Abstract
A 10-year-old Quarter Horse gelding presented to the Texas A&M University Veterinary Teaching Hospital with a six month-history of ataxia and lameness in the hind limbs. The horse was treated presumptively for equine protozoal myeloencephalitis (EPM) based on clinical signs but was ultimately euthanized after its condition worsened. Gross lesions were limited to a small area of reddening in the gray matter of the thoracic spinal cord. Histologically, trypanosome amastigotes morphologically similar to Trypanosoma cruzi, the agent of Chagas disease in humans and dogs, were sporadically detected within segments of the thoracic spinal cord surrounded by mild lymphoplasmacytic inflammation. Ancillary testing for Sarcocystis neurona, Neospora spp., Toxoplasma gondii and Leishmania spp. was negative. Conventional and real time polymerase chain reaction (PCR) of affected paraffin embedded spinal cord were positive for T. cruzi, and sequencing of the amplified T. cruzi satellite DNA PCR fragment from the horse was homologous with various clones of T. cruzi in GenBank. While canine Chagas disease cases have been widely reported in southern Texas, this is the first report of clinical T. cruzi infection in an equid with demonstrable amastigotes in the spinal cord. In contrast to previous instances of Chagas disease in the central nervous system (CNS) of dogs and humans, no inflammation or T. cruzi amastigotes were detected in the heart of the horse. Based on clinical signs, there is a potential for misdiagnosis of Chagas disease with other infectious diseases that affect the equine CNS. T. cruzi should be considered as a differential diagnosis in horses with neurologic clinical signs and histologic evidence of meningomyelitis that originate in areas where Chagas disease is present. The prevalence of T. cruzi in horses and the role of equids in the parasite life cycle require further study.
Keywords: Chagas disease, Equine, Amastigotes, Trypanosomiasis, Polymerase chain reaction, Formalin fixed paraffin embedded tissue
1. Introduction
Chagas disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi (Gutiérrez et al., 2013; Kjos et al., 2008). It is endemic in many non-Caribbean Latin American countries with a purported 5.7 million people currently infected (Anon, 2010). The protozoan multiplies within arthropod vectors of the Reduviidae family (Rhodnius spp., Panstrongylus spp. and Triatoma spp.), also known as cone-nose or kissing bugs, which harbor the protozoan epimastigote and metacyclic trypomastigote life stages within the midgut and hindgut, respectively (Gutiérrez et al., 2013). Triatomine bugs transmit T. cruzi by defecating infectious metacyclic trypomastigotes around cutaneous bite wounds or mucous membranes, thereby allowing access to the host’s blood stream where the trypomastigotes can either infect host cells to form amastigotes or infect other kissing bugs feeding on the host (Gutiérrez et al., 2013). Humans can become infected transplacentally, via blood or organs through transplantation (Rassi et al., 2012). While autochthonous cases of acute Chagas disease in people remain rare in the United States (US), there are an estimated 300,000 chronic cases in immigrants from endemic countries living in the US (Bern and Montgomery, 2009). Chagas disease has been frequently reported in dogs from the southeastern US where T. cruzi persists through a sylvatic life cycle that involves infection of raccoons, opossums, armadillos, and skunks (Bern et al., 2011). While the arthropod vector will readily feed on horses and other live stock in experimental situations (Grundemann, 1947), the various life stages of T. cruzi have never been reported before in peripheral blood or tissues from horses.
2. Case report
A 10-year-old, Quarter Horse gelding, used for barrel racing, presented in mid-summer to the Texas A&M University Veterinary Medical Teaching Hospital with a six-month history of lameness and ataxia in the hind limbs. The horse lived in southern Bexar County, Texas and had been previously diagnosed with chronic impar ligament desmitis in the right front foot. Four months prior to referral, the horse was seen by a veterinarian when the owner noted a new left hind limb lameness. At that time, the gelding was ataxic in both hind limbs, with the right hind more severely affected than the left hind. Cerebrospinal fluid (CSF) was tested for antibodies against Sarcocystis neurona, the causative agent of equine protozoal myeloencephalitis (EPM), via surface antigen 2, 4/3 enzyme-linked immunosorbent assay (ELISA), Western Blot and indirect fluorescent antibody test (IFAT). All tests on the CSF were negative for S. neurona, and a Neospora hughesi IFAT was also negative. However, due to the difficulty of ante-mortem diagnosis of EPM in horses, treatment with ponazuril (5 mg/kg, per os) for 28 days was initiated. An additional 10-day course of levamisole and tapering course of dexamethasone (starting at 0.1 mg/kg) were administered after the horse failed to improve with ponazuril treatment. Nuclear scintigraphy (bone scan) did not reveal any significant musculoskeletal abnormalities.
