Abstract
Objective This study described the prospective relationship between pharmacological and behavioral measures of 6-mercaptopurine (6MP) medication adherence in a multisite cohort of pediatric patients diagnosed with cancer (N = 139). Methods Pharmacological measures (i.e., metabolite concentrations) assessed 6MP intake. Behavioral measures (e.g., electronic monitoring) described adherence patterns over time. Results Three metabolite profiles were identified across 15 months: one group demonstrated low levels of both metabolites (40.8%) consistent with nonadherence and/or suboptimal therapy; two other groups demonstrated metabolite clusters indicative of adequate adherence (59.2%). Those patients whose metabolite profile demonstrated low levels of both metabolites had consistently lower behavioral adherence rates. Conclusions To our knowledge, this was the first study to prospectively validate a pharmacological measure of medication adherence with a behavioral adherence measure in a relatively large sample of pediatric patients with cancer. Using multiple methods of adherence measurement could inform clinical care and target patients in need of intervention.
Keywords: adherence, cancer, metabolites, pediatrics, pharmacology, metabolites
Maintenance therapy is considered vital for survival and long-term outcomes for pediatric patients diagnosed with acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL). Relapse prevention is the primary goal of maintenance treatment, which necessitates patient adherence to a lengthy and complex course of therapy that could be difficult for many patients and their parents to implement (Bhatia et al., 2012; Davies & Lilleyman, 1995). Rates of nonadherence to prescribed treatment regimens during the maintenance phase of pediatric cancer treatment are high, yet variable, ranging from 10% to 94% (Bhatia et al., 2012; Davies & Lilleyman, 1995; Kato, Cole, Bradlyn, & Pollock, 2008; Lau, Matsui, Greensberg, & Koren, 1998). Research has documented that nonadherence during the maintenance phase of treatment contributes to morbidity (e.g., worse disease prognosis, disease relapse, adverse side effects) and mortality in pediatric patients diagnosed with ALL and LBL (Bhatia et al., 2012; Davies & Lilleyman, 1995; Lau et al., 1998; Lennard, Welch, & Lilleyman, 1995). Bhatia and colleagues (2012) found that adherence rates to 6-mercaptopurine (6MP) of <95% based on electronic monitoring were associated with an increased risk of relapse. In fact, nonadherent patients were 2.5 times more likely to relapse compared with adherent patients (Bhatia et al., 2012). The authors also illustrated that 6MP adherence enhanced the therapeutic efficacy of the medication, which was critical for disease remission (Bhatia et al., 2012). However, Bhatia et al. (2012) did not describe the relationship between multiple measures of adherence (e.g., electronic monitoring versus metabolites of 6MP). These findings heighten the need for accurate and valid measurement of medication adherence.
Accurate measurement of medication adherence in pediatric patients diagnosed with ALL and LBL necessitates a methodologically sound study using multiple objective measures. Despite the concern that self-report measures typically overestimate adherence, a large number of studies in pediatric ALL and LBL have used physician-, parent-, or patient-reported adherence measures (Kenna, Labbé, Barrett, & Pfister, 2005; Lau et al., 1998; Riekert & Rand, 2002). Given limitations with self-reported adherence, Kenna et al. (2005) suggested examining the relationship between multiple, objective adherence measures such as behavioral adherence (i.e., electronic monitoring: an indirect, objective measure of daily adherence that provides information about the date/time a pill bottle containing the medication was opened) and pharmacological adherence (i.e., metabolites of 6MP: a direct, objective adherence measure).
Electronic monitoring has been used in a number of studies in pediatric cancer for monitoring medication adherence, and provides an objective estimate of patient adherence behavior (Bhatia et al., 2012; Kato et al., 2008; Kenna et al., 2005; Lau et al., 1998; Riekert & Rand, 2002). However, electronic monitoring does not provide a direct measure of adherence because patients might remove the medication from the monitored bottle, but might not ingest the medication (Ingerski, Hente, Modi, & Hommel, 2011; Kenna et al., 2005; Lau et al., 1998). Moreover, a direct pharmacological measure has special advantages in characterizing adherence. When 6MP is ingested, it is metabolized into two primary metabolites: thioguanine nucleotides (TGN) and methylated mercaptopurine (MMP) (Davies, Lennard, & Lilleyman, 1993). Metabolite concentration profiles of 6MP provide unique information regarding 6MP medication adherence for the period immediately before the blood draw (i.e., 5 days before the blood draw), which could be integrated with the information obtained from behavioral adherence measures (Kenna et al., 2005; Lau et al., 1998). To our knowledge, previous research in pediatric cancer has not described longitudinal adherence patterns to 6MP medication in the context of multiple, objective adherence measures.Analyses investigating the prospective relationship between metabolite profiles and behavioral adherence are important for describing 6MP adherence patterns in pediatric cancer.
It has been suggested that low concentrations of both metabolites (TGN and MMP) could reflect poor bioavailability of the drug (e.g., inadequate dosing) or nonadherence to prescribed 6MP (Lennard et al., 1995; Lilleyman & Lennard, 1994). Low levels of both metabolites were associated with poor disease prognosis in children with ALL and LBL, including higher risk for disease relapse (Hawwa et al., 2009; Lilleyman & Lennard, 1994; Traore et al., 2006). Thus, it is important to identify which patients present with low levels of both metabolites. Two previous studies used cluster analysis to identify subgroups of individuals who present with similar levels of 6MP metabolite concentrations (TGN and MMP) (Hawwa et al., 2009; Traore et al., 2006). Traore et al. (2006) identified four metabolite clusters in a sample of pediatric patients with ALL (n = 48 patients, Mage = 15 years): (1) high TGN–low MMP (n = 6), (2) low TGN–high MMP (n = 25), (3) low TGN–low MMP (n = 39), and (4) low TGN–very high MMP (n = 4). Hawwa et al. (2009) identified five metabolite profiles in a sample of 19 patients diagnosed with ALL (Mage = 10 years): (1) high TGN–low MMP (n = 8), (2) low TGN–high MMP (n = 25), (3) low TGN–low MMP (n = 11), (4) very high TGN–low MMP (n = 4), and (5) very high MMP–low TGN (n = 16). These findings suggested that cluster analysis could be a useful analytic technique to identify adherent and nonadherent patients, in addition to those patients being treated with suboptimal therapy. Given that cluster analysis relies heavily on the research sample and empirically derives metabolite clusters by identifying patients with similar patterns of metabolite values, it is important to validate these metabolite profiles with an independent, objective measure of medication adherence (e.g., behavioral adherence).
