Abstract
Background:
Small cell carcinoma of the bladder is a rare, aggressive and poorly differentiated neoplasm. There has been no standard treatment guideline for the disease.
Methods:
Retrospective chart analysis was performed for the patients who were diagnosed and treated for small cell carcinoma of the bladder at our institution during the period between 1995 and 2015. Survivals were compared between different treatment periods and among different treatment modalities.
Results:
38 patients were diagnosed and treated for small cell carcinoma of the bladder at Fox Chase Cancer Center during a 20-year period. Median survival was 11.8 months. Overall survival rates after 1, 3, 5 years were 46.6%, 26.2% and 14%, progression free rates after 1, 3, 5 years were 36.9%, 19.5% and 7.3% respectively. There was no tendency in improvement of survival at our institution before and after 2010(p=0.63). In survival analysis with adjustment of age, histology and stage, there was no single treatment strategy that was significantly superior than others(95% CI 0.27-2.6;p=0.783 for surgery, 95% CI 0.31-3.01;p=0.963 for neoadjuvant chemotherapy, 95% CI 0.67-5.16;p=0.233 for radiation treatment). Existence of nodal or organ metastases was the only significant indicator of survival(HR 2.92;95% CI 1.14-7.51;p=0.026). Almost all of the lengthy survivors received platinum-based neoadjuvant chemotherapy and radical cystectomy. Down staging occurred at the time of surgery after neoadjuvant chemotherapy(≤T2N0M0).
Conclusions:
Like its counterpart in the lung, small call carcinoma of bladder is rapidly growing but sensitive to platinum-based chemotherapy. Neoadjuvant chemotherapy provides clinical benefit as it may halt the progression or even down stage of the disease until the time of cystectomy. At our institution, the best treatment response was observed in patients who had neoadjuvant chemotherapy followed by radical cystectomy.
Keywords: Small cell carcinoma of bladder, neoadjuvant chemotherapy, platinum-based chemotherapy, Fox Chase Cancer Center
Introduction
Small cell carcinoma of the bladder(SCCB) is an aggressive, poorly differentiated neuroendocrine malignancy, with clinical characteristics and prognosis similar to small cell lung cancer(SCLC). Despite some evidences that SCCB shares the same clonal origin with urothelial cancer1, disease behavior is significantly different in the two bladder cancer types, raising the need for development of more disease-specific treatment strategy.
SCCB is a rare disease, comprising only 0.5-0.7% of all bladder tumors2,3. Because of the low incidence and lack of sufficient data, there has been no consensus on the standard treatment of SCCB. Thus, treatment of the disease has been largely dependent on extrapolation of its equivalent in the lung. Under the current treatment modalities, survival rate of the patients with SCCB has been very poor. In an analysis of Surveilance, Epidemiology, and End Results(SEER) database, median length of survival of the patients with SCCB was 11 months, and survival rate was worse with patients with metastatic disease, as expected4. A retrospective study at Mayo clinic demonstrated that 5 year survival rates for patients with Stage 2, 3, 4 SCCB were 63.6%, 15.4% and 10.5% respectively5. Surprisingly, survival rates were also discouraging among the patients with localized and surgically resectable tumor; most them died of the disease within 1 year from the diagnosis6.
With the understanding of aggressive behavior of the disease, multiple strategies incorporating different treatment approaches have been tried. However, there has been few randomized clinical trial conducted so far and therefore no consensus regarding effective treatment for SCCB. Conventional strategy for the treatment of SCCB has been surgical resection of the tumor regardless of the muscle invasion, because of the disease propensity to metastasize even when the cancer is localized. Chemotherapy and/or radiotherapy have been main treatment options for the patients whose functional status is not suitable for invasive surgery. Combinations of multiple treatment modalities were also attempted to prolong survival. Some retrospective analyses have suggested promising insight to the treatment effect of neoadjuvant chemotherapy. A recent study by Lynch and colleagues demonstrated that neoadjuvant chemotherapy was associated with prolonged overall survival(OS) and progression free survival(PFS), and resulted in pathologic down staging to <pT1N0 in 62% of tumors7.
