Abstract

Near-infrared (NIRS) spectroscopy, coupled with partial least squares regression, was used to predict adenosine and cordycepin concentrations in fruiting bodies of Cordyceps militaris. The fruiting body samples were prepared in four different sample formats, which were intact fruiting bodies, chopped fruiting bodies, dried powder, and dried crude extract. The actual amount of the adenosine and cordycepin concentrations in fresh fruiting bodies was analyzed by high-performance liquid chromatography. Results showed that the prediction models developed from the chopped samples provided excellent accuracy in both parameters with minimal sample preparation. These optimum models provided a coefficient of determination of prediction, standard error of prediction, bias, and residual predictive deviation, which were respectively 0.95, 16.60 mg kg–1, –8.57 mg kg–1, and 5.04 for adenosine prediction, and 0.98, 181.56 mg kg–1, –1.05 mg kg–1, and 8.9 for cordycepin prediction. The accuracy and performance of the model were determined by ISO12099:2017(E). It was found that these two equations can be considered to be acceptable at a probability level of 95% confidence. The NIRS technique, therefore, has the potential to be an objective method for determining the adenosine and cordycepin concentrations in C. militaris fruiting bodies.
1. Introduction
Cordyceps militaris is an entomopathogenic fungus in the class Ascomycetes.1 It has traditionally been used as a tonic and as a traditional folk medicine, especially in East Asia.2 Various bioactive compounds are found in Cordyceps spp.3 The main active constituents are adenosine and cordycepin (3′-deoxyadenosine).4 Adenosine is a nucleoside that is composed of adenine and d-ribose (Figure 1a). It is considered as being the main active component of Cordyceps mushrooms when used in traditional Chinese medicines (TCMs).5 It has a number of actions that merit its use as a possible cardioprotective and therapeutic agent for chronic heart failure.6 Cordycepin is a derivative of the nucleoside adenosine, differing from the latter by the absence of the hydroxy group in the 3′ position of its ribose moiety (Figure 1b). Cordycepin was first extracted from C. militaris by Cunningham and colleagues.7 It is a nucleoside analogue, which exhibits a broad spectrum of biological activities including neuroprotection,8 lung and kidney protection,9 antitumor/anticancer/antileukemic activity,10−12 high antioxidant concentration,13 antibacterial and antifungal14 and anti-inflammatory properties,15 immunomodulatory effects,15,16 and prosexual activity.17C. militaris has become a valuable substitute for Cordyceps sinensis because it can be cultivated in various media and can be maintained under laboratory conditions more readily than C. sinensis.18 However, the concentrations of both adenosine and cordycepin vary depending on the type of culture media used.19
Figure 1.
Chemical structure of adenosine and cordycepin.
Because of its medicinal uses, the demand for fruiting bodies of C. militaris is increasing. The production of C. militaris mushrooms is required to produce high-quality fruiting bodies. Traditional approaches for the determination of major bioactive components in Cordyceps, such as adenosine and cordycepin, involve sample preparation using an extraction technique followed by an analytical technique to determine the concentration, such as high-performance liquid chromatography (HPLC).18,20 Another detection method is also sometimes used: ultrahigh-performance liquid chromatography.21 Although these chromatographic-based methods display high accuracy and sensitivity, they are time-consuming, labor-intensive, complicated, and expensive; moreover, they produce considerable quantities of solvent waste.22 Therefore, these traditional techniques are not suitable for the rapid onsite detection of adenosine and cordycepin concentrations in complex matrices. A faster, more efficient, and environmentally friendly method of determining these concentrations in C. militaris mushrooms is needed.
The near-infrared spectroscopic (NIRS) technique is widely applied in industries, such as agriculture,23−25 food industry,26−28 cosmetics, and pharmaceuticals.29 This method is fast, nondestructive, inexpensive, simple, and accurate and requires little or no sample preparation. NIRS records the multifrequency and cofrequency information of different organic molecules, which involves the responses of molecular bonds such as C–H, N–H, C–O, and O–H.30 Partial least squares (PLS) is one of the most effective chemometric methods,31 which is used for the development of a calibration equation. Although there are many reports of the use of NIRS on a range of samples, the use of this technique to determine the composition of fungi appears to be limited. In a previously published report, the Fourier transform near-infrared (FT-NIR) spectroscopy technique was successful in determining the content of arginine in dried fermented C. sinensis mycelia. The results showed accurate arginine prediction models with the highest values of the coefficient of determination of prediction (Rp2 = 0.8370) and residual predictive deviation (RPD = 2.4741) and the lowest value of root mean square error of prediction (RMSEP = 0.0841 g/100 g).32 NIR reflectance spectroscopy was reported for achieving excellent prediction of adenosine and polysaccharide contents in Cordyceps militaris mycelium powder, with a correlation coefficient (R) of 0.8820 and 0.8859 and RMSEP of 0.6720 mg/g and 0.0083 g/g, respectively,33 including the protein content with an R of 0.9868 and RMSEP of 0.0149 g/g.34 Although previous studies have showed promising results for the analysis of the C. militaris constituent, they needed to prepare the samples into dried powder and homogenize samples. However, no reports appear to have been published on the determination of adenosine and cordycepin concentrations of intact or minimally processed C. militaris fruiting bodies using NIRS. The objective of this work was, therefore, to investigate the feasibility of using the NIRS technique to determine the adenosine and cordycepin concentrations in either intact or minimally processed C. militaris fruiting bodies.
