Abstract
Background
The Preparedness Plan for Surveillance and Interventions on Emerging Vector-Borne Diseases (VBDs) in Southern Switzerland outlines the strategy for preventing and managing potential outbreaks, as well as the surveillance and control activities with a specific focus on Aedes-borne diseases transmitted by Aedes albopictus mosquitoes. The objective of the plan is to provide Public Health Authorities with a framework of preventive and control measures according to the situation and level of epidemic risks.
Material and methods
The plan is divided into various phases representing the different steps for all potential situations, ranging from no vectors and no transmission risk to epidemic levels with multiple autochthonous/local cases of hospitalization (and deaths) until the end of the epidemic. An algorithm presents how decisions are taken to move from one phase of the plan to another, with detailed activities for different partners and strategies for each specific phase.
Results
The different phases of the plan include activities on disease surveillance and clinical case management, on vector surveillance and control, communication and coordination of activities. The plan is divided into five phases of activities and decision levels. From phase 0 (no cases) to phase 1 (low number of local cases, less than 5), phase 2 (small outbreak with more than 5 local cases), phase 3 (epidemic) and phase 4 (return to no more cases).
Conclusion
The plan has been approved by the cantonal authorities and will be submitted to federal authorities. The required implementation tests will begin shortly.
Keywords: Aedes albopictus, Arboviruses, Canton Ticino, Emergency, Preparedness plan
1. Introduction
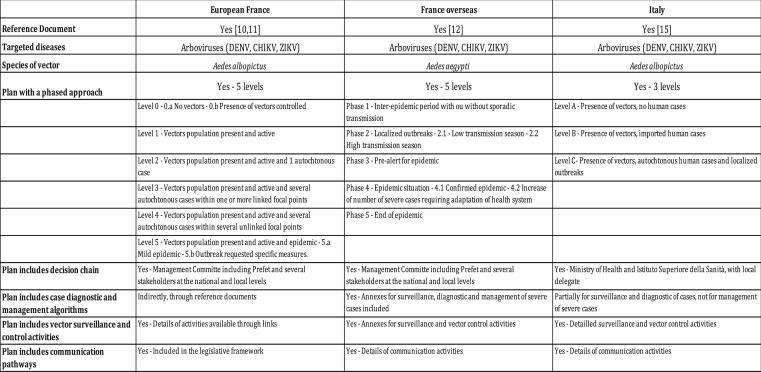
Several arthropod species can act as vectors of disease in the Canton Ticino in Southern Switzerland, although no mosquito-borne diseases autochtonous transmission have been confirmed in the past 50 years. Nevertheless, a preparedness plan to prevent and control the transmission of Vector-Borne Diseases (VBDs) is an essential component of the Public Health System. The VBDs preparedness plan developed in this document focuses on risks related to the Aedes albopictus mosquito. This species of mosquito is competent for transmitting at least 26 arboviruses such as dengue, chikungunya, Zika and yellow fever [1]. Ae. albopictus was introduced in Europe in Albania in 1979 and then in Italy during the early 1990s. In the past 30 years, it has spread continuously, establishing increased populations in all southern European countries [2]. This situation results in a substantial risk of autochthonous transmission and epidemics to all countries infested by Ae. albopictus mosquitoes. Further, the current situation of Aedes-borne diseases in Europe is becoming quite alarming due to a succession of dengue and chikungunya epidemics across various countries since the years 2000s [3] with the first chikungunya epidemic in Emilia-Romagna (Italy) in 2007, which reported more than 200 cases [4] and small chikungunya and dengue outbreaks regularly reported from different European countries since 2010 [5,6]. A dengue epidemic on Madeira Island in 2012 caused 122 severe cases that were hospitalized [7] and recently another chikungunya epidemic in Lazio and Calabria (Italy) in 2017 reported more than 300 cases [8]. Surveillance for the early detection of pathogens, as well as monitoring and controlling vector populations are thus essential for preventing outbreaks and allowing for timely adoption of effective control measures [9]. For that purpose, some European countries have developed intervention strategies against Aedes-borne diseases (Box 1), such as France [[10], [11], [12]] and Italy [[13], [14], [15]]. The structures of the different strategies are almost similar as they all describe a series of specific measures to be taken progressively, based on the surveillance of mosquito vectors and human cases. Other countries also have publicly available plans such as Greece, Portugal and Spain, but the writing of the plans in their country language limits their wide use. However, these strategies still have different approaches for risk definitions and levels as well as the identified pathways to move from one level to the next, and no harmonization has been achieved at a European level.
Box 1. Comparison of the main elements for response plans for France and Italy.
Alt-text: Box 1
In Switzerland, Ae. albopictus has been present in the Canton of Ticino since 2003 [16]. The spread of this vector has actively been surveyed by the cantonal Working Group for Mosquitoes (Gruppo Lavoro Zanzare, GLZ) [17]. Ae. albopictus populations are now abundant in Ticino, however no infected mosquitoes have ever been found [18]. The spread of Ae. albopictus in other Cantons of Switzerland has now been confirmed, even in the northern Canton of Zurich. The GLZ, in collaboration with more than 80 municipalities in the Canton of Ticino, are surveying mosquito populations and implementing control measures both to prevent the establishment of Ae. albopictus in areas not yet colonized and to reduce population densities in infested areas. The current strategy to prevent and control Aedes-borne diseases in Canton Ticino is based on the surveillance and reports of human cases and the fight against the Ae. albopictus mosquito [19]. Until now, no autochthonous cases of disease have been reported from Ticino or elsewhere in Switzerland. Nevertheless, the possibility of VBDs transmissions in Southern Switzerland remains significant [20], due to the climate and history of an established summer malaria transmission in the region until the beginning of the 20th century [21]. After the malaria eradication, no VBDs transmissions were reported in the Canton of Ticino or in neighboring areas of northern Italy, until the arrival of Lyme disease [22,23] and more recently the West Nile Virus (WNV) and Usutu Virus (USUV) [24]. Moreover, the yearly amount of imported cases of dengue increased in Switzerland from a few reported cases in the early 2000s to about 201 cases in 2016. For chikungunya imported cases, a peak was reached in 2014 with 78 cases, and for Zika virus 54 cases were reported in 2016 (https://www.bag.admin.ch/). This data clearly shows that the number of imported VBDs cases in Switzerland is increasing regularly, creating the need for adequate preparedness [25]. In the Canton of Ticino, the presence of imported viruses each year increases the risks of local transmission since a potential vector is now established and more and more abundant.
