Abstract
Social connections are vital to survival throughout the animal kingdom and are dynamic across the lifespan. There are debilitating consequences of social isolation and loneliness, and social support is increasingly a primary consideration in healthcare, disease prevention, and recovery. Considering social connection as an “innate need,” it is hypothesized that evolutionarily conserved neural systems underlie the maintenance of social connections: alerting the individual to their absence and coordinating effector mechanisms to restore social contact. This is reminiscent of a homeostatic system designed to maintain social connection. Here, we explore the identity of neural systems regulating “social homeostasis.” We review findings from rodent studies evaluating the rapid response to social deficit (in the form of acute social isolation) and propose that parallel, overlapping circuits are engaged to adapt to the vulnerabilities of isolation and restore social connection. By considering the neural systems regulating other homeostatic needs, such as energy and fluid balance, we discuss the potential attributes of social homeostatic circuitry. We reason that uncovering the identity of these circuits/mechanisms will facilitate our understanding of how loneliness perpetuates long-term disease states, which we speculate may result from sustained recruitment of social homeostatic circuits.
Keywords: social homeostasis, neural circuits, social isolation, loneliness, social rank, motivational state
Graphical abstract:
Here, we explore the identity of neural systems regulating “social homeostasis.” We review findings from rodent studies evaluating the rapid response to social deficit (in the form of acute social isolation) and propose that parallel, overlapping circuits are engaged to adapt to the vulnerabilities of isolation and restore social connection. By considering the neural systems regulating other homeostatic needs, such as energy and fluid balance, we discuss the potential attributes of social homeostatic circuitry.
Introduction
The 21st century has unleashed a tsunami of opportunities for social engagement and accelerated the flow of social information. Yet as our outlets for social sustenance proliferate, along with the global population1, there is a paradoxical increase in social isolation within society2. The proportion of the population who live alone has risen3 and an increasing number of people experience loneliness4,5. Social isolation presents itself in multiple forms including social rejection, exclusion, ostracism, discrimination, social loss, or neglect—all of which have a significant negative impact on emotional state. Across the animal kingdom, social isolation can threaten survival—individuals lack protection from predators, assistance foraging, support raising offspring, opportunities for social play, and mating prospects. Similarly, in humans, deficits in either objective quantity and/or subjective quality of social relationships can compromise longevity6. Lower social integration (assessed by network size/participation, living arrangements, and frequency of close social contact) is predictive of elevated mortality6–9, and even just the perception of isolation (colloquially referred to as loneliness) is associated with poor physical and mental health10,11 and higher mortality rates6,8,12,13.
However, beyond just constituting an unwelcome emotional side effect of social isolation, loneliness is theorized to represent an “adaptive predisposition” providing the motivational drive to maintain social contact and prevent the aversive consequences of isolation14,15. This adaptive response to deviation from an expected quantity/quality of social connections is reminiscent of negative feedback mechanisms triggered by challenges to physiological homeostasis, such as energy balance, thermoregulation, and others.
In our review, we introduce the idea that coordinated adaptations across discrete neural circuits function to maintain “social homeostasis.” The term social homeostasis has previously been applied to the maintenance of stable organization within a large group of animals, typically social insects, such as ants, termites, and bees. This “supraorganismal” structure requires tight regulation to maintain stable hierarchical social organization when met with changes in the environment or internal composition16,17. Here we propose to extend this concept to the individual level, in order to encourage a mechanistic understanding of how deficiencies in social connection are detected and evaluated, and how effector systems are activated to compensate for perturbations.
Social homeostasis: a widespread phenomenon
Homeostasis classically refers to physiological processes wherein stable states are maintained through compensatory mechanisms18. Homeostatic systems are known to exist for a number of physiological needs essential to survival such as thermoregulation, energy balance, and osmoregulation. These rely upon detection of a deviation from a defined homeostatic “set-point,” followed by central coordination of a response in a “control center”, and the recruitment of “effector systems” that interact with the environment to correct the deviation (Fig. 1). Challenges to physiological homeostasis can also elicit motivated behaviors associated with strong negative “drive” states, such as overheating, thirst, and hunger, designed to appropriately adapt/direct behavior19–21.
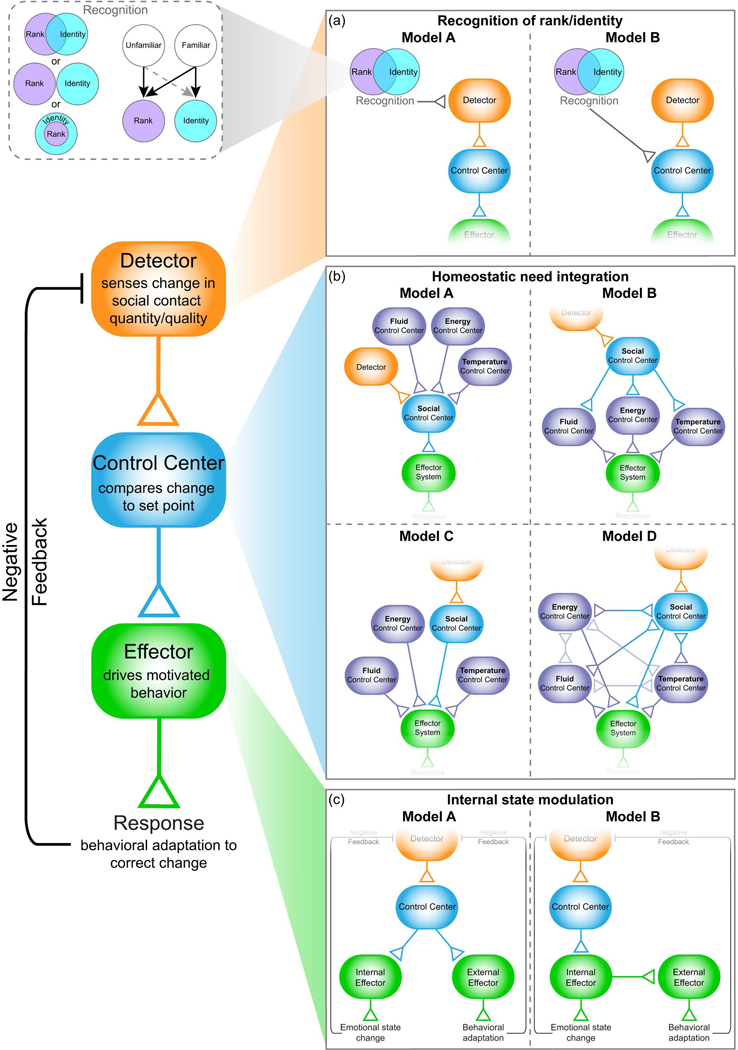
Figure 1.
Proposed model for social homeostasis. Based on Cannon’s classic model for homeostatic regulation18 we propose that a social homeostatic system consists of a detector to sense a change in overall quantity/quality of social contact, a control center to compare this deviation to the individual’s set-point, and effector systems to correct the change. (a) Detection of social signals (both their quantity and quality) would require social recognition. in order to facilitate recall of previous social encounters and determine the expectation for interaction. Information relevant to the identity of the social agent (recognizing that individual as such) as well as estimation of their relative social rank would be required for appropriate evaluation of a deviation. Integration of this information may occur at the level of the detector (model A) or the control center (model B) stage of processing. Identity and rank information may be represented in an overlapping or nonoverlapping fashion (callout box). For a familiar animal both these variables may be incorporated to set social expectation, but for an unfamiliar animal only rank perception would be available. (b) Deviations from the set-point would be evaluated within the control center by comparing the current social input to the homeostatic set-point for quantity and/or quality of social contact. The social control center may integrate information pertinent to other homeostatic needs (e.g., energy balance, fluid balance, and thermoregulation) in a “hub and spoke” fashion (model A), or the social control center may be subservient to other homeostatic control systems (model B). Alternatively, integration of homeostatic needs may occur in a convergent arrangement onto shared effector systems (model C), with interconnections between control centers (model D). (c) If a deviation from set-point is determined, effector systems may be engaged to correct the change. This process could include activation of “external” effectors to promote behavioral adaptation (e.g., social approach/avoidance) along with “internal” effectors to adjust internal/emotional state (model A). Alternatively, engagement of internal effector systems, and a change in emotional state, may itself promote behavioral adaptation (model B).
While a change in social connection may not appear to constitute an immediate challenge to internal stability, individuals on the social perimeter are vulnerable and becoming isolated can threaten survival. Even in controlled laboratory environments (where external threats to survival are absent), the presence of social contact is associated with increased lifespan across a range of social species including honeybees, ants, Drosophila melanogaster22,23, mice24,25, and rats26–28, as well as in free-ranging groups of macaques29, and baboons30. Therefore, an emerging social neuroscience model posits that evolutionarily conserved neurophysiological mechanisms underlie the adaptive, short-term, self-preservation mode triggered by a lack of social connections/mutual protection14,31. This model proposes that loneliness operates as an aversive signal designed to promote adaptation to the vulnerabilities of being alone and motivate reconnection32. Thus, the long-term disease states perpetuated by chronic loneliness may result from the continued engagement of neural systems that were intended for short-term preservation.
To begin unravelling how the chronic state of loneliness emerges, it is necessary to first understand the neural mechanisms underlying the response to social deficit. Conceptualizing this as the response of a homeostatic system would apply certain defined principles (Fig. 1). A social homeostatic system would be required to (1) monitor social conditions; (2) detect deviation from a homeostatic “set point” in control centers; and (3) activate effector systems to elicit an appropriate response (e.g., strategies to promote social contact). A deficit in social connections (whether perceived or actual) would be predicted to engage this system. In animals, one way to create a social deficit is to remove social contact entirely. While this only captures the objective component of social isolation, it offers controlled conditions for assessing rapid neurophysiological adaptations. Chronic social isolation, particularly in rodents, has been used as a developmental model of early life stress since many of the long-term maladaptive changes resemble features of human neuropsychiatric disease33. This rich body of work has been comprehensively reviewed elsewhere for both rodents33–37 and nonhuman primates38–40.
Alternatively, here we examine the response to acute social isolation (using under 1 week as an arbitrary operational definition of “acute” for the purpose of the review) in order to identify candidate neural circuits involved in the rapid response to social deficit. We focus primarily on experiments in social rodents, including laboratory mice (Mus musculus), rats (Rattus norvegicus), and prairie voles (Microtus ochrogaster), which are social species, adapted to group living, but with different styles of social behavior. The wild species of mice and rats from which laboratory strains were derived are promiscuous and territorial, but show greater social tolerance in high-density living environments and adopt linear dominance hierarchies that promote group stability41. In a laboratory setting, mice and rats prefer social company (even that of other males) over a solitary existence42,43. They show conditioned preference for regions previously associated with social contact44, make nests in close proximity to conspecifics when partially separated43,45, and will actively work to obtain social contact46,47. Alternatively, prairie voles are socially monogamous and form an enduring, selective bond with their partner following mating. They show biparental care towards offspring, tend to live in extended families48,49, and are well-utilized in the study of social bonding and isolation.
