Abstract
In this study, the natural zeolite and rice husk biochar were mixed as a combination amendment for metal immobilization in a Cd, Pb, As, and W co-contaminated soil. A 90 day incubation study was conducted to investigate the effects of amendments on toxic metal in soil. Zeolite, biochar, and their combination application increased the soil pH and cation exchange capacity. A combination of amendments decreased the bioavailability of Cd, Pb, As, and W. Besides, the potential drawback of biochar application on As and W release was overcome by the combination agent. Zeolite, biochar, and combination treatment decreased total bioavailability toxicity from 335.5 to 182.9, 250.5, and 143.4, respectively, which means that combination was an optimum amendment for soil remediation. The results of the Community Bureau of Reference sequential extraction and scanning electron microscopy–energy-dispersive spectrometry images confirmed the Cd and Pb adsorption onto biochar. However, As and W immobilization was dominantly controlled by zeolite. It appears that the combination of amendments is an efficient amendment to remediate Cd, Pb, As, and W co-contamination in soil, although the combination of amendments has a lower stabilization rate for W than for zeolite.
1. Introduction
The pollution of toxic metals in cultivated soil can pose considerable threats to the environment and human body because of its nonbiodegradable and persistent nature.1,2 Mining activity is a major anthropogenic source of toxic metals, especially Cd, Pb, and As, which produce serious environmental problems, including soil contamination, ecosystem degradation, and food contamination.3,4 Toxic metals in mining waste could contaminate adjacent farmland and groundwater, after transportation by wind or rain. Thus, toxic metal pollution is a major factor in restricting the opportunities for agricultural land use. As a consequence of the rapid development of mining and metal processing in China for decades,5,6 contamination of soil with toxic metals has become a widespread concern. Previous study showed that over 19% of cultivated land in China suffered different degrees of metal pollution.7 In China, about 20 million hectares of farmland have been contaminated by toxic metals, producing 12 million tons of contaminated grains per year approximately.8 Multiple studies certified that metals have negative effects on germination, growth, yield, and food safety in crops, particularly Cd, Pb, and As.9,10 Therefore, it is urgent to find an efficient remediation method for reducing the bioavailability of toxic metals.
The transportation of toxic metals from soil to the food chain can be reduced by chemical means. For instance, Cd can be stabilized and converted into nonavailable forms by amendments’ application, which reduces bioavailability but does not remove toxic metals from the soil.10 It can be figured out that the application of stabilizing agents is a safe and cost-efficient method to remediate its pollution. Remediation of soils containing toxic metals with stabilizing agents represents a suitable method owing to the provision of large surface area, high cation exchange capacity (CEC), and the presence of functional groups.11,12 Several stabilizing agents have been applied to remediate contaminated soil, which includes mineral and organic amendments. Biochar is a promising organic amendment to immobilize toxic metals and its impacts on soil pH, CEC, mineral composition, and organic carbon13 were produced by slow pyrolysis of biowaste materials in the absence of oxygen (O2). Meanwhile, biochar application has a positive impact on soil carbon sequestration and mitigation of global climate change.
Biochar has been studied for its ability to immobilize toxic metals. For instance, Zhan et al. used rice straw biochar to immobilize Cd and subsequent reduction in the bioaccumulation of metal in maize plants and grains.14 Kiran compared the stabilization rate of rice husk ash and rice husk biochar in lead-spiked soils.15 Most contaminated sites might contain multimetals; the number of mixed-metal-contaminated sites is more than single-metal-contaminated sites.2 Most studies revealed that diverse types of toxic metals might lead to different results of biochar, especially for soils containing mutimetals.13 For instance, biochar significantly decreased Pb and Cd bioavailability but had no depicted effect on Zn.16 Biochar addition to a contaminated soil decreased the Cd mobility but increased As concentrations in the pore water.17,18 It has been reported that biochar immobilized metals, namely, Pb, Zn, and Cu, but mobilized As and Sb.19
Thus, it is reasonable to assume that combination agents might be more suitable for remediation of multimetal-contaminated soil.20 Meanwhile, for single-metal-polluted soil, the combination of amendments was generally having a greater stabilization rate than a single amendment. For example, Ran shown that a mixed agent slightly affects the grain yields of rice but significantly decreased the diethylene triamine pentaacetic acid (DTPA)-extractable Cd content compared to single agent.21 Biochar application (3%) combined with acidified manure (B2 + acidified manure solid (AMS)) significantly minimized Cr mobility and thereby reduced the uptake by maize plant.22 Huang figured out that the mixture of biochar and phosphate has good immobilization capacity on Cd, Cu, Pb, and Zn in contaminated soil.23 Besides, the advantages of zeolite for the remediation of toxic-metal-contaminated soil have been certified.24 For instance, zeolite has been used for soil remediation in the Copsa Mica area, Romania.25 Shi et al. suggested that the zeolite was a high effective amendment for toxic-metal-polluted garden soils, which significantly decreased the availability of Pb.26
Consequently, the above literature indicated that the application of biochar and zeolite could be an effective method to stabilize toxic metals in soil. Especially in the case where application of biochar on soils with a high level of As concentrations could be a potential drawback,17 single biochar amendment does not satisfy the requirements for metal immobilization in specific multicontaminated soil. For example, abundant tungsten mines are distributed in Jiangxi Province, China, where the soil contains Cd, Pb, As, and W contamination.27 The combined use of biochar and zeolite mitigated the emissions of ammonia and nitrogen dioxide. It reduced the mobility of the toxic metal during pig manure composting,28 but the combination of biochar and zeolite has not been used for metal immobilization in contaminated soils. Therefore, the purpose of this study was (1) assessing the immobilization efficiency of biochar, zeolite, and their mixture on toxic metals in a field soil sample, (2) assessing the remediation rate of the amendment via total bioavailable toxicity (TBT), and (3) assessing the speciation changes in Cd, Pb, As, and W in the soil after amendment application.