In this context, great efforts are constantly carried out in the Canton of Ticino for the monitoring and control of Ae. albopictus. Nevertheless, the densities of this mosquito species are constantly increasing. To better prepare the Canton of Ticino to prevent and control the emergence of VBDs that could be transmitted by Ae. albopictus, a preparedness plan was developed. This plan is an extension of the current strategy on how to deal with the worst-case scenario of an enlarged epidemic. The preparedness plan proposes an organizational chart for all partners involved in the prevention and control against VBDs, which includes the available tools required to perform necessary surveillance, prevention, control, case management and communication. The plan also sets the target to integrate the actions and to propose a strategy for several levels/phases of vector presence/abundance and virus circulation. The general objectives of the plan are to survey and control the development of vector populations, to prevent and control the emergence of clinical cases, to prevent the emergence of an epidemic and/or to limit the extension of the transmission and to decrease the health and economic impacts. The operational objectives are to articulate surveillance, alert and response actions, within a gradual framework moving from the less severe to the more severe situations and to propose concrete actions to partners, with a final operational objective of avoiding social disruption due to inappropriate action/communication. The plan will allow all partners to have access to all information on tools and activities.
2. Methods
2.1. The different levels of the plan and how the activities are moving from one level to the next
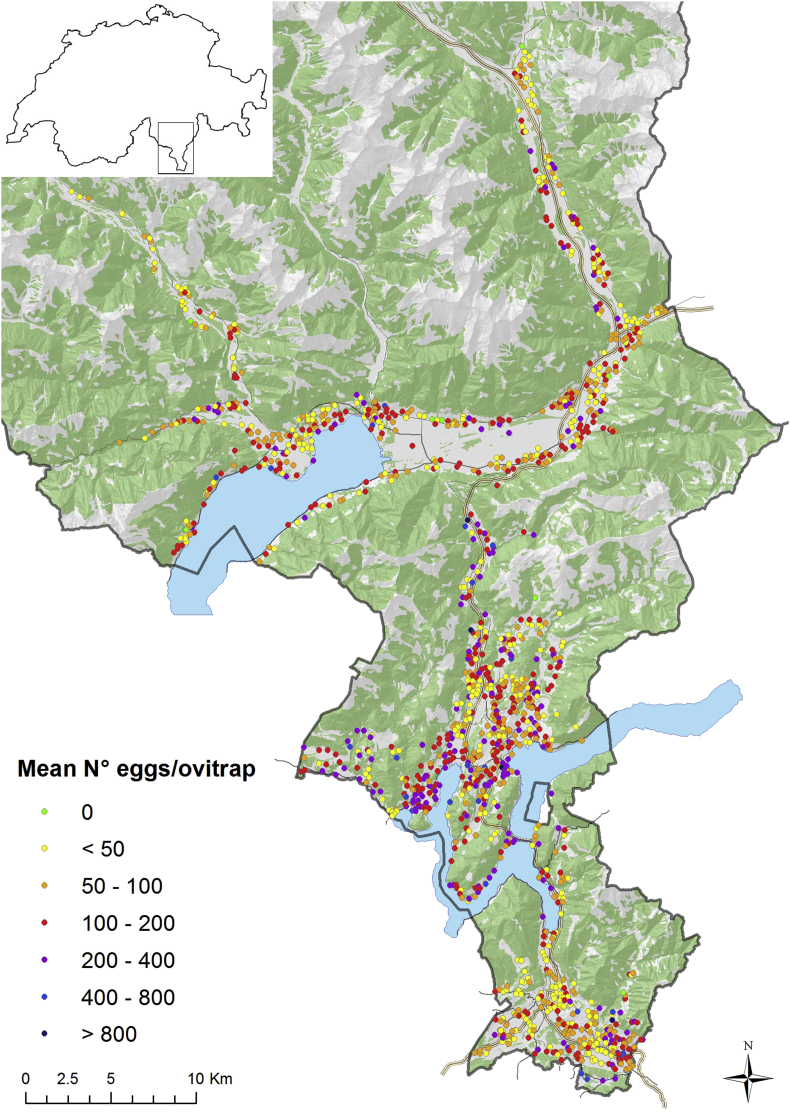
The plan is based on a classification of territories according to their status in terms of vector's presence/absence, abundance and suitability for disease transmission or risks. This classification has been established for the Canton of Ticino according to the mapping of egg densities in ovitraps (Fig. 1) and this classification is updated regularly. The plan includes five levels divided into sub-sections as well as two committees, one decision committee and one committee of experts for making recommendations. The first phase of the plan is based on the vector absence/presence/abundance when no clinical cases are reported. The next two phases of the plan are based on the number of clinical cases with an increased level of risk for epidemics from imported to autochthonous clinical cases and according to the number and severity of cases. The last part of the plan includes the phases of transition from mild to severe outbreak until the epidemic is over. The actions included in the plan are grouped into four strategies: for coordination, vectors, cases of disease and communication. For each phase of the plan, the activities are detailed into Standard Operating Procedures and the frequency of committee meetings is assigned. Moving through the phases of the plan according to the algorithms, is decided by the decision committee, based on the recommendations of the committee of experts.
