Abstract
In the U.S., causes of racial differences in stroke and its risk factors remain only partly understood, and there is a long-standing disparity in stroke incidence and mortality impacting Black Americans. Only half of the excess risk of stroke in U.S. Black populations is explained by traditional risk factors, suggesting potential effects of other factors including genetic and biological characteristics. Here, we non-systematically reviewed candidate laboratory biomarkers for stroke and their relationships to racial disparities in stroke. Current evidence indicates that interleukin-6, a proinflammatory cytokine, mediates racial disparities in stroke through its association with traditional risk factors. Only one reviewed biomarker, lipoprotein(a) (Lp(a)), is a race-specific risk factor for stroke. Lp(a) is highly genetically determined and levels are substantially higher in Black than White people; clinical and pharmaceutical ramifications for stroke prevention remain uncertain. Other studied stroke risk biomarkers did not explain racial differences in stroke. More research on Lp(a) and other biological and genetic risk factors is needed to understand and mitigate racial disparities in stroke.
Keywords: Stroke, Racial and Ethnicity, Biomarker, Disparities, Lipoprotein a, Interleukin-6
Subject terms: Cardiovascular Disease, Race and Ethnicity, Cerebrovascular disease/Stroke
Introduction
There are racial and ethnic disparities in the distribution of stroke and its risk factors in the United States. These disparities extend to different aspects of prevention, care, treatment and prognosis of stroke.1 Traditional risk factors including hypertension, diabetes mellitus, smoking, atrial fibrillation and heart disease are prevalent in the population and disproportionally affect other racial/ethnic populations as compared to White people.2-5 Importantly, the disparities are more marked among the younger population and the differences fade away in people 80 years and older.6, 7 What is striking is that about 50% of the excess stroke is explained by the overrepresentation of these traditional risk factors among underrepresented racial/ethnic populations.5, 6 Factors that may explain the higher prevalence of traditional risk factors in underrepresented racial/ethnic groups include poor diet, high salt intake, obesity and the presence of a higher predisposition to inflammation besides the added effect of social determinants such as low socioeconomic status, lower education and lack of access to care.6-8
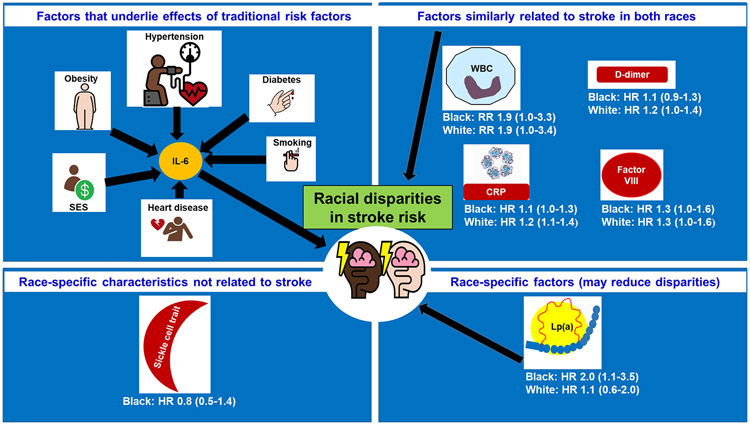
Since traditional risk factors cannot explain the remaining half of the racial disparity, the impact of biological and genetic factors warrants investigation. This line of thought has led to the search for candidate biomarkers that can moderate or mediate racial differences in stroke. Mediation may be by virtue of being in the pathway to the disease by directly affecting the disease, or by affecting it via the impact or traditional risk factors. Moderation would occur when a risk marker has a stronger relationship in one group compared to another. Here, we review current evidence on the role of biological and genetic marker sin mediating or moderating racial disparities in stroke, with results summarized in the Figure 1. Most evidence derives from the Atherosclerosis Risk in Communities (ARIC) cohort, which enrolled participants for long term follow up in 1987-9, the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study, which enrolled 30,239 Black and White men and women from across the United States in 2003-7, and the Northern Manhattan Study (NOMAS), which enrolled a multi-ethnic cohort for long term follow-up in 1993-2001.
Figure 1.
Overview of genetic and biological determinants of racial disparities in stroke risk
HR: Hazard ratio; RR: relative risk; SES: socio-economic status; IL-6: interleukin-6, WBC: white blood cell count; CRP: C-reactive protein; Lp(a): Lipoprotein(a)
Inflammation
Epidemiologic evidence on whether inflammation explains racial disparities in stroke is limited, although it has been known for some time that Black people have higher levels than other groups of a number of inflammation markers implicated in cardiovascular risk, including C-reactive protein (CRP) and interleukin-6 (IL-6).