2. Results and Discussion
2.1. Adenosine and Cordycepin Concentrations in Fruiting Bodies
The statistical parameters of adenosine and cordycepin concentrations in C. militaris fruiting bodies, measured by HPLC, in the calibration set and the validation set are listed in Table 1. The standard error of laboratory (SEL) of the adenosine and cordycepin determination was 36.19 and 289.66 mg kg–1, respectively. The concentration ranges of adenosine and cordycepin were 254.6–769.8 and 700–9677.2 mg kg–1 for the calibration, respectively. For validation, they were ranged at 255.8–670.8 and 734.9–9336 mg kg–1, respectively. The average and standard deviation of the samples in the calibration set and the validation set were similar, and so the samples in the validation set were suitable for evaluating the accuracy of the calibration equation. The same sample sets were scanned using a spectrometer.
Table 1. Characteristics of Adenosine and Cordycepin Concentrations in the Calibration Set and Validation Seta.
| bioactive compound | SEL | groups | max | min | mean | SD |
|---|---|---|---|---|---|---|
| adenosine (mg kg–1) | 36.19 | calibration | 769.8 | 254.6 | 516.8 | 115.6 |
| validation | 670.8 | 255.8 | 497.0 | 132.3 | ||
| cordycepin (mg kg–1) | 289.66 | calibration | 9677.2 | 700.0 | 2750.7 | 2759.9 |
| validation | 9336.0 | 734.9 | 2564.6 | 2530.5 |
SEL: standard error of laboratory, max: maximum value of data, min: minimum value of data, and SD: standard deviation of data.
2.2. Near-Infrared Measurement
Figure 2 shows raw absorbance spectral scans for each of the four sample forms (fresh intact fruiting bodies, fresh chopped fruiting bodies, fruiting bodies prepared as a dried powder, and dried crude extract) that are associated with the main constituents within the fruiting bodies. The spectra of the fresh fruiting bodies (Figure 2a) were similar to those of the fresh chopped fruiting bodies (Figure 2b). There are four dominant absorption regions in the raw spectra, that is, at approx. 10,253, 8384, 6904, and 5188 cm–1 for the fresh fruiting bodies and 10,246, 8392, 6919, and 5137 cm–1 for the fresh chopped fruiting bodies. There are more than four dominant absorption regions in the raw spectra when the fruiting bodies were prepared as a dried powder (Figure 2c) or in the dried crude extract (Figure 2d). The shift in the position of the bands at approx. 10,253 and 10,246 cm–1 in both of these samples may have been the result of the removal of water during drying. All selected analytical wavenumbers from raw absorbance spectra of C. militaris fruiting bodies are shown in Table 2.
Figure 2.
Raw absorbance spectral scans of the four sample forms: (a) fresh intact fruiting bodies, (b) fresh chopped fruiting bodies, (c) fruiting bodies prepared as a dried powder, and (d) dried crude extract.
Table 2. Near-IR Wavenumbers and Approximate Description of Vibrational Modes for C. militaris Fruiting Bodiesa.
| wavenumber (cm–1) | bond vibration | compound | refs. |
|---|---|---|---|
| 10,253, 10,246 | OH anti-sym. stret. +OH sym. stret. second overtone | water | (35) |
| 8392, 8384 | –CH second overtone | carbohydrate | (35) and (36) |
| 7341, 7235, 7232 | 2 × CH stret. +CH bend. | carbohydrate | (35) and (36) |
| 6919, 6904 | combination of 2 × CH stret. +CH bend. | carbohydrate | (35) |
| OH first overtone | water | (35) | |
| 6728, 6334 | –NH2 first overtone | protein | (35) and (36) |
| 5772 | –CH first overtone | carbohydrate | (35) |
| 5188, 5164, 5160, 5137 | combination of OH stret. +OH bend. | water, carbohydrate | (35) and (37) |
| 4752, 4746 | combination of NH stret.+amid | protein | (35) |
| 4377, 4312, 4305 | combination of CH stret. +CH bend. | protein | (35) and (36) |
Stret. = stretching and bend. = bending.
The preprocessing spectra, which were directly calculated from the original NIR spectra, offer further information about the contributions from specific chemical components within the NIR spectra. Two preprocessing methods were used in this study including constant offset elimination (COE) and vector normalization (SNV). It is obvious that the absorbance spectrum was smoother when using COE and SNV methods. For example, the preprocessing spectra from the fresh fruiting body forms had a reduced number of peak bands and moved more closely (Figure 3). This pattern was similar to the spectrum from the fresh chopped fruiting bodies, fruiting body dried powder, and dried crude extract. However, the peak bands of these samples had similar absorption regions as those in the raw absorbance spectra.
Figure 3.

Preprocessing absorbance spectral scans of fresh fruiting body forms using (a) COE and (b) SNV methods.
From these overall results, it can be seen that there is low molecular absorbance in the region from 9000 to 8500 cm–1. Furthermore, there is higher absorbance in the first overtone region from 8500 to 6700 cm–1 with the highest absorbance value occurring in the combination region from 6000 to 4000 cm–1. The most dominant absorption bands in typical NIR spectra are due to hydrogen bonds such as C–H, O–H, N–H, S–H, and P–H, as they can give strong overtone and combination.38 The strong absorptions observed between the wavenumbers of 7500 to 4000 cm–1 correspond to C=O, N–H, C–H, and C–C bonds. The bands from 7500 to 4000 cm–1 are related to C–H first overtone stretch vibration modes in CH3 and CH2 groups. The absorption bands from 5000 to 4000 cm–1 are due to −NH2 primary amines, −CONH2 primary amide combination bands, and −CONH– secondary amide combination bands,35 which are characteristic bands for proteins and amino acids. Xie and colleagues32 reported that the bands from 7500 to 5500 cm–1 and from 5000 to 4000 cm–1 using the FT–NIR spectroscopy technique are characteristic bands for proteins and amino acids in fermented C. sinensis mycelia. Therefore, it may be possible that the absorbance NIR spectra obtained in this study are related to the structural chemicals of adenosine and cordycepin, which consist of a protein that is bound to a sugar molecule. The structural formula of adenosine and cordycepin is shown in Figure 1, and the chemical groups can be seen directly and clearly.
