Abstract
We report the first synthesis of 225Ac (t1/2 = 10 days) endohedral fullerenes,225Ac@C60. The 225Ac@C60 was produced with a 12 ± 2% efficiency by applying an electrical arc discharge between a source of α-particle emitter 225Ac (∼1 mCi, electroplated on a Pt disk) and a thin coat of “preformed” C60 on an Al disk (C60 thickness of ∼0.25 mg/cm2). After formation by electrical arc discharge, the resulting radiofullerenes on the Al disk were dissolved in toluene under anaerobic conditions and converted to a malonate derivative using the Bingel reaction. Subsequent to repeated washings of the organic phase with dilute acidic solutions to remove exohedral 225Ac, ∼45% of 225Ac activity was retained in the organic phase, which resisted extraction into the aqueous phase. Failure to extract the 225Ac from the organic phase provided definitive evidence that the 225Ac is located inside of the fullerene. The formation of 225Ac@C60 was further confirmed using a classical hot-atom chemistry technique in which the organic phase containing purified endohedral 225Ac@C60 malonate was contacted with fresh dilute acid to repeatedly extract the ionic 4.8 m 221Fr and 45.6 m 213Bi activities (decay daughters of 225Ac), which were released by molecular disruption due to nuclear recoil. The result from the extraction experiments was further supported by a series of thin-layer chromatography and high-pressure liquid chromatography analysis of the organic phase containing 225Ac@C60 or 225Ac@C60 malonate. Taken together, studies show that, like polydentate chelators, single-wall fullerenes are not capable of retaining the 225Ac decay daughters.
Introduction
Since the first successful synthesis, endohedral metallofullerenes have attracted special attention due to their novel properties, which differ from empty fullerenes.1−5 Novel applications of endohedral metallofullerenes include the feasibility of using 166Ho metallofullerenes as radiotracers,6 water-solubilized Gd metallofullerenes as MRI contrast agents,7 and 212Pb@C60 metallofullerenes for radioimmunotherapy.8 Related studies also include the hot-atom incorporation of tritium in C60,9 the potential use of functionalized metallofullerenes for drug delivery systems, like brain tumor brachytherapy,10 and 64Cu-labeled C60 for toxicology studies.11 Endohedral metallofullerenes have been prepared for all of the lanthanides (except Pm) and a few of the actinides.12,13 Further, fullerenes exhibit physical and chemical properties that make them well suited as nanocarriers of α-emitting actinides such as 225Ac, a medically useful radionuclide. Extensive preclinical and clinical studies have shown the potential applications of α-particle-emitting radionuclides in a variety of cancer systems and targeted radiotherapy.14−17 In particular, the clinical trials for the treatment of acute myeloid leukemia,18−20 a therapy for recurrent glioblastoma,21,22 and treatment of metastatic melanoma23 have all demonstrated the effectiveness of the α-emitter 225Ac (t1/2 = 10 days) and its decay daughter, 213Bi (t1/2 = 46 min), in killing cancer cells. The significant radiotoxicity associated with the actinides, particularly 225Ac, emphasizes the need for special facilities for the safe handling of actinides. Lack of dedicated soft ionization mass spectrometers for use with actinides, however, is the main obstacle in exploring the novel applications of actinofullerenes. Further, because of the lack of stable chelating agents for actinium, the in vivo targeting of this promising radioisotope remains a challenge. Therefore, exploring the potential applications of endohedral 225Ac in targeted α therapy is timely.
The techniques for the insertion of a stable atom or a small cluster of atoms into fullerenes include in situ arc combustion of graphite, glow discharge reactor, laser vaporization, ion beam implantation, and high-pressure/high-temperature incorporation.2,24−26 Neutron activation of preformed endohedral fullerenes was used to produce radioendohedral fullerenes (radiofullerenes).6,27−29 However, these studies showed that a significant fraction of the trapped radioendohedral atoms are ejected out of the fullerene from the recoil caused by the prompt γ-ray emission that follows the neutron capture process similar to the well-known Szilard–Chalmers process.30,31 It was later realized that external atoms could also be recoiled into a fullerene using this process.6,32−37 Indeed, one of the few demonstrated methods for placing an atom inside of the fullerene cage is by ion implantation, whereby atoms or ions accelerated to about 100 eV or greater are able to penetrate and remain lodged inside of fullerenes.38,39 The efficiencies of the two processes are comparable, and the techniques are viable methods for the production of radiofullerenes even though the overall yield of the encapsulation process with respect to the number of captured neutrons is only on the order of 1–2%. In addition to its simplicity, the recoil implantation process has the advantage of allowing for the production of carrier-free radiofullerenes because most endohedral fullerenes exhibit different chemical behavior from that of the empty fullerenes (starting material) and are therefore chemically separable. In this paper, we report the first synthesis of actinium endohedral fullerenes, 225Ac@C60, produced by electrical arc discharge between a source of 225Ac and a thin coat of “preformed” C60 on an Al disk (i.e., electrical arc discharge was used to insert 225Ac into the existing molecular fullerene). The synthesized 225Ac@C60 was stabilized by conversion to 225Ac@C60 malonic ester by the Bingel–Hirsch nucleophilic reaction for surface (exohedral) functionalization of fullerene using the Bingel cyclo-propanation reaction.40,41 Liquid–liquid extraction (organic/aqueous system) was used to study the stability of the synthesized 225Ac@C60 and its malonic ester, and results were further confirmed by a series of high-pressure liquid chromatography (HPLC) and thin-layer chromatography (TLC) studies. The much higher stability of the malonic ester-derivatized 225Ac@C60 cage allowed us to document the leakage of both 221Fr and 213Bi (the α-decay daughters of 225Ac) from the cage as a result of nuclear recoil.
Materials and Methods
Chemicals, Radioisotopes, and Radioactivity Measurement
Fullerene C60 (99%, Sigma-Aldrich, Burlington, MA) was used without further purification. Nitric acid (HNO3, Optima Grade, Fisher Scientific, Waltham, MA), tetrahydrofuran (THF), diethylbromomalonate (DBM), HPLC-grade toluene, and sodium hydride (NaH) were purchased from Sigma-Aldrich (Burlington, MA) and were used as received. Actinium-225 (2 mCi batches) was obtained from the U.S. Department of Energy National Isotope Development Center (www.isotopes.gov/catalog). This material was extracted from a stock of 229Th at Oak Ridge National Laboratory, and a detailed procedure for these separations is given by Boll.42 γ-ray spectrometry was used to measure all radioisotopes used in this study. The γ-ray spectrometer consisted of a calibrated intrinsic Ge detector (crystal active volume of ∼100 cm3) and a PC-based multichannel analyzer (Canberra Industries, Meriden, CT). The detector had a resolution of 0.8 at 5.9, 1.0 at 123, and 1.9 keV at 1332 keV. Energy and efficiency calibrations were determined with γ-ray sources traceable to the National Institute of Standard and Technology. Since the intensity of the γ-ray from 225Ac at 188.1 keV is only 0.47%, 225Ac was quantified by 218.0 keV (11.58%) and 440.4 keV (26.1%) γ-rays from its 4.9 min 221Fr and 45.6 min 213Bi daughters at secular equilibrium (values in parentheses are the absolute emission rates per 100 decays), respectively. All relevant nuclear data are taken from the Table of Radioisotopes.43 Gross radioactivity was measured in an ionization chamber (CRC-7, Capintec Inc., Florham, NJ), which was cross-calibrated with the γ-ray spectrometer described above. An automated γ-ray scintillation counter consisting of a well-type NaI(Tl) detector (Wallac Wizard 1470, Perkin Elmer, Waltham, MA) was used for the assay of radioactivity in the chromatography samples. A radioactivity TLC imaging scanner (AR-2000 with Winscan 2D Imaging Software, Bioscan Inc., Washington DC) was used for the direct counting of radioactivity on developed TLC plates. In a single run, a 20–25 min period was required to develop the TLC plate and assayed for radioactivity.