Here, we evaluate social isolation–induced adaptations in these rodents, in light of the phenotype of human loneliness, which may also represent a state of activation of “social homeostatic systems.” We have categorized the behavioral and neural adaptations into three broad themes: (1) hypervigilance/arousal; (2) social motivation; and (3) passive coping. We propose that parallel, overlapping circuits mediate the response to social deficit (the output of homeostatic “effector” systems) in an effort to heighten attention to environmental stimuli, motivate social reconnection, and limit emotional distress (Fig. 2). While we can only speculate as to the neural identity of the detector, control, and effector systems in a social homeostatic network, we anticipate that a cohesive understanding of the response to social deficit will help unmask candidate neural substrates.
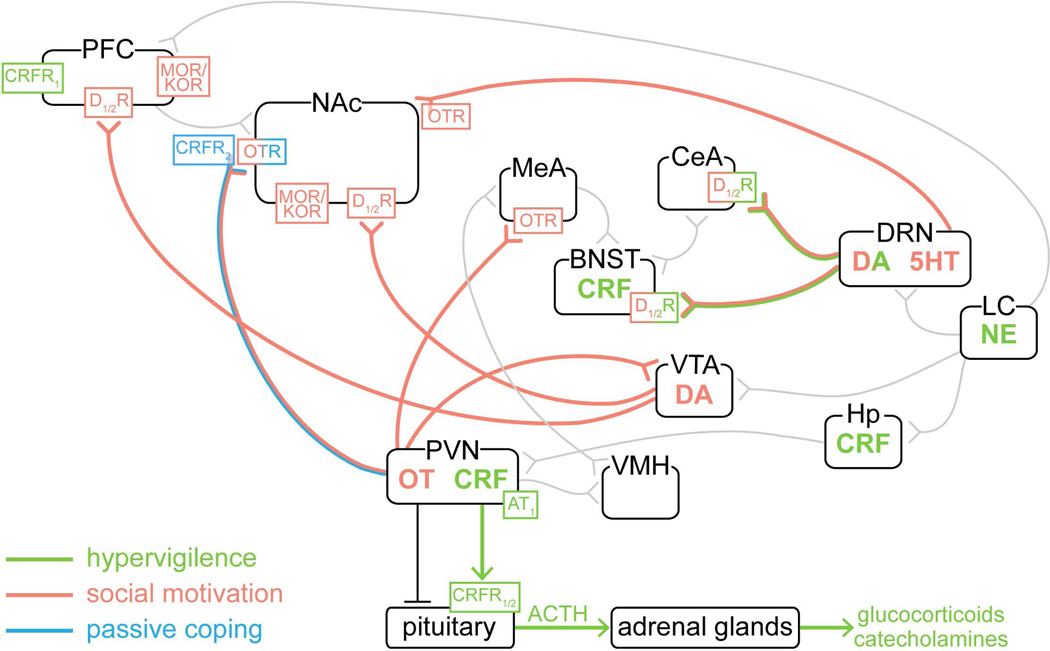
Figure 2.
Neural circuit components implicated in the response to social deficit. Pathways, neuromodulators, neuropeptides, and receptors showing modifications following acute social isolation in rodents. Circuit components are colored based on their involvement in hypervigilance, social motivation, and passive coping. Other prominent projections/connections are shown in grey. Coordinated activity across these parallel, overlapping circuits may function to maintain social homeostasis by heightening attention to environmental stimuli, motivating social reconnection, and limiting emotional distress. 5-HT, 5-hydroxytryptamine (serotonin); ACTH, adrenocorticotropic hormone; AT1, angiotensin II receptor 1; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; CRF, corticotropin-releasing factor; CRFR1/2, corticotropin-releasing factor receptor; DA, dopamine; D1/2, dopamine D1/2 receptor; DRN, dorsal raphe nucleus; Hp, hippocampus; KOR, ĸ-opioid receptor; LC, locus coeruleus; MeA, medial amygdala; MOR, μ-opioid receptor; NAc, nucleus accumbens; NE, norepinephrine; OT, oxytocin; PFC, prefrontal cortex; PVN, paraventricular hypothalamic nucleus; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
Homeostatic response to social deficit: promoting hypervigilance
An evolutionary perspective on the origins of loneliness proposes that the vulnerabilities of isolation promote hypervigilance to guard against potential threats50. Lonely individuals often show high levels of anxiety51,52, and hypervigilant responses to negative social stimuli, suggestive of heightened recruitment of attentional and self-preservation mechanisms53. In rodents, acute isolation can promote behaviors suggestive of enhanced arousal and heightened vigilance. For example, adult rats show an increase in escape-related behaviors over 1–4 days of isolation54, along with a reduction in exploratory behavior and an increase in self-grooming55–57. Targeted manipulations in rodents have unveiled that anxiety-related behaviors arise from activity across distributed, interconnected corticolimbic circuitry, which interpret and evaluate incoming environmental stimuli (reviewed in Ref. 58). One major output system is the hypothalamic–pituitary–adrenocortical (HPA) axis, which regulates arousal, vigilance, and attention, in concert with central arousal circuits including the lateral hypothalamic (LH) orexin/hypocretin system, locus coeruleus (LC) noradrenergic neurons, basal forebrain cholinergic neurons, dorsal raphe nucleus (DRN) serotonergic neurons, and midbrain dopaminergic neurons59,60. Several of these neural circuits exhibit rapid adaptations following acute social isolation. Here, we briefly outline the nature of these changes and their potential role in the response of a social homeostatic system.
HPA axis
Glucocorticoid production is initiated by paraventricular hypothalamic nucleus (PVN) secretion of corticotropin-releasing factor (CRF) into the hypophyseal portal system, triggering adrenocorticotropic hormone (ACTH) release by the anterior pituitary that in turn acts on the adrenal cortex to secrete glucocorticoids. The HPA axis is regulated by a negative feedback loop, wherein glucocorticoids bind to receptors in the pituitary and other brain regions including the hippocampus, which subsequently inhibits CRF and ACTH production. While acute activation of the HPA axis can be an adaptive physiological response to salient events, chronic activation of this system, particularly by continued psychosocial stressors, is implicated in the progression of multiple disease states and psychopathologies61. Consistent with this, high self-reported loneliness in humans has been associated with elevated daily cortisol output62–66 and a flattening of diurnal cortisol rhythm65, suggesting poor regulation of the HPA axis67.
Heightened HPA axis activity (evidenced by a robust increase in circulating corticosterone and ACTH) is observed after 1–5 days of social isolation in juvenile (3–5 weeks old)68,69 or pair-bonded adult70–72 prairie voles. Peripheral corticosterone levels are also reportedly increased in male mice isolated for 12 h,73 and both adrenal ACTH and corticosterone are increased in male rats isolated for 24 h in a novel environment74,75. This recruitment of the HPA axis during acute periods of isolation may reflect the increased need for vigilance and attention to salient stimuli.
CRF signaling
CRF pathways are a prominent point of convergence for isolation-induced adaptations. Aside from their role in initiating the neuroendocrine response to stress, PVN CRF neurons are pivotal in orchestrating the rapid, complex behavioral adaptations that occur following acute stress (potentially via glutamate coreleasing projections to neighboring hypothalamic regions)76. CRF-producing neurons are also widely distributed in extrahypothalamic regions, including the bed nucleus of the stria terminalis (BNST), central amygdala (CeA), nucleus accumbens (NAc), and hippocampus77–79, which have, likewise, been implicated in the behavioral and physiological responses to stress. Isolation of preadolescent female (but not male) mice for <24 h decreased the excitability of PVN CRF neurons in a glucocorticoid-dependent manner80. This finding may reflect glucocorticoid feedback–induced suppression of CRF activity. Consistent with this interpretation, a 24-hour isolation in adult rats decreased CRF mRNA and protein in the PVN75 and decreased cortical CRF1 receptor levels81. Moreover, these changes in CRF were accompanied by enhanced angiotensin II (ATII) AT1 receptor expression in the PVN74. ATII is a circulating endocrine factor that can trigger CRF production in response to stress82. This factor may be necessary for isolation-induced adaptations within the hypothalamic CRF system, as the isolation-induced decrease in CRF mRNA in male rats could be prevented by an AT1 receptor antagonist75. Conversely, a shorter period (1 h) of social isolation in adult male and female prairie voles housed with same-sex siblings, resulted in increased hypothalamic and hippocampal CRF mRNA83. This discrepancy may reflect the shorter duration of isolation or the different species under study. However, it highlights the growing need for a thorough understanding of the timeline of adaptations following social isolation.
LC noradrenergic system
The LC is the sole source of noradrenergic innervation to the central nervous system, best known for its role in arousal and vigilance, but more broadly thought to be recruited to combat environmental challenges84,85. In adult rats, a 24-hour isolation increased tyrosine hydroxylase (TH; the rate-limiting enzyme in catecholamine synthesis) mRNA in the LC, an effect which could be blocked by an AT1 receptor antagonist81. Thus, the acute response to isolation involves coordination across both peripheral and central neuromodulatory systems.
Homeostatic response to social deficit: engaging social motivational systems
In humans, a deficit in social connections is conceptualized to engage the “social monitoring system”86 with the purpose of directing attention towards socially relevant information. Accordingly, lonely individuals show enhanced sensitivity to social cues and increased socially affiliative motivation86–90. Enhanced social motivation is similarly evident in acutely isolated rodents: when given the opportunity, previously isolated (2–48-hour duration) juvenile and adult rodents spend more time engaged in social behaviors91–98. It is suggested that up to 7 days of isolation promotes affiliative social behavior and social interest in rats57,92, whereas in adult mice, a significant increase in aggressive behavior was observed after 48 h, but not 24 h of social isolation99.
For many social species, the inherently rewarding nature of social interactions is a major driving force for social contact. In rodents, one method to evaluate the positive reinforcing properties of social interaction is the social conditioned place preference (social CPP) assay—an adaptation of a test traditionally employed to measure the rewarding properties of drugs of abuse44. In this task, animals typically demonstrate preference for a region previously paired with social housing over one paired with isolate housing (~24-hour duration44). Notably, therefore, the conditioned approach to a socially conditioned context is a product of both “social reward” and “isolation aversion.”44 Several neuromodulatory systems (including dopamine, oxytocin, and opioid circuits) have been posited to underlie the motivation for social reward. These circuits are also prominent sites of rapid adaptation following social isolation, which we discuss below. Notably, however, it remains to be determined whether the motivational processes of “social reward” and “isolation aversion” are encoded in separable or overlapping neural circuits (see also below).