An investigation of inflammatory cytokines [interleukin (IL)-6, IL-8 and IL-10], in the REGARDS cohort, showed IL-6 was the only cytokine that increased stroke risk in both Black and White participants. The relationship was strong, with the relative risk in the top compared to the bottom quartile of 2.0 (95% CI 1.2–3.1) after adjusting for demographic factors and stroke risk factors.9 IL-6 was also the only cytokine that mediated racial differences in stroke risk. However, adjustment for risk factors nullified the mediation. These results indicate IL-6 might explain racial disparities in stroke risk because of the well-documented proinflammatory effects of traditional risk factors.9, 10 Assessing for racial differences in the involvement of IL-6 in risk factor development and severity will help clarify how this cytokine mediates racial disparities in stroke through stroke risk factors. In addition, future research should determine whether IL-6 affects stroke-related tissues and organs, i.e. blood vessels in the brain.
Like IL-6, C-reactive protein (CRP) is associated with a higher risk of ischemic stroke in both racial groups. The relationship of CRP and incidence stroke in REGARDS was weaker in Black than White persons; a CRP concentration of ≥ 3mg/L, a traditional cutoff used in clinical practice, increased the stroke risk in White people whereas the risk only became elevated at CRP concentration >10mg/L in Black people.11 While CRP is one of the most important clinically used markers of inflammation, it does not seem to explain racial differences in stroke.
Interestingly, in the Northern Manhattan Study (NOMAS), when inflammation was characterized based on dominant quartile values of IL-6 and CRP, the effects CRP dominance (i.e, CRP being higher relative to IL-6) on ischemic stroke risk were modified by race-ethnicity. Compared to the referent group (codominance or congruent CRP and IL-6), CRP dominance was associated with a higher risk of stroke in White [HR:9.5, 95% CI (2.7-33.3)], and Hispanic [HR: 2.5, 95% CI (1.1-5.7)], but not Black participants [HR:1.2, 95% CI(0.5-2.7)]12. It is possible these results merely reflect data biases and that there were only 113 stroke cases in the analysis, but this study highlights the need for more study on the multivariate inflammatory constructs in studies of racial disparities in stroke.
Another inflammatory marker that ought to be examined is higher white blood cell count (WBC), as this is readily available in clinical practice and it relates broadly to cardiovascular risk, including stroke risk.13-17 Based on data from the ARIC cohort, the risk of stroke with higher WBC was similar across racial groups.18-20 In an analysis of ARIC data that was well-powered for race-specific analyses, when comparing the highest quartile of WBC to the lowest quartile, the risk of stroke increased by twofold in Black and White participants alike.14 These findings indicate that WBC, like CRP, is a risk factor for stroke in both racial groups, but it does not mediate racial disparities.
Overall, there is some evidence to indicate that inflammation contributes to the excess risk of stroke in Black people. More research is needed with larger samples to evaluate racial differences in the context of the interaction of inflammatory markers with each other and traditional risk factors for stroke. Future investigations should examine the way inflammation particularly IL-6, mediates risk factor impacts. Beyond traditional risk factors of stroke, much uncertainty still exists about the relationship of inflammation, social determinants of health (i.e. diet, psychosocial stressors) and racial disparities in stroke.
Coagulation
The final pathologic process for most strokes is thrombosis, the occlusion of an artery by a blood clot leading to tissue ischemia and death.21 Prior research has demonstrated that Black and White people have different thrombotic biomarker profiles. Compared to other groups, Black people have higher levels of procoagulant proteins, especially factor VIII and fibrinogen, and lower levels of anticoagulant proteins such as protein C, all culminating in higher D-dimer, a marker of coagulation activation.22, 23 Anticoagulation is proven to reduce stroke risk in those with atrial fibrillation23 and as well as for secondary prevention in people with a prior stroke24, proving a causal role for thrombosis in some strokes.
The impact of novel coagulation biomarkers, in general, is less studied for stroke than coronary heart disease25, and minimally studied as a reason for racial disparities in stroke risk, especially among younger Black people. The REGARDS study assessed the association of thrombotic biomarkers with stroke, and assessed whether these biomarkers explained any of the increased risk.26-31 Among the thrombotic biomarkers studied, factor VIII30 and D-dimer28 were the only biomarkers associated with stroke risk after adjusting for traditional stroke risk factors (Table 1). Both D-dimer and factor VIII were significantly higher in Black people than White people, and so were prime candidates for potentially explaining some of the racial difference in stroke risk between Black and White people. However, D-dimer did not attenuate the excess risk of stroke in Black people.28
Table 1.