2.3. Development of Calibration Models
The results for the developed equations among the different forms and preprocessing methods are shown in Table 3. The optimum preprocessing spectral method for the determination of adenosine concentration in the fresh fruiting bodies and the fresh chopped fruiting bodies was COE spectra and without preprocessing spectra, respectively, while for the dried powder form of the fruiting bodies and the dried crude extract of the fruiting bodies, it was SNV spectra. The best PLS equation was obtained from fresh chopped fruiting bodies without preprocessing spectra with the highest coefficient of determination (R2) of 0.95 and lowest SEP 27.50 of mg kg–1 with seven factors, while the bias and RPD were −8.57 mg kg–1 and 5.04, respectively.
Table 3. Calibration and Validation Results for the Adenosine and Cordycepin Concentrations in C. militaris Fruiting Bodies Prepared in Four Different Formsa.
| calibration set |
validation set |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| bioactive compound | sample preparations | preprocessing methods | PLS factors | Rc2 | SEC | Rp2 | RMSEP | SEP | bias | RPD |
| adenosine (mg kg–1) | fresh | no preprocess | 8 | 0.98 | 19.61 | 0.85 | 48.40 | 49.61 | –1.13 | 2.66 |
| SNV | 10 | 0.98 | 21.15 | 0.85 | 49.10 | 49.86 | –5.74 | 2.64 | ||
| COE | 9 | 0.98 | 16.94 | 0.91 | 38.10 | 38.42 | –7.55 | 3.45 | ||
| fresh chopped | no preprocess | 7 | 0.94 | 32.63 | 0.95 | 26.90 | 27.50 | –8.57 | 5.04 | |
| SNV | 8 | 0.98 | 18.81 | 0.93 | 33.99 | 34.43 | 3.71 | 3.83 | ||
| COE | 8 | 0.98 | 19.19 | 0.94 | 30.80 | 30.36 | –10.40 | 4.44 | ||
| dried powder | no preprocess | 9 | 0.96 | 31.88 | 0.92 | 35.2 | 36.12 | 0.12 | 3.66 | |
| SNV | 7 | 0.92 | 38.44 | 0.93 | 32.60 | 28.75 | –10.30 | 4.17 | ||
| COE | 9 | 0.95 | 33.01 | 0.92 | 34.80 | 35.56 | –3.50 | 3.72 | ||
| dried crude extract | no preprocess | 6 | 0.86 | 74.44 | 0.86 | 46.60 | 47.33 | –3.82 | 2.77 | |
| SNV | 7 | 0.88 | 48.50 | 0.88 | 43.50 | 44.40 | 3.35 | 2.97 | ||
| COE | 5 | 0.76 | 65.24 | 0.84 | 50.20 | 51.56 | –1.43 | 2.56 | ||
| cordycepin (mg kg–1) | fresh | no preprocess | 10 | 0.99 | 275.23 | 0.95 | 502.00 | 512.87 | –76.1 | 4.96 |
| SNV | 9 | 0.99 | 331.66 | 0.97 | 386.00 | 386.83 | 86.30 | 6.54 | ||
| COE | 7 | 0.97 | 528.49 | 0.95 | 533.00 | 534.42 | –115.00 | 4.74 | ||
| fresh chopped | no preprocess | 7 | 0.99 | 308.62 | 0.97 | 365.00 | 356.39 | –36.60 | 6.78 | |
| SNV | 8 | 0.98 | 455.58 | 0.97 | 397.00 | 378.85 | –147.00 | 6.68 | ||
| COE | 8 | 0.99 | 248.66 | 0.98 | 277.00 | 283.69 | –1.05 | 8.90 | ||
| dried powder | no preprocess | 7 | 0.99 | 181.44 | 0.99 | 226.00 | 230.31 | –28.10 | 11.00 | |
| SNV | 10 | 0.99 | 158.23 | 0.99 | 218.00 | 223.99 | –22.50 | 11.30 | ||
| COE | 10 | 0.99 | 188.42 | 0.99 | 196.00 | 198.78 | –29.30 | 12.70 | ||
| dried crude extract | no preprocess | 9 | 0.99 | 185.30 | 0.98 | 308.00 | 312.89 | –69.90 | 8.21 | |
| SNV | 6 | 0.99 | 303.46 | 0.98 | 339.00 | 313.92 | –66.90 | 7.40 | ||
| COE | 9 | 0.99 | 142.68 | 0.98 | 337.00 | 335.33 | –85.60 | 7.54 | ||
Rc2: determination coefficient in calibration, SEC: standard error of calibration, Rp2: determination coefficient in prediction, RMSEP: root mean square error of prediction, SEP: standard error of prediction, and RPD: ratio of prediction to deviation (RPD = SD/SEP).
For cordycepin concentration, the optimum preprocessing spectral method in the fresh fruiting bodies and the dried crude extract of the fruiting bodies was SNV spectra and without preprocessing spectra, respectively, while for the fresh chopped fruiting bodies and the dried powder form of the fruiting bodies, it was COE spectra. The best PLS equation was obtained from the dried powder form of the fruiting bodies using COE pretreatment spectra with the highest coefficient of determination (R2) of 0.99 and lowest SEP of 198.78 mg kg–1 with 10 factors, while the bias and RPD were −29.30 mg kg−1 and 12.70, respectively. The obtained results are similar to previous reports, which normally focused on the dried powder form in the use of NIRS for determination of various substances in Cordyceps mushrooms.32−34
However, it is observed that the prediction results for both fresh and chopped samples in scatter plots are quite similar in other sample forms. The scatter plots for the adenosine and cordycepin concentrations of the four sample forms are shown in Figure 4. It can be seen that most samples were dispersed nearby around the target line in all validation sets, which meant that the predicted adenosine and cordycepin values were no different from the actual adenosine and cordycepin values. With further analysis, the equations that are established for the fresh and chopped samples will be evaluated again in independent samples.
