameter set obtained for a considered compound class. Potential energy value can be calculated as a sum of interaction potentials for bonded (covalent bond stretching, angle bending, proper torsions) and non-bonded (electrostatic and van der Waals interactions) terms, and can include other terms (e.g., improper torsions, solvation, hydrogen bonds [151], nonconventional hydrogen bonds [101], for protein-carbohydrate complexes—CH-π stacking interactions [152,153,154,155], CHI Carbohydrate Intrinsic (CHI) energy contribution [156,157]).
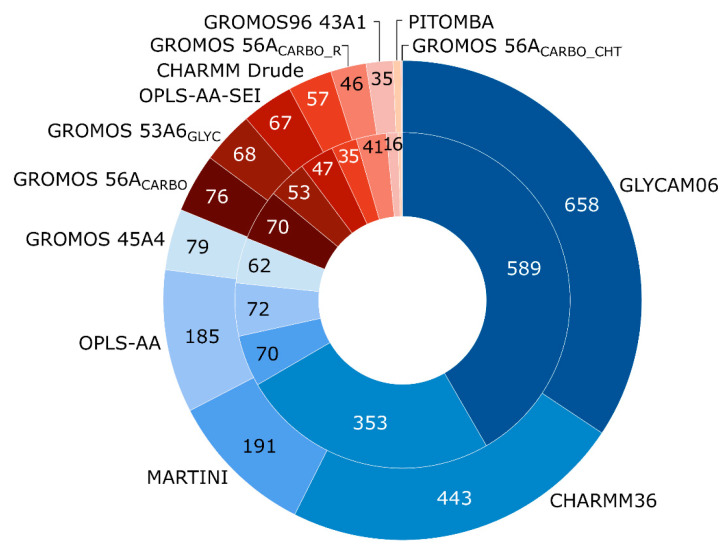
Several force fields developed for general representation of wide range of organic compounds (e.g., Allinger’s MM2, MM3, MM4) can be applied to carbohydrate 3D modeling [151,158,159]. Of them, despite being a universal force field, MM3 [160,161] still exhibits good performance on glycans [162,163,164] (Reviews), [165,166] (exemplary Articles). However, a number of force fields specially tuned for carbohydrates have been developed (Figure 4). In Supplementary Table S2, we provided citation metrics of articles reporting carbohydrate-dedicated and selected general force fields that could be applied to carbohydrate structure modeling. Unfortunately, usage of general force fields could not be adequately estimated via number of citations. Automated full-text analysis and retrieval of data, needed to confirm employment of force fields for carbohydrate molecules, is beyond the scope of this review. Nevertheless, statistical data obtained for general force fields supported in popular MD software packages (e.g., AMBER, CHARMM, GROMACS, Tinker) shows obsolescence of modern force fields above Allinger’s ones, and MM3 in particular (see more detailed data, references to original publications and absolute values in Supplementary Table S2).
Figure 4.
Citations of dedicated force fields in carbohydrate studies for the recent five years, according to Scopus. Outer circle shows total citations (number of citing publications) of force fields in 2015–2020. Inner circle shows citations in articles filtered by a carbohydrate topic. See detailed data, references to original publications, absolute values, and carbohydrate filer details in Supplementary Table S2.
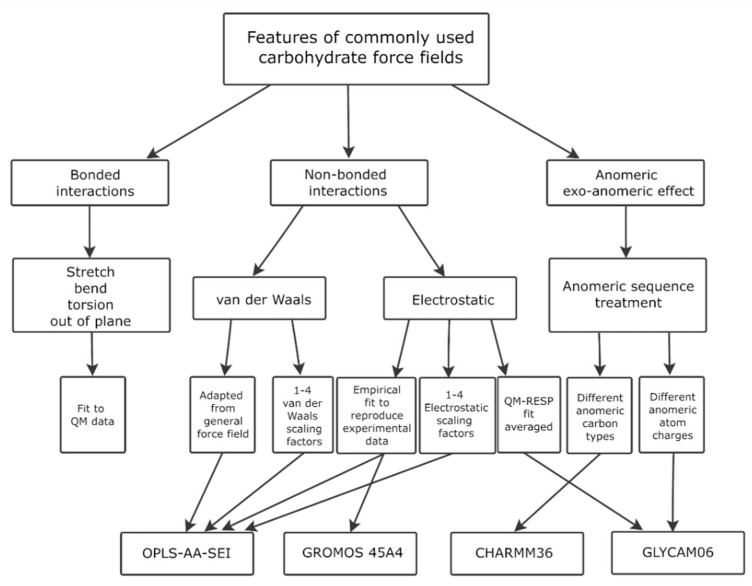
Detailed comparisons of all-chemical and dedicated force fields in a context of glycan modeling have been published [114,139,151,167]. CHARMM36, GLYCAM06, GROMOS and OPLS-AA-SEI were reported as commonly used force fields for handling carbohydrate or glycoconjugate molecules. More details are provided in Figure 5.
Figure 5.
Digest of the most commonly used carbohydrate force fields with parameterization protocol comparison. Reproduced with permission from [138], © 2020 Elsevier Inc.
CHARMM36 force field with modern carbohydrate parameter table (C36 [168]) was derived from CHARMM all-atom biomolecular force field [169,170]. Currently, CHARMM36 parameterization features include monosaccharides in furanose [171] and pyranose [172] forms, glycosidic linkages between monosaccharides [171,173], complex carbohydrates and glycoproteins [174], monosaccharide-linked sulfate and phosphate groups [175], acyclic carbohydrates and alditols [171], as well as carbohydrate simulations in aqueous solution [176].
GLYCAM06 force field is compatible with carbohydrates of all ring sizes and conformations for both mono- and oligosaccharides built of residues common for mammalian glycans, such as widespread aldoses, N-acetylated amino-sugars, sialic, glucuronic and galacturonic acids [177]. Parameter set was extended to non-carbohydrate moieties such as lipids [178], glycolipids [179,180], lipopolysaccharides [181], proteins and nucleic acids. Parameterization of GLYCAM06 for glycosaminoglycans was reported [182].
GROMOS represents a broad family of carbohydrate force fields. Having been a classic one since 2005, GROMOS 45A4 [183] parameter set is used for explicit-solvent simulation of hexopyranose-based saccharides. In the recent decade, several parameters of 45A4 were optimized in GROMOS 56ACARBO [184] including lipopolysaccharides [185]. GROMOS 53A6GLYC was improved for explicit-solvent simulations [186] and extended for glycoproteins [187]. GROMOS 56ACARBO_R [188] was designed to improve description of ring conformational equilibria in hexopyranose-based saccharide chains as compared to the previous 56ACARBO version. Another modification of 56ACARBO named 56ACARBO_CHT [189] was developed for chitosan and its derivatives. Recently, extensions of GROMOS 56ACARBO/CARBO_R parameter set were adapted towards charged, protonated and esterified urinates [190] and furanose-based carbohydrates [191]. GROMOS96 43A1 was reported to have good performance on glycan structure simulation in glycoproteins [192,193].
OPLS-AA scaling of electrostatic interactions (SEI) force field [194] consists of improved parameters for conformational changes associated with φ-ψ dihedrals combined with enhanced accuracy of QM relative energy calculation in carbohydrate molecules refined for OPLS-AA biomolecular force field [195,196]. Additionally OPLS force field was improved for explicit-water simulations [197].
Rapidly developing CHARMM Drude polarizable force field for carbohydrates based on classical Drude oscillator has to be mentioned. Parameter sets obtained for hexapyranoses [198] and their aqueous solutions [199], aldopentafuranoses and methyl-aldopentafuranosides [200], carboxylate and N-acetylamine saccharide derivatives [201], alditols [202] and glycosidic linkages [203] demonstrated significant improvement of QM data reproduction compared to CHARMM additive force field.
MARTINI coarse-grained (CG) force field [204] can be used alternatively to all-atom (AA) level simulations with advantage of modeling large carbohydrate systems (solutions of oligo-, polysaccharides, glycolipids [205,206,207]) on a long time scale at reasonable computational cost. Blocked ring puckering (only 4C1 conformation is allowed) and restrictions on the anomeric effect and glycosidic bond flexibility cumulatively provide reduction of available degrees of freedom. Another CG model PITOMBA [208] for carbohydrate simulations was developed based on GROMOS 53A6GLYC force field.
Docking methods for carbohydrate ligands utilize molecular modeling approaches for protein-carbohydrate complexes for initial geometry generation, conformational sampling, grafting, active site mapping and binding affinity estimation [129,137,209,210,211]. Accurate reproduction of experimental data requires application of particular scoring function parameterization (empirical, force fields or knowledge-based [212]) and docking protocols, which depend on the interaction types present in a system (CH-π interactions, CHI-energy, hydrogen bonding, solvent model, influence of solvent molecules inclusion effects, charged moiety etc.) [8,213,214,215,216,217,218,219]. Extension of several docking software packages to handle carbohydrate molecules was reported to improve modeling of biologically relevant systems such as lectin-glycan [220,221], GAG-protein [222,223,224], or antibody-carbohydrate [225].
4. Model Building and Analysis Tools
Currently available web-based tools along with standalone software packages were developed to facilitate work with carbohydrate 3D structure. Versatile online services for in silico molecular modeling allow users to start from a user-friendly structure input, and to automatize further procedures (see Table 2 for references). GLYCAM-Web provides tools for glycan structure prediction, glycosylated protein 3D model generation, grafting and docking. CHARMM-GUI modeler offers options for 3D structure generation and modeling of glycans including N-/O-glycoproteins and glycolipids [226,227]. Biological membranes can be simulated with the assistance of CHARMM-GUI Membrane Builder (by combining features of LPS and glycolipid CHARMM-GUI Modelers) and GNOMM (a tool for building lipopolysaccharide-rich membranes). Noteworthy standalone programming frameworks for structure modeling are Glycosylated (modeling of glycans, glycoproteins and glycosylation) and Rosetta Carbohydrate (loop modeling [228], glycan-to-protein docking, and glycosylation modeling).
Table 2.
Informatics tools for carbohydrate and glycoprotein modeling, 3D structure prediction and analysis.
| Tool | Description | Type a | Reference |
|---|---|---|---|
| Structure modeling | |||
| CHARMM-GUI Glycan Modeler | In silico N-/O-glycosylation of proteins;modeling of carbohydrate-only systems | Web-service | [230] (http://www.charmm-gui.org/?doc=input/glycan) |
| CHARMM-GUI Glycolipid/LPS Modeler | Glycolipid and lipoglycan structure modeling | Web-service | [230] (http://charmm-gui.org/?doc=input/glycolipid) (http://charmm-gui.org/?doc=input/lps) |
| Glycosylator | Rapid modeling of glycans and glycoproteins (including glycosylation) based on CHARMM force field | Python framework | [231] (https://github.com/tlemmin/glycosylator) |
| RosettaCarbohydrate | Modeling a wide variety of saccharide and glycoconjugate structures (including loop modeling, glyco-ligand docking and glycosylation) | Python framework | [228,232,233,234] (https://www.rosettacommons.org/docs/latest/application_documentation/carbohydrates/WorkingWithGlycans) |
| Azahar | Monte Carlo conformational search and trajectory analysis of glycans | Python framework; PyMol plugin | [235] (https://github.com/BIOS-IMASL/Azahar) |
| Shape | Carbohydrate-dedicated fully automated MM3-based conformation simulation | Standalone software | [236] (https://sourceforge.net/projects/shapega/) |
| Glydict | MM3-based N-glycan structure prediction based on MD simulations | Web-service | [237] (http://www.glycosciences.de/modeling/glydict/) |
| GLYGAL | MM3-based conformational analysis of oligosaccharides | Standalone software | [238] |
| Fast Sugar Structure Prediction Software (FSPS) | Automatic structure prediction tool for oligo- and polysaccharides in solution | Standalone software | [239,240,241,242] |
| Glycosylation modeling and grafting | |||
| GLYCAM-Web Glycoprotein Builder | Attaching a glycan (user input) to a protein (PDB file) | Web-service | (http://glycam.org/gp) |
| GlyProt | In silico generation of N-glycosylated 3D models of proteins | Web-service | [243] (http://www.glycosciences.de/modeling/glyprot/php/main.php) |
| Phenix CarboLoad | Loading a carbohydrate structure into protein model and PDB file generation | Python framework | [244] (https://www.phenix-online.org/documentation/reference/carbo_load.html) |
| GLYCAM-Web GlySpec (Grafting) | Prediction of glycan specificity by integrating glycan array screening data and 3D structure | Web-service | [245,246,247,248,249] (http://glycam.org/djdev/grafting/) |
| Biological membranes and micelles | |||
| CHARMM-GUI Membrane Builder | Building complex glycolipid-/LPS-/LOS-containing biological membrane systems | Web-service | [230,250,251,252,253] (http://www.charmm-gui.org/?doc=input/membrane.bilayer) |
| GNOMM (gram-negative outer membrane modeler) | Automated building of lipopolysaccharide-rich bacterial outer membranes (3D model preparation for MD simulations in GROMACS) | Standalone software | [254] (http://thalis.biol.uoa.gr/GNOMM/) |
| Micelle Maker | Micelle building based on broad range of starting lipids and glycolipids (3D model preparation using AMBER software package and GLYCAM library) | Web-service | [255] (http://micelle.icm.uu.se/) |
| Carbohydrate moiety identification | |||
| Cheminformatics Tool for Probabilistic Identification of Carbohydrates (CTPIC) | Identification of small saccharides and their derivatives (input in SDF or MOL format) | Web-service | [256] (http://ctpic.nmrfam.wisc.edu/) (https://github.com/htdashti/ctpic) |
| Sails | Automated identification of linked sugars | Python framework | (https://github.com/glycojones/sails) |
| GlyFinder | Locating relevant carbohydrate-containing structures in Protein Data Bank | Part of web-service pipeline | [257,258] (https://dev.glycam.org/portal/gf_home/) |
| pdb2linucs | Extraction of carbohydrate data from a PDB file | Web-tool | [259] (http://www.glycosciences.de/tools/pdb2linucs/) |
| GLYCAM-Web PDB-preprocessor | Processing of PDB files with (glyco-)proteins for AMBER-style output | Web-service | (http://glycam.org/pdb) |
| Sugar identification program | Identifying the residue names of carbohydrates in a PDB file | Standalone software | (http://glycam.org/docs/othertoolsservice/downloads/downloads-software/) |
| Glycan Reader | Automated sugar identification and simulation preparation for carbohydrates and glycoproteins in PDB files | Web-service | [260,261] (http://glycanstructure.org/glycanreader/) (http://www.charmm-gui.org/?doc=input/glycan) |
| Structure building and model preparation | |||
| doGlycans | Preparing carbohydrate structures (including polysaccharides, glycolipids and glycoproteins) for GROMACS atomistic simulations | Python framework | [262] (https://bitbucket.org/biophys-uh/doglycans/src/master/) |
| GLYCAM-Web Carbohydrate builder | 3D structure prediction of carbohydrates and related macromolecules using GLYCAM06 force field and MD in AMBER (successor of GLYCAM Biomolecule Builder (http://glycam.org/old/biombuilder/biomb_index.jsp)) | Web-service | [177] (http://glycam.org/) |
| SWEET-II | Rapid 3D model construction of oligo- and polysaccharides with MM3 optimization | Web-service | [263,264] (http://www.glycosciences.de/modeling/sweet2/) |
| REStLESS API | 3D structure generation of carbohydrates and derivatives from CSDB Linear notation with MMFF94 optimization (including aglycone moiety) | Web-service | [265] (http://csdb.glycoscience.ru/database/core/translate.html#from) |
| Polysaccharide builders | |||
| POLYS | 3D structure generation of poly- and complex oligosaccharides from MM2-precalculated glycosidic linkage torsions and energy minimization | Web-service | [266,267] (https://bitbucket.org/polys/polys/src/default/) (http://glycan-builder.cermav.cnrs.fr/) |
| CarbBuilder | Building of 3D structures of polysaccharides in CHARMM force field from pre-calculated glycosidic linkage torsions | Standalone software | [268,269] (https://people.cs.uct.ac.za/~mkuttel/Downloads.html) |
| GAG-builder | Translating of GAG sequences into 3D models based on POLYS glycan builder | Web-service | [270] (http://glycan-builder.cermav.cnrs.fr/gag/) (http://matrixdb.univ-lyon1.fr/) |
| GLYCAM-Web GAG Builder | Modeling of GAG 3D structure in GLYCAM06 force field using AMBER MD package | Web-service | [271] (http://glycam.org/gag) |
| Docking | |||
| BALLDock/SLICK | Protein-carbohydrate complex docking software | Standalone software, a module in docking software | [272,273] (https://ball-project.org/download/) |
| HADDOCK | Modeling of biomolecular complexes with support of glycosylated proteins | Web-service | [274] (https://wenmr.science.uu.nl/haddock2.4/library) |
| Vina-Carb | CHI-energy functions implemented in AutoDock Vina software | Standalone software | [156,157] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/vina-carb/) |
| GLYCAM-Web Antibody docking | Docking of an antibody (from a PDB file) to a glycan antigen (from a library or user input) | Web- service | (http://glycam.org/ad) |
| Cluspro | Sulfated GAG docking (as one of options) | Web-service | [275,276] (https://cluspro.bu.edu/login.php) |
| GAGDock (DarwinDock) | Modification of DarwinDock method for sulfated glycosaminoglycans | Algorithm | [277] |
| GlycoTorch Vina | Docking of sulfated glycosaminoglycans based on Vina-Carb | Standalone software | [278] (http://ericboittier.pythonanywhere.com/) |
| Structural data analysis | |||
| Conformational Analysis Tool (CAT) | Analysis of carbohydrate molecular trajectory data derived from MD simulations | Standalone software | [279] (http://www.md-simulations.de/CAT/) |
| Best-fit, Four-Membered Plane (BFMP) | Analysis of conformational data from crystal structures and MD simulations of carbohydrates | Standalone software | [280] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/bfmp/) |
| Distance Mapping | Estimation of nuclear Overhauser effects in disaccharides | Web-tool | (http://www.glycosciences.de/modeling/distmap/) |
| MD2NOE | Calculation of Nuclear Overhauser effect build-up curves from long MD trajectories | Standalone software | [281] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/md2noe/) |
| GS-align | Glycan structure alignment and similarity calculation | Standalone software | [282] (http://www.glycanstructure.org/gsalign) |
| GlyTorsion | Analysis of torsion angles in carbohydrates from Protein Data Bank | Web-tool | [283] (http://www.glycosciences.de/tools/glytorsion/) |
| GlyVicinity | Analysis of amino acids in the vicinity of carbohydrate residues derived from Protein Data Bank | Web-tool | [284] (http://www.glycosciences.de/tools/glyvicinity/) |
a Web-service implies an automated pipeline for running a specific software (e.g., molecular modeling, structure building, carbohydrate coordinate extraction, format conversion). It results in 3D structural data output starting from primary structure input or atomic coordinate file upload. Web-tool is employed for 3D structural data processing and analysis without 3D structural data output; it is a simpler application designed primarily for statistics and visualization. Other types are self-explanatory.
