Abstract
The management and treatment of chronic wounds or acute wounds remain a major challenge in modern medicine. The application of autologous platelet-rich plasma (PRP) has become a promising adjuvant therapy to promote wound healing. PRP is derived from centrifuged whole blood to extract concentrated platelets, and a large amount of cytokines and growth factors are released upon activation. These bioactive molecules can enhance angiogenesis and tissue regeneration. Herein, PRP-loaded gelatin microspheres were prepared by the emulsion cross-linking method. Scanning electron microscopy results showed that the prepared microspheres are completely spherical, with an average particle size of 15.95 ± 3.79 μm and having a uniform particle size. Among them, the surface of a single microsphere is smooth and has a microporous structure, which may be the main channel for drug diffusion. Results of drug release measurements show that the prepared microspheres can slowly release the vascular endothelial growth factor for more than 7 days. In vitro cell experiments show that the prepared microspheres can promote proliferation and migration of L929 mouse fibroblast cells. In summary, the prepared PRP-loaded gelatin microspheres with high and long-term activity can provide experimental and theoretical knowledge for the development of the clinical long-acting injectable formulations.
1. Introduction
The treatment of chronic wounds or impaired acute wounds represents a major public healthcare burden affecting a relatively large population.1,2 Wound healing is a complex and orderly physiological process that includes inflammation, proliferation, regeneration, and remodeling, which is coordinated by a large number of cells, cytokines, and various extracellular matrix components.3−5 As an important participant in the process of wound repair, the application of cytokines can often play a critical role in a small dose.6,7 Therefore, cytokines are an ideal bioactive drug for treating trauma and promoting wound repair.8
Platelet-rich plasma (PRP) is a mixture of highly concentrated platelets and associated growth factors including the platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), and stromal cell-derived factor, all of which play crucial roles in regulating a variety of physiological processes such as inflammation response, angiogenesis, and wound healing.9,10 VEGF and bFGF are effective pro-angiogenic factors, which can promote neovascularization in models of tissue ischemia.11,12 Moreover, bFGF and VEGF, as mitogens in the process of wound healing, can regulate various cells during wound healing.13 However, the half-life of growth factors is extremely short, which will need the dosage and frequency of administration to be increased to maintain its effect, resulting in the high cost of treatment.14 Consequently, discovery and development of a controlled-release drug system with controlled release and prolonging activity is an urgent global need.
Recently, a variety of drug-controlled release systems have attracted increasing interest in the treatment of various diseases.15,16 Polymer microspheres with low cost, good plasticity, good biocompatibility, and biodegradability have become ideal raw materials for the preparation of biological and tissue engineering materials.17 Drug-loaded microspheres based on high molecular polymers have been successfully used in many studies.18,19 These drug-loaded microspheres have good biocompatibility and protective and slow controlled release effects.20−22
In this study, PRP-loaded gelatin microspheres were successfully prepared by the emulsion cross-linking method to promote wound healing. Their physical properties, drug release, and proliferation effect on the L929 mouse fibroblast cells in vitro were evaluated. The results show that the average diameter of the microspheres is 15.95 ± 3.79 μm and the particle size is uniform. The effect of PRP concentration in microspheres on cell proliferation was detected by CCK-8, and the microspheres were determined to be noncytotoxic and have good biocompatibility. Meanwhile, the 100 μg/mL PRP-loaded gelatin microspheres are beneficial in promoting cell proliferation and migration.