Figure 4.
Validation plots of the NIR spectroscopic models in the best preprocessing methods of each of the fruiting body sample forms: the fresh fruiting bodies (a,b), fresh chopped fruiting bodies (c,d), dried powder form of the fruiting bodies (e,f), and dried crude extract of the fruiting bodies (g,h) for the adenosine (on the left) and cordycepin concentrations (on the right).
2.4. Application of Developed Calibration Equations on Fresh Samples
Because the results of the developed equations tend to be well applied to the fresh samples, a more external set of data is required to test their results. Based on data from Table 3 (in bold), the correlation coefficients and RPD of the four models for intact and chopped samples are higher than 0.91 and 3.45, respectively. A total of 10 independent samples were the new test set used to evaluate the equations. These samples were prepared and then measured with a spectrometer according to the abovementioned method. Table 4 shows the predicted values of adenosine and cordycepin concentrations of the test set using the optimum equation. The best equation for determining adenosine concentration was obtained from the fresh chopped samples without preprocessing. This result was similar to the use of this preprocessing method for scanned spectra in other reports. For example, Chang-ji and co-workers33 reported previously that raw unprocessed spectra provided the highest prediction ability for determining the polysaccharide content in dried C. militaris mycelia. Xie and colleagues32 similarly reported that raw spectral data without any preprocessing performed best for determining the content of arginine in dried C. sinensis mycelia. Hassan and co-workers39 reported that total flavonoid contents in Chinese wild rice could be determined by NIR spectroscopy with no preprocessing. For cordycepin concentration, the best prediction values were those that used the equation from the fresh chopped samples based on spectra adjusted using the COE method.
Table 4. Predicted Values of the Adenosine and Cordycepin Concentrations Using the New Sample Test Set Based on the Optimum Established Equations of Four Sample Forms and Actual Values Determined by HPLCa.
| adenosine
concentration (mg kg–1) |
cordycepin
concentration (mg kg–1) |
|||||
|---|---|---|---|---|---|---|
| NIR
predicted |
NIR
predicted |
|||||
| sample test | HPLC | intact | chopped | HPLC | intact | chopped |
| 1 | 596.52 | 722.87 | 613.75 | 6249.79 | 11,302.33 | 6270.76 |
| 2 | 495.51 | 810.21 | 454.41 | 5719.98 | 12,175.00 | 5253.70 |
| 3 | 650.71 | 466.70 | 697.11 | 3777.77 | 6043.80 | 4416.10 |
| 4 | 652.16 | 534.04 | 601.20 | 4308.65 | 10,575.66 | 4565.96 |
| 5 | 727.99 | 684.59 | 603.76 | 3510.89 | 8799.03 | 4457.20 |
| 6 | 639.52 | 670.31 | 648.52 | 5214.33 | 8714.43 | 5755.06 |
| 7 | 730.58 | 907.95 | 790.17 | 3984.07 | 11,363.00 | 3558.70 |
| 8 | 861.28 | 670.16 | 571.82 | 5237.84 | 8995.96 | 5042.56 |
| 9 | 748.32 | 649.25 | 780.00 | 9081.82 | 9355.56 | 4696.36 |
| 10 | 936.32 | 802.80 | 913.68 | 6157.45 | 10,337.03 | 6156.26 |
| SEP | 141.62 | 100.06 | SEP | 5571.44 | 1505.12 | |
| Bias | 12.00 | 36.44 | bias | 444.19 | 306.99 | |
SEP: standard error of prediction.
Reproducibility was calculated for expressing precision of NIR and HPLC methods. In this case, standard deviation was determined by analyzing a sample three times and then, the average standard deviation of 10 samples was shown as reproducibility. The reproducibility of NIR and HPLC methods was 16.06 and 32.98 mg kg–1, respectively, for adenosine prediction and 232.21 and 245.31 mg kg–1, respectively, for cordycepin prediction. The NIR method seemed to be more precise than the HPLC method. These results confirmed that the equations established from the fresh chopped samples can be used to determine the adenosine and cordycepin concentrations for process control. In addition, the result is new and important as it demonstrates the possibility of using the NIRS technique to determine the adenosine and cordycepin concentrations in the fresh chopped samples. This suggests that particle size and firmness of the fresh fruiting bodies affect the reflection of near infrared; thus, it is possible to use these sample forms for adenosine and cordycepin determinations, which saves time in sample preparation compared with that needed for the dried form.
2.5. Statistics for Performance Measurement
The statistic values obtained from the best equations, bias, SEP, and slope were tested according to ISO 12099:2017(E) for equation performance checking and the results are shown in Table 5. From Table 5, it can be seen that all statistics obtained from both the best equations could pass the test based on such criteria completely. The bias was lower than Tb obtained from calculations, thus confirming that the bias was not significantly different from zero. SEP was lower than TUE, thus indicating that SEP was low enough to make it acceptable in a practical sense. In addition, tobs calculated from the equation for the slope test was lower than t(1−α/2), which was obtained from value of the t-distribution with a probability of α = 0.05. This indicates that the slope was not significantly different from 1. The results showed that the NIR-predicted values obtained from NIR spectrometers were not significantly different from the actual values in the 95% confident interval.