Adjusted Association of Hemostasis Biomarkers with Stroke Risk, REGARDS cohort (hazard ratios and 95% confidence intervals)*
| All | Black people | White people | |
|---|---|---|---|
| Factor VIII30 | |||
| Per SD Higher (44%) | 1.26 (1.08, 1.46) | 1.26 (1.02, 1.55) | 1.26 (1.02, 1.56) |
| Factor IX27 | |||
| Per SD higher (22%) | 1.03 (0.89, 1.19) | 1.00 (0.80, 1.25) | 1.05 (0.88, 1.26) |
| Factor XI27 | |||
| Per SD higher (25%) | 1.08 (0.94, 1.24) | 1.01 (0.83, 1.24) | 1.12 (0.93, 1.35) |
| Protein C30 | |||
| Per SD lower (22%) | 1.04 (0.88, 1.23) | 0.99 (0.77, 1.26) | 1.09 (0.87, 1.35) |
| D-dimer28 | |||
| Per doubling | 1.15 (1.01, 1.31) | 1.12 (0.92, 1.34) | 1.19 (0.99, 1.42) |
Adjusted for: Age, sex, race, age*race interaction, systolic blood pressure, taking blood pressure medications, diabetes, current smoking, atrial fibrillation, left ventricular hypertrophy, and baseline cardiovascular disease (coronary heart disease or peripheral vascular disease)
p-values for race*biomarker interaction were all >0.10 (non-significant)
Higher D-dimer levels in Black compared to White people may be partially explained by ancestry-specific genetic factors. The Cardiovascular Health study reported each standard deviation higher in European ancestry was associated with about 10% lower mean D-dimer levels in Black people. The association held after adjustment for confounders including traditional cardiovascular risk factors.32 However, these results may be somewhat limited by the small number of Black participants and residual confounding.
People with non-O blood group have higher factor VIII level 33, 34 because blood group oligosaccharides, which are attached to the von Willebrand factor (vWF; the carrier protein for factor VIII), determine vWF clearance.35 The ABO glycosytransferase gene (ABO) defines the sequence of oligosaccharides, which determines the presence of A, B or neither antigen on the surfaces of red blood cells.36 ABO blood group is also very consistently associated with cardiovascular diseases across a number of studies. 37-42 Blood type AB is more common in Black people, and AB is also associated with the highest factor VIII level compared to other blood types.26 As such, ABO blood group was hypothesized to be associated with stroke risk and to mediate racial disparities in stroke. In REGARDS, after adjustment for demographic factors and stroke risk factors, people with blood type AB had 83% higher risk of stroke compared to those with blood type O [HR: 1.83, 95% CI(1.01-3.30). FVIII explained 60% of the excess risk in stroke for blood type AB, but racial differences in ABO blood group did not mediate racial disparities in stroke.26
Overall, on a population level, procoagulant biomarkers (factor VIII) and markers of coagulation activation (D-dimer) were related modestly with stroke risk but not differ by race and did not explain the Black-White disparity in stroke risk at any age.
Stroke is not one disease43, 44, and hemostasis is not measured by one biomarker45. For stroke, understanding how race may impact the stroke subtype could help reveal whether different etiologies underpin stroke risk (and stroke prevention) in different populations. Likewise, thrombosis is a complex interplay of multiple proteins and cellular elements; reducing hemostasis to assessing one or two biomarkers does not do justice to the complexity of coagulation in vivo. The future lies is refining our definition of stroke based on pathophysiology and measuring thrombosis as a concept rather than through individual biomarkers. Only then might these complex relationships be disentangled.
Sickle cell trait
Sickle cell trait (SCT) and sickle cell anemia result from a point mutation in the β chain of hemoglobin, which leads to a switch from glutamic acid to valine. The SCT denotes an inheritance of both normal (HbA) and mutated (HbS) hemoglobin. Individuals who inherit the HbS type hemoglobin from both parents develop sickle cell anemia. In the U.S., the trait is relatively common in the Black population, with a prevalence of 9%, and about 1 out 601 Black newborns have the HbSS genotype.46-49
Both SCT and sickle cell anemia are race-specific risk factors for venous thrombosis. As many as 25% of patients with sickle cell anemia or SCT have a confirmed history of venous thromboembolism,50 and SCT is associated with higher D-dimer concentration.51 An investigation of middle-aged, Black participants showed that HbSA raised the risk of venous thromboembolism 2-fold. 52
Despite the associated hypercoagulability of SCT, a recent meta-analysis involving 19,464 Black people, followed for 7-14 years, revealed that SCT was not associated with a higher risk of ischemic stroke in Black persons. Upon adjustment for stroke risk factors, the overall hazard ratio (HR) was 0.80 (95% CI: 0.47-1.35) for Black adults with SCT compared to those without.53 These results indicate that despite the fact SCT is largely a race-specific characteristic in the US, and associated with thromboembolism and hypercoagulability, it is not associated with stroke.
