Abstract
Because melatonin has strong antioxidant activity and wine is an alcoholic beverage of economic relevance, in the present work, the impact of some variable parameters that may occur in the winemaking process on the concentrations of melatonin and its precursors in Romanian wines was studied. Therefore, a sensitive and selective high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was developed for the simultaneous analysis of melatonin, serotonin, and l-tryptophan, and some method performance parameters including selectivity, detection limit, precision (by comparing with an alternative HPLC-FL method), accuracy, and robustness were validated. These determinations are significant and the final amounts of analytes are dependent on the microorganisms involved in the winemaking process, the grape variety, geographic regions of vineyards, and aging of wines. In the future, the method may be useful to increase the melatonin content and the antioxidant activity in wines by improved steps in the winemaking process, especially based on application of selected yeasts and improved fermentation conditions.
Introduction
Melatonin is an important indole-amine hormone that modulates several physiological processes and has powerful antioxidant activity.1 Tryptophan, an essential amino acid, and serotonin, a monoamine neurotransmitter, which plays a role in controlling the circadian rhythm of sleep2 and the response to stress,3 are precursors of melatonin. Although for many years, the pineal gland of vertebrates was considered the only source of melatonin, in the last 20 years, it has been found in many food and beverages from plants4 and now it can be considered a component of dietary.5 It has been demonstrated that the consumption of foods with melatonin increases its amount and the number of derived metabolites from melatonin in plasma.6 In contradistinction to polyphenols, melatonin is able to cross physiological barriers and reduce the oxidative damage in cell environments.7 The presence of melatonin in wines8 is because it is present in grapes9 and because its precursors are biosynthesized into melatonin by the yeasts during the alcoholic fermentation process.10 At this stage, l-tryptophan is released in the wine and contributes to the development of wine’s aroma. The most used yeast as a starter for wine fermentation is Saccharomyces cerevisiae, but in some conditions, other Saccharomyces species can be chosen such as Saccharomyces bayanus or Saccharomyces uvarum. Moreover, recently winemaking technologies have started to use coinnoculation of Saccharomyces strain with other yeast strains of non-Saccharomyces yeasts, also selected from grapes in particular vineyards, such as Pichia, Torulopsis, and so on, to improve the aroma of wines and simulate the natural process of wine fermentation. At some stage in the winemaking process, mainly during fermentation, important modifications in the concentration of melatonin and its precursors occur and the synthesis of these indolic compounds strongly depends on the sugars present in the growth medium and the yeast strain or species and on the cell’s metabolic state.11,12 Serotonin is derived from the decarboxylation of l-tryptophan and it may be formed by the microorganisms’ (yeast, lactic acid bacteria) action during malolactic and alcoholic fermentation.13
Wine contains many bioactive compounds, which have antioxidant properties and make an important contribution to a healthy diet.14,15 Until recently, polyphenols were the main antioxidants in wine components, and research has been largely focused on these bioactive compounds.16−22 The health benefits attributed to wine due to its antioxidant activity depend on additive and synergistic effects of phytochemicals and are related not only to the concentration of endogenous polyphenolic constituents and melatonin but also to the reactions between them.23 Besides their polyphenol content, the presence of melatonin and its precursors in wines should be taken into consideration when their potential health benefits are evaluated, and the availability of an appropriate method for quantitative analysis of these compounds is very important. Compounds like melatonin and serotonin are present in low concentrations in beverages and require complex and expensive analytical techniques for their analysis. Various analytical methods were developed for the analysis of melatonin, l-tryptophan, and serotonin in wines using capillary electrochromatography24 or high-performance liquid chromatography (HPLC)25 with different detection techniques such as fluorescence (FL)8 and mass spectrometry (MS).26−30 Also, a voltammetric technique was proposed for the determination of melatonin but the HPLC analysis is better because it has huge discriminating capability and unambiguous identification.31 Enzyme-linked immunosorbent assay (ELISA) is used to identify serum melatonin, but for wine samples, it is not a reliable method for quantifying melatonin, and the results revealed that HPLC-tandem mass spectrometry (HPLC-MS/MS) is suitable for assaying melatonin and its precursors in wines.32 According to the existing experimental data, HPLC-MS/MS technology is the optimal analytical method for analyzing melatonin levels in wine and it is essential to develop more accurate analysis and detection methods in future studies.33
The goal of this work is to study the impact of some variable parameters that may occur in the winemaking process on the concentrations of melatonin and its precursors in wines. To accomplish this, a sensitive and selective HPLC-MS/MS method was developed for the simultaneous analysis of melatonin, serotonin, and l-tryptophan in wine samples. To be sure that we have obtained a reliable and fast analytical tool for monitoring the contents of melatonin and its precursors in wine samples, the method was validated and the results obtained for wines were compared with those acquired by an alternative HPLC-FL method.
Materials and Methods
Reagents
Melatonin and formic acid, both of analytical grade, were obtained from Sigma-Aldrich, Germany. l-Tryptophan and serotonin were obtained from Fluka, Germany. Acetic acid, methanol, and acetonitrile (ACN) were obtained from Riedel-de-Haen. Ultrapure water was obtained using an Elix 3 (Millipore) system. All of the solutions were protected from light and stored at 4 °C. Syringe-driven filter units (0.2 μm) (Chromafil, PTFE, Macherey-Nagel) were used for the filtration of all samples before HPLC analysis.