Table 5. Statistics for Performance Measurement Following ISO 12099.
| best equations | parameters | calculated values | criterion | results |
|---|---|---|---|---|
| adenosine (mg kg–1) | Bias | –8.57 | Tb = ±13.27 | pass |
| SEP | 27.5 | TUE = 52.20 | pass | |
| tobs | 0.40 | t(1-α/2) = 2.10 | pass | |
| cordycepin (mg kg–1) | Bias | –1.05 | Tb = ±136.95 | pass |
| SEP | 283.69 | TUE = 406.31 | pass | |
| tobs | 1.10 | t(1-α/2) = 2.10 | pass |
The regression coefficient plot for the best equation using the NIR spectrometer is shown in Figure 5. The important variable of the calibration equation was between the wavenumbers of 7500 to 4000 cm–1. This variable corresponds to an absorption band of protein and sugar molecules,35 related to the structural chemicals of adenosine and cordycepin consisting of the protein bound to sugar molecules.
Figure 5.
Regression coefficient plot for the PLS equation for (a) adenosine determination and (b) cordycepin determination in fresh chopped fruiting bodies.
3. Conclusions
This study was carried out to evaluate the feasibility of using NIR spectroscopy, which covers the spectral range of 12,500–3500 cm–1, to determine adenosine and cordycepin concentrations in C. militaris fruiting bodies. The results indicate that NIR spectroscopy could be used as a rapid and powerful alternative for evaluating these aspects of quality in the fruiting bodies. The model developed from fresh chopped fruiting bodies, where there was no preprocessing of the raw spectra, performed best for adenosine prediction, while a model using COE of the raw spectra performed best for cordycepin prediction. Therefore, application of NIR spectroscopy appears to be promising for determining adenosine and cordycepin concentrations in C. militaris fruiting bodies. However, further improvement in the sensing configuration, spectral preprocessing, and modelling methods is needed. Other algorithms for selecting optimal wavenumbers with higher accuracy and fewer variables should also be considered in order to achieve the robustness and accuracy of the predictive ability.
4. Materials and Methods
4.1. Preparation of Samples
A total of 39 samples of C. militaris fruiting bodies were prepared by cultivating the mycelia of C. militaris from different strains in a range of different media as follows. The mycelia of each strain were initially cultivated on sterilized potato dextrose agar medium in a Petri dish under static conditions and at 22 °C in the dark for 14 days. The resultant culture was then transferred to sterilized potato dextrose broth (PDB) medium in 250 mL flasks and then incubated on a rotary shaker incubator at 120 rpm at 22 °C for 14 days. Finally, 2 mL of each mycelium growing in PDB medium was transferred to different formulas of sterilized rice culture medium in a 30 mL bottle in order to alter the adenosine and cordycepin concentrations in the developing fruiting bodies. This provided a range of concentrations in the samples that were subsequently evaluated using the NIRS technique. The incubated medium was cultured at 22 °C under 12 h light and 12 h dark and 60–70% humidity for 60 days for fruiting body formation. After harvesting, the fruiting bodies produced from the cultures were prepared in four different forms as follows before being scanned by NIRS:
-
(1)
Intact fresh fruiting bodies (i.e., nondestructive) with moisture content 87.3%.
-
(2)
Fresh fruiting bodies chopped with moisture content 87.9% prepared using a kitchen knife into small pieces.
-
(3)
Fruiting bodies prepared as a dried powder with moisture content 11.6%; fresh mushrooms were dried in a hot air oven (UF55 Memmert, Germany) at 50 °C for 48 h and then ground into a powder using a chopper (HR2118, Philips, China) for about 30 s.
-
(4)
Dried crude extract; a 1.0 g sample of dried mushroom powder was extracted with 10 mL of methanol/water (50/50, V/V) solution in a 50 mL centrifuge tube. The tube was placed in an ultrasonic machine (Mettler Electronic ME 5.5, Canada) for 30 min, followed by centrifugation at 9900g for 15 min for crude extraction. The supernatant obtained from the centrifugation was filtered through a 0.45 μm filter. A 1 mL sample of this crude extract was then dropped onto a glass microfiber filter paper (GF/C Johnson, UK) and then dried in a hot air oven (UF55 Memmert, Germany) at 50 °C for 10 min.
4.2. Near-Infrared Spectroscopy Experiment
The spectra of the fruiting body samples were measured using a FT-NIR spectrometer with fiber-coupled sensor heads (Matrix-F duplex, Bruker, German) in the reflection mode at 12,500–3500 cm–1 (Figure 6) at room temperature (25 ± 1 °C) and relative humidity (58 ± 3%). Three sample forms (10 g of fresh fruiting bodies, 10 g of chopped fresh fruiting bodies, and 5 g of dried fruiting body powder) were presented to the spectrometer in 60 mm-diam. aluminum moisture cans, while the crude extract form was presented in a 90 mm-diam. Petri dish. The NIR illumination was over the entire can or dish area. Each sample was scanned three times and an average NIR spectrum was determined.
Figure 6.

FT-NIR spectrometer with the reflection mode for the spectrum acquisition in the wavenumber range of 12,500–3500 cm–1.
4.3. Measurement of Adenosine and Cordycepin by HPLC
The crude extract samples were used to determine the adenosine and cordycepin concentrations using HPLC according to the method of Huang and co-workers18 with some modifications at room temperature (25 ± 1 °C) and relative humidity (58 ± 3%). Standards of cordycepin and adenosine were purchased from Sigma Chemical Corporation (St. Louis, MO, USA). A mixture of the two standards was consecutively injected into a high-performance liquid chromatograph five times to prepare calibration curves. The HPLC analyzer (Shimadzu, Japan) used a Restek, Ultra IBD (150 mm × 4.6 mm, 5 μm particle size) column and a UV–vis detector. The mobile phase was a mixture of water and methanol (90:10, V/V). The injection volume was 20 μL, the column temperature was 35 °C, the flow rate was set at 1 mL min–1, and the eluent was monitored at 254 nm. The amount of adenosine and cordycepin analyzed by HPLC was regarded as being the actual values in the samples. Adenosine and cordycepin concentrations were determined in three duplicates. The SEL was used to verify the accuracy of the reference method and was calculated using the following formula
 |
1 |
where xi is the reference method mean value of all replicates of the i th sample; xij is the reference value of the j th replicate of the i th sample; R is the number of replicates; and N is the number of samples.