Based on insufficient knowledge and sparse clinical data, we decided to analyze survivals of the patients who underwent treatment for SCCB at Fox Chase Cancer Center(FCCC). We examined the trend in the length of survival to see if there was any improvement, given recent advancement in treatment modalities. We also compared the survival benefit of each treatment option for SCCB with an attempt to identify optimal treatment strategy. From the results of this study, we sought to generate hypothesis for future prospective trials which will lead to establishing more effective treatment guidelines for SCCB.
Methods
Study Population
Patients who had pathologic diagnosis of small cell cancer, mixed urothelial and small cell bladder cancer or neuroendocrine carcinoma of the bladder were included in the data analyses. Primary tumor specimen for pathologic exam was obtained from either transurethral resection of bladder tumor(TURBT) or cystectomy during the period between 01/01/1995 and 12/31/2015. Cases were identified from our institutional Bladder Cancer Database, Tumor Registry and Radiation Oncology Registry at FCCC. All of the pathologic diagnoses were confirmed by Department of Pathology at FCCC. After database, registry review, 38 cases were identified and included in this analysis.
Data Analysis
Data from the electronic medical record was entered onto a password-protected spreadsheet and stored in the FCCC shared drive. Access to the data was only available to the investigators. Information on patient demographics, smoking history, performance status, stage, treatment received and time of death or last contact was obtained. Stage was dichotomized into 1/2/3 and 4 during the analysis, based on the two tier staging system of small cell lung cancer4,8. Survival was estimated as the time between the diagnosis of SCCB and death or last contact. Primary endpoints of this study were overall survival(OS) and progression free survival(PFS). Median OS and PFS were calculated and survivorship was estimated using Kaplan-Meier curves. Survival comparison between the patients who were treated before January 2010 and after January 2010 was done using log rank test. Total number of patients was divided into equal halves when 12/31/2010 was used as a cutoff time for the date of diagnosis. Survivals were also compared in patients who received different treatment modalities - surgery, chemotherapy or radiation as a single or combination therapy. Proportional hazards regression was used for comparison of survival in different treatment groups. Statistical analyses were performed with R 3.1.0 and STATA/IC 11.2.
HIPAA waiver and waiver of informed consent were obtained prior to data collection. This study was approved by the Institutional Review Board at Fox Chase Cancer Center.
Results
Demographics and Clinical Features
From March 1999 to January 2014, a total of 38 patients with SCCB were treated at FCCC. Median age was 68 years, 28 out of 38 patients were male. 22 patients(57.9%) were current smokers, 6 patients(15.8%) had previous smoking history. There was one patient of Indian descent, rest of the patients were Caucasian. Staging computerized tomography(CT) was done in all cases. 22 patients(57.9%) had stage 2 disease at the time of diagnosis. 7 patients had stage 4 disease. 36.8% of the patients had mixed small cell and urothelial cell carcinoma based on the pathology report. Most common presenting symptom was hematuria(71.1%), most common sites of metastases were liver, bone and lung. Rest of the patient and disease characteristics are summarized in Table 1.
Table 1.