2. Materials and Methods
2.1. Materials
Gelatin (type A, from porcine skin) was purchased from Sigma-Aldrich Industrial Corporation (Shanghai, China). Span 80, 25% glutaraldehyde solution, isopropyl alcohol, and petroleum ether were obtained from National Medicine Group Chemical Reagent Co., Ltd. (Shanghai, China). The L929 fibroblast cell line was purchased from Beogene Biotechnology Co., Ltd. (Guangzhou, China). Cell Counting Kit-8 (CCK-8) and LIVE/DEAD cell imaging kit were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
2.2. Preparation of PRP-Loaded Gelatin Microspheres
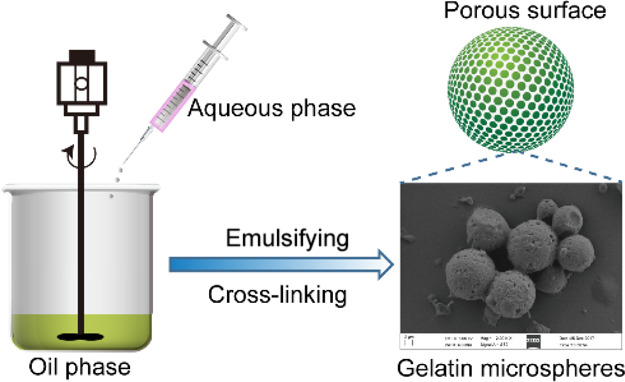
Gelatin microspheres (GMs) were prepared by the water–oil (W/O) method.23 Briefly, 3 g of gelatin is dissolved in 20 mL of ultrapure water. The solution was added into 100 mL of liquid paraffin containing 1.5 mL of SPAN-80 with a stirring rate of 800 rpm at 50 °C. After emulsification for 10 min, the emulsion was then cooled to 4 °C in an ice bath. Glutaraldehyde (0.1 mL, 25% v/v) was added into the solution, and the reaction was stirred at 4 °C for 24 h. After fixation for 24 h, the supernatant was removed and 100 mL of isopropanol was added to dehydrate. Finally, the obtained microspheres were washed with isopropyl alcohol, petroleum, and deionized water ether three times alternately, then filtered by a filter, and dried in a drying oven at 60 °C to obtain a light yellow powdered gelatin microsphere. After screening with a 20 μm aperture pore size sieve, it is bottled and stored in a desiccator.
2.3. Characterization of the Microspheres
The microspheres were placed onto a microscope slide and deionized water was added on top. Samples were made for observation by an optical microscope. The morphology of gelatin microspheres was visualized using optical microscopy and scanning electron microscopy (SEM, HITACHI S-3700N, Tokyo, Japan). The corresponding size distribution of gelatin microspheres was measured and quantified by Nano measure software.
2.4. Preparation of PRP-Loaded Gelatin Microspheres
2.4.1. Preparation of PRP
Whole blood (type O positive) acquired from eight young volunteers was stored with ethylenediaminetetraacetic acid at 4 °C. Extraction of autologous PRP was prepared by a two-step centrifugation technique. The first step was centrifuged at 1500 rpm for 10 min, and the second step was centrifuged at 3000 rpm for 20 min, and the platelet count was normalized to 1019.13 ± 10.93 × 109/L. The platelet activator (100 mg/mL calcium gluconate solution and 100 U/mL thrombin) was mixed with PRP in a volume ratio of 10:1 and then incubated in a water bath at 37 °C for 30 min. The PRP was centrifuged to obtain the platelet compound growth factor and other bioactive factors. The PRP was stored in an ultralow temperature refrigerator at −80 °C.
2.4.2. Preparation of PRP-Loaded Gelatin Microspheres
Briefly, 100 μL of PRP was added to the 1.5 mL sterile EP tube containing 10 mg gelatin microspheres and then fully infiltrated and expanded at 4 °C for 24 h. Then, the sample was lyophilized for 24 h, sealed, and placed at −20 °C for storage.
2.5. In Vitro Growth Factor Release Studies
2.5.1. Quantification of the Growth Factor in PRP
PRP (100 μL) was added to the 1.5 mL sterile EP tube without microspheres. After fully infiltrating and swelling at 4 °C for 24 h, all the supernatants were centrifuged at 8090 rpm for 15 min and collected. Then, the three EP tubes were mixed with 200 μL phosphate-buffered saline (PBS) and centrifuged at 8090 rpm for 5 min. The supernatants were repeatedly washed three times, marked each time, and stored in an ultralow temperature refrigerator at −80 °C. Total amounts of PDGF-BB, TGF-β, VEGF, IGF-1, bFGF, and EGF in PRP were evaluated by enzyme-linked immunosorbent assay (ELISA) from RayBio (Norcross, GA, USA).