To build diverse saccharide 3D models online, one can use such tools as REStLESS and SWEET-II. doGlycans standalone framework can be used for preparation of the atomistic models of glycopolymers, glycolipids and glycoproteins. Complex polysaccharide 3D models can be generated via POLYS and CarbBuilder. Another special class of polysaccharide builders is dedicated to glycosaminoglycans (GAGs) which can be accessed using POLYS GAG-builder and GLYCAM-Web GAG-builder. Recently, another approach for building GAG molecules was reported [229] (exemplary data pipeline only). Unfortunately, application scope of the majority of the existing structure building and modeling services is limited to rigidly defined set of supported sugar residues, and lacks non-carbohydrate moiety support.
Tools for locating and identification of a carbohydrate moiety (e.g., pdb2linucs, GlyFinder, Glycan Reader) are useful for the atomic coordinate analysis and extraction of glycoproteins and protein-carbohydrate complexes deposited in Protein Data Bank (PDB). Automated molecular geometry processing facilities can be accessed via glycoinformatics tools designed for conformational data analysis (CAT, BFMP), nuclear Overhauser effect (NOE) calculation (MD2NOE, Distance Mapping) and 3D structural data analysis related to glycan moieties from PDB (GlyTorsion, GlyVicinity, GS-align).
In Table 2, we summarized freely available tools for generation and processing carbohydrate 3D structural data and divided them into eight categories of application.
5. Experimental Data Validation
Vast variety of methods provide information about 3D structure of individual glycans and glycan moieties of glycoproteins and protein-carbohydrate complexes (Figure 6) [285,286]. The following approaches are most utilized for 3D structural data validation [287,288,289]:
Ccombination of carbohydrate simulated geometry data with X-ray crystallographic data analysis [225,290];
Analysis of inter-glycosidic NMR spin couplings, which depend on glycosidic bond torsions [114,291,292];
Deriving nuclear Overhauser effects (NOEs) from relative populations of the interatomic distances, with subsequent comparison to the experimental NOEs in solution [99,293,294];
Purely informatic detection of errors, such as incompatible atomic coordinates originating from incorrect processing or simulation [295,296,297,298];
Simulation by other computational methods at higher levels of theory [102,103,105,108].
Figure 6.
Interplay of the instrumental and computational methods in the 3D structure determination of carbohydrates, proteins, and protein–glycoconjugate complexes. Reproduced from [285] © 2020 The authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Unfortunately, most of the data obtained on the basis of crystallographic experiments can dramatically differ from glycan conformations in solution or have poor resolution which needs further adjustment [299,300]. Moreover, not all of the objects of interest can be obtained as a single crystal. Electron cryo-microscopy gains popularity for carbohydrate 3D structural research [301], however, this method requires additional refinement procedures due to resolution restrictions of the obtained density maps [302,303,304]. Recently, cryo-EM data were used for the refinement of SARS-CoV-2 spike glycoprotein stucture using Privateer (see Table 3 for references) software [305,306].
Table 3.
Tools for structural validation of carbohydrates.
| Tool | Description | Type a | Reference |
|---|---|---|---|
| CNS | Macromolecular structure determination and refinement (including carbohydrates and glycoproteins) based on X-ray and NMR data | Standalone software | [327,328,329,330] (http://cns-online.org/v1.3/) |
| pdb-care | Identification and assigning carbohydrate structures using atom types and coordinates from PDB files | Web-tool | [326] (http://www.glycosciences.de/tools/pdb-care/) |
| CARP | Glycoprotein 3D quality evaluation based on the analysis of glycosidic torsion angles from PDB | Web-tool | [283] (http://www.glycosciences.de/tools/carp/) |
| GlyProbity | Accuracy and internal consistency check of carbohydrate 3D structures | Part of web-service pipeline | [257] (https://dev.glycam.org/portal/gf_home/) |
| PDB2Glycan | 3D structure analysis and validation of glycoprotein PDB entries | Part of web-service pipeline | [61] (https://glyconavi.org/TCarp/) (https://gitlab.com/glyconavi/pdb2glycan) |
| PDB-REDO | Glycoprotein structure model improvement and validation | Web-service; standalone software | [295,325] (https://pdb-redo.eu/) |
| Coot | Refinement and validation of glycoprotein 3D structure from cryoEM and X-ray crystallography data | Standalone software | [298,331] (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/) |
| Rosetta Carbohydrate | Refinement of glycoprotein 3D structure from cryoEM and X-ray crystallography data, based on correction of conformational and configurational errors in carbohydrates | Python framework | [296] (https://www.rosettacommons.org/docs/latest/application_documentation/carbohydrates/WorkingWithGlycans) |
| Privateer | Automated validation of carbohydrate conformation data based on 3D structure analysis | Standalone software | [297,332] (https://smb.slac.stanford.edu/facilities/software/ccp4/html/privateer.html) |
| Phenix | Determination, refinement and validation of macromolecular structure (including carbohydrates and glycoproteins) from cryoEM, X-ray diffraction and neutron diffraction crystallography data | Standalone software | [244] (http://phenix-online.org/) |
| Motive Validator | Automatic custom residue validation in biomolecules, including carbohydrates | Web-service | [333] (https://webchem.ncbr.muni.cz/Platform/MotiveValidator) |
| ValidatorDB | Pre-computed validation results of ligands and non-standard residues in PDB (including carbohydrates) | Web-service | [334] (http://webchem.ncbr.muni.cz/Platform/ValidatorDb) |
a See footnote a to Table 2.
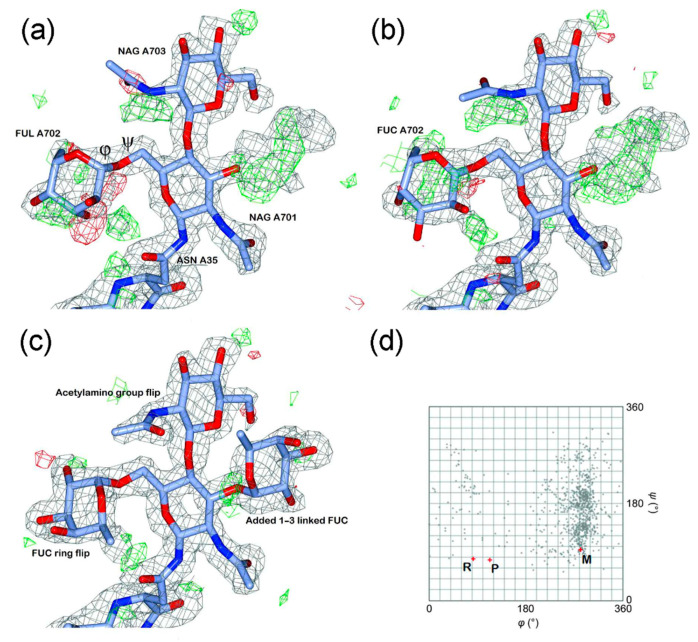
Van Beusekom et al., illustrated [295] quality improvement of the PDB glycan structure model with incorrect (1–6)-linked fucose annotation, poor fit to the electron density, and missing (1–3)-linked fucose (Figure 7a) with the help of PDB-REDO (Figure 7b) and CARP (Figure 7d) tools (see Table 3 for references). Structure model obtained by PDB-REDO treatment was further manually inspected (Figure 7c): corrections were made for acetylamino group geometry, distorted (1–6)-linked fucose ring conformation, and (1–3)-linked fucose residue was added. Despite successful automated resolution of residue annotation problem and poor electron density refinement, complete revision could not be achieved without manual intervention.
Figure 7.
X-ray diffraction data refinement of N-glycan moiety from PDB ID 2Z62. 2mFo–DFc electron density map contoured at 1σ is displayed in grey; positive and negative mFo–DFc difference electron density maps contoured at 3σ are displayed in green and red, respectively. (a) Original glycan structure model from the PDB entry. (b) PDB-REDO model with properly renamed fucose residue and improved fit to the electron density. (c) Manually rebuilt model based on PDB-REDO results. (d) CARP distribution plot for glycosidic φ-ψ torsions of FUC(1-6)NAG (from panel (a)) in PDB. Characteristic points: R, model refined with PDB-REDO; P, original PDB model; M, manually rebuilt model. Reproduced from [295], © 2020 The authors. Published by John Wiley & Sons, Inc.
NMR techniques are a powerful approach to investigate conformational and dynamic behavior of carbohydrate moieties in biomolecules [307,308,309,310]. However, the nature of NOE enhancement factor has been hampering obtaining the sufficient number of distance restrains [99]. In the case of saccharides with their multiple rotatable bonds, the stable 3D structure was difficult to define, making molecular modeling essential for this class of compounds. Adjustment of experimental conditions helped to overcome the mentioned limitation and to reproduce crystal structures of oligosaccharides by modeling with NOE-derived distance restraints [100,101].
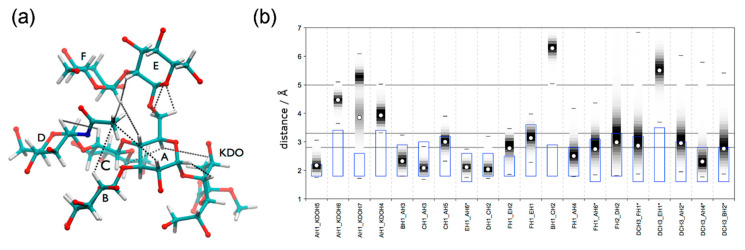
Since there is no direct way to derive detailed three-dimensional representation from the observed NOE intensities, additional molecular modeling protocols are required to establish comprehensive view of conformational space at the atomic level [311,312,313]. Frank et al., demonstrated conformation filtering based on the observed NOE obtained by molecular dynamics in explicit solvent [314]. As a representative example, Figure 8 depicts 1H-1H spatial contacts and conformation selection criteria illustrated by Moraxella catarrhalis lgt2Δ bacterium heptasaccharide, which adopts an unusual conformation.
Figure 8.
M. catarrhalis lgt2Δ structure validation based on NOE data analysis. (a) Characteristic proton-proton contacts; (b) NOE-filtered (blue boxes) sampling of proton-proton distances from MD simulation (grey shades). Reproduced from [314], © 2020 The authors. Licensee MDPI, Basel, Switzerland.
6. Protein Data Bank and Its Validation
Protein Data Bank (PDB) [315] and Cambridge Structural Database (CSD) [316] are historically considered the main repositories of experimentally determined carbohydrate three-dimensional structures. CSD is reported to deposit over 4000 crystal structures of oligosaccharides [93]. Unlike Cambridge Structural Database, Protein Data Bank provides open access to the entire structural archive. Carbohydrate moieties deposited in PDB are usually represented as covalently bound to protein or imply non-covalently bound protein-carbohydrate complex formation [302]. According to recent reports, as at November 18, 2019 Protein Data Bank contained ~13500 carbohydrate structures representing ~9.4% of total database records [317].
Despite being a valuable source of 3D structural data for glycoscientists, PDB lacks convenient search facilities for glycan structures. Some projects have developed data-mining tools capable of retrieving bioglycan molecular geometry data from PDB: Glycan Reader (GlycanStructure.org) [260,261] (http://www.glycanstructure.org/), pdb2linucs (GLYCOSCIENCES.de) [47,259,318] (http://www.glycosciences.de/database/start.php?action=form_pdb_data), GlycoNAVI TCarp [61] (https://glyconavi.org/TCarp/) (https://gitlab.com/glyconavi/pdb2glycan) and GlyFinder (GLYCAM-Web) [257,258] (https://dev.glycam.org/portal/gf_home/).
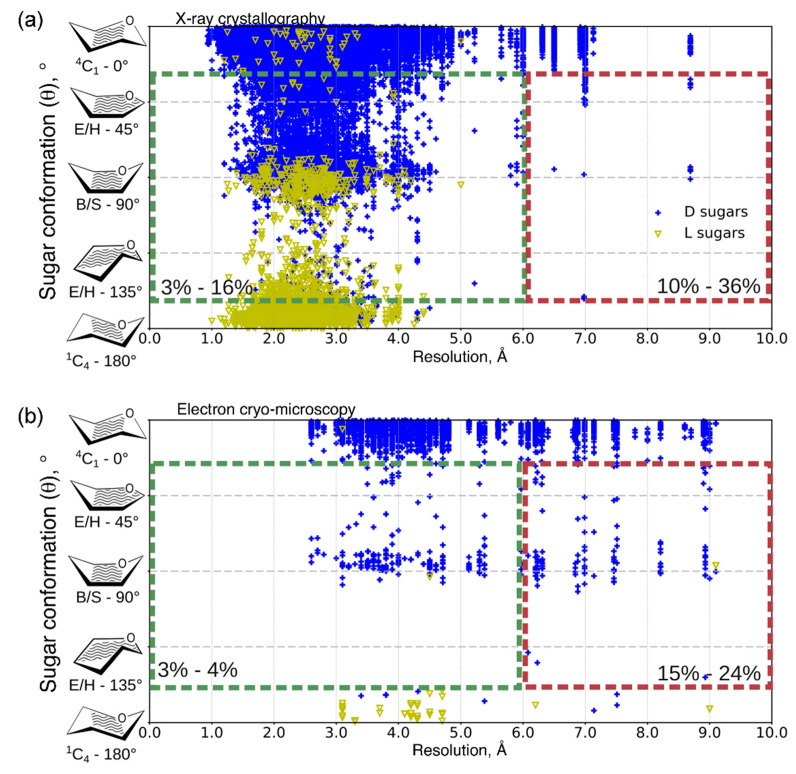
Another issue of concern related to Protein Data Bank is large proportion of errors in deposited coordinates, leading to requirement for a thorough checkup and development of data remediation services [319]. Commonly occurring problems associated with nomenclature, poor glycan geometry, linkage errors, missing or surplus atoms can seriously decline the quality of the obtained 3D structures [300,320,321]. Using Privateer software, it was discovered [299],[301] that PDB deposits significant number of erroneous N-glycosylated structures with pyranose ring distortions, considering preferred adoption of 4C1 conformation for D-sugars and 1C4 conformation for L-sugars (Figure 9). In most cases, poor electron density of carbohydrate moiety results in anomalous high-energy pyranose ring conformations (envelopes, half-chairs, boats, skew boats, etc.). To obtain a reasonable structure model, experimental data refinement programs should be applied to derive geometric restraints for sugar monomers. Notably, despite a cryo-EM method has a resolution limit disadvantage, observed results indicate larger content of atypical conformations solved by X-ray crystallography, as compared to cryo-EM data.
Figure 9.
Distribution of D- (shown in blue) and L-pyranoside (shown in yellow) ring conformations as function of resolution for all sugar moieties in N-glycosylated proteins in PDB (on April 2019) solved with (a) X-ray crystallography and (b) electron cryo-microscopy. Non-chair conformations are bordered by dotted line boxes for 0.0-6.0 Å (green) and 6.0-10.0 Å (red) resolution ranges; the percentage of structures is given in the boxes. Reproduced with permission from [301], © 2020 Elsevier Ltd.
Exceptions for the relevancy of high-energy conformations were found in complexes involving carbohydrate-active enzymes, which force pyranose ring distortion enabling catalytic transformation of a carbohydrate substrate via transition states (e.g., glycosydic bond hydrolysis) [322]. Fushinobu has performed glycosidic torsion analysis for a set of PDB entries of crystal structure complexes bound to ligands bearing lacto-N-biose I (LNB, both α- and β-anomers) disaccharide unit presented in type-1 antigens. The study was supported by GlycoMaps DB (see Table 1 for references) [323]. Obtained φ-ψ data for LNBs bound to various proteins was plotted against corresponding free energy maps. Distortion of the energetically favored ring conformation strongly depended on substrate catalytic and recognition mechanisms.
To date, existing tools for carbohydrate structural error detection and correction in PDB files (Table 3) cannot be used directly as an integral part of Protein Data Bank. Nevertheless, initiative aimed at improvement of quality at wwPDB was carried out via collaboration with glycoscience community in July 2020 [324] (https://www.wwpdb.org/documentation/carbohydrate-remediation). It includes data annotation and validation of carbohydrate-containing records.
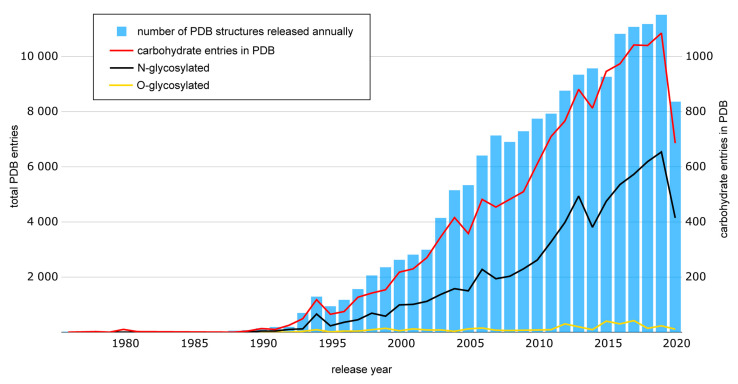
Proportion of carbohydrate-containing structures in PDB has been recently reported in [302]. Figure 10 presents our analysis of data published in the framework of Protein Data Bank carbohydrate remediation project. 14117 PDB entries from carbohydrate remediation list (https://cdn.rcsb.org/wwpdb/docs/documentation/carbohydrateRemediation/PDB_carbohydrate_list.list) were sorted by release year and plotted against the growth of PDB structures released annually (https://www.rcsb.org/stats/growth/growth-released-structures) (as on August 10, 2020; 167,327 PDB entries were available). Obtained results indicated that ~8.4% of PDB records contained a carbohydrate moiety. Additionally, each PDBx/mmCIF file corresponding to PDB ID from carbohydrate remediation list was parsed to reveal the presence of N- or O-glycosylation site annotations, which resulted in ~4.2% (7076 N-glycosylated entries) and 0.2% (362 O-glycosylated entries) of total database records. A few S- and C-glycans (24 entries in total) were neglected.
Figure 10.
Deposition statistics of carbohydrate-containing structures in Protein Data Bank based on carbohydrate remediated list data. Data for 2020 cover seven of twelve months. See detailed data in Supplementary Tables S3–S4.
Statistics on glycans in Protein Data Bank was reported [259,302,317,325], as well as tools that could facilitate collection of statistical data (Glycan Reader [70,260,261], GlyFinder [258], pdb2linucs and pdb-care [326]).