2. Results and Discussion
2.1. Characteristics of Amendments
The following bands were observed in the FTIR result of biochar (Figure 1). The broad strong absorption bands at 3430–1 could be attributed to the presence of −OH groups of alcohols, phenols, or carboxyl functional groups.29 Simultaneous absorption peaks at the range of 3500–3100–1 and 1600–1 were assigned to the presence of −NH in amides.7 Besides, it can be found that there are strong peaks at 1420–1 and 1090–1, which correspond to CO32–and PO43–. Abundant CO32– and PO43– in biochar has been reported.30 The band at 800 is due to the presence of −(CH2)n– (n > 4) and indicates that a long carbon chain existed.
Figure 1.
Infra spectrogram of rice husk biochar.
Some properties of biochar, zeolite, and their combination are listed in Table 1. In general, zeolite has higher pH and CEC than biochar (8.95 vs 7.81 and 137.68 cmol/kg vs 35.32 cmol/kg, respectively). Besides, biochar contained a high amount of OM. Both biochar and zeolite with a higher specific surface area (SSA) were a major contributor to metal stabilization. All toxic metals (Cd, As, Pb, and W) in amendments were very low, which means the amendments were not a metal risk for soil.
Table 1. Properties of Amendments and Test Soila.
| property | zeolite | biochar | combination | soil |
|---|---|---|---|---|
| texture | loam (red soil) | |||
| pH | 8.95 | 7.81 | 8.33 | 5.60 |
| CEC (cmol·kg–1) | 137.68 | 35.32 | 61.28 | 6.5 |
| OM (g·kg–1) | — | 485.77 | 443.50 | 35.2 |
| SSA (m2·g–1) | 178.24 | 89.06 | 112.66 | — |
| total N | — | 0.79 | 0.42 | — |
| total C | — | 45.15 | 27.84 | — |
| total Cd (mg·kg–1) | 0.02 | 0.05 | 0.03 | 7.20 |
| total Pb (mg·kg–1) | 1.12 | 0.78 | 1.01 | 91.00 |
| total As (mg·kg–1) | 1.60 | 1.04 | 1.27 | 45.32 |
| total W (mg·kg–1) | 2.51 | 0.65 | 2.02 | 118.50 |
OM, organic matter; SSA, specific surface area. —, not detected.
2.2. Changes in Soil Properties
After incubation, the effects of amendments on soil pH and CEC are shown in Figure 2. As per many studies, zeolite and biochar could increase the pH of acidic soil because of the original alkalinity.31 Compared to control, after zeolite, biochar, and their combination treatment, the pH of test soil was increased by 0.59, 0.15, and 0.29 units, respectively. Besides, the CEC of soil was increased by 35.3, 11.8, and 23.5%, respectively. Both CEC and pH were crucial parameters for metal mobility in soil. A critical review figured out that increased soil pH and CEC were a major mechanism for metal stabilization via zeolite and biochar application, particularly metal cations.32
Figure 2.
Effects of amendments on soil properties. (a) pH and (b) CEC.
2.3. Transformation of Metal Fraction after Amendment Application
2.3.1. Cadmium
The percentage of each fraction of Cd in soil samples is shown in Figure 3. After zeolite, biochar, and their combination treatment, F1 of Cd decreased from 39.2 to 25.8, 22.4, and 18.1%, respectively. Zeolite application increased F4 of Cd by 14.3%. Metal retention could take place at either pH value because of its high CEC. However, biochar and its combination agent increased both F3 (1.1–1.8%) and F4 (13.3–19.2%) fraction. The increased F3 may be dominantly controlled by biochar application. Walker revealed that biochar application could transform soluble metals into the insoluble forms that bind with organic matter (OM).33 Meanwhile, CO32– in biochar plays a role in Cd immobilization.34
Figure 3.
ercentage of the metal fraction with different amendments.