Upon neurologic examination at referral, no cranial nerve deficits were noted and mentation was appropriate. A standing musculoskeletal exam was unremarkable and no muscle atrophy was appreciated. Range of motion of the cervical vertebrae was within normal limits and the cutaneous trunci reflex was intact. During a moving examination, the patient had difficulty pivoting on his hind limbs and would swing his rear legs into wide arcs (circumduction) when circled tightly. The horse also had difficulty navigating around low obstacles, such as curb stones, and would scrape the tops of his hind hooves on the pavement (knuckling) when transitioning from a walk to a trot. The hind end deficits were consistent with upper motor neuron paresis, as on tail pull the gelding had good resting muscle tone with adequate extensor strength while standing, but was easily pulled to the side while walking. His ataxia was asymmetric, with the right side more severely affected than the left, and he was assessed as Grade 3/5 and 4/5 ataxic in the left and right hind limbs, respectively. Assessment of the fore limbs was complicated by the severity of ataxia in the hind limbs, but they were apparently unaffected. Lesion localization was presumed to be within the T3-L3 region based on neurologic examination. Radiographs of the cervical and lumbar vertebrae revealed no abnormalities. Ultrasonography of the caudal lumbar vertebrae, sacrum and pelvis (both transcutaneous and transrectal) was within normal limits. Serum vitamin E concentration was within the normal range (3.32 μg/mL; reference 2.5–4.0 μg/mL) and there were no hematologic or serum chemistry abnormalities. Euthanasia was elected three months after neurologic evaluation due to worsening condition and poor prognosis for recovery.
A necropsy was conducted at the Texas A&M Pathology Diagnostic Laboratory. A focally extensive area of the dorsal and ventral horn gray matter of the T8-T13 spinal cord segments was dark red (congestion and hemorrhage) (Fig. 1a). No lesions were apparent in the heart or other major parenchymal organs. An unfixed section of the pons and cerebellum sent to the Texas State Department of Health was negative for rabies via immunofluorescence assay (IFA). Tissues from all major organ systems were preserved in 10% neutral buffered formalin and routinely processed for paraffin embedding. Slides (5 μm sections) were stained with hematoxylin and eosin (HE). Histologically, small perivascular clusters of lymphocytes, fewer plasma cells and macrophages were scattered randomly throughout all levels of the spinal cord meninges, gray and white matter. A few axons near inflamed areas were mildly swollen with dilated myelin sheaths (spheroids). Lesions were most severe in the thoracic spinal cord within the reddened area observed at necropsy. Inflammatory cells occasionally surrounded pseudocysts in the white matter that contained a few 2–3 μm, round protozoan amastigotes (Fig. 1b) characterized by an outer thin periplasmic membrane, 1 μm basophilic nucleus and a rod-shaped kinetoplast (Fig. 1b inset) that was sometimes oriented parallel to nucleus. Amastigotes were not observed in the brainstem, cerebrum or cerebellum, and no histologic lesions were apparent in the heart and other parenchymal organs. The protozoa were not stained by periodic acid Schiff or Gomori methenamine silver stains. Unstained sections of affected areas of the spinal cord were sent to additional diagnostic laboratories for further identification of the protozoa. Although clusters of organisms were apparent in sections, the protozoa did not stain on immunohistochemistry for Sarcocystis spp. (University of Georgia Veterinary Diagnostic Laboratory; Fig. 1c), Neospora spp. or Toxoplasma gondii (United States Department of Agriculture, Beltsville). DNA in situ hybridization for Leishmania spp. (Michigan State University Diagnostic Center for Population and Animal Health) was also negative. Electron microscopy of the affected spinal cord was attempted, but the paucity of the protozoa and fixation artifacts prevented a thorough examination.