7. 3D Structure Input and Visualization
Carbohydrate structure visualization in publications and computer interfaces is extremely important in terms of perception universality, unambiguity, and machine-readability. Hence, carbohydrate input [335,336,337] and visualization [338,339] tools are actively developing. Feature comparison of glycan sketchers, builders and viewers (occasionally including 3D ones) was reported in a recently published review [340]. In our review, we gave more emphasis to 3D visualization approaches.
Being informative to represent glycan primary structure, most of graphical input tools such as GlycanBuilder [341], DrawRINGS [342], SugarSketcher [343], DrawGlycan-SNFG [344,345] and GlycoGlyph [337] are inappropriate for obtaining 3D structural models and their visualization due to lack of underlying modeling and insufficient data conversion functionality.
At present, glycan 3D molecular models can be built in user-friendly software allowing constructing glycans from individual saccharide components. Free web-tools, such as GLYCAM-Web, CHARMM-GUI, POLYS glycan builder, GAG-builder, SWEET-II should be noted (more references are listed in Table 2). A few commercial molecular modeling software is equipped with special plugins for glycan 3D structure building based on a list of predefined monosaccharide templates, e.g., Sugar Builder tool in HyperChem (http://www.hyper.com/?tabid=360) software [346] or Azahar [235] plugin in PyMol package (Schrödinger software) (https://pymol.org/2/)[347].
To render 3D glycan structure and its conformational features, it should be recorded using a notation which includes atomic coordinates, such as MOL [348] or PDB [349]. All-atom visualization based on atomic coordinates is supported by the majority of existing molecular modeling software. Several carbohydrate structure databases utilize interactive 3D visualization using open-source software engines. As one of the pioneers, GLYCOSCIENCES.de portal developed PDB2MultiGIF [350] (http://www.glycosciences.de/modeling/pdb2mgif/) visualization pipeline which generates an animated image of 3D model from a PDB file using RasMol [351] (http://www.openrasmol.org/). RasMol visualization was included in W3-SWEET [263] (ancestor of SWEET-II) pipeline developed by same project. Nowadays, more advanced interactive visualization applications have been developed for carbohydrate 3D molecule presentation. Jmol/JSmol [352] (http://www.openrasmol.org/) visualization applet is useful to display 3D models of carbohydrate molecules applied in numerous projects, such as CSDB, GLYCOSCIENCES.de, GLYCAM-Web and EK3D (see references in Table 1). NGL [353,354] (http://nglviewer.org/), LiteMol [355] (https://www.litemol.org/) and Mol* [356] (https://www.rcsb.org/news?year=2020&article=5efe0f606378d876901146f8) (https://molstar.org/) 3D viewers are handy for processing macromolecular PDB data stored in glycoproteomics databases (UniLectin3D, Glycan Binding Site DB, ProCarbDB, GlycoNAVI, ProCaff, etc.; see references in Table 1) and general proteomics repositories such as PDB [315] (http://www.wwpdb.org/), UniProtKB [357] (https://www.uniprot.org/) or SWISS-MODEL [90] (https://swissmodel.expasy.org/repository).
NGL viewer was developed mainly for convenient protein macromolecule structure processing. It allows only ball-stick representation for small molecules or non-peptide fragments, such as saccharide residues. LiteMol (and its successor, Mol*) viewer could be applied for the visualization of an arbitrary glycan with facility of highlighting carbohydrate fragments or displaying specific interactions in protein-carbohydrate complex structure. Due to these features, it was implemented in multiple carbohydrate structure databases (e.g., CSDB, Glyco3D, MatrixDB, and EPS-DB).
Despite the absence of the experimental 3D structural data, a number of carbohydrate databases have opportunity to simulate 3D atomic coordinates for deposited or inputted compounds from primary structure owing to tools developed by glycoinformatics community. CSDB (REStLESS API [265]), GLYCOSCIENCES.de (SWEET-II [264,350]) and GLYCAM-Web (http://glycam.org/) portals make it possible to generate 3D atomic coordinates recorded in PDB (all) and MOL (CSDB) file formats. POLYS developed by Glyco3D project enables the construction of polysaccharides in PDB format; it was introduced in MatrixDB and EPS-DB databases. More details are provided in Table 2.
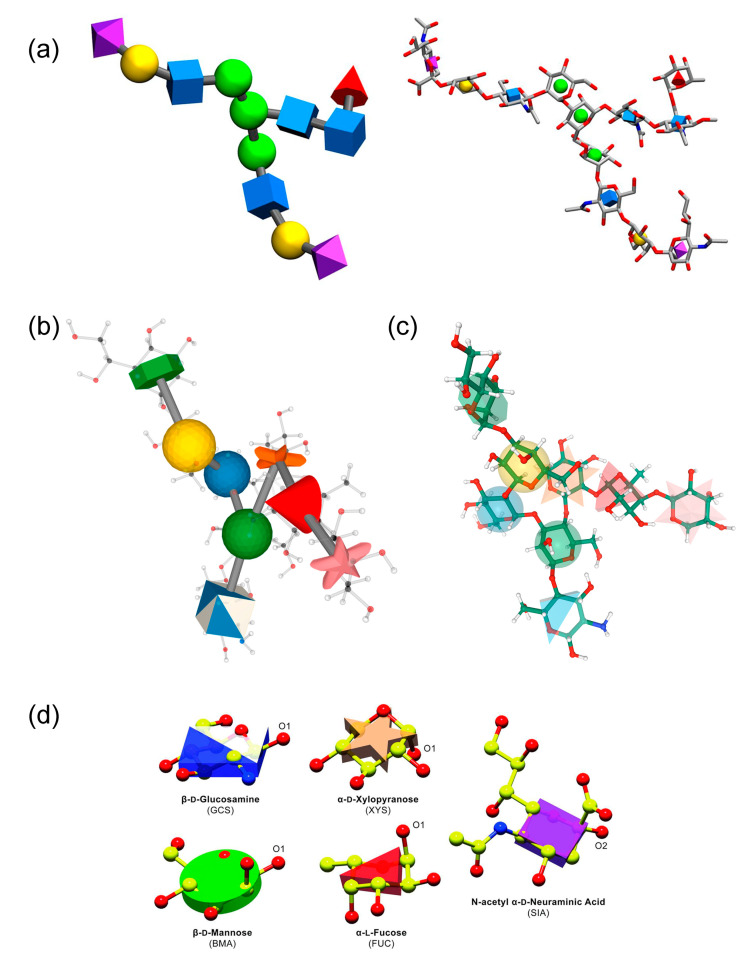
Atomic coordinates and all-atom molecular models have not been popular in publications due to a lack of human readability. First attempts [358,359] of prof. Kuttel et al., to visualize carbohydrate molecules in an efficient and simple way were made by developing PaperChain and Twister graphic algorithms as a part of CarboHydra [360] and Visual Molecular Dynamics [361] software packages. Later, group of prof. Pé rez suggested to restrict visualized molecule to skeletal atoms via conditional cycle plane coloring in accordance with the color code adopted in SNFG [338] visualization scheme (SweetUnityMol software [362], Figure 11a). Another UnityMol visualization approach called Umbrella Visualization [363,364] was tailored for N-glycan structures. Azahar plugin for PyMol [235] affords cartoon models with polygons and rods. Several solutions for convenient visualization came up with the development of SNFG notation [339]. Thus, group of prof. Woods proposed to combine molecular structure elements with 3D SNFG icons (Figure 12a). Such convenient visualization technique was integrated in LiteMol (Figure 12b) [365] and Mol* (Figure 12c) [324,356]. 3D SNFG visualization plugins are available via Visual Molecular Dynamics platform [366] (http://glycam.org/docs/othertoolsservice/2016/06/03/3d-symbol-nomenclature-for-glycans-3d-snfg/) and UCSF Chimera [367] visualization software Tangram plugin (https://github.com/insilichem/tangram_snfg). Designed as part of CCP4mg [368] molecular-graphics software, Glycoblocks [369] representation of monosacchrides uses shapes and colors, identical to those in SNFG (Figure 12d). Available as PyMol plugin developed by Widmalm group (http://www.organ.su.se/gw/doku.php?id=3dcfg), 3D-CFG representation [370] based on CFG notation [371] (often referred to as a predecessor of SNFG) should also be noted as earlier approach to interpretation of carbohydrate 3D structures based on a symbol library.
Figure 11.
Glycan structure colored according to SNFG, or superimposed with 3D SNFG, as implemented in SweetUnityMol (a), GLYCOSCIENCES.de (via JSmol) (b), and CSDB (via JSmol) (c,d), see text. Panel (a) was reproduced with permission from [372], © Springer Japan 2017.
Figure 12.
Glycan structure colored according to SNFG, or superimposed with 3D SNFG, as implemented in 3D-SNFG (a), LiteMol (b), Mol* (c); monosaccharide presentation in Glycoblocks (d). Panel (a) was reproduced with permission from [366], © 2020, Oxford University Press. Panel (d) was reproduced from [369], © 2020 The authors. Published by John Wiley & Sons, Inc.
Considering efficiency and usability of 3D representation based on SNFG concept, which grows popular among glycoscientists, the development of alternative solutions in carbohydrate 3D structure representations has a potential for application in glycoinformatics projects. Support of colored residues in 3D structures implemented via JSmol on GLYCOSCIENCES.de portal was reported [47] (Figure 11b). Similarly, CSDB project has developed a 3D viewer (http://csdb.glycoscience.ru/database/core/show_3d.php?csdb=-3)aDManp(1-3)[Ac(1-2)?DGlcpN(1-6)]bDGal?(1-) with carbohydrate residue coloring according to the SNFG notation in the framework of a modeling module based on REStLESS API. In this tool, user can visualize input structure with help of sticks, balls and sticks, or van der Waals spheres (Figure 11c). Options for aglycone moiety (white) and pseudo-atoms (polymeric repeats, blue caps) are supported (Figure 11d).
8. Conclusions
Development of glycoinformatics resources makes great impact on treating enormous masses of data sets produced by glyco-related research. Tools for carbohydrate 3D structural information retrieval provide a framework for experimental and computational data quality validation. Data sources based on conformational ensemble generation and analysis assist structure–function and structure–activity relationship prediction of biologically relevant bioglycans and glycoconjugates. In this review, we have summarized existing facilities on working with glycan spatial features that can provide harmonious network of structural databases, web-services, tools and standalone software for modeling and processing structural data. Further advances in this field will help building better understanding of glycan participation in biological processes and supply glycoscience community with user-friendly access to voluminous data collections.
Abbreviations
| 3D | Three-dimensional |
| AA | All-atom |
| CAZy | Carbohydrate-Active Enzyme |
| CD | Cluster of Differentiation |
| CFG | Consortium for Functional Glycomics |
| CG | Coarse-grained |
| CHI | Carbohydrate Intrinsic |
| CRD | Carbohydrate Recognition Site |
| Cryo-EM | Electron cryo-microscopy |
| CSD | Cambridge Structural Database |
| DFT | Density Functional Theory |
| FUC | α-L-fucopyranose |
| GAG | Glycosaminoglycan |
| GAMD | Gaussian Accelerated MD |
| GBP | Glycan-Binding Protein |
| GM9 | Glc1Man9GlcNAc2 |
| HPLC | High Performance Liquid Chromatography |
| HREX | Hamiltonian Replica-Exchange MD |
| INIOM | Our own N-layered integrated molecular orbital and molecular mechanics |
| LNB | Lacto-N-biose I |
| MD | Molecular Dynamics |
| MM | Molecular Mechanics |
| MS | Mass-spectrometry |
| msesMD | Multidimensional swarm-enhanced sampling MD |
| NAG | 2-acetamido-2-deoxy-β-D-glucopyranose |
| NMR | Nuclear Magnetic Resonance |
| NOE | Nuclear Overhauser Effect |
| PDB | Protein Data Bank |
| PDBe | Protein Data Bank Europe |
| PDBj | Protein Data Bank Japan |
| PDBsum | Database of Structural Summaries of PDB Entries |
| QM | Quantum Mechanics |
| RCSB PDB | Research Collaboratory for Structural Bioinformatics Protein Data Bank |
| REMD | Replica-exchange MD |
| SNFG | Symbol Nomenclature for Glycans |
| UniProtKB | UniProt Knowledgebase |
| wwPDB | Worldwide Protein Data Bank |
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/1422-0067/21/20/7702/s1. Supplementary Tables: S1. Application rate for quantum mechanics and molecular mechanics methods in 2015–2020. S2. Citing of force fields in 2015-2020. S3. Protein Data Bank statistics. S4. PDB carbohydrate remediation list data.
Author Contributions
S.I.S. and P.V.T. contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
The work with carbohydrate molecular modeling and PDB data was funded by Russian Foundation for Basic Research grant 18-04-00094. The work with structural databases, glycoinformatic tools and visualization was funded by Russian Science Foundation grant 18-14-00098.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hricovini M. Structural Aspects of Carbohydrates and the Relation with their Biological Properties. Curr. Med. Chem. 2004;11:2565–2583. doi: 10.2174/0929867043364414. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buddhadeb M., Dimitrios M. Applications of Molecular Dynamics Simulations in Immunology: A Useful Computational Method in Aiding Vaccine Design. Curr. Proteom. 2006;3:259–270. [Google Scholar]
- 4.Kuttel M.M., Ravenscroft N. Carbohydrate-Based Vaccines: From Concept to Clinic. Volume 1290. American Chemical Society; Washington, DC, USA: 2018. The Role of Molecular Modeling in Predicting Carbohydrate Antigen Conformation and Understanding Vaccine Immunogenicity; pp. 139–173. [Google Scholar]
- 5.Mishra S.K., Calabró G., Loeffler H.H., Michel J., Koča J. Evaluation of Selected Classical Force Fields for Alchemical Binding Free Energy Calculations of Protein-Carbohydrate Complexes. J. Chem. Theory Comput. 2015;11:3333–3345. doi: 10.1021/acs.jctc.5b00159. [DOI] [PubMed] [Google Scholar]
- 6.Woods R.J., Tessier M.B. Computational glycoscience: Characterizing the spatial and temporal properties of glycans and glycan–protein complexes. Curr. Opin. Struct. Biol. 2010;20:575–583. doi: 10.1016/j.sbi.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadden J.A., Tessier M.B., Fadda E., Woods R.J. Calculating Binding Free Energies for Protein–Carbohydrate Complexes. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 431–465. [DOI] [PubMed] [Google Scholar]
- 8.Woods R.J. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem. Rev. 2018;118:8005–8024. doi: 10.1021/acs.chemrev.8b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank M. Computational Docking as a Tool for the Rational Design of Carbohydrate-Based Drugs. In: Seeberger P.H., Rademacher C., editors. Carbohydrates as Drugs. Springer; Cham, Switzerland: 2014. pp. 53–72. [Google Scholar]
- 10.Purcell S.C., Godula K. Synthetic glycoscapes: Addressing the structural and functional complexity of the glycocalyx. Interface Focus. 2019;9:20180080. doi: 10.1098/rsfs.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagae M., Yamaguchi Y. Function and 3D Structure of the N-Glycans on Glycoproteins. Int. J. Mol. Sci. 2012;13:8398–8429. doi: 10.3390/ijms13078398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki-Kinoshita K.F., Lisacek F., Mazumder R., York W.S., Packer N.H. The GlySpace Alliance: Toward a collaborative global glycoinformatics community. Glycobiology. 2020;30:70–71. doi: 10.1093/glycob/cwz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada I., Shiota M., Shinmachi D., Ono T., Tsuchiya S., Hosoda M., Fujita A., Aoki N.P., Watanabe Y., Fujita N., et al. The GlyCosmos Portal: A unified and comprehensive web resource for the glycosciences. Nat. Methods. 2020;17:649–650. doi: 10.1038/s41592-020-0879-8. [DOI] [PubMed] [Google Scholar]
- 14.Mariethoz J., Alocci D., Gastaldello A., Horlacher O., Gasteiger E., Rojas-Macias M., Karlsson N.G., Packer N.H., Lisacek F. Glycomics@ExPASy: Bridging the Gap. Mol. Cell. Proteom. 2018;17:2164. doi: 10.1074/mcp.RA118.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahsay R., Vora J., Navelkar R., Mousavi R., Fochtman B., Holmes X., Pattabiraman N., Ranzinger R., Mahadik R., Williamson T., et al. GlyGen data model and processing workflow. Bioinformatics. 2020;36:3941–3943. doi: 10.1093/bioinformatics/btaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda M., Fujita N., Suzuki Y., Sawaki H., Shikanai T., Narimatsu H. JCGGDB: Japan Consortium for Glycobiology and Glycotechnology Database. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 161–179. [DOI] [PubMed] [Google Scholar]
- 17.Tiemeyer M., Aoki K., Paulson J., Cummings R.D., York W.S., Karlsson N.G., Lisacek F., Packer N.H., Campbell M.P., Aoki N.P., et al. GlyTouCan: An accessible glycan structure repository. Glycobiology. 2017;27:915–919. doi: 10.1093/glycob/cwx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.York W.S., Agravat S., Aoki-Kinoshita K.F., McBride R., Campbell M.P., Costello C.E., Dell A., Feizi T., Haslam S.M., Karlsson N., et al. MIRAGE: The minimum information required for a glycomics experiment. Glycobiology. 2014;24:402–406. doi: 10.1093/glycob/cwu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comelli E.M., Head S.R., Gilmartin T., Whisenant T., Haslam S.M., North S.J., Wong N.-K., Kudo T., Narimatsu H., Esko J.D., et al. A focused microarray approach to functional glycomics: Transcriptional regulation of the glycome. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]
- 20.Aoki-Kinoshita K.F. RINGS: A Web Resource of Tools for Analyzing Glycomics Data. In: Aoki-Kinoshita K.F., editor. A Practical Guide to Using Glycomics Databases. Springer; Tokyo, Japan: 2017. pp. 299–334. [Google Scholar]
- 21.Gourdine J.-P.F., Brush M.H., Vasilevsky N.A., Shefchek K., Köhler S., Matentzoglu N., Munoz-Torres M.C., McMurry J.A., Zhang X.A., Robinson P.N., et al. Representing glycophenotypes: Semantic unification of glycobiology resources for disease discovery. Database. 2019;2019 doi: 10.1093/database/baz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alocci D., Ghraichy M., Barletta E., Gastaldello A., Mariethoz J., Lisacek F. Understanding the glycome: An interactive view of glycosylation from glycocompositions to glycoepitopes. Glycobiology. 2018;28:349–362. doi: 10.1093/glycob/cwy019. [DOI] [PubMed] [Google Scholar]
- 23.Barnett C.B., Aoki-Kinoshita K.F., Naidoo K.J. The Glycome Analytics Platform: An integrative framework for glycobioinformatics. Bioinformatics. 2016;32:3005–3011. doi: 10.1093/bioinformatics/btw341. [DOI] [PubMed] [Google Scholar]
- 24.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katoh T., Yamamoto K. Glycoenzymes in Glycan Analysis and Synthesis. In: Taniguchi N., Endo T., Hart G.W., Seeberger P.H., Wong C.-H., editors. Glycoscience: Biology and Medicine. Springer; Tokyo, Japan: 2015. pp. 379–389. [Google Scholar]
- 26.Montgomery A.P., Xiao K., Wang X., Skropeta D., Yu H. Chapter Two—Computational Glycobiology: Mechanistic Studies of Carbohydrate-Active Enzymes and Implication for Inhibitor Design. In: Karabencheva-Christova T., editor. Advances in Protein Chemistry and Structural Biology. Volume 109. Academic Press; Cambridge, MA, USA: 2017. pp. 25–76. [DOI] [PubMed] [Google Scholar]