Lipoprotein (a)
Lipoprotein(a) [Lp(a)] consists of a modified low-density lipoprotein (LDL) bound to a apolipoprotein(a). Higher circulating level is associated with increased risk of coronary and cerebrovascular disease54, so Lp(a) measurement in those screened for cardiovascular risk is recommended.55, 56 Approximately 1.5 billion people worldwide have elevated Lp(a), so there a tremendous potential for disease prevention leveraging this biomarker.57 Apolipoprotein (a) size determines the size of Lp(a); larger size correlates with lower Lp(a). Black people have smaller Lp(a) isoforms, and much higher Lp(a) compared to other race-ethnic groups. Lp(a) concentration is about 80% genetically determined, with little influence by lifestyle or other factors. There is a stronger and unique genetic influence in Black compared to White individuals, and a stronger relationship of genetic variation in Lp(a) with subclinical atherosclerosis in Black people.58 These findings in sum suggest that Lp(a) is a causal cardiovascular and stroke risk factor that might be more important in Black than White people.
There are few studies of Lp(a) and stroke risk58, with only two reporting on race differences in this relationship. Using a race-sex-specific threshold level representing Lp(a) in the 4th quartile of the distribution, the REGARDS investigators reported that elevated Lp(a) was weakly associated with ischemic stroke; HR 1.45 (95% CI 0.96, 2.19). The relationship was only present in Black participants, with a HR of 1.96 (95% CI 1.10, 3.46), while in White participants the HR was only 1.14 (95% CI 0.64, 2.04).59 Similarly, in the NOMAS, elevated Lp(a) was more strongly related to stroke in Black people compared to other groups; among Black people the odds ratio (OR was 2.7 (95% CI 1.2-6.2) while this was 2.1 (95% CI (0.7-6.6) in White people, and only 1.5 (95% CI 0.8-2.5) in Hispanic people.60 This study was limited by its smaller sample size in race subgroups, and because Lp(a) was measured after stroke occurred, which might bias the findings in unpredictable ways. Overall, these findings suggest, from the point of view of stroke, that Lp(a) is a race-specific risk factor, or a stronger risk factor among Black people. The clinical ramifications of this are uncertain.
Current cardiovascular prevention medications do not lower Lp(a). With the mounting evidence that Lp(a) is a causal risk factor for atherosclerotic disease, drugs are in development to lower Lp(a),61-63. For example, an antisense oligonucleotide, AKCEA-APO(a)-LRx reduced elevated Lp(a) in a dose dependent manner up to 80% in patients with cardiovascular disease and elevated Lp(a). Phase 3 trials are underway. The potential for Lp(a) inhibition to reduce the burden of stroke, in particular among Black individuals, warrants intensive study based on the epidemiologic findings above.
Investigation of Biological Factors Influencing Stroke Outcome in Pre-Clinical Studies
Racial differences are difficult to study in pre-clinical stroke models, as part of the value of using animals is the removal of “background” genetic variation to reduce variability and confirm the importance of a specific pathway or biomarker. However, even rodents with more restricted genetic backgrounds can be used to model some aspects of risk factors that are more prevalent in one race than another, such as hypertension, hypercoagulability or elevated Lp(a). For example, commonly used models include the spontaneously hypertensive rat (SHR), which is a model of essential hypertension, bred from a line of Wistar-Kyoto rats in the 1960s by Okamoto and colleagues.64 The stroke prone SHR (SHR-SP) is a further development of SHR that has even higher blood pressure than SHR and has a high incidence of stroke and intracerebral hemorrhage.65 Despite decades of study, the genetics of these strains are still relatively unknown, and their value for studying complex polygenetic disorders such as hypertension or stroke has been questioned,66 especially with the growing availability of genetic information from human studies. However, there is value in the use of animal models to understand basic mechanisms related to stroke, as so many other factors that influence stroke risk can be controlled, such as dietary and environmental factors.
Additionally, animal studies have identified several potential targets67 that can now be validated in patients, many of which involve inflammatory signaling that also contribute to stroke outcomes in humans. Understanding the underlying biology could identify new targets in specific racial populations at risk for poor outcomes after stroke.
Clinical implications
There are numerous clinical factors that likely contribute to the racial disparities in stroke incidence and outcomes. Biological and genetic factors, like the higher prevalence of traditional risk factors such as hypertension and diabetes, or racial differences in Lp(a) levels, likely have a complex interactive relationship with stroke risk. Other social determinants of health (SDOH), like access to care, socioeconomic status (SES), compliance, and environmental/dietary factors also play a role.