Wine Sampling
The aim of this study was to observe the differences, both qualitatively and quantitatively, between compounds of interest from a wide range of wine samples, which led to the selection of some different wine samples from the point of view of winemaking process, production year, varieties of grapes, and different geographical regions of cultivation. Two different types of wine samples were tested: commercial Romanian wines (four red, one rose, two white, and one sparkling) acquired from markets and 17 Romanian wines (seven red, six rose, and four white) homemade by people with small vineyards. The wines were produced in various geographic Romanian regions (Moldavia, Muntenia, Oltenia, and Dobruja) and in different years (from 2009 to 2018; production years are shown in Table 2). In the winemaking process of commercial samples, commercial yeast starters were used to control the fermentation process and to obtain standardized products. The commercial wines were produced from grapes grown in vineyards with noble vine (Riesling, Cabernet Sauvignon, Merlot, and Pinot Noir). The homemade wines were obtained with grapes from noble vines (Riesling, Feteasca, Cabernet Sauvignon, Chasselas, Merlot, and Babeasca neagra) but also with grapes from hybrid vine varieties (Noah, Lidia, Seibel 1, Isabelle, and Othello), which were made for consumption in the family and not for sale. For realization of homemade wines (with grapes grown by producers not with grapes bought, which ensures the geographical origin of these wines), classical maceration was used in the vinification process and the yeasts used in the fermentation stage were natural ambient yeasts from grapes or from air, and all parameters could not be controlled.
Table 2. Concentrations of Melatonin, Serotonin, and l-Tryptophan in Wine Samples Quantified with HPLC-MS/MS.
| origin of wines | geographic Romanian regions | production year | grape variety | melatonin (ng mL–1) | serotonin (ng mL–1) | l-tryptophan (ng mL–1) |
|---|---|---|---|---|---|---|
| markets | Muntenia | 2018 | White, Sparkling | 19.6 ± 0.78 | 66.6 ± 0.36 | 700.6 ± 8.69 |
| 2017 | White, Riesling | 11.8 ± 0.15 | 24.5 ± 0.21 | 1450.0 ± 15.24 | ||
| 2018 | White, Riesling | 17.0 ± 0.1 | 33.6 ± 0.52 | 1768.2 ± 12.36 | ||
| Oltenia | 2018 | Rose, Cabernet Sauvignon | 8.4 ± 0.29 | 5.8 ± 0.32 | 976.8 ± 5.89 | |
| Moldavia | 2018 | Red, Cabernet Sauvignon | 17.7 ± 0.15 | 23.3 ± 0.45 | 550.3 ± 4.25 | |
| Red, Merlot & Pinot Noir | 23.0 ± 0.84 | 12.8 ± 0.08 | 890.4 ± 7.48 | |||
| Oltenia | 2014 | Red, Merlot | 18.0 ± 0.21 | 5.0 ± 0.02 | 1262.0 ± 10.25 | |
| Dobruja | 2014 | Red, Cabernet Sauvignon & Merlot | 11.6 ± 0.67 | 83.4 ± 0.82 | 1773.5 ± 15.23 | |
| small vineyards | Muntenia | 2018 | White, Noah | 12.3 ± 0.08 | 3.5 ± 0.05 | |
| White, Noah | 35.4 ± 0.43 | 13.2 ± 0.04 | 1385.2 ± 1.14 | |||
| Moldavia | 2014 | White, Riesling & Feteasca | 1.0 ± 0.07 | 16.8 ± 0.06 | 49.4 ± 0.07 | |
| 2018 | White, Cabernet Sauvignon | 12.5 ± 0.69 | 13.2 ± 0.03 | 1125.4 ± 16.36 | ||
| Muntenia | 2016 | Rose, Lidia | 30.8 ± 0.64 | 21.2 ± 0.05 | 3038.2 ± 20.12 | |
| 2018 | Rose, Lidia | 32.6 ± 0.57 | 89.0 ± 1.42 | 2382.6 ± 19.27 | ||
| Rose, Lidiaa | 82.6 ± 2.12 | 265.6 ± 5.25 | 17 001.0 ± 124.36 | |||
| Rose, Riesling & Chasselas | 2.0 ± 0.07 | 72.8 ± 0.11 | 21.8 ± 0.08 | |||
| Moldavia | 2018 | Rose, Cabernet Sauvignon | 5.8 ± 0.07 | 11.0 ± 0.27 | 275.0 ± 1.36 | |
| Rose, Cabernet Sauvignon | 1.4 ± 0.09 | 36.4 ± 0.72 | 5.5 ± 0.04 | |||
| Muntenia | 2009 | Red, Merlot | 2.0 ± 0.06 | 43.2 ± 0.73 | 5.5 ± 0.03 | |
| 2012 | Red, Babeasca neagra | 3.8 ± 0.08 | 16.8 ± 0.09 | 330.4 ± 1.02 | ||
| 2018 | Red, Seibel 1 | 10.0 ± 0.25 | 53.6 ± 0.85 | 805.2 ± 5.78 | ||
| Red, Isabelle & Babeasca neagra | 11.6 ± 0.33 | 22.2 ± 0.09 | 2467.6 ± 18.24 | |||
| Red, Seibel 1 | 1.0 ± 0.02 | 10.4 ± 0.07 | 100.5 ± 0.87 | |||
| Moldavia | 2018 | Red, Othello | 66.6 ± 1.13 | 68.8 ± 1.35 | 6137.0 ± 5.47 | |
| Red Cabernet Sauvignon | 4.2 ± 0.07 | 25.5 ± 1.14 | 76.0 ± 0.057 |
Bold values present the wine with the highest concentration analytes of interest.