- 27.Copoiu L., Malhotra S. The current structural glycome landscape and emerging technologies. Curr. Opin. Struct. Biol. 2020;62:132–139. doi: 10.1016/j.sbi.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Toukach P.V., Egorova K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016;44:D1229–D1236. doi: 10.1093/nar/gkv840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egorova K.S., Toukach P.V. Glycoinformatics: Bridging isolated islands in the sea of data. Angew. Chem. Int. Ed. 2018;57:14986–14990. doi: 10.1002/anie.201803576. [DOI] [PubMed] [Google Scholar]
- 30.Lisacek F., Mariethoz J., Alocci D., Rudd P.M., Abrahams J.L., Campbell M.P., Packer N.H., Ståhle J., Widmalm G., Mullen E., et al. Databases and Associated Tools for Glycomics and Glycoproteomics. In: Lauc G., Wuhrer M., editors. High-Throughput Glycomics and Glycoproteomics: Methods and Protocols. Humana Press; New York, NY, USA: 2017. pp. 235–264. [DOI] [PubMed] [Google Scholar]
- 31.Abrahams J.L., Taherzadeh G., Jarvas G., Guttman A., Zhou Y., Campbell M.P. Recent advances in glycoinformatic platforms for glycomics and glycoproteomics. Curr. Opin. Struct. Biol. 2020;62:56–69. doi: 10.1016/j.sbi.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Xu Z., Hong X., Zhang Y., Zou X. Databases and Bioinformatic Tools for Glycobiology and Glycoproteomics. Int. J. Mol. Sci. 2020;21:6727. doi: 10.3390/ijms21186727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell M.P., Peterson R., Mariethoz J., Gasteiger E., Akune Y., Aoki-Kinoshita K.F., Lisacek F., Packer N.H. UniCarbKB: Building a knowledge platform for glycoproteomics. Nucleic Acids Res. 2014;42:D215–D221. doi: 10.1093/nar/gkt1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doubet S., Bock K., Smith D., Darvill A., Albersheim P. The Complex Carbohydrate Structure Database. Trends Biochem. Sci. 1989;14:475–477. doi: 10.1016/0968-0004(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 35.Doubet S., Albersheim P. CarbBank. Glycobiology. 1992;2:505–507. doi: 10.1093/glycob/2.6.505. [DOI] [PubMed] [Google Scholar]
- 36.von der Lieth C.W., Freire A.A., Blank D., Campbell M.P., Ceroni A., Damerell D.R., Dell A., Dwek R.A., Ernst B., Fogh R., et al. EUROCarbDB: An open-access platform for glycoinformatics. Glycobiology. 2011;21:493–502. doi: 10.1093/glycob/cwq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranzinger R., Herget S., Wetter T., von der Lieth C.W. GlycomeDB—Integration of open-access carbohydrate structure databases. BMC Bioinf. 2008;9:384. doi: 10.1186/1471-2105-9-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranzinger R., Frank M., von der Lieth C.W., Herget S. Glycome-DB.org: A portal for querying across the digital world of carbohydrate sequences. Glycobiology. 2009;19:1563–1567. doi: 10.1093/glycob/cwp137. [DOI] [PubMed] [Google Scholar]
- 39.Nakahara T., Hashimoto R., Nakagawa H., Monde K., Miura N., Nishimura S. Glycoconjugate Data Bank: Structures—An annotated glycan structure database and N-glycan primary structure verification service. Nucleic Acids Res. 2008;36:D368–D371. doi: 10.1093/nar/gkm833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper C.A., Harrison M.J., Wilkins M.R., Packer N.H. GlycoSuiteDB: A new curated relational database of glycoprotein glycan structures and their biological sources. Nucleic Acids Res. 2001;29:332–335. doi: 10.1093/nar/29.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper C.A., Joshi H.J., Harrison M.J., Wilkins M.R., Packer N.H. GlycoSuiteDB: A curated relational database of glycoprotein glycan structures and their biological sources. 2003 update. Nucleic Acids Res. 2003;31:511–513. doi: 10.1093/nar/gkg099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toukach P.V. Bacterial carbohydrate structure database 3: Principles and realization. J. Chem. Inf. Model. 2011;51:159–170. doi: 10.1021/ci100150d. [DOI] [PubMed] [Google Scholar]
- 43.Egorova K.S., Toukach P.V. CSDB_GT: A new curated database on glycosyltransferases. Glycobiology. 2017;27:285–290. doi: 10.1093/glycob/cww137. [DOI] [PubMed] [Google Scholar]
- 44.Toukach P.V., Knirel Y.A. New database of bacterial carbohydrate structures. Glycoconj. J. 2005;22:216–217. [Google Scholar]
- 45.Lütteke T., Bohne-Lang A., Loss A., Goetz T., Frank M., Von Der Lieth C.W. GLYCOSCIENCES.de: An Internet portal to support glycomics and glycobiology research. Glycobiology. 2006;16:71R–81R. doi: 10.1093/glycob/cwj049. [DOI] [PubMed] [Google Scholar]
- 46.Lütteke T. Glycan data retrieval and analysis using GLYCOSCIENCES. de applications. In: Aoki-Kinoshita K.F., editor. A Practical Guide to Using Glycomics Databases. 1st ed. Springer; Tokyo, Japan: 2017. pp. 335–350. [Google Scholar]
- 47.Bohm M., Bohne-Lang A., Frank M., Loss A., Rojas-Macias M.A., Lutteke T. Glycosciences.DB: An annotated data collection linking glycomics and proteomics data (2018 update) Nucleic Acids Res. 2019;47:D1195–D1201. doi: 10.1093/nar/gky994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez S., Sarkar A., Rivet A., Breton C., Imberty A. Glyco3D: A Portal for Structural Glycosciences. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 241–258. [DOI] [PubMed] [Google Scholar]
- 49.Pérez S., Sarkar A., Rivet A., Drouillard S., Breton C., Imberty A. Glyco3D: A Suite of Interlinked Databases of 3D Structures of Complex Carbohydrates, Lectins, Antibodies, and Glycosyltransferases. In: Aoki-Kinoshita K.F., editor. A Practical Guide to Using Glycomics Databases. Springer; Tokyo, Japan: 2017. pp. 133–161. [Google Scholar]
- 50.Sarkar A., Pérez S. PolySac3DB: An annotated data base of 3 dimensional structures of polysaccharides. BMC Bioinf. 2012;13:302. doi: 10.1186/1471-2105-13-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunduru B.R., Nair S.A., Rathinavelan T. EK3D: An E. coli K antigen 3-dimensional structure database. Nucleic Acids Res. 2016;44:D675–D681. doi: 10.1093/nar/gkv1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veluraja K., Selvin J.F.A., Venkateshwari S., Priyadarzini T.R.K. 3DSDSCAR—A three dimensional structural database for sialic acid-containing carbohydrates through molecular dynamics simulation. Carbohydr. Res. 2010;345:2030–2037. doi: 10.1016/j.carres.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Veluraja K., Fermin Angelo Selvin J., Jasmine A., Hema Thanka Christlet T. Three Dimensional Structures of Carbohydrates and Glycoinformatics: An Overview. In: Wadhwa G., Shanmughavel P., Singh A.K., Bellare J.R., editors. Current Trends in Bioinformatics: An Insight. Springer; Singapore: 2018. pp. 55–87. [Google Scholar]
- 54.Chautard E., Fatoux-Ardore M., Ballut L., Thierry-Mieg N., Ricard-Blum S. MatrixDB, the extracellular matrix interaction database. Nucleic Acids Res. 2011;39:D235–D240. doi: 10.1093/nar/gkq830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Launay G., Salza R., Multedo D., Thierry-Mieg N., Ricard-Blum S. MatrixDB, the extracellular matrix interaction database: Updated content, a new navigator and expanded functionalities. Nucleic Acids Res. 2015;43:D321–D327. doi: 10.1093/nar/gku1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clerc O., Deniaud M., Vallet S.D., Naba A., Rivet A., Perez S., Thierry-Mieg N., Ricard-Blum S. MatrixDB: Integration of new data with a focus on glycosaminoglycan interactions. Nucleic Acids Res. 2019;47:D376–D381. doi: 10.1093/nar/gky1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birch J., Van Calsteren M.R., Perez S., Svensson B. The exopolysaccharide properties and structures database: EPS-DB. Application to bacterial exopolysaccharides. Carbohydr. Polym. 2019;205:565–570. doi: 10.1016/j.carbpol.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 58.Cao Y., Park S.-J., Mehta A.Y., Cummings R.D., Im W. GlyMDB: Glycan Microarray Database and analysis toolset. Bioinformatics. 2020;36:2438–2442. doi: 10.1093/bioinformatics/btz934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raman R., Venkataraman M., Ramakrishnan S., Lang W., Raguram S., Sasisekharan R. Advancing glycomics: Implementation strategies at the consortium for functional glycomics. Glycobiology. 2006;16:82R–90R. doi: 10.1093/glycob/cwj080. [DOI] [PubMed] [Google Scholar]
- 60.Venkataraman M., Sasisekharan R., Raman R. Glycan Array Data Management at Consortium for Functional Glycomics. In: Lütteke T., Frank M., editors. Glycoinformatics. 1st ed. Humana Press; New York, NY, USA: 2015. pp. 181–190. [DOI] [PubMed] [Google Scholar]
- 61.Yamada I., Angata K., Watanabe Y., Ono T. Databases for glycoconjugates (GlyCosmos Glycoproteins and Glycolipids, GlycoProtDB, GlycoNAVI:TCarp, GlycoPOST) Glycoforum. 2020;23:A2. [Google Scholar]
- 62.Yamada I., Aoki-Kinoshita K.F. Program and Abstracts for 2017 Annual Meeting of the Society for Glycobiology, Portland, OR, USA, 5–8 November 2017. Oxford University Press Inc.; Cary, NC, USA: 2017. Integration of Glycoscience Data in GlyCosmos Using Semantic Web Technologies; p. 61. [Google Scholar]
- 63.Shiota M., Tsuchiya S., Ono T., Kuoka T., Miura N., Hiraki A., Yamada I., Shinmachi D., Aoki N.P., Kim J.-D. The GlyCosmos Web Portal: Glycan structures, glycogenes, glycoproteins, pathways, diseases and more! In Program and Abstracts for 2018 Annual Meeting of the Society for Glycobiology, New Orleans, LA, USA, 5–8 November 2018. Oxford University Press Inc.; Cary, NC, USA: 2018. pp. 1070–1071. [Google Scholar]
- 64.Mariethoz J., Khatib K., Alocci D., Campbell M.P., Karlsson N.G., Packer N.H., Mullen E.H., Lisacek F. SugarBindDB, a resource of glycan-mediated host–pathogen interactions. Nucleic Acids Res. 2016;44:D1243–D1250. doi: 10.1093/nar/gkv1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alocci D., Mariethoz J., Gastaldello A., Gasteiger E., Karlsson N.G., Kolarich D., Packer N.H., Lisacek F. GlyConnect: Glycoproteomics Goes Visual, Interactive, and Analytical. J. Proteome Res. 2019;18:664–677. doi: 10.1021/acs.jproteome.8b00766. [DOI] [PubMed] [Google Scholar]
- 66.Choudhary P., Nagar R., Singh V., Bhat A.H., Sharma Y., Rao A. ProGlycProt V2.0, a repository of experimentally validated glycoproteins and protein glycosyltransferases of prokaryotes. Glycobiology. 2019;29:461–468. doi: 10.1093/glycob/cwz013. [DOI] [PubMed] [Google Scholar]
- 67.Bhat A.H., Mondal H., Chauhan J.S., Raghava G.P.S., Methi A., Rao A. ProGlycProt: A repository of experimentally characterized prokaryotic glycoproteins. Nucleic Acids Res. 2012;40:D388–D393. doi: 10.1093/nar/gkr911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Copoiu L., Torres P.H.M., Ascher D.B., Blundell T.L., Malhotra S. ProCarbDB: A database of carbohydrate-binding proteins. Nucleic Acids Res. 2020;48:D368–D375. doi: 10.1093/nar/gkz860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siva Shanmugam N.R., Jino Blessy J., Veluraja K., Michael Gromiha M. ProCaff: Protein–carbohydrate complex binding affinity database. Bioinformatics. 2020;36:3615–3617. doi: 10.1093/bioinformatics/btaa141. [DOI] [PubMed] [Google Scholar]
- 70.Cao Y., Park S.-J., Im W. A Systematic Analysis of Protein-Carbohydrate Interactions in the PDB. Glycobiology. 2020 doi: 10.1093/glycob/cwaa062. [DOI] [PubMed] [Google Scholar]
- 71.Malik A., Firoz A., Jha V., Ahmad S. PROCARB: A Database of Known and Modelled Carbohydrate-Binding Protein Structures with Sequence-Based Prediction Tools. Adv. Bioinf. 2010;2010:1–9. doi: 10.1155/2010/436036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonnardel F., Mariethoz J., Salentin S., Robin X., Schroeder M., Perez S., Lisacek F., Imberty A. UniLectin3D, a database of carbohydrate binding proteins with curated information on 3D structures and interacting ligands. Nucleic Acids Res. 2019;47:D1236–D1244. doi: 10.1093/nar/gky832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonnardel F., Perez S., Lisacek F., Imberty A. Structural Database for Lectins and the UniLectin Web Platform. In: Hirabayashi J., editor. Lectin Purification and Analysis: Methods and Protocols. Humana; New York, NY, USA: 2020. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 74.Hirabayashi J., Tateno H., Shikanai T., Aoki-Kinoshita K.F., Narimatsu H. The Lectin Frontier Database (LfDB), and data generation based on frontal affinity chromatography. Molecules. 2015;20:951–973. doi: 10.3390/molecules20010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandra N.R., Kumar N., Jeyakani J., Singh D.D., Gowda S.B., Prathima M.N. Lectindb: A plant lectin database. Glycobiology. 2006;16:938–946. doi: 10.1093/glycob/cwl012. [DOI] [PubMed] [Google Scholar]
- 76.Kawasaki T., Nakao H., Takahashi E., Tominaga T. GlycoEpitope: The integrated database of carbohydrate antigens and antibodies. Trends Glycosci. Glycotechnol. 2006;18:267–272. doi: 10.4052/tigg.18.267. [DOI] [Google Scholar]
- 77.Kawasaki T., Nakao H., Tominaga T. GlycoEpitope: A database of carbohydrate epitopes and antibodies. In: Taniguchi N., Suzuki A., Ito Y., Narimatsu H., Kawasaki T., Hase S., editors. Experimental Glycoscience. Springer; Tokyo, Japan: 2008. pp. 429–431. [Google Scholar]
- 78.Aoki-Kinoshita K.F. Using Databases and Web Resources for Glycomics Research. Mol. Cell. Proteom. 2013;12:1036. doi: 10.1074/mcp.R112.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar S., Lütteke T., Schwartz-Albiez R. GlycoCD: A repository for carbohydrate-related CD antigens. Bioinformatics. 2012;28:2553–2555. doi: 10.1093/bioinformatics/bts481. [DOI] [PubMed] [Google Scholar]
- 80.Allcorn L.C., Martin A.C.R. SACS—Self-maintaining database of antibody crystal structure information. Bioinformatics. 2002;18:175–181. doi: 10.1093/bioinformatics/18.1.175. [DOI] [PubMed] [Google Scholar]
- 81.Dunbar J., Krawczyk K., Leem J., Baker T., Fuchs A., Georges G., Shi J., Deane C.M. SAbDab: The structural antibody database. Nucleic Acids Res. 2014;42:D1140–D1146. doi: 10.1093/nar/gkt1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantarel B.L., Coutinho P.M., Rancurel C., Bernard T., Lombard V., Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terrapon N., Lombard V., Drula E., Coutinho P.M., Henrissat B. The CAZy Database/the Carbohydrate-Active Enzyme (CAZy) Database: Principles and Usage Guidelines. In: Aoki-Kinoshita K.F., editor. A Practical Guide to Using Glycomics Databases. Springer; Tokyo, Japan: 2017. pp. 117–131. [Google Scholar]
- 85.Lee T.-Y., Huang H.-D., Hung J.-H., Huang H.-Y., Yang Y.-S., Wang T.-H. dbPTM: An information repository of protein post-translational modification. Nucleic Acids Res. 2006;34:D622–D627. doi: 10.1093/nar/gkj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang K.-Y., Su M.-G., Kao H.-J., Hsieh Y.-C., Jhong J.-H., Cheng K.-H., Huang H.-D., Lee T.-Y. dbPTM 2016: 10-year anniversary of a resource for post-translational modification of proteins. Nucleic Acids Res. 2016;44:D435–D446. doi: 10.1093/nar/gkv1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang K.-Y., Lee T.-Y., Kao H.-J., Ma C.-T., Lee C.-C., Lin T.-H., Chang W.-C., Huang H.-D. dbPTM in 2019: Exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019;47:D298–D308. doi: 10.1093/nar/gky1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kopp J., Schwede T. The SWISS-MODEL Repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32:D230–D234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiefer F., Arnold K., Künzli M., Bordoli L., Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bienert S., Waterhouse A., de Beer T.A.P., Tauriello G., Studer G., Bordoli L., Schwede T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017;45:D313–D319. doi: 10.1093/nar/gkw1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank M., Lutteke T., Von Der Lieth C.W. GlycoMapsDB: A database of the accessible conformational space of glycosidic linkages. Nucleic Acids Res. 2007;35:287–290. doi: 10.1093/nar/gkl907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jo S., Im W. Glycan fragment database: A database of PDB-based glycan 3D structures. Nucleic Acids Res. 2013;41:D470–D474. doi: 10.1093/nar/gks987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez S. X-Ray Diffraction and Crystallography of Oligosaccharides and Polysaccharides. In: Roberts G.C.K., editor. Encyclopedia of Biophysics. Springer; Berlin/Heidelberg, Germany: 2013. pp. 2767–2777. [Google Scholar]
- 94.Gimeno A., Valverde P., Ardá A., Jiménez-Barbero J. Glycan structures and their interactions with proteins. A NMR view. Curr. Opin. Struct. Biol. 2020;62:22–30. doi: 10.1016/j.sbi.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blaum B.S., Neu U., Peters T., Stehle T. Spin ballet for sweet encounters: Saturation-transfer difference NMR and X-ray crystallography complement each other in the elucidation of protein-glycan interactions. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018;74:451–462. doi: 10.1107/S2053230X18006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Battistel M.D., Azurmendi H.F., Yu B., Freedberg D.I. NMR of glycans: Shedding new light on old problems. Prog. Nucl. Magn. Reson. Spectrosc. 2014;79:48–68. doi: 10.1016/j.pnmrs.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Schubert M. Insights into Carbohydrate Recognition by 3D Structure Determination of Protein–Carbohydrate Complexes Using NMR. In: Kato K., Peters T., editors. NMR in Glycoscience and Glycotechnology. The Royal Society of Chemistry; London, UK: 2017. pp. 101–122. [Google Scholar]
- 98.Cléry A., Schubert M., Allain F.H.T. NMR Spectroscopy: An Excellent Tool to Understand RNA and Carbohydrate Recognition by Proteins. Chimia. 2012;66:741–746. doi: 10.2533/chimia.2012.741. [DOI] [PubMed] [Google Scholar]