Other Instrumentation
A general-purpose artist’s air brush (such as Air Brush Kit 1601by Air Brush City) was used for applying a thin coat of C60 to Al catcher disks. An HPLC instrument was employed for characterization of fullerene, endohedral 225Ac fullerene, and endohedral 225Ac fullerene malonate complex. The HPLC setup was similar to the system described by Komatsu et al. for the separation of H2@C60 from C60 employing two tandem semipreparative Cosmosil Buckyprep columns.25 Our HPLC system was equipped with a Waters millennium work station, a 996 in-line photodiode detector (PDA) ultraviolet–visible (UV–vis) detector (fullerenes were monitored at 330 nm), a flow splitter, and two Waters high-pressure 515 pumps and was equipped with one Cosmosil Buckyprep analytical column (10 × 200 mm2, Nacalai USA, Inc., San Diego, CA). The mobile phase was 100% toluene, which was filtered before use and was pumped at a fixed flow rate of ∼1 mL/min. Under isocratic conditions, typically 5 μL of the sample was injected using a 10 μL glass syringe. A radioactivity TLC imaging scanner (AR-2000 with Winscan 2D Imaging Software, Bioscan Inc., Washington, DC) was used for the direct counting of radioactivity on developed TLC plates. Typically, 10 μL of the sample was spotted on the TLC plate at 10 mm from the bottom edge and was developed with toluene or ethyl acetate (organic solvent); the TLC plates of 225Ac @C60 were developed with toluene, and the TLC plates of 225Ac @C60 malonate were developed against ethyl acetate. In a single run, a 20–25 min period was required to develop the TLC plate and assayed for radioactivity, which is enough time to establish a 90% equilibrium between 221Fr from 225Ac. The control TLC plates of C60, malonic ester, mixture of C60, and malonic ester in toluene were separately developed with ethyl acetate. The control TLC plates of 225Ac and C60 were developed in their respective organic solvents to confirm that exohedral 225Ac (unbound actinium) does not migrate or interact with fullerene on the TLC plate. This was achieved by applying a 10 μL of 225Ac in 0.01 M HNO3 on a TLC plate, allowing it to dry, then applying 10 μL of C60 in toluene or C60 malonate in ethyl acetate on top of the 225Ac spot, and allowing it to dry before developing the plate. A low-potential and high-current dc power supply (20 V, 5 A) (Hewlett Packard 6205C, model, HP E3610A DC) was used for electroplating 225Ac onto Pt electrodes. A high-potential and low-current dc power supply (5 kV, 5 mA) (ORTEC model 459) was used to generate an electric arc between electrodes. Mass measurement of stable Gd (which, in a few instances, was used as the surrogate for 225Ac) was performed by quadrupole inductively coupled plasma mass spectrometry (ICP-MS, Thermo X-SeriesII, Thermo Fisher Scientific, Franklin, MA). The sample introduction system consisted of a cooled spray chamber inlet system (PC3, Elemental Scientific Inc., Omaha, NB). The system was further optimized for signal response and stability by monitoring oxide levels, mass responses, and detector dead time determinations using a tuning solution designed for this optimization process.
Electrical Arc Discharge Apparatus
The dc electrical arc discharge apparatus (arc chamber) consisted of two pieces of glass tubing (each 6 cm o.d., ∼14 cm length) attached through flat ground joints and held together with three standard laboratory clamps. The other ends of the glass tubing were attached to an ACE #7 glass/Teflon bushing adapter (ACE Glass Inc., Vineland, NJ), as depicted in Figure 1. The arc chamber had a provision for a He inlet and outlet located on opposite sides of the chamber. Two brass rods (6 mm o.d.) provided the mechanism to suspend the C60 catcher disk and 225Ac source inside the chamber as well as provided electrical connections. The C60 catcher disk, attached to one of the brass rods, was inserted into the chamber through the left ACE adapter. The 225Ac source, attached to a second brass rod, was inserted into the chamber through the right ACE adapter. Helium gas at a slightly above atmospheric pressure was allowed to leak into the chamber with just enough flow rate (≤10 mL/min) to support the arcing. Vacuum grease was used between flat joints to minimize He leaks (Figure 1).
Figure 1.
Electrical arc discharge apparatus. (1) Discharge chamber (Pyrex glass, 6 cm o.d., 14 cm long), (2) flat ground joint (Pyrex), (3) ACE #7 Teflon fitting, (4) Al-coated C60, (5) 225Ac-deposited Pt disk, (6) brass rod (6 mm o.d.), (7) Teflon jacket, (8) power supply, and (9) electrical leads.
Preparation of the C60 Catcher Disk
A solution of fullerene (C60) was prepared by suspending 1 g of C60 in 10 mL of toluene. The solution was then transferred to a glass container connected to an Air Brush apparatus and sprayed onto an Al disk (2 cm diameter). To facilitate evaporation of the solvent, the Al disk was slightly heated by placing it on a hot plate (at the lowest setting) while spraying the C60 solution. This process was repeated several times until a 1.5–2.0 mg uniform layer of C60 was deposited on the Al disk. The C60 catcher disk was inserted and tightly attached to a 6 mm o.d. brass rod using a small set screw (Figure 1).
Preparation of 225Ac Source
The 225Ac sources were prepared using an electrodeposition technique. The apparatus for electrodeposition of 225Ac was the conventional design, consisting of a 20 mL Pyrex vial and a Pt working electrode (10 mm diameter, 2 mm thickness) silver-soldered to a 6 mm o.d. brass rod to a Pt rod encased in a Teflon tube (∼8 mm o.d. and 6 mm i.d.). The counter electrode was a Pt mesh disk (∼1 cm o.d.) spot-welded to a 1 mm o.d. Pt rod encased in a 6 mm o.d. glass tube. The distance between the two electrodes was ∼5 mm. The electrolyte, 10 mL of 0.01 M HNO3, was stirred magnetically during the electrodeposition. In a typical experiment, 2 mCi of 225Ac as dried nitrate was dissolved in 200 μL of 1.2 M HNO3, and the mixture was transferred to a 20 mL scintillation vial and evaporated to dryness on a hot plate. Upon cooling, the radioactivity was dissolved in 10 mL of 0.01 M HNO3, and the solution was transferred to the electrolytic cell. The working electrode was connected to a negative terminal on the low-voltage dc power supply, while a counter electrode was connected to the positive terminal with alligator clamps. Electrodeposition was typically conducted under a constant potential of 10–11 V. Under our experimental setup, the initial current was 1.56–1.80 A, which dropped to ∼0.5 A over a 2 h period. The electroplating rate was monitored by analyzing 100 μL aliquots of the electrolyte at 1 h intervals for 225Ac activity. After 3 h, when the assay of the electrolyte indicated the removal of 225Ac from solution, the working electrode was carefully removed from the electrolysis cell without opening the electrical circuit. The working electrode was then left to dry in air for 2 h and assayed for radioactivity. The 2 h waiting period was sufficient for 221Fr and 213Bi to regain equilibrium with 225Ac.