2.5.2. Release of VEGF from Gelatin Microspheres
PBS (500 μL) was added to each EP tube containing PRP-loaded gelatin microspheres with a stirring speed of 100 rpm at 37 °C. At predetermined time points (1, 2, 3, 4, 5, 6, and 7 days), three EP tubes were centrifuged at 1500 rpm for 15 min, and the supernatant was marked and stored in a cryogenic refrigerator at −80 °C for 15 min. The ELISA kit was used to detect the collected samples and the supernatants containing the unabsorbed growth factor in microspheres. Meanwhile, the cumulative release rate of VEGF in vitro was calculated.
2.6. Biocompatibility In Vitro
2.6.1. Cell Culture
L929 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) complete medium containing 10% fetal bovine serum (FBS), and 1% double antibodies (100 mol/mL penicillin, 100 μg/mL streptomycin) in 37 °C and 5% CO2 incubator were added for 24 h until they reached the logarithmic phase of growth. The cells were digested and dispersed with 0.25% trypsin, centrifuged, and resuscitated at 1500 rpm for 3min, and cell suspension (1 × 104 cells/mL) was prepared.
2.6.2. Evaluation of the Cell Proliferation Rate
The cell proliferation rate of microspheres was evaluated according to the National Standard of China GB/T16886.12 using L929 cells. The prepared microspheres were soaked in DMEM at the ratio of 0.1 g:1 mL to prepare leaching solution at 37 °C for 24 h. Afterward, 100 μL of previous cell suspension was added in a 96-well culture plate. After 12 h of incubation, the initial culture medium was taken out and replaced with 100 μL of leaching solution. The control group is DMEM complete medium containing 10% FBS, and 0 μg/mL GMs/PRP group is leaching solution of pure gelatin microspheres without PRP. At each culture time interval (24, 48, and 72 h), the cell proliferation rate was assessed by CCK-8 assay and live/dead staining. For CCK-8 assay, the culture medium in 96-well plates was replaced with DMEM containing 10% of CCK-8 solution and incubated at 37 °C for 30–60 min. The absorbance at 450 nm was determined using a microplate reader (SH1000, Corona, Japan). The cell proliferation rate was calculated according to the equation.
where ODs, ODc, and ODb represent the absorbance of the experimental wells of microspheres treated, the control wells, and the blank wells, respectively.
For live/dead staining, the medium was removed and washed three times with PBS. Then, 100 μL of live/dead staining stock solution was added and incubated at 4 °C for 15 min. Finally, the fluorescent images of cultured cells were viewed under an inverted fluorescence microscope (TE2000-S, Nikon, Japan).
2.6.3. Cell Migration Study-Scratch Assay
Scratch-wound assays were performed to assess the effects of PRP-loaded gelatin microspheres on cell migration. L929 were seeded in 24-well plates at a density of 1 × 104 cells per well and allowed to grow until 90–95% confluence was reached. Then, the cells were washed twice with PBS and grown in serum-free medium for 24 h. Subsequently, the cell monolayer was carefully scratched with sterile 200 μL pipet tips. Following scratch injury, the cells were washed with PBS to remove cell debris and the culture media were replaced with 100 μL of leach liquor in DMEM medium without FBS. Serum-free medium was used as the control group. After being incubated at 0, 24, and 48 h time points, the cells were re-photographed using an optical microscope. The wound gap distance was quantified by measurement of photographic images using IPP 6.0 software.
2.7. Statistical Analysis
All data represent the mean ± standard deviation. Statistical analysis was conducted by one-way ANOVA with SPSS 20.0. Significant difference was considered at * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
3. Results
3.1. Characterization of the Gelatin Microspheres
Optical microscopy images of the prepared gelatin microspheres are shown in Figure 1A. The prepared gelatin microspheres exhibited a highly porous surface, uniform particle size, and good dispersion (Figure 1B,C). Statistical analysis showed that 88.23% of the microspheres were distributed in the range of 7–20.5 μm, and the average particle size was 15.95 ± 3.79 μm (Figure 1D). The mean hydrodynamic diameter obtained by dynamic light scattering was 18.92 μm (Figure 1E), which is quite close to the average particle size of the SEM result. Freeze-dried gelatin microsphere powder was a white loose powder without collapse and shrinkage.
Figure 1.