Fig. 1.
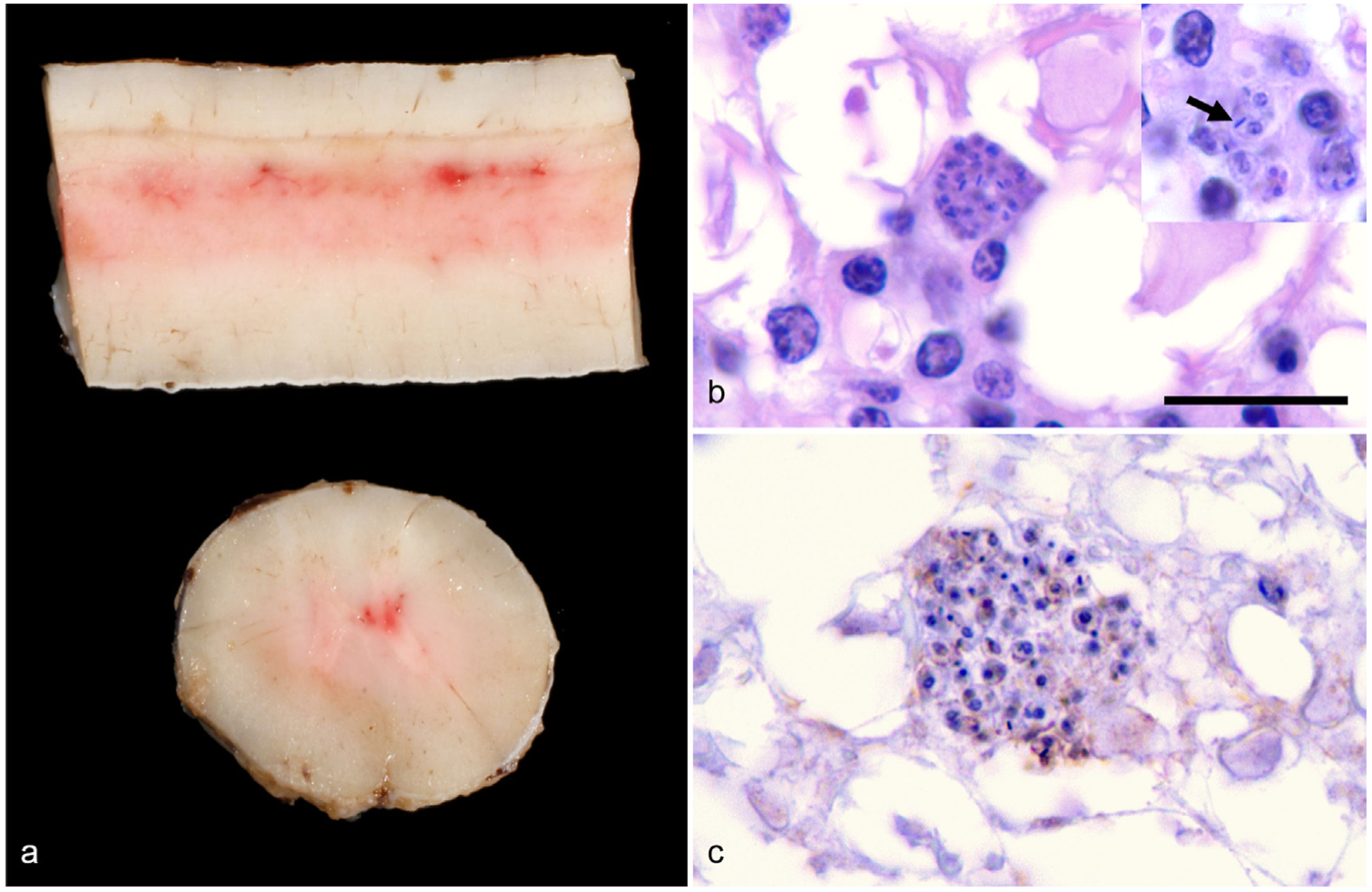
Gross and histologic lesions within the spinal cord of a 10-year-old Quarter Horse gelding from Texas. (a) Longitudinal and transverse sections of the thoracic spinal cord reveal multifocal areas of congestion and mild hemorrhage within the dorsal and ventral horn gray matter. (b) Histologically, clusters of 2–3 μm in diameter, protozoan amastigotes surrounded by a few plasma cells are in the hyperemic areas of spinal cord identified in panel a. In the insert, amastigotes have a thin outer membrane, contain a 1 μm basophilic nucleus and have a bar-shaped kinetoplast (arrow) that is often oriented parallel to the nucleus; HE, 1000×, Bar = 10 μm. (c) Large clusters of amastigotes are negative with Sarcocystis spp.—specific immunohistochemical staining, which would stain organisms bright red if positive; IgG antibody conjugated with horseradish peroxidase, 1000×.
Sections (containing approximately 50 μm of tissue) from affected spinal cord and heart formalin-fixed paraffin embedded (FFPE) tissue blocks were processed for DNA extraction using two different methods: the E.Z.N.A® Tissue Extraction (Omega Bio-Tek, USA) and BiOstic® FFPE Tissue DNA Isolation (MO-BIO, USA) kits as per the manufacturers’ instructions. DNA from the extracted horse samples was quantified using an Epoch spectrophotometer (Biotek, USA) with readings of 34–128 ng/μL DNA for the spinal cord samples and 800 ng/μL for the heart sample. Conventional polymerase chain reaction (PCR) was conducted using the TCZ1/TCZ2 (Moser et al., 1989) and Tc121/Tc122 (Virreira et al., 2003; Wincker et al., 1994) primer sets, which target the nuclear satellite and kinetoplast minicircle DNA sequences, respectively (Fig. 2). Quantitative real-time PCR (qPCR) was performed with the Cruzi 1/2 primer set with 6-carboxyfluorescein (FAM)-labeled Cruzi 3 probe (Duffy et al., 2013; Piron et al., 2007) as previously described except with an initial denaturation time of 3 min. FFPE heart tissue from a canine clinical isolate confirmed via histopathology and T. cruzi Sylvio X10 strain (ATCC©, USA) were used as positive controls, while negative controls consisted of distilled water and reaction mix with no template added. Positive bands were seen for the horse spinal cord samples with both conventional PCR primer sets. For qPCR, two separate extractions from a thoracic spinal cord block had positive cycle threshold (Ct) values of 27 and 24. Additional spinal cord sections from the thoracolumbar region had borderline positive Ct values (32 and 33). The heart sample was negative using all conventional and qPCR primer sets evaluated. The TCZ1/2 DNA fragment from the horse and the FFPE canine clinical isolate control were purified using a Wizard® SV Gel and PCR Clean-Up System (Promega, USA) and were sequenced offsite (Eton Biosciences, USA). The sequences were analyzed with the nucleotide Basic Local Alignment Search Tool (NCBI, USA) to determine homology. Both sequences had 98% identity with multiple T. cruzi accessions (AY520047.1, HM015662.1 and HM015648.1) in GenBank and corresponded to the T. cruzi satellite sequence as previously described (Moser et al., 1989). Further attempts to determine the T. cruzi discrete typing unit of the positive samples through amplification and DNA sequencing of the TcSC5D gene (Cosentino and Agüero, 2012) were unsuccessful, but the low sensitivity of this assay in samples that are not from pure parasite culture may have contributed to the lack of amplification.