Preparation of 225Ac@C60 by Arc Discharge
After insertion of the C60 catcher disk and 225Ac source, the arc chamber (described in the Electrical Arc Discharge Apparatus section) was closed and flushed with He (through inlet and outlet valves). The positive terminal of the dc power supply was connected to the electrode attached to the 225Ac source, and the negative terminal was connected to the C60 catcher disk. The electrical connections were made using alligator clips. A constant potential of 4000–4500 V (low current ≤5 mA) was applied to generate an electric arc between the electrodes, which was maintained for 2 h. Every 30 min, the power supply was turned off and the 225Ac–Pt electrode was manually rotated to alter the striking position of the arc. After 2 h, the power was turned off, and the C60 catcher disk was removed and transferred to a clean 20 mL scintillation vial. After the assay of radioactivity, the 225Ac–C60 catcher disk was immersed in 5 mL of toluene, and after 5 min, the Al disk was removed from the solution and transferred to a clean empty container.
In developing the arc discharge apparatus for the insertion of 225Ac in C60, we used stable Gd as a surrogate for Ac. Gadolinium was quantified by ICP-MS as described by Zheng et al.45 for quantitative analysis of Gd@C82(OH)22 and by Cagle et al.6 for characterization of endohedral metallofullerene radiotracers. In our experiment, the organic layer containing Gd/C60 was digested with 2 mL of concentrated HNO3 and 0.5 mL of 30% H2O2 and then gently evaporated to dryness. The residue was dissolved in 1 mL of 0.1 M HNO3, and the Gd content was determined by ICP-MS. The ICP/MS of the Gd samples clearly shows the mass distribution of naturally occurring Gd isotopes (this information is readily available in the literature).
Extraction of Unbound 225Ac and Its Decay Daughters from the Organic Phase
The toluene solution acquired in the previous section containing C60 and 225Ac was assayed for radioactivity and then subjected to a series of liquid–liquid extraction processes by contacting with 5 mL of 0.01 M HNO3. The immiscible liquid bilayer was intensively mixed for 2 min using a stir vortex and then allowed to settle. Using a glass pipette, the aqueous layer was carefully removed and transferred to a clean vial. The aqueous layer was then backwashed using 1 mL of fresh toluene to extract the residual organic phase. The typical time interval between the start of extraction to the beginning of sample analysis was 11–12 min. Separation and backwashing processes were repeated six times using fresh 5 mL portions of 0.01 M HNO3 followed by fresh 1 mL portions of toluene for the backwash. All fractions were assayed for radioactivity.
Fullerene Surface Modification
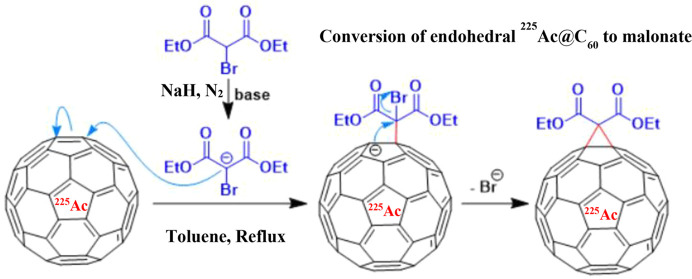
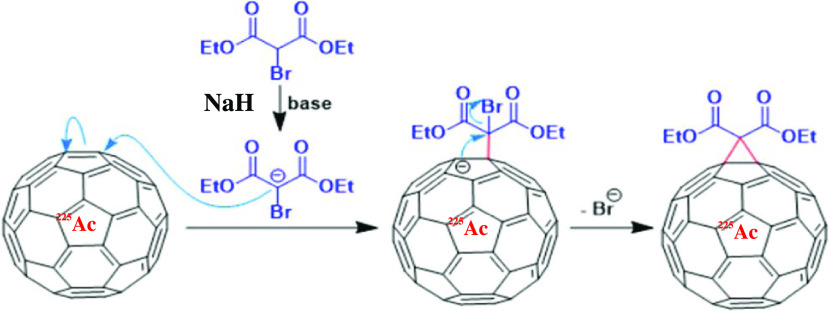
As stated earlier, the resulting radiofullerenes on the Al disk were dissolved in toluene under anaerobic conditions and converted to a malonate derivative using the Bingel reaction in two steps.40,41 In the first step, the toluene fraction, discussed in the Preparation of 225Ac@C60 by Arc Discharge section, containing endohedral 225Ac fullerene was transferred to a two-neck 10 mL pear-shaped flask. The flask was purged with N2 and 0.02 mL of DBM, and then 3 mg of NaH suspended in 4 mL of THF was added. Using a syringe, the THF slurry was transferred into a 15 mL polypropylene centrifuge tube and the mixture was centrifuged for 2 min at ∼1000g to remove the sodium salt. Using a syringe, the supernatant was transferred to a clean three-neck flask and evaporated to dryness under a flow of N2. In the second step, 1 mL of DBM and 10 mL of toluene containing 3 mg of suspended NaH were added. The mixture was refluxed at 80 °C for 6 h while purging with N2. As endohedral metallofullerene adducts formed, the color of the reaction mixture changed from dark purple (color of fullerene) to red (color of C60 malonate) (Figure 2). The progress of the reaction was monitored by withdrawing 5 μL aliquots from the reaction mixture and analyzing using HPLC. Note that the addition of a small amount of DBM in step 1 was part of an overall strategy to optimize the yield.46
Figure 2.
Conversion of 225Ac@C60 fullerene to its malonate derivative.
Results
Electrodeposition of Actinium
In a 0.01 M HNO3 electrolyte containing 2 mCi of 225Ac and under a constant potential of 10.5 V and an initial current of 1.6 A, ∼70 ± 10% of 225Ac was electroplated on the Pt electrode within 2 h. Under the above experimental conditions, the electrical current through the electrolyte drops very quickly from 1.5 to 0.5 A in the first 20 min of electrolysis, and then it decreases very slowly with time. In our setup, the gap between the electrodes, acid concentration, and speed of mixing have significant effects on the electrical current between the two electrodes and on the overall efficacy of electroplating. Since the mass of 2 mCi of 225Ac is only 0.03 μg, the fast drop in the current is not due to a decrease in the mass of 225Ac in the electrolyte during the coarse electrodeposition. Instead, it is most likely due to the presence of ion impurities in the system. In a typical experiment, starting with 1.34 mCi of 225Ac in the electrolyte, 80.2% of 225Ac (1.07 mCi) was electroplated within 90 min. The remaining 17.0% of the activity (0.23 mCi) was found in the electrolyte for an activity recovery of 97%. In this case, because of the high level of activity, we used an ionization chamber for gross measurement of the activity. Because it is an alkali metal, 221Fr (the α-decay daughter of 225Ac) is not expected to be electroplated. Consequently, after removal of the electrode from the electrodeposition cell, the measured gross activity on the electrode actually increases with time and reaches a maximum value (i.e., 221Fr gains equilibrium with 225Ac) within 1 h (Table 1). With a 4.8 min half-life, 221Fr reaches 95% equilibrium with 225Ac within 20 min. The somewhat longer equilibrium time given in Table 1 reflects the fact that some of the 46 min 213Bi generated from the in situ decay of 221Fr is not plated at the time the electrode was removed from the electrodeposition cell.
Table 1. Electrodeposition of 225Aca.
| activity
in electrolyte |
activity on electrode |
||||
|---|---|---|---|---|---|
| events | time (min) | mCi | % | mCi | % |
| start of electroplating | 0 | 1.34 | 100 | ||
| end of electroplating | 98 | 0.228 | 17.0 | 1.07 | 80.2 |
| measurement of activities on the electrode as a function of time after the end of electroplating | 2 | 0.677 | 63.0 | ||
| 7 | 0.775 | 72.2 | |||
| 18 | 0.836 | 77.8 | |||
| 41 | 0.982 | 91.4 | |||
| 56 | 1.05 | 97.8 | |||
| 62 | 1.07 | 100 | |||
The electrolyte (10 mL of 0.01 M HNO3) was magnetically stirred during the electrodeposition. Both working and counter electrodes were Pt (see the Preparation of 225Ac Source section for details). Electrodeposition was conducted under a constant dc potential of 10–11 V. In this experiment, gross radioactivity was measured in an ionization chamber (see the Chemicals, Radioisotopes, and Radioactivity Measurement section).