Fig. 1.
Map with classification of territories according to their status in terms of Aedes albopictus presence/absence, abundance and suitability for disease transmission in 2018 [32].
2.2. The strategies for coordination and decision, clinical cases, vectors and communication
All activities included in the different levels of the plan are attached to a specific area or strategy, and four areas are defined. The first strategy is on the coordination and decision activities describing the committees involved in the coordination process, the frequency of their meetings according to the level of the plan and the decision process. The second strategy is on disease surveillance and case management which describes the activities related to the surveillance and report of clinical cases, including the process for requested epidemiological investigations along with methods and standards of diagnosis. The management of clinical cases using specific procedures according to the severity of the disease also pertains to this second strategy. The third strategy describes all activities related to vector surveillance and control according to the situation. The entomological investigations are part of this strategy and are adapted to the situation. The fourth and final strategy included in the plan is for communication activities. This will define not only who will communicate but how communication will be done according to the level of the plan, with communication templates for various audiences ranging from the medical sector to the general population at national and international level.
2.3. The partners and stakeholders of the committees with the required expertise
Two committees are included in the plan, a Decision Committee (DC) with Swiss Health authorities and Cantonal authorities involved in the decision process, and an Expert Committee (EC) for providing expertise on different topics and including representatives from different sectors such as the health sector, the civil society, the environmental sector and any other sector with relevance to the situation, as well as experts in related scientific disciplines and some members of the DC. The role of the EC is to support the DC with the relevant multi-sectoral information, including scientific evidence.
3. Results
3.1. The plan
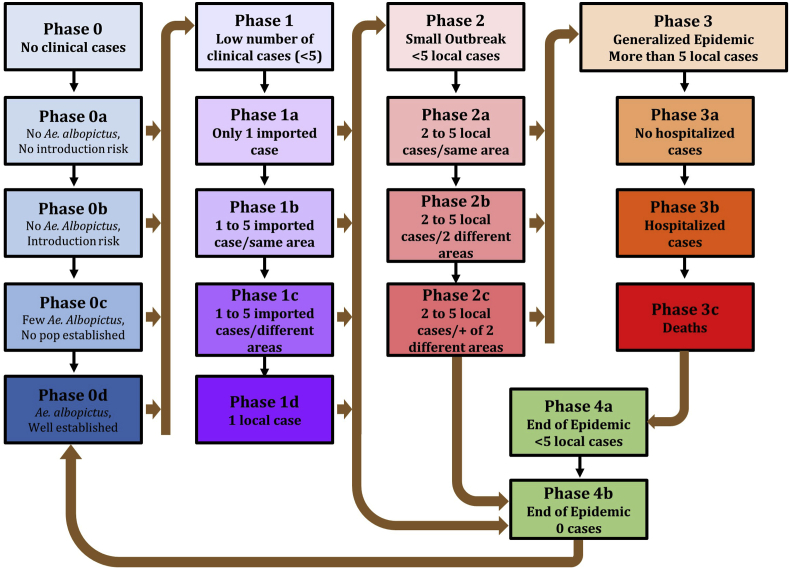
The four phases of the plan are established at the cantonal level and are moving from phase 0 with no clinical cases to phases 1–3 with increased numbers of confirmed clinical cases and epidemic situation, until phase 4 when the epidemic is over (Fig. 2). In phase 0, the sub-phases are related to the presence and abundance of the vectors (Box 2).
Fig. 2.
Algorithm of the preparedness plan with the different phases for each of the four levels, with the directions for moving from one phase to the next.(Phase 4a can move back to phase 3 without passing to phase 4b, and phase 4b can move directly to phases 0 and 2, according to the evolution of the numbers of clinical cases)
Box 2. Phase 0.
Sub-phase 0a with no vectors and no risk of introduction.
Sub-phase 0b with no vectors but risk of introduction.
Sub-phase 0c with presence of a few vectors but no established populations.
Sub-phase 0d when Ae. albopictus populations are well established and potentially abundant.
Alt-text: Box 2
If a few clinical cases (less than 5) are reported then the plan will move to phase 1 (Box 3). But the plan can move from each of the sub-phases 0a–d to any of the sub-phase 1a–d, according to the situation.
Box 3. Phase 1.
Sub-phase 1a when only 1 imported case is reported.
Sub-phase 1b if 2–5 imported clinical cases are reported from the same area.
Sub-phase 1c if 2–5 imported clinical cases are reported from different areas.
Sub-phase 1d if one autochthonous case is reported.
Alt-text: Box 3
When more than 1 autochthonous clinical case is confirmed, the plan will move to phase 2 (Box 4). Again, the plan can move from each of the sub-phases 1a–d to any of the sub-phase 2a–c, according to the situation.
Box 4. Phase 2.
Sub-phases 2a with 2–5 autochthonous clinical cases confirmed from the same area.
Sub-phase 2b with 2–5 autochthonous clinical cases confirmed from 2 different areas.
Sub-phase 2c with 2–5 autochthonous clinical cases confirmed from more than 2 areas.
Alt-text: Box 4
Then when more than 5 autochthonous clinical cases are confirmed, the epidemic situation is declared and the plan enters into phase 3 (Box 5), with sub-phases 3a–c, based on the severity of the cases. This number of 5 autochthonous cases was established by the Cantonal authorities based on the capacity of the Canton to deal with local transmission. As above, the plan can move from each of the sub-phases 2a–c to any of the sub-phase 3a–c, depending on the situation.
Box 5. Phase 3.
Sub-phase 3a with no severe forms.
Sub-phases 3b with severe and/or haemorrhagic cases.
Sub-phase 3c with deaths.