Because the concentrations of melatonin and its precursors are low in beverages, after the sample bottles were opened (all of 2019), the wines were subjected to a solid-phase extraction (SPE) to concentrate the compounds of interest. The SPE was made with a Bond Elut C18 (500 mg and 3 mL, Agilent) cartridge. The SPE cartridges were first activated with 2 mL of methanol and then equilibrated with 2 mL of water. On the top of the cartridges, 1 mL of samples was loaded and their washing was done with 1 mL of methanol. Afterward, the eluted samples were dried completely using an Eppendorf concentrator Plus and redissolved in 0.2 mL of methanol. To remove as much interference from matrices as possible, the tubes were centrifuged for 5 min at 12 500 rot min–1 and the supernatant was then transferred into filter units (0.2 μm PTFE membrane filter), filtered, and injected into the HPLC system.
HPLC Analysis
The analyses of melatonin, serotonin, and l-tryptophan were performed with a Shimadzu (Kyoto, Japan) HPLC system coupled with a mass detector, LCMS 8045 Shimadzu. The system consisted of an SIL-20ACHT autosampler, two LC-20AD pumps, a DGU-20A5 degasser, a CTO-20A column oven, and a Peak Scientific nitrogen generator. Also, another Shimadzu HPLC system, equipped with an RF-10AXL fluorescence detector and LC Solution software, was used. For the reverse-phase HPLC-MS/MS method by which the compounds of interest were identified and quantified, a mobile phase composed of 5% formic acid in water, solvent A, and 5% formic acid in ACN, solvent B, was used.
In the HPLC system, 1 μL of the samples was injected into a 100–3C18 column (Luna, Phenomenex, 50 × 4.6 mm). The experiments were effectuated at 20 °C (room temperature) for 12 min with an elution gradient (0 min ϕ = 0.2 mL min–1; 0–3.75 min 80% solvent A; 3.75–4 min 80–60% solvent A; 4 min ϕ = 0.3 mL min–1; 4–9 min 60% solvent A; 9.01–12 min 80% solvent A). Before injections, the column was equilibrated for 15 min. The mass spectrometer parameters were as follows: positive multiple reaction monitoring mode, MRM (+), electrospray ionization (ESI), 10 L min–1 heating gas flow, 300 °C interface temperature, 3 L min–1 nebulizing gas flow, 250 °C DL temperature, 400 °C heat block temperature, and 10 L min–1 drying gas flow.
The identification and quantification of melatonin and its precursors using HPLC-FL were performed according to a method previously published by our group.34
The results obtained after the triplicate injection of each wine sample and calculations (Microsoft Excel 2016) are reported as mean ± standard error.
HPLC Method Validation
The HPLC-MS/MS method for the determination of melatonin and its precursors was validated for some method performance parameters including selectivity, detection limit, precision, accuracy, and robustness.35 For selectivity, the chromatograms of two wine samples were compared with those of two wine samples spiked with analytes to evaluate any potentially interfering compounds. With seven different concentrations of standards in triplicate (10–5000 ng mL–1), the regression curves, the specific correlation coefficients, and the linear range of the response were estimated. Limits of detection (LoD) were calculated as 3 times the signal/noise ratio. The method precision was determined by estimating the repeatability and reproducibility of the method. To evaluate the injection repeatability using the same method and the same instrument in 1 day, results of 10 repetitive injections (50 ng mL–1 standard mixture) were used for calculation of coefficients of variation (CV). The method’s repeatability was assessed by effectuating 10 consecutive analysis runs for a wine sample in a day. For between-day reproducibility of the method, five consecutive injections for each standard mixture accomplished everyday on three different days for 2 months were made. The intermediate precision of the HPLC-MS/MS method was obtained by comparison of results obtained for 25 wine samples injected three times with the data values achieved by an HPLC-FL method for the same wine samples. To assess the method’s accuracy (recovery), three wine samples spiked with standards were analyzed. For evaluation of the method’s robustness, standard mixture analyses were performed with small variations in the operational parameters, for example, temperature (at 20 °C or room temperature) controlled with air condition and the use of two different columns with the same dimensions (Phenomenex and Kromasil).