Patient and Disease Characteristics
| Patient characteristics | No. of Pts (%) | Disease characteristics | No. of Pts (%) |
|---|---|---|---|
| Total number of patients | 38 (100) | Clinical stage at diagnosis | |
| Age at the diagnosis (years) | 1 | 3 (7.9) | |
| Median | 68 | 2 | 22 (57.9) |
| Range | 46-89 | 3 | 6 (15.8) |
| 4 | 7 (18.4) | ||
| Sex | Histology | ||
| Male | 28 (73.6) | Small cell | 24 (63.2) |
| Female | 10 (26.3) | Small cell + Urothelial cell | 14 (36.8) |
| Follow up period (days) | Presenting symptom | ||
| Median | 336 | Hematuria | 27 (71.1) |
| Range | 76-3974 | Pain | 2 (5.3) |
| Race | Urinary frequency | 1 (2.6) | |
| Caucasian | 37 (97.4) | Frequent UTI | 1 |
| Indian | 1 (2.6) | Incidental mass | 1 |
| Seizure | 1 | ||
| Smoking status | Sites of metastases | ||
| Current smoker | 22 (57.9) | Liver | 10 (26.3) |
| Former smoker | 6 (15.8) | Bone | 8 (21.1) |
| Never | 10 (26.3) | Lung | 7 (18.4) |
| Peritoneum | 6 (15.8) | ||
| Brain | 2 (5.3) | ||
| Other* | 5 (13.2) |
Abdominal wall(1), adrenal gland(1), uterus(1), ureter(1), urethra(1)
Treatments
Treatments provided to the patients are summarized in Table 2. As cystectomy is considered a mainstay treatment for SCCB, treatment modalities were largely divided into surgical and non-surgical. 25 patients(65.8%) underwent radical cystectomy at FCCC, and 1 patient had partial cystectomy at another medical facility. 12 patients(31.6%) were deemed ineligible for surgery and underwent palliative chemotherapy with or without radiation treatment. 1 patient had cystectomy planned initially, but the surgery was cancelled because of overall decline in performance status. When the cases were divided into local vs. metastatic disease, 74.2% and 42.9% in each group underwent surgery(p=0.176 on Fisher’s exact test)
Table 2.
Treatment Modalities
| Treatment | No. of Pts (%) |
|---|---|
| Non-surgical therapy | |
| Chemotherapy | 7 (18.4) |
| Chemotherapy + Radiation | 5 (13.1) |
| Cystectomy with Chemotherapy | |
| Neo-adjuvant chemotherapy + Cystectomy | 19 (50.0) |
| Cystectomy + Adjuvant Chemotherapy | 4 (10.5) |
| Cystectomy with Radiation therapy with/without chemotherapy | |
| Cystectomy + Radiation | 1 (2.6) |
| Neo-adjuvant chemotherapy + Cystectomy + Radiation | 1 (2.6) |
| Cystectomy + Adjuvant chemotherapy + Radiation | 1 (2.6) |
There have been promising reports regarding neoadjuvant chemotherapy in the treatment of SCCB, as it may halt the progression of cancer until the day of surgery or even down stage the disease6,7. In FCCC, 52.6% of the patients underwent neoadjuvant chemotherapy prior to cystectomy, comprising the largest treatment group. Radiation treatment was used in 8 patients.
Platinum-based chemotherapy was used in almost all of the patients(37 out of 38 patients). Combination of platinum and etoposide was most commonly used. The rest of the regimens used are listed in Table 3. 1 patient participated in phase 1B clinical trial and received pembrolizumab(MK-3475) as a 4th line systemic therapy. He was withdrawn from the study after 2 months because of progression of disease. Another patient participated in phase 1 clinical trial with JAK inhibitor(AZD-1480) as a 2nd line systemic therapy and died after 1 month.
Table 3.
Chemotherapy Regimen
| Chemotherapy | Neo-adjuvant | Adjuvant | Palliative | Total |
|---|---|---|---|---|
| Cisplatin + Etoposide | 17 | 1 | 5 | 23 |
| Carboplatin + Etoposide | 4 | 2 | 6 | 12 |
| Paclitaxel | 2 | 3 | 5 | |
| Topotecan | 3 | 3 | ||
| Gemcitabine | 3 | 3 | ||
| Cisplatin + Gemcitabine | 2 | 2 | ||
| Cisplatin + Pemetrexed | 1 | 1 | ||
| Cisplatin + Irinotecan | 1 | 1 | ||
| Cisplatin | 1 | 1 | ||
| Gemcitabine + Taxol + Carboplatin | 1 | 1 | ||
| Pembrolizumab (MK-3475) | 1 | 1 | ||
| Mitomicin | 1 | 1 | ||
| AZD-1480 | 1 | 1 |
Survivals
For the total of 38 patients who were treated at FCCC during the 20-year period, median OS was 11.8 months, survival rates after 1, 3, 5 years were 46.6%, 26.2% and 14%. Median PFS was 10.1 months, PFS rates after 1, 3, 5 years were 36.9%, 19.5% and 7.3%.