2.3.2. Lead
For the control group, the Pb concentration of F1 was 17.6 mg/kg, which accounts for 19.4% of total Pb concentration, approximately. After amendment application, the F1 fraction of Pb was decreased by 27.2, 43.1, and 51.3%. Compared to the control group, after zeolite application, a significant decrease in F1 and F3 fraction was observed, most of which was converted to an F4 fraction (increased from 11.9 to 30.2%). However, the Pb in the soil after biochar application was considered to be in a more stable form (F3 > 30%). Meanwhile, the percentage of F4 fraction for biochar treatment was higher than that for the control group (11.9 vs 15.5%). Generally, the combination agent application decreased the F1 and F2 fraction percentage but simultaneously increased the F3 and F4 fraction percentage. Compared to BC and CO groups, CO has a higher F4 percentage. The explanations for that result are as follows. Higher pH in the CO group of soil can prompt the formation of Ca2Pb8(PO4)6(OH)2 and Pb5(PO4)3OH.
2.3.3. Arsenic
Compared to control, the F1 fraction of As was increased after biochar treatment. However, after zeolite and combination agent application, the F1 fraction of As decreased by 26.7 and 8.0%, respectively. The high Fe content in zeolite could be the possible explanations for that. Gu et al. reported that the combination of biochar and zeolite could reduce the exchangeable As in soil.35 Siljeg certificated that As could be adsorbed onto the iron oxyhydroxide in the zeolite surface.36 Besides, after biochar application, the F4 fraction was decreased from 31.9 to 21.2%. That could be regarded as the part of As adsorbed on soil particle was mobilized by biochar treatment.
2.3.4. Tungsten
Similar to As, the F4 fraction of W was decreased after biochar application and increased F1 fraction. It means that a single biochar may pose a threat to specific soil. Most W was considered to be in a more stable form (F3 > 40%), and the F3 fraction of W in CO accounted for 52.6%. Besides, after the combination of amendment application, the F2 fraction of W was decreased to 8.4% compared to control. In general, the limitation of a single biochar amendment was overcome.
2.4. Assessment of Remediation
2.4.1. Effect of Amendments on Metal Bioavailability (Ethylenediaminetetraacetic Acid Extraction Results)
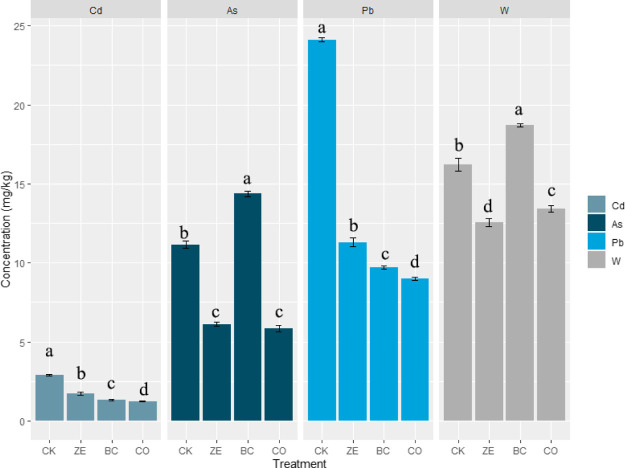
As expected, metal concentrations in ethylenediaminetetraacetic acid (EDTA) extracts were significantly changed (Figure 4). The bioavailability of Cd and Pb in the control group was 2.91 and 24.1 mg/kg, respectively. After treatment, Cd and Pb in EDTA extracts decreased by 40.5–57.4% and 53.1–62.7%, respectively. Besides, the largest decrease was observed in CO for both Cd and Pb. This result suggested that the combination agent has a more effective remediation rate than a single agent for specific metal. Meanwhile, biochar has a higher remediation rate for Cd and Pb than zeolite. Both zeolite and biochar supplying alkalinity to the soil and prompt the precipitation of insoluble particles, which contained metal elements. Cao reported that Pb reacted with phosphorus contained in biochar to form insoluble hydroxy pyromorphite.30 CO32– plays a role in Cd immobilization by prompting the formation of cadmium carbonate.37 High CEC and large surface area of amendments were major contributors to Pb and Cd immobilization.
Figure 4.
Effect of amendments on metal bioavailability.
In contrast, it should be noted that biochar application mobilized As in test soil. As well, the highest W concentration in EDTA extracts was observed in biochar treatment. This result was consistent with a previous study.38 Wu et al. reported that increased soil OM content could suppress the adsorption of As onto soil particles, which was attributed to the competition of soil OM and As for the retention sites.39 Increased W concentration was observed in biochar treatment that could be regarded as W being mobilized by increased soil pH.140 However, both As and W concentrations in extracts were decreased after the application of zeolite and a combination of amendments. It could be revealed that the combination of amendments reduced As and W concentrations in extracts by 56.4 and 22.5%, respectively. The largest reduction for As and W was observed in CO and ZE, respectively. He et al. reported that As was stabilized by forming precipitation with magnesium and calcium after zeolite application.40 Siljeg revealed that As could be adsorbed onto the iron oxyhydroxide in the zeolite surface.36 Generally, the immobilization of As and W may be dominantly controlled by zeolite rather than by biochar. Considering the element composition of zeolite, the immobilization of As and W could be explained as a high level of iron. The iron in the zeolite surface could be transformed to ferric hydroxide, which was positively charged after the protonation process. Thus, the metallic anion could be adsorbed by ferric hydroxide, such as arsenate and tungstate.