Results and Discussion
At first, the optimal conditions for operational parameters of ESI-tandem mass spectrometry (ESI-MS/MS) were established. Positive and negative modes were tried for the optimization of mass spectrometry conditions. For the positive mode, lower background and better intensity for analytes were obtained. Next, to be sure that the analytes of interest were properly resolved, the chromatographic conditions were optimized using a C18 column and a standard mixture of melatonin, l-tryptophan, and serotonin. Numerous experiments (different elution phases and corresponding chromatographic gradients) have been performed to achieve distinctive symmetrical peaks and a suitable resolution, and also, in the mass detector, a better signal. The most favorable conditions obtained are those presented in the HPLC analysis section. The peaks were properly resolved (see Figure 1) and the retention times (tR) were (precursor m/z 177.20 and product m/z 160.20, 115.20, 117.10) 2.66 ± 0.03 min for serotonin, (precursor m/z 205.20 and product m/z 188.10, 146.15, 118.10) 3.52 ± 0.10 min for l-tryptophan, and (precursor m/z 233.00 and product m/z 174.10, 159.10, 130.10) 8.60 ± 0.10 min for melatonin.
Figure 1.
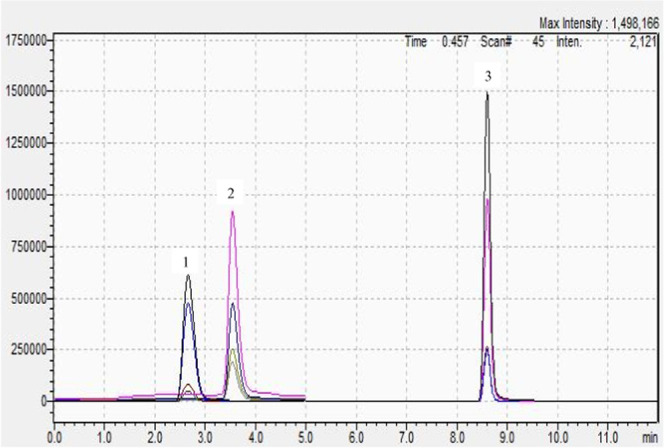
Chromatograms obtained for different mixtures of standards (10, 25, 100, 150 ng mL–1) by HPLC-MS/MS (ion extracted chromatograms, 1: serotonin peak, 2: l-tryptophan peak, and 3: melatonin peak).
The CVs obtained for injection repeatability retention time are 0.23% for melatonin, 0.63% for l-tryptophan, and 0.62% for serotonin, which are less than 1%, as suggested for the method validation. The chromatograms used to evaluate the selectivity of the method indicate that there is no significant interference from the wine matrix. The concentration range previously reported32,36 for melatonin in wines is between 5 and 423.01 ng mL–1. The results obtained for the linearity range of the response for all analytes were several decades and 0.9999 for the correlation coefficients (R) (Table 1). The calculated LoD values attest that the method is competent to be used for quantitative analysis of melatonin, serotonin, and l-tryptophan in wine samples. The CV values calculated for the method’s repeatability were 1.29 for melatonin, 1.17% for l-tryptophan, and 2.49% for serotonin, lower than 2.5%. For between-day reproducibility of the method, the CV values are smaller than 2.15% for all compounds. The CV values resulting from the analysis of 25 wine samples with two different methods and two different HPLCs (less than 1.63% for melatonin, 5.07% for l-tryptophan, and 1.17% for serotonin) demonstrate that the intermediate precision of the method is appropriate.
Table 1. Some Performance Characteristics of the HPLC-MS/MS Method for Melatonin, l-Tryptophan, and Serotonin.
| compound | precursor ion m/z | product ions m/z | tR (min) | linear regression equations | linearity range of response (ng mL–1) | R | LoD (ng mL–1) |
|---|---|---|---|---|---|---|---|
| melatonin | 233.00 | 174.10 | 8.60 ± 0.10 | A = 6.62428e + 006xC – 675.693 | 10–5000 | 0.9999 | 0.6 |
| 159.10 | |||||||
| 130.10 | |||||||
| l-tryptophan | 205.20 | 188.10 | 3.52 ± 0.10 | A = 4.52570e + 006xC + 139 057 | 10–5000 | 0.9999 | 0.5 |
| 146.15 | |||||||
| 118.10 | |||||||
| serotonin | 177.2 | 160.20 | 2.66 ± 0.03 | A = 6.13544e + 006xC + 37 040.6 | 10–5000 | 0.9999 | 0.2 |
| 115.20 | |||||||
| 117.10 |
In the case of method recovery, the resulting values are in the range 93–108% and demonstrate that at a low concentration level (0.5 mg L–1 for l-tryptophan and 0.05 mg L–1 for melatonin and serotonin), the HPLC-MS/MS method is sufficiently accurate and does not introduce errors that can considerably interfere with the analysis results. The results for the evaluation of the robustness of the method confirmed that the modifications appearing in the retention times and the peak area values of each standard with a known concentration (melatonin and serotonin, 0.05 mg L–1, and l-tryptophan, 0.5 mg L–1) do not affect the linearity response and yet allow the identification of compounds with retention time criteria.
Because wine represents a source of melatonin and is an alcoholic beverage of economic relevance, the developed and validated HPLC-MS/MS method was used for identification and quantification of melatonin, l-tryptophan, and serotonin from 25 wine samples (red, rose, and white) from diverse producers (commercial or homemade wines) but also from different years or geographic Romanian regions (Figure 2 and Table 2). The reasons for the variation of melatonin and its precursor concentrations in wines are complex and numerous. The compound concentrations depend on the grape variety, geographic regions (climatic conditions and soil, which differ even in a small area) of vineyards, and aging of wines.