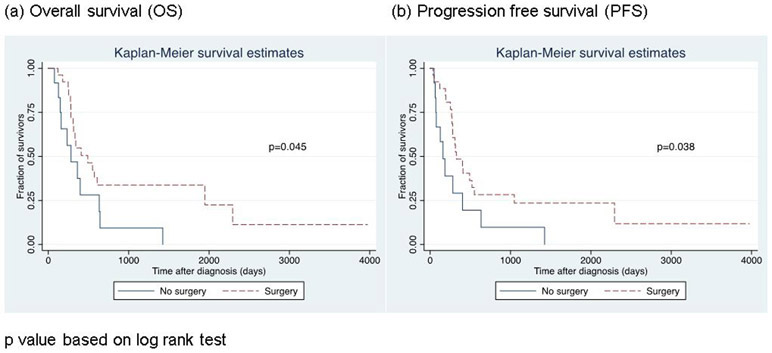
For the patients who underwent surgery, median OS was 16.1 months. Patients who underwent cystectomy survived longer based on log rank test(p=0.045). Progression free survival was also longer for patients who underwent cystectomy(p=0.038). (Figure 1) However, surgery did not significantly affect OS(95% CI 0.27-2.6;p=0.753) nor PFS(95% CI 0.38-3.63;p=0.783) when adjusted for age, histology (pure small cell vs. mixed with urothelial cell) and stage. Neither the use of neoadjuvant chemotherapy nor radiation treatment affected OS(95% CI 0.31-3.01;p=0.963 for neoadjuvant chemotherapy, 95% CI 0.67-5.16;p=0.233 for radiation treatment). Histology(pure small cell vs mixed with urothelial cell) did not significantly affect survival either(95% CI 0.20-1.37;p=0.191). In multivariable analysis, the only variable that significantly increased OS was stage(localized vs. metastatic to lymph nodes or distant organs) with p value of 0.026(HR 2.92;95% CI 1.14-7.51).
Figure 1.
Survival curves by surgical treatment
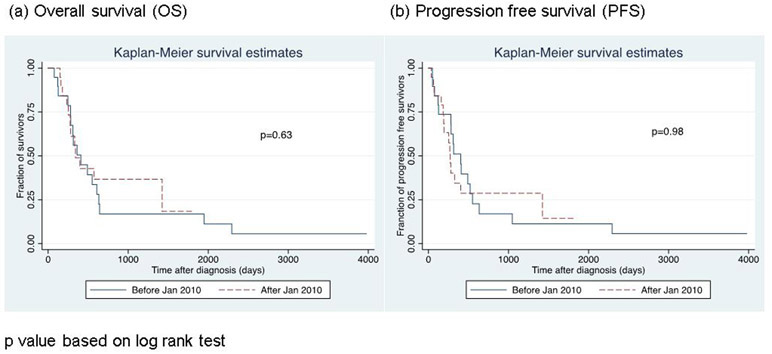
We sought to see if there was any difference in OS or PFS between the first half of the patients who were treated before January 2010 and the other half treated afterwards. OS and PFS were not significantly different between the patients treated before 2010 and after 2010(p=0.63 for OS, p=0.96 for PFS). (Figure 2)
Figure 2.