The difference in stroke mortality between Black and White people may be a result of either a higher incidence of stroke in Black than White people, or a higher case fatality among Black people suffering stroke than white people suffering stroke. There is strong evidence that the former is the driver of the epidemiological differences in stroke. There is a higher incidence of stroke in the Black population than the white population, as documented in the NOMAS68, the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS)69, the REGARDS study70 and the ARIC study71. The Black-to-White incidence ratio ranges from 1.0-3.0, which mirrors mortality differences. In contrast, there is little evidence of a higher case fatality among Black relative to White individuals.70 Therefore, it seems likely that the Black-to-White disparity in stroke is largely attributable to a racial difference in stroke incidence. Contributions of risk factors that are both more prevalent in the black population and strongly associated with stroke risk may be substantial contributors to disparities in stroke incidence. For example, Black people are at a disadvantage for many measures of SES, and lower SES is strongly associated with stroke risk (but more weakly associated with case-fatality). Much work remains to be done to investigate to what extent SES and other SDOH such as stress, health behavior (ie., smoking) depression, isolation etc. contributes to racial disparities in stroke, and if these are mediated by specific biological pathways like thrombosis and inflammation. A greater understanding is needed on the impact of individual/societal factors and unique biological pathways that interact with traditional risk factors and lead to the higher stroke incidence in Black people.
Future directions
Although the Black–White disparities in stroke have been known for at least a half century, only recently have studies focused on biological and genetic factors that contribute to racial disparities in stroke. Understanding the causes of these large disparities is the first step to design and implement interventions to reduce the unequal distribution of the public health burden of stroke. More research is needed; both for broader genomics data, study of interactions among biomarkers, risk factors and SDOH, origins of stroke risk factors by race, and preclinical models. Table 2 summarizes research questions that can fill knowledge gaps on biomarkers and racial disparities in stroke. Additionally, the framework outlined here (Figure 1) to study both the moderating and mediating effects of risk factors on racial disparities in stroke should provide useful direction for future investigation.
Table 2.
Knowledge gaps in the association of biomarkers and racial disparities in stroke in the U.S.
| How can inflammation (IL-6) mediate the impact of traditional risk factors and social determinants of health (stress, diet, health behavior, isolation) on racial disparities in stroke? |
| How does IL-6 affect stroke-related tissues and organs? How does it relate to racial disparities in stroke? |
| How do biomarkers, in particular IL-6, affect racial differences in risk factor incidence? |
| How will the interaction of multiple thrombotic markers impact racial disparities in stroke? |
| How do stress markers (markers of the hypothalamic-pituitary-adrenal axis and other neuroendocrine markers) impact racial disparities in stroke? |
| Does Lp(a) inhibition reduces the excess burden of stroke in Black people? |
| Can newer mouse strains be developed to better assess biological mechanisms of stroke disparities? |
| To what extent do socioecononomic status and social determinants of health contribute to racial disparities in stroke? How do they interact with biomarkers of stroke to increase racial disparities? |
| To what extent are racial disparities due to genetic factors? Do these genetic determinants interact with traditional risk factors, socioecononomic status, social determinants of health and other non-traditional risk factors to increase racial disparities in stroke? |
Abbreviations: IL-6: interleukin-6; SLp(a); Lipoprotein(a)
Acknowledgements
The authors wish to acknowledge the organizers of the Health Equity and Actionable Disparities in Stroke: Understanding and Problem-solving (HEADS-UP) Symposium, the research community working on stroke epidemiology and disparities in the United States, and the committed participants of studies on stroke disparities.
Sources of funding
The authors had funding support from U01 NS041588 (DKM, MC), P20 GM135007 (MC) and K08 HL096841 (NAZ).
Non-standard abbreviations
- IL
Interleukin
- CRP
C-reactive protein
- vWF
von Willebrand factor
- Lp(a)
Lipoprotein (a)
- WBC
White blood cell count
- SCT
Sickle cell trait
- SHR
Spontaneously hypertensive rat
- ARIC
Atherosclerosis Risk in Communities
- REGARDS
REasons for Geographic And Racial Differences in Stroke
- NOMAS
Northern Manhattan Study
- GCNKSS
Greater Cincinnati/Northern Kentucky Stroke Study
Footnotes
Disclosures:
Dr. Salvador Cruz-Flores consulted for Novo Nordisk and Sunovion. The other authors reported no relevant relationships.