Figure 2.
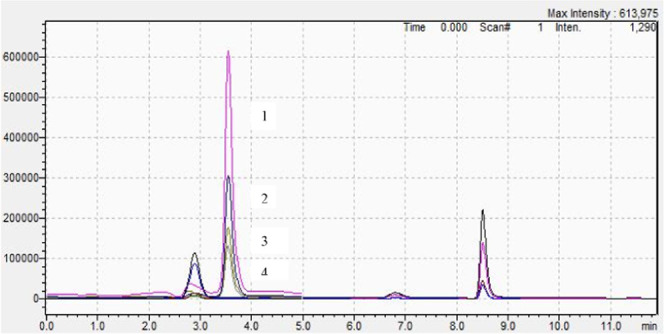
Overlapped chromatograms of wine samples obtained with the HPLC-MS/MS method (ion extracted chromatograms: 1–2018, Isabelle and Babeasca neagra wine from Muntenia; 2–2018, Riesling wine from Muntenia; 3–2012, Babeasca neagra from Muntenia; and 4–2018, Cabernet Sauvignon wine from Moldovia).
From the results obtained for the commercial wine samples, it can be noticed that in the composition of wines, the concentrations of melatonin are in the range 8.4–23 ng mL–1, serotonin between 5.0 and 83.4 ng mL–1, and l-tryptophan in the range of 700.6–1773.5 ng mL–1. Because the samples were bought, we do not know the used selected yeast species and cannot conclude which was the most productive microorganism. In homemade wines, produced in small vineyards, the vinification process was carried out without controlling fermentation with selected yeasts and did not have the most favorable conditions for wine production. The concentration values obtained were between 0 and 82.6 ng mL–1 for melatonin, 10.4 and 265.6 ng mL–1 for serotonin, and 5.5 and 17 001 ng mL–1 for l-tryptophan. In this case, melatonin and its precursors are present in wine due to concentrations contained in grapes or of the yeasts existing in grapes and musts from spontaneous flora. However, the final concentration of melatonin depends not only on the microorganisms used in winemaking technology but also on the fermentation conditions, as well as the climatic conditions during the cultivation of vines.37
In Europe, in the 19th century, a consequence of the Phylloxera vastatrix attack was hybridization, namely, the crossing of European sensitive vine varieties with resistant American vine varieties, resulting in direct producer hybrids (HPD). At present, due to the risk of HPD spreading in areas advantageous to noble vines, which are very easy to propagate and maintain culture, prohibition measures are being taken in Europe to plant the HPD in vine-growing areas, as well as to market the wine produced. On comparing the data values obtained according to the grape variety, it was observed that the highest values of melatonin concentration, and corresponding values of its precursors, are found in wines made with HPD for white (Noah, 35.4 ng mL–1 for melatonin), rose (Lidia, 82.6 ng mL–1 for melatonin, 265.6 ng mL–1 for serotonin, and 17 001.0 ng mL–1 for l-tryptophan), or red (Othello, 66.6 ng mL–1 for melatonin and 6137 ng mL–1 for l-tryptophan) wines. Likewise, it is remarkable that melatonin and its precursor concentrations are depended on the color of the wine type and the rose wines have the largest amount of compounds followed by red and white wines (Figure 3).
Figure 3.
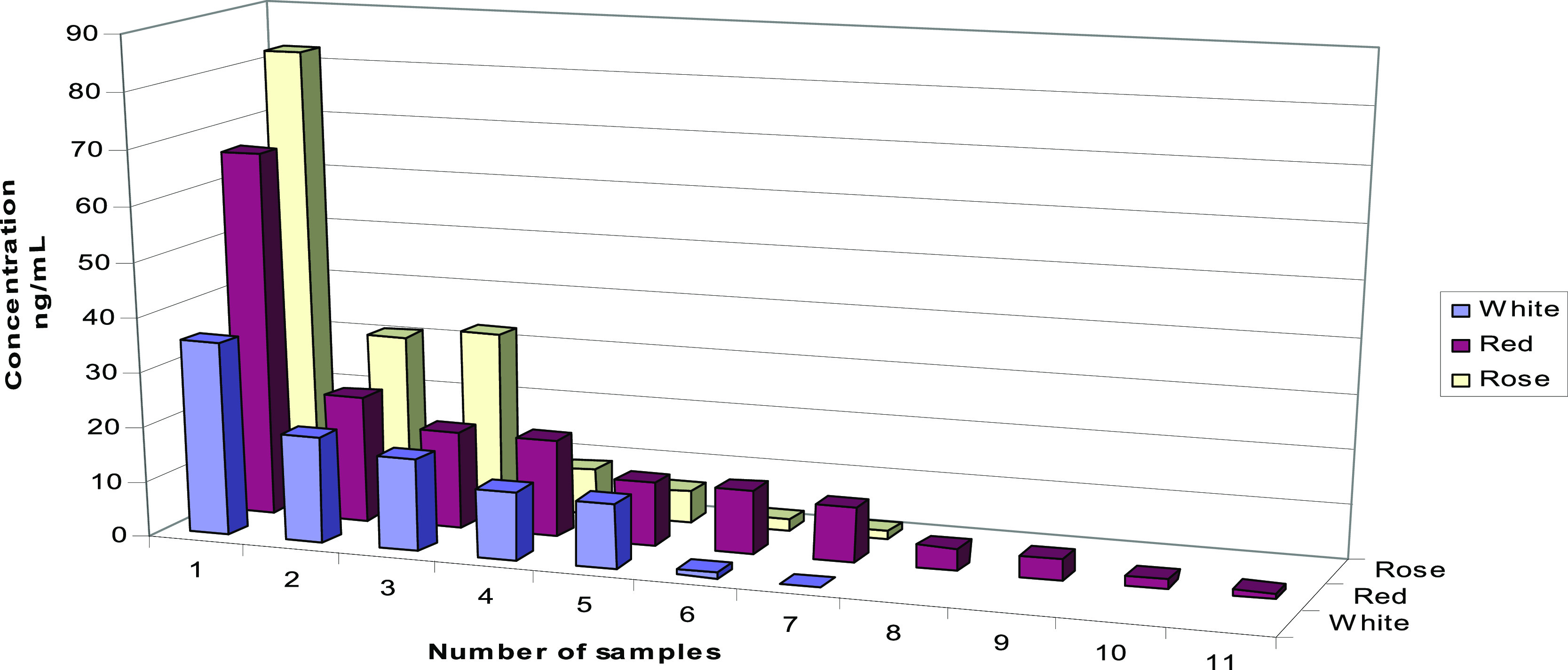
Melatonin concentrations versus different wine varieties.