VTA dopamine system
The midbrain dopamine system has a long-standing role in reward processing100 and affiliative social behavior101, and is frequently reported as a site of isolation-induced adaptation. The ventral tegmental area (VTA) dopamine neurons project to multiple regions including the striatum, prefrontal cortex (PFC), and basolateral amygdala (BLA), with the VTA-NAc pathway being particularly well-associated with social reward102,103. In juvenile rats, isolation-induced social play was suppressed by D1- or D2-receptor blockade in the NAc104, while in adult rats, 24 h or 4 days of isolation decreased striatal D2-receptor density105 and increased mesostriatal TH activity106, respectively. Isolation-induced changes do not appear to be limited to the mesostriatal pathway, however, as adolescent mice isolated for 1–7 days showed an increase in both striatal and cortical dopamine metabolism107. Additionally, in the PFC, decreased GABAA-stimulated chloride influx was evident in a membrane preparation from 24-hour isolated rats108 along with reduced benzodiazepine binding81, indicative of reduced cortical GABAA expression and/or function. Given the functional diversity of dopamine input to striatal subregions109,110 and dopamine’s divergent effects on cortical projector populations111, further work is necessary to elucidate precisely how these rapid isolation-induced changes to dopamine neurotransmission influence downstream activity.
DRN dopamine system
Another component of the midbrain dopamine system—the DRN dopamine neurons—also exhibits acute isolation-induced adaptations. These dopamine neurons were historically considered a caudal extension of the VTA, but accumulating evidence has revealed distinct downstream projections and functional roles112–118. In adult male mice, 24 h social isolation potentiated glutamatergic synapses onto DRN dopamine neurons, and also heightened their activity in vivo in response to a novel mouse118. Artificially enhancing activity of DRN dopamine neurons with optogenetic stimulation was sufficient to increase social preference. However, in the absence of a social stimulus, mice chose to avoid receiving stimulation of DRN dopamine neurons (demonstrated by real-time and conditioned place avoidance), which suggests the induction of a negative affective state118. DRN dopamine neurons may, therefore, be recruited following acute isolation to elicit “negative drive”-induced social motivation, in a manner distinct from the reward-related social motivation mediated by the VTA-NAc dopaminergic pathway103. Consistent with this assertion, optogenetic inhibition of the DRN dopamine population had no effect on sociability in group-housed animals, but it suppressed social preference following 24 h of isolation118.
The DRN dopamine neurons lie directly upstream of several regions, most notably the BNST and CeA116,118. While the explicit role of dopamine in these regions in social behavior remains to be determined, dopamine receptor signaling can modulate synaptic transmission and activity in both the BNST and CeA115,118–120. Specifically, in the dorsolateral BNST blunting of long-term potentiation (LTP) is evident just after 24 h of social isolation121. Given that dopamine in the BNST can facilitate glutamatergic transmission, via a CRF-dependent process119, it is tempting to speculate that increased dopamine neurotransmission following acute isolation may occlude LTP.
Interestingly, it was recently revealed that an intermediate duration of isolation in mice (2 weeks) is associated with upregulation of the neuropeptide tachykinin 2 (TAC2; also known as neurokinin B) in several regions including the anterodorsal BNST (adBNST), CeA, and dorsomedial hypothalamus (DMH), with levels gradually increasing from just 30 min post-isolation122. Behavioral changes observed following this period of isolation appeared to be mediated by TAC2 upregulation in discrete sites, as chemogenetic silencing of TAC2-expressing neurons in the adBNST, CeA, or DMH selectively prevented persistent freezing, acute freezing, or aggression, respectively122. Notably, CRF co-expression was observed in ~50% of TAC2-expressing neurons in the adBNST and CeA122, which again highlights the involvement of CRF circuits in isolation-induced adaptation. A substantial body of work supports a role for the BNST in mediating sustained responses, and the CeA in mediating rapid responses, to potential/unpredictable threats123. This behavioral control is enabled by the far-reaching connections of the BNST and CeA, particularly with hypothalamic and brainstem structures, which underlies their ability to influence autonomic and neuroendocrine functions124,125. These regions are therefore well-positioned to drive isolation-induced adaptive responses, under modulatory control from upstream regions, including the DRN dopamine neurons.
This collection of findings compels the hypothesis that dopaminergic signaling may be involved in the initial response to social isolation, but that downstream regions (including the BNST and CeA) might exhibit longer-term remodeling/plasticity following chronic isolation. Indeed, there is considerable evidence to support a similar model for the stages of drug-evoked plasticity in the mesocorticolimbic dopamine system. Specifically, a single dose of cocaine is sufficient to potentiate glutamatergic transmission onto VTA dopamine neurons after 24 hours126. Synaptic strength returns to baseline levels within a week, however, this VTA plasticity is required for the persistent changes that occur downstream in the NAc following prolonged cocaine exposure127 (reviewed in Ref. 128). This permissive role of synaptic plasticity in VTA dopamine neurons could similarly be a feature of DRN dopamine neurons in the response to social isolation. Such a feature would predict that acute isolation-induced synaptic changes in DRN dopamine neurons precede, and are necessary for, chronic isolation-induced adaptations in downstream regions. In this way, the myriad of maladaptive behavioral changes associated with long-term social isolation37 might result from chronic engagement of neural circuits mediating the acute response to social isolation and persistent remodeling in downstream regions.
Opioid system
The opioid system exerts a broad influence on neural activity through widespread expression of opioid peptides and receptors, most notably within regions connected to positive reinforcement (reviewed in Ref. 129). Opioid signaling plays well-documented roles in regulating pain/analgesia130, reward processing129, and social bonding131, and has also been implicated in isolation-induced social behavior. In vivo autoradiography revealed changes to opioid receptor binding, with 7 days of isolation in juvenile rats associated with upregulation of opioid receptor number or affinity in the PFC57. Additionally, isolation-induced social play in juvenile rats was attenuated by systemic administration of a μ-opioid (MOR) antagonist97,132 or a ĸ-opioid receptor (KOR) agonist132, but enhanced by administration of a MOR agonist97. Furthermore, in the CeA, infusion of an ACTH analog suppressed isolation-enhanced social interest in 7-day isolated rats, but this could be prevented by administration of naltrexone (a MOR and KOR antagonist)56,133. Therefore, both opioid and dopamine receptor signaling may be necessary for the heightened sociability evoked by acute isolation.
Hypothalamic oxytocin system
Oxytocin-producing neurons of the PVN, along with the closely related vasopressin (AVP) neurons, are intimately involved in the regulation of social affiliation134 and have been particularly well-studied in the monogamous prairie vole, as they play a pivotal role in pair bonding135. Oxytocin neurons project not only to the posterior pituitary where they release oxytocin into the bloodstream but also to distinct targets within the brain for direct modulation of neuronal activity. One important site for oxytocin action is the NAc, which is a critical hub for the integration of motivationally relevant information and relays information to elicit motor responses136. In male prairie voles, 3 days of isolation from a bonded female partner, but not a male sibling, decreased oxytocin mRNA in the PVN and oxytocin receptor binding in the NAc shell137. Notably, oxytocin signaling in the NAc is reportedly essential for the expression of social CPP in adult male mice138. Specifically, it was elegantly demonstrated that social CPP required activation of oxytocin receptors on presynaptic terminals in the NAc arising from DRN serotonergic neurons—facilitating serotonin release138. In addition, social CPP was shown to be dependent on the PVN oxytocin projection to the VTA139. Either suppressing activity in the PVN-VTA pathway or VTA dopamine-specific knockout of oxytocin receptors prevented social CPP in male mice139. Collectively, these findings illustrate the dynamic balancing of dopamine, oxytocin, and serotonin signaling that is required for the reinforcing properties of social interactions.
Acute isolation has also been demonstrated to disrupt social recognition memory140. Rodents possess an innate tendency to investigate novel rather than familiar social stimuli and typically reduce their investigation of a familiar conspecific on repeated exposure141. This effect is absent in mice isolated for 24 h or 7 days, who display equivalent investigation of a familiar juvenile compared with the first exposure142,143. This lack of social recognition memory is associated with a suppression of oxytocin-dependent synaptic plasticity in the medial amygdala (MeA) following 7 days of isolation in rats144. It has been further revealed that suppression of social recognition memory may result from elevated hippocampal Ras-related C3 botulinum toxin substrate 1 (from the Rho family of small GTPases), which is evident in male mice isolated for 24 h or 7 days145. Thus, isolation-induced modifications to oxytocin signaling, in a pathway-specific manner, may contribute to changes in both social motivation and social recognition memory.
Homeostatic response to social deficit: a self-protective coping strategy?
Individuals that self-identify as lonely frequently exhibit features of negative affective state or depression10,50,146–149. Furthermore, individuals swayed towards feelings of future loneliness (by receipt of false feedback following a questionnaire) show a reduction in physical pain sensitivity and emotional sensitivity89. This suggests the adoption of self-protective strategies to minimize further emotional distress. While we cannot directly measure emotional state in rodents, we can assay motivated behavior as a proxy58,150. In rodents, immobility in the forced swim and tail suspension tests is thought to reflect passive coping and/or behavioral despair151,152 but see Ref. 153. There is a general lack of agreement over whether acute isolation alters immobility in these assays in mice55,73,154. However, more consistent results have been obtained in monogamous prairie voles, wherein females or males isolated from their bonded partner (but not from a male sibling) for 3–5 days show an increase in immobility time70,71,137.
There is also evidence for disruption of reward-related behavior in acutely isolated rodents, specifically in the response to addictive drugs. In rats, a 24-hour social isolation increased preference for ethanol and opioid intake, which was reversed with social housing155,156. Reduced pain sensitivity has also been reported, with male mice and juvenile rats exhibiting higher thermal and mechanical pain thresholds following 2–3 days of social isolation157,158. The prominent role of dopamine and opioid signaling in mediating the effects of drugs of abuse159 and analgesia130,160 makes them strong contenders for underlying these adaptations. In particular, chemogenetic activation of ventrolateral periaqueductal grey (vlPAG)/DRN dopamine neurons can promote antinociception117, while a lesion of these neurons suppresses both the antinociceptive161,162 and rewarding163 properties of exogenous opioids. Furthermore, inhibition of VTA dopamine signaling in mice can induce depression-related behaviors164, while KOR antagonists are proposed to have antidepressive effects in rodents (see Refs. 165–167). In this way, interactions between the dopamine and opioid systems may underlie isolation-induced changes to reward processing, pain sensitivity, and emotional affect.