- 99.Valverde P., Delgado S., Martínez J.D., Vendeville J.-B., Malassis J., Linclau B., Reichardt N.-C., Cañada F.J., Jiménez-Barbero J., Ardá A. Molecular Insights into DC-SIGN Binding to Self-Antigens: The Interaction with the Blood Group A/B Antigens. ACS Chem. Biol. 2019;14:1660–1671. doi: 10.1021/acschembio.9b00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aeschbacher T., Zierke M., Smieško M., Collot M., Mallet J.-M., Ernst B., Allain F.H.-T., Schubert M. A Secondary Structural Element in a Wide Range of Fucosylated Glycoepitopes. Chem. Eur. J. 2017;23:11598–11610. doi: 10.1002/chem.201701866. [DOI] [PubMed] [Google Scholar]
- 101.Zierke M., Smieško M., Rabbani S., Aeschbacher T., Cutting B., Allain F.H.T., Schubert M., Ernst B. Stabilization of Branched Oligosaccharides: Lewisx Benefits from a Nonconventional C–H···O Hydrogen Bond. J. Am. Chem. Soc. 2013;135:13464–13472. doi: 10.1021/ja4054702. [DOI] [PubMed] [Google Scholar]
- 102.Sattelle B.M., Almond A. Is N-acetyl-d-glucosamine a rigid 4C1 chair? Glycobiology. 2011;21:1651–1662. doi: 10.1093/glycob/cwr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.França B.A., da Silva C.O. Specific rotation of monosaccharides: A global property bringing local information. Phys. Chem. Chem. Phys. 2014;16:13096–13102. doi: 10.1039/c4cp01316f. [DOI] [PubMed] [Google Scholar]
- 104.Ling Z., Edwards J.V., Nam S., Xu F., French A.D. Conformational analysis of xylobiose by DFT quantum mechanics. Cellulose. 2020;27:1207–1224. doi: 10.1007/s10570-019-02874-3. [DOI] [Google Scholar]
- 105.McMahon C.M., Isabella C.R., Windsor I.W., Kosma P., Raines R.T., Kiessling L.L. Stereoelectronic Effects Impact Glycan Recognition. J. Am. Chem. Soc. 2020;142:2386–2395. doi: 10.1021/jacs.9b11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W., Meredith R., Pan Q., Wang X., Woods R.J., Carmichael I., Serianni A.S. Use of Circular Statistics To Model αMan-(1→2)-αMan and αMan-(1→3)-α/βMan O-Glycosidic Linkage Conformation in 13C-Labeled Disaccharides and High-Mannose Oligosaccharides. Biochemistry. 2019;58:546–560. doi: 10.1021/acs.biochem.8b01050. [DOI] [PubMed] [Google Scholar]
- 107.Saraboji K., Håkansson M., Genheden S., Diehl C., Qvist J., Weininger U., Nilsson U.J., Leffler H., Ryde U., Akke M., et al. The Carbohydrate-Binding Site in Galectin-3 Is Preorganized To Recognize a Sugarlike Framework of Oxygens: Ultra-High-Resolution Structures and Water Dynamics. Biochemistry. 2012;51:296–306. doi: 10.1021/bi201459p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turupcu A., Bowen A.M., Di Paolo A., Matagne A., Oostenbrink C., Redfield C., Smith L.J. An NMR and MD study of complexes of bacteriophage lambda lysozyme with tetra- and hexa-N-acetylchitohexaose. Proteins Struct. Funct. Bioinf. 2020;88:82–93. doi: 10.1002/prot.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turupcu A., Oostenbrink C. Modeling of Oligosaccharides within Glycoproteins from Free-Energy Landscapes. J. Chem. Inf. Model. 2017;57:2222–2236. doi: 10.1021/acs.jcim.7b00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang W., Turney T., Meredith R., Pan Q., Sernau L., Wang X., Hu X., Woods R.J., Carmichael I., Serianni A.S. Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics. J. Phys. Chem. B. 2017;121:3042–3058. doi: 10.1021/acs.jpcb.7b02252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sattelle B.M., Almond A. Shaping up for structural glycomics: A predictive protocol for oligosaccharide conformational analysis applied to N-linked glycans. Carbohydr. Res. 2014;383:34–42. doi: 10.1016/j.carres.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frank M. Conformational Analysis of Carbohydrates—A Historical Overview. In: Von Der Lieth C.W., Lütteke T., Frank M., editors. Bioinformatics for Glycobiology and Glycomics: An Introduction. Wiley; Hoboken, NJ, USA: 2009. pp. 335–357. [Google Scholar]
- 113.Frank M. Predicting Carbohydrate 3D Structures Using Theoretical Methods. In: Von Der Lieth C.W., Lütteke T., Frank M., editors. Bioinformatics for Glycobiology and Glycomics: An Introduction. Wiley; Hoboken, NJ, USA: 2009. pp. 359–388. [Google Scholar]
- 114.Toukach F.V., Ananikov V.P. Recent advances in computational predictions of NMR parameters for the structure elucidation of carbohydrates: Methods and limitations. Chem. Soc. Rev. 2013;42:8376–8415. doi: 10.1039/c3cs60073d. [DOI] [PubMed] [Google Scholar]
- 115.French A.D. Computerized Models of Carbohydrates. In: Ramawat K.G., Mérillon J.-M., editors. Polysaccharides: Bioactivity and Biotechnology. Springer; Cham, Switzerland: 2015. pp. 1397–1440. [Google Scholar]
- 116.French A.D., Johnson G.P. Computerized Molecular Modeling of Carbohydrates. In: Popper Z.A., editor. The Plant Cell Wall: Methods and Protocols. Humana; New York, NY, USA: 2020. pp. 513–539. [DOI] [PubMed] [Google Scholar]
- 117.Feng T., Li M., Zhou J., Zhuang H., Chen F., Ye R., Campanella O., Fang Z. Application of molecular dynamics simulation in food carbohydrate research—A review. Innov. Food Sci. Emerg. Technol. 2015;31:1–13. doi: 10.1016/j.ifset.2015.06.015. [DOI] [Google Scholar]
- 118.Dowd M.K., Kiely D.E., Zhang J. Monte Carlo-based searching as a tool to study carbohydrate structure. Carbohydr. Res. 2011;346:1140–1148. doi: 10.1016/j.carres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 119.Zhang W., Howell S.C., Wright D.W., Heindel A., Qiu X., Chen J., Curtis J.E. Combined Monte Carlo/torsion-angle molecular dynamics for ensemble modeling of proteins, nucleic acids and carbohydrates. J. Mol. Graph. Modell. 2017;73:179–190. doi: 10.1016/j.jmgm.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Rahal-Sekkal M., Sekkal N., Kleb D.C., Bleckmann P. Structures and energies of D-galactose and galabiose conformers as calculated by ab initio and semiempirical methods. J. Comput. Chem. 2003;24:806–818. doi: 10.1002/jcc.10223. [DOI] [PubMed] [Google Scholar]
- 121.Barnett C.B., Naidoo K.J. Ring Puckering: A Metric for Evaluating the Accuracy of AM1, PM3, PM3CARB-1, and SCC-DFTB Carbohydrate QM/MM Simulations. J. Phys. Chem. B. 2010;114:17142–17154. doi: 10.1021/jp107620h. [DOI] [PubMed] [Google Scholar]
- 122.Govender K., Gao J., Naidoo K.J. AM1/d-CB1: A Semiempirical Model for QM/MM Simulations of Chemical Glycobiology Systems. J. Chem. Theory Comput. 2014;10:4694–4707. doi: 10.1021/ct500372s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Govender K.K., Naidoo K.J. Evaluating AM1/d-CB1 for Chemical Glycobiology QM/MM Simulations. J. Chem. Theory Comput. 2014;10:4708–4717. doi: 10.1021/ct500373p. [DOI] [PubMed] [Google Scholar]
- 124.Gould I.R., Bettley H.A.-A., Bryce R.A. Correlated ab initio quantum chemical calculations of di- and trisaccharide conformations. J. Comput. Chem. 2007;28:1965–1973. doi: 10.1002/jcc.20738. [DOI] [PubMed] [Google Scholar]
- 125.French A.D., Johnson G.P., Cramer C.J., Csonka G.I. Conformational analysis of cellobiose by electronic structure theories. Carbohydr. Res. 2012;350:68–76. doi: 10.1016/j.carres.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 126.Schnupf U., Momany F.A. DFT Energy Optimization of a Large Carbohydrate: Cyclomaltohexaicosaose (CA-26) J. Phys. Chem. B. 2012;116:6618–6627. doi: 10.1021/jp208927v. [DOI] [PubMed] [Google Scholar]
- 127.Devarajan A., Markutsya S., Lamm M.H., Cheng X., Smith J.C., Baluyut J.Y., Kholod Y., Gordon M.S., Windus T.L. Ab Initio Study of Molecular Interactions in Cellulose Iα. J. Phys. Chem. B. 2013;117:10430–10443. doi: 10.1021/jp406266u. [DOI] [PubMed] [Google Scholar]
- 128.Chan B. Aqueous-Phase Conformations of Lactose, Maltose, and Sucrose and the Assessment of Low-Cost DFT Methods with the DSCONF Set of Conformers for the Three Disaccharides. J. Phys. Chem. A. 2020;124:582–590. doi: 10.1021/acs.jpca.9b10932. [DOI] [PubMed] [Google Scholar]
- 129.Ishida T. Computational analysis of carbohydrate recognition based on hybrid QM/MM modeling: A case study of norovirus capsid protein in complex with Lewis antigen. Phys. Chem. Chem. Phys. 2018;20:4652–4665. doi: 10.1039/C7CP07701G. [DOI] [PubMed] [Google Scholar]
- 130.Tafazzoli M., Ghiasi M. Structure and conformation of α-, β- and γ-cyclodextrin in solution: Theoretical approaches and experimental validation. Carbohydr. Polym. 2009;78:10–15. doi: 10.1016/j.carbpol.2009.02.020. [DOI] [Google Scholar]
- 131.Ardèvol A., Rovira C. Reaction Mechanisms in Carbohydrate-Active Enzymes: Glycoside Hydrolases and Glycosyltransferases. Insights from ab Initio Quantum Mechanics/Molecular Mechanics Dynamic Simulations. J. Am. Chem. Soc. 2015;137:7528–7547. doi: 10.1021/jacs.5b01156. [DOI] [PubMed] [Google Scholar]
- 132.Tvaroška I. Atomistic insight into the catalytic mechanism of glycosyltransferases by combined quantum mechanics/molecular mechanics (QM/MM) methods. Carbohydr. Res. 2015;403:38–47. doi: 10.1016/j.carres.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 133.Johnson G.P., Petersen L., French A.D., Reilly P.J. Twisting of glycosidic bonds by hydrolases. Carbohydr. Res. 2009;344:2157–2166. doi: 10.1016/j.carres.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 134.Chung L.W., Sameera W.M.C., Ramozzi R., Page A.J., Hatanaka M., Petrova G.P., Harris T.V., Li X., Ke Z., Liu F., et al. The ONIOM Method and Its Applications. Chem. Rev. 2015;115:5678–5796. doi: 10.1021/cr5004419. [DOI] [PubMed] [Google Scholar]
- 135.Burnham J.F. Scopus database: A review. Biomed. Digit. Libr. 2006;3:1. doi: 10.1186/1742-5581-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frank M., Schloissnig S. Bioinformatics and molecular modeling in glycobiology. Cell. Mol. Life Sci. 2010;67:2749–2772. doi: 10.1007/s00018-010-0352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sapay N., Nurisso A., Imberty A. Simulation of Carbohydrates, from Molecular Docking to Dynamics in Water. In: Monticelli L., Salonen E., editors. Biomolecular Simulations: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2013. pp. 469–483. [DOI] [PubMed] [Google Scholar]
- 138.Pérez S., Tvaroška I. Chapter 1—Carbohydrate–Protein Interactions: Molecular Modeling Insights. In: Horton D., editor. Advances in Carbohydrate Chemistry and Biochemistry. Volume 71. Academic Press; Cambridge, MA, USA: 2014. pp. 9–136. [DOI] [PubMed] [Google Scholar]
- 139.Fadda E., Woods R.J. Molecular simulations of carbohydrates and protein–carbohydrate interactions: Motivation, issues and prospects. Drug Discov. Today. 2010;15:596–609. doi: 10.1016/j.drudis.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yongye A.B., Gonzalez-Outeiriño J., Glushka J., Schultheis V., Woods R.J. The Conformational Properties of Methyl α-(2,8)-Di/Trisialosides and Their N-Acyl Analogues: Implications for Anti-Neisseria meningitidis B Vaccine Design. Biochemistry. 2008;47:12493–12514. doi: 10.1021/bi800431c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Re S., Nishima W., Miyashita N., Sugita Y. Conformational flexibility of N-glycans in solution studied by REMD simulations. Biophys. Rev. 2012;4:179–187. doi: 10.1007/s12551-012-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patel D.S., Pendrill R., Mallajosyula S.S., Widmalm G., MacKerell A.D. Conformational Properties of α- or β-(1→6)-Linked Oligosaccharides: Hamiltonian Replica Exchange MD Simulations and NMR Experiments. J. Phys. Chem. B. 2014;118:2851–2871. doi: 10.1021/jp412051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mishra S.K., Kara M., Zacharias M., Koča J. Enhanced conformational sampling of carbohydrates by Hamiltonian replica-exchange simulation. Glycobiology. 2014;24:70–84. doi: 10.1093/glycob/cwt093. [DOI] [PubMed] [Google Scholar]
- 144.Mallajosyula S.S., MacKerell A.D. Influence of Solvent and Intramolecular Hydrogen Bonding on the Conformational Properties of O-Linked Glycopeptides. J. Phys. Chem. B. 2011;115:11215–11229. doi: 10.1021/jp203695t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alibay I., Burusco K.K., Bruce N.J., Bryce R.A. Identification of Rare Lewis Oligosaccharide Conformers in Aqueous Solution Using Enhanced Sampling Molecular Dynamics. J. Phys. Chem. B. 2018;122:2462–2474. doi: 10.1021/acs.jpcb.7b09841. [DOI] [PubMed] [Google Scholar]
- 146.Alibay I., Bryce R.A. Ring Puckering Landscapes of Glycosaminoglycan-Related Monosaccharides from Molecular Dynamics Simulations. J. Chem. Inf. Model. 2019;59:4729–4741. doi: 10.1021/acs.jcim.9b00529. [DOI] [PubMed] [Google Scholar]
- 147.Balogh G., Komáromi I., Bereczky Z. The mechanism of high affinity pentasaccharide binding to antithrombin, insights from Gaussian accelerated molecular dynamics simulations. J. Biomol. Struct. Dyn. 2019;38:4718–4732. doi: 10.1080/07391102.2019.1688194. [DOI] [PubMed] [Google Scholar]
- 148.Balogh G., Gyöngyösi T., Timári I., Herczeg M., Borbás A., Fehér K., Kövér K.E. Comparison of Carbohydrate Force Fields Using Gaussian Accelerated Molecular Dynamics Simulations and Development of Force Field Parameters for Heparin-Analogue Pentasaccharides. J. Chem. Inf. Model. 2019;59:4855–4867. doi: 10.1021/acs.jcim.9b00666. [DOI] [PubMed] [Google Scholar]
- 149.Suzuki T., Kajino M., Yanaka S., Zhu T., Yagi H., Satoh T., Yamaguchi T., Kato K. Conformational Analysis of a High-Mannose-Type Oligosaccharide Displaying Glucosyl Determinant Recognised by Molecular Chaperones Using NMR-Validated Molecular Dynamics Simulation. ChemBioChem. 2017;18:396–401. doi: 10.1002/cbic.201600595. [DOI] [PubMed] [Google Scholar]
- 150.Yamaguchi T., Sakae Y., Zhang Y., Yamamoto S., Okamoto Y., Kato K. Exploration of Conformational Spaces of High-Mannose-Type Oligosaccharides by an NMR-Validated Simulation. Angew. Chem. Int. Ed. 2014;53:10941–10944. doi: 10.1002/anie.201406145. [DOI] [PubMed] [Google Scholar]
- 151.Foley B.L., Tessier M.B., Woods R.J. Carbohydrate force fields. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012;2:652–697. doi: 10.1002/wcms.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kozmon S., Matuška R., Spiwok V., Koča J. Dispersion interactions of carbohydrates with condensate aromatic moieties: Theoretical study on the CH–π interaction additive properties. Phys. Chem. Chem. Phys. 2011;13:14215–14222. doi: 10.1039/c1cp21071h. [DOI] [PubMed] [Google Scholar]
- 153.Hudson K.L., Bartlett G.J., Diehl R.C., Agirre J., Gallagher T., Kiessling L.L., Woolfson D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015;137:15152–15160. doi: 10.1021/jacs.5b08424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsu C.-H., Park S., Mortenson D.E., Foley B.L., Wang X., Woods R.J., Case D.A., Powers E.T., Wong C.-H., Dyson H.J., et al. The Dependence of Carbohydrate–Aromatic Interaction Strengths on the Structure of the Carbohydrate. J. Am. Chem. Soc. 2016;138:7636–7648. doi: 10.1021/jacs.6b02879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stanković I.M., Blagojević Filipović J.P., Zarić S.D. Carbohydrate—Protein aromatic ring interactions beyond CH/π interactions: A Protein Data Bank survey and quantum chemical calculations. Int. J. Biol. Macromol. 2020;157:1–9. doi: 10.1016/j.ijbiomac.2020.03.251. [DOI] [PubMed] [Google Scholar]
- 156.Nivedha A.K., Makeneni S., Foley B.L., Tessier M.B., Woods R.J. Importance of ligand conformational energies in carbohydrate docking: Sorting the wheat from the chaff. J. Comput. Chem. 2014;35:526–539. doi: 10.1002/jcc.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nivedha A.K., Thieker D.F., Hu H., Woods R.J. Vina-Carb: Improving Glycosidic Angles during Carbohydrate Docking. J. Chem. Theory Comput. 2016;12:892–901. doi: 10.1021/acs.jctc.5b00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pérez S. 2.11—Molecular Modeling in Glycoscience. In: Kamerling H., editor. Comprehensive Glycoscience. Volume 2. Elsevier; Oxford, UK: 2007. pp. 347–388. [Google Scholar]
- 159.Stortz C.A., French A.D. Disaccharide conformational maps: Adiabaticity in analogues with variable ring shapes. Mol. Simul. 2008;34:373–389. doi: 10.1080/08927020701663339. [DOI] [Google Scholar]
- 160.Allinger N.L., Yuh Y.H., Lii J.H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 1989;111:8551–8566. doi: 10.1021/ja00205a001. [DOI] [Google Scholar]
- 161.Allinger N.L., Rahman M., Lii J.H. A molecular mechanics force field (MM3) for alcohols and ethers. J. Am. Chem. Soc. 1990;112:8293–8307. doi: 10.1021/ja00179a012. [DOI] [Google Scholar]
- 162.Stortz C.A. Comparative performance of MM3(92) and two TINKER™ MM3 versions for the modeling of carbohydrates. J. Comput. Chem. 2005;26:471–483. doi: 10.1002/jcc.20185. [DOI] [PubMed] [Google Scholar]
- 163.Stortz C.A., Johnson G.P., French A.D., Csonka G.I. Comparison of different force fields for the study of disaccharides. Carbohydr. Res. 2009;344:2217–2228. doi: 10.1016/j.carres.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 164.Taha H.A., Richards M.R., Lowary T.L. Conformational analysis of furanoside-containing mono- and oligosaccharides. Chem. Rev. 2013;113:1851–1876. doi: 10.1021/cr300249c. [DOI] [PubMed] [Google Scholar]
- 165.Stortz C.A. Additive effects in the modeling of oligosaccharides with mm3 at high dielectric constants: An approach to the ‘multiple minimum problem’. Carbohydr. Res. 2006;341:663–671. doi: 10.1016/j.carres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 166.Stortz C.A. mm3 Potential energy surfaces of trisaccharide models of λ-, μ-, and ν-carrageenans. Carbohydr. Res. 2006;341:2531–2542. doi: 10.1016/j.carres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 167.Xiong X.M., Chen Z.Q., Cossins B.P., Xu Z.J., Shao Q., Ding K., Zhu W.L., Shi J.Y. Force fields and scoring functions for carbohydrate simulation. Carbohydr. Res. 2015;401:73–81. doi: 10.1016/j.carres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 168.CHARMM Force Field Files. [(accessed on 31 July 2020)]; Available online: https://www.charmm.org/charmm/resources/charmm-force-fields/#charmm.