2.4.2. Assessment of Remediation Rate via Bioavailable Toxicity
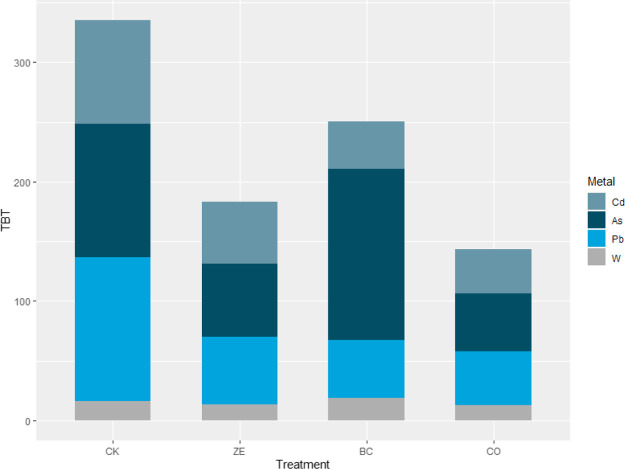
TBT was used to assess the remediation rate of multimetal-contaminated soil (Figure 5). Considering the contribution of the metals, single bioavailable toxicity (BT) was also performed. The soil in CK, ZE, and CO has a similar structure of BT. After the biochar application, As was a major contributor to the TBT of soils, which account for 57.5% of TBT. Besides, compared to the control group, all treatment could decrease the TBT. After the combination of amendment application, the TBT of soils decreased by 57.3%. The TBT of soils for different treatment in all treatments were decreased in the order of CK (335.5) > BC (250.5) > ZE (182.9) > CO (143.4). Compared to biochar treatment, the combination of amendments can reduce the risk of As and W release, which indicates the advantage of combined application.
Figure 5.
Assessment of remediation rate by TBT.
2.5. Retention of Trace Elements by Biochar
Lead and a lesser proportion of Cd were adsorbed to biochar, indicated by scanning electron microscopy/energy-dispersive spectrometry (SEM/EDS) element mapping (Figure 6). It was an explanation for the decreased Cd and Pb concentrations in EDTA extracts for the BC and CO group (although this is a part of the reason for CO). The Pb concentration in the biochar surface was increased after incubation (Figure 6b) compared to that of fresh biochar (Figure 6a). In the case of Cd, a lesser proportion was retained, which could be attributed to lower orders of magnitude of total Cd concentration compared to Pb (7.2 vs 91 mg/kg). Unlike Cd and Pb, the concentrations of W and As in the biochar surface were observed to lack retention. There could be some possible explanations for that. A high level of dissolved P in biochar was competing with As after biochar was added to the soil. Hartley et al. reported that As was mobilized in biochar-treated soils, which contribute to the competition of P and As.41 Meanwhile, higher pH in biochar-treated soil may lead to As mobilization rather than that adsorbed to the biochar surface. Krol revealed that metal anion release increases toward high pH.42 Tungsten and As are chemically analogous. These explanations were also acceptable for a lack of retention for W in biochar.
Figure 6.
SEM/EDS images of the metals spatial distribution in biochar.
Metal retention may occur in both biochar surface and network of pores. When the retention sites in the surface were effectively saturated, metals were further adsorbed to the pore structure. The porous structure of biochar leads to a large surface area, providing more retention sites. Besides, the precipitation process was also a mechanism for metal immobilization, especially Pb and Cd. The macro-, micro-, and nanoporous structures in biochar may prompt the precipitation.43
3. Conclusions
In this study, the stabilization rate of zeolite, biochar, and their combination for Cd, Pb, As, and W in a specific soil was assessed. Zeolite application decreased the bioavailability of Cd, Pb, As, and W. Meanwhile, biochar could immobilize Cd and Pb but mobilized As and W. Thus, after the combination of amendment application, the risk of biochar for anion mobilization was overcome. The combination of amendment application significantly decreased the bioavailability of Cd, Pb, As, and W by 57.4, 62.7, 56.4, and 22.5%, respectively. The Community Bureau of Reference(BCR) extraction further confirmed that the combination amendment could transform the activate faction of metals (Cd, Pb, As, and W) into the stable fraction. Zeolite, biochar, and their combination decreased the TBT from 335.5 to 182.9, 250.5, and 143.4, respectively. The result means that the combination of amendments has the highest remediation rate for the test soil. The SEM/EDS images confirmed that Pb and a less proportion of Cd were adsorbed onto biochar. It appears that the combination of zeolite and biochar is an efficient and environmentally friendly amendment to remediate multimetal-contaminated soil.
4. Materials and Methods
4.1. Soil and Amendment Characterization
Topsoil (0–20 cm) was obtained from a vegetable field adjacent to the Dangping tungsten mining area in Ganzhou City, China (114.3191° E, 25.4647° N). The soil was passed through a 2 mm sieve after air-drying. Biochar was produced by the pyrolysis of rice husk at 400 °C in the absence of O2 (4 h) and broken and passed through a #60 sieve after oven-drying, and the pH of biochar was 7.81 (1:20 solid/water).44 Zeolite was purchased from the Yusong water treatment equipment factory in Gongyi City, China, and broken and passed through a #60 sieve, and the pH of zeolite was 8.95 (1:20 solid/water).