In addition to dopaminergic and opioidergic mechanisms, isolation-induced depressive-like behavior may result from changes in the balance of CRF and oxytocin neurotransmission. Specifically, the passive coping behavior observed in pair-bonded prairie voles isolated for 3 days was prevented by NAc shell infusion of a CRF2 receptor antagonist, or oxytocin, throughout the period of isolation137. Microdialysis experiments suggest a mechanism by which this effect is mediated via presynaptic CRF2 receptor activation on oxytocin terminals in the NAc, which serves to reduce oxytocin release137. These findings point towards a confluence of isolation-induced adaptations in the NAc. The NAc receives strong glutamatergic input from thalamic and cortical regions, enabling it to integrate motivationally relevant information from neuromodulatory nuclei with higher cognitive and sensory input168. Thus, this region is aptly poised to adapt goal-directed behavior in response to social deficit.
Proposed attributes of components within a social homeostatic system.
Flexibility
Animals are frequently faced with conflicting signals in the environment, which can elicit competing motivational drives. To ensure survival, animals must appropriately weigh environmental cues and evaluate them in light of current homeostatic need state. Selecting the appropriate behavioral response under these conditions requires dynamic balancing and coordination of neural activity169. Thus, a key requirement for a social homeostatic system is its capacity to flexibly adapt to meet other physiological needs. Specifically, a change in environmental conditions and/or need state (e.g., hunger and thirst) may require a shift in the “set point” for social contact in the control center (Figs. 1b and 3). This will be heavily influenced by dynamic factors, such as resource availability, predator threat, mating prospects, and the presence of offspring.
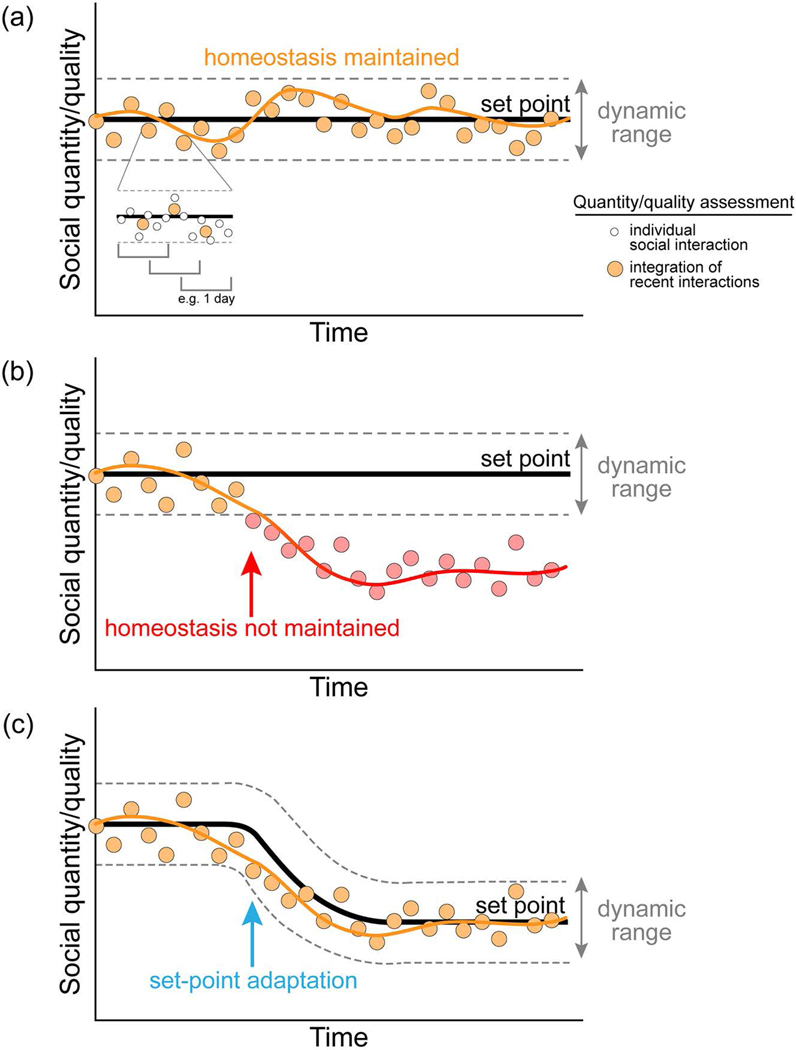
Figure 3.
Long-term integration of social experience within a homeostatic system. (a) Under normal conditions, appropriate functioning of a social homeostatic system would maintain social contact quantity/quantity within an acceptable dynamic range. Experienced social interactions may be assimilated and assessed (compared with set-point) in a sliding window fashion across time (e.g., days/months). Social quality and quantity information might be weighted differently depending on the individual (traits) and current environmental conditions. The set-point could be determined by a combination of factors including age, sex, species-typical behavior, and past history of social encounters. (b) Failure of homeostatic system to correct deviations in social contact quantity/quality might result in a chronic deficit. This deficit (whether perceived or actual) may be associated with chronic engagement of homeostatic effector systems, and experienced as a state of loneliness. (c) Major life or environmental changes, such as moving away from home, switching jobs, and others, might provoke the need for a shift in set-point. A stable shift in set-point, and acceptance of a new expected quantity/quality for social contact, could represent an adaptive change. This shift may prevent social homeostatic effector systems from being chronically recruited and promote continued balance.
For example, in a state of hypernatremia (elevated plasma sodium, which is associated with the perception of thirst), rats exhibit greater social investigation of a novel intruder170. This effect is proposed to have evolved to suppress anxiety in social situations, such as those encountered at a communal source of water, in order to promote social approach and allow drinking behavior170. This observation suggests that social motivation can be altered by other physiological needs. Intriguingly, acute hypernatremia in rats is also associated with increased plasma oxytocin, increased c-Fos expression in magnocellular PVN oxytocin neurons, and suppression of ATII production170. Given that ATII signaling can drive HPA axis activity, these data suggest that hypernatremia concomitantly suppresses activity within stress-related circuitry, while promoting activity in social reward-related oxytocin pathways, thereby inhibiting stress/anxiety-related behavior and facilitating social interaction. This relationship illustrates overlap and coordination across homeostatic systems and also demonstrates the flexibility of effector systems.
The motivational state of hunger is another essential homeostatic drive which promotes rapid neural adaptations171,172. Specifically, the agouti-related protein (AgRP) neurons in the arcuate nucleus of the hypothalamus are essential in the maintenance of energy balance, and their activity rapidly drives feeding behavior in a state of hunger173. Interestingly, optically evoked feeding behavior in isolated mice persists, even in the presence of a novel male or female mouse171. This finding suggests that AgRP activity in a state of hunger is sufficient to override a competing social motivational drive. Conceptually, it is possible that competing homeostatic drives are integrated into a social homeostatic system at the level of the control center in a “hub-and-spoke” fashion, or they may form an interconnected hierarchical arrangement that converges on effector systems (Fig. 1b). While it is possible that one homeostatic system may be subservient to another, an interconnected network would permit flexible control in a state of motivational need competition, prior to convergence on effector systems. Precisely how these homeostatic systems interface with one another remains to be elucidated.
Maintaining physiological variables, such as core body temperature and energy levels, within an appropriate dynamic range relies heavily upon a functioning immune system. Inflammation is theorized to be a unifying feature of homeostatic perturbation: providing a protective response to extreme deviations from the homeostatic set point174,175. Adverse conditions, including social isolation, can provoke a shift in immune function: enhancing expression of proinflammatory genes and reducing expression of antiviral/antibody-related genes in circulating leukocytes176. Social isolation may recruit this response to appropriately prepare an individual for the susceptibilities of being alone, which might include an increased need for a rapid inflammatory response to combat bacterial infections sustained through physical injury, but the reduced need for protection against socially transmitted viral infections15,176. This immune response also appears to be recruited in a state of perceived isolation—humans with high self-reported loneliness show enhanced proinflammatory activity but reduced antiviral response66,177–179. Immune system changes can also predict subsequent loneliness, suggesting a reciprocal relationship between these two phenomena64. Remarkably, classifying rhesus macaques as putatively high in loneliness (by sociability levels and social initiation attempts)180 revealed leukocyte gene expression changes similar to those observed in lonely human subjects64. Mounting evidence for recruitment of inflammatory processes under conditions of actual or perceived social isolation31 suggests that this state is recognized as a threat to an essential variable. As such, this supports the assertion that social contact may be regulated in a homeostatic manner.
Mixed selectivity
Considering the neural processing of “social homeostatic” information, one possibility is the existence of dedicated neural circuitry. An alternative model would feature overlap between neural systems governing social homeostasis and other highly conserved neural circuitry. This scenario would predict that certain nodes in a social homeostatic network display “mixed selectivity,” similar to neurons underlying other complex cognitive processes181. In particular, this is conceivably a feature of “effector” regions in a homeostatic system. The disinhibition of VTA dopamine neurons, for example, has been shown to enhance motivation towards a variety of stimuli ranging from social stimuli to novel objects182,183. Furthermore, activation of BLA input to the ventral hippocampus or mPFC not only induces robust anxiety-like behavior in exploratory assays but also suppress social investigation in the resident–intruder assay184–186. Perturbations in social behavior are often, but not always, co-expressed with anxiety-related behaviors, and there is significant overlap in underlying neural correlates, which suggests a tight relationship between these two forms of behavioral expression58,187.
The DRN dopamine system is another prime example of overlapping circuit function. Monitoring fluorescent calcium activity in vivo revealed that these neurons are active in response to social stimuli, and this activity is heightened following acute isolation118. However, these neurons are also responsive to other salient stimuli, including palatable food and unexpected foot shock, and show greater activity during wakefulness compared with sleep, suggesting an arousal-promoting function112,115,188. This diversity of sensitivities is consistent with the notion that neural circuits regulating social homeostasis may promote attention to a variety of salient stimuli, in an effort to scan the environment for potential threats or opportunities for social engagement. It was also recently reported that optogenetic or chemogenetic inhibition of DRN dopamine neurons during fear conditioning suppressed freezing in response to a footshock-predictive cue, suggesting an additional role in aversive responding115. Taken together, these findings illustrate the existence of multiple mechanisms through which DRN dopamine neurons may limit the vulnerabilities of being alone: increasing social motivation, promoting vigilance/arousal, and enhancing responsivity to aversive stimuli.
Considering the potential for mixed selectivity, the recent observation that activation of DRN dopamine neurons not only increases social preference118 but can promote antinociception117 could be reconciled in a number of ways. One possibility is that functional heterogeneity exists within this cell population. Another possibility is biological convergence in the representation of emotional pain and nociceptive pain in DRN dopamine neurons. In accordance with this notion, an emerging hypothesis posits that social and physical pain are processed by overlapping neural circuitry189. This is supported by human imaging studies revealing that social disconnection engages brain regions including the dorsal anterior cingulate cortex and anterior insula cortex190, which also process the affective component of physical pain191. This dual role of DRN dopamine neurons also points towards a potential mechanism through which acute isolation reduces pain perception157,158. Opioidergic signaling might contribute to this effect as a MOR agonist has been shown to disinhibit DRN dopamine neurons117. It is also interesting to note that inflammatory pain is suppressed by activation of AgRP neurons, suggesting a general reduction of chronic pain perception by strong motivational drive states192. Mixed selectivity may, therefore, be a common feature within neural circuits regulating homeostatic needs, and cross-talk between these systems might facilitate the activation or suppression of appropriate “effector” systems (Fig. 1).