- 169.MacKerell A.D., Bashford D., Bellott M., Dunbrack R.L., Evanseck J.D., Field M.J., Fischer S., Gao J., Guo H., Ha S., et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 170.Mackerell A.D., Jr., Feig M., Brooks C.L., III Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 171.Guvench O., Hatcher E., Venable R.M., Pastor R.W., MacKerell A.D. CHARMM Additive All-Atom Force Field for Glycosidic Linkages between Hexopyranoses. J. Chem. Theory Comput. 2009;5:2353–2370. doi: 10.1021/ct900242e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guvench O., Greene S.N., Kamath G., Brady J.W., Venable R.M., Pastor R.W., Mackerell A.D., Jr. Additive empirical force field for hexopyranose monosaccharides. J. Comput. Chem. 2008;29:2543–2564. doi: 10.1002/jcc.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Raman E.P., Guvench O., MacKerell A.D. CHARMM Additive All-Atom Force Field for Glycosidic Linkages in Carbohydrates Involving Furanoses. J. Phys. Chem. B. 2010;114:12981–12994. doi: 10.1021/jp105758h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guvench O., Mallajosyula S.S., Raman E.P., Hatcher E., Vanommeslaeghe K., Foster T.J., Jamison F.W., MacKerell A.D. CHARMM Additive All-Atom Force Field for Carbohydrate Derivatives and Its Utility in Polysaccharide and Carbohydrate—Protein Modeling. J. Chem. Theory Comput. 2011;7:3162–3180. doi: 10.1021/ct200328p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mallajosyula S.S., Guvench O., Hatcher E., MacKerell A.D. CHARMM Additive All-Atom Force Field for Phosphate and Sulfate Linked to Carbohydrates. J. Chem. Theory Comput. 2012;8:759–776. doi: 10.1021/ct200792v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cloutier T., Sudrik C., Sathish H.A., Trout B.L. Kirkwood–Buff-Derived Alcohol Parameters for Aqueous Carbohydrates and Their Application to Preferential Interaction Coefficient Calculations of Proteins. J. Phys. Chem. B. 2018;122:9350–9360. doi: 10.1021/acs.jpcb.8b07623. [DOI] [PubMed] [Google Scholar]
- 177.Kirschner K.N., Yongye A.B., Tschampel S.M., González-Outeiriño J., Daniels C.R., Foley B.L., Woods R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008;29:622–655. doi: 10.1002/jcc.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tessier M.B., DeMarco M.L., Yongye A.B., Woods R.J. Extension of the GLYCAM06 biomolecular force field to lipids, lipid bilayers and glycolipids. Mol. Simul. 2008;34:349–364. doi: 10.1080/08927020701710890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.DeMarco M.L., Woods R.J. Atomic-resolution conformational analysis of the GM3 ganglioside in a lipid bilayer and its implications for ganglioside-protein recognition at membrane surfaces. Glycobiology. 2009;19:344–355. doi: 10.1093/glycob/cwn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.DeMarco M.L. Molecular Dynamics Simulations of Membrane- and Protein-Bound Glycolipids Using GLYCAM. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 379–390. [DOI] [PubMed] [Google Scholar]
- 181.Kirschner K.N., Lins R.D., Maass A., Soares T.A. A Glycam-Based Force Field for Simulations of Lipopolysaccharide Membranes: Parametrization and Validation. J. Chem. Theory Comput. 2012;8:4719–4731. doi: 10.1021/ct300534j. [DOI] [PubMed] [Google Scholar]
- 182.Singh A., Tessier M.B., Pederson K., Wang X., Venot A.P., Boons G.-J., Prestegard J.H., Woods R.J. Extension and validation of the GLYCAM force field parameters for modeling glycosaminoglycans. Can. J. Chem. 2016;94:927–935. doi: 10.1139/cjc-2015-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lins R.D., Hünenberger P.H. A new GROMOS force field for hexopyranose-based carbohydrates. J. Comput. Chem. 2005;26:1400–1412. doi: 10.1002/jcc.20275. [DOI] [PubMed] [Google Scholar]
- 184.Hansen H.S., Hünenberger P.H. A reoptimized GROMOS force field for hexopyranose-based carbohydrates accounting for the relative free energies of ring conformers, anomers, epimers, hydroxymethyl rotamers, and glycosidic linkage conformers. J. Comput. Chem. 2011;32:998–1032. doi: 10.1002/jcc.21675. [DOI] [PubMed] [Google Scholar]
- 185.Pontes F.J.S., Rusu V.H., Soares T.A., Lins R.D. The Effect of Temperature, Cations, and Number of Acyl Chains on the Lamellar to Non-Lamellar Transition in Lipid-A Membranes: A Microscopic View. J. Chem. Theory Comput. 2012;8:3830–3838. doi: 10.1021/ct300084v. [DOI] [PubMed] [Google Scholar]
- 186.Pol-Fachin L., Rusu V.H., Verli H., Lins R.D. GROMOS 53A6GLYC, an Improved GROMOS Force Field for Hexopyranose-Based Carbohydrates. J. Chem. Theory Comput. 2012;8:4681–4690. doi: 10.1021/ct300479h. [DOI] [PubMed] [Google Scholar]
- 187.Pol-Fachin L., Verli H., Lins R.D. Extension and validation of the GROMOS 53A6glyc parameter set for glycoproteins. J. Comput. Chem. 2014;35:2087–2095. doi: 10.1002/jcc.23721. [DOI] [PubMed] [Google Scholar]
- 188.Plazinski W., Lonardi A., Hünenberger P.H. Revision of the GROMOS 56A6CARBO force field: Improving the description of ring-conformational equilibria in hexopyranose-based carbohydrates chains. J. Comput. Chem. 2016;37:354–365. doi: 10.1002/jcc.24229. [DOI] [PubMed] [Google Scholar]
- 189.Naumov V.S., Ignatov S.K. Modification of 56ACARBO force field for molecular dynamic calculations of chitosan and its derivatives. J. Mol. Model. 2017;23:244. doi: 10.1007/s00894-017-3421-x. [DOI] [PubMed] [Google Scholar]
- 190.Panczyk K., Gaweda K., Drach M., Plazinski W. Extension of the GROMOS 56a6CARBO/CARBO_R Force Field for Charged, Protonated, and Esterified Uronates. J. Phys. Chem. B. 2018;122:3696–3710. doi: 10.1021/acs.jpcb.7b11548. [DOI] [PubMed] [Google Scholar]
- 191.Nester K., Gaweda K., Plazinski W. A GROMOS Force Field for Furanose-Based Carbohydrates. J. Chem. Theory Comput. 2019;15:1168–1186. doi: 10.1021/acs.jctc.8b00838. [DOI] [PubMed] [Google Scholar]
- 192.Pol-Fachin L., Fernandes C.L., Verli H. GROMOS96 43a1 performance on the characterization of glycoprotein conformational ensembles through molecular dynamics simulations. Carbohydr. Res. 2009;344:491–500. doi: 10.1016/j.carres.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 193.Fernandes C.L., Sachett L.G., Pol-Fachin L., Verli H. GROMOS96 43a1 performance in predicting oligosaccharide conformational ensembles within glycoproteins. Carbohydr. Res. 2010;345:663–671. doi: 10.1016/j.carres.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 194.Kony D., Damm W., Stoll S., Van Gunsteren W.F. An improved OPLS–AA force field for carbohydrates. J. Comput. Chem. 2002;23:1416–1429. doi: 10.1002/jcc.10139. [DOI] [PubMed] [Google Scholar]
- 195.Damm W., Frontera A., Tirado–Rives J., Jorgensen W.L. OPLS all-atom force field for carbohydrates. J. Comput. Chem. 1997;18:1955–1970. doi: 10.1002/(SICI)1096-987X(199712)18:16<1955::AID-JCC1>3.0.CO;2-L. [DOI] [Google Scholar]
- 196.Jorgensen W.L., Maxwell D.S., Tirado-Rives J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996;118:11225–11236. doi: 10.1021/ja9621760. [DOI] [Google Scholar]
- 197.Jamali S.H., Westen T.V., Moultos O.A., Vlugt T.J.H. Optimizing Nonbonded Interactions of the OPLS Force Field for Aqueous Solutions of Carbohydrates: How to Capture Both Thermodynamics and Dynamics. J. Chem. Theory Comput. 2018;14:6690–6700. doi: 10.1021/acs.jctc.8b00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Patel D.S., He X., MacKerell A.D. Polarizable Empirical Force Field for Hexopyranose Monosaccharides Based on the Classical Drude Oscillator. J. Phys. Chem. B. 2015;119:637–652. doi: 10.1021/jp412696m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang M., Aytenfisu A.H., MacKerell A.D. Proper balance of solvent-solute and solute-solute interactions in the treatment of the diffusion of glucose using the Drude polarizable force field. Carbohydr. Res. 2018;457:41–50. doi: 10.1016/j.carres.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jana M., MacKerell A.D. CHARMM Drude Polarizable Force Field for Aldopentofuranoses and Methyl-aldopentofuranosides. J. Phys. Chem. B. 2015;119:7846–7859. doi: 10.1021/acs.jpcb.5b01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pandey P., Aytenfisu A.H., MacKerell A.D., Mallajosyula S.S. Drude Polarizable Force Field Parametrization of Carboxylate and N-Acetyl Amine Carbohydrate Derivatives. J. Chem. Theory Comput. 2019;15:4982–5000. doi: 10.1021/acs.jctc.9b00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.He X., Lopes P.E.M., MacKerell A.D., Jr. Polarizable Empirical Force Field for Acyclic Polyalcohols Based on the Classical Drude Oscillator. Biopolymers. 2013;99:724–738. doi: 10.1002/bip.22286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aytenfisu A.H., Yang M., MacKerell A.D. CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses. J. Chem. Theory Comput. 2018;14:3132–3143. doi: 10.1021/acs.jctc.8b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.López C.A., Rzepiela A.J., De Vries A.H., Dijkhuizen L., Hünenberger P.H., Marrink S.J. Martini Coarse-Grained Force Field: Extension to Carbohydrates. J. Chem. Theory Comput. 2009;5:3195–3210. doi: 10.1021/ct900313w. [DOI] [PubMed] [Google Scholar]
- 205.Schmalhorst P.S., Deluweit F., Scherrers R., Heisenberg C.-P., Sikora M. Overcoming the Limitations of the MARTINI Force Field in Simulations of Polysaccharides. J. Chem. Theory Comput. 2017;13:5039–5053. doi: 10.1021/acs.jctc.7b00374. [DOI] [PubMed] [Google Scholar]
- 206.Shivgan A., Marzinek J.K., Huber R.G., Krah A., Henchman R.H., Matsudaira P.T., Verma C.S., Bond P.J. Extending the Martini Coarse-Grained Forcefield to N-Glycans. J. Chem. Inf. Model. 2020;60:3864–3883. doi: 10.1021/acs.jcim.0c00495. [DOI] [PubMed] [Google Scholar]
- 207.López C.A., Sovova Z., van Eerden F.J., De Vries A.H., Marrink S.J. Martini Force Field Parameters for Glycolipids. J. Chem. Theory Comput. 2013;9:1694–1708. doi: 10.1021/ct3009655. [DOI] [PubMed] [Google Scholar]
- 208.Rusu V.H., Baron R., Lins R.D. PITOMBA: Parameter Interface for Oligosaccharide Molecules Based on Atoms. J. Chem. Theory Comput. 2014;10:5068–5080. doi: 10.1021/ct500455u. [DOI] [PubMed] [Google Scholar]
- 209.Spiwok V., Lipovová P., Skálová T., Vondráčková E., Dohnálek J., Hašek J., Králová B. Modelling of carbohydrate–aromatic interactions: Ab initio energeticsand force field performance. J. Comput. Aided Mol. Des. 2005;19:887–901. doi: 10.1007/s10822-005-9033-z. [DOI] [PubMed] [Google Scholar]
- 210.Wimmerová M., Kozmon S., Nečasová I., Mishra S.K., Komárek J., Koča J. Stacking interactions between carbohydrate and protein quantified by combination of theoretical and experimental methods. PLoS ONE. 2012;7:e46032. doi: 10.1371/journal.pone.0046032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Makeneni S., Thieker D.F., Woods R.J. Applying Pose Clustering and MD Simulations To Eliminate False Positives in Molecular Docking. J. Chem. Inf. Model. 2018;58:605–614. doi: 10.1021/acs.jcim.7b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang S.-Y., Grinter S.Z., Zou X. Scoring functions and their evaluation methods for protein–ligand docking: Recent advances and future directions. Phys. Chem. Chem. Phys. 2010;12:12899–12908. doi: 10.1039/c0cp00151a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vandenbussche S., Díaz D., Fernández-Alonso M.C., Pan W., Vincent S.P., Cuevas G., Cañada F.J., Jiménez-Barbero J., Bartik K. Aromatic–Carbohydrate Interactions: An NMR and Computational Study of Model Systems. Chem. Eur. J. 2008;14:7570–7578. doi: 10.1002/chem.200800247. [DOI] [PubMed] [Google Scholar]
- 214.Hill A.D., Reilly P.J. A Gibbs free energy correlation for automated docking of carbohydrates. J. Comput. Chem. 2008;29:1131–1141. doi: 10.1002/jcc.20873. [DOI] [PubMed] [Google Scholar]
- 215.Malik A., Baig M.H., Manavalan B. Protein-Carbohydrate Interactions. In: Ranganathan S., Gribskov M., Nakai K., Schönbach C., editors. Encyclopedia of Bioinformatics and Computational Biology. Volume 3. Elsevier; Oxford, UK: 2019. pp. 666–677. [Google Scholar]
- 216.Samsonov S.A., Teyra J., Pisabarro M.T. Docking glycosaminoglycans to proteins: Analysis of solvent inclusion. J. Comput. Aided Mol. Des. 2011;25:477–489. doi: 10.1007/s10822-011-9433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Samsonov S.A., Gehrcke J.-P., Pisabarro M.T. Flexibility and Explicit Solvent in Molecular-Dynamics-Based Docking of Protein–Glycosaminoglycan Systems. J. Chem. Inf. Model. 2014;54:582–592. doi: 10.1021/ci4006047. [DOI] [PubMed] [Google Scholar]
- 218.Gerlits O.O., Coates L., Woods R.J., Kovalevsky A. Mannobiose Binding Induces Changes in Hydrogen Bonding and Protonation States of Acidic Residues in Concanavalin a As Revealed by Neutron Crystallography. Biochemistry. 2017;56:4747–4750. doi: 10.1021/acs.biochem.7b00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuriev E., Holien J., Ramsland P.A. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. J. Mol. Recognit. 2015;28:581–604. doi: 10.1002/jmr.2471. [DOI] [PubMed] [Google Scholar]
- 220.Mishra N.K., Kříž Z., Wimmerová M., Koča J. Recognition of selected monosaccharides by Pseudomonas aeruginosa Lectin II analyzed by molecular dynamics and free energy calculations. Carbohydr. Res. 2010;345:1432–1441. doi: 10.1016/j.carres.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 221.Mishra S.K., Adam J., Wimmerová M., Koča J. In Silico Mutagenesis and Docking Study of Ralstonia solanacearum RSL Lectin: Performance of Docking Software to Predict Saccharide Binding. J. Chem. Inf. Model. 2012;52:1250–1261. doi: 10.1021/ci200529n. [DOI] [PubMed] [Google Scholar]
- 222.Raghuraman A., Mosier P.D., Desai U.R. Finding a Needle in a Haystack: Development of a Combinatorial Virtual Screening Approach for Identifying High Specificity Heparin/Heparan Sulfate Sequence(s) J. Med. Chem. 2006;49:3553–3562. doi: 10.1021/jm060092o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Samsonov S.A., Pisabarro M.T. Computational analysis of interactions in structurally available protein–glycosaminoglycan complexes. Glycobiology. 2016;26:850–861. doi: 10.1093/glycob/cww055. [DOI] [PubMed] [Google Scholar]
- 224.Gehrcke J.-P., Pisabarro M.T. Identification and characterization of a glycosaminoglycan binding site on interleukin-10 via molecular simulation methods. J. Mol. Graph. Modell. 2015;62:97–104. doi: 10.1016/j.jmgm.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 225.Agostino M., Sandrin M.S., Thompson P.E., Yuriev E., Ramsland P.A. In silico analysis of antibody–carbohydrate interactions and its application to xenoreactive antibodies. Mol. Immunol. 2009;47:233–246. doi: 10.1016/j.molimm.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 226.Lee J., Cheng X., Swails J.M., Yeom M.S., Eastman P.K., Lemkul J.A., Wei S., Buckner J., Jeong J.C., Qi Y., et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee J., Hitzenberger M., Rieger M., Kern N.R., Zacharias M., Im W. CHARMM-GUI supports the Amber force fields. J. Chem. Phys. 2020;153:035103. doi: 10.1063/5.0012280. [DOI] [PubMed] [Google Scholar]
- 228.Labonte J.W., Adolf-Bryfogle J., Schief W.R., Gray J.J. Residue-centric modeling and design of saccharide and glycoconjugate structures. J. Comput. Chem. 2017;38:276–287. doi: 10.1002/jcc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Whitmore E.K., Vesenka G., Sihler H., Guvench O. Efficient Construction of Atomic-Resolution Models of Non-Sulfated Chondroitin Glycosaminoglycan Using Molecular Dynamics Data. Biomolecules. 2020;10:537. doi: 10.3390/biom10040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Park S.J., Lee J., Qi Y.F., Kern N.R., Lee H.S., Jo S., Joung I., Joo K., Lee J., Im W. CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology. 2019;29:320–331. doi: 10.1093/glycob/cwz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lemmin T., Soto C. Glycosylator: A Python framework for the rapid modeling of glycans. BMC Bioinf. 2019;20:513. doi: 10.1186/s12859-019-3097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Alford R.F., Leaver-Fay A., Jeliazkov J.R., O’Meara M.J., DiMaio F.P., Park H., Shapovalov M.V., Renfrew P.D., Mulligan V.K., Kappel K., et al. The Rosetta All-Atom Energy Function for Macromolecular Modeling and Design. J. Chem. Theory Comput. 2017;13:3031–3048. doi: 10.1021/acs.jctc.7b00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roy Burman S.S., Nance M.L., Jeliazkov J.R., Labonte J.W., Lubin J.H., Biswas N., Gray J.J. Novel sampling strategies and a coarse-grained score function for docking homomers, flexible heteromers, and oligosaccharides using Rosetta in CAPRI rounds 37–45. Proteins Struct. Funct. Bioinf. 2020;88:973–985. doi: 10.1002/prot.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Leman J.K., Weitzner B.D., Lewis S.M., Adolf-Bryfogle J., Alam N., Alford R.F., Aprahamian M., Baker D., Barlow K.A., Barth P., et al. Macromolecular modeling and design in Rosetta: Recent methods and frameworks. Nat. Methods. 2020;17:665–680. doi: 10.1038/s41592-020-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Arroyuelo A., Vila J.A., Martin O.A. Azahar: A PyMOL plugin for construction, visualization and analysis of glycan molecules. J. Comput. Aided Mol. Des. 2016;30:619–624. doi: 10.1007/s10822-016-9944-x. [DOI] [PubMed] [Google Scholar]