Change in survival curves over time
Lengthy survivors
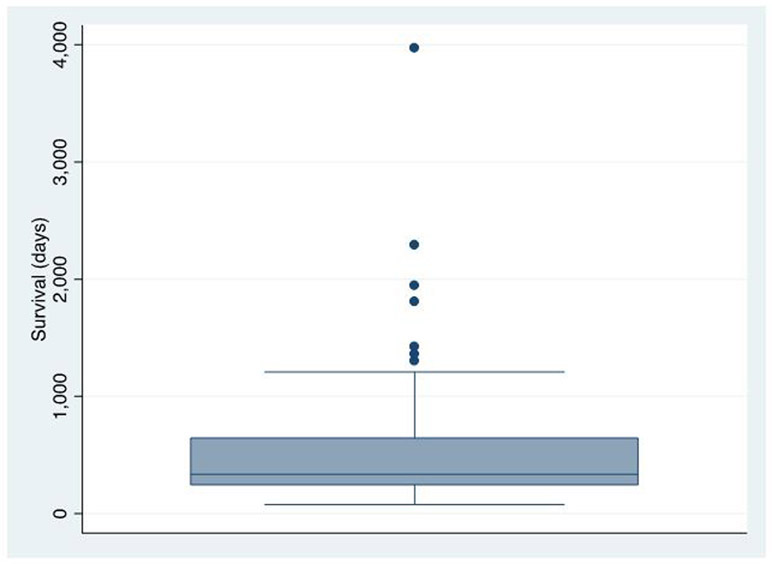
When survivals were analyzed in a box and whisker plot, we were able to identify 7 outlying lengthy survivors. (Figure 3) Disease characteristics and treatments provided for these patients are summarized in Table 4.
Figure 3.
Distribution of overall survival
Table 4.
Disease characteristics of lengthy survivors and treatments received
| Patient | Survival | Age* | Sex | Histology | Stage | Neoadjuvant Chemotherapy |
Surgery | Adjuvant Chemotherapy | Radiation |
|---|---|---|---|---|---|---|---|---|---|
| A | 3974¶ | 72 | F | Small cell | cT2N0M0 pT0N0M0 |
Cisplatin + Etoposide | Cystectomy | None | None |
| B | 2294 | 67 | M | Small cell + urothelial | cTisN0M0 pTisN0M0 |
Cisplatin + Etoposide | Cystoprostatectomy | Gemcitabine + Paclitaxel§ | None |
| C | 1947 | 73 | M | Small cell | pT2N0M0 | None | Partial cystectomy | Gemcitabine + Paclitaxel + Carboplatin, Cisplatin, pemetrexed⌘, pembrolizumab⌘ | None |
| D | 1809 | 46 | M | Small cell + urothelial | cT1N0M0 pT0N0M0 |
Cisplatin + Etoposide | Cystoprostatectomy | None | None |
| E | 1425 | 76 | M | Small cell | cT2N0M0 pT2N0M0 |
Cisplatin + Etoposide | Cystoprostatectomy | None | None |
| F | 1363 | 49 | M | Small cell + urothelial | cT1N0M0 pT1N0M0 |
Cisplatin + Etoposide | Cystoprostatectomy | None | None |
| G | 1303 | 74 | M | Small cell + urothelial | cT1N0M0 pT2N0M0 |
Cisplatin + Etoposide | Cystoprostatectomy | None | None |
Patient alive at the time of analysis
Age at the time of diagnosis
Chemotherapy for second primary pancreatic cancer
Palliative chemotherapy for small cell carcinoma of bladder
The patient who survived for 3974 days(still alive at the time of analysis) had received 4 cycles of neoadjuvant cisplatin and etoposide, and subsequently underwent cystectomy with ileal conduit urinary diversion. Initial stage was cT2N0M0, but the pathologic exam at the time of surgery demonstrated no carcinoma (ypT0N0M0).
Another patient who survived 2294 days had 3 cycles of neo-adjuvant cisplatin and etoposide and underwent radical cystoprostatectomy and neobladder construction. Initial clinical stage was cTisN0M0, pathologic stage at the time of surgery was the same. Histology was mixed urothelial and small cell carcinoma. Pancreatic cancer was incidentally found during the urologic surgery. The patient died of pneumonitis as a complication of chemotherapy for pancreatic cancer.
The patient who survived 1947 days had initial partial cystectomy and had recurrence in lymph nodes 3 years later. The patient had not had neoadjuvant chemotherapy. After recurrence, patient received multiple lines of chemotherapy with gemcitabine, taxol, carboplatin, cisplatin and pemetrexed, but they were all discontinued because of disease progression. The patient participated in a clinical trial for immunotherapy(pembrolizumab) but the medication was discontinued shortly after disease progression.