Previous research that described 6MP metabolic profiles in children with ALL (Hawwa et al., 2009; Traore et al., 2006) was limited by small sample sizes (Ns = 19, 48) and restricted monitoring periods (e.g., 4–6 months), which limited the clinical significance of the research for understanding prospective patterns of medication adherence (Lau et al., 1998). In addition, the measurement and description of thiopurine methyltransferase (TPMT) activity and optimal versus suboptimal 6MP dosing and its relative influence on metabolite profiles were not conducted. TPMT is an enzyme that metabolizes 6MP into MMP and TGN (Relling et al., 2011). Patients present with one of three TPMT phenotypes (Relling et al., 2011): homozygous deficient (1:300; present with low or deficient TPMT activity); heterozygous (10% of population; present with intermediate levels of TPMT activity); or homozygous wild type (90% of patients; present with normal or high TPMT activity). TPMT phenotypes can influence the pharmacokinetics of TGN, which could impact the therapeutic levels of TGN and would likely influence the generation of metabolite profiles (Relling et al., 2011). Similarly, suboptimal dosing could influence metabolite levels and should be examined in research investigating pharmacological measures of adherence. Validation of a pharmacological measure of medication adherence using data obtained from behavioral adherence measures extends the clinical utility of using objective measures of 6MP adherence in routine follow up of pediatric patients with ALL and LBL. For this reason, it is important to describe, evaluate, and validate patterns of nonadherence as measured by multiple, objective measures of adherence and to differentiate between nonadherence and inadequate dosing.
To address the limitations of previous research, the current study used a prospective research design to describe 6MP medication adherence patterns across 15 months based on pharmacological and behavioral measures of 6MP adherence. Based on previous research (Hawwa et al., 2009; Traore et al., 2006), it was hypothesized that there would be three unique metabolite profiles across 15 months: a group that demonstrated low levels of both TGN and MMP metabolites, which could indicate suboptimal dosing or nonadherence to 6MP; and two groups that demonstrated a negative correlation between the two metabolites, which could reflect adherence to 6MP: high TGN–low MMP and low TGN–high MMP (Lilleyman & Lennard, 1994). The primary aim of the current study was to validate the metabolite profiles with results obtained from a behavioral adherence measure (electronic monitoring) during a 15-month period, including correction of dosing and TPMT activity, which has not been examined in previous research. Measurement of TPMT activity and dosing was included given earlier evidence that both factors could influence metabolite concentrations and hence metabolite profiles (Relling et al., 2011). It was hypothesized that patients who were identified as potentially nonadherent to 6MP based on results obtained using pharmacological measures of adherence (i.e., low levels of both metabolites) would also have the lowest behavioral adherence rates.
Methods
Study Design
This study was a secondary analysis of data that were collected as part of a prospective, multisite randomized controlled trial. Adherence and medical data were collected as part of the 15-month longitudinal study of a family-centered problem-solving intervention to promote medication adherence for pediatric cancer. This is the first description of prospective measurement patterns of behavioral adherence and pharmacological adherence measures. Baseline results of behavioral adherence monitoring were discussed in Rohan et al. (2015).
Participants
Participants were 139 patients, aged 7–19 years, diagnosed with ALL or LBL, and their primary caregivers, followed at six medical centers in the United States. Baseline demographic characteristics are provided in Table I. The four young adult patients (18 years: n = 3; 19 years: n = 1) lived with their primary caregivers at the time of study participation. Ethnicity was representative of each clinic’s sample. Institutional review boards at each site approved the study. Patients and their parents were compensated for their time and study participation at baseline, 6 months, and 15 months.
Table I.
Demographic and Medical Characteristics of Baseline Sample (M ± SD) or n (%)
| Demographic and Medical Characteristics | Entire sample (n = 139) | High TGN–low MMP (n = 26) | Low TGN–high MMP (n = 58) | Low TGN–low MMP (n = 55) |
|---|---|---|---|---|
| Patient’s age at baseline (years) | 12.29 years ± 3.44 | 11.55 ± 3.44 | 11.99 ± 3.42 | 12.95 ± 3.42 |
| Type of cancer diagnosis | ||||
| ALL | 133 (95.7) | 24 (92.3) | 56 (96.6) | 53 (96.4) |
| LBL | 6 (4.3) | 2 (7.7) | 2 (3.4) | 2 (3.6) |
| Duration of cancer diagnosis (years) | 1.29 years ± 0.35 | 1.28 years ± 0.39 | 1.29 years ± 0.36 | 1.30 years ± 0.32 |
| Child’s gender | ||||
| Male | 94 (67.6) | 18 (69.2) | 38 (65.5) | 38 (69.1) |
| Female | 45 (32.4) | 8 (30.8) | 20 (34.5) | 17 (30.9) |
| Child’s ethnicity/race | ||||
| Non-Hispanic, Caucasian | 