- 236.Rosen J., Miguet L., Pérez S. Shape: Automatic conformation prediction of carbohydrates using a genetic algorithm. J. Cheminf. 2009;1:16. doi: 10.1186/1758-2946-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Frank M., Bohne-Lang A., Wetter T., Von Der Lieth C.-W. Rapid Generation of a Representative Ensemble of N-Glycan Conformations. In Silico Biol. 2002;2:427–439. [PubMed] [Google Scholar]
- 238.Nahmany A., Strino F., Rosen J., Kemp G.J.L., Nyholm P.-G. The use of a genetic algorithm search for molecular mechanics (MM3)-based conformational analysis of oligosaccharides. Carbohydr. Res. 2005;340:1059–1064. doi: 10.1016/j.carres.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 239.Xia J., Daly R.P., Chuang F.-C., Parker L., Jensen J.H., Margulis C.J. Sugar Folding: A Novel Structural Prediction Tool for Oligosaccharides and Polysaccharides 1. J. Chem. Theory Comput. 2007;3:1620–1628. doi: 10.1021/ct700033y. [DOI] [PubMed] [Google Scholar]
- 240.Xia J., Daly R.P., Chuang F.-C., Parker L., Jensen J.H., Margulis C.J. Sugar Folding: A Novel Structural Prediction Tool for Oligosaccharides and Polysaccharides 2. J. Chem. Theory Comput. 2007;3:1629–1643. doi: 10.1021/ct700034q. [DOI] [PubMed] [Google Scholar]
- 241.Xia J., Margulis C. A tool for the prediction of structures of complex sugars. J. Biomol. NMR. 2008;42:241–256. doi: 10.1007/s10858-008-9279-6. [DOI] [PubMed] [Google Scholar]
- 242.Xia J., Margulis C.J. Computational Study of the Conformational Structures of Saccharides in Solution Based on J Couplings and the “Fast Sugar Structure Prediction Software”. Biomacromolecules. 2009;10:3081–3088. doi: 10.1021/bm900756q. [DOI] [PubMed] [Google Scholar]
- 243.Bohne-Lang A., Von Der Lieth C.-W. GlyProt: In silico glycosylation of proteins. Nucleic Acids Res. 2005;33:W214–W219. doi: 10.1093/nar/gki385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liebschner D., Afonine P.V., Baker M.L., Bunkoczi G., Chen V.B., Croll T.I., Hintze B., Hung L.-W., Jain S., McCoy A.J., et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D: Struct. Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tessier M.B., Grant O.C., Heimburg-Molinaro J., Smith D., Jadey S., Gulick A.M., Glushka J., Deutscher S.L., Rittenhouse-Olson K., Woods R.J. Computational screening of the human TF-glycome provides a structural definition for the specificity of anti-tumor antibody JAA-F11. PLoS ONE. 2013;8:e54874. doi: 10.1371/journal.pone.0054874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Grant O.C., Smith H.M., Firsova D., Fadda E., Woods R.J. Presentation, presentation, presentation! Molecular-level insight into linker effects on glycan array screening data. Glycobiology. 2014;24:17–25. doi: 10.1093/glycob/cwt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Grant O.C., Woods R.J. Recent advances in employing molecular modelling to determine the specificity of glycan-binding proteins. Curr. Opin. Struct. Biol. 2014;28:47–55. doi: 10.1016/j.sbi.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Grant O.C., Xue X., Ra D., Khatamian A., Foley B.L., Woods R.J. Gly-Spec: A webtool for predicting glycan specificity by integrating glycan array screening data and 3D structure. Glycobiology. 2016;26:1027–1028. doi: 10.1093/glycob/cww094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Grant O.C., Tessier M.B., Meche L., Mahal L.K., Foley B.L., Woods R.J. Combining 3D structure with glycan array data provides insight into the origin of glycan specificity. Glycobiology. 2016;26:772–783. doi: 10.1093/glycob/cww020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jo S., Kim T., Im W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE. 2007;2:e880. doi: 10.1371/journal.pone.0000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jo S., Lim J.B., Klauda J.B., Im W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009;97:50–58. doi: 10.1016/j.bpj.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu E.L., Cheng X., Jo S., Rui H., Song K.C., Dávila-Contreras E.M., Qi Y., Lee J., Monje-Galvan V., Venable R.M., et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014;35:1997–2004. doi: 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gao Y., Lee J., Widmalm G., Im W. Modeling and Simulation of Bacterial Outer Membranes with Lipopolysaccharides and Enterobacterial Common Antigen. J. Phys. Chem. B. 2020;124:5948–5956. doi: 10.1021/acs.jpcb.0c03353. [DOI] [PubMed] [Google Scholar]
- 254.Baltoumas F.A., Hamodrakas S.J., Iconomidou V.A. The gram-negative outer membrane modeler: Automated building of lipopolysaccharide-rich bacterial outer membranes in four force fields. J. Comput. Chem. 2019;40:1727–1734. doi: 10.1002/jcc.25823. [DOI] [PubMed] [Google Scholar]
- 255.Krüger D.M., Kamerlin S.C.L. Micelle Maker: An Online Tool for Generating Equilibrated Micelles as Direct Input for Molecular Dynamics Simulations. ACS Omega. 2017;2:4524–4530. doi: 10.1021/acsomega.7b00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dashti H., Westler W.M., Wedell J.R., Demler O.V., Eghbalnia H.R., Markley J.L., Mora S. Probabilistic identification of saccharide moieties in biomolecules and their protein complexes. Sci. Data. 2020;7:210. doi: 10.1038/s41597-020-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Woods R. Time-Proof Perspectives on Glycoscience—Beilstein Glyco-Bioinformatics Symposium, Limburg, Germany, 25–27 June 2019. Beilstein-Institut; Frankfurt, Germany: 2019. GlyFinder and GlyProbity: New Online Tools for Locating and Curating Carbohydrate Structures in wwPDB; pp. 82–83. [Google Scholar]
- 258.Woods R.J., Montgomery D.W., Young J., Grant O.C., Wentworth D., Foley B.L. Tools to Find Glycoproteins in the Protein Data Bank and Generate Realistic 3D Structures for Them. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.09703. [DOI] [Google Scholar]
- 259.Lütteke T., Frank M., von der Lieth C.-W. Data mining the protein data bank: Automatic detection and assignment of carbohydrate structures. Carbohydr. Res. 2004;339:1015–1020. doi: 10.1016/j.carres.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 260.Jo S., Song K.C., Desaire H., MacKerell A.D., Jr., Im W. Glycan reader: Automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J. Comput. Chem. 2011;32:3135–3141. doi: 10.1002/jcc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Park S.-J., Lee J., Patel D.S., Ma H., Lee H.S., Jo S., Im W. Glycan Reader is improved to recognize most sugar types and chemical modifications in the Protein Data Bank. Bioinformatics. 2017;33:3051–3057. doi: 10.1093/bioinformatics/btx358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Danne R., Poojari C., Martinez-Seara H., Rissanen S., Lolicato F., Róg T., Vattulainen I. doGlycans–Tools for Preparing Carbohydrate Structures for Atomistic Simulations of Glycoproteins, Glycolipids, and Carbohydrate Polymers for GROMACS. J. Chem. Inf. Model. 2017;57:2401–2406. doi: 10.1021/acs.jcim.7b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bohne A., Lang E., von der Lieth C.-W. W3-SWEET: Carbohydrate Modeling by Internet. J. Mol. Model. 1998;4:33–43. doi: 10.1007/s008940050068. [DOI] [Google Scholar]
- 264.Bohne A., Lang E., von der Lieth C.W. SWEET—WWW-based rapid 3D construction of oligo- and polysaccharides. Bioinformatics. 1999;15:767–768. doi: 10.1093/bioinformatics/15.9.767. [DOI] [PubMed] [Google Scholar]
- 265.Chernyshov I.Y., Toukach P.V. REStLESS: Automated translation of glycan sequences from residue-based notation to SMILES and atomic coordinates. Bioinformatics. 2018;34:2679–2681. doi: 10.1093/bioinformatics/bty168. [DOI] [PubMed] [Google Scholar]
- 266.Engelsen S.B., Cros S., Mackie W., Pérez S. A molecular builder for carbohydrates: Application to polysaccharides and complex carbohydrates. Biopolymers. 1996;39:417–433. doi: 10.1002/(SICI)1097-0282(199609)39:3<417::AID-BIP13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 267.Engelsen S.B., Hansen P.I., Pérez S. POLYS 2.0: An open source software package for building three-dimensional structures of polysaccharides. Biopolymers. 2014;101:733–743. doi: 10.1002/bip.22449. [DOI] [PubMed] [Google Scholar]
- 268.Kuttel M., Mao Y., Widmalm G., Lundborg M. CarbBuilder: An Adjustable Tool for Building 3D Molecular Structures of Carbohydrates for Molecular Simulation; Proceedings of the 2011 IEEE Seventh International Conference on eScience; Stockholm, Sweden. 5–8 December 2011; pp. 395–402. [Google Scholar]
- 269.Kuttel M.M., Stahle J., Widmalm G. CarbBuilder: Software for building molecular models of complex oligo- and polysaccharide structures. J. Comput. Chem. 2016;37:2098–2105. doi: 10.1002/jcc.24428. [DOI] [PubMed] [Google Scholar]
- 270.Clerc O., Mariethoz J., Rivet A., Lisacek F., Pérez S., Ricard-Blum S. A pipeline to translate glycosaminoglycan sequences into 3D models. Application to the exploration of glycosaminoglycan conformational space. Glycobiology. 2019;29:36–44. doi: 10.1093/glycob/cwy084. [DOI] [PubMed] [Google Scholar]
- 271.Singh A., Montgomery D., Xue X., Foley B.L., Woods R.J. GAG Builder: A web-tool for modeling 3D structures of glycosaminoglycans. Glycobiology. 2019;29:515–518. doi: 10.1093/glycob/cwz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kerzmann A., Neumann D., Kohlbacher O. SLICK—Scoring and Energy Functions for Protein−Carbohydrate Interactions. J. Chem. Inf. Model. 2006;46:1635–1642. doi: 10.1021/ci050422y. [DOI] [PubMed] [Google Scholar]
- 273.Kerzmann A., Fuhrmann J., Kohlbacher O., Neumann D. BALLDock/SLICK: A New Method for Protein-Carbohydrate Docking. J. Chem. Inf. Model. 2008;48:1616–1625. doi: 10.1021/ci800103u. [DOI] [PubMed] [Google Scholar]
- 274.van Zundert G.C.P., Rodrigues J.P.G.L.M., Trellet M., Schmitz C., Kastritis P.L., Karaca E., Melquiond A.S.J., van Dijk M., de Vries S.J., Bonvin A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 275.Mottarella S.E., Beglov D., Beglova N., Nugent M.A., Kozakov D., Vajda S. Docking Server for the Identification of Heparin Binding Sites on Proteins. J. Chem. Inf. Model. 2014;54:2068–2078. doi: 10.1021/ci500115j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sankaranarayanan N.V., Nagarajan B., Desai U.R. So you think computational approaches to understanding glycosaminoglycan–protein interactions are too dry and too rigid? Think again! Curr. Opin. Struct. Biol. 2018;50:91–100. doi: 10.1016/j.sbi.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Griffith A.R., Rogers C.J., Miller G.M., Abrol R., Hsieh-Wilson L.C., Goddard W.A. Predicting glycosaminoglycan surface protein interactions and implications for studying axonal growth. Proc. Natl. Acad. Sci. USA. 2017;114:13697–13702. doi: 10.1073/pnas.1715093115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Eric B., Jed B., Neha G., Vito F. GlycoTorch Vina: Improved Docking of Sulfated Sugars Using QM-derived Scoring Functions. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12279251.v1. [DOI] [Google Scholar]
- 279.Frank M. Conformational Analysis of Oligosaccharides and Polysaccharides Using Molecular Dynamics Simulations. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 359–377. [DOI] [PubMed] [Google Scholar]
- 280.Makeneni S., Foley B.L., Woods R.J. BFMP: A Method for Discretizing and Visualizing Pyranose Conformations. J. Chem. Inf. Model. 2014;54:2744–2750. doi: 10.1021/ci500325b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chalmers G., Glushka J.N., Foley B.L., Woods R.J., Prestegard J.H. Direct NOE simulation from long MD trajectories. J. Magn. Reson. 2016;265:1–9. doi: 10.1016/j.jmr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lee H.S., Jo S., Mukherjee S., Park S.-J., Skolnick J., Lee J., Im W. GS-align for glycan structure alignment and similarity measurement. Bioinformatics. 2015;31:2653–2659. doi: 10.1093/bioinformatics/btv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lütteke T., Frank M., von der Lieth C.-W. Carbohydrate Structure Suite (CSS): Analysis of carbohydrate 3D structures derived from the PDB. Nucleic Acids Res. 2005;33:D242–D246. doi: 10.1093/nar/gki013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rojas-Macias M.A., Lütteke T. Statistical Analysis of Amino Acids in the Vicinity of Carbohydrate Residues Performed by GlyVicinity. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 215–226. [DOI] [PubMed] [Google Scholar]
- 285.Marchetti R., Perez S., Arda A., Imberty A., Jimenez-Barbero J., Silipo A., Molinaro A. Rules of Engagement of Protein-Glycoconjugate Interactions: A Molecular View Achievable by using NMR Spectroscopy and Molecular Modeling. ChemistryOpen. 2016;5:274–296. doi: 10.1002/open.201600024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yu Y., Delbianco M. Conformational Studies of Oligosaccharides. Chem. Eur. J. 2020;26:9814–9825. doi: 10.1002/chem.202001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Imberty A., Pérez S. Structure, Conformation, and Dynamics of Bioactive Oligosaccharides: Theoretical Approaches and Experimental Validations. Chem. Rev. 2000;100:4567–4588. doi: 10.1021/cr990343j. [DOI] [PubMed] [Google Scholar]
- 288.Wormald M.R., Petrescu A.J., Pao Y.-L., Glithero A., Elliott T., Dwek R.A. Conformational Studies of Oligosaccharides and Glycopeptides: Complementarity of NMR, X-ray Crystallography, and Molecular Modelling. Chem. Rev. 2002;102:371–386. doi: 10.1021/cr990368i. [DOI] [PubMed] [Google Scholar]
- 289.Lutteke T. Analysis and validation of carbohydrate three-dimensional structures. Acta Crystallogr. Sect. D Struct. Biol. 2009;65:156–168. doi: 10.1107/S0907444909001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Blanco Capurro J.I., Di Paola M., Gamarra M.D., Martí M.A., Modenutti C.P. An efficient use of X-ray information, homology modeling, molecular dynamics and knowledge-based docking techniques to predict protein–monosaccharide complexes. Glycobiology. 2019;29:124–136. doi: 10.1093/glycob/cwy102. [DOI] [PubMed] [Google Scholar]
- 291.Coxon B. Advances in Carbohydrate Chemistry and Biochemistry. Volume 62. Academic Press; Cambridge, MA, USA: 2009. Chapter 3 Developments in the Karplus Equation as they Relate to the NMR Coupling Constants of Carbohydrates; pp. 17–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Widmalm G. A perspective on the primary and three-dimensional structures of carbohydrates. Carbohydr. Res. 2013;378:123–132. doi: 10.1016/j.carres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 293.Slynko V., Schubert M., Numao S., Kowarik M., Aebi M., Allain F.H.T. NMR Structure Determination of a Segmentally Labeled Glycoprotein Using In vitro Glycosylation. J. Am. Chem. Soc. 2009;131:1274–1281. doi: 10.1021/ja808682v. [DOI] [PubMed] [Google Scholar]
- 294.Soares P.A.G., Queiroz I.N.L., Pomin V.H. NMR structural biology of sulfated glycans. J. Biomol. Struct. Dyn. 2017;35:1069–1084. doi: 10.1080/07391102.2016.1171165. [DOI] [PubMed] [Google Scholar]
- 295.van Beusekom B., Lutteke T., Joosten R.P. Making glycoproteins a little bit sweeter with PDB-REDO. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018;74:463–472. doi: 10.1107/S2053230X18004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Frenz B., Ramisch S., Borst A.J., Walls A.C., Adolf-Bryfogle J., Schief W.R., Veesler D., DiMaio F. Automatically Fixing Errors in Glycoprotein Structures with Rosetta. Structure. 2019;27:134–139. doi: 10.1016/j.str.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bagdonas H., Ungar D., Agirre J. Leveraging glycomics data in glycoprotein 3D structure validation with Privateer. Beilstein J. Org. Chem. 2020;16:2523–2533. doi: 10.3762/bjoc.16.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Casañal A., Lohkamp B., Emsley P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 2020;29:1055–1064. doi: 10.1002/pro.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Agirre J., Davies G., Wilson K., Cowtan K. Carbohydrate anomalies in the PDB. Nat. Chem. Biol. 2015;11:303. doi: 10.1038/nchembio.1798. [DOI] [PubMed] [Google Scholar]