Alt-text: Box 5
The final phase of the plan is phase 4 when the epidemic is over, and less than 5 autochthonous clinical cases are reported in phase 4a to no more reported cases in phase 4b. For each phase and sub-phase of the plan, the requested actions are well established and defined. Finally, the plan includes pathways (Fig. 2) that not only take into account the increased number of incidences and/or outbreaks, but also shows how to react appropriately when the situation is returning to a lower level.
3.2. The actors and a description of the committees and their roles
3.2.1. The decision committee (DC)
The Stato Maggiore Cantonale di Condotta (SMCC) is the DC and includes members nominated according to the Ticino and Swiss legislation on protection of the population. The Epidemics Act of September 28, 2012 (EpidA; SR 818.101) and the Epidemics Ordinance of April 29, 2015 (EpV; SR 818.101.1) form the statutory foundation for the control of communicable diseases in Switzerland. These two pieces of legislation define powers and responsibilities. This legislation is in line with the World Health Organization's (WHO) International Health Regulations of May 23, 2005 (IGV; SR0.818.103). The EpidA also specifies the exchange of information, the collaboration and the harmonization of measures within international partners such as WHO and the European Union (EU). The DC is also in charge of making sure that activities are following an ethical assessment according to the national and international rules and legislations.
3.2.2. The Expert Committee (EC)
The EC includes stakeholders such as the Chief medical officer, the vector control manager, the delegate from the civil protection organization, the biologists in charge of the hospital laboratory as well the reference diagnostic laboratory, delegates of physicians, civil society and municipalities and qualified experts on vector-borne diseases epidemiology, diagnostic, surveillance, medical entomology, clinical management, infectious diseases, virology, communication and any other expertise needed.
3.3. The strategies
3.3.1. Strategy for coordination
At phase 0 of the plan, the DC (SMCC) and EC are established and each meets at least once a year. SMCC will take the decision to move from one phase to the next according to the situation.
The coordination from phase 1 to the following phases will be different according to the time of year:
–During the season with low temperatures, from 1 October to 30 April, the coordination is the same as at phase 0.
–During the favorable season from 1 May to 30 September, for sub-phases 1a–d, if the imported case(s) resides in an area where mosquitoes are present, the committees will meet ad hoc according to the situation.
In Phase 1 and during the favorable season, the coordination between the Chief medical officer and the epidemiological surveillance is enforced to take the necessary actions as reported in Table 1. In the same way, the coordination between the Chief medical officer and the Vector Control Agency (Gruppo Vettori LMA) is also enforced to take the appropriate actions as reported in Table 2. A coordination map with an updated report of the areas showing positive results for mosquitoes is updated and made available for the SMCC and EC.
Table 1.
Activities in diagnostic, passive and active surveillance of human cases, as well as clinical management according to the phases and levels of the plan.
| Activities | Phases of the plan |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 0 | Phase 1 |
Phase 2 |
Phase 3 |
Phase 4 |
|||||||
| 1a, 1b, 1c | 1d | 2a | 2b | 2c, 2d | 3a | 3b | 3c | 4a | 4b | ||
| Diagnostic | |||||||||||
| Declaration of case within 24 h as per CH recommendations | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory |
| Information sent to Medico Cantonale within 24 h | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory |
| List of reference laboratories | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | |
| SOPs for diagnostic | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | |
| Laboratory confirmation | Optional | Optional | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Optional |
| Increased diagnostic capacity | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Choice of the Laboratory for diagnostic by the physician | Optional | Optional | Optional | Optional | Optional | Optional | Optional | Optional | Optional | Optional | Optional |
| Federal Reference Laboratories supporting diagnostic | Optional | Optional | Optional | Optional | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Optional | |
| Virus isolation and sequencing | Optional | Optional | Optional | Optional | Optional | Optional | Optional | Mandatory | Mandatory | Optional | Optional |
| Passive surveillance | |||||||||||
| According to travel history of cases | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | Algorithm Fig. 3 | |
| Epidemiological investigation | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| Informed consent filled by patient or responsible family member | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| Information given by Medico Cantonale to vector control | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| Increased epidemiological investigations | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Active surveillance | |||||||||||
| Evolution of the number of cases followed in real-time | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| Research of suspected cases in the family environment | Optional | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Optional | |
| Information given to the physicians of the area of the cases | Optional | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Optional | |
| Alert given to the physicians in the area of the cases | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Surveillance activities and alert to physicians extended to the cantonal level | No | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Surveillance activities and alert extended to the federal level | No | No | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Clinical case management | |||||||||||
| SOPs for case management | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | |
| Treatment and materials for severe cases | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | |
| Isolation of cases in areas where vectors are present | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| Hospital capacity prepared and reinforced | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Sessions of information for case detection and management, given to physicians | No | No | No | No | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | No | |
| Follow up on hospitalized cases | No | No | No | No | No | No | Mandatory | Mandatory | No | No | |
| Follow up on deceased cases | No | No | No | No | No | No | No | Mandatory | No | No | |
The activities are detailed into specific SOPs and are considered as not required when there is a “no” in the box, as optional or mandatory. (* the algorithm for passive surveillance according to travel history is represented in Fig. 3).
Table 2.