The highest melatonin concentrations were obtained in the wines produced in Muntenia, followed by those obtained in the wines from Oltenia and Dobruja. The lowest concentrations were quantified from wines produced in Moldavia. Even in the same geographic regions, significant differences in concentrations of melatonin and its precursors were observed, for example, in wines produced in Muntenia from Noah grapes (0–35.4 ng mL for melatonin, 12.3–13.2 ng mL for serotonin, and 3.5–1385.2 ng mL for l-tryptophan) or Lidia grapes (30.8–82.6 ng mL for melatonin, 21.2–265.6 ng mL for serotonin, and 2382.6–17 001 ng mL for l-tryptophan) and in Moldavia from Cabernet Sauvignon grapes (1.4–5.8 ng mL for melatonin, 11–36.4 ng mL for serotonin, and 5.5–275 ng mL for l-tryptophan).
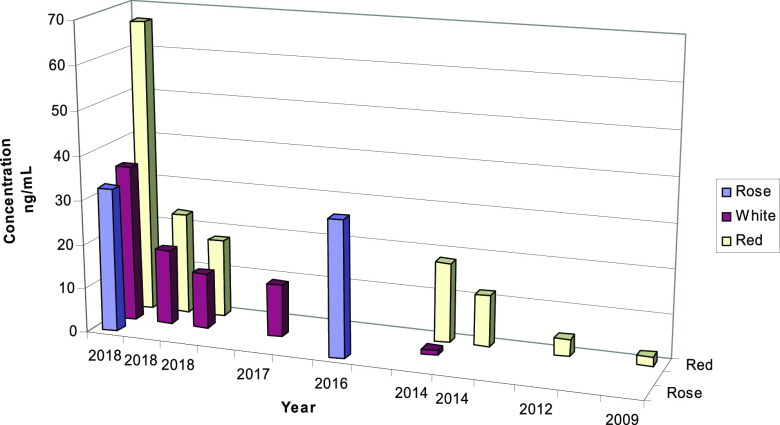
Also, it was observed that changes occurred according to the aging of wines, noting that the stability of the compounds was influenced by the age of the wines and the analyte concentrations decreased with time (Figure 4).
Figure 4.
Influence of aging on melatonin concentration in wines.
Compared to data from the literature, the melatonin content found from Cabernet Sauvignon red wine samples is in the range 4.2–17.7 ng mL–1, similar to that measured by HPLC-MS/MS32 and at least 10 times higher than those measured by ELISA32 or capillary electrochromatography,24 and this proves that the newly developed and optimized method may be used to obtain an accurate quantification of melatonin.
Conclusions
In this study, the analysis of melatonin and its precursors in different Romanian wines is reported for the first time. A variety of wines produced in different vineyards with various growing conditions, management practices, grape varieties, and localized ecosystems were analyzed. These determinations are significant as they have proven that the final amounts of analytes from wines are dependent on the microorganisms involved in the winemaking process, the grape variety, geographic regions of vineyards, and aging of wines. To realize the aim of this work, an HPLC-MS/MS method was developed and validated for the simultaneous analysis of melatonin and its precursors. Because the method has proper performance characteristics, it can be used as an optimal analytical tool for analysis of these compounds in beverage routine analysis. In the future, the method may be useful to increase the melatonin content and the antioxidant activity in wines by improved steps in the winemaking process, especially based on application of selected yeasts and improved fermentation conditions. Due to the findings of this study on the target analyte levels from HPD wines and the role of melatonin as an antioxidant, it is necessary to study melatonin concentrations in HPD wines as compared to noble wines.