1 patient survived 1809 days. This patient had 4 cycles of neoadjuvant cisplatin and etoposide and underwent cystoprostatectomy and neobladder construction. Initial stage was cT1N0M0, and the disease pathologically down staged to ypT0N0M0.
The other 3 patients who each survived 1425 days, 1363 days and 1303 days all received neo-adjuvant chemotherapy with cisplatin and etoposide and underwent radical cystectomy. Their disease stages were either 1 or 2. None of them underwent adjuvant chemotherapy.
Discussion
Treatment of SCCB remains a predicament for oncologists because of the aggressive behavior of the disease and lack of consensus treatment guideline. At Fox Chase Cancer Center, survival rates were dismal as anticipated. Median survival was 11.8 months, 1 and 5 year overall survival rates were 46.6% and 14% respectively. Apparently, treatment was not more effective in recently treated patients either. In our analysis of patients treated at FCCC, there was no evidence in improvement of survival before and after 2010.
Because of rarity of the disease, there have been few clinical trials for the treatment of SCCB. However, several articles analyzed patient survivals retrospectively and attempted to compare treatment outcomes. Survival outcomes at other cancer centers were similarly poor. An article from the University of Southern California reported that median survival of SCCB patients was 13 months and 5 year survival rate was 10%9. In Mayo clinic experience, median OS of SCCB patients was 20 months and 5 year survival rates for patients with Stage 2, 3, 4 diseases were 63.3%, 15.4% and 10.5% respectively5. In a SEER database analysis, median survival for the nation-wide SCCB patients from 1991 to 2005 was 11 months4.
Based on the experience from treatment of transitional cell bladder cancer, surgery was advocated as a primary armamentarium for treatment of SCCB. However, after curative attempts with radical cystectomy alone, even patients with localized disease without metastasis died within 1 or 2 years.10,11. As with small cell cancer of the lung, SEER data analysis demonstrated that presence of lymph nodal or distant metastasis predicts prognosis more accurately, especially in terms of survival4. Our analysis also revealed that metastatic disease is a poor prognostic indicator. When controlled for age, histology and treatments received, patients without any nodal or distal metastasis survived significantly longer.
Neoadjuvant chemotherapy was associated with improved survival outcome in a study at M.D. Anderson Cancer Center. 5 year survival rate for the patients who underwent neoadjuvant chemotherapy was 78%, which was significantly higher as compared to 36% for the patients who had initial cystectomy(p=0.026). A subsequent analysis at the same center which included larger patient population and longer observation period demonstrated even more impressive survival benefit with neoadjuvant chemotherapy. Median OS was 159.5 months when patients received neoadjuvant chemothearpy as compared to 18.3 months when treated with initial cystectomy(p<0.001)7. Survival benefit of neoadjuvant chemotherapy was thought to be from the immediate control of tumor progression for this aggressive disease. Many patients who received neoadjuvant chemotherapy were down staged later at the time of surgery (pathologic stage lower than clinical stage or ≤T1N0). In contrast, for some cases of tumors initially deemed surgically resectable, cystectomy was aborted when distant metastasis was found intraoperatively.
In our analysis, 5 year survival rate for the patients who received neoadjuvant chemotherapy was 16.9% and it did not improve survival significantly as compared to initial cystectomy. Survival improvement was not apparent in our study probably due to the small sample size and small proportion of patients who did not receive neoadjuvant chemotherapy. Medical oncologists at FCCC recommend in their practice to start with chemotherapy for the patients with new diagnosis of SCCB while they are waiting to be evaluated and scheduled for surgery. Only 6 out of 26 patients who underwent cystectomy at our institution did not receive neoadjuvant chemotherapy. Out of 6 patients who did not have neoadjuvant chemotherapy, 5 patients received adjuvant chemotherapy after surgery, as previous studies reported lengthy survivors with initial cystectomy and consolidative chemotherapy12,13. 1 patient who underwent cystectomy did not receive any neoadjuvant or adjuvant chemotherapy because of poor renal function. Initial clinical staging was T2N0M0 for that patient, but the stage progressed to T4aN0M1 at the time of surgery. The patient died 179 days after the initial diagnosis.