Activities in entomological surveillance and vector control according to the phases and levels of the plan.
| Activities | Phases of the plan |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 0 |
Phase 1 |
Phase 2 |
Phase 3 |
Phase 4 |
||||||||
| 0a | 0b | 0c | 0d | 1a, 1b, 1c | 1d | 2a, 2b | 2c | 2d | 3a, 3b, 3c | 4a | 4b | |
| Vector surveillance | ||||||||||||
| SOPs for surveillance of mosquitoes breeding-sites | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available |
| Ovitrap surveillance for potential new infestation, controlled each 14 days | No | yes | No | No | No | |||||||
| Ovitraps regularly deposited each 14 days by MW* | No | yes | No | No | No | |||||||
| Ovitraps regularly deposited each 14 days by MW* according to a matrix** | yes | yes | yes | yes | No | No | No | yes | yes | |||
| Eggs collected in the ovitraps analyzed by TEG*** | yes | yes | yes | yes | yes | yes | No | No | No | yes | yes | |
| Monitoring of the sensibility of the mosquitoes to the products at best each 2 years | yes | yes | yes | yes | No | No | No | yes | yes | |||
| Coordination of vector surveillance with epidemiological data in locations where clinical cases are reported | yes | yes | yes | No | No | No | yes | |||||
| Coordination of vector surveillance with epidemiological data about 500 m around locations where cases are reported, and at commune level | yes | yes | No | No | No | yes | ||||||
| Collection of entomological data such as densities, GIS, infection rates, sensibility and any other relevant data | yes | yes | yes | yes | yes | yes | yes | |||||
| Vector control | ||||||||||||
| SOPs for treatment of mosquitoes breeding-sites | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available | Available |
| Positive traps removed or treated by larvicide product | yes | yes | yes | yes | yes | yes | yes | No | No | yes | yes | |
| Positive breeding sites removed or treated by larvicide product | yes | yes | yes | yes | yes | yes | yes | No | No | yes | yes | |
| Peri-focal treatment on potential breeding sites about 200 m around positive sites | yes | yes | yes | yes | yes | yes | No | No | yes | yes | ||
| Monitoring of the efficiency of the treatment in peri-focal environment by TEG | yes | yes | yes | yes | yes | yes | No | No | yes | yes | ||
| Regular treatment in all potential breeding sites in public spaces, from 01/05 to 30/09 each year | yes | yes | yes | yes | yes | No | No | yes | yes | |||
| Recommendations to remove or treat breeding-sites in private spaces | yes | yes | yes | yes | yes | No | No | yes | yes | |||
| Monitoring of the efficiency of the treatment in public spaces by TEG | yes | yes | yes | yes | yes | No | No | yes | yes | |||
| SOPs for spraying of adulticide | Available | Available | Available | Available | Available | Available | Available | Available | Available | |||
| In location at risk of transmission, reinforcement of vector control with adulticide sprayings | yes | yes | yes | yes | No | No | yes | |||||
| Monitoring of the efficiency of the breeding-site elimination campaign and adulticide sprayings | yes | yes | yes | yes | No | No | yes | |||||
| Recommendations for use of personal protection measures (repulsive, fumigants and other) | yes | yes | yes | yes | No | No | yes | |||||
| Vector control activities deployed around houses with more than one clinical case | yes | yes | yes | No | No | yes | ||||||
| Regular treatment in all potential breeding sites in public spaces, each 7 days | yes | yes | yes | No | No | yes | ||||||
| Control activities deployed in all infected areas with intensive adulticide sprayings | yes | yes | ||||||||||
The activities are detailed into specific SOPs and are considered as not required when there is a “no” in the box, as optional or to be implemented when there is a “yes” in the box.
*MW, Municipality Workers.
**The deployment of the traps is made according to a matrix in which the number of traps is estimated as a function of the density of the mosquito population, in the area under surveillance.
***TEG, Trained Entomologist Group.
When phase 2 is declared by the SMCC, the members of SMCC meet at least once a week for follow up and adaptation of the plan and actions. An EC meeting is convened as soon as possible when phase 2 is declared, and recommendations are made available for SMCC. Further meetings can be convened ad hoc for the SMCC, and for EC upon request from SMCC.
When phase 3 is declared by the SMCC, the members of SMCC meet daily for an update on the situation in order to follow-up on actions and adapt the plan. The EC meetings are convened at least once a week and ad hoc according to the situation, including through teleconference to provide the required support to the SMCC, and to the different strategies.
3.3.2. Strategy for diseases surveillance and case management
The activities performed during different phases of the plan for the diagnosis of suspected clinical cases, for surveillance of cases, epidemiological investigations and management of clinical cases, from mild to severe cases are detailed in Table 1. For each phase of the plan, the declaration of cases follows the obligatory declaration in Switzerland and must be made by the laboratory within 24 h to the Chief medical officer and to the Federal Office of Public Health [26]. The declaration of cases is mandatory for several vector-borne diseases, some of them transmitted by Aedes mosquitoes, such as dengue, chikungunya and Zika viruses. Further, starting at Phase 2 and for subsequent phases, the declaration of cases is recommended within less than 24 h.
At phase 0 of the plan, the surveillance is passive and for each suspected case, the family physician will decide eventually but not obligatorily for laboratory confirmation, as well as for the choice of the laboratory that will perform the diagnostic or send the sample for diagnostic to a reference laboratory.
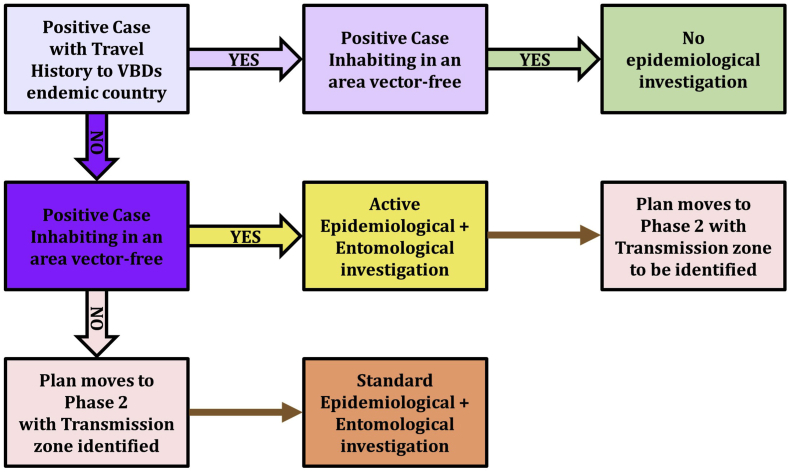
At phase 1, when one imported clinical case is confirmed, the surveillance of the clinical cases as well as the epidemiological investigations will follow the process described in the algorithm represented in Fig. 3.