Subjective nature of social experience
A third element in conceptualizing a social homeostatic system is the integration of subjective experience. There is mounting support for the notion that subjective or “perceived” isolation (the quality of social relationships) is a stronger predictor of poor health and emotional state in humans than objective isolation (the number/frequency of social contacts)11,62,146. Consistent with this, loneliness—independent of social network size—is associated with higher mortality13,193 increased blood pressure11,194, higher rate of diabetes, hypertension, arthritis, emphysema195, and Alzheimer’s disease196, along with poor health habits stemming from a lack of self-control197,198. Thus, in evaluating social needs, a homeostatic system would need to incorporate a subjective assessment of social experience, in addition to its overall objective nature), which may be heavily influenced by interoceptive signals and internal state (Figs. 1 and 3).
There is an ongoing debate as to whether animals experience emotions in the same way as humans150,199. However, it has been reasoned that emotions constitute an internal state, encoded by specific neural circuits, which can give rise to externally observable behaviors150. These internal brain states may be subjectively perceived as feelings by the individual150. Although the traditional concept of homeostasis refers to a purely automatic physiological control system, motivational drive states (guided by “homeostatic feelings”) play a significant role in the maintenance of homeostasis200. Homeostatic feelings act as “informative regulatory interfaces”—providing means for an animal to sense its physiological state and guaranteeing attention to relevant stimuli200. While this process can be adaptive and introduces greater flexibility into homeostatic regulation, it also passes an element of control to the individual, taking homeostatic regulation beyond purely automatic mechanisms200.
In order to understand the neural mechanisms of social homeostasis, a major hurdle lies in the ability to infer subjective social experience in animals14. Although we can never truly know the emotional experience of a rodent, one method of differentiating between individuals is by exploiting the natural variability introduced into groups of animals by social hierarchy. Grouped living can lead to the establishment of social hierarchies in multiple species including fish, birds, rodents, and primates201–204. Hierarchies create a scenario in which grouped individuals might have divergent perceptions of their social experience. Social rank can influence access to essential resources including food, territory, and mates205, and thus a more dominant rank is often a coveted position associated with higher quality of life. Although subordination in animal societies is not always directly related to low social connectedness or unmet social needs, social rank bestows variability in subjective social experience without removing support structure for safety, warmth and other nonsocial benefits of a group.
Strikingly, studies on social hierarchy in mice and rats have revealed underlying neural correlates in the same circuits implicated in the response to social deficit (Fig. 2). These findings include differences between subordinates and dominants in CRF expression in the BNST, CeA, MeA, and mPOA206, mitochondrial function and dopamine signaling in the NAc207,208, and glutamatergic synaptic strength in the mPFC209. The mPFC, in particular, is frequently implicated in the representation of social rank. Most recently, “winning”-induced plasticity in the tube test was localized to a mediodorsal thalamic (MDT) projection to the dorsomedial PFC (dmPFC), as phasic optogenetic stimulation of the dmPFC, or the MDT–dmPFC projection, immediately induced winning against a previously dominant cagemate210. Notably, social rank also predicted the magnitude of behavioral effects elicited upon DRN dopamine manipulations in mice118. Optogenetic activation of these neurons promoted social preference and real-time place avoidance, whereas inhibition reduced isolation-induced social preference. However, the behavioral change observed in these assays was greater in dominant animals relative to subordinates118. This observation suggests that prior social experience may influence the ability of the DRN dopamine neurons to modulate behavior. Collectively, these findings illustrate that rank-related information may be integrated into multiple neural circuits that respond to social deficit (Fig. 1A). This organization would permit flexible control over homeostatic regulation and adjustment of goal-directed behavior depending on the social opportunities available.
Moving forward, several questions remain in elucidating how social information might be processed through a homeostatic system. For example, is the individual’s “expectation” for social contact encoded upstream in detector regions, or at the level of the control center? And how are different categories of social contact represented? To speculate on this last point, one possibility is that a social homeostatic system is category blind. Another potential arrangement would involve separate processing streams for the regulation of different social relationships, such as same-sex affiliative, opposite sex, mother–offspring, unfamiliar conspecifics, and others. Indeed, specialized circuits, within discrete hypothalamic nuclei, underlie the expression of parental behavior211, aggression212, male intruder-specific behavior213, opposite sex approach,214 and mating212. If we conceptualize these as discrete “effector systems,” then this raises the possibility that decentralized processing of different social “needs” may occur in separable nodes215. However, the precise organization of social homeostatic elements remains a topic of conjecture.
Valence of motivational drive
A fourth consideration is the valence of motivational drives that direct social interaction216. Motivated behaviors regulating food intake are often distinguished as homeostatic (essential for maintaining energy balance and survival) or hedonic (driven by sensory perception or pleasure in the absence of a need state)217. Feeding behavior, therefore, is directed by motivational drives of opposing valence: the negative sensation of hunger and the positive hedonic value of palatable food. In extrapolating to social behavior, equivalent opposing motivational drives may promote social interaction: the aversive state of isolation and the hedonic value of social reward. However, while mechanistic differences exist, the neural systems mediating homeostatic and hedonic feeding are proposed to be intertwined, and highly overlapping with reward circuitry217,218. Similarly, social reward circuitry is heavily recruited in isolated animals. Engagement of reward circuitry in situations of social deficit may enhance the rewarding value of social contact—potentially similar to how food deprivation enhances the rewarding properties of food219–222. In support of this concept, functional magnetic resonance imaging in humans has revealed that more lonely individuals show greater activation of the ventral striatum in response to familiar social cues223, but contrastingly reduced activation in response to unfamiliar social cues224. Similarly, ventral striatal activity is initially high in response to palatable food but diminishes as individuals consume beyond satiety225.
The coordination of social behavior to meet homeostatic needs may, therefore, recruit both positive and negative motivational processes. The DRN dopamine system might be one source of negative motivational drive in response to social deficit118. Recruitment of this system aligns with the “drive reduction” hypothesis, in which internal state elicits goal-directed behaviors in order to reduce the intensity of an aversive/negative motivational drive (e.g., hunger and thirst)226 (Fig.1c). A potentially similar function has been described for arcuate nucleus AgRP neurons and nitric oxide synthase 1 (NOS1) neurons in the subfornical organ (SFO). These neurons show heightened activity during hunger (AgRP) and thirst (NOS1), their activity elicits an aversive state (place avoidance), and they are essential for driving feeding and drinking behaviors, respectively21,227.
However, the role of valence processing in homeostatic feeding behavior is complex. AgRP neurons are activated in a state of energy deficit228, and their optical stimulation can voraciously promote food consumption, but also elicits real-time place avoidance (indicative of an aversive state) in the absence of food173,227,229. However, AgRP activity is rapidly suppressed on sensory detection of food227,228,230, which, surprisingly, suggests that ongoing AgRP activity is dispensable for food consumption. This paradox is potentially reconciled by the observation that brief optical stimulation, as little as 1 min, prior to food availability was sufficient to promote robust, sustained feeding in well-fed mice once food was made available231. Furthermore, mice performed operant responses to stimulate AgRP neurons in the presence, but not the absence of food, suggesting that AgRP activity can be positively reinforcing231. Therefore, an alternative hypothesis proposes that AgRP activity provides a sustained positive valence signal, that potentiates the incentive value of food, and supports transition from foraging to feeding behavior via persistent changes in downstream circuitry231. Intriguingly, this is not a feature of SFO NOS1 neurons, as prestimulation was insufficient to drive drinking behavior when water was subsequently made available231. Therefore, the relationship between neuronal activity and behavioral regulation may depend on a specific homeostatic need.
While the precise role of DRN dopamine activity in social motivation and valence processing remains to be fully elucidated, drawing insight from other neural circuits that participate in maintaining homeostatic balance provides mechanistic clues into their mode of operation. However, an important consideration for social behavior is that (unlike food detection) initial social contact does not necessarily guarantee a rewarding social experience. Therefore immediate suppression of neural activity on social contact may be inappropriate for DRN dopamine neurons, and activity may persist until a stable relationship has been achieved. Moving forward, it will be important to determine the temporal dynamics of activity within and across neural circuits during the response to social deficit and to understand how valence is represented in these systems.
Outlook
Moving forward, we propose that improving the evaluation of subjective social experience, and standardizing parameters used in studies of social behavior (Table 1), will accelerate the assembly of a cohesive model for social homeostasis. Studies in rodents are continuing to move towards approaches that capture larger, more naturalistic group living206, and the incorporation of automated tracking is permitting a deeper longitudinal analysis of complex social interaction dynamics233. Great promise has arisen from detailed behavioral observations on semifree-ranging groups of nonhuman primates, facilitating classification of social relationship quality in females chacma baboons30 and putative loneliness in male rhesus macaques180. Across the animal kingdom, we may see conservation in neuromodulatory systems for social behavior all the way to invertebrate systems, as recent groundbreaking work in the octopus demonstrates232.
Table 1.
Experimental conditions to report in the methodology of studies on social behavior and/or social isolation to facilitate informative interpretation and reproducibility.
| Experimental conditions | |
|---|---|
| Housing conditions | |
| Grouped/control animals | Isolated animals |
| Number of cagemates | Prior number of cagemates |
| Cagemate relationship (siblings/age-matched, etc.) | Cagemate relationship (siblings/age-matched, etc.) |
| Sexual experience | Sexual experience |
| Age at isolation | |
| Duration of isolation | |
| Extent of experimenter handling | Extent of experimenter handling |
| Housing type (size, bedding material, etc.) | Housing type (size, bedding material, etc.) |
| Social rank (if known) | Social rank (if known) |
| Environmental enrichment | Environmental enrichment |
| Proximity of other animals | |
| Normal or reverse light/dark cycle | Normal or reverse light/dark cycle |
| Measurement of behavioral/neurophysiological parameters | |
| Time of testing | |
| Age at testing | |
| Conditions of behavioral assays | |
| Stress exposure | |
| Food/water restriction | |
| Timeline of conducted experiments | |
Current technological approaches in rodents now provide unprecedented temporal and spatial resolution with which to scrutinize neural circuits and have already yielded fascinating results identifying discrete systems mediating specific social behaviors including parental behavior211, social reward103,138,139, male intruder-specific behavior213, opposite-sex approach214, and social observational learning234. The new millennium has brought with it a rapid rise in opportunities for social nourishment together with a growing prevalence of loneliness and social isolation. Given the protective effects of social contact on a vast array of physical and mental health measures, there has never been a more important time to understand the neural mechanisms underlying the need for social connection.