References
- 1.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, et al. Racial-Ethnic Disparities in Stroke Care: The American Experience: A statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116 [DOI] [PubMed] [Google Scholar]
- 2.Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10-year Stroke Risk by Region and Race in the United States: Geographic and Racial Differences in Stroke Risk. Ann Neurol. 2008;64(5):507–513. doi: 10.1002/ana.21493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-Ethnic Disparities in the Impact of Stroke Risk Factors: The Northern Manhattan Stroke Study. Stroke. 2001;32:1725–1731 [DOI] [PubMed] [Google Scholar]
- 5.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, et al. Association of Race and Sex With Risk of Incident Acute Coronary Heart Disease Events. JAMA. 2012;308:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, et al. Traditional Risk Factors as the Underlying Cause of Racial Disparities in Stroke: Lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeger BC, Anstey DE, Bress AP, Booth JN 3rd, Butler M, Clark D 3rd, , Howard G, Kalinowski J, Long DL, Ogedegbe G, et al. Cardiovascular Disease and Mortality in Adults Aged >/=60 years According to Recommendations by the American College of Cardiology/American Heart Association and American College of Physicians/American Academy of Family Physicians. Hypertension. 2019;73:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2015;132:873–898 [DOI] [PubMed] [Google Scholar]
- 9.Jenny NS, Callas PW, Judd SE, McClure LA, Kissela B, Zakai NA, Cushman M. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology. 2019;92:e2375–e2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of Increased C-Reactive Protein for Cardiovascular Risk Stratification in Black and White Men and Women in the US. Clin Chem. 2009;55:1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans CR, Long DL, Howard G, McClure LA, Zakai NA, Jenny NS, Kissela BM, Safford MM, Howard VJ, Cushman M. C-reactive protein and stroke risk in blacks and whites: The Reasons for Geographic and Racial Differences in Stroke cohort. Am Heart J. 2019;217:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna JM, Moon YP, Liu KM, Spitalnik S, Paik MC, Cheung K, Sacco RL, Elkind MS. High-Sensitivity C-Reactive Protein and Interleukin-6-Dominant Inflammation and Ischemic Stroke Risk: The Northern Manhattan Study. Stroke. 2014;45:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkind MS, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology. 2005;64:2121–2125 [DOI] [PubMed] [Google Scholar]
- 14.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White Blood Cell Count and Incidence of Coronary Heart Disease and Ischemic Stroke and Mortality from Cardiovascular Disease in African-American and White Men and Women: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154:758–764 [DOI] [PubMed] [Google Scholar]
- 15.Noto D, Barbagallo CM, Cavera G, Cefalu AB, Caimi G, Marino G, Lo Coco L, Caldarella R, Notarbartolo A, Averna MR. Leukocyte count, diabetes mellitus and age are strong predictors of stroke in a rural population in southern italy: An 8-year follow-up. Atherosclerosis. 2001;157:225–231 [DOI] [PubMed] [Google Scholar]
- 16.Prentice RL, Szatrowski TP, Kato H, Mason MW. Leukocyte counts and cerebrovascular disease. J Chronic Dis. 1982;35:703–714 [DOI] [PubMed] [Google Scholar]
- 17.Wu TH, Chien KL, Lin HJ, Hsu HC, Su TC, Chen MF, Lee YT. Total white blood cell count or neutrophil count predict ischemic stroke events among adult taiwanese: Report from a community-based cohort study. BMC Neurol. 2013;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkind MS, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction [supplementary table e-1]. Neurology. 2005;64:2121–2125 [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, Rasmussen ML, Wu KK, The Atherosclerosis Risk in Communities (Aric) Study Investigators. Prospective Study of Markers of Hemostatic Function with Risk of Ischemic Stroke.. Circulation. 1999;100:736–742 [DOI] [PubMed] [Google Scholar]
- 20.Gillum RF, Ingram DD, Makuc DM. White Blood Cell Count and Stroke Incidence and Death: The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1994;139:894–902 [DOI] [PubMed] [Google Scholar]
- 21.Petty GW, Brown RD Jr., Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic Stroke Subtypes: A Population-Based Study of Incidence and Risk Factors. Stroke. 1999;30:2513–2516 [DOI] [PubMed] [Google Scholar]
- 22.Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: The MESA study. J Thromb Haemost. 2006;4:2629–2635 [DOI] [PubMed] [Google Scholar]
- 23.Gaines KJ, Chesney C, Vander Zwaag R, Cape C. Racial differences in coagulation studies in stroke. Neurol Res. 1992;14:103–108 [DOI] [PubMed] [Google Scholar]