Fig. 3.
Algorithm for passive and active surveillance of the clinical cases according to their travel history and the status of the absence/presence of mosquito vectors in the places/environment where the cases are living and/or working.
The activities are then adapted to the different phases of the plan (Table 1) moving from different diagnostic options, with the choice of the diagnostic laboratory left as optional by the physician until phase 2a and then decided by SMCC from phase 2b to the end of the epidemic. From phase 2b of the plan to all subsequent phases, each case will have to be reported in less than 24 h.
The isolation of the circulating virus strain is also mandatory when the epidemic with severe cases is declared in phase 3b. The passive surveillance activities are mandatory when first cases are reported including imported cases, and the active surveillance turns into mandatory when the first local case is reported.
The last part of the plan on case management also moves from the preparation of the facilities starting as early as phase 2a, when the first local case is reported to the full follow up of all cases during the epidemic phase 3.
3.3.3. Strategy for vector surveillance and control
The deployment of different types of activities in mosquito surveillance and control is adapted to the preparedness plan (Fig. 2) and starts from phase 0a where there is no vector and no risk of new infestation, however surveillance is in place to face environmental changes, such a climate which could affect this risk. The entomological activities will then move to different phases according to the presence of mosquito vectors and clinical cases (Table 2). Vector surveillance is mainly performed by trapping mosquito eggs in specific devices (ovitraps) and they are deployed from phase 0 to phase 2b, which is when the situation is not epidemic. If the vector density is abundant, the surveillance follows a matrix planning determined as a function of the vector density. When the situation becomes an epidemic in phase 3, the surveillance activities are limited to the essential ones with collection of important data since resources in entomology are mostly used for vector control. In the same way, vector control activities are increasing according to the situation in the first part of the plan until phase 2c. However, when the epidemic situation is declared in phase 3, only control activity with adulticide spraying is maintained. However, the recommendation to the general population to eliminate all potential breeding sites for mosquito vectors are maintained throughout the plan. In the same way, the recommendations to use repellents for self-protection are maintained from the first human case in phase 1a until the end of the epidemic. The entomological activities are consequently adapted to the different phases of the plan and also to the resources available to manage all activities. Some of them are kept optional since they would provide a supplementary control if maintained but cannot be considered as priorities in this context. The strategy for vector control also includes the monitoring and evaluation of activities, as well as product testing, and Standard Operating Procedures (SOPs) are updated every 2 years to take into account the evolution of insecticide resistance, and also new vector control technologies that may become available.
3.4. Strategy for communication
Communication to all actors, including the health sector and medical professionals as well as to the general population is under the responsibility of the SMCC and has specific processes and procedures. In the first phases of the plan, from phase 0 to phase 1c, no dedicated communication on the imported clinical cases is planned. However, some documentation for frequently asked questions (FAQ) on the disease and its transmission is available from the Office of the Chief medical officer. Documentation on vectors for FAQ is also available from the LMA. The first communication on the cases is planned at phase 1d when the first local human case is confirmed. This communication will include prepared messages from the office of Chief medical officer to inform the different sectors on the situation, and on the activities related to the situation, including on vector control activities. At this stage, specific recommendations for increased personal protection will be given to the population. From phases 2a until phase 4b, on the way to ending the epidemic, the SMCC will decide, prepare and deliver all communication related to the situation, with a frequency that will be determined ad-hoc, and using the means and media as decided by the committee.
4. Discussion and conclusion
It is always best to prevent the emergence of an epidemic when possible. Nevertheless, when an epidemic occurs, the management of the epidemic cannot be improvised. In this event, a plan must be prepared to detect cases as early as possible to be able to respond adequately in the optimal way according to the level of the situation, so that the impact of the event on health and other sectors are minimized. The preparedness plan should include and describe all activities, links and elements to be done/established before, during, and after epidemics. The countries and health systems are already used to developing similar preparedness plans for various types of events including natural disasters and pandemic diseases such as Influenza [27]. However, the challenges of this type of plan is not only to predict the unpredictable, but rather to make all sectors work together in an efficient way.
Among the specific issues that a preparedness plan needs to overcome, the first one is the early detection of an event through a sensitive surveillance system. The second challenge is to coordinate the rapid investigation detailing the outbreak and identifying the interventions required according to the level of the outbreak. The third challenge is then to deploy an effective response. The management of the event will not be complete without a post-action review, with the evaluation and identification of what went right and wrong before, during and after the outbreak [28].
For the emerging arboviruses, only two preparedness plans have been published in scientific reviews [29,30] showing some lack of interest by the scientific community for this type of activity. Consequently, the development of plans by many countries are at the initiative of the health sector, which is facing the complexity of the multi-sectorial approach without the help of a theoretical framework. This often results in truncated views where some important elements of the plan are missing.
In the case of the preparedness plan for emerging arboviruses transmitted by Ae. albopictus in the Canton Ticino in southern Switzerland, the activities will be updated on a two-year schedule and the process for monitoring and evaluating the plan will be included. The coordinating body is already multi-sectorial, but the functioning of the plan will be used to better understand the dynamic of this approach, which is strongly needed for prevention of diseases and Universal Health Coverage [31].
Credit role for the authors
The authors, Florence Fouque, Valeria Guidi, Mario Lazzaro, Damiana Ravasi, Gladys Martinetti-Lucchini, Giorgio Merlani, Mauro Tonolla and Eleonora Flacio, all participated to the conceptualization of the project, design of the plan, acquisition of information, drafting of the article, critically revising and final approval of the version submitted. All authors approve the development of the plan and concur with/approve the submission and any subsequent revisions submitted by the corresponding author, Florence Fouque.
Funding source
Funding from the respective institutions through the time of the staff.