Acknowledgements
We thank Gwendolyn G. Calhoon and Ruihan Zhang for comments on the manuscript and all members of the Tye Lab for helpful discussion. K.M.T. is a New York Stem Cell Foundation - Robertson Investigator and McKnight Scholar and this work was supported by funding from the JPB Foundation, the Picower Institute Innovation Fund (PIIF) and PIIF Engineering Award, the Picower Neurological Disorder Research Grant, a Junior Faculty Development Program, the Alfred P. Sloan Foundation, the New York Stem Cell Foundation, the McKnight Foundation, and by the following grants from the National Institutes of Health: NIMA R01-MH102441–01, NIA RF1-AG047661–01, NIAAA R01-AA023305–01, the NIH Director’s New Innovator Award DP2-DK-102256–01 (NIDDK), and the NIH Director’s Pioneer Award DP1-AT009925 (NCCIH). G.A.M. was supported by a fellowship from the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., cotrustees.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.United Nations. The World Population Prospects: 2015 Revision. (2015). [Google Scholar]
- 2.McPherson M, Smith-Lovin L. & Brashears ME Social Isolation in America: Changes in Core Discussion Networks over Two Decades. Am. Sociol. Rev. 71, 353–375 (2006). [Google Scholar]
- 3.United Nations. United Nations, Department of Economic and Social Affairs, Population Division (2017). Household Size & Composition, 2017 (2017). Available at: https://population.un.org/Household/index.html#/countries. (Accessed: 9th September 2018) [Google Scholar]
- 4.Cacioppo JT & Patrick W. Loneliness: Human Nature and the Need for Social Connection. (W. W. Norton & Company, 2008). [Google Scholar]
- 5.Victor CR & Yang K. The Prevalence of Loneliness Among Adults: A Case Study of the United Kingdom. J. Psychol. 146, 85–104 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Holt-Lunstad J, Smith TB & Layton JB Social Relationships and Mortality Risk: A Meta-analytic Review. PLoS Med 7, e1000316 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkman LF & Syme SL Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 109, 186–204 (1979). [DOI] [PubMed] [Google Scholar]
- 8.House JS, Landis KR & Umberson D. Social relationships and health. Science 241, 540–545 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Steptoe A, Shankar A, Demakakos P. & Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl. Acad. Sci. U. S. A. 110, 5797–5801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC & Thisted RA Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging 21, 140–151 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Hawkley LC, Masi CM, Berry JD & Cacioppo JT Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol. Aging 21, 152–164 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Holwerda TJ et al. Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL). Psychol. Med. 42, 843–853 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Perissinotto CM, Stijacic Cenzer I. & Covinsky KE Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med. 172, 1078–1083 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cacioppo JT et al. Loneliness Across Phylogeny and a Call for Comparative Studies and Animal Models. Perspect. Psychol. Sci. J. Assoc. Psychol. Sci. 10, 202–212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberger NI & Cole SW Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 15, 669–674 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Emerson AE Regenerate Behavior and Social Homeostasis of Termites. Ecology 37, 248–258 (1956). [Google Scholar]
- 17.Wheeler WM The ant-colony as an organism. J. Morphol. 22, 307–325 (1911). [Google Scholar]
- 18.Cannon WB THE SYMPATHETIC DIVISION OF THE AUTONOMIC SYSTEM IN RELATION TO HOMEOSTASIS. Arch. Neurol. Psychiatry 22, 282–294 (1929). [Google Scholar]
- 19.Betley JN, Cao ZFH, Ritola KD & Sternson SM Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oka Y, Ye M. & Zuker CS Thirst Driving and Suppressing Signals Encoded by Distinct Neural Populations in the Brain. Nature 520, 349–352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman CA et al. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey JR Demographic mechanisms for the evolution of long life in social insects. Exp. Gerontol. 36, 713–722 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Omholt SW & Amdam GV Epigenetic regulation of aging in honeybee workers. Sci. Aging Knowl. Environ. SAGE KE 2004, pe28 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Chida Y, Sudo N. & Kubo C. Social isolation stress exacerbates autoimmune disease in MRL/lpr mice. J. Neuroimmunol. 158, 138–144 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa-Toyama Y, Zhang S. & Krieger M. Dietary Manipulation and Social Isolation Alter Disease Progression in a Murine Model of Coronary Heart Disease. PLOS ONE 7, e47965 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermes GL et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl. Acad. Sci. U. S. A. 106, 22393–22398 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menich SR & Baron A. Social housing of rats: life-span effects on reaction time, exploration, weight, and longevity. Exp. Aging Res. 10, 95–100 (1984). [DOI] [PubMed] [Google Scholar]
- 28.Yee JR, Cavigelli SA, Delgado B. & McClintock MK Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom. Med. 70, 1050–1059 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brent LJN, Ruiz-Lambides A. & Platt ML Family network size and survival across the lifespan of female macaques. Proc. Biol. Sci. 284, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silk JB et al. Strong and Consistent Social Bonds Enhance the Longevity of Female Baboons. Curr. Biol. 20, 1359–1361 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Cacioppo JT & Cacioppo S. Chapter Three - Loneliness in the Modern Age: An Evolutionary Theory of Loneliness (ETL). in Advances in Experimental Social Psychology (ed. Olson JM) 58, 127–197 (Academic Press, 2018). [Google Scholar]
- 32.Cacioppo JT, Cacioppo S. & Boomsma DI Evolutionary Mechanisms for Loneliness. Cogn. Emot 28, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fone KCF & Porkess MV Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 32, 1087–1102 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Marco EM et al. The maternal deprivation animal model revisited. Neurosci. Biobehav. Rev. 51, 151–163 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Tractenberg SG et al. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 68, 489–503 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Lapiz MDS et al. Influence of Postweaning Social Isolation in the Rat on Brain Development, Conditioned Behavior, and Neurotransmission. Neurosci. Behav. Physiol. 33, 13–29 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Robbins TW Neurobehavioural sequelae of social deprivation in rodents revisited: Modelling social adversity for developmental neuropsychiatric disorders. J. Psychopharmacol. (Oxf.) 30, 1082–1089 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Harlow HF, Dodsworth RO & Harlow MK Total social isolation in monkeys. Proc. Natl. Acad. Sci. U. S. A. 54, 90–97 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney WT Primate Social Isolation: Psychiatric Implications. Arch. Gen. Psychiatry 31, 422–426 (1974). [DOI] [PubMed] [Google Scholar]
- 40.Suomi SJ, Mineka S. & Harlow HF Social Separation in Monkeys as Viewed from Several Motivational Perspectives in Motivation (eds. Satinoff E. & Teitelbaum P) 543–583 (Springer US, 1983). doi: 10.1007/978-1-4684-4286-1_14 [DOI] [Google Scholar]
- 41.Lott DF Intraspecific Variation in the Social Systems of Wild Vertebrates. Behaviour 88, 266–325 (1984). [Google Scholar]
- 42.Balcombe JP Laboratory environments and rodents’ behavioural needs: a review. Lab. Anim. 40, 217–235 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Loo PLPV, Groot A. C. de, Zutphen BFMV & Baumans V. Do Male Mice Prefer or Avoid Each Other’s Company? Influence of Hierarchy, Kinship, and Familiarity. J. Appl. Anim. Welf. Sci. 4, 91–103 (2001). [Google Scholar]
- 44.Panksepp JB & Lahvis GP Social reward among juvenile mice. Genes Brain Behav. 6, 661–671 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Loo PLP, Van de Weerd HA, Van Zutphen LFM & Baumans V. Preference for social contact versus environmental enrichment in male laboratory mice. Lab. Anim. 38, 178–188 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Patterson-Kane EG, Hunt M. & Harper D. Rats Demand Social Contact. Anim. Welf. 11, 327–332 (2002). [Google Scholar]
- 47.Sherwin CM Laboratory mice persist in gaining access to resources: a method of assessing the importance of environmental features. Appl. Anim. Behav. Sci. 48, 203–213 (1996). [Google Scholar]
- 48.Carter CS, DeVries AC & Getz LL Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 19, 303–314 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Getz LL & Hofmann JE Social organization in free-living prairie voles, Microtus ochrogaster. Behav. Ecol. Sociobiol. 18, 275–282 (1986). [Google Scholar]
- 50.Cacioppo JT et al. Loneliness within a nomological net: An evolutionary perspective. J. Res. Personal. 40, 1054–1085 (2006). [Google Scholar]
- 51.Ginter EJ, Glauser A. & Richmond BO Loneliness, Social Support, and Anxiety among Two South Pacific Cultures. Psychol. Rep. 74, 875–879 (1994). [DOI] [PubMed] [Google Scholar]
- 52.Stednitz JN & Epkins CC Girls’ and mothers’ social anxiety, social skills, and loneliness: associations after accounting for depressive symptoms. J. Clin. Child Adolesc. Psychol. Off. J. Soc. Clin. Child Adolesc. Psychol. Am. Psychol. Assoc. Div. 53 35, 148–154 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Cacioppo S. et al. Loneliness and implicit attention to social threat: A high-performance electrical neuroimaging study. Cogn. Neurosci. 7, 138–159 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Hurst null, Barnard null, Tolladay null, Nevision null & West null. Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Anim. Behav. 58, 563–586 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Hilakivi LA, Ota M. & Lister RG Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ‘despair’. Pharmacol. Biochem. Behav. 33, 371–374 (1989). [DOI] [PubMed] [Google Scholar]
- 56.Hol T. & Spruijt BM The MSH/ACTH(4–9) analog Org2766 counteracts isolation-induced enhanced social behavior via the amygdala. Peptides 13, 541–544 (1992). [DOI] [PubMed] [Google Scholar]
- 57.Vanderschuren LJ, Stein EA, Wiegant VM & Van Ree JM Social isolation and social interaction alter regional brain opioid receptor binding in rats. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 5, 119–127 (1995). [DOI] [PubMed] [Google Scholar]
- 58.Calhoon GG & Tye KM Resolving the neural circuits of anxiety. Nat. Neurosci. 18, 1394–1404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chrousos GP Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Tyree SM & de Lecea L. Optogenetic Investigation of Arousal Circuits. Int. J. Mol. Sci. 18, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coplan JD et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc. Natl. Acad. Sci. 93, 1619–1623 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adam EK, Hawkley LC, Kudielka BM & Cacioppo JT Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. U. S. A. 103, 17058–17063 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cacioppo JT et al. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 35, 143–154 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Cole SW et al. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc. Natl. Acad. Sci. 112, 15142–15147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doane LD & Adam EK Loneliness and Cortisol: Momentary, Day-to-day, and Trait Associations. Psychoneuroendocrinology 35, 430–441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pressman SD et al. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 24, 297–306 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Cacioppo JT, Hawkley LC, Norman GJ & Berntson GG Social isolation. Ann. N. Y. Acad. Sci. 1231, 17–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruscio MG, Sweeny T, Hazelton J, Suppatkul P. & Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm. Behav. 51, 54–61 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Kim JW & Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol. Psychiatry 40, 918–922 (1996). [DOI] [PubMed] [Google Scholar]