Synthesis of 225Ac@C60 by Arc Discharge
Passage of electrons from the cathode to anode results in the reverse transfer of 225Ac from the cathode to anode. In general, the transferable amount depends on the electrical potential and current applied between the two electrodes, arcing time, size of the gap between the two electrodes, as well as electrodes’ material, size, and shape. In practice, however, since the two electrodes are not absolutely parallel, it is not possible to predict the strike position of the arc. By trial and error, the highest transfer efficiency was obtained when the arc strikes different positions on the C60-covered anode, and this was achieved by rotating one electrode relative to the other (see Figure 1). Every 30 min, the power to the arc chamber was turned off and the anode was slightly rotated clockwise. Under our experimental conditions, typically, on average, 12 ± 3% of electroplated 225Ac was transferred to the C60-coated catcher electrode during each single arc discharge event, lasting 2 h.
Malonic Acid Derivatization of Synthesized 225Ac@C60
As stated, the extent of conversion of the endohedral fullerene starting material to the malonate derivative was monitored by HPLC analysis, and at various times, 5 μL of the reaction mixture was withdrawn and injected into the HPLC. The time-lapsed HPLC spectra of the reaction mixture at 5 min, 30 min, and 6 h are shown in Figure 3a–c. As shown, the spectra at reaction times of 5 and 30 min consist of three predominate peaks with retention times (RTs) of 5, 7, and 14 min. As the reaction time increases from 5 min to 6 h, the progress for conversion of C60 to its malonate derivative can be seen by the decrease in the magnitude of the peak with an RT of 14 min and by an increase in the magnitude of the peak with an RT of 5 min (Figure 3a–c). Earlier, we established an RT of 14 min for pure C60 and an RT of 5 min for C60 malonate under our experimental conditions. The corresponding HPLC chromatograms, shown in Figure S1 in the Supporting Data, indicate RTs that are consistent with the reported values (see, for example, Gasper and Armstrong47 and Ogawa et al.48). Therefore, the peak with an RT of 14 min corresponds to either C60 or Ac@C60 (both have the same RT; see also Figure S1), and the peak with an RT of 5 min corresponds to either C60 malonate or Ac@C60 malonate. After 6 h, the magnitude of the peak with an RT of 5 min reaches a maximum (Figure 3c), indicating that >95% of Ac@C60 has been converted to its malonate derivative within 6 h. Characterization of the peak with an RT of 7 min was not investigated, but its size diminished as the reaction advanced, suggesting that the species responsible for this peak are an intermediate or unstable product.
Figure 3.
Time-lapsed HPLC analysis of the reaction mixtures for the conversion of 225Ac@C60 to its malonate derivative at (a: blue) 5 min, (b: green) 30 min, and (c: red) 6 h. Mobile phase was 100% toluene.
Stability of 225Ac@C60 and Its Malonic Acid Derivative
225Ac@C60
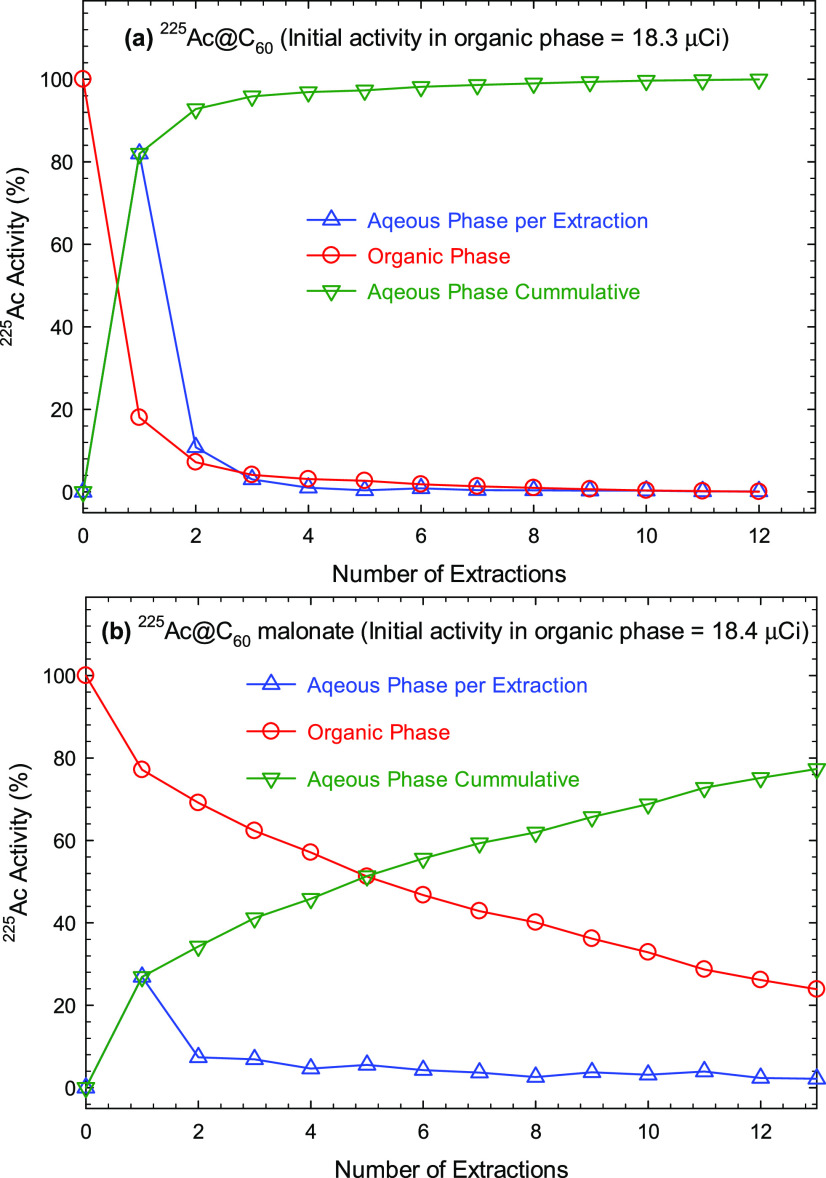
As stated, liquid–liquid extractions were used to study the stability of the synthesized 225Ac@C60 and its malonic acid derivative. The toluene fraction containing 18.3 μCi of 225Ac@C60 (dissolved and recovered from the catcher electrode) was contacted with 5 mL portions of 0.01 M HNO3. The exohedral 225Ac ions were expected to be extracted into the aqueous phase, while the endohedral 225Ac atoms remained in the organic phase (toluene). The fraction of 225Ac extracted into the aqueous phase as a function of the number of extractions is shown in Figure 4a. As indicated in the Chemicals, Radioisotopes, and Radioactivity Measurement section, the activity of 225Ac was based on the activity of 221Fr and 213Bi, the decay daughters of 225Ac in secular equilibrium, which is attained in 3 h. In the initial extraction, >80% of 225Ac was extracted into the aqueous phase. Subsequent washing of the organic phase with nitric acid removed the remainder of 225Ac. However, after six washes, ≤1% of 225Ac remained in the organic phase and resisted extraction and presumably was encapsulated in the fullerene cage. Rapid chemical dissociation of the 225Ac@C60 molecule and significantly reduced radioactivity, however, do not allow for further investigation of the nature of residual activity in the organic phase. In this case, the TLC studies of small portions (10 μL) of the organic phase after the sixth extraction indicated that radioactivity remained at the origin and did not move with the fullerene (Figure 5). Consistent with the TLC results, the HPLC studies of 225Ac@C60 in the organic phase also showed a single peak with an RT of ∼14 min, which is identical to that of empty C60 (Figure S1).