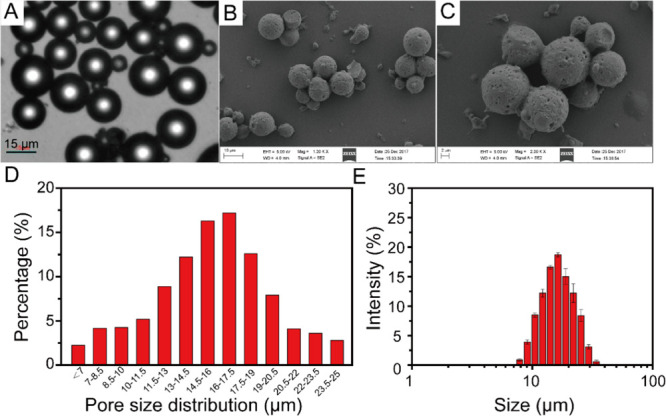
(A) Optical microscopy images of gelatin microspheres, (B,C) scanning electron microscopy of gelatin microspheres, (D) particle size distribution of gelatin microspheres, and (E) mean hydrodynamic diameter of gelatin microspheres.
3.2. In Vitro Growth Factor Release Studies
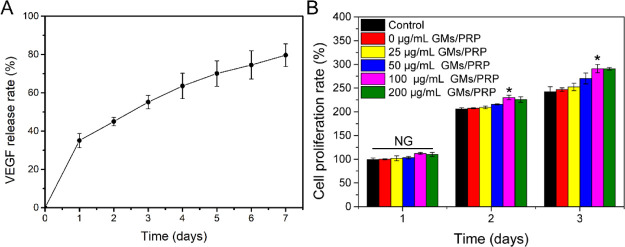
The contents of PDGF-BB, TGF-β, VEGF, IGF-1, bFGF, and EGF in the PRP were 17.033 ± 0.089, 8.716 ± 0.804, 285.089 ± 6.342 ng/mL, 275.80 ± 1.00, 136.527 ± 9.154, and 80.113 ± 2.156 pg/mL, respectively. The contents of PDGF-BB, TGF-β, and VEGF loaded on gelatin microspheres were 123.63, 72.335, and 2.50 pg/mg, respectively. The adsorption rates of PDGF-BB, TGF-β, and VEGF growth factors on microspheres were 72.58, 82.99, and 87.84%, respectively. Figure 2A shows the in vitro release ratio of VEGF from gelatin microspheres. The release rate of VEGF was slower and exhibited a steady growth. Results indicated that the gelatin microspheres could provide a sustained delivery of VEGF.
Figure 2.
(A) Sustained release of VEGF in gelatin microspheres. (B) Proliferation rate of the L929 cells at 1, 2, and 3 days of different concentrations of GMs/PRP treated. N.S., not significant, *p < 0.05.
3.3. Evaluation of Cell Proliferation
Figure 2B shows the relative cell proliferation rate of PRP-loaded gelatin microsphere group at 1, 2, and 3 days. With the increasing culture time, it can be clearly shown that the cell proliferation rate of all GM/PRP microsphere-treated groups exhibited an increasing trend, indicating that prepared GM/PRP microspheres had good biocompatibility. It is worth noting that the cell proliferation rate of microspheres containing PRP was higher than that of the pure gelatin microspheres and control group. Meanwhile, the cell proliferation rate of the pure gelatin microsphere group was similar to the control group, indicating that the prepared gelatin microspheres was no cytotoxicity. Overall, it can be considered that the PRP-loaded gelatin microspheres have good biocompatibility and can significantly promote cell proliferation.