- 70.Bosch OJ, Nair HP, Ahern TH, Neumann ID & Young LJ The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 34, 1406–1415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNeal N. et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci. Basic Clin. 180, 9–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun P, Smith AS, Lei K, Liu Y. & Wang Z. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res. 265, 22–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takatsu-Coleman AL et al. Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: mood-congruent memory in male mice? J. Psychiatry Neurosci. JPN 38, 259–268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armando I. et al. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation Stress. Endocrinology 142, 3880–3889 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Armando I, Volpi S, Aguilera G. & Saavedra JM Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 1142, 92–99 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP & Bains JS Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 7, 11937 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deussing JM & Chen A. The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol. Rev. 98, 2225–2286 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Peng J. et al. A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Front. Neuroanat. 11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swanson LW, Sawchenko PE, Rivier J. & Vale WW Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36, 165–186 (1983). [DOI] [PubMed] [Google Scholar]
- 80.Senst L, Baimoukhametova D, Sterley T-L & Bains JS Sexually dimorphic neuronal responses to social isolation. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saavedra JM et al. A Centrally Acting, Anxiolytic Angiotensin II AT1 Receptor Antagonist Prevents the Isolation Stress-Induced Decrease in Cortical CRF1 Receptor and Benzodiazepine Binding. Neuropsychopharmacology 31, 1123–1134 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Aguilera G, Young WS, Kiss A. & Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61, 437–444 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Pournajafi-Nazarloo H. et al. Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology 36, 780–789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berridge CW & Waterhouse BD The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Sara SJ & Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012). [DOI] [PubMed] [Google Scholar]
- 86.Gardner WL, Pickett CL, Jefferis V. & Knowles M. On the outside looking in: loneliness and social monitoring. Pers. Soc. Psychol. Bull. 31, 1549–1560 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Gardner WL, Pickett CL & Brewer MB Social Exclusion and Selective Memory: How the Need to belong Influences Memory for Social Events. Pers. Soc. Psychol. Bull. 26, 486–496 (2000). [Google Scholar]
- 88.Pickett CL, Gardner WL & Knowles M. Getting a Cue: The Need to Belong and Enhanced Sensitivity to Social Cues. Pers. Soc. Psychol. Bull. 30, 1095–1107 (2004). [DOI] [PubMed] [Google Scholar]
- 89.DeWall CN & Baumeister RF Alone but feeling no pain: Effects of social exclusion on physical pain tolerance and pain threshold, affective forecasting, and interpersonal empathy. J. Pers. Soc. Psychol. 91, 1–15 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Maner JK, DeWall CN, Baumeister RF & Schaller M. Does social exclusion motivate interpersonal reconnection? Resolving the ‘porcupine problem.’ J. Pers. Soc. Psychol. 92, 42–55 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Latane B, Nesbitt P, Eckman J. & Rodin J. Long- and short-term social deprivation and sociability in rats. J. Comp. Physiol. Psychol. 81, 69–75 (1972). [Google Scholar]
- 92.Niesink RJ & van Ree JM Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol. Behav. 29, 819–825 (1982). [DOI] [PubMed] [Google Scholar]
- 93.Panksepp J. & Beatty WW Social deprivation and play in rats. Behav. Neural Biol. 30, 197–206 (1980). [DOI] [PubMed] [Google Scholar]
- 94.Terranova ML, Laviola G. & Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev. Psychobiol. 26, 467–481 (1993). [DOI] [PubMed] [Google Scholar]
- 95.Varlinskaya EI, Spear LP & Spear NE Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol. Behav. 67, 475–482 (1999). [DOI] [PubMed] [Google Scholar]
- 96.Vanderschuren LJMJ, Stein EA, Wiegant VM & Van Ree JM Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 680, 148–156 (1995). [DOI] [PubMed] [Google Scholar]
- 97.Niesink RJ & Van Ree JM Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 28, 411–418 (1989). [DOI] [PubMed] [Google Scholar]
- 98.Achterberg EJM et al. Contrasting Roles of Dopamine and Noradrenaline in the Motivational Properties of Social Play Behavior in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 41, 858–868 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lister RG & Hilakivi LA The effects of novelty, isolation, light and ethanol on the social behavior of mice. Psychopharmacology (Berl.) 96, 181–187 (1988). [DOI] [PubMed] [Google Scholar]
- 100.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gunaydin LA & Deisseroth K. Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb. Symp. Quant. Biol. 79, 221–227 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Robinson DL, Heien MLAV & Wightman RM Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J. Neurosci. Off. J. Soc. Neurosci. 22, 10477–10486 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gunaydin LA et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manduca A. et al. Dopaminergic Neurotransmission in the Nucleus Accumbens Modulates Social Play Behavior in Rats. Neuropsychopharmacology 41, 2215–2223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rilke O, May T, Oehler J. & Wolffgramm J. Influences of housing conditions and ethanol intake on binding characteristics of D2, 5-HT1A, and benzodiazepine receptors of rats. Pharmacol. Biochem. Behav. 52, 23–28 (1995). [DOI] [PubMed] [Google Scholar]
- 106.Segal DS, Knapp S, Kuczenski RT & Mandell AJ The effects of environmental isolation on behavior and regional rat brain tyrosine hydroxylase and tryptophan hydroxylase activities. Behav. Biol. 8, 47–53 (1973). [DOI] [PubMed] [Google Scholar]
- 107.Rilke O, Freier D, Jähkel M. & Oehler J. Dynamic Alterations of Serotonergic Metabolism and Receptors During Social Isolation of Low- and High-Active Mice. Pharmacol. Biochem. Behav. 59, 891–896 (1998). [DOI] [PubMed] [Google Scholar]
- 108.Thielen RJ, McBride WJ, Lumeng L. & Li TK Housing conditions alter GABAA receptor of alcohol-preferring and -nonpreferring rats. Pharmacol. Biochem. Behav. 46, 723–727 (1993). [DOI] [PubMed] [Google Scholar]
- 109.Jong J. W. de et al. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 0, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Menegas W, Babayan BM, Uchida N. & Watabe-Uchida M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife 6, e21886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vander Weele CM et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho JR et al. Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 94, 1205–1219.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Dougalis AG et al. Functional properties of dopamine neurons and co-expression of vasoactive intestinal polypeptide in the dorsal raphe nucleus and ventro-lateral periaqueductal grey. Eur. J. Neurosci. 36, 3322–3332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dougalis AG, Matthews GAC, Liss B. & Ungless MA Ionic currents influencing spontaneous firing and pacemaker frequency in dopamine neurons of the ventrolateral periaqueductal gray and dorsal raphe nucleus (vlPAG/DRN): A voltage-clamp and computational modelling study. J. Comput. Neurosci. 42, 275–305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groessl F. et al. Dorsal tegmental dopamine neurons gate associative learning of fear. Nat. Neurosci. 21, 952–962 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hasue RH & Shammah-Lagnado SJ Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J. Comp. Neurol. 454, 15–33 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Li C. et al. Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 41, 2122–2132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matthews GA et al. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kash TL, Nobis WP, Matthews RT & Winder DG Dopamine Enhances Fast Excitatory Synaptic Transmission in the Extended Amygdala by a CRF-R1-Dependent Process. J. Neurosci. 28, 13856–13865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D. & Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog. Neurobiol. 90, 198–216 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Conrad KL, Louderback KM, Gessner CP & Winder DG Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol. Behav. 104, 248–256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zelikowsky M. et al. The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 173, 1265–1279.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis M, Walker DL, Miles L. & Grillon C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crestani CC et al. Mechanisms in the Bed Nucleus of the Stria Terminalis Involved in Control of Autonomic and Neuroendocrine Functions: A Review. Curr. Neuropharmacol. 11, 141–159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Keifer OP, Hurt RC, Ressler KJ & Marvar PJ The Physiology of Fear: Reconceptualizing the Role of the Central Amygdala in Fear Learning. Physiology 30, 389–401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ungless MA, Whistler JL, Malenka RC & Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001). [DOI] [PubMed] [Google Scholar]
- 127.Mameli M. et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 12, 1036–1041 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Creed MC & Lüscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr. Opin. Neurobiol. 23, 553–558 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Merrer JL, Becker JAJ, Befort K. & Kieffer BL Reward Processing by the Opioid System in the Brain. Physiol. Rev. 89, 1379–1412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Basbaum AI & Fields HL Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 7, 309–338 (1984). [DOI] [PubMed] [Google Scholar]
- 131.Aj M. & Rim D. The brain opioid theory of social attachment: A review of the evidence. Behaviour 148, 985–1025 (2011). [Google Scholar]
- 132.Vanderschuren LJ, Niesink RJ, Spruijt BM & Van Ree JM Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur. J. Pharmacol. 276, 257–266 (1995). [DOI] [PubMed] [Google Scholar]
- 133.Niesink RJ & van Ree JM Normalizing effect of an adrenocorticotropic hormone (4–9) analog ORG 2766 on disturbed social behavior in rats. Science 221, 960–962 (1983). [DOI] [PubMed] [Google Scholar]
- 134.Young LJ The neuroendocrinology of the social brain. Front. Neuroendocrinol. 30, 425–428 (2009). [DOI] [PubMed] [Google Scholar]
- 135.Young LJ & Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054 (2004). [DOI] [PubMed] [Google Scholar]
- 136.Goto Y. & Grace AA Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 31, 552–558 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bosch OJ et al. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dölen G, Darvishzadeh A, Huang KW & Malenka RC Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hung LW et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leser N. & Wagner S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 124, 97–103 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Thor D. & Holloway W. Social Memory of the Male Laboratory Rat. J. Comp. Physiol. Psychol. 96, 1000–1006 (1982). [Google Scholar]
- 142.Gusmão ID et al. Odor-enriched environment rescues long-term social memory, but does not improve olfaction in social isolated adult mice. Behav. Brain Res. 228, 440–446 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Kogan JH, Frankland PW & Silva AJ Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56 (2000). [DOI] [PubMed] [Google Scholar]
- 144.Gur R, Tendler A. & Wagner S. Long-Term Social Recognition Memory Is Mediated by Oxytocin-Dependent Synaptic Plasticity in the Medial Amygdala. Biol. Psychiatry 76, 377–386 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, Lv L, Wang L. & Zhong Y. Social Isolation Induces Rac1-Dependent Forgetting of Social Memory. Cell Rep. 25, 288–295.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Cacioppo JT, Hawkley LC & Thisted RA Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging 25, 453–463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo Y, Hawkley LC, Waite LJ & Cacioppo JT Loneliness, Health, and Mortality in Old Age: A National Longitudinal Study. Soc. Sci. Med. 1982 74, 907–914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oxman TE, Berkman LF, Kasl S, Freeman DH & Barrett J. Social Support and Depressive Symptoms in the Elderly. Am. J. Epidemiol. 135, 356–368 (1992). [DOI] [PubMed] [Google Scholar]