Acknowledgments
The authors acknowledge the support from the Shimadzu company, Romania, for providing the mass detector for doing the experiments.
This work was supported by the Core-Program, developed with the support of the Romanian Ministry of Research and Innovation (projects 25N/19270101/2019 and PERFORM-PDI 22 PFE/2018).
The authors declare no competing financial interest.
References
- Galano A.; Tan D. X.; Reiter R. J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011, 51, 1–16. 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Yogman M. W.; Zeisel S. H.; Roberts C. Assessing effects of serotonin precursors on newborn behaviour. J. Psychiatr. Res. 1982, 17, 123–133. 10.1016/0022-3956(82)90014-0. [DOI] [PubMed] [Google Scholar]
- Anisman H.; Zacharko R. M. Multiple neurochemical and behavioural consequences of stressors: implications for depression. Pharmacol. Ther. 1990, 46, 119–136. 10.1016/0163-7258(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Iriti M.; Varoni E. M. Melatonin in Mediterranean diet, a new perspective. J. Sci. Food Agric. 2015, 95, 2355–2359. 10.1002/jsfa.7051. [DOI] [PubMed] [Google Scholar]
- Verde A.; Míguez J. M.; Gallardo M. Melatonin and related bioactive compounds in commercialized date palm fruits (Phoenix dactylifera L.): correlation with some antioxidant parameters. Eur. Food Res. Technol. 2019, 245, 51–59. 10.1007/s00217-018-3139-8. [DOI] [Google Scholar]
- Garrido M.; Paredes S. D.; Cubero J.; Lozano M.; Toribio-Delgado A. F.; Muñoz J. L.; Reiter R. J.; Barriga C.; Rodríguez A. B. Jerte valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol., Ser. A 2010, 65A, 909–914. 10.1093/gerona/glq099. [DOI] [PubMed] [Google Scholar]
- Reiter R. J.; Tan D. X.; Gitto E.; Sainz R. M.; Mayo J. C.; Leon J.; Manchester L. C.; Vijayalaxmi; Kilic E.; Kilic U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol. J. Pharmacol. 2004, 56, 159–170. [PubMed] [Google Scholar]
- Mercolini L.; Addolorata Saracino M.; Bugamelli F.; Ferranti A.; Malaguti M.; Hrelia S.; Raggi M. A. HPLC-Fl analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Sep. Sci. 2008, 31, 1007–1014. 10.1002/jssc.200700458. [DOI] [PubMed] [Google Scholar]
- Iriti M.; Rossoni M.; Faoro F. Melatonin content in grape: Myth or panacea?. J. Sci. Food Agric. 2006, 86, 1432–1438. 10.1002/jsfa.2537. [DOI] [Google Scholar]
- Rodriguez-Naranjo M. I.; Torija M. J.; Mas A.; Cantos-Villar E.; Garcia-Parrilla C. Production of melatonin by Saccharomyces strains under growth and fermentation conditions. J. Pineal. Res. 2012, 53, 219–224. 10.1111/j.1600-079X.2012.00990.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Cruz E.; Álvarez-Fernández M. A.; Valero E.; Troncoso A. M.; García-Parrilla M. C. Melatonin and derived l-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. 10.1016/j.foodchem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Vilela A. The importance of yeasts on fermentation quality and human health-promoting compounds. Fermentation 2019, 5, 46 10.3390/fermentation5020046. [DOI] [Google Scholar]
- Fernandez-Cruz E.; González B.; Muñiz-Calvo S.; Morcillo-Parra M. A.; Bisquert R.; Troncoso A. M.; Garcia-Parrilla M. C.; Torija M. J.; Guillamón J. M. Intracellular biosynthesis of melatonin and other indolic compounds in Saccharomyces and non-Saccharomyces wine yeasts. Eur. Food Res. Technol. 2019, 245, 1553–1560. 10.1007/s00217-019-03257-5. [DOI] [Google Scholar]
- Castaldo L.; Narváez A.; Izzo L.; Graziani G.; Gaspari A.; Minno G. D.; Ritieni A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626 10.3390/molecules24193626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan R.; Gepner Y.; Shai I. Wine and Health–New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. 10.1038/s41430-018-0309-5. [DOI] [PubMed] [Google Scholar]
- Alecu A.; Albu C.; Litescu S. C.; Eremia S. A. V.; Radu G. L. Phenolic and anthocyanin profile of Valea Calugareasca red wines by HPLC-PDA-MS and MALDI-TOF analysis. Food Anal. Methods 2016, 9, 300–310. 10.1007/s12161-015-0197-4. [DOI] [Google Scholar]
- Albu C.; Eremia S. A. V.; Penu R.; Vasilescu I.; Litescu S. C.; Radu G. L. Characterization of the phenolics and free-radical scavenging of Romanian red wine. Anal. Lett. 2017, 50, 591–606. 10.1080/00032719.2016.1192641. [DOI] [Google Scholar]