Mackey et al. reported in 1998 that platinum-based chemotherapy, aimed for neuroendocrine cells, is associated with more favorable prognosis14. A phase II clinical trial of neoadjuvant chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin demonstrated a promising result in 18 patients with surgically resectable diseases, with median OS of 58 months15. 37 out of 38 of the patients who were treated at FCCC received either cisplatin or carboplatin at least once during the treatment course. 1 patient who did not receive platinum based chemotherapy did not receive chemotherapy at all. This precluded meaningful analysis of effect of platinum-based chemotherapy in our study.
Several reports presented cases of SCCB which were well controlled with local radiation treatment16,17. However, most of those patients in those reports also received chemotherapy and those studies failed to prove clinical benefit of the radiation alone. In a recent nation-wide, retrospective analysis of treatment distributions among the patients who had advanced SCCB(TxN+M0 or TxNxM1), only 5.3% of the patient population(total n=960) had radiation alone and 13.9% had radiation combined with chemotherapy18. Another analysis using the same database(NCDB-National Cancer Database) suggested a potential role of multi-modality approach with radiation treatment for patients with localized or locally advanced SCCB(cTis-cT4, cN0 or cM0)19. The analysis reported that bladder preservation therapy (TURBT or partial cystectomy) with chemotherapy and/or radiation was associated with prolonged overall survival when compared to bladder preservation therapy alone. However, the report could not separate out the role of radiation treatment in the survival benefit, and the longest survival was again demonstrated in the group of patients who had neoadjuvant chemotherapy and radical cystectomy.
By the nature of retrospective study design, we cannot draw any definite conclusion from our study. We attempted to minimize selection bias for each treatment groups by controlling variables in regression analyses, but we could not completely eliminate hidden confounding factors. Sample sizes were small too. Initial power analysis was based on the goal to identify any tendency of survival improvement among the patients treated at FCCC. Data from 38 patients may not have been sufficient to catch any significant survival benefit from different treatment modalities. Our study, however, identified several lengthy survivors who received neoadjuvant, platinum-based chemotherapy followed by radical cystectomy, which substantiates previous studies supporting the benefit of neoadjuvant chemotherapy. 6 out of the 7 longest survivors had platinum-based preoperative chemotherapy and had their disease down staged at the time of radical cystectomy(≤T2N0M0). The other patient had initial cystectomy and adjuvant chemotherapy postoperatively. As previously reported12,13, consolidative chemotherapy is recommended for optimal treatment outcome, if preoperative chemotherapy had been unavailable.
Conclusion
Treatment of SCCB has been a challenge for oncologists because of aggressive behavior of the disease and lack of standard treatment strategy. Surgery has been a mainstay of treatment to prevent recurrence of the disease, but surgical treatment alone has failed to provide long term survival. Like its counterpart in the lung, small cell carcinoma in the bladder is also sensitive to platinum-based chemotherapy aimed for rapidly growing, neuroendocrine cells. Accumulating evidence suggest that integration of platinum-based neoadjuvant chemotherapy and radical cystectomy is associated with prolonged survival. In our experience, the same treatment strategy was effective in achieving lengthy survivals in some SCCB patients. Although there are difficulties in conducting prospective clinical trials for this rare disease, further studies are necessary to elucidate optimal treatment plan.