4.4. Spectral Preprocessing
Data preprocessing is an important stage in calibrating the spectra prior to their use in the prediction. By preprocessing the data, a good correlation between the spectral data and the concentration values can be ensured. In this study, the spectra from all samples were preprocessed by two mathematic methods: COE and SNV. In COE, the spectrum is shifted in order to set the y minimum value to zero. In SNV, the spectrum was normalized by first calculating the average intensity value and subsequently subtracting that value from the spectrum. The sum of the squared intensities was then calculated and the spectrum was divided by the square root of this sum.39,40 The calibration models were developed on both the original unprocessed spectra and the preprocessed spectra using PLS regression.
4.5. PLS Regression Quantitative Analysis Model
In this study, the 39 spectra were divided into a calibration set to establish the PLS model (20 spectra) and into an independent validation set to predict the robustness of the model (19 spectra) using OPUS software (version 7.2, Bruker Optics, Germany). The prediction ability was evaluated on the validation set by the coefficient of determination (R2), RMSEP, standard error of prediction (SEP), bias, and residual predictive deviation (RPD).41 A robust and accurate model should have low values of bias, RMSEP, and SEP and high values of R2 and RPD.42 The R2, RMSEP, RPD, SEP, and bias could be calculated by the following equations
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
where xi is the measurement value of the sample i; x̅ is the average value of xi; yi is the predicted value of the sample i; y̅ is the average value of yi; STD is the standard deviation of the measurement value; and n is the number of samples.
4.6. Testing the Accuracy of the Equations
The best model, which was obtained from the optimum prediction results, was further evaluated for accuracy. Many previous reports suggest that model performance using NIRS should be verified according to the standard ISO method.25,43 ISO 12099:2017 gives guidelines for near-infrared spectrometry44 of animal feeding stuffs, cereals, and milled cereal products, which state that the performance of a prediction model shall be determined from a set of validation samples and based on the statistical parameters, bias, SEP, and slope. The significance of the bias is checked by a t-test using the following formula
| 7 |
where α is the probability of making a type I error; t is the appropriate student t-value for a two-tailed test with degrees of freedom associated with SEP and the selected probability of a type I error; n is the number of independent samples; and SEP is the standard error of prediction. If the bias value is lower than Tb, the bias is not significantly different from zero.
SEP expresses the accuracy of routine NIR results corrected for the mean difference (bias) between routine NIR and reference methods. If the SEP is lower than the unexplained error confidence limits (TUE), the SEP can be accepted, whereas TUE can be calculated from an F-test using the following formula
| 8 |
where SEC is the standard error of calibration; α is the probability of making a type I error; and ν = n – 1 is the numerator degrees of freedom associated with the SEP for the test set, in which n is the number of samples in the validation process. M = nc – p – 1 is the denominator degrees of freedom associated with the standard error of calibration (SEC), in which nc is the number of calibration samples and p is the number of terms or PLS factors for the model.
The slope, b, of the simple regression Y = a + bŷ is often reported in NIR publications. The slope must be calculated with the reference values as the dependent variables and the predicted NIR values as the independent variables, if the calculated slope is intended to be used for adjustment of the NIR result. As for the bias, a t-test can be calculated to check the hypothesis that b = 1
| 9 |
where b is the slope; n is the number of independent samples; Sŷ2 is the variance of the n predicted values; and Sres is the residual standard deviation as defined in the equation.
| 10 |
where a is the intercept
of the equation 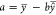 , where
, where 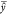 is the mean of the predicted value; y̅ is the mean of reference values; b is the slope; yi is
the i th reference value; and ŷi is the i th predicted
value obtained when applying the multivariate NIR model. The slope, b, is considered as different from 1 when tobs ≥ t(1−α/2), where tobs is the observed t-value, calculated according to the above mentioned equation,
and t(1−α/2) is the t-value obtained from the table t-distribution
for a probability of α = 0.05.
is the mean of the predicted value; y̅ is the mean of reference values; b is the slope; yi is
the i th reference value; and ŷi is the i th predicted
value obtained when applying the multivariate NIR model. The slope, b, is considered as different from 1 when tobs ≥ t(1−α/2), where tobs is the observed t-value, calculated according to the above mentioned equation,
and t(1−α/2) is the t-value obtained from the table t-distribution
for a probability of α = 0.05.
Acknowledgments
The authors acknowledge the financial support from Research and Researchers for Industries-RRI under the Thailand Research Fund and Tang Chao Tongkham company, Ltd. We are grateful to The Center of Excellence in Posthavest Technology, Naresuan University and faculty of Agriculture Natural Resources and Environment, Naresuan University, Phitsanulok, Thailand, for scientific instruments and facilities.
The authors declare no competing financial interest.