Declaration of competing interest
There is no conflict of interest concerning the development of the plan, the results or the publication of the manuscript. The authors, Florence Fouque, Valeria Guidi, Mario Lazzaro, Damiana Ravasi, Gladys Martinetti-Lucchini, Giorgio Merlani, Mauro Tonolla and Eleonora Flacio, all participated in the development of the plan and concur with the submission and any subsequent revisions submitted by the corresponding author, Florence Fouque.
Acknowledgements
The authors want to thank the Special Programme for Research and Training in Tropical Diseases (TDR) of the World Health Organization (WHO) and its Director Dr. John Reeder for having allowed the development of this work. The authors also want to sincerely thank all collaborators from the LMA in Canton Ticino for constructive discussion. And lastly the authors are very grateful to Daniel Hollies from TDR for English editing. This work was funded by the respective institutions through allocating specific time to the staff for developing this plan.
Contributor Information
Florence Fouque, Email: fouquef@who.int.
Valeria Guidi, Email: valeria.guidi@supsi.ch.
Mario Lazzaro, Email: Mario.Lazzaro@ti.ch.
Damiana Ravasi, Email: damiana.ravasi@supsi.ch.
Gladys Martinetti-Lucchini, Email: Gladys.MartinettiLucchini@eoc.ch.
Giorgio Merlani, Email: Giorgio.Merlani@ti.ch.
Mauro Tonolla, Email: mauro.tonolla@supsi.ch.
Eleonora Flacio, Email: eleonora.flacio@supsi.ch.
References
- 1.Bonizzoni M., Gasperi G., Chen X., James A.A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29(9):460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunze S., Kochmann J., Koch L.K., Klimpel S. Aedes albopictus and its environmental limits in Europe. PloS One. 2016;11(9):e0162116. doi: 10.1371/journal.pone.0162116. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzon L. Ongoing and emerging arbovirus threats in Europe. J Clin Virol. 2018;107:38–47. doi: 10.1016/j.jcv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A.C., Panning M., Cordioli P., Fortuna C., Boros S., Magurano F., Silvi G., Angelini P., Dottori M., Ciufolini M.G., Majori G.C., Cassone A., CHIKV study group Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 5.Calba C., Guerbois-Galla M., Franke F., Jeannin C., Auzet-Caillaud M., Grard G., Pigaglio L., Decoppet A., Weicherding J., Savaill M.C., Munoz-Riviero M., Chaud P., Cadiou B., Ramalli L., Fournier P., Noël H., De Lamballerie X., Paty M.C., Leparc-Goffart I. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22(39) doi: 10.2807/1560-7917.ES.2017.22.39.17-00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pem-Novosel I., Vilibic-Cavlek T., Gjenero-Margan I., Kaic B., Babic-Erceg A., Merdic E., Medic A., Ljubic M., Pahor D., Erceg M. Dengue virus infection in Croatia: seroprevalence and entomological study. New Microbiol. 2015;38(1):97–100. [PubMed] [Google Scholar]
- 7.Auerswald H., de Jesus A., Seixas G., Nazareth T., Mao S., Duong V., Silva A.C., Paul R., Dussart P., Sousa C.A. First dengue virus seroprevalence study on Madeira Island after the 2012 outbreak indicates unreported dengue circulation. Parasit Vectors. 2019;12(1):103. doi: 10.1186/s13071-019-3357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezza G. Chikungunya is back in Italy: 2007-2017. J Travel Med. 2018;25(1) doi: 10.1093/jtm/tay004. [DOI] [PubMed] [Google Scholar]
- 9.Tomasello D., Schlagenhauf P. Chikungunya and dengue autochthonous cases in Europe, 2007-2012. Travel Med Infect Dis. 2013;11(5):274–284. doi: 10.1016/j.tmaid.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Souarès Y. Le Plan anti-dissémination du chikungunya et de la dengue en France métropolitaine. Revue ADSP. 2011;76:40–42. [Google Scholar]
- 11.Ministère des Solidarités et de la Santé . 2016. Cartes de présence du moustique tigre (Aedes albopictus) en France métropolitaine.http://solidarites-sante.gouv.fr/sante-et-environnement/risques-microbiologiques-physiques-et-chimiques/especes-nuisibles-et-parasites/article/cartes-de-presence-du-moustique-tigre-aedes-albopictus-en-france-metropolitaine [Google Scholar]
- 12.Cassadou S., Gustave J., Chaud P., Yebakima A. 2007. Programme de surveillance, d'alerte et de gestion des épidémies de dengue en Guadeloupe continentale et îles proches (Psage dengue)https://www.santepubliquefrance.fr/regions/antilles/documents/rapport-synthese/2007/programme-de-surveillance-d-alerte-et-de-gestion-des-epidemies-de-dengue-en-guadeloupe-continentale-et-iles-proches-psage-dengue-.-version-1-du-18/09/2007 Version 1 du 18/09/2007, Santé Publique France. [Google Scholar]
- 13.2018. Dipartimento di Sanità Pubblica dell' Azienda Unità Sanitaria Locale in Relazione al controllo degli organismi infestanti e potenziali vettori di arbovirosi in Regione Emilia-Romagna.http://www.zanzaratigreonline.it/Portals/zanzaratigreonline/Documenti/Piano%20sorveglianza%20arbovirosi%202018%20Emilia-Romagna.pdf [Google Scholar]
- 14.Regione Autonoma Friuli Venezia Giulia. Piano regionale di sorveglianza e gestione delle arbovirosi trasmesse da zanzare (Aedes sp.) con particolare riferimento a virus Chikungunya, Dengue e virus Zika – 2016. 2016. https://www.regione.fvg.it/rafvg/export/sites/default/RAFVG/salute-sociale/zanzara-tigre/allegati/ARBOVIRUS_FVG_VERSIONE_2_0_21_6_2016.pdf Versione 1.0 del 20 giugno. [Google Scholar]