List of abbreviations
- SCCB
small cell carcinoma of the bladder
- SCLC
small cell lung cancer
- SEER
Surveilance, Epidemiology, and End Results
- FCCC
fox chase cancer center
- TURBT
transurethral resection of bladder tumor
- OS
overall survival
- PFS
progression free survival
- CT
computerized tomography
- NCDB
national cancer database
Footnotes
Competing interests
None declared
Contributor Information
Kyungsuk Jung, Department of Medicine, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA
Pooja Ghatalia, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA
Samuel Litwin, Department of Statistics, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA
Marijo Bilusic, Department of Medical Oncology, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA
References
- 1.Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT, Lopez-Beltran A, Yang XJ, Koch MO, Zhang S, Pan CX, Baldridge LA. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol. 2005;166(5):1533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomjous CE, Vos W, De Voogt HJ, Van der Valk P, Meijer CJ. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989;64(6):1347–57. [DOI] [PubMed] [Google Scholar]
- 3.Holmäng S, Borghede G, Johansson SL. Primary small cell carcinoma of the bladder: a report of 25 cases. J Urol. 1995;153(6):1820–2. [PubMed] [Google Scholar]
- 4.Koay EJ, Teh BS, Paulino AC, Butler EB. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer. 2011. December;117(23):5325–33. [DOI] [PubMed] [Google Scholar]
- 5.Choong NW, Quevedo JF, Kaur JS. Small Cell Carcinoma of the urinary bladder: The Mayo clinic experience. Cancer. 2005;1-3(6):1172–8 [DOI] [PubMed] [Google Scholar]
- 6.Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, Grossman HB, Swanson DA, Millikan RE. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172(2):481–4. [DOI] [PubMed] [Google Scholar]
- 7.Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, Dinney CP, Siefker-Radtke A. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64(2):307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretto P, Wood L et al. Management of small cell carcinoma of the bladder. Consensus guidelines from the Canadian Association of Genitourinary Medical Oncologists (CAGMO). Can Urol Assoc J. 2013;7(1-2):44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quek ML, Nicholas PW, Yamzon J, Daneshmand S, Miranda G, Cai J, Groshen S, Stein JP, Skinner DG. Radial cystectomy for primary neuroendocrine tumors of the bladder: The University of Southern California experience. J Urol. 2005;174:93–96 [DOI] [PubMed] [Google Scholar]
- 10.Trias I, Algaba F, Condom E, Espanol I, Sequi J, Orsola I, Villavicencio H, Garcia Del Muro X. Small cell carcinoma of the urinary bladder. Presentation of 23 cases and review of 134 published cases. Eur Urol. 2001;39(1):85–90 [DOI] [PubMed] [Google Scholar]
- 11.Lopez JI, Anqulo JC, Flores N, Toledo JD. Smal cell carcinoma of the urinary bladder. A clinicopathological study of six cases. Br J Urol. 1994;73(1):43–9 [DOI] [PubMed] [Google Scholar]
- 12.Grignon DJ, Ro YJ, Ayala AG, Shum DT, Ordonez NG, Logothetis CJ, Johnson DE, Mackay B. Small cell carcinoma of the urinary bladder. A clinicopathologic analysis of 22 cases. Cancer. 1992;69(2):527–36 [DOI] [PubMed] [Google Scholar]
- 13.Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–30 [DOI] [PubMed] [Google Scholar]
- 14.Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998;159:1624–1998 [DOI] [PubMed] [Google Scholar]
- 15.Siefker-Radtke AO, Kamat AM, Grossman B, Williams DL, Qiao W, Thall PF, Dinney CP, Millikan RE. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattes MD, Kan C, Dalbagni G, Zelefsky MJ, Kollmeier MA. External beam radiation therapy for small cell carcinoma of the urinary bladder. Pract Radiat Oncol. 2015:5(1);17–22 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Neufeld S, Kroczak TJ, Bashir B, Ahmed N, Czaykowski P, Aljada I, Koul R, Galloway K, Drachenberg DE. Small cell cancer of the bladder and prostate: A retrospective review from a tertiary cancer center. Cureus 7(8):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geynisman DM, Handorf E, Wong YN, Doyle J, Plimack ER, Horwitz EM, Canter DJ, Uzzo RG, Kutikov A, Smaldone MC. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med. 2016:5(2):192–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SG, Stimson CJ, Zaid HB, Resnick MJ, Cookson MS, Barocas DA, Chang SS. Locoregional small cell carcinoma of the bladder: clinical characteristics and treatment patterns. J urol. 2014:191(2);329–34 [DOI] [PubMed] [Google Scholar]