Table 2 lists the element composition of soil and amendments determined by X-ray fluorescence. The properties of soil and amendments were characterized (Table 1). The pH values of soil were measured using a pH meter at a soil/water ratio of 1:2.5. The ammonium acetate extraction procedure was used for CEC determination.45 The Stuanes method was used for exchangeable acidity determination.46 The average pore diameter and SSA of amendments were measured using a BSD-BET400 surface area analyzer (Beishide, China). Total N and C of biochar were determined by an elemental analyzer. The total Cd, Pb, and As concentrations were applied to acid digestion (6:3:1 ratio of HNO3, HCl, and HF) using a microwave-accelerated digestion system (TK-100).47
Table 2. Element Composition of Soil and Amendments (wt %)a.
| Si | Al | Fe | Ca | Mg | Na | K | S | P | O | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| soil | 31.74 | 11.78 | 1.00 | 0.20 | 0.09 | 0.08 | 0.42 | 0.03 | 0.02 | 28.30 | 26.2 |
| zeolite | 27.12 | 11.32 | 4.87 | 0.15 | 0.41 | 0.10 | 1.38 | 0.05 | 0.09 | 27.22 | 26.5 |
| biochar | 16.33 | 0.79 | 0.59 | 1.08 | 0.19 | — | 2.67 | 0.26 | 0.85 | 7.90 | 68.8 |
| combination | 22.04 | 6.17 | 3.29 | 1.31 | 0.25 | — | 1.88 | 0.13 | 0.45 | 16.63 | 46.7 |
LOI, loss on ignition. —, not detected.
4.2. Treatment
The experiments were conducted in a plastic container (height 9.6 cm, top and bottom diameter of 17 and 12.3 cm, respectively). A meta-analysis indicated that 5% was the optimal application rate for soil remediation.48 Thus, 1.5 kg of soil was placed into a container and combined with 5% ZE, 5% BC, and 5% CO. The combination agent was labeled as CO, which was a mixture of zeolite and biochar with a weight ratio of 1:1.28 Soil without amendment was designated as the control group (CK) for comparison purposes. Three replicates were conducted. Thus, in total, there were 12 pots, which were incubated at a 20 ± 5% moisture content by weight for 90 days. The soils were stored in a chamber at 25 °C. After 90 days of incubation, approximately 100 g of soil was collected from each container for CEC, pH, EDTA extraction, and sequential extraction determination.
4.3. EDTA Extraction
EDTA extraction is closely related to toxic metals’ bioavailability to plants and other organisms.49 Therefore, the EDTA extraction procedure was used to assess the metals’ (Cd, Pb, and As) mobility and the efficiency of remediation. The EDTA extraction was conducted in a 50 mL centrifugal tube; 10 g of soil was mixed with 50 mL of 0.05 mol/L EDTA (pH = 7.0), and the mixture was shaken (180 rpm/min) for 2 h. After that, the mixture was filtered and analyzed. The concentrations of metal in the filtrate were determined by inductively coupled plasma mass spectrometry (ICP–MS, Agilent 8800, SureCycler).
4.4. Sequential Extraction Procedure
After 90 days of incubation, the BCR sequence extract procedure was adopted to determine the metal form present in the soil sample under treatment of different amendments.50 The following is a list of the sequence extraction procedures performed on the soil metals.
4.4.1. F1—Acid Extractable Fraction
Soil (1.0 g; through a #100 sieve)was extracted with 40 mL of 0.11 mol/L acetic acid with continuous shaking for 16 h at 25 °C. Then, centrifuging was performed at 3000 rpm for 2 h at room temperature, filtering of the supernatant fluid, and saving for the next step of the experiment.
4.4.2. F2—Reducible Fraction
The residual of F1 was washed with deionized water and extracted with 40 mL of 0.5 mol/L hydroxylamine hydrochloride, with continuous shaking for 16 h at 25 °C. Then, centrifuging was performed at 3000 rpm for 2 h at room temperature, filtering of the supernatant fluid, and saving for the next step of the experiment.
4.4.3. F3—Oxidizable Fraction
The residual of F2 was washed with deionized water and extracted with 10 mL of hydrogen peroxide with pH 2–3, with continuous digesting for 1 h at room temperature, and 1 h at 85 °C, respectively. Ammonium acetate (50 mL, 1 mol/L) was added for extraction with continuous shaking for 16 h at 25 °C. Then, centrifuging was performed at 3000 rpm for 2 h at room temperature, filtering of the supernatant fluid, and saving for the next step of the experiment.
4.4.4. F4—Residual Fraction
The residual of F3 was washed with deionized water and digested as the procedure of total metal analysis.