Figure 4.
Radioactivity of 225Ac in organic and aqueous phases as a function of the number of extractions. (a) 225Ac@C60 and (b) 225Ac@C60 malonate. Wash 0 is the initial radioactivity in the toluene faction before extraction. Error bars representing the uncertainty of the radioactivity measurement are smaller than the size of the data points.
Figure 5.
Thin-layer chromatograms of endohedral 225Ac@C60 in the organic phase (red plot) and exohedral 225Ac/C60 (blue plot). The exohedral 225Ac/C60 was prepared as noted in the Other Instrumentation section describing the preparation of the control TLC plates.
225Ac@C60 Malonate
For the case of 225Ac@C60 malonate, the 225Ac@C60 on the catcher electrode was dissolved in toluene under a flow of N2, and then it was converted to a malonate as described in the Fullerene Surface Modification section. The toluene fraction containing 225Ac@C60 malonate (17.5 μCi of 225Ac) was contacted with 5 mL portions of 0.01 M HNO3. Only 29% of 225Ac was initially extracted into the aqueous phase, and after the first six extractions, ∼45% of 225Ac was still retained in the organic phase (Figure 4b). High levels of 225Ac in the organic phase indicate that the 225Ac@C60 malonate is chemically more stable than 225Ac@C60. The higher stability of 225Ac@C60 malonate allowed the use of hot-atom chemistry to verify that 225Ac was inside of the C60 molecule. This was achieved by following the activity of 4.8 min 221Fr (α-decay daughter of 225Ac) in the aqueous washes of extraction numbers 7, 9, 11, and 13 as a function of time after contact with the toluene (Figure 6a). The fluctuation in the level of 221Fr activities in these samples is due to the presence of a minute amount of 225Ac in the aqueous washes as a result of incomplete extraction. Figure 6b shows the observed decay of the 218 keV γ-ray from 221Fr from extraction number 13, which is fitted with two components: an initially large component with a slope of 0.1444 (min–1) corresponding to a half-life of 4.8 min (i.e., 221Fr initially present in the aqueous phase) and a second smaller component with a slope of ∼10 days. The second component represents 221Fr created from the decay of 225Ac present in the aqueous phase as a result of the chemical dissociation of 225Ac@C60 malonate and from incomplete separation of the aqueous and organic layers. The fraction of 221Fr activities found in the aqueous phase in extraction number 13 was 40 ± 2% (Figure 7b). Similar treatment of the data for 221Fr activities found in the aqueous phase for extraction numbers 7, 9, and 11 produced similar values but with larger uncertainty, 44 ± 5, 38 ± 6, and 41 ± 6%, respectively. Collectively, these data indicate that a significant amount of 221Fr becomes accessible to the aqueous solution during the time between each wash.
Figure 6.
(a) Decay of 4.8 m 221Fr (α-decay daughter of 225Ac) in extraction nos. 7, 9, 11, and 13 of 225Ac@C60 malonate (linear scale). (b) Analysis of the decay of 4.8 m 221Fr in extraction no. 13 of 225Ac@C60 malonate (log scale). Error bars represent the uncertainty of the radioactivity measurement.
Figure 7.
TLC chromatogram of the acid-washed toluene fraction containing 225Ac@C60 malonate (a) 30 min after synthesis and (b) 3 days later.
A typical TLC spectrum of the 225Ac@C60 malonate in toluene taken within 30 min after extraction number 6 is shown in Figure 7a. In contrast to the TLC spectrum of 225Ac@C60 in which no activity moved with the fullerenes, the TLC spectrum of the 225Ac@C60 malonate indicated that ∼42% of the activity moved with fullerenes with a peak position or retention distance (RD) of 150–200 mm. Consistent with the results from the extraction experiments discussed in the preceding paragraph, almost no radioactivity remained at the origin. As shown, radioactivity is distributed in three main peaks with RDs of 20–50 mm (33%), 50–65 mm (9.5%), and 100–110 mm (43%). Analysis of this TLC plate, shortly after it was developed, indicated that the peak with an RD of 50–65 mm decayed with the characteristic 4.8 min half-life of 221Fr, and the peak with an RD of 100–110 mm diminished with the characteristic 45.6 min half-life of 213Bi. Analysis of the same TLC plate 3 days later showed only two peaks at 20–50 and 150–200 mm, and their relative intensities were generally unchanged (Figure 7b). The peak at 20–50 mm most likely is 225Ac attached to fragments of C60 malonate formed as a consequence of nuclear decay or chemical dissociation. We cannot offer any information about the chemical nature of these fragments except that they are soluble in toluene.
Discussion
In the preceding sections, we provided experimental evidence on the formation of endohedral 225Ac@C60. This was made possible by a significant improvement in the chemical stability of a surface-modified C60 cage and conversion of 225Ac@C60 to its malonate derivative. After the formation by electrical arc discharge, the resulting radiofullerene on an Al catcher disk was dissolved in toluene under anaerobic conditions and converted to a malonate derivative. After repeated washings of the organic phase with dilute acidic solutions to remove the ionic or aqueous-soluble 225Ac, ∼45% of 225Ac activity was retained in the organic phase and resisted extraction into the aqueous phase. Failure to extract the 225Ac from the organic phase provides definitive evidence that the 225Ac is located inside of the fullerene. In contrast, before functionalization of 225Ac@C60, ∼99% of radioactivity was extracted into the aqueous phase from the organic solution, and only <1% of the activity remained in the organic phase. In a typical experiment, the organic phase contained ∼200 μCi of 225Ac corresponding to a mass of ∼3 ng of 225Ac. Consequently, the concentration of 225Ac used in our study is well below the detection limits of the standard techniques typically employed for the characterization of endohedral fullerenes, including mass spectrometry. In our experiment, the product formed after arc strikes was soluble in toluene, and the solution was completely transparent with no indication of aggregates, and samples were not filtered prior to proceeding to the next step. Note that in the extraction step, the mixture was centrifuged at 1000g, and again, both phases were transparent with no aggregates. Obviously, the transparent solution does not exclude the presence of subnanometer aggregates; however, the movement of radioactivity in the TLC plates of the 225Ac@C60 malonate (Figure 7) clearly supports the fact that Ac products in the toluene fraction were not aggregates; otherwise, 100% of the radioactivity would remain in the origin.
The formation of 225Ac@C60 was further confirmed by a classical hot-atom chemistry technique.49,52 After initial washings of the organic phase with dilute acidic solutions to remove ionic or aqueous-soluble 225Ac, the organic phase was then allowed to stand for at least 3 h to re-establish the equilibrium between 225Ac and its decay daughters, 4.8 min 221Fr and 45.6 min 213Bi. Next, the organic phase containing the endohedral 225Ac@C60 malonate was contacted with fresh dilute acid to extract the ionic Fr and Bi formed by molecular disruption due to nuclear recoil. The disappearance of 221Fr and 213Bi from the organic phase also complemented the results obtained from the aqueous phase analysis. This experiment was repeated every few hours after equilibrium between 225Ac, 221Fr, and 213Bi was re-established. The appearance of a significant fraction of 221Fr activity in the aqueous phase indicated that the molecular dissociation proceeds through the metal recoil mechanism.