- 24.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, et al. A Comparison of Warfarin and Aspirin for the Prevention of Recurrent Ischemic Stroke. N Engl J Med. 2001;345:1444–1451 [DOI] [PubMed] [Google Scholar]
- 25.Bhatia M, Rothwell PM. A Systematic Comparison of the Quality and Volume of Published Data available on Novel Risk Factors for Stroke versus Coronary Heart Disease. Cerebrovasc Dis. 2005;20:180–186 [DOI] [PubMed] [Google Scholar]
- 26.Zakai NA, Judd SE, Alexander K, McClure LA, Kissela BM, Howard G, Cushman M. ABO blood type and stroke risk: the REasons for Geographic And Racial Differences in Stroke Study. J Thromb Haemost. 2014;12:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson NC, Cushman M, Judd SE, Kissela BM, Safford MM, Howard G, Zakai NA. Associations of coagulation factors IX and XI levels with incident coronary heart disease and ischemic stroke: the REGARDS study. J Thromb Haemost. 2017;15:1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakai NA, McClure LA, Judd SE, Kissela B, Howard G, Safford M, Cushman M. D-dimer and the Risk of Stroke and Coronary Heart Disease. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Thromb Haemost. 2017;117:618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakai NA, Olson NC, Judd SE, Kleindorfer DO, Kissela BM, Howard G, Cushman M. Haemostasis Biomarkers and Risk of Intracerebral Haemorrhage in the REasons for Geographic and Racial Differences in Stroke Study. Thromb Haemost. 2017;117:1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakai NA, Judd SE, Kissela B, Howard G, Safford MM, Cushman M. Factor VIII, Protein C and Cardiovascular Disease Risk: The REasons for Geographic and Racial Differences in Stroke Study (REGARDS). Thromb Haemost. 2018;118:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffield LM, Lu AT, Szeto MD, Little A, Grinde KE, Shaw J, Auer PL, Cushman M, Horvath S, Irvin MR, et al. Coagulation factor VIII: Relationship to cardiovascular disease risk and whole genome sequence and epigenome-wide analysis in African Americans. J Thromb Haemost. 2020;18:1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange LA, Reiner AP, Carty CL, Jenny NS, Cushman M, Lange EM. Common genetic variants associated with plasma fibrin d-dimer concentration in older European- and African-American adults. J Thromb Haemost. 2008;6:654–659 [DOI] [PubMed] [Google Scholar]
- 33.Song J, Chen F, Campos M, Bolgiano D, Houck K, Chambless LE, Wu KK, Folsom AR, Couper D, Boerwinkle E, et al. Quantitative Influence of ABO Blood Groups on Factor VIII and Its Ratio to von Willebrand Factor, Novel Observations from an ARIC Study of 11,673 Subjects. PLoS One. 2015;10:e0132626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirado I, Mateo J, Soria JM, Oliver A, Martínez-Sánchez E, Vallvé C, Borrell M, Urrutia T, Fontcuberta J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93:468–474 [DOI] [PubMed] [Google Scholar]
- 35.Franchini M, Capra F, Targher G, Montagnana M, Lippi G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb J. 2007;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dean L Blood Groups and Red Cell Antigens. Bethesda, MD: National Center for Biotechnology Information (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK2261/ [Google Scholar]
- 37.Dentali F, Sironi AP, Ageno W, Crestani S, Franchini M. ABO Blood Group and Vascular Disease: An Update. Semin Thromb Hemost. 2014;40:49–59 [DOI] [PubMed] [Google Scholar]
- 38.Dentali F, Sironi AP, Ageno W, Turato S, Bonfanti C, Frattini F, Crestani S, Franchini M. Non-O Blood Type Is the Commonest Genetic Risk Factor for VTE: Results from a Meta-Analysis of the Literature. Semin Thromb Hemost. 2012;38:535–548 [DOI] [PubMed] [Google Scholar]
- 39.Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014;112:1103–1109 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Mooney CJ, Reilly MP. ABO Blood Groups and Cardiovascular Diseases. Int J Vasc Med. 2012;2012:64191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, Qi L. ABO Blood Group and Risk of Coronary Heart Disease in Two Prospective Cohort Studies. Arterioscler Thromb Vasc Biol. 2012;32:2314–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauer AJ, Ruigrok YM, Algra A, van Dijk EJ, Koudstaal PJ, Luijckx GJ, ederkoorn PJ, van Oostenbrugge RJ, Visser MC, Wermer MJ. Age-Specific Vascular Risk Factor Profiles According to Stroke Subtype. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lioutas VA, Beiser A, Himali J, Aparicio H, Romero JR, DeCarli C, Seshadri S. Lacunar Infarcts and Intracerebral Hemorrhage Differences: A Nested Case-Control Analysis in the FHS (Framingham Heart Study). Stroke. 2017;48:486–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tynngård N, Lindahl TL, Ramström S. Assays of different aspects of haemostasis - what do they measure? Thrombosis journal. 2015;13:8–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashorobi D, Bhatt R. Sickle Cell Trait In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK537130/ [PubMed] [Google Scholar]