4.5. Assessment of Immobilization
For single-metal immobilization, the immobilization rate can be assessed by the following equation
where R % is the immobilization rate; C0 is the extraction of metal concentration in the control group; and Ci is the extraction of metal concentration in the treatment group. However, there was no method for the assessment of the remediation rate on multimetal-contaminated soil. Thus, the TBT index and single BT index were used in this study for immobilization assessment. The single BT has been defined as
where BT is single BT of metal; Ci is the extraction of metal concentration, and EDTA extraction result was used in this study; Tr is the toxic factor. The Tr of Cd, As, and Pb was 30, 10, and 5, respectively.51 The Tr for W was defined as 1 in the previous study.27 The TBT was the sum of BT for each metal.
where TBT is total bioavailable toxicity; four kinds of toxic metals were considered in this study.
4.6. Scanning Electron Microanalysis
Scanning electron microanalysis was used to compare the metal distribution in the biochar surface before and after incubation. Fresh biochar and biochar after incubation in CO treatment were treated for scanning electron microscopy.18 The treatment details for the sample could be checked in the reference.
4.7. Instrumental Analysis and Quality Assurance
All chemicals were of analytical grade reagents, and all containers were soaked in 5% HNO3 for more than 24 h for cleaning. ICP–MS was used for metal analysis. The standard sample (GSB07) obtained from the Ministry of Environmental Protection Standard Sample Research Institute was used for quality control. The relative standard deviation of all elements was <10% for the triplicated test, which means that the results were following the requirements.
Acknowledgments
This work was funded by the National Natural Science Foundation of China [nos. 51664025, 41861002], the Ganzhou Science and Technology Program [authorization number GSKF201850], and the National Key R&D Program of China [authorization number 2019YFC1805100].
The authors declare no competing financial interest.
References
- Habiba U.; Ali S.; Farid M.; Shakoor M. B.; Rizwan M.; Ibrahim M.; Abbasi G. H.; Hayat T.; Ali B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 1534–1544. 10.1007/s11356-014-3431-5. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.; Li G.; Chan F. K. S.; Kaye P.; Liu X.-X.; Firbank L.; Xu Y.-Y. Biochars effects potentially toxic elements and antioxidant enzymes in Lactuca sativa L. grown in multi-metals contaminated soil. Environ. Technol. Innov. 2019, 15, 100427. 10.1016/j.eti.2019.100427. [DOI] [Google Scholar]
- Qu L.; Xie Y.; Lu G.; Yang C.; Zhou J.; Yi X.; Dang Z. Distribution, fractionation, and contamination assessment of heavy metals in paddy soil related to acid mine drainage. Paddy Water Environ. 2017, 15, 553–562. 10.1007/s10333-016-0572-9. [DOI] [Google Scholar]
- Zhao H.; Xia B.; Fan C.; Zhao P.; Shen S. Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Sci. Total Environ. 2012, 417–418, 45–54. 10.1016/j.scitotenv.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Guo X.; Wang K.; He M.; Liu Z.; Yang H.; Li S. Antimony smelting process generating solid wastes and dust: Characterization and leaching behaviors. J. Environ. Sci. 2014, 26, 1549–1556. 10.1016/j.jes.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Yu H.-Y.; Liu C.; Zhu J.; Li F.; Deng D.-M.; Wang Q.; Liu C. Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. 10.1016/j.envpol.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Liu S.-J.; Jiang J.-Y.; Wang S.; Guo Y.-P.; Ding H. Assessment of water-soluble thiourea-formaldehyde (WTF) resin for stabilization/solidification (S/S) of heavy metal contaminated soils. J. Hazard. Mater. 2018, 346, 167–173. 10.1016/j.jhazmat.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Teng Y.; Ni S.; Wang J.; Zuo R.; Yang J. A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area, China. J. Geochem. Explor. 2010, 104, 118–127. 10.1016/j.gexplo.2010.01.006. [DOI] [Google Scholar]
- Hamid Y.; Tang L.; Yaseen M.; Hussain B.; Zehra A.; Aziz M. Z.; He Z.-l.; Yang X. Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice-wheat cropping system. Chemosphere 2019, 214, 259–268. 10.1016/j.chemosphere.2018.09.113. [DOI] [PubMed] [Google Scholar]
- Kamran M.; Malik Z.; Parveen A.; Zong Y.; Abbasi G. H.; Rafiq M. T.; Shaaban M.; Mustafa A.; Bashir S.; Rafay M.; Mehmood S.; Ali M. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manage. 2019, 250, 109500. 10.1016/j.jenvman.2019.109500. [DOI] [PubMed] [Google Scholar]