3.4. Live/Dead Staining Assay
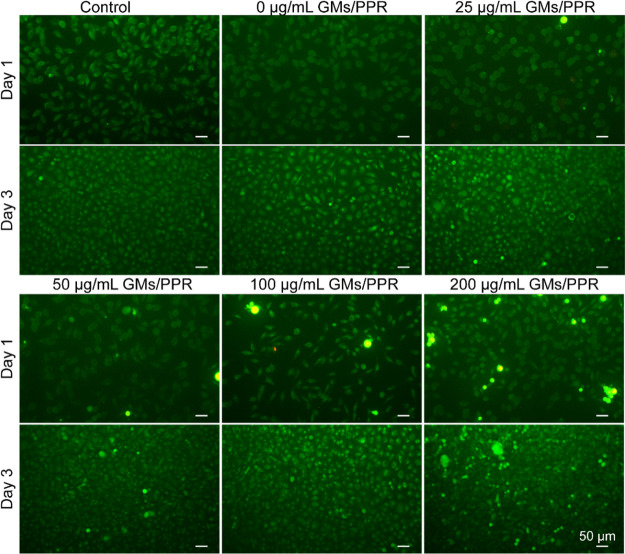
To further clearly and intuitively observe the cell proliferation effect of PRP-loaded gelatin microspheres, the cultured L929 cells were stained with a dead-and-live staining kit, and then the live (green) and dead (red) cells were visualized with an inverted fluorescence microscope. As shown in Figure 3, it can be seen that most of the cells survived in all gelatin microsphere groups. At day 1, only a small amount of cells adhered and proliferated on the well plate. At day 2, a larger number of cells began to migrate and proliferate. At day 3, with the extension of the culture time, a large number of cells could be seen densely growing on the well plate, the cells being close to saturation, and the cells densely arranged, basically covering the entire bottom surface. It is worth mentioning that the cell density gradually increased with increasing the concentration of PRP in gelatin microspheres. All results showed that the prepared PRP-loaded gelatin microspheres showed no cytotoxicity, indicating that the released PRP possesses good biocompatibility, can effectively promote cell proliferation, and has a great potential in biological applications.
Figure 3.
Live/dead staining fluorescence images of the L929 cells at 1 and 3 days of different concentrations of GM/PRP treated. The scale bar is 50 μm.
3.5. Evaluation of Scratch
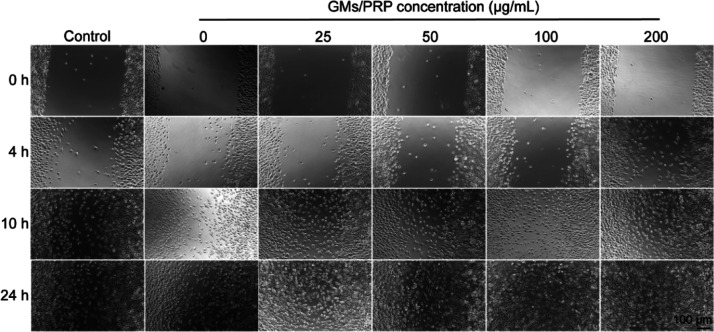
The scratch wound assay can quickly and easily evaluate the effect of materials on cell behavior. As shown in Figure 4, scratch effects followed the same trend as the cell proliferation rate. Because of the proliferation of cells in the microsphere group and the control group, the cell mass slightly widened at 4 h and then gradually extended to the middle. With the increasing concentration of PRP in the gelatin microspheres, the scratches closed faster, indicating that PRP-loaded gelatin microspheres play an important role in promoting cell proliferation.
Figure 4.
Representative photographs of cell migration in different concentrations of GM/PRP treated using the scratch assay. The scale bar is 100 μm.
After incubation for 24 h, cells exposed to 100 and 200 μg/mL showed higher migration (85.96 ± 3.68%) and (86.38 ± 4.52%), followed by those exposed to GMs (55.29 ± 1.78%) and control (59.24 ± 2.53%) (Figure 5). Statistical analysis of the change in the scratch area was performed to validate the cell proliferation rate, which was consistent with the previous reports stating that growth factors in PRP could promote cell migration.24
Figure 5.
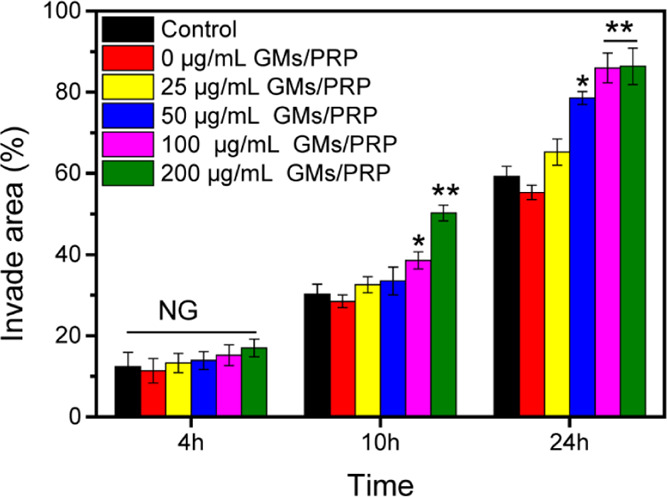
Migration area results in different concentrations of GM/PRP treated using the scratch assay. N.S., not significant, *p < 0.05, **p < 0.01.