Fig. 2.
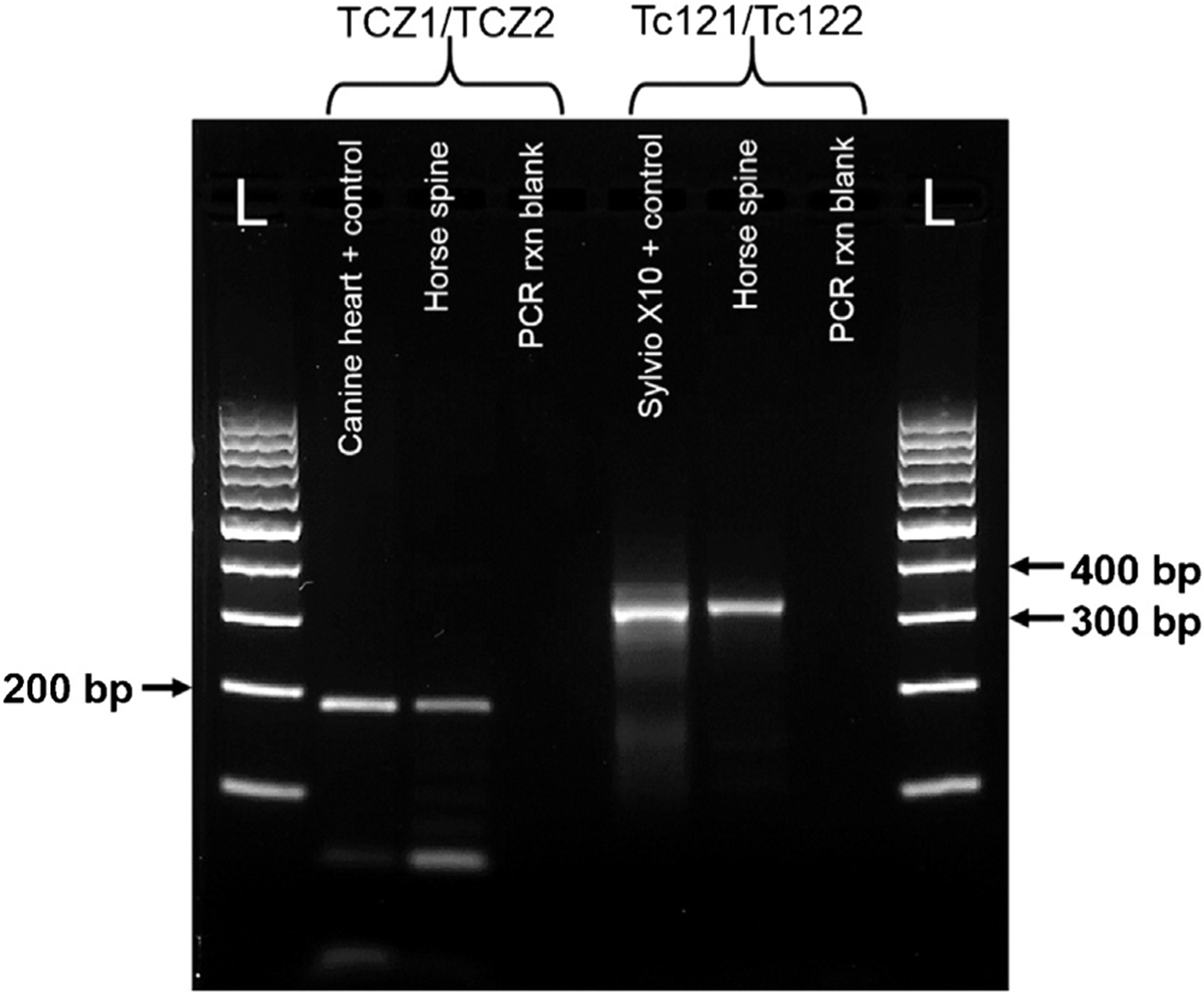
DNA gel electrophoresis of PCR amplicons from the horse and control samples using the T. cruzi TCZ1/TCZ2 and Tc121/Tc122 PCR primer sets, 2% agarose gel, 100 V for 30 min. Each band of the DNA ladder (L) represents 100 bp. From left to right for the TCZ1/TCZ2 primer set: formalin-fixed paraffin embedded (FFPE) heart from a canine patient with IFA confirmed Chagas disease; FFPE horse spinal cord (BiOstic® extraction) and PCR reaction blank. For the Tc121/Tc122 primer set: T. cruzi Sylvio X10 strain positive control; FFPE horse spinal cord (E.Z.N.A® extraction) and PCR reaction blank.