- Friesl W.; Friedl J.; Platzer K.; Horak O.; Gerzabek M. H. Remediation of contaminated agricultural soils near a former Pb/Zn smelter in Austria: batch, pot and field experiments. Environ. Pollut. 2006, 144, 40–50. 10.1016/j.envpol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Lahori A. H.; Guo Z.; ZHANG Z.; Li R.; Mahar A.; Awasthi M. K.; Shen F.; Sial T. A.; Kumbhar F.; Wang P.; Jiang S. Use of biochar as an amendment for remediation of heavy metal-contaminated soils: prospects and challenges. Pedosphere 2017, 27, 991–1014. 10.1016/s1002-0160(17)60490-9. [DOI] [Google Scholar]
- He L.; Zhong H.; Liu G.; Dai Z.; Brookes P. C.; Xu J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. 10.1016/j.envpol.2019.05.151. [DOI] [PubMed] [Google Scholar]
- Zhan F.; Zeng W.; Yuan X.; Li B.; Li T.; Zu Y.; Jiang M.; Li Y. Field experiment on the effects of sepiolite and biochar on the remediation of Cd- and Pb-polluted farmlands around a Pb-Zn mine in Yunnan Province, China. Environ. Sci. Pollut. Res. 2019, 26, 7743–7751. 10.1007/s11356-018-04079-w. [DOI] [PubMed] [Google Scholar]
- Kiran B. R.; Prasad M. N. V. Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicol. Environ. Saf. 2019, 183, 109574. 10.1016/j.ecoenv.2019.109574. [DOI] [PubMed] [Google Scholar]
- Khan S.; Reid B. J.; Li G.; Zhu Y.-G. Application of biochar to soil reduces cancer risk via rice consumption: a case study in Miaoqian village, Longyan, China. Environ. Int. 2014, 68, 154–161. 10.1016/j.envint.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Beesley L.; Marmiroli M.; Pagano L.; Pigoni V.; Fellet G.; Fresno T.; Vamerali T.; Bandiera M.; Marmiroli N. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci. Total Environ. 2013, 454–455, 598–603. 10.1016/j.scitotenv.2013.02.047. [DOI] [PubMed] [Google Scholar]
- Beesley L.; Moreno-Jiménez E.; Gomez-Eyles J. L.; Harris E.; Robinson B.; Sizmur T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. 10.1016/j.envpol.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; Lee S. S.; Lee S. E.; Al-Wabel M. I.; Tsang D. C. W.; Ok Y. S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. 10.1007/s11368-015-1339-4. [DOI] [Google Scholar]
- Du C.; Wang P.; Du J.; Zhu H.; Bao L.; Guo Y.; Zhang N.; Pan Y. Influence of Fixed Addition of Biochar, Zeolite and Bentonite on Growth and Cd, Pb, Zn Uptake by Maize. Ecol. Environ. Sci. 2019, 28, 190–198. [Google Scholar]
- Ran H.; Guo Z.; Shi L.; Feng W.; Xiao X.; Peng C.; Xue Q. Effects of mixed amendments on the phytoavailability of Cd in contaminated paddy soil under a rice-rape rotation system. Environ. Sci. Pollut. Res. 2019, 26, 14128–14136. 10.1007/s11356-019-04477-8. [DOI] [PubMed] [Google Scholar]
- Abbas A.; Azeem M.; Naveed M.; Latif A.; Bashir S.; Ali A.; Bilal M.; Ali L. Synergistic use of biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int. J. Phytorem. 2020, 22, 52–61. 10.1080/15226514.2019.1644286. [DOI] [PubMed] [Google Scholar]
- Huang L.; Liu C.; Liu X.; Chen Z. Immobilization of Heavy Metals in e-Waste Contaminated Soils by Combined Application of Biochar and Phosphate Fertilizer. Water, Air, Soil Pollut. 2019, 230, 26. 10.1007/s11270-019-4179-9. [DOI] [Google Scholar]
- Misaelides P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. 10.1016/j.micromeso.2011.03.024. [DOI] [Google Scholar]
- Vrînceanu N. O.; Motelică D. M.; Dumitru M.; Calciu I.; Tănase V.; Preda M. Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copşa Mică case study (Romania). Catena 2019, 176, 336–342. 10.1016/j.catena.2019.01.015. [DOI] [Google Scholar]
- Shi W.-y.; Shao H.-b.; Li H.; Shao M.-a.; Du S. Co-remediation of the lead-polluted garden soil by exogenous natural zeolite and humic acids. J. Hazard. Mater. 2009, 167, 136–140. 10.1016/j.jhazmat.2008.12.092. [DOI] [PubMed] [Google Scholar]
- Zheng X.-J.; Chen M.; Wang J.-F.; Li F.-G.; Liu Y.; Liu Y.-C. Ecological Risk Assessment of Heavy Metals in the Vicinity of Tungsten Mining Areas, Southern Jiangxi Province. Soil Sediment Contam. 2020, 29, 665–679. 10.1080/15320383.2020.1763912. [DOI] [Google Scholar]
- Wang Q.; Awasthi M. K.; Ren X.; Zhao J.; Li R.; Wang Z.; Chen H.; Wang M.; Zhang Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245, 300–308. 10.1016/j.biortech.2017.08.158. [DOI] [PubMed] [Google Scholar]
- Keiluweit M.; Nico P. S.; Johnson M. G.; Kleber M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. 10.1021/es9031419. [DOI] [PubMed] [Google Scholar]
- Cao X.; Ma L.; Liang Y.; Gao B.; Harris W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. 10.1021/es103752u. [DOI] [PubMed] [Google Scholar]
