Introduction
National recommendations for cancer screening have changed significantly in recent years for older adults.1 Changes have been related to an increased recognition that cancer screening decisions are often complex in adults who are older than 75 years. While many older adults have substantial life expectancy and are in good health, the majority of randomized controlled trials (RCTs) of cancer screening tests have not included adults over the age of 75 years old.2 Consequently, it can be unclear when it is appropriate to extrapolate potential benefits to individual older adults seen in clinic. Moreover, there is accumulating evidence of potential harms of cancer screening.3 For example, older adults who are frail or who have many co-morbid medical conditions may experience greater rates of complications from follow-up procedures to screening tests and be unaware of “diagnostic cascades” following positive tests. Overdiagnosis, or the diagnosis and treatment of a cancer that would not have caused symptoms during an individual’s remaining lifetime, occurs more frequently among older adults with less than a 10-year life expectancy, potentially exposing them to the harms of testing and treatment (including surgeries, chemotherapy, and radiation) without benefits.4 Moreover, in typical time-limited primary care settings, discussions about cancer screening may take time away from discussing interventions for treating known comorbid diseases, such as heart disease, or reducing polypharmacy.
In response to the need to balance the potential benefits and harms, cancer screening should not follow the “check-box” approach based solely on age. Rather, cancer screening is a medical procedure which requires thoughtful individualized decision making in older adults prior to undergoing testing. In practice, however, it can be challenging to communicate cancer screening recommendations while reconciling different, and often inconsistent, cancer screening guidelines. This can lead to both missed opportunities to refer older adults who would benefit from screening, and situations where screening potentially leads to more harm than benefit. We therefore have two objectives in this paper. First, we discuss a framework for individualized decision making for prostate, lung, breast, and colon cancer screening. Second, we provide guidance on how to communicate cancer screening recommendations, including recommendations to stop screening when appropriate.
Approach to individualized decision-making
Individualized decision making involves accounting for the risks and benefits of cancer screening among older adults, as well as perspectives and values that influence their decisions.5 We suggest a structured framework focused on three key areas to develop an individualized recommendation: 1) The person’s overall health and estimated life expectancy, 2) individual preferences and values, and 3) how health, life expectancy and individual preferences impact the potential benefits and harms of screening tests. We discuss each factor in more detail below.
Overall health and life expectancy.
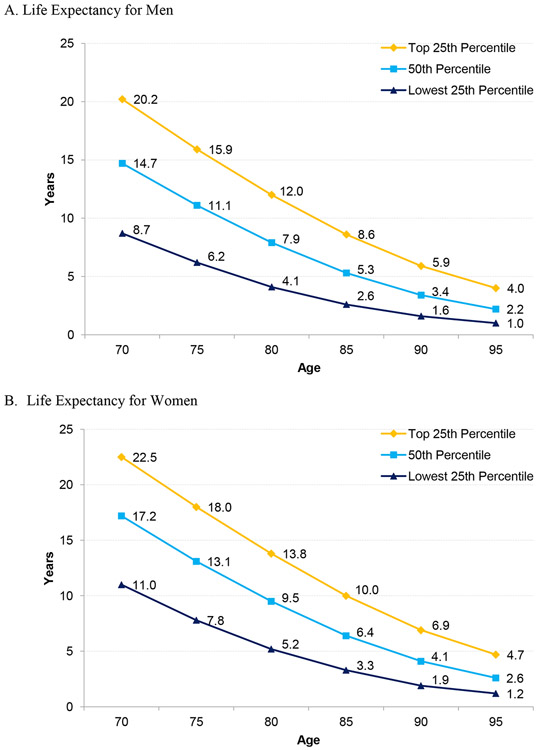
Clinicians should make an assessment of a person’s overall health and whether an individual has a life expectancy of at least 10 years, since the harms of screening outweigh the benefits for those with less than a 10 year life expectancy.5 There are several potential approaches to estimating health and life expectancy. First, clinicians can use their clinical judgment to determine whether an individual is in the highest quartile, middle two quartiles, or lowest quartile of life expectancy for their age group and match this to life table data (Figure 1).5, 6 These data, for example, show that women age 80 in the top or middle two quartiles might benefit from cancer screening, women age 85 need to be in the top quartile of health to potentially benefit, and women age 90 are unlikely to benefit in any quartile. In addition, the majority of men over age 85 are unlikely to benefit from cancer screening. Approaches to determining the quartile of health for each person includes conducting a clinical exam to assess gait speed,7 self-rated health, and/or the severity of multiple chronic medical conditions.5 For example, persons diagnosed with moderate or severe dementia on average have less than a 10-year life expectancy and often cannot tolerate invasive downstream interventions. For another example, a 70-year-old with poorly controlled heart failure experiencing frequent hospitalizations is less likely to benefit from cancer screening, compared to a 77-year-old with well-controlled heart failure.
Fig 1.
Upper, Middle, and Lower Quartiles of Life Expectancy for Men and Women at Selected Ages Based on 2017 United States Life Tables. Data from Arias E, Xu J. United States Life Tables, 2017. National Vital Statistics Reports. 2017;68(7). Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_07-508.pdf
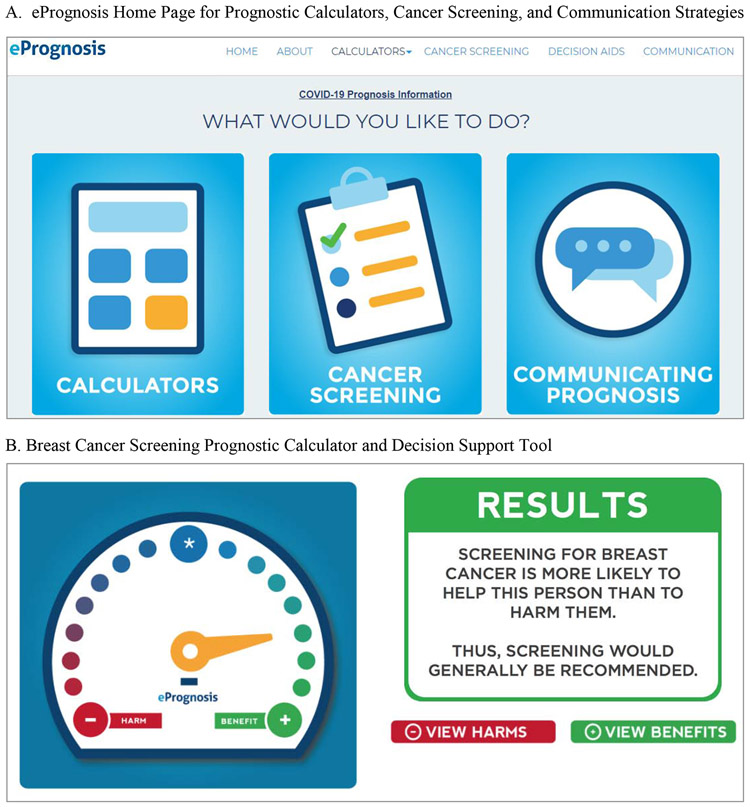
Second, clinicians can enhance their clinical judgment of estimated life expectancy by using online prognostic tools. A collection of prognostic calculators is provided online (see: eprognosis.org) which specifically estimate 10-year life expectancy (Figure 2A).8 These short online calculators include measures of age, medical conditions, functional disabilities, and health behaviors, such as smoking status, to provide percentage estimates of 10-year mortality. Having more than a 50% likelihood of 10-year mortality indicates an individual has less than a 10-year life expectancy and is unlikely to benefit from cancer screening.
Fig 2.
The ePrognosis Online Application (Source: www.eprognosis.org). After arriving to the website main page (A), users can choose the “Cancer Screening” option which will prompt users to select the cancer screening test they would like decision support for. Options include breast cancer screening, colorectal cancer screening, or both. After selecting a cancer screening test, users can complete an online prognostic calculator and results of this prognostic calculator are integrated into a decision support tool with multiple visual representations of data to facilitate shared-decision making. An example of part of the breast cancer screening decision aid is shown in (B).
Individual Preferences and Values.
It is important to understand a person’s preferences for cancer screening and values that guide their decision making. Older adults should be asked, for example, about if they have undergone screening in the past and their experience with it. In general, clinicians can solicit perspectives on whether individuals prefer to have more medical information and testing done compared to a general preference to avoid medical testing. Individuals who feel they have other pressing health priorities might reasonably feel a need to control these conditions before pursuing cancer screening.9 In addition, preferences include the willingness to undergo downstream invasive diagnostic procedures with a realistic understanding of potential risks and benefits. The risks of prostate biopsies, lung biopsies, or colonoscopies, for example, may not be considered tolerable to some individuals. Other individuals may have experienced false positive tests requiring biopsies in the past, and be less willing to undergo such procedures again.10 In addition, it can be helpful to have a general sense of willingness to undergo more invasive or major treatments such as surgery or chemotherapy. Older adults who would not want or tolerate further work-up or treatment after a positive screening test should not be screened.
Risks and Benefits of Individual Cancer Screening Tests.
Individualized decisions should take into account the risks and benefits of individual cancer screening tests in the context of a person’s health, life expectancy and preferences. Below, we present a brief summary of evidence related to common cancer screening tests in older adults in which individualized decisions based on life expectancy are recommended (e.g., breast, prostate, colorectal, and lung cancer screening) as well as in national guidelines. We discuss how to incorporate potential risks, benefits, and guidelines of each cancer screening test into individualized decision making. Risks and benefits are further summarized in the Table.
Breast Cancer Screening
Potential Benefits.
Trial-based evidence of benefit in older women is uncertain because RCTs of mammography for breast cancer screening included few women over 70 years old. Only one of 8 RCTs examining mammography included a small number of women 70-74 years old and found no reduction in breast cancer-specific mortality in this age group. No trials have included women over 75 years old.11 Observational studies have shown potential benefit of screening mammography in older women, including the detection of earlier-stage breast cancer and reduced breast cancer-specific mortality, particularly for women in better health (Charlson comorbidity scores <2 or living for a median of 10 years).12-15 However, these results should be interpreted with caution as they may reflect effects of length-time, lead-time, or selection bias rather than a benefit of cancer screening. Simulation models have been used to generate potential benefits of mammography among older women. These estimate 1-2 fewer breast cancer deaths per 1000 women in their 70s screened biennially for 10 years.16, 17 In addition, mammography may detect early cancers more frequently and more accurately (higher sensitivity and specificity) in older women.18, 19 Taken together, it is reasonable to extrapolate the modest benefits of mammography to older women who have at least a 10 year life expectancy.
Potential Harms.
With one screening mammography test, false positive results occur in approximately 7% of women age 70-79 and 6.5% of women age 80-89, which often causes anxiety or downstream testing.20 Biopsies are recommended in 1.8% of women age 70-79 and 1.6% of women age 80-89.20 Breast biopsies more frequently detect cancer in older adults, but may be distressing or uncomfortable, particularly in women with dementia who may not understand what is being done to them. In model-based simulation studies of overdiagnosis, rates of overdiagnosis increase as routine cancer screening continues into older ages and for women with more comorbid medical conditions.21, 22 One simulation study estimated rates of overdiagnosis ranging 12-29% for women who stop biennial screening at 74 years old, 17-41% for women who stop at 80 years old, and 32-48% for women who stop screening at 90 years old.22 The harms of overdiagnosis are especially relevant to older women given the increased risks of cancer-related treatment toxicity with age.23
Guidelines.
The American Cancer Society (ACS) recommends women ≥55 have biennial screening if they have a life expectancy ≥10 years, and does not have an age cut-off to stop screening.1 The United States Preventive Services Task Force (USPSTF) recommends biennial mammograms for women 55-74 years, and that current evidence is insufficient about screening women ≥75 years old.24
Individualized Decisions.
Among older women with less than a 10-year life expectancy, we recommend discussions about stopping mammography and prioritizing preventive care towards treating known health conditions or health behaviors. When older women have at least a 10-year life expectancy, we suggest discussing risks and benefits of mammography and reaching a shared-decision. The harm of overdiagnosis in older women may be of most concern,25 and so we suggest explanations of risks using concrete numbers and visual presentations of data (Figure 2B).8 The ACS recommends decision aids be used to assist in shared decision making, and a peer-reviewed decision aid with visual representations of data specific to women age 75-84 and ≥85 years old is available and was recently tested in an RCT.26, 27
Colorectal Cancer Screening
Potential Benefits.
Several trials have demonstrated CRC-specific mortality benefits in older adults. Among trials of guaiac-based fecal occult blood tests (FOBTs), hereafter referred to as FOBT and distinct from fecal immunochemical tests (FIT), four RCTs included a combined 50,144 participating adults age 70-80 years old.2 Three European trials found reductions in CRC-specific mortality of 11-16%,28-30 and a large US trial found reductions of 22-32% overall, with a 53% reduction among adults > 70 years old.31, 32 For sigmoidoscopies, the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial included 20,726 adults >70 years old, and found a 35% reduced CRC mortality for adults age 65-74 when screened every 3-5 years.33 For colonoscopies, considered the definitive test for detection of CRC and pre-cancerous lesions, there are no published RCTs. However, one large prospective cohort study found adults >75 years old had a 50% reduction in incident CRC diagnoses in both the proximal and distal colon if >5 years since the last endoscopy and 63% reduction if <5 years from last endoscopy.34 In addition, colonoscopies prevent CRC in addition to early detection of CRC, and the CRC-specific mortality lag-time to benefit of both prevention and early detection is approximately 10 years.35
Potential Harms.
False positives can occur with FOBT or FIT screening tests; Hubbard, et al. estimated up to 23% of individuals receiving annual FOBT screenings over a 10 year period had at least one false positive.36 In cases of sigmoidoscopy, perforation (0.1 per 1000 sigmoidoscopies) is a rare complication and there can be challenges achieving adequate depth in older adults.37 Colonoscopies have higher rates of adverse events in adults 65 or older, and include GI adverse events (26 in 1000), perforation (1 in 1000), post-polypectomy bleeding (3.6 in 1000), severe cardiac or pulmonary events (12.1 in 1000) and death (1 in 1000).38 Challenges with bowel prep in older adults are common and include dizziness, abdominal pain, fecal incontinence, and nausea, and individuals can experience confusion and falls with sedation post-procedure.39 There is limited data on overdiagnosis in CRC screening, especially since CRC screening contributes to prevention of CRC. The possibility of overdiagnosis appears to be lower compared to other cancer screening tests. Autopsy studies show a rate of 2-3% of individuals have undiagnosed CRC unrelated to cause of death, 2 RCT data on FOBTs suggests a rate of approximately 6% in 40- to 60-year-olds,4 and a population-based study in Germany found a rate of approximately 1% in older adults.40
National Guidelines.
Several tests are recommended for CRC screening including high-sensitivity FOBT, FIT, multi-target stool DNA test, sigmoidoscopy, computed tomography (CT) colonography, and colonoscopy. The USPSTF recommends routine screening for adults age 55-75 years old, and to consider screening as an individualized decision for adults age 76-85 years old.41 The ACS recommends routine screening starting at age 45, that screening continue until age 75 for individuals with >10 year life expectancy, that clinicians individualize decisions for adults age 76-85, and discourage screening for individuals older than 85 years.42
Individualized Decisions.
Among individuals with less than a 10-year life expectancy, CRC screening should be discouraged as the procedural risks of colonoscopy, either as a screening test, or as a diagnostic test for positive non-colonoscopy CRC screening tests, likely outweigh the benefits. We discuss communication strategies below since many older adults remain enthusiastic about continuing screening even when the tests are low-value and unlikely to help them live longer.43 CRC screening has the greatest potential for benefit among older adults if they never were screened before, they are healthy enough to undergo treatment of colorectal cancer, and/or they have at least a 10-year life expectancy. In these individuals, prior to a FOBT or FIT, it is important to discuss the risk of a false positive and whether individuals would be willing to undergo a colonoscopy in the event of a positive result. For colonoscopies, individuals should receive information about both the procedural risks as well as the burdens of bowel prep, sedation, and need for arranging transportation in the context of an older adult’s health. Decision aids are effective at improving knowledge and reducing decisional conflict,44 and decision aids are available which are tailored to CRC screening in older adults (Figure 2B).45
Lung Cancer Screening
Potential Benefits.
Several trials have evaluated the benefits of lung cancer screening using chest x-rays or low-dose computed tomography (LDCT) among adults age 55-74 years old. For chest x-rays, the PLCO trial found no lung cancer mortality benefit among 154,942 adults age 55-74 years with no eligibility requirement regarding smoking.46 In contrast, the National Lung Cancer Screening Trial (NLST) in the US examined the efficacy of LDCT in 53,454 participants age 55-74 years with a history of at least 30 pack years of smoking who were current smokers or had quit in the past 15 years. This trial found a 20% relative reduction in lung cancer mortality compared to chest x-rays alone after 6.5 years of follow-up. Extended follow-up found an overall NNS of 303 to prevent one death from lung cancer after a lag-time of 11 years.47 In addition, the Dutch-Belgian Lung Cancer Screening (NELSON) Trial of LDCT was conducted on 15,792 current or former smokers (quit <10 years ago) age 50-74 years old who had smoked at least 15 cigarettes/day for 25 years or 10 cigarettes/day for 30 years. This trial found a 24% reduction in lung-cancer specific mortality at 10 year follow-up.48
Potential Harms.
False positive results are common with LDCT screening; in the NLST, 39% of people in the LDCT group had at least 1 positive test result and 96% of positive results were false positives.49 After positive tests most individuals had follow-up imaging, 4.2% had surgical procedures, 2.2% had biopsies, and there was an 8.5 to 9.8% complication rate after invasive diagnostic procedures.50, 51 Complication rates after invasive procedures may be higher among the general population of older adults compared with the specialty centers in the NLST. One retrospective study of 344,510 individuals aged 55-77 years old undergoing diagnostic pulmonary procedures showed complication rates of 22% (more than twice that of NLST).52 Moreover, false positive results and complications from diagnostic interventions are higher among older adults and among those in worse health compared to those who are younger or healthier.53-55 The reported rates of overdiagnosis ranged from 3% in the NLST trial with extended follow-up to 8.9% in the NELSON trial, although this is an area of active study.47, 48, 56 Additional harms include radiation exposure, financial strain, and anxiety from false-positive results.51
National Guidelines.
The USPSTF and ACS recommend annual LDCT for lung cancer screening in adults 55-74 years old (or up to 80 in USPSTF guidelines) who have a 30 pack year history and currently smoke or quit within the last 15 years.57, 58 Guidelines suggest avoiding screening in older adults with a short life expectancy (<10 years) or comorbidities that would make curative surgery or cancer-directed therapies not a reasonable option.
Individualized decisions.
LDCT may be of most benefit when an older adult is at high risk of lung cancer (calculators available),59-61 has a smoking history comparable to the NLST or NELSON trials, and has a low risk of a competing cause of death.62 Older adults should be counseled about the possibility of frequent follow-up nodule tracking, false positive results, including lesions detected by LDCT in the thyroid and other organs, and downstream diagnostic or therapeutic medical interventions. Medicare currently requires shared decision making between individuals and their clinicians, although in practice such conversations seem to rarely occur.63,64 The lack of shared-decision making may be expected as lung cancer screening is relatively new and clinicians may be less comfortable discussing risks and benefits. This highlights the need for more informational content or decision aids which balance the risks and benefits of lung cancer screening. A decision support pamphlet developed by the VA is available to help educate adults about the risks and benefits of LDCT screening.65
Prostate Cancer Screening
Potential Benefits.
RCTs of prostate specific antigen (PSA) screening have provided limited evidence of benefit in men ≥70 years old. The US PLCO trial examined annual PSA screening over 6 years in 76,685 men aged 55-74 years old (approximately 10,000 men over age 70),66 and found no prostate cancer mortality reduction even at 15 years of follow-up, although there were high rates of contamination in the control arm.67 The European Randomized Study of Screening for Prostate Cancer (ERSPC) trial randomized men 50-74 years to PSA screening every 2-4 years and the control group received no PSA screening.68 Results indicated an overall 20% reduction in prostate cancer-specific mortality after a lag-time of 13 years,69 however, benefits of screening were only found among men 55-69 years at randomization. In addition, a recent UK trial of a single PSA screening test was conducted in 419,582 men 55-69 years old and found no prostate cancer-specific mortality benefit after 10 years of follow-up.70
Potential Harms.
False positive results are common after PSA tests (30-40% of tests) and can lead to both anxiety and unneeded prostate biopsies.71 Prostate biopsies are associated with anxiety, moderate to severe pain (7%) during and immediately after the procedure, moderate to severe hematuria (6%), infections requiring hospitalization (0.4-1.3%), and hospitalizations (7%).72-74 In addition, overdiagnosis represents a significant harm since prostate cancers detected through PSA screening are typically slow growing and may remain asymptomatic during an individual’s lifetime; in the ERSPC and PLCO trials, it is estimated that 40-60% of screen-detected cancers were cases of overdiagnosis.75, 76 Using the ERSPC data there were approximately 24 cases of overdiagnosis for every 1 prostate-cancer related death prevented after 14 years of follow-up. Overdiagnosis of prostate cancer is associated with anxiety during watchful waiting for low-risk cancers and adverse effects from cancer-directed treatments (including prostatectomy, androgen deprivation therapy, and radiation), which include bowel dysfunction, urinary incontinence, erectile dysfunction, premature death, and others.77
National Guidelines.
The most recent USPSTF guidelines encourage men 55-69 years old to make an individualized decision about PSA screening after discussion with a clinician and men >70 years old not to be screened.78 The ACS recommends men over age 50 with at least a 10 year life expectancy have an opportunity to make an informed decision about whether to be screened after receiving information about the uncertainties, risks, and potential benefits of PSA screening.1
Individualized Decisions.
PSA screening in men >70 years old should be rare and only considered in men with at least a 10-15 year life expectancy after a shared decision. Short-term harms from prostate biopsies should be discussed, as should the substantial harm of overdiagnosis in older men using easy to understand language and visual data. Decision aids are available, and a recent systematic review of 19 decision aids found reductions in decisional conflict to screen. However, there was little evidence that decision aids facilitate shared decision making or impact screening choice.79
Communicating Cancer Screening Recommendations
Individualized decision making has been a recommended strategy for improving cancer screening decisions for nearly two decades.5 Yet, overscreening remains common among older adults with limited life expectancy.39, 80 Conversely, screening may not be offered to older adults with inadequate prior screening (as in colorectal cancer or cervical cancer screening) or with >10 year life expectancies despite potential benefit. One possible contributor to overscreening is that individuals tend to overestimate the benefits of screening and underestimate the potential harms.81, 82 This may contribute to misplaced enthusiasm for screening or requests of health providers for screening tests when they may no longer be in the patient’s best interest.80 In response, health providers might feel uncomfortable managing these expectations or having difficult conversations about life expectancy, and even order investigations they know to be unnecessary.83 Clinicians similarly may overestimate benefits of tests,84 or may have difficulty translating statistical or highly numerical concepts in ways that are understandable to individuals. Finally, there are limitations in time for clinicians to incorporate estimates of life expectancy into recommendations or to have careful shared decisions with older adults. Consequently, it is essential to have insight into strategies which can enhance effective communication of cancer screening recommendations.
A recent systematic review discussed several strategies to improve discussions of risks and benefits of medical tests.85 First, using visual displays of data can improve accurate recall of conversations and comprehension among adults. Visual displays of cancer screening risk are available online (see: eprognosis.org, Figure 2) and are in several decision aids specific to older adults.8, 86 Second, it can be helpful to provide context for cancer screening outcomes in relation to competing medical priorities or risks. For example, in an individual with poorly controlled heart disease, a discussion might contextualize that interventions to control heart disease will help the individual live longer and better than cancer screening tests. Third, we suggest avoiding positive framing, or framing testing results as gains rather than losses, as it is associated with increased acceptance of harmful interventions. Positive framing can be reduced by asking questions about individual values and preferences before discussing the risks and benefits of medical tests so as to frame testing outcomes in the context of what is important to that person.
When clinicians recommend screening, this recommendation should include a clear plan for re-evaluating the need to continue screening at specified time points and an explanation of what might make cancer screening less of a priority in the future. Providing anticipatory guidance might lead to more comfort with eventual stopping of cancer screening when harms outweigh the benefits.
If clinicians feel that stopping cancer screening is most appropriate for an individual, there are several phrases that can be used to improve acceptability of the recommendation and comprehension of the reasoning. For example, a survey of older adults found that phrases such as “your other health issues should take priority,” “this [screening test] is not recommended for you by medical guidelines,” or “you are at high risk for harms from [this screening test],” are preferred to phrases such as “you may not live long enough to benefit from [screening].”87 Similarly, discussing that the “risks outweigh the benefits” when considering their overall health status may be more acceptable than using the term “life expectancy.” Of note, while some clinicians or older adults might find it acceptable to not offer cancer screening tests when the harms clearly outweigh potential benefits,88 we suggest open and shared decisions.
Conclusion
Cancer screening recommendations for older adults should be individualized to account for overall health, life expectancy, values and preferences, and how these impact the risk-benefit ratio of individual cancer screening tests. Moreover, there are strategies that clinicians should consider to best communicate these recommendations, address misperceptions, and align treatment goals between clinicians and older adults. By combining the process of individualized decision making with thoughtful communication, we may be able to shift current screening trends towards ensuring older adults who may benefit have the opportunity to be screened, and those where harms outweigh the benefits avoid harmful screening.
Table 1.
Benefits and harms of cancer screening among older adults. Data from Refs 2, 4, 12-23, 28-40, 47-49, 50-52, 71-74, 76
| Screening | Harms | Benefits |
|---|---|---|
| Breast cancer Screening (Mammography) |
Overdiagnosis: Model-based simulations of overdiagnosis suggest rates increase with age, with rates ranging 12-29% for women 74 years old, 17-41% for women 80 years old, and 32-48% for women 90 years old [21, 22]. The harms of overdiagnosis increase with age due to cancer related treatment toxicity [23]. False Positive recall following mammography: Cumulative probability of 7% in adults age 70-79 and 6.5% in women age 80-89 [20]. False positives are less common in older women. Biopsies: Biopsy rate of 1.8% in women age 70-79 and 1.6% in women age 80-89 [20]. Other: Anxiety, distress from false positives, financial impact of screening |
Breast-cancer specific mortality reduction: No RCTs showing mortality reduction in women >70. Observational studies suggest benefit for women in good health, although results may reflect lead-time, length-time, or selection bias [12-15]. Mammography is more accurate in detecting cancer in older adults [18, 19]. Simulation models indicate 1-2 fewer breast cancer deaths per 1000 women in their 70s screened biennially for 10 years [16, 17]. Women should have >10 year life expectancy to extrapolate the benefits of screening seen at younger ages which may outweigh harms of screening |
| Colorectal cancer screening |
Overdiagnosis: Low risk, ranging 0.1-6% of screen-detected cases [2, 4, 40]. False Positives requiring colonoscopies: Up to 23% over 10 yrs of annual FOBT testing [36]. Sigmoidoscopy procedural complications: inadequate depth, perforation in 0.1 per 1000 sigmoidoscopies [37]. Colonoscopy procedural complications: GI adverse events (26 in 1000), perforation (1 in 1000), post-polypectomy bleeding (3.6 in 1000), severe cardiac or pulmonary events (12.1 in 1000) and death (1 in 1000) [38]. Colonoscopy prep: dizziness, abdominal pain, fecal incontinence, and nausea, and individuals can experience confusion and falls with sedation post-procedure [39]. |
Colorectal cancer specific mortality reduction: 11-53% CRC-specific mortality reduction with annual FOBTs [28-32]. 35% mortality reduction from sigmoidoscopies every 3-5 years [33]. No RCTs for colonoscopy, but observational studies suggest 50% reduction in incident CRCs and 50-63% reduced CRC mortality in adults >75 years old [34]. Older adults with >10 year life expectancy are more likely to experience benefits > harms. Colorectal cancer prevention: removal of colonic adenomas can reduce CRC incidence. Lag-time to benefit of removal of adenomas of 10 years [35]. |
| Prostate cancer screening (Prostate specific antigen (PSA)-tests) |
Overdiagnosis: Approximately 40-60% of screen-detected cancers based on RCT data. 24 cases of overdiagnosis for 1 case of avoided prostate-cancer death for age 50-69. Overdiagnosis rate increases with older age, as does cancer-treatment adverse effects including bowel dysfunction, urinary incontinence, erectile dysfunction, and premature death [76]. False positives requiring biopsy: 30-40% of PSA tests [71]. Biopsy related complications: anxiety, moderate to severe pain (7%) during and immediately after the procedure, moderate to severe hematuria (6%), infection requiring hospitalization (0.4-1.3%), and hospitalizations (7%) [72-74]. Other: Anxiety, distress from false positives |
Prostate cancer specific mortality reduction: RCTs of PSA screening have provided limited evidence of benefit in men >70 years old, and have not included men >75 years old. Men should have a life expectancy of at least 10-15 years to potentially experience benefits > harms from screening. |
| Lung cancer screening (Low-dose tomography (LDCT)) |
Overdiagnosis: Can occur in 3-9% of screen-detected cancers [47-49]. Overdiagnosis rates increase with older age and limited life expectancy. False positives: 39% of people in the LDCT group had at least 1 positive test result and 96% of positive results were false positives [49]. Biopsy-related complications: 8-20% rate of complications after invasive diagnostic procedures [50-52]. Other: Burden of nodule tracking, radiation, anxiety. |
Lung cancer specific mortality reduction: Two RCTs of LDCTs indicate a 20%-24% lung-cancer specific mortality reduction among adults age 55-80 with >30 pack year smoking history and either currently smoke or quit within the past 15 years [47-49]. Older adults should have a 10 year life expectancy to potentially experience benefits > harms of screening. |
Definitions: Overdiagnosis - detection of a cancer that would never progress to cause symptoms in a person's lifetime, which can lead to overtreatment (surgery, radiation, chemotherapy) that provides no benefits and only adverse effects.
Key Points:
The benefits of cancer screening are uncertain in older adults due to lack of inclusion of adults over 75 years old in the majority of randomized controlled trials.
There are several known harms of cancer screening in older adults including risks of overdiagnosis, false positive results, and procedural complications from downstream diagnostic interventions that increase with decreasing life expectancy.
Cancer screening recommendations should be individualized for older adults by accounting for overall health and life expectancy, values and preferences, and how these affect specific risks and benefits of cancer screening tests.
Communicating screening recommendations should incorporate visual data when possible, provide context in terms of competing medical priorities, and use phrases considered more acceptable and easy to understand by older adults.
Synopsis:
Cancer screening decisions in older adults can be complex due to the unclear cancer-specific mortality benefits of screening and several known harms including false positives, overdiagnosis, and procedural complications from downstream diagnostic interventions. In this review, we provide a framework for individualized cancer screening decisions among older adults, involving accounting for overall health and life expectancy, individual values, and the risks and benefits of specific cancer screening tests. We then discuss strategies for effective communication of recommendations during clinical visits which are considered more effective, easy to understand, and acceptable by older adults and clinicians.
Clinics Care Points.
Cancer screening recommendations should be individualized for older adults by accounting for overall health and life expectancy, values and preferences, and how these affect specific risks and benefits of cancer screening tests.
The benefits of cancer screening are uncertain in older adults due to lack of inclusion of adults over 75 years old in the majority of randomized controlled trials.
Harms of cancer screening in older adults include the risk of overdiagnosis, false positive results, and procedural complications from downstream diagnostic interventions.
Older adults with less than a 10 year life expectancy are unlikely to benefit from cancer screening and may be more likely experience harms of testing.
Communicating screening recommendations should incorporate visual data when possible, provide context in terms of competing medical priorities, and use phrases considered more acceptable and easy to understand by older adults.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: a cancer journal for clinicians. 2018;68(4):297–316. [DOI] [PubMed] [Google Scholar]
- 2.Walter LC, Lewis CL, Barton MB. Screening for colorectal, breast, and cervical cancer in the elderly: a review of the evidence. The American journal of medicine. 2005;118(10):1078–1086. [DOI] [PubMed] [Google Scholar]
- 3.Kotwal AA, Schonberg MA. Cancer Screening in the Elderly: A Review of Breast, Colorectal, Lung, and Prostate Cancer Screening. The Cancer Journal. 2017;23(4):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. Bmj. 2015;350:g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285(21):2750–2756. [DOI] [PubMed] [Google Scholar]
- 6.Arias E, Xu J. United States Life Tables, 2017. National Vital Statistics Reports. 2017;68(7). [PubMed] [Google Scholar]
- 7.Kotwal AA, Mohile SG, Dale W. Remaining life expectancy measurement and PSA screening of older men. Journal of geriatric oncology. 2012;3(3):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ePrognosis: Lee Schonberg Index. http://eprognosis.ucsf.edu/leeschonberg.php. Accessed April 5th 2017.
- 9.Schoenborn NL, Xue Q-L, Pollack CE, et al. Demographic, health, and attitudinal factors predictive of cancer screening decisions in older adults. Preventive medicine reports. 2019;13:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabbous FM, Dolecek TA, Berbaum ML, et al. Impact of a false-positive screening mammogram on subsequent screening behavior and stage at breast cancer diagnosis. Cancer Epidemiology and Prevention Biomarkers. 2017;26(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taáar L, Vitak B, Chen H-H, et al. The Swedish Two-County Trial twenty years later: updated mortality results and new insights from long-term follow-up. Radiologic Clinics of North America. 2000;38(4):625–651. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. Journal of the American Geriatrics Society. 2000;48(10):1226–1233. [DOI] [PubMed] [Google Scholar]
- 13.McPherson CP, Swenson KK, Lee MW. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. Journal of the American Geriatrics Society. 2002;50(6):1061–1068. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson H, Törnberg S, Nyström L, Lenner P. Service screening with mammography of women aged 70–74 years in Sweden: Effects on breast cancer mortality. Cancer Detection and Prevention. 2003;27(5):360–369. [DOI] [PubMed] [Google Scholar]
- 15.Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer. JNCI: Journal of the National Cancer Institute. 2014;106(11). [DOI] [PubMed] [Google Scholar]
- 16.Barratt A, Howard K, Irwig L, Salkeld G, Houssami N. Model of outcomes of screening mammography: information to support informed choices. Bmj. April 23 2005;330(7497):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. April 2 2014;311(13):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Annals of internal medicine. 2003;138(3):168–175. [DOI] [PubMed] [Google Scholar]
- 19.Lee CS, Sengupta D, Bhargavan-Chatfield M, Sickles EA, Burnside ES, Zuley ML. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA oncology. 2017;3(8):1134–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson HD, O'Meara ES, Kerlikowske K, Balch S, Miglioretti D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data. Annals of internal medicine. 2016;164(4):226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Annals of internal medicine. 2016;164(4):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Ravesteyn NT, Stout NK, Schechter CB, et al. Benefits and harms of mammography screening after age 74 years: model estimates of overdiagnosis. Journal of the National Cancer Institute. 2015;107(7):djv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurria A, Brogan K, Panageas KS, et al. Patterns of toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Breast cancer research and treatment. 2005;92(2):151–156. [DOI] [PubMed] [Google Scholar]
- 24.Siu AL. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 25.Hersch J, Jansen J, Barratt A, et al. Women’s views on overdiagnosis in breast cancer screening: a qualitative study. Bmj. 2013;346:f158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. March 1 2014;174(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonberg MA, Kistler CE, Pinheiro A, et al. Effect of a Mammography Screening Decision Aid for Women 75 Years and Older: A Cluster Randomized Clinical Trial. JAMA internal medicine. 2020. [DOI] [PMC free article] [PubMed]
- 28.Scholefield J, Moss S, Mangham C, Whynes D, Hardcastle J. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61:1036–1040. [DOI] [PubMed] [Google Scholar]
- 29.Faivre J, Dancourt V, Denis B, et al. Comparison between a guaiac and three immunochemical faecal occult blood tests in screening for colorectal cancer. European Journal of Cancer. 2012;48(16):2969–2976. [DOI] [PubMed] [Google Scholar]
- 30.Kronborg O, Jørgensen O, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scandinavian journal of gastroenterology. 2004;39(9):846–851. [DOI] [PubMed] [Google Scholar]
- 31.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine. 2000;343(22):1603–1607. [DOI] [PubMed] [Google Scholar]
- 32.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. New England Journal of Medicine. 2013;369(12):1106–1114. [DOI] [PubMed] [Google Scholar]
- 33.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366(25):2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New England Journal of Medicine. 2013;369(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard RA, Johnson E, Hsia R, Rutter CM. The cumulative risk of false-positive fecal occult blood test after 10 years of colorectal cancer screening. Cancer Epidemiology and Prevention Biomarkers. 2013;22(9):1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. Jama. 2016;315(23):2576–2594. [DOI] [PubMed] [Google Scholar]
- 38.Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2011;74(4):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonberg MA, Breslau ES, Hamel MB, Bellizzi KM, McCarthy EP. Colon cancer screening in US adults aged 65 and older according to life expectancy and age. Journal of the American Geriatrics Society. 2015;63(4):750–756. [DOI] [PubMed] [Google Scholar]
- 40.Brenner H, Altenhofen L, Stock C, Hoffmeister M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clinical gastroenterology and hepatology. 2015;13(4):717–723. [DOI] [PubMed] [Google Scholar]
- 41.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 42.Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians. 2018;68(4):250–281. [DOI] [PubMed] [Google Scholar]