4. Discussion
The instability of the released growth factor in PRP is a major factor limiting its broad clinical application.25 Growth factors such as VEGF or bFGF have poor stability in vivo, short-lasting biological activity, and poor biological membrane permeability.26 When the growth factor is applied, the local fluid environment in vivo is more complex with a variety of influence factors, such as protease degradation, tissue cell phagocytosis, and so on, which will affect its activity and their functional interaction.27 In addition, most of the growth factors are released after activation for a short period of time, which will lead to the high concentration of growth factors locally. However, the high concentration of growth factors cannot be effectively used by tissues during short duration, and some of them diffuse with body fluids.
Gelatin is an ideal natural bioactive material for designing the microsphere growth factor release system.28,29 It is amenable to various surface modifications and reactions, contains short peptides of amino acid biological activity, and can be combined with cell surface receptors, which is conducive to cell surface adhesion molecule recognition. The gelatin molecules form a stable three-dimensional network structure under the action of a curing agent (glutaraldehyde).30 The PRP and gelatin are combined to prepare slow-release microspheres. The basic principle is that the growth factor is a peptide structure, which has a certain affinity between them. The gelatin microspheres swell, absorb, and encapsulate the growth factor, ensuring the adsorption rate and loading rate of the growth factor. With gelatin microsphere degradation, the slow release of growth factors in vivo is achieved. Gelatin can replace water to form a hydrogen bond with the polypeptide during lyophilization, stabilize the natural conformation of the polypeptide, and thereby protect the activity of the growth factor.
In this study, the prepared PRP-loaded gelatin microsphere shows a high adsorption rate of growth factor, the loaded amount of growth factor, and slow-release effect in vitro. One possible reason for the evaluation of the sustained release effect of microspheres in terms of the amount of VEGF detected is that the activity of VEGF is relatively long at 37 °C in vitro, and it still maintains a high activity after 7 days. However, the activity of PDGF began to decline after 1 day; the activity of TGF-β began to decrease after 45 min. The activity of EGF, bFGF, and IGF-1 is also relatively long, but the concentration is low, which is not easy to accurately measure after dilution. Another possible contributing factor is that VEGF, as a pro-angiogenic factor or vascular permeability factor, can specifically bind to the surface receptors of vascular endothelial cells. It can promote the proliferation and migration of vascular endothelial cells, stimulate angiogenesis, and improve vascular permeability and so on. We performed further in vitro experiments to study the indirect effects of the different concentrations of PRP-loaded gelatin microspheres on L929 fibroblasts. The result indicated that prepared PRP-loaded gelatin microspheres can effectively promote cell proliferation and migration, which will have a great potential in biological applications.
5. Conclusions
Chronic wounds are a great burden on patients, their families, society, and the healthcare system. PRP, as a natural source of growth factors, plays a critical role in tissue repair and regeneration. However, there might be rapid degradation following the direct injection of PRP at the site of injury, which will reduce the utility. Herein, we reported that gelatin microspheres, as a delivery system for slow-release growth factors, exhibit a relatively smooth surface, uniform particle size, and good dispersion. Our result indicated that prepared microspheres show the slow-release effect in vitro and slowly release VEGF for more than 7 days in vitro at 37 °C. In vitro experiments indicated that the prepared gelatin microspheres possess good biocompatibility and can effectively promote cell proliferation and migration, which will have a great potential in biological applications.
Acknowledgments
The authors wish to thank the military logistics. This work was supported by the fund from preparation of composite absorbable wound adjuvant loaded with platelet gel dressing (CWH17C019).
Author Contributions
§ J.Y. and M.Z. contributed equally to this work.
The authors declare no competing financial interest.