Taken together, studies to date show that, like polydentate chelators, single-wall fullerenes are not capable of retaining the 225Ac decay daughters. Little evidence is available to elucidate the chemistry that accompanies this breakup. Considering the ∼100 keV recoil energy imparted to the nucleus of 221Fr following the α-decay of 225Ac and the estimated 3–7 eV carbon–carbon bond energy in fullerene,50,51 these results are not surprising. Further, studies by Thrash et al.28 on 166Ho@C60 system clearly indicated that recoil energies ≤200 eV are sufficient to remove a 166Ho atom from the inside of C60. The 5.75 MeV α particle following the decay of 225Ac imparts a maximum nuclear recoil energy of 104 keV to the 221Fr daughter, which can be calculated from the law of conservation of momentum by Er = 103(mα/M)Eα, where Er is the recoil energy (keV), mα is mass (amu), and Eα is the kinetic energy (MeV) of the α particle, and M is the mass of the recoiling atom (amu). Because 221Fr is incorporated into and is a part of the C60 molecule, the recoil energy will be distributed among the various degrees of freedom of the molecule. Thus, not all of the recoil excitation is actually available for complex dissociation. At this point, it is not clear how the magnitude of this fraction could be estimated. Furthermore, for diatomic molecules, the fraction of energy that contributes to bond-breaking is the effective recoil energy, Ereff, and can be calculated as Ereff = ErmaxM1/(M1 + M2), where M1 and M2 are the atomic weights of the recoiling atom and the attached atom, respectively.52 As a first approximation, the Ereff for a polyatomic molecule, like 225Ac@C60, can also be calculated from the above expression by considering the complex as a pseudo-diatomic molecule and by taking M2 = Mcomplex– M1, where M2 is the mass of the C60 malonate molecule. This results in a net reduction of ∼87% of the recoil energy to ∼13 keV, which is still 3 orders of magnitude larger than the estimated 3–7 eV for the carbon–carbon bond energy in fullerenes (for calculation purposes, we assumed five ethyl malonates per C60).
The result obtained from the extraction experiments was further supported by a series of chromatographic analysis of the organic phase containing 225Ac@C60 or 225Ac@C60 malonate employing both TLC and HPLC techniques. The TLC chromatogram of the organic phase containing 225Ac@C60 showed that ≥99% of radioactivity remained at the origin as a single spot, confirming that almost all 225Ac was in ionic form and was released from the C60 cage. In contrast, the TLC chromatogram of the organic phase containing 225Ac@C60 malonate indicated that 42% of 225Ac moved with the peak associated with C60, and ∼33% of radioactivity remained very close to the origin (i.e., 225Ac that was not incorporated into or released from the C60 cage). Further, analysis of the corresponding TLC plate indicated three smaller peaks in the radio-chromatogram; two of the peaks that were assigned to 4.8 min 221Fr and 45.6 min 213Bi disappeared as a function of time. The third small peak, which decayed with the half-life of 225Ac and accounted for <3% of the total activity, most likely is 225Ac attached to fragments of C60 malonate formed as a consequence of nuclear decay or chemical dissociation. The corresponding HPLC results from 225Ac@C60 or 225Ac@C60 malonate were also consistent with the TLC data; the HPLC chromatograms of 225Ac@C60 and 225Ac@C60 malonate showed a single peak corresponding to either C60 or 225Ac@C60 and a predominant peak corresponding to either C60 malonate or Ac@C60 malonate. The similarity of data with the previously reported data on 212Pb experiment8 and data on ITC and hot-atom chemistry studies presented in the current work supports the formation of 225Ac@C60, which was soluble in toluene. The reason that Ac@C60 is different from Gd@C60 or other lanthanides previously studied perhaps, in part, is because of the limited solubility of Ln@C60 in the solvent and the vast difference in concentrations of Ac@C60 (nM) and empty C60 (mM). A plausible mechanism, which was offered by one of the reviewers of the manuscript, is that each Ac@C60 is surrounded by empty C60 and perhaps it dimerizes or is stabilized by the nearby empty C60 and rendered soluble.
Our studies also showed that endohedral 225Ac fullerenes can be produced by applying an electrical arc discharge between a source of 225Ac (∼1 mCi, electroplated on a Pt electrode) and a thin coat of preformed C60 on an Al disk (C60 thickness of ∼0.25 mg/cm2). In dc arc discharge, the steady electric field drains the electrons from the cathode to anode resulting in the opposite reverse transfer of 225Ac cations from the cathode to anode either in vacuum or under ∼1 atm pressure of He. It is estimated that ions drifting toward the anode pick up energies of the order of 100 eV or more when striking the anode.53 The process can be thought of as a sort of sputtering technique where an arc of high-energy electrons dislodges 225Ac atoms from the surface of the Pt electrode. The dislodged or sputtered 225Ac now constitutes a vapor stream, which accelerates toward and hits the C60-coated anode. Theoretically, the range of 225Ac3+ ions with a kinetic energy of 100 eV in graphite is ∼0.6 μm, corresponding to roughly 500 C60 molecules. Therefore, the 225Ac ions likely smash through more than 100 fullerenes before losing enough energy to stay encapsulated. This may account for the 12% encapsulation efficiency. Material transfer through the dc arc discharge depends on several variables including arcing gap and time, position and movement of the arc on the electrode surfaces, and the electrodes’ size, shape, and material. A comprehensive study of the effects of these variables is beyond the scope of the present work, but as stated under our experimental conditions, on average, 12 ± 2% of the total 225Ac was transferable during each 2 h arcing event. The inherent simplicity of this method is its main advantage over the standard electric arc or laser ablation processes used for encapsulating radioisotopes in fullerenes. This process can be performed in a Class C radiochemical hood on a very small scale and without aerosolizing radioisotopes, and the process does not require a sophisticated vacuum system. As presented, the molecular plating of carrier-free 225Ac is fairly efficient, and typically 70% of the 225Ac was electroplated onto the Pt electrode within a short time using a very simple setup and a simple electrolysis cell.
Conclusions
For the first time, endohedral metallofullerenes were prepared containing 225Ac, an α-particle-emitting radionuclide with broad applications in radioimmunotherapy. In a new preparation route, 225Ac@C60 was produced with an ∼12% efficiency by applying an electrical arc discharge between a source of 225Ac (electroplated on a Pt disk) and a thin coat (∼0.25 mg/cm2) of preformed C60 on an Al disk. The synthesized 225Ac@C60 was stabilized by the addition of malonic esters to the surface of the radiofullerenes. After repeated wash steps, ∼45% of the 225Ac activity was retained in the organic phase and resisted extraction into the aqueous phase, providing definitive evidence that the 225Ac is located inside of the fullerene. The formation of 225Ac@C60 was further confirmed by the repeated extraction of the decay daughters of 225Ac (4.8 min 221Fr1+ and 45.6 min 213Bi3+ released by molecular disruption due to nuclear recoil) into the aqueous phase from the organic phase containing purified endohedral 225Ac@C60 malonate. A series of TLC and HPLC chromatographic analyses of the organic phase containing 225Ac@C60 or 225Ac@C60 malonate further supported the result obtained from extraction experiments. This study confirms the endohedral encapsulation of 225Ac within the C60 molecule due to enhanced structural stability of 225Ac@C60 malonate and also shows that, similar to polydentate chelators, single-wall fullerenes are not capable of retaining the 225Ac decay daughters.
Acknowledgments
The authors acknowledge Dr. Roy Copping, Benjamin Lewis, Jr., and James Gaugler (Oak Ridge National Laboratory) for their critical review of the manuscript. This research was supported in part by the U.S. Department of Energy Isotope Program, managed by the Office of Science for Isotope R&D and Production. The manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the U.S. Department of Energy (DOE).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01659.