- Fernandes I.; Pérez-Gregorio R.; Soares S.; Mateus N.; De Freitas V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292 10.3390/molecules22020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quirós A. R. B.; Lage-Yusty M. A.; López-Hernández J. HPLC-analysis of polyphenolic compounds in Spanish white wines and determination of their antioxidant activity by radical scavenging assay. Food Int. Res. 2009, 42, 1018–1022. 10.1016/j.foodres.2009.04.009. [DOI] [Google Scholar]
- Mulero J.; Zafrilla P.; Cayuela J. M.; Martínez-Cachá A.; Pardo F. Antioxidant activity and phenolic compounds in organic red wine using different winemaking techniques. J. Food Sci. 2011, 76, C436–C440. 10.1111/j.1750-3841.2011.02104.x. [DOI] [PubMed] [Google Scholar]
- Samoticha J.; Wojdyło A.; Chmielewska J.; Oszmiański J. The effects of flash release conditions on the phenolic compounds and antioxidant activity of Pinot noir red wine. Eur. Food Res. Technol. 2017, 243, 999–1007. 10.1007/s00217-016-2817-7. [DOI] [Google Scholar]
- Jara-Palacios M. J. Wine lees as a source of antioxidant compounds. Antioxidants 2019, 8, 45 10.3390/antiox8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Yue Q.; Bian F.; Zhai H.; Yao Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 144–150. 10.1016/j.hpj.2018.05.004. [DOI] [Google Scholar]
- Stege P. W.; Sombra L. L.; Messina G.; Martinez L. D.; Silva M. F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. 10.1002/elps.200900782. [DOI] [PubMed] [Google Scholar]
- Fracassetti D.; Vigentini I.; Lo Faro A. F. F.; De Nisi P.; Foschino R.; Tirelli A.; Orioli M.; Iriti M. Assessment of tryptophan, tryptophan ethylester, and melatonin derivatives in red wine by SPE-HPLC-FL and SPE-HPLC-MS methods. Foods 2019, 8, 99 10.3390/foods8030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudela R.; Ribas-Agustí A.; Buxaderas S.; Riu-Aumatell M.; Castellari M.; López-Tamames E. Ultrahigh-Performance Liquid Chromatography (UHPLC)-Tandem Mass Spectrometry (MS/MS) Quantification of Nine Target Indoles in Sparkling Wines. J. Agric. Food Chem. 2016, 64, 4772–4776. 10.1021/acs.jafc.6b01254. [DOI] [PubMed] [Google Scholar]
- Vitalini S.; Gardana C.; Simonetti P.; Fico G.; Iriti M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal. Res. 2013, 54, 322–333. 10.1111/jpi.12028. [DOI] [PubMed] [Google Scholar]
- Gardana C.; Iriti M.; Stuknyte M.; De Noni I.; Simonetti P. ’Melatonin isomer’ in wine is not an isomer of the melatonin but tryptophan-ethylester. J. Pineal. Res. 2014, 57, 435–441. 10.1111/jpi.12183. [DOI] [PubMed] [Google Scholar]
- Boccalandro H. E.; González C. V.; Wunderlin D. A.; Silva M. F. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: Evidence of its antioxidant role in fruits. J. Pineal. Res. 2011, 51, 226–232. 10.1111/j.1600-079X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- Gomez F. J. V.; Hernández I. G.; Martinez L. D.; Silva M. F.; Cerutti S. Analytical tools for elucidating the biological role of melatonin in plants by LC-MS/MS. Electrophoresis 2013, 34, 1749–1756. 10.1002/elps.201200569. [DOI] [PubMed] [Google Scholar]
- Muñiz-Calvo S.; Guillamón J. M.; Domínguez I.; Doménech-Carbó A. Detecting and monitoring the production of melatonin and other related indole compounds in different Saccharomyces strain by solid-state electrochemical techniques. Food Anal. Methods 2017, 10, 1408–1418. 10.1007/s12161-016-0699-8. [DOI] [Google Scholar]
- Rodriguez-Naranjo M. I.; Gil-Izquierdo A.; Troncoso A. M.; Cantos E.; Garcia-Parrilla M. C. Melatonin: A new bioactive compound in wine. J. Food Compos. Anal. 2011, 24, 603–608. 10.1016/j.jfca.2010.12.009. [DOI] [Google Scholar]
- Meng J. F.; Shi T. C.; Song S.; Zhang Z. W.; Fang Y. L. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem. 2017, 231, 185–191. 10.1016/j.foodchem.2017.03.137. [DOI] [PubMed] [Google Scholar]
- Albu C.; Radu G. L. Development and Application of a HPLC-PDA-FL Method for the Determination of Melatonin and its Precursors in Infant Formulas. Food Anal. Methods 2018, 11, 951–958. 10.1007/s12161-017-1068-y. [DOI] [Google Scholar]
- Eurachem. The Fitness for Purpose of Analytical Methods. A Laboratory Guide to MethodValidation and Related Topics. 2014.
- Rodriguez-Naranjo M. I.; Gil-Izquierdo A.; Troncoso A. M.; Cantos-Villar E.; Garcia-Parrilla M. C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. 10.1016/j.foodchem.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Naranjo M. I.; Ordóñez J. L.; Callejón R. M.; Cantos-Villar E.; Garcia-Parrilla M. C. Melatonin is formed during winemaking at safe levels of biogenic amines. Food Chem. Toxicol. 2013, 57, 140–146. 10.1016/j.fct.2013.03.014. [DOI] [PubMed] [Google Scholar]