3. Discussion
In light of the histologic and molecular findings, the scattered protozoa in the spinal cord of the horse were identified as T. cruzi. Histologically, T. cruzi forms large pseudocysts within host cells that are filled with amastigotes (Fig. 3a) that have a distinctive morphology and features that are not apparent in the circulatory life stages (Taylor and Authié, 2004). A prominent feature of Trypanosoma spp. is the presence of a kinetoplast, which is housed in the mitochondrion of the basal body and is composed of a network of circular DNA that encodes the mitochondrial genome (Rassi et al., 2012). Known trypanosome species that infect horses include Trypanosoma evansi and Trypanosoma equiperdum. A third species, Trypanosoma equinum, is now thought to be a variant of T. evansi or equiperdum (Gutiérrez et al., 2013). T. evansi is widespread in South America and causes the neurologic disease Mal de Cadeiras (surra) in horses with sporadic infections in cattle and dogs (Desquesnes et al., 2007). T. evansi is genetically similar to T. equiperdum, the cause of the venereal disease, dourine in equids. Both species are considered to be mutants of Trypanosoma brucei, the agent of African sleeping sickness, that have lost kinetoplast DNA (Lai et al., 2008). T. evansi does not form amastigotes (Rodrigues et al., 2009), and the infective trypomastigotes (Fig. 3b) are often not visible in section without immunolabeling (Fig. 3c). T. equiperdum trypomastigotes localize to capillaries within the mucous membranes of the urogenital tract and do not form pseudocysts within tissues (Taylor and Authié, 2004). Additional protozoa that can infect the CNS of horses and cause clinical disease include S. neurona and N. hughesi (Dubey et al., 2001; Marsh et al., 1996). T. gondii has also been reported incidentally in the CNS of horses at slaughter without appreciable clinical signs (Evers et al., 2013). These agents do not have kinetoplasts (Dubey et al., 2001) and produce tissue stages that are morphologically distinct from trypanosome amastigotes. S. neurona does not form sarcocysts in equine tissues (Dubey et al., 2001), and schizonts in the CNS are composed of 3–5 μm long, slender merozoites (Fig. 3c). Neospora spp. (Fig. 3d) and T. gondii (Fig. 3e) are histologically indistinguishable from each other and form large tissue cysts (Dubey et al., 2001). Ancillary testing was negative for these agents as well as the closest morphological alternative to T. cruzi, visceral Leishmania spp., which have been reported to cause cutaneous lesions in horses in Europe, Puerto Rico and Florida but are not known to affect the equine CNS (Reuss et al., 2012). Further supporting description of the equine CNS protozoa as a Trypanosoma spp., the T. cruzi qPCR primer and probe set used in this case has also been validated to not cross-react with L. infantum (Piron et al., 2007).
Fig. 3.
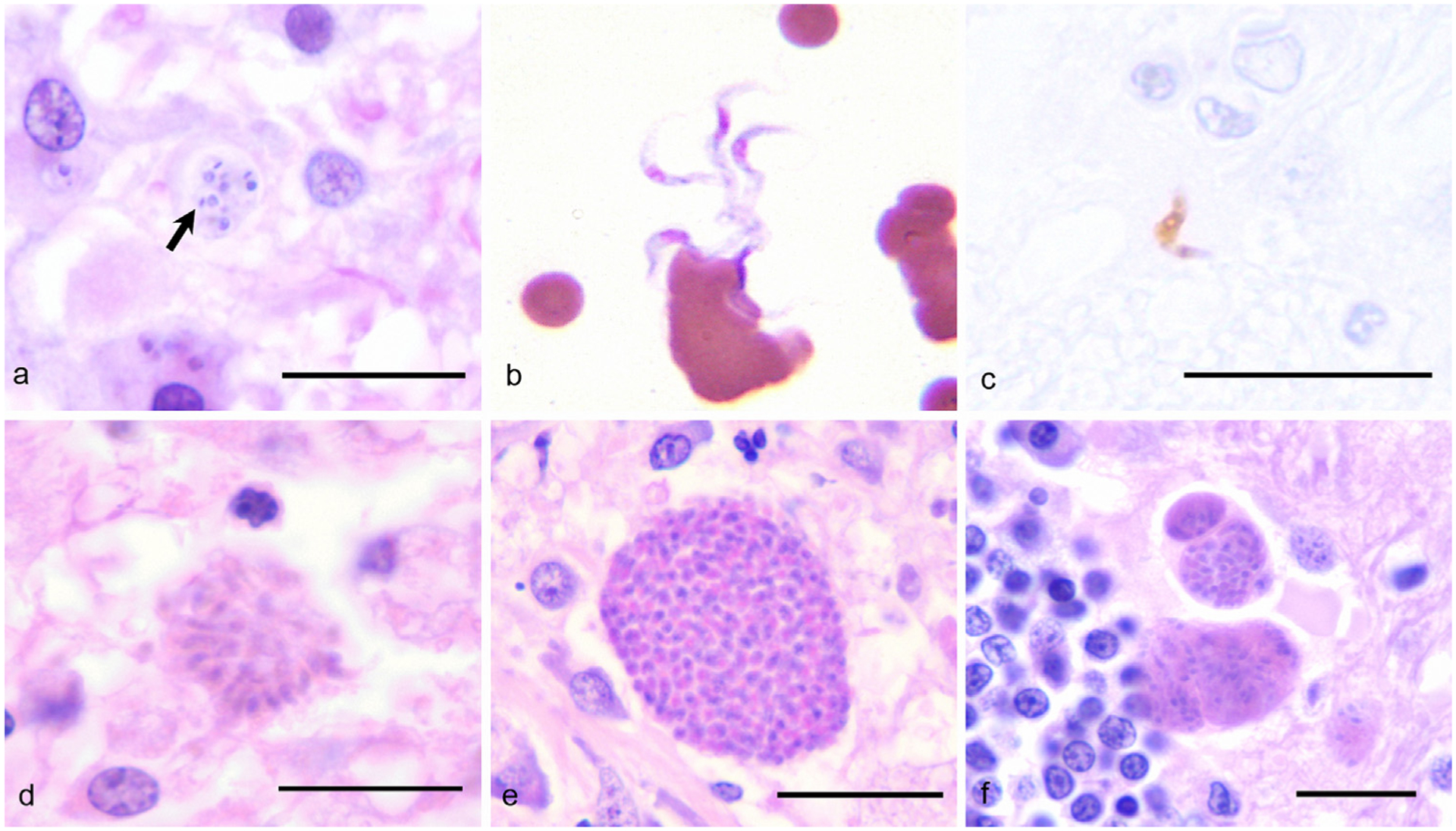