- Trgo M.; Peric J.; Medvidovic N. A comparative study of ion exchange kinetics in zinc/lead - modified zeolite-clinoptilolite systems. J. Hazard. Mater. 2006, 136, 938–945. 10.1016/j.jhazmat.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Lahori A. H.; Guo Z.; Zhang Z.; Li R.; Mahar A.; Awasthi M. K.; Shen F.; Sial T. A.; Kumbhar F.; Wang P.; Jiang S. Use of Biochar as an Amendment for Remediation of Heavy Metal-Contaminated Soils: Prospects and Challenges. Pedosphere 2017, 27, 991–1014. 10.1016/s1002-0160(17)60490-9. [DOI] [Google Scholar]
- Uchimiya M.; Lima I. M.; Klasson K. T.; Wartelle L. H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. 10.1016/j.chemosphere.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Xu X.; Tsang D. C. W.; Cao X. Contrasting impacts of pre-and post-application aging of biochar on the immobilization of Cd in contaminated soils. Environ. Pollut. 2018, 242, 1362–1370. 10.1016/j.envpol.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Xia J.; Gu J.; Zhou H.; Yang W.; Zen M.; Peng P.; Zhang P. Effect of Combined Soil Amendment Regulating Chemical Forms of Cadmium and Arsenic in Paddy Soil and Their Bioaccumulation and Translocation in Rice. Acta Pedol. Sin. 2016, 53, 1576–1585. [Google Scholar]
- Šiljeg M.; Foglar L.; Gudelj I. The removal of arsenic from water with natural and modified clinoptilolite. Chem. Ecol. 2012, 28, 75–87. 10.1080/02757540.2011.619531. [DOI] [Google Scholar]
- Cui L.; Noerpel M. R.; Scheckel K. G.; Ippolito J. A. Wheat straw biochar reduces environmental cadmium bioavailability. Environ. Int. 2019, 126, 69–75. 10.1016/j.envint.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley L.; Marmiroli M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. 10.1016/j.envpol.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Wu J.; Li Z.; Huang D.; Liu X.; Tang C.; Parikh S. J.; Xu J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. 10.1016/j.jhazmat.2019.122010. [DOI] [PubMed] [Google Scholar]
- Harita Y.; Hori T.; Sugiyama M. Release of trace oxyanions from littoral sediments and suspended particles induced by pH increase in the epilimnion of lakes. Limnol. Oceanogr. 2005, 50, 636–645. 10.4319/lo.2005.50.2.0636. [DOI] [Google Scholar]
- He Y.; Lin H.; Jin X.; Dong Y.; Luo M. Simultaneous reduction of arsenic and cadmium bioavailability in agriculture soil and their accumulation in Brassica chinensis L. by using minerals. Ecotoxicol. Environ. Saf. 2020, 198, 110660. 10.1016/j.ecoenv.2020.110660. [DOI] [PubMed] [Google Scholar]
- Hartley W.; Dickinson N. M.; Riby P.; Lepp N. W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 2009, 157, 2654–2662. 10.1016/j.envpol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Król A.; Mizerna K.; Bozym M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2019, 384, 121502. 10.1016/j.jhazmat.2019.121502. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Munroe P.; Joseph S.; Kimber S.; Van Zwieten L. Nanoscale organo-mineral reactions of biochars in ferrosol: an investigation using microscopy. Plant Soil 2012, 357, 369–380. 10.1007/s11104-012-1169-8. [DOI] [Google Scholar]
- Sun J.; Lian F.; Liu Z.; Zhu L.; Song Z. Biochars derived from various crop straws: Characterization and Cd(II) removal potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. 10.1016/j.ecoenv.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Lusiba S.; Odhiambo J.; Ogola J. Effect of biochar and phosphorus fertilizer application on soil fertility: soil physical and chemical properties. Arch. Agron. Soil Sci. 2016, 63, 477–490. 10.1080/03650340.2016.1218477. [DOI] [Google Scholar]
- Stuanes A. O.; Ogner G.; Opem M. Ammonium nitrate as extractant for soil exchangeable cations, exchangeable acidity and aluminum. Commun. Soil Sci. Plant Anal. 1984, 15, 773–778. 10.1080/00103628409367516. [DOI] [Google Scholar]
- Ahmad M.; Lee S. S.; Lim J. E.; Lee S.-E.; Cho J. S.; Moon D. H.; Hashimoto Y.; Ok Y. S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. 10.1016/j.chemosphere.2013.09.077. [DOI] [PubMed] [Google Scholar]
- Huang M.; Liu X.; Zhu C.; Huang X.; Tong X.; Yang L. A meta-analysis of effects of biochar application on the availability of Cd and Pb in soils. Huanjing Kexue Xuebao 2019, 39, 560–569. [Google Scholar]
- Li S.; Wang M.; Zhao Z.; Li X.; Han Y.; Chen S. Alleviation of cadmium phytotoxicity to wheat is associated with Cd re-distribution in soil aggregates as affected by amendments. RSC Adv. 2018, 8, 17426–17434. 10.1039/c8ra03066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C. M.; Duncan A. L.; Littlejohn D.; Ure A. M.; Garden L. M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 1998, 363, 45–55. 10.1016/s0003-2670(98)00057-9. [DOI] [Google Scholar]
- Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. 10.1016/0043-1354(80)90143-8. [DOI] [Google Scholar]