References
- Shiekh P. A.; Singh A.; Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]
- Qiao Y.; He J.; Chen W.; Yu Y.; Li W.; Du Z.; Xie T.; Ye Y.; Hua S. Y.; Zhong D.; Yao K.; Zhou M. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano 2020, 14, 3299–3315. 10.1021/acsnano.9b08930. [DOI] [PubMed] [Google Scholar]
- Loh E. Y. X.; Fauzi M. B.; Ng M. H.; Ng P. Y.; Ng S. F.; Mohd Amin M. C. I. Insight into delivery of dermal fibroblast by non-biodegradable bacterial nanocellulose composite hydrogel on wound healing. Int. J. Biol. Macromol. 2020, 159, 497. 10.1016/j.ijbiomac.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Rai R.; Verma S. K.; Kim D.; Ramirez V.; Lux E.; Li C.; Sahoo S.; Wilsbacher L. D.; Vaughan D. E.; Quaggin S. E.; Ghosh A. K. A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis. Epigenetics 2017, 12, 1004. 10.1080/15592294.2017.1370173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Zhang Q.; Wu Y.; Branch-Brooks C. D.; Butler C. E. Short-term influences of radiation on musculofascial healing in a laparotomy rat model. Sci. Rep. 2019, 9, 11896. 10.1038/s41598-019-48201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise N.; Rountree I.; Polucha C.; Montagna G.; Visai L.; Coulombe K. L. K.; Munarin F. Engineering immunomodulatory biomaterials for regenerating the infarcted myocardium. Front. Bioeng. Biotech. 2020, 8, 292. 10.3389/fbioe.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara C. P.; Mendes N. F.; Prado T. P. d.; de Araújo E. P. Bioactive fatty acids in the resolution of chronic inflammation in skin wounds. Adv. Wound Care 2020, 9, 472. 10.1089/wound.2019.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X.; Falcon D. M. The role of immune cells and cytokines in intestinal wound healing. Int. J. Mol. Sci. 2019, 20, 6097. 10.3390/ijms20236097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis B.; D’Autilio M. F. L. M.; Orlandi F.; Pepe G.; Garcovich S.; Scioli M. G.; Orlandi A.; Cervelli V.; Gentile P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. 10.3390/jcm8091486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria M.; Vasileff W. K.; Miller M.; Borchers J.; Flanigan D. C.; Durgam S. S. Cellular components and growth factor content of platelet-rich plasma with a customizable commercial system. Am. J. Sports. Med. 2019, 47, 1216–1222. 10.1177/0363546519827947. [DOI] [PubMed] [Google Scholar]
- Qi J. H.; Bell B.; Singh R.; Batoki J.; Wolk A.; Cutler A.; Prayson N.; Ali M.; Stoehr H.; Anand-Apte B. Sorsby fundus dystrophy mutation in tissue inhibitor of metalloproteinase 3 (TIMP3) promotes choroidal neovascularization via a fibroblast growth factor-dependent mechanism. Sci. Rep. 2019, 9, 17429. 10.1038/s41598-019-53433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Mei F. C.; Yang W.; Wang H.; Wong E.; Cai J.; Toth E.; Luo P.; Li Y.-M.; Zhang W.; Cheng X. Epac1 inhibition ameliorates pathological angiogenesis through coordinated activation of Notch and suppression of VEGF signaling. Sci. Adv. 2020, 6, eaay3566 10.1126/sciadv.aay3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede F.; Marconi G. D.; Fonticoli L.; Pizzicanella J.; Merciaro I.; Bramanti P.; Mazzon E.; Trubiani O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. 10.3390/ijms21093242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Mou S.; Xiao P.; Li G.; Li J.; Tong J.; Wang J.; Yang J.; Sun J.; Wang Z. Delayed two steps PRP injection strategy for the improvement of fat graft survival with superior angiogenesis. Sci. Rep. 2020, 10, 5231. 10.1038/s41598-020-61891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath Udeni Gunathilake T. M.; Ching Y. C.; Chuah C. H.; Rahman N. A.; Liou N.-S. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. 10.1016/j.ijbiomac.2020.05.010. [DOI] [PubMed] [Google Scholar]
- Cano A.; Sánchez-López E.; Ettcheto M.; López-Machado A.; Espina M.; Souto E. B.; Galindo R.; Camins A.; García M. L.; Turowski P. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine 2020, 15, 1239. 10.2217/nnm-2019-0443. [DOI] [PubMed] [Google Scholar]