HPLC chromatograms of C60 and C60 malonate in toluene. Also, the chromatogram of 225Ac@C60 with an RT identical to empty C60 is shown (Figure S1) (PDF)
Author Present Address
§ USARMY MEDCOM BMACH (USA). jofa.mwakisege2.mil@mail.mil.
The authors declare no competing financial interest.
Supplementary Material
References
- Heath J. R.; O’Brien S. C.; Zhang Q.; Liu Y.; Curl R. F.; Tittel F. K.; Smalley R. E. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1985, 107, 7779–7780. 10.1021/ja00311a102. [DOI] [Google Scholar]
- McElvany S. W. Production of Endohedral Yttrium-Fullerene Cations by Direct Laser Vaporization. J. Phys. Chem. A 1992, 96, 4935. 10.1021/j100191a039. [DOI] [Google Scholar]
- Nagase S.; Kobayashi K. Structural study of endohedral dimetallofullerenes Sc2@C84 and Sc2@C74. Chem. Phys. Lett. 1997, 276, 55. 10.1016/S0009-2614(97)88034-7. [DOI] [Google Scholar]
- Endofullerenes: A New Family of Carbon Clusters; Akasaka T.; Nagase S., Eds.; Kluwer Academic Publishers: Doordrecht, The Netherlands, 2002. Later Published by Springer Science & Business Media. [Google Scholar]
- Endohedral Fullerenes, from Fundamentals to Applications; Yang S.; Wang C.-R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2014. [Google Scholar]
- Cagle D. W.; Kennel S. J.; Mirzadeh S.; Alford M. J.; Wilson L. J. In vivo Studies of Fullerene-based Materials Using Endohedral Metallofullerene Radiotraces. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 5182–5187. 10.1073/pnas.96.9.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolskar R. D.; Benedetto A. F.; Husebo L. O.; Price R. E.; Jackson E. F.; Wallace S.; Wilson L. J.; Alford J. M. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J. Am. Chem. Soc. 2003, 125, 5471–5478. 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- Diener M. D.; Alford J. M.; Kennel S. J.; Mirzadeh S. 212Pb@C60 and its Water-Soluble Derivatives: Synthesis, Stability and Suitability for Radioimmunotherapy. J. Am. Chem. Soc. 2007, 129, 5131–5138. 10.1021/ja068639b. [DOI] [PubMed] [Google Scholar]
- Khong A.; Cross R. J.; Saunders M. From 3He@C60 to 3H@C60: Hot-Atom Incorporation of Tritium in C60. J. Phys. Chem. A 2000, 104, 3940. 10.1021/jp994289m. [DOI] [Google Scholar]
- Shultz M. D.; Wilson J. D.; Fuller C. E.; Zhang J.; Dorn H. C.; Fatouros P. P. Metallofullerene-based Nanoplatform for Brain Tumor Brachytherapy and Longitudinal Imaging in a Murine Orthotopic Xenograft Model. Radiology 2011, 261, 136. 10.1148/radiol.11102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaibaq N. G.; Pollard A. C.; Collins M. J.; Pisaneschi F.; Pagel M. D.; Wilson L. J. Evaluation of the Biodistribution of Serinolamide-Derivatized C60 Fullerene. Nanomaterials 2020, 10, 143 10.3390/nano10010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T.; Diener M.; Chai Y.; Alford M.; Haufler R.; McClure S.; Ohno T.; Weaver J.; Scuseria G.; Smalley R. Uranium stabilization of C28: a tetravalent fullerene. Science 1992, 257, 1661. 10.1126/science.257.5077.1661. [DOI] [PubMed] [Google Scholar]
- Cai W.; Chen C.-H.; Chen N.; Echegoyen L. Fullerenes as Nanocontainers that Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity. Acc. Chem. Res. 2019, 52, 1824. 10.1021/acs.accounts.9b00229. [DOI] [PubMed] [Google Scholar]
- Morgenstern A.; Apostolidis C.; Kratochwil C.; Sathekge M.; Krolicki L.; Bruchertseifer F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200. 10.2174/1874471011666180502104524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poty S.; Francesconi L. C.; McDevitt M. R.; Morris M. J.; Lewis J. S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 1. J. Nucl. Med. 2018, 59, 878. 10.2967/jnumed.116.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K.; Brechbiel M. Application of 212Pb for Targeted α-particle Therapy (TAT): Preclinical and Mechanistic Understanding through to Clinical Translation. AIMS Med. Sci. 2015, 2, 228. 10.3934/medsci.2015.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgqvist J.; Frost S.; Pouget J.-P.; Albertsson P. The potential and hurdles of targeted alpha therapy – clinical trials and beyond”. Front. Oncol. 2014, 3, 324 10.3389/fonc.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcic J. G.; Larson S. M.; Sgouros G.; McDevitt M. R.; Finn R. D.; Divgi C. R.; Ballangrud A. M.; Hamacher K. A.; Ma D.; Humm J. L.; Brechbiel M. W.; Molinet R.; Scheinberg D. A. Targeted alpha particle immunotherapy for myeloid leukemia”. Blood 2002, 100, 1233. 10.1182/blood.V100.4.1233.h81602001233_1233_1239. [DOI] [PubMed] [Google Scholar]
- Rosenblat T. L.; McDevitt M. R.; Mulford D. A.; Pandit-Taskar N.; Divgi C. R.; Panageas K. S.; Heaney M. L.; Chanel S.; Morgenstern A.; Sgouros G.; Larson S. M.; Scheinberg D. A.; Jurcic J. G. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213 lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303. 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcic J. Alpha-Particle Therapy for Acute Myeloid Leukemia. J. Med. Imaging Radiat. Sci. 2019, 50, S18. 10.1016/j.jmir.2019.03.058. [DOI] [PubMed] [Google Scholar]
- Krolicki L.; Bruchertseifer F.; Kunikowska J.; Koziara H.; Królicki B.; Jakuciński M.; Pawlak D.; Apostolidis C.; Mirzadeh S.; Rola R.; Merlo A.; Morgenstern A. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. 10.1007/s00259-018-4015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Królicki L.; Bruchertseifer F.; Kunikowska J.; Koziara H.; Królicki B.; Jakuciński M.; Pawlak P.; Apostolidis C.; Mirzadeh S.; Rola R.; Merlo A.; Morgenstern A. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. 10.1007/s00259-018-4225-7. [DOI] [PubMed] [Google Scholar]
- Brown M. P.; Bezak E.; Allen B. J. The potential complementary role of targeted alpha therapy in the management of metastatic melanoma. Melanoma Manage. 2015, 2, 353. 10.2217/mmt.15.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki H.; Fujiwara Y.; Minowa Y.; Ikehara Y.; Kaneko T.; Okazaki T.; Lizumi Y.; Kim J.; Sakakita H. Synthesis of endohedral-fullerenes using laser ablation plasma from solid material and vaporized fullerenes. AIP Adv. 2019, 9, 075324 10.1063/1.5100980. [DOI] [Google Scholar]
- Komatsu K.; Murata M.; Murata Y. Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 2005, 307, 238. 10.1126/science.1106185. [DOI] [PubMed] [Google Scholar]
- Gan L.; Yang D.; Zhang Q.; Huang H. Preparation of Open-Cage Fullerenes and Incorporation of Small Molecules Through Their Orifices. Adv. Mater. 2010, 22, 1498. 10.1002/adma.200903705. [DOI] [PubMed] [Google Scholar]
- Kobayashi K.; Nagase S. Structures and electronic states of M@C82 (M = Sc, Y, La and lanthanides). Chem. Phys. Lett. 1998, 282, 325. 10.1016/S0009-2614(97)01328-6. [DOI] [Google Scholar]
- Thrash T. P.; Cagle D. W.; Alford J. M.; Wright K.; Ehrhardt G. J.; Mirzadeh S.; Wilson L. J. Toward Fullerene-Based Radiopharmaceuticals: High Yield Neutron Activation of Endohedral 165Ho Metallofullerenes. Chem. Phys. Lett. 1999, 308, 329–336. 10.1016/S0009-2614(99)00581-3. [DOI] [Google Scholar]
- Wilson L. J.; Cagle D. W.; Thrash T. P.; Kennel S. J.; Mirzadeh S.; Alford M. J.; Ehrhardt G. J. Metallofullerene Drug Design”. Coord. Chem. Rev. 1999, 190–192, 199. 10.1016/S0010-8545(99)00080-6. [DOI] [Google Scholar]
- Capture of a neutron by a nucleus is followed by an emission of 6–8 MeV prompt gamma-rays, and to conserve the momentum, the product nucleus has to recoil. For a mass of 100 amu, the maximum recoil energy following the capture of a neutron by a nucleus is on the order of ∼200 eV.