- 300.Hendrickx J., Tran V., Sanejouand Y.-H. Numerous severely twisted N-acetylglucosamine conformations found in the protein databank. Proteins Struct. Funct. Bioinf. 2020;88:1376–1383. doi: 10.1002/prot.25957. [DOI] [PubMed] [Google Scholar]
- 301.Atanasova M., Bagdonas H., Agirre J. Structural glycobiology in the age of electron cryo-microscopy. Curr. Opin. Struct. Biol. 2020;62:70–78. doi: 10.1016/j.sbi.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 302.Agirre J. Strategies for carbohydrate model building, refinement and validation. Acta Crystallogr. Sect. D Struct. Biol. 2017;73:171–186. doi: 10.1107/S2059798316016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pallesen J., Murin C.D., De Val N., Cottrell C.A., Hastie K.M., Turner H.L., Fusco M.L., Flyak A.I., Zeitlin L., Crowe J.E., et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat. Microbiol. 2016;1:16128. doi: 10.1038/nmicrobiol.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lee J.H., Ozorowski G., Ward A.B. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016;351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 306.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bubb W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A. 2003;19A:1–19. doi: 10.1002/cmr.a.10080. [DOI] [Google Scholar]
- 308.Ardá A., Coelho H., de Toro B.F., Galante S., Gimeno A., Poveda A., Sastre J., Unione L., Valverde P., Cañada F.J., et al. Carbohydrate Chemistry. Volume 42. The Royal Society of Chemistry; London, UK: 2017. Recent advances in the application of NMR methods to uncover the conformation and recognition features of glycans; pp. 47–82. [Google Scholar]
- 309.Ardá A., Jiménez-Barbero J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. 2018;54:4761–4769. doi: 10.1039/C8CC01444B. [DOI] [PubMed] [Google Scholar]
- 310.Valverde P., Quintana J.I., Santos J.I., Ardá A., Jiménez-Barbero J. Novel NMR Avenues to Explore the Conformation and Interactions of Glycans. ACS Omega. 2019;4:13618–13630. doi: 10.1021/acsomega.9b01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang M., Angles d’Ortoli T., Säwén E., Jana M., Widmalm G., MacKerell A.D. Delineating the conformational flexibility of trisaccharides from NMR spectroscopy experiments and computer simulations. Phys. Chem. Chem. Phys. 2016;18:18776–18794. doi: 10.1039/C6CP02970A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Säwén E., Hinterholzinger F., Landersjö C., Widmalm G. Conformational flexibility of the pentasaccharide LNF-2 deduced from NMR spectroscopy and molecular dynamics simulations. Org. Biomol. Chem. 2012;10:4577–4585. doi: 10.1039/c2ob25189b. [DOI] [PubMed] [Google Scholar]
- 313.Turupcu A., Blaukopf M., Kosma P., Oostenbrink C. Molecular Conformations of Di-, Tri-, and Tetra-α-(2→8)-Linked Sialic Acid from NMR Spectroscopy and MD Simulations. Int. J. Mol. Sci. 2020;21:30. doi: 10.3390/ijms21010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Frank M., Collins P.M., Peak I.R., Grice I.D., Wilson J.C. An unusual carbohydrate conformation is evident in Moraxella catarrhalis oligosaccharides. Molecules. 2015;20:14234–14253. doi: 10.3390/molecules200814234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Burley S.K., Berman H.M., Kleywegt G.J., Markley J.L., Nakamura H., Velankar S. Protein Data Bank (PDB): The single global macromolecular structure archive. In: Wlodawer A., Dauter Z., Jaskolski M., editors. Protein Crystallography. Humana Press; New York, NY, USA: 2017. pp. 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Groom C.R., Bruno I.J., Lightfoot M.P., Ward S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016;72:171–179. doi: 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.De Meirelles J.L., Nepomuceno F.C., Peña-García J., Schmidt R.R., Pérez-Sánchez H., Verli H. Current Status of Carbohydrates Information in the Protein Data Bank. J. Chem. Inf. Model. 2020;60:684–699. doi: 10.1021/acs.jcim.9b00874. [DOI] [PubMed] [Google Scholar]
- 318.Lütteke T., von der Lieth C.W. Data Mining the PDB for Glyco-Related Data. In: Packer N.H., Karlsson N.G., editors. Glycomics: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2009. pp. 293–310. [DOI] [PubMed] [Google Scholar]
- 319.Agirre J., Davies G.J., Wilson K.S., Cowtan K.D. Carbohydrate structure: The rocky road to automation. Curr. Opin. Struct. Biol. 2017;44:39–47. doi: 10.1016/j.sbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 320.Crispin M., Stuart D.I., Jones E.Y. Building meaningful models of glycoproteins. Nat. Struct. Mol. Biol. 2007;14:354. doi: 10.1038/nsmb0507-354a. [DOI] [PubMed] [Google Scholar]
- 321.Joosten R.P., Lütteke T. Carbohydrate 3D structure validation. Curr. Opin. Struct. Biol. 2017;44:9–17. doi: 10.1016/j.sbi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 322.Speciale G., Thompson A.J., Davies G.J., Williams S.J. Dissecting conformational contributions to glycosidase catalysis and inhibition. Curr. Opin. Struct. Biol. 2014;28:1–13. doi: 10.1016/j.sbi.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fushinobu S. Conformations of the type-1 lacto-N-biose I unit in protein complex structures. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018;74:473–479. doi: 10.1107/S2053230X18006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zardecki C., Shao C., Feng Z., Westbrook J.D., Persikova I., Burley S.K., Young J.Y. Collaborating with Glycoscience Community To Improve Data Representation of Carbohydrates in the Protein Data Bank. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.03289. [DOI] [Google Scholar]
- 325.Van Beusekom B., Wezel N., Hekkelman M.L., Perrakis A., Emsley P., Joosten R.P. Building and rebuilding N-glycans in protein structure models. Acta Crystallogr. Sect. D Struct. Biol. 2019;75:416–425. doi: 10.1107/S2059798319003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lütteke T., von der Lieth C.W. pdb-care (PDB carbohydrate residue check): A program to support annotation of complex carbohydrate structures in PDB files. BMC Bioinf. 2004;5:69. doi: 10.1186/1471-2105-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Brunger A.T., Adams P.D., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.-S., Kuszewski J., Nilges M., Pannu N.S., et al. Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallogr. Sect. D Struct. Biol. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 328.Brunger A.T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 329.Emsley P., Brunger A.T., Lütteke T. Tools to Assist Determination and Validation of Carbohydrate 3D Structure Data. In: Lütteke T., Frank M., editors. Glycoinformatics. Humana Press; New York, NY, USA: 2015. pp. 229–240. [DOI] [PubMed] [Google Scholar]
- 330.Feng Y. Compatible topologies and parameters for NMR structure determination of carbohydrates by simulated annealing. PLoS ONE. 2017;12:e0189700. doi: 10.1371/journal.pone.0189700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Emsley P., Crispin M. Structural analysis of glycoproteins: Building N-linked glycans with Coot. Acta Crystallogr. Sect. D Struct. Biol. 2018;74:256–263. doi: 10.1107/S2059798318005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Agirre J., Iglesias-Fernandez J., Rovira C., Davies G.J., Wilson K.S., Cowtan K.D. Privateer: Software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 2015;22:833–834. doi: 10.1038/nsmb.3115. [DOI] [PubMed] [Google Scholar]
- 333.Vařeková R.S., Jaiswal D., Sehnal D., Ionescu C.-M., Geidl S., Pravda L., Horský V., Wimmerová M., Koča J. MotiveValidator: Interactive web-based validation of ligand and residue structure in biomolecular complexes. Nucleic Acids Res. 2014;42:W227–W233. doi: 10.1093/nar/gku426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sehnal D., Svobodová Vařeková R., Pravda L., Ionescu C.-M., Geidl S., Horský V., Jaiswal D., Wimmerová M., Koča J. ValidatorDB: Database of up-to-date validation results for ligands and non-standard residues from the Protein Data Bank. Nucleic Acids Res. 2015;43:D369–D375. doi: 10.1093/nar/gku1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Aoki-Kinoshita K.F. A Practical Guide to Using Glycomics Databases. 1st ed. Springer; Tokyo, Japan: 2017. 370p [Google Scholar]
- 336.Tsuchiya S., Aoki N.P., Shinmachi D., Matsubara M., Yamada I., Aoki-Kinoshita K.F., Narimatsu H. Implementation of GlycanBuilder to draw a wide variety of ambiguous glycans. Carbohydr. Res. 2017;445:104–116. doi: 10.1016/j.carres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 337.Mehta A.Y., Cummings R.D. GlycoGlyph: A glycan visualizing, drawing and naming application. Bioinformatics. 2020;36:3613–3614. doi: 10.1093/bioinformatics/btaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D., Stanley P., Hart G., Darvill A., Kinoshita T., et al. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25:1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Neelamegham S., Aoki-Kinoshita K., Bolton E., Frank M., Lisacek F., Lütteke T., O’Boyle N., Packer N., Stanley P., Toukach P., et al. Updates to the Symbol Nomenclature For Glycans (SNFG) Guidelines. Glycobiology. 2019;29:620–624. doi: 10.1093/glycob/cwz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lal K., Bermeo R., Perez S. Computational tools for drawing, building and displaying carbohydrates: A visual guide. Beilstein J. Org. Chem. 2020;16:2448–2468. doi: 10.3762/bjoc.16.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Damerell D., Ceroni A., Maass K., Ranzinger R., Dell A., Haslam S.M. The GlycanBuilder and GlycoWorkbench glycoinformatics tools: Updates and new developments. Biol. Chem. 2012;393:1357–1362. doi: 10.1515/hsz-2012-0135. [DOI] [PubMed] [Google Scholar]
- 342.Akune Y., Hosoda M., Kaiya S., Shinmachi D., Aoki-Kinoshita K.F. The RINGS Resource for Glycome Informatics Analysis and Data Mining on the Web. OMICS. 2010;14:475–486. doi: 10.1089/omi.2009.0129. [DOI] [PubMed] [Google Scholar]
- 343.Alocci D., Suchánková P., Costa R., Hory N., Mariethoz J., Svobodová Vařeková R., Toukach P., Lisacek F. SugarSketcher: Quick and intuitive online glycan drawing. Molecules. 2018;23:3206. doi: 10.3390/molecules23123206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cheng K., Zhou Y., Neelamegham S. DrawGlycan-SNFG: A robust tool to render glycans and glycopeptides with fragmentation information. Glycobiology. 2017;27:200–205. doi: 10.1093/glycob/cww115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Cheng K., Pawlowski G., Yu X., Zhou Y., Neelamegham S. DrawGlycan-SNFG and gpAnnotate: Rendering glycans and annotating glycopeptide mass spectra. Bioinformatics. 2020;36:1942–1943. doi: 10.1093/bioinformatics/btz819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hypercube, Inc. HyperChem. [(accessed on 31 July 2020)]; Available online: http://www.hyper.com/?tabid=360.
- 347.Schrödinger, Inc. The PyMOL Molecular Graphics System. [(accessed on 31 July 2020)]; Available online: https://pymol.org/2/
- 348.Dalby A., Nourse J.G., Hounshell W.D., Gushurst A.K.I., Grier D.L., Leland B.A., Laufer J. Description of several chemical structure file formats used by computer programs developed at Molecular Design Limited. J. Chem. Inf. Comput. Sci. 1992;32:244–255. doi: 10.1021/ci00007a012. [DOI] [Google Scholar]
- 349.Callaway J., Cummings M., Deroski B., Esposito P., Forman A., Langdon P., Libeson M., McCarthy J., Sikora J., Xue D., et al. Protein Data Bank Contents Guide: Atomic Coordinate Entry Format Description. [(accessed on 20 December 1996)];Brookhaven Natl. Lab. 1996 Available online: https://cdn.rcsb.org/wwpdb/docs/documentation/file-format/PDB_format_Dec_1996.pdf.
- 350.Bohne A. PDB2MultiGIF: A Web Tool to Create Animated Images of Molecules. J. Mol. Model. 1998;4:344–346. doi: 10.1007/s008940050092. [DOI] [Google Scholar]
- 351.Sayle R.A., Milner-White E.J. RASMOL: Biomolecular graphics for all. Trends Biochem. Sci. 1995;20:374–376. doi: 10.1016/S0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- 352.Willighagen E., Howard M. Fast and Scriptable Molecular Graphics in Web Browsers without Java3D. Nat. Prec. 2007 doi: 10.1038/npre.2007.50.1. [DOI] [Google Scholar]
- 353.Rose A.S., Hildebrand P.W. NGL Viewer: A web application for molecular visualization. Nucleic Acids Res. 2015;43:W576–W579. doi: 10.1093/nar/gkv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rose A.S., Bradley A.R., Valasatava Y., Duarte J.M., Prlić A., Rose P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics. 2018;34:3755–3758. doi: 10.1093/bioinformatics/bty419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sehnal D., Deshpande M., Vařeková R.S., Mir S., Berka K., Midlik A., Pravda L., Velankar S., Koča J. LiteMol suite: Interactive web-based visualization of large-scale macromolecular structure data. Nat. Methods. 2017;14:1121–1122. doi: 10.1038/nmeth.4499. [DOI] [PubMed] [Google Scholar]
- 356.Sehnal D., Rose A., Koca J., Burley S., Velankar S. Mol: Towards a Common Library and Tools for Web Molecular Graphics. In: Byska J., Krone M., Sommer B., editors. Workshop on Molecular Graphics and Visual Analysis of Molecular Data, Brno, Czech Republic, 4 June 2018. Eurographics Association; Geneva, Switzerland: 2018. pp. 29–33. [Google Scholar]
- 357.Consortium T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kuttel M., Gain J., Burger A., Eborn I. Techniques for visualization of carbohydrate molecules. J. Mol. Graph. Modell. 2006;25:380–388. doi: 10.1016/j.jmgm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 359.Cross S., Kuttel M.M., Stone J.E., Gain J.E. Visualisation of cyclic and multi-branched molecules with VMD. J. Mol. Graph. Modell. 2009;28:131–139. doi: 10.1016/j.jmgm.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Eborn I., Burger A., Kuttel M., Gain J. Carbohydra: Rendering Carbohydrate Cartoons. University of Cape Town; Cape Town, South Africa: 2004. [Google Scholar]
- 361.Humphrey W., Dalke A., Schulten K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 362.Perez S., Tubiana T., Imberty A., Baaden M. Three-dimensional representations of complex carbohydrates and polysaccharides—SweetUnityMol: A video game-based computer graphic software. Glycobiology. 2015;25:483–491. doi: 10.1093/glycob/cwu133. [DOI] [PubMed] [Google Scholar]
- 363.Besançon C., Guillot A., Blaise S., Dauchez M., Belloy N., Prévoteau-Jonquet J., Baud S. New visualization of dynamical flexibility of N-Glycans: Umbrella Visualization in UnityMol; Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); Madrid, Spain. 3–6 December 2018; pp. 291–298. [Google Scholar]
- 364.Besançon C., Guillot A., Blaise S., Dauchez M., Belloy N., Prévoteau-Jonquet J., Baud S. Umbrella Visualization: A method of analysis dedicated to glycan flexibility with UnityMol. Methods. 2020;173:94–104. doi: 10.1016/j.ymeth.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sehnal D., Grant O.C. Rapidly Display Glycan Symbols in 3D Structures: 3D-SNFG in LiteMol. J. Proteome Res. 2019;18:770–774. doi: 10.1021/acs.jproteome.8b00473. [DOI] [PubMed] [Google Scholar]
- 366.Thieker D.F., Hadden J.A., Schulten K., Woods R.J. 3D implementation of the symbol nomenclature for graphical representation of glycans. Glycobiology. 2016;26:786–787. doi: 10.1093/glycob/cww076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 368.McNicholas S., Potterton E., Wilson K.S., Noble M.E.M. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. Sect. D Struct. Biol. 2011;67:386–394. doi: 10.1107/S0907444911007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.McNicholas S., Agirre J. Glycoblocks: A schematic three-dimensional representation for glycans and their interactions. Acta Crystallogr. Sect. D Struct. Biol. 2017;73:187–194. doi: 10.1107/S2059798316013553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pendrill R., Jonsson K.H.M., Widmalm G. Glycan synthesis, structure, and dynamics: A selection. Pure Appl. Chem. 2013;85:1759. doi: 10.1351/pac-con-12-10-17. [DOI] [Google Scholar]
- 371.Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Marth J.D., Bertozzi C.R., Hart G.W., Etzler M.E. Symbol nomenclature for glycan representation. Proteomics. 2009;9:5398–5399. doi: 10.1002/pmic.200900708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Perez S., Aoki-Kinoshita K.F. Development of Carbohydrate Nomenclature and Representation. In: Aoki-Kinoshita K.F., editor. A Practical Guide to Using Glycomics Databases. Springer; Tokyo, Japan: 2017. pp. 7–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.