- 15.Ministero della Salute . Piano nazionale di sorveglianza e risposta alle arbovirosi trasmesse da zanzare invasive (aedes sp.) con particolare riferimento ai virus chikungunya, dengue e zika. 2018. Direzione Generale della prevenzione sanitaria, Prevenzione delle malattie trasmissibili e profilassi internazionale.http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=64314&parte=1&seriehttp://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=64314&parte=1&serie= [Google Scholar]
- 16.Flacio E., Engeler L., Tonolla M., Lüthy P., Patocchi N. Strategies of a thirteen years surveillance programme on Aedes albopictus (Stegomyia albopicta) in southern Switzerland. Parasit Vectors. 2015;8:208. doi: 10.1186/s13071-015-0793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flacio E., Engeler L., Tonolla M., Müller P. Spread and establishment of Aedes albopictus in southern Switzerland between 2003 and 2014: an analysis of oviposition data and weather conditions. Parasit Vectors. 2016;9(1):304. doi: 10.1186/s13071-016-1577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suter T.T., Flacio E., Feijoó Fariña B., Engeler L., Tonolla M., Regis L.N., de Melo Santos M.A., Müller P. Surveillance and control of Aedes albopictus in the Swiss-Italian border region: differences in egg densities between intervention and non-intervention areas. PLoS Negl Trop Dis. 2016;10(1):e0004315. doi: 10.1371/journal.pntd.0004315. eCollection 2016 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wipf N.C., Guidi V., Tonolla M., Ruinelli M., Müller P., Engler O. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasit Vectors. 2019;12(1):554. doi: 10.1186/s13071-019-3798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravasi D., Parrondo Monton D., Guidi V., Flacio E. Evaluation of the public health risk for autochthonous transmission of mosquito-borne viruses in southern Switzerland. Short Commun. 2019 doi: 10.1111/mve.12421. [DOI] [PubMed] [Google Scholar]
- 21.SUPSI (Scuola Universitaria Professionale della Svizzera italiana) Dipartimento ambiente costruzioni e design, Laboratorio microbiologia applicata (LMA) 2019. Sorveglianza e controllo della zanzara tigre (Aedes albopictus) in Canton Ticino.http://www.supsi.ch/lma/ricerca-servizi/vettori/zanzare/documentazione-sulla-zanzara-tigre.html Rapporto di attività 2018. [Google Scholar]
- 22.Borrani . University of Lausanne; 1937. Osservazioni e ricerche sulla distribuzione dei culicidi e soprattutto delle anofelini del cantone ticino in relazione con gli antichi focolai malarici. dissertation. [Google Scholar]
- 23.Bernasconi M.V., Valsangiacomo C., Balmelli T., Péter O., Piffaretti J.C. Tick zoonoses in the southern part of Switzerland (Canton Ticino): occurrence of Borrelia burgdorferi sensu lato and Rickettsia sp. Eur J Epidemiol. 1997;13(2):209–215. doi: 10.1023/a:1007394901846. [DOI] [PubMed] [Google Scholar]
- 24.Beltrame A., Laroche M., Degani M., Perandin F., Bisoffi Z., Raoult D., Parola P. Tick-borne pathogens in removed ticks Veneto, northeastern Italy: a cross-sectional investigation. Travel Med Infect Dis. 2018;26:58–61. doi: 10.1016/j.tmaid.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Calzolari M., Chiapponi C., Bonilauri P., Lelli D., Baioni L., Barbieri I., Lavazza A., Pongolini S., Dottori M., Moreno A. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect Genet Evol. 2017;51:255–262. doi: 10.1016/j.meegid.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Reusken C.B., Ieven M., Sigfrid L., Eckerle I., Koopmans M. Laboratory preparedness and response with a focus on arboviruses in Europe. Clin Microbiol Infect. 2018;24(3):221–228. doi: 10.1016/j.cmi.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Medina A. Promoting a culture of disaster preparedness. J Bus Contin Emer Plan. 2016;9(3):281–290. [PubMed] [Google Scholar]
- 28.Ayoola Fatiregun Akinola, Efe Isere Elvis. Epidemic preparedness and management: a guide on Lassa fever outbreak preparedness plan. Niger Med J. 2017;58(1):1–6. doi: 10.4103/0300-1652.218414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortuna C., Remoli M.E., Rizzo C., Benedetti E., Fiorentini C., Bella A., Argentini C., Farchi F., Castilletti C., Capobianchi M.R., Zammarchi L., Bartoloni A., Zanchetta N., Gismondo M.R., Nelli L.C., Vitale G., Baldelli F., D'Agaro P., Sodano G., Rezza G., Arbovirus Working Group. Venturi G. Imported arboviral infections in Italy, July 2014-October 2015: a National Reference Laboratory report. BMC Infect Dis. 2017;17(1):216. doi: 10.1186/s12879-017-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadot L., Segondy M., Foulongne V. Laboratory surveillance of arboviral infections in a southern France region colonized by Aedes albopictus. Epidemiol Infect. 2017 Mar;145(4):710–714. doi: 10.1017/S0950268816002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamison D.T., Alwan A., Mock C.N., Nugent R., Watkins D., Adeyi O., Anand S., Atun R., Bertozzi S., Bhutta Z., Binagwaho A., Black R., Blecher M., Bloom B.R., Brouwer E. Universal health coverage and intersectoral action for health: key messages from disease control priorities, third ed. Lancet. 2018;391(10125):1108–1120. doi: 10.1016/S0140-6736(17)32906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://www4.ti.ch/fileadmin/DSS/DSP/ICM/PDF/Rapporto_di_attivita___2018-Sorveglianza_e_controllo_della_zanzara_tigre_in_Ticino.pdf