- Braun T.; Thege I. K.; Rausch H.; Suvegh K.; Vertes A. Dose effect in neutron-irradiated C60: a positron lifetime spectroscopy and DSC study. Chem. Phys. Lett. 1995, 238, 290. 10.1016/0009-2614(95)00429-8. [DOI] [Google Scholar]
- a Braun T.; Rausch H. Endohedral Incorporation of Argon Atoms into C60 by Neutron Irradiation. Chem. Phys. Lett. 1995, 237, 443–447. 10.1016/0009-2614(95)00352-5. [DOI] [Google Scholar]; b Braun T.; Rausch H. Nuclear Recoil Implosion for Generating Radioisotopically Labeled Fullerene Endohedrals and Cages. J. Radioanal. Nucl. Chem. 2000, 243, 27–30. 10.1023/A:1006798709868. [DOI] [Google Scholar]
- Gadd G. E.; Blackford M.; Moricca S.; Webb N.; Evans P. J.; Smith A. M.; Jacobsen G.; Leung S.; Day A.; Hua Q. The World’s Smallest Gas Cylinders. Science 1997, 277, 933. 10.1126/science.277.5328.933. [DOI] [Google Scholar]
- Gadd G. E.; Schmidt P.; Bowles C.; McOrist G.; Evans P. J.; Wood J.; Smith L.; Dixon A.; Easey J. Evidence for Rare Gas Endohedral Fullerene Formation from γ Recoil from HPLC Studies. J. Am. Chem. Soc. 1998, 120, 10322–10325. 10.1021/ja9806276. [DOI] [Google Scholar]
- Sueki K.; Kikuchi K.; Tomura K.; Nakahara H. Stability of Metallofullerenes Following Neutron Capture Reaction on the Metal Ion. J. Radioanal. Nucl. Chem. 1998, 234, 95. 10.1007/BF02389754. [DOI] [Google Scholar]
- Sueki K.; Akiyama K.; Kikuchi K.; Nakahara H.; Tomura K. Stability of Radio-Metallofullerenes Against Beta Decay. J. Radioanal. Nucl. Chem. 1999, 239, 179. 10.1007/BF02349553. [DOI] [Google Scholar]
- Ohtsuki T.; Ohno K.; Shiga K.; Kawazoe Y.; Maruyama Y.; Masumoto K. J. Systematic study of foreign-atom-doped fullerenes by using a nuclear recoil method and their MD simulation. J. Chem. Phys. 2000, 112, 2834–2842. 10.1063/1.480858. [DOI] [Google Scholar]
- Tellgmann R.; Krawez N.; Lin S.; et al. Endohedral fullerene production. Nature 1996, 382, 407. 10.1038/382407a0. [DOI] [Google Scholar]
- Pietzak B.; Waiblinger M.; Almeida-Murphy T.; Weidinger A.; Höhneb M.; Dietelc E.; Hirsch A. Buckminsterfullerene C60: a chemical Faraday cage for atomic nitrogen. Chem. Phys. Lett. 1997, 279, 259. 10.1016/S0009-2614(97)01100-7. [DOI] [Google Scholar]
- Nakamura Y.; Suzuki M.; Imai Y.; Nishimura J. Synthesis of [60]Fullerene Adducts Bearing Carbazole Moieties by Bingel Reaction and Their Properties. Org. Lett. 2004, 6, 2797. 10.1021/ol048952n. [DOI] [PubMed] [Google Scholar]
- Alegret N.; Chaur M. N.; Santos E.; Rodríguez-Fortea A.; Echegoyen L.; Poblet J. M. Bingel–Hirsch Reactions on Non-IPR Gd3N@C2n (2n = 82 and 84). J. Org. Chem. 2010, 75, 8299. 10.1021/jo101620b. [DOI] [PubMed] [Google Scholar]
- Boll R. A.; Malkemus D.; Mirzadeh S. Production of Actinium-225 for Alpha Particle Mediated Radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. 10.1016/j.apradiso.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Table of Isotopes, 8th ed.; Firestone R. B.; Shirley V. S., Eds.; John Wiley & Sons Inc: New York, 1996; p 1. [Google Scholar]
- Zheng L.-N.; Wang M.; Zhao L. C.; Sun B. Y.; Wang B.; Chen H. Q.; Zhao Y. L.; Chai Z. F.; Feng W. Y. Quantitative analysis of Gd@C82(OH)22 and cisplatin uptake in single cells by inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2383. 10.1007/s00216-014-8422-3. [DOI] [PubMed] [Google Scholar]
- Fullerenes, Chemistry, Physics, and Technology; Kadish K. M.; Ruoff R. S., Eds.; John Wiley & Sons Inc: New York, 2000; pp149–156. [Google Scholar]
- Gasper M.; Armstrong D. A Comparative Study of Buckministerfullerene and Higher fullerene Separations by HPLC. J. Liq. Chromatogr. 1995, 18, 1047–1076. 10.1080/10826079508009276. [DOI] [Google Scholar]
- Ogawa T.; Sugai T.; Shiinohara H. Isolation and characterization of Er@C60. J. Am. Chem. Soc. 2000, 122, 3538–3539. 10.1021/ja992665a. [DOI] [Google Scholar]
- The process known as “hot atom chemistry” relates to the fact that following the nuclear decay, the kinetic and electronic excitations (originated from the release of nuclear energy) may cause molecular bond disruption. See, for example, reference 54.
- Mirzadeh S.; Kumar K.; Gansow O. A. The Chemical Fate of 212Bi-DOTA Formed by ß-Decay of 212Pb(DOTA)2- Complex. Radiochim. Acta 1993, 60, 1–10. 10.1524/ract.1993.60.1.1. [DOI] [Google Scholar]
- Saunders M.; Jimenez-Vazquez H. A.; Cross R. J.; Poreda R. J. Stable compounds of helium and neon: He@C60 and Ne@C60. Science 1993, 259, 1428. 10.1126/science.259.5100.1428. [DOI] [PubMed] [Google Scholar]
- Murray R. L.; Scuseria G. E. Theoretical evidence for a C60 “window” mechanism. Science 1994, 263, 791. 10.1126/science.263.5148.791. [DOI] [PubMed] [Google Scholar]
- Fite W. L.Chemical Physics of Discharges In Chemical Reactions in Electrical Discharges, Advances in Chemistry; Blaustein, Ed.; American Chemical Society: Washington, DC, 1969. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.