- Dou D. D.; Zhou G.; Liu H. W.; Zhang J.; Liu M. L.; Xiao X. F.; Fei J. J.; Guan X. L.; Fan Y. B. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: Design and characterization. Int. J. Biol. Macromol. 2019, 123, 622–628. 10.1016/j.ijbiomac.2018.11.099. [DOI] [PubMed] [Google Scholar]
- Metaxa A.-F.; Efthimiadou E. K.; Boukos N.; Fragogeorgi E. A.; Loudos G.; Kordas G. Hollow microspheres based on - Folic acid modified - Hydroxypropyl Cellulose and synthetic multi-responsive bio-copolymer for targeted cancer therapy: controlled release of daunorubicin, in vitro and in vivo studies. J. Colloid Interface Sci. 2014, 435, 171–181. 10.1016/j.jcis.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Ramazani F.; Chen W.; van Nostrum C. F.; Storm G.; Kiessling F.; Lammers T.; Hennink W. E.; Kok R. J. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Int. J. Pharm. 2016, 499, 358–367. 10.1016/j.ijpharm.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Chen N.; Johnson M. M.; Collier M. A.; Gallovic M. D.; Bachelder E. M.; Ainslie K. M. Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J. Control Release 2018, 273, 147–159. 10.1016/j.jconrel.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żywicka B.; Krucińska I.; Garcarek J.; Szymonowicz M.; Komisarczyk A.; Rybak Z. Biological properties of low-toxic PLGA and PLGA/PHB fibrous nanocomposite scaffolds for osseous tissue regeneration. Evaluation of potential bioactivity. Molecules 2017, 22, 1852. 10.3390/molecules22111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H.-B.; Zeng Y.; Lu Y.-R.; Cheng J.-Q.; Shen B. Control-released basic fibroblast growth factor-loaded poly-lactic-co-glycolic acid microspheres promote sciatic nerve regeneration in rats. Exp. Ther. Med. 2017, 13, 429–436. 10.3892/etm.2016.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Xing X.; Tan H.; Jia Y.; Zhou T.; Chen Y.; Ling Z.; Hu X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 70, 287–295. 10.1016/j.msec.2016.08.086. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Chang Q.; Lu F.; Xing M. Injectable mussel-inspired immobilization of platelet-rich plasma on microspheres bridging adipose micro-tissues to improve autologous fat transplantation by controlling release of PDGF and VEGF, angiogenesis, stem cell migration. Adv. Healthc. Mater. 2017, 6, 1700131. 10.1002/adhm.201700131. [DOI] [PubMed] [Google Scholar]
- McClain A. K.; McCarrel T. M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC. Vet. Res. 2019, 15, 292. 10.1186/s12917-019-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Zhang Y. L.; Wang H. F.; Zhao B.; Wang J. L.; Yan G. L.; Xu S. Y.; Zhou Y. Y.; Liu H. Y.; Zheng Y. F.; Quan W.; Zhou J. Y.; Liu Y.; Zhen M. C.; Zhu X.; Zhao Y. L. The development of an extra-anatomic tissue-engineered artery with collateral arteries for therapeutic angiogenesis in ischemic hind limb. Sci. Rep. 2018, 8, 4627. 10.1038/s41598-018-22799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. C.; Sun H. J.; Li K. H.; Fu C.; Liu M. Z. Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro. Exp. Ther. Med. 2014, 8, 1528–1534. 10.3892/etm.2014.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikina A. D.; Strobel H. A.; Lai B. P.; Rolle M. W.; Alsberg E. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials 2015, 52, 452–462. 10.1016/j.biomaterials.2015.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio L. D.; Dhami C. D.; Dang P. N.; Vieregge E. L.; Alsberg E. Spatiotemporal Regulation of Chondrogenic Differentiation with Controlled Delivery of Transforming Growth Factor-β1 from Gelatin Microspheres in Mesenchymal Stem Cell Aggregates. Stem Cells Transl. Med. 2012, 1, 632–639. 10.5966/sctm.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Feng Z.; You C.; Jin Y.; Hu X.; Wang X.; Han C. In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan-gelatin microsphere scaffolds for angiogenesis. Biomed. Eng. Online 2013, 12, 134. 10.1186/1475-925x-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]