- 47.Gibson JS, Rees DC. How benign is sickle cell trait? EBioMedicine. 2016;11:21–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassell KL. Population Estimates of Sickle Cell Disease in the U.S. Am J Prev Med. 2010;38:S512–521 [DOI] [PubMed] [Google Scholar]
- 49.Sedrak A, Kondamudi NP. Sickle cell disease In: Statpearls. Treasure Island, FL; 2020. https://www.ncbi.nlm.nih.gov/books/NBK482384/ [Google Scholar]
- 50.Naik RP, Streiff MB, Haywood C Jr., Nelson JA, Lanzkron S. Venous Thromboembolism in Adults with Sickle Cell Disease: A Serious and Under-recognized Complication. Am J Med. 2013;126:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naik RP, Wilson JG, Ekunwe L, Mwasongwe S, Duan Q, Li Y, Correa A, Reiner AP. Elevated D-dimer levels in African Americans with sickle cell trait. Blood. 2016;127:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folsom AR, Tang W, Roetker NS, Kshirsagar AV, Derebail VK, Lutsey PL, Naik R, Pankow JS, Grove ML, Basu S, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyacinth HI, Carty CL, Seals SR, Irvin MR, Naik RP, Burke GL, Zakai NA, Wilson JG, Franceschini N, Winkler CA, et al. Association of Sickle Cell Trait With Ischemic Stroke Among African Americans: A Meta-analysis. JAMA Neurol. 2018;75:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Emerging Risk Factors Collaboration. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA. 2009;302:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langlois MR, Chapman MJ, Cobbaert C, Mora S, Remaley AT, Ros E, Watts GF, Borén J, Baum H, Bruckert E, et al. Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem. 2018;64:1006–1033 [DOI] [PubMed] [Google Scholar]
- 56.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018:CIR0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeedi R, Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clin Diabetes Endocrinol. 2016;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arora P, Kalra R, Callas PW, Alexander KS, Zakai NA, Wadley V, Arora G, Kissela BM, Judd SE, Cushman M. Lipoprotein(a) and Risk of Ischemic Stroke in the REGARDS Study. Arterioscler Thromb Vasc Biol. 2019;39:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boden-Albala B, Kargman DE, Lin IF, Paik MC, Sacco RL, Berglund L. Increased Stroke Risk and Lipoprotein(a) in a Multiethnic Community: The Northern Manhattan Stroke Study. Cerebrovasc Dis. 2010;30:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM,et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. New England Journal of Medicine. 2015;372:1500–1509 [DOI] [PubMed] [Google Scholar]
- 62.Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. Journal of lipid research. 2016;57:340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253 [DOI] [PubMed] [Google Scholar]
- 64.Okamoto K, Aoki K. Development of a Strain of Spontaneously Hypertensive Rats. Jpn Circ J. 1963;27:282–293 [DOI] [PubMed] [Google Scholar]
- 65.Yamori Y, Tomimoto K, Ooshima A, Hazama F, Okamoto K. Proceedings: Developmental course of hypertension in the SHR-substrains susceptible to hypertensive cerebrovascular lesions. Jpn Heart J. 1974;15:209–210 [DOI] [PubMed] [Google Scholar]
- 66.Nabika T, Ohara H, Kato N, Isomura M. The stroke-prone spontaneously hypertensive rat: Still a useful model for post-gwas genetic studies? Hypertens Res. 2012;35:477–4844 [DOI] [PubMed] [Google Scholar]
- 67.Verma R, Ritzel RM, Harris NM, Lee J, Kim T, Pandi G, Vemuganti R, McCullough LD. Inhibition of miR-141-3p Ameliorates the Negative Effects of Poststroke Social Isolation in Aged Mice. Stroke. 2018;49:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. S Stroke Incidence among White, Black, and Hispanic Residents of an Urban Community: The Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268 [DOI] [PubMed] [Google Scholar]
- 69.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, et al. Stroke in a Biracial Population: The Excess Burden of Stroke among Blacks. Stroke. 2004;35:426–43 [DOI] [PubMed] [Google Scholar]
- 70.Howard G, Moy CS, Howard VJ, McClure LA, Kleindorfer DO, Kissela BM, Judd SE, Unverzagt FW, Soliman EZ, Safford MM, et al. Where to Focus Efforts to Reduce the Black-White Disparity in Stroke Mortality: Incidence Versus Case Fatality? Stroke. 2016;47:1893–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke Incidence and Survival Among Middle-Aged Adults: 9-Year Follow-Up of the Atherosclerosis Risk in Communities (ARIC) Cohort. Stroke. 1999;30:736–74 [DOI] [PubMed] [Google Scholar]