A comparison of selected protozoal agents that affect the CNS. The details of the cases in panels b and c have been previously published (Rodrigues et al., 2009), and the cases were re-photographed for this report. (a) Trypanosoma cruzi amastigotes (arrow) within the cerebrum of a dog; HE, 1000×, Bar = 10 μm. (b) Trypanosoma evansi trypomastigotes in the peripheral blood of a horse; Dif-quick, 1000×. (c) Immunohistochemistry identifies a Trypanosoma evansi trypomastigote in the brain of a horse; Avidin-biotin-peroxidase complex, 1000×, Bar = 10 μm. (d) Sarcocystis neurona merozoites within the brain of a horse; HE, 1000×, Bar = 10 μm. (e) Neospora spp. tissue cyst in the brain of a dog; HE, 1000×, Bar = 10 μm. (f) Toxoplasma gondii tissue cyst within the brain of a cat; HE, 1000×, Bar = 10 μm.
Chagas disease has been frequently reported in dogs from the southern US, and especially in Texas, where one study (Kjos et al., 2008) identified 537 positive cases over a 14-year period in 48 of 254 Texan counties (20.3% prevalence) either through serology or histopathology at necropsy. In that study, more than 25 cases of canine Chagas disease were reported in Bexar County, where this horse originated, and 60% of the cases occurred within the south-central or coastal regions of Texas (Kjos et al., 2008). Dogs are important reservoir hosts for T. cruzi in Central and South America, but their role in the domestic transmission cycle in the US is less certain (Bern et al., 2011). While horses could also have the potential to be reservoir hosts in the US and Latin America, only a few studies have evaluated T. cruzi seroprevalence in horses and the sample size has been small (Fujita et al., 1994; Ikenga and Richerson, 1984). In serologic surveys of domestic animals and livestock conducted in endemic regions of Central and South America, the majority of examined horses were seronegative for T. cruzi antibodies (Fujita et al., 1994). A few T. cruzi seropositive horses were identified in Argentina, but T. cruzi antibody detection is complicated in regions that are also endemic for T. evansi where there may be significant antibody cross-reactivity between the two species (Desquesnes et al., 2007). A study of potential T. cruzi hosts in Brewster County, Texas conducted in the early 1980s identified two clinically normal horses that were seropositive for T. cruzi via indirect hemagglutination assay (Ikenga and Richerson, 1984). While the seropositivity in the Texan horses may also be due to antibody cross reaction with another Trypanosoma spp., the most likely confounder, T. evansi, has never been reported in the US. Blood was not retained from the horse of this report after initial examination, so it is unknown if the patient was seropositive for T. cruzi or had trypomastigotes in circulation. Discrete typing could not be performed on the equine FFPE spinal cord sample to determine the lineage of the T. cruzi strain infecting the horse and whether it represented a unique haplotype. The presence of seropositive horses in Texas coupled with evidence of amastigotes in tissues in this case report suggests that horses can be reservoir hosts for Chagas disease, but additional study is needed to determine the overall impact on the human population and if the CNS lesions observed in this case represent a unique feature of T. cruzi infection in horses or are related to a specific haplotype.
In humans, Chagas disease occurs in acute and chronic forms where initial infection is characterized by trypomastigote invasion of leukocytes and hyperplasia of peripheral lymph nodes draining lymph from the area of the insect bite wound (Rassi et al., 2012) with most patients experiencing no symptoms. Direct invasion of the CNS by the parasite is rare, and severe encephalitis is most frequently observed in patients with acquired immunodeficiency syndrome (Py, 2011). In a previous case of neurologic Chagas disease in a dog from Texas, the patient also presented with hind limb paresis and proprioceptive deficits with minimal signs of cardiac compromise (Berger et al., 1991). Inflammation in the brain and spinal cord consisted primarily of perivascular and parenchymal aggregates of macrophages, plasma cells and lymphocytes with occasional amastigotes that had a similar morphology to those observed in the horse of this report (Berger et al., 1991). This particular pattern of inflammation is similar to the presentation of EPM, which is a more common protozoal neurologic disease in horses that is often diagnosed presumptively post-mortem due to difficulty in detecting S. neurona merozoites in tissue (Marsh et al., 1996). The present case is an unusual presentation of Chagas disease in that inflammation and amastigotes were not observed in the myocardium or other organs outside of the CNS. However, the presence of T. cruzi amastigotes in tissue without apparent cardiac involvement is not without precedent; another dog from Texas had T. cruzi PCR-positive amastigotes within a peripheral lymph node without significant heart lesions (Nabity et al., 2006).
The anti-protozoal drugs benznidazole and nifurtimox are used in Latin America for the treatment of acute and chronic forms of Chagas disease in humans (Gutiérrez et al., 2013). These drugs are not widely available in the US but are provided by the Centers for Disease Control for use in treatment of confirmed human cases and have had some success in treating experimental infections in dogs (Bern et al., 2011). However, most clinical canine cases are treated only with supportive therapy (Bern et al., 2011). The possible side effects of these drugs in horses are unknown, but in humans, peripheral neuritis and dermatitis are potential adverse reactions to nifurtimox and benznidazole, respectively. Itraconazole and allopurinol have also been evaluated with mixed results (Rassi et al., 2012). The effect of ponazuril and levamisole treatment, the standard EPM therapeutics used in this case report, on mitigating T. cruzi infection has not been investigated.
This report represents the first documented incidence of clinical disease related to T. cruzi infection in equids, and is the first histologic description of T. cruzi amastigotes in the tissues of a horse. The diagnosis of Chagas disease in the horse of this report was confirmed via conventional and qPCR from FFPE tissue blocks. While the use of FFPE tissues can be problematic due to increased incidence of fixation artifacts and potential fragmentation of DNA (Ludyga et al., 2012), this report shows that FFPE tissues can be used for molecular confirmation of Chagas disease in equine tissues. In addition to retrospective analysis of archival specimens for T. cruzi, FFPE tissues could also be useful in determining the prevalence of Chagas disease in horses from areas that cannot process fresh tissues due to limited access to refrigeration or lack of nearby veterinary diagnostic laboratories. Three different primer sets reported to be specific for T. cruzi in samples derived from human patients were used in this report. While there is a possibility that the primer sets developed for use in people could be cross reacting with another Trypansoma spp. found in animals, the histopathologic morphology of the protozoa and geographic location from which the horse originated support a diagnosis of Chagas disease. The similarity of the spinal cord lesions to the more common cause of protozoal myelitis, S. neurona, suggests that there is a potential for misdiagnosis and T. cruzi should now be considered as a differential diagnosis in horses with similar neurologic clinical signs and lesions, especially in areas where canine Chagas disease is present. Further study to determine the prevalence of Chagas disease in horses and their role in the parasite life cycle is warranted.
Acknowledgement
The authors would like to thank Dr. Aline Rodrigues Hoffmann for her assistance.
References
- Anon, 2010. Chagas disease in Latin America: an epidemiological update based on estimates. Wkly. Epidemiol. Rec 90, 33–43. [PubMed] [Google Scholar]
- Berger SL, Palmer RH, Hodges CC, Hall DG, 1991. Neurologic manifestations of trypanosomiasis in a dog. J. Am. Vet. Med. Assoc 198, 132–134. [PubMed] [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP, 2011. Trypanosoma cruzi and Chagas’ disease in the United States. Clin. Microbiol. Rev 24, 655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Montgomery SP, 2009. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis 49, e52–e54. [DOI] [PubMed] [Google Scholar]
- Cosentino RO, Agüero F, 2012. A simple strain typing assay for Trypanosoma cruzi: discrimination of major evolutionary lineages from a single amplification product. PLoS Negl. Trop. Dis 6, e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquesnes M, Bosseno M-F, Brenière SF, 2007. Detection of Chagas infections using Trypanosoma evansi crude antigen demonstrates high cross-reactions with Trypanosoma cruzi. Infect. Genet. Evol 7, 457–462. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJA, Reed SM, Granstrom DE, Speer CA, 2001. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet. Parasitol 95, 89–131. [DOI] [PubMed] [Google Scholar]
- Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, Bello ZD, Velazquez E, Muñoz-Calderon A, Juiz NA, Basile J, Garcia L, Riarte A, Nasser JR, Ocampo SB, Yadon ZE, Torrico F, de Noya BA, Ribeiro I, Schijman AG, 2013. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl. Trop. Dis 7, e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers F, Garcia JL, Navarro IT, Zulpo DL, Nino B.d.S.L., Ewald M.P.d.C., Pagliari S, Almeida J.C.d., Freire RL, 2013. Diagnosis and isolation of Toxoplasma gondii in horses from Brazilian slaughterhouses. Rev. Bras. Parasitol 22, 58–63. [DOI] [PubMed] [Google Scholar]
- Fujita O, Sanabria L, Inchaustti A, De Arias AR, Tomizawa Y, Oku Y, 1994. Animal reservoirs for Trypanosoma cruzi infection in an endemic area in Paraguay. J. Vet. Med. Sci 56, 305–308. [DOI] [PubMed] [Google Scholar]
- Grundemann AW, 1947. Studies on the biology of Triatoma sanguisuga (Leconte) in Kansas, (Reduviidae, Hemiptera). J. Kans. Entomol. Soc 20, 77–85. [Google Scholar]
- Gutiérrez C, González-Martín M, Corbera JA, Junco M, 2013. Chemotherapeutic agents against pathogenic animal Trypanosomes In: Mendez-Vilas A (Ed.), Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education Formatex Research Center, Madrid, pp. 1564–1573. [Google Scholar]
- Ikenga JO, Richerson JV, 1984. Trypanosoma cruzi (Chagas) (protozoa: kinetoplastida: trypanosomatidae) in invertebrate and vertebrate hosts from Brewster County in Trans-Pecos Texas. J. Econ. Entomol 77, 126–129. [DOI] [PubMed] [Google Scholar]
- Kjos SA, Snowden KF, Craig TM, Lewis B, Ronald N, Olson JK, 2008. Distribution and characterization of canine Chagas disease in Texas. Vet. Parasitol 152, 249–256. [DOI] [PubMed] [Google Scholar]
- Lai D-H, Hashimi H, Lun Z-R, Ayala FJ, Lukeš J, 2008. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. U. S. A 105, 1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludyga N, Grünwald B, Azimzadeh O, Englert S, Höfler H, Tapio S, Aubele M, 2012. Nucleic acids from long-term preserved FFPE tissues are suitable for downstream analyses. Virchows Arch 460, 131–140. [DOI] [PubMed] [Google Scholar]
- Marsh A, Barr B, Madigan J, Lakritz J, Nordhausen R, Conrad P, 1996. Neosporosis as a cause of equine protozoal myeloencephalitis. J. Am. Vet. Med. Assoc 209, 1907–1913. [PubMed] [Google Scholar]
- Moser DR, Kirchhoff L, Donelson J, 1989. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol 27, 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity MB, Barnhart K, Logan KS, Santos RL, Kessell A, Melmed C, Snowden KF, 2006. An atypical case of Trypanosoma cruzi infection in a young English Mastiff. Vet. Parasitol 140, 356–361. [DOI] [PubMed] [Google Scholar]
- Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, Gascón J, i Prat JG, Portús M, Sauleda S, 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta. Trop 103, 195–200. [DOI] [PubMed] [Google Scholar]
- Py MO, 2011. Neurologic manifestations of Chagas disease. Curr. Neurol. Neurosci. Rep 11, 536–542. [DOI] [PubMed] [Google Scholar]
- Rassi A Jr., Rassi A, Marcondes de Rezende J, 2012. American trypanosomiasis (Chagas Disease). Infect. Dis. Clin. North Am 26, 275–291. [DOI] [PubMed] [Google Scholar]
- Reuss SM, Dunbar MD, Mays MBC, Owen JL, Mallicote MF, Archer LL, Wellehan JF Jr., 2012. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg. Infect. Dis 18, 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A, Fighera RA, Souza TM, Schild AL, Barros CSL, 2009. Neuropathology of naturally occurring Trypanosoma evansi infection of horses. Vet. Pathol 46, 251–258. [DOI] [PubMed] [Google Scholar]
- Taylor K, Authié EM-L, 2004. Pathogenesis of animal trypanosomiasis In: Maudlin I, Holmes PH, Miles MA (Eds.), The Trypanosomiases CABI Publishing, Wallingford, UK, pp. 331–353. [Google Scholar]
- Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, Svoboda M, 2003. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg 68, 574–582. [DOI] [PubMed] [Google Scholar]
- Wincker P, Britto C, Pereira JB, Cardoso MA, Oelemann W, Morel CM, 1994. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg 51, 771–777. [DOI] [PubMed] [Google Scholar]