- 149.Bruce ML & Hoff RA Social and physical health risk factors for first-onset major depressive disorder in a community sample. Soc. Psychiatry Psychiatr. Epidemiol. 29, 165–171 (1994). [DOI] [PubMed] [Google Scholar]
- 150.Anderson DJ & Adolphs R. A Framework for Studying Emotions Across Phylogeny. Cell 157, 187–200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cryan JF, Valentino RJ & Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 29, 547–569 (2005). [DOI] [PubMed] [Google Scholar]
- 152.Steru L, Chermat R, Thierry B. & Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 85, 367–370 (1985). [DOI] [PubMed] [Google Scholar]
- 153.Molendijk ML & de Kloet ER Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62, 389–391 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Kwak C, Lee S-H & Kaang B-K Social Isolation Selectively Increases Anxiety in Mice without Affecting Depression-like Behavior. Korean J. Physiol. Pharmacol. 13, 357–360 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heyne A. The development of opiate addiction in the rat. Pharmacol. Biochem. Behav. 53, 11–25 (1996). [DOI] [PubMed] [Google Scholar]
- 156.Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl.) 101, 233–239 (1990). [DOI] [PubMed] [Google Scholar]
- 157.Naranjo JR & Fuentes JA Association between hypoalgesia and hypertension in rats after short-term isolation. Neuropharmacology 24, 167–171 (1985). [DOI] [PubMed] [Google Scholar]
- 158.Konecka AM & Sroczynska I. Stressors and pain sensitivity in CFW mice. Role of opioid peptides. Arch. Int. Physiol. Biochim. 98, 245–252 (1990). [DOI] [PubMed] [Google Scholar]
- 159.Koob GF & Volkow ND Neurocircuitry of Addiction. Neuropsychopharmacology 35, 217–238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wood PB Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 8, 781–797 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Flores JA, El Banoua F, Galán-Rodríguez B. & Fernandez-Espejo E. Opiate antinociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain 110, 205–214 (2004). [DOI] [PubMed] [Google Scholar]
- 162.Meyer PJ, Morgan MM, Kozell LB & Ingram SL Contribution of dopamine receptors to periaqueductal gray-mediated antinociception. Psychopharmacology (Berl.) 204, 531–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S. & Fernandez-Espejo E. Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 31, 1475–1488 (2006). [DOI] [PubMed] [Google Scholar]
- 164.Tye KM et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carlezon WA et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 316, 440–447 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Shirayama Y. et al. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem. 90, 1258–1268 (2004). [DOI] [PubMed] [Google Scholar]
- 167.Todtenkopf MS, Marcus JF, Portoghese PS & Carlezon WA Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl.) 172, 463–470 (2004). [DOI] [PubMed] [Google Scholar]
- 168.Kelley AE Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 27, 765–776 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Burgos-Robles A. et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krause EG et al. Hydration state controls stress responsiveness and social behavior. J. Neurosci. Off. J. Soc. Neurosci. 31, 5470–5476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burnett CJ et al. Hunger-Driven Motivational State Competition. Neuron 92, 187–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calhoon GG et al. Acute Food Deprivation Rapidly Modifies Valence-Coding Microcircuits in the Amygdala. bioRxiv 285189 (2018). doi: 10.1101/285189 [DOI] [Google Scholar]
- 173.Krashes MJ et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chovatiya R. & Medzhitov R. Stress, Inflammation, and Defense of Homeostasis. Mol. Cell 54, 281–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kotas ME & Medzhitov R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 160, 816–827 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cole SW Human Social Genomics. PLOS Genet. 10, e1004601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cole SW et al. Social regulation of gene expression in human leukocytes. Genome Biol. 8, R189 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Creswell JD et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain. Behav. Immun. 26, 1095–1101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Glaser R, Kiecolt-Glaser JK, Speicher CE & Holliday JE Stress, loneliness, and changes in herpesvirus latency. J. Behav. Med. 8, 249–260 (1985). [DOI] [PubMed] [Google Scholar]
- 180.Capitanio JP, Hawkley LC, Cole SW & Cacioppo JT A Behavioral Taxonomy of Loneliness in Humans and Rhesus Monkeys (Macaca mulatta). PLOS ONE 9, e110307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rigotti M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nieh EH et al. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell 160, 528–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nieh EH et al. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron 90, 1286–1298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Felix-Ortiz AC & Tye KM Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. Off. J. Soc. Neurosci. 34, 586–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Felix-Ortiz AC et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA & Tye KM Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Allsop SA, Weele V, M C, Wichmann R. & Tye KM Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front. Behav. Neurosci. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu J, Jhou TC & Saper CB Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J. Neurosci. Off. J. Soc. Neurosci. 26, 193–202 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eisenberger NI The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. (2012). doi: 10.1038/nrn3231 [DOI] [PubMed] [Google Scholar]
- 190.Eisenberger NI, Lieberman MD & Williams KD Does Rejection Hurt? An fMRI Study of Social Exclusion. Science 302, 290–292 (2003). [DOI] [PubMed] [Google Scholar]
- 191.Price DD Psychological and Neural Mechanisms of the Affective Dimension of Pain. Science 288, 1769–1772 (2000). [DOI] [PubMed] [Google Scholar]
- 192.Alhadeff AL et al. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173, 140–152.e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Andrew MK Reported loneliness rather than social isolation is a risk factor for 10-year mortality in older men. Evid. Based Ment. Health 15, 87–87 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Hawkley LC, Thisted RA, Masi CM & Cacioppo JT Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol. Aging 25, 132–141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tomaka J, Thompson S. & Palacios R. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J. Aging Health 18, 359–384 (2006). [DOI] [PubMed] [Google Scholar]
- 196.Wilson RS et al. Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry 64, 234–240 (2007). [DOI] [PubMed] [Google Scholar]
- 197.Baumeister RF, Nathan C, Ciarocco NJ & Twenge JM Social Exclusion Impairs Self-Regulation. J. Pers. Soc. Psychol. 88, 589–604 (2005). [DOI] [PubMed] [Google Scholar]
- 198.DeWall CN, Baumeister RF & Vohs KD Satiated with Belongingness? Effects of Acceptance, Rejection, and Task Framing on Self-Regulatory Performance. J. Pers. Soc. Psychol. 95, 1367–1382 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.LeDoux J. Rethinking the emotional brain. Neuron 73, 653–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Damasio A. & Damasio H. Exploring the concept of homeostasis and considering its implications for economics. J. Econ. Behav. Organ. 126, 125–129 (2016). [Google Scholar]
- 201.Grosenick L, Clement TS & Fernald RD Fish can infer social rank by observation alone. Nature 445, 429–432 (2007). [DOI] [PubMed] [Google Scholar]
- 202.Morgan D. et al. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am. J. Primatol. 52, 115–131 (2000). [DOI] [PubMed] [Google Scholar]
- 203.Schjelderup-Ebbe T. Beiträge zur Sozialpsychologie des Haushuhns. [Observation on the social psychology of domestic fowls.]. Z. Für Psychol. Physiol. Sinnesorgane Abt 1 Z. Für Psychol. 88, 225–252 (1922). [Google Scholar]
- 204.Uhrich J. The social hierarchy in albino mice. J. Comp. Psychol. 25, 373–413 (1938). [Google Scholar]
- 205.Syme GJ Competitive orders as measures of social dominance. Anim. Behav. 22, Part 4, 931–940 (1974). [Google Scholar]
- 206.So N, Franks B, Lim S. & Curley JP A Social Network Approach Reveals Associations between Mouse Social Dominance and Brain Gene Expression. PLOS ONE 10, e0134509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hollis F. et al. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. 112, 15486–15491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jupp B. et al. Social dominance in rats: Effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology (Berl.) 233, 579–589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang F. et al. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science 334, 693–697 (2011). [DOI] [PubMed] [Google Scholar]
- 210.Zhou T. et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168 (2017). [DOI] [PubMed] [Google Scholar]
- 211.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M. & Dulac CG Galanin neurons in the medial preoptic area govern parental behavior. Nature 509, 325–330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lin D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Soden ME et al. Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior. Cell Rep. 16, 304–313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McHenry JA et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 20, 449–458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Swan M. Blockchain Thinking : The Brain as a Decentralized Autonomous Corporation [Commentary]. IEEE Technol. Soc. Mag. 34, 41–52 (2015). [Google Scholar]
- 216.Tye KM Neural Circuit Motifs in Valence Processing. Neuron 100, 436–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rossi MA & Stuber GD Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 27, 42–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Castro DC, Cole SL & Berridge KC Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Berridge KC Motivation concepts in behavioral neuroscience. Physiol. Behav. 81, 179–209 (2004). [DOI] [PubMed] [Google Scholar]
- 220.Berridge KC, Robinson TE & Aldridge JW Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 9, 65–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lockie SH & Andrews ZB The hormonal signature of energy deficit: Increasing the value of food reward. Mol. Metab. 2, 329–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rolls ET, Burton MJ & Mora F. Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res. 194, 339–357 (1980). [DOI] [PubMed] [Google Scholar]
- 223.Inagaki TK et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc. Cogn. Affect. Neurosci. nsv076 (2015). doi: 10.1093/scan/nsv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cacioppo JT, Norris CJ, Decety J, Monteleone G. & Nusbaum H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J. Cogn. Neurosci. 21, 83–92 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Small DM, Zatorre RJ, Dagher A, Evans AC & Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain J. Neurol. 124, 1720–1733 (2001). [DOI] [PubMed] [Google Scholar]
- 226.Hull CL Principles of Behavior: An Introduction to Behavior Theory. (D. Appleton-Century Company, Incorporated, 1943). [Google Scholar]
- 227.Betley JN et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mandelblat-Cerf Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife (2015). doi: 10.7554/eLife.07122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aponte Y, Atasoy D. & Sternson SM AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Y, Lin Y-C, Kuo T-W & Knight ZA Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell 160, 829–841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen Y, Lin Y-C, Zimmerman CA, Essner RA & Knight ZA Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife (2016). doi: 10.7554/eLife.18640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Edsinger E. & Dölen G. A Conserved Role for Serotonergic Neurotransmission in Mediating Social Behavior in Octopus. Curr. Biol. CB (2018). doi: 10.1016/j.cub.2018.07.061 [DOI] [PubMed] [Google Scholar]
- 233.Shemesh Y. et al. High-order social interactions in groups of mice. eLife (2013). doi: 10.7554/eLife.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Allsop SA et al. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173, 1329–1342.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]