- 43.Piper MS, Maratt JK, Zikmund-Fisher BJ, et al. Patient Attitudes Toward Individualized Recommendations to Stop Low-Value Colorectal Cancer Screening. JAMA network open. 2018;1(8):e185461–e185461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. American Journal of Preventive Medicine. 2016;51(5):779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis CL, Golin CE, DeLeon C, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: development and results of an uncontrolled trial. BMC medical informatics and decision making. 2010;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. Jama. 2011;306(17):1865–1873. [DOI] [PubMed] [Google Scholar]
- 47.Team NLSTR. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. Journal of Thoracic Oncology. 2019;14(10):1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. New England Journal of Medicine. 2020;382(6):503–513. [DOI] [PubMed] [Google Scholar]
- 49.Aberle D, Adams A, Berg C, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. Jama. 2012;307(22):2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris RP, Sheridan SL, Lewis CL, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA internal medicine. 2014;174(2):281–286. [DOI] [PubMed] [Google Scholar]
- 52.Huo J, Xu Y, Sheu T, Volk RJ, Shih Y-CT. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA internal medicine. 2019;179(3):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Annals of internal medicine. 2011;155(3):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozower BD, Sheng S, O'brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. The Annals of thoracic surgery. 2010;90(3):875–883. [DOI] [PubMed] [Google Scholar]
- 55.Pinsky PF, Gierada DS, Hocking W, Patz EF, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Annals of internal medicine. 2014;161(9):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the Danish lung cancer screening trial. JAMA internal medicine. 2018;178(10):1420–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 58.Wender R, Fontham ET, Barrera E Jr., et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. Mar-Apr 2013;63(2):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MSKCC. Lung Cancer Screening Decision Tool. 2014; http://nomograms.mskcc.org/Lung/Screening.aspx. Accessed January 27, 2017.
- 60.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. New England Journal of Medicine. 2013;369(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. Jama. 2016;315(21):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caverly TJ, Fagerlin A, Wiener RS, et al. Comparison of observed harms and expected mortality benefit for persons in the Veterans Health Affairs Lung Cancer Screening Demonstration Project. JAMA internal medicine. 2018;178(3):426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodwin JS, Nishi S, Zhou J, Kuo Y-F. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA internal medicine. 2019;179(5):716–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision making for lung cancer screening. JAMA internal medicine. 2018;178(10):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.VA. Screening for Lung Cancer. 2014; http://www.prevention.va.gov/docs/LungCancerScreeningHandout.pdf. Accessed January 26, 2017.
- 66.Andriole GL, Crawford ED, Grubb III RL, et al. Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine. 2009;360(13):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;123(4):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl j Med. 2009;2009(360):1320–1328. [DOI] [PubMed] [Google Scholar]
- 69.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. The Lancet. 2014;384(9959):2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. Jama. 2018;319(9):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brawer MK. Prostate-specific antigen: Current status. CA: a cancer journal for clinicians. 1999;49(5):264–281. [DOI] [PubMed] [Google Scholar]
- 72.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. The Journal of urology. 2011; 186(5):1830–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. European urology. 2013;64(6):876–892. [DOI] [PubMed] [Google Scholar]
- 74.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. Bmj. 2012;344:d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welch HG, Black WC. Overdiagnosis in cancer. Journal of the National Cancer Institute. 2010;102(9):605–613. [DOI] [PubMed] [Google Scholar]
- 76.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101(6):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roussel B, Ouellet GM, Mohile SG, Dale W. Prostate cancer in elderly men: Screening, active surveillance, and definitive therapy. Clinics in geriatric medicine. 2015;31(4):615–629. [DOI] [PubMed] [Google Scholar]
- 78.Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Jama. 2018;319(18):1901–1913. [DOI] [PubMed] [Google Scholar]
- 79.Riikonen JM, Guyatt GH, Kilpeläinen TP, et al. Decision aids for prostate cancer screening choice: A systematic review and meta-analysis. JAMA internal medicine. 2019. [DOI] [PMC free article] [PubMed]
- 80.Kotwal AA, Walter LC, Lee SJ, Dale W. Are We Choosing Wisely? Older Adults’ Cancer Screening Intentions and Recalled Discussions with Physicians About Stopping. Journal of general internal medicine. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. Jama. 2004;291(1):71–78. [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA internal medicine. 2015;175(2):274–286. [DOI] [PubMed] [Google Scholar]
- 83.Campbell EG, Regan S, Gruen RL, et al. Professionalism in medicine: results of a national survey of physicians. Annals of internal medicine. 2007;147(11):795–802. [DOI] [PubMed] [Google Scholar]
- 84.Krouss M, Croft L, Morgan DJ. Physician understanding and ability to communicate harms and benefits of common medical treatments. JAMA internal medicine. 2016;176(10):1565–1567. [DOI] [PubMed] [Google Scholar]
- 85.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Annals of internal medicine. 2014;161(4):270–280. [DOI] [PubMed] [Google Scholar]
- 86.Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA internal medicine. 2014;174(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schoenborn NL, Janssen EM, Boyd CM, Bridges JF, Wolff AC, Pollack CE. Preferred clinician communication about stopping cancer screening among older us adults: Results from a national survey. JAMA oncology. 2018;4(8):1126–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schoenborn NL, Boyd CM, Lee SJ, Cayea D, Pollack CE. Communicating about stopping cancer screening: Comparing clinicians’ and older adults’ perspectives. The Gerontologist. 2019;59(Supplement_1):S67–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]