References
- Das S. K.; Masuda M.; Sakurai A.; Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia 2010, 81, 961–968. 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Bhandari A. K.; Negi J. S.; Bisht V. K.; Rana C. S.; Bharti M. K.; Singh N. Chemical constituent, inorganic elements and properties of Cordyceps sinensis – a review. Nat. Sci. 2010, 8, 253–256. [Google Scholar]
- Shashidhar M. G.; Giridhar P.; Udaya Sankar K.; Manohar B. Bioactive principles from Cordyceps sinensis: A potent food supplement - A review. J. Funct. Foods 2013, 5, 1013–1030. 10.1016/j.jff.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H.; Li S. P.; Xiang J. J.; Lai C. M.; Yang F. Q.; Gao J. L.; Wang Y. T. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS). Anal. Chim. Acta 2006, 567, 218–228. 10.1016/j.aca.2006.03.032. [DOI] [Google Scholar]
- Dong J. Z.; Liu M. R.; Lei C.; Zheng X. J.; Wang Y. Effects of selenium and light wavelengths on liquid culture of Cordyceps militaris link. Appl. Biochem. Biotechnol. 2012, 166, 2030–2036. 10.1007/s12010-012-9628-5. [DOI] [PubMed] [Google Scholar]
- Kitakaze M.; Hori M. Adenosine therapy: a new approach to chronic heart failure. Expert Opin. Invest. Drugs 2000, 9, 2519–2535. 10.1517/13543784.9.11.2519. [DOI] [PubMed] [Google Scholar]
- Cunningham K. G.; Hutchinson S. A.; Manson W.; Spring F. S. 508. Cordycepin, a metabolic product from cultures of Cordyceps militaris(Linn.) link. Part I. Isolation and characterisation. J. Chem. Soc. 1951, 2299–2300. 10.1039/jr9510002299. [DOI] [Google Scholar]
- Schmidt K.; Li Z.; Schubert B.; Huang B.; Stoyanova S.; Hamburger M. Screening of entomopathogenic Deuteromycetes for activities on targets involved in degenerative diseases of the central nervous system. J. Ethnopharmacol. 2003, 89, 251–260. 10.1016/j.jep.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Konoha K.; Yoshikawa N.; Yamaguchi Y.; Kagota S.; Shinozuka K.; Kunitomo M. Effect of cordycepin (3’-deoxyadenosine) on hematogenic lung metastatic model mice. In Vivo 2005, 19, 137–141. [PubMed] [Google Scholar]
- Dai G.; Bao T.; Xu C.; Cooper R.; Zhu J.-S. CordyMax Cs-4 improves steady-state bioenergy status in mouse liver. J. Altern. Complementary Med. 2001, 7, 231–240. 10.1089/107555301300328106. [DOI] [PubMed] [Google Scholar]
- Yoshikawa N.; Nakamura K.; Yamaguchi Y.; Kagota S.; Shinozuka K.; Kunitomo M. Antitumour activity of cordycepin in mice. Clin. Exp. Pharmacol. Physiol. 2004, 31, S51–S53. 10.1111/j.1440-1681.2004.04108.x. [DOI] [PubMed] [Google Scholar]
- Weil M. K.; Chen A. P. PARP inhibitor treatment in ovarian and breast cancer. Curr. Probl. Canc. 2011, 35, 7–50. 10.1016/j.currproblcancer.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Li Z.; Fan M.; Cheng W.; Long Y.; Ding T.; Ming L. The composition of Hirsutella sinensis, anamorph of Cordyceps sinensis. J. Food Compos. Anal. 2006, 19, 800–805. 10.1016/j.jfca.2006.04.007. [DOI] [Google Scholar]
- Lee H. J.; Burger P.; Vogel M.; Friese K.; Brüning A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Invest. New Drugs 2012, 30, 1917–1925. 10.1007/s10637-012-9859-x. [DOI] [PubMed] [Google Scholar]
- Yu H. M.; Wang B.-S.; Huang S. C.; Duh P.-D. Comparison of Protective Effects between CulturedCordyceps militarisand NaturalCordyceps sinensisagainst Oxidative Damage. J. Agric. Food Chem. 2006, 54, 3132–3138. 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Hong E. K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. 10.1016/j.intimp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Lim L.; Lee C.; Chang E. Optimization of Solid State Culture Conditions for the Production of Adenosine, Cordycepin, and D-mannitol in Fruiting Bodies of Medicinal Caterpillar Fungus Cordyceps militaris (L.:Fr.) Link (Ascomycetes). Int. J. Med. Mushrooms 2012, 14, 181–187. 10.1615/intjmedmushr.v14.i2.60. [DOI] [PubMed] [Google Scholar]
- Huang L.; Li Q.; Chen Y.; Wang X.; Zhou X. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr. J. Microbiol. Res.. 2009, 3, 957–961. [Google Scholar]
- Singpoonga N.; Chaiprasart P.; Sangon B. Effect of artificial culture media on Cordyceps militaris fruiting body formation and bioactive compound production. Songklanakarin J. Pl. Sci. 2016, 3, 34–46. [Google Scholar]
- Zhao J.; Xie J.; Wang L. Y.; Li S. P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. 10.1016/j.jpba.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Fu F.; Chen L.; Cao Q. Separation and determination of cordycepin and adenosine in artificial Cordyceps sinensis mycelium by gradient UPLC. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 022019(1–5) 10.1088/1757-899x/394/2/022019. [DOI] [Google Scholar]
- Zhang Y.; Xiang B.; Dong Y.; Xu J. Rapid determination of prochloraz in orange juice by near-infrared spectroscopy. Anal. Lett. 2013, 46, 2739–2751. 10.1080/00032719.2013.811678. [DOI] [Google Scholar]
- Shenk J. S.; Workman J. J.; Westerhaus M. O.. Application of NIR spectroscopy to agricultural products. In Handbook of Near-Infrared Analysis; Burns D. A., Ciurczak E. W., Eds.; Practical Spectroscopy Series; Marcel Dekker Inc.: New York, 1992; Vol. 13 pp 383–431. [Google Scholar]
- Ritthiruangdej P.; Ritthiron R.; Shinzawa H.; Ozaki Y. Non-destructive and rapid analysis of chemical compositions in Thai steamed pork sausages by near-infrared spectroscopy. Food Chem. 2011, 129, 684–692. 10.1016/j.foodchem.2011.04.110. [DOI] [PubMed] [Google Scholar]
- Narongwongwattana S.; Rittiron R.; Hock L. C. Rapid determination of alkalinity (ammonia content) in para rubber latex using portable and fourier transform-near infrared spectrometers. J. Near Infrared Spectrosc. 2015, 23, 181–188. 10.1255/jnirs.1160. [DOI] [Google Scholar]
- Ghosh S.; Rodgers J.. NIR analysis of textiles. In Handbook of Near-Infrared Analysis; Burns D. A., Ciurczak E. W., Eds.; Practical Spectroscopy Series; Marcel Dekker Inc.: New York, 1992; Vol. 13 pp 495–525. [Google Scholar]
- Cen H.; He Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. 10.1016/j.tifs.2006.09.003. [DOI] [Google Scholar]
- Prieto N.; Pawluczyk O.; Dugan M. E. R.; Aalhus J. L. A review of the principles and applications of near-infrared spectroscopy to characterize meat, fat, and meat products. Appl. Spectrosc. 2017, 71, 1403–1426. 10.1177/0003702817709299. [DOI] [PubMed] [Google Scholar]
- Prajapati P.; Solanki R.; Modi V.; Basuri T. A brief review on NIR spectroscopy and its pharmaceutical applications. Int. J. Pharm. Chem. Anal. 2016, 3, 117–123. 10.5958/2394-2797.2016.00018.6. [DOI] [Google Scholar]
- Osborne B. G.; Fearn T.; Hindle P. H.. Theory of near infrared spectrophotometry. In Practical NIR Spectroscopy with Applications in Food and Beverage Analysis, 2nd ed.; Longman Singapore Publisher (Pte) Ltd.: Singapore, 1993; pp 22–33. [Google Scholar]
- Liu L. Y.; Chen H. Z. Prediction of maize stover components with near infrared reflectance spectroscopy. Spectrosc. Spect. Anal. 2007, 27, 275–278. [PubMed] [Google Scholar]
- Xie C.; Xu N.; Shao Y.; He Y. Using FT-NIR spectroscopy technique to determine arginine content in fermented Cordyceps sinensis mycelium. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 149, 971–977. 10.1016/j.saa.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Chang-ji Y.; Shi-jie L.; Guo-dong Y.; Di W.; Jia-hui L.; Qing-fan M.. Application of near infrared spectroscopy in rapid determination of adenosine and polysaccharide in Cordyceps militaris. Proceedings of 2009 Fifth International Conference on Natural Computation: Tianjin, Chaina, Aug 14-16, 2009.
- Jia-hui L.; Di W.; Qing-fan M.; Hong-ru T.; Li-rong T.. Determination of the protein content in Cordyceps militaris by near infrared spectroscopy quantitative model based on partial least squares method. Proceedings of 2009 WRI World Congress on Computer Science and Information Engineering: Los Angeles, CA, Mar-Apr 31-2, 2009.
- Bokobza L.Origin of Near-Infrared Absorption Bands. In Near-Infrared Spectroscopy: Principles, Instruments, Applications; Siesler H. W., Ozaki Y.; Kawata S., Heise H. M., Eds.; WILEY-VCH: Germany, 2001; pp 11–41. [Google Scholar]
- Türker-Kaya S.; Huck C. W. A review of mid-infrared and near-infrared imaging: principles, concepts and applications in plant tissue analysis. Molecules 2017, 22, 1–20. 10.3390/molecules22010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M. G.; García-González A. S.; Franco-Robles E. Carbohydrate analysis by NIRS-Chemometrics. Dev. Near-Infrared Spectrosc. 2017, 10, 67208. 10.5772/67208. [DOI] [Google Scholar]
- Ghosh P. K.; Jayas D. S. Use of spectroscopic data for automation in food processing industry. Sens. & Instrumen. Food Qual. 2009, 3, 3–11. 10.1007/s11694-008-9068-7. [DOI] [Google Scholar]
- Hassan H.; Fan M.; Zhang T.; Yang K. Prediction of total phenolics and flavonoids contents in Chinese wild rice (Zizania latifolia) using FT-NIR spectroscopy. Am. J. Food Technol. 2015, 10, 109–117. [Google Scholar]
- Tripathi S.; Mishra H. N. A rapid FT-NIR method for estimation of aflatoxin B1 in red chili powder. Food Contr. 2009, 20, 840–846. 10.1016/j.foodcont.2008.11.003. [DOI] [Google Scholar]
- Gómez A. H.; He Y.; Pereira A. G. Non-destructive measurement of acidity, soluble solids and firmness of Satsuma mandarin using Vis/NIR-spectroscopy techniques. J. Food Eng. 2006, 77, 313–319. 10.1016/j.jfoodeng.2005.06.036. [DOI] [Google Scholar]
- Li X.-l.; He Y. Evaluation of least squares support vector machine regression and other multivariate calibrations in determination of internal attributes of tea beverages. Food Bioprocess Technol. 2010, 3, 651–661. 10.1007/s11947-008-0101-y. [DOI] [Google Scholar]
- Bantadjan Y.; Rittiron R.; Malithong K.; Narongwongwattana S. Rapid starch evaluation in fresh cassava root using a developed portable visible and near-infrared spectrometer. ACS Omega 2020, 5, 11210–11216. 10.1021/acsomega.0c01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Standard . Statistics for performance measurement. In ISO 12099:2017(E): Animal Feeding Stuffs, Cereals and Milled Cereal Products–Guidelines for Application of Near Infrared Spectrometer: Switzerland, 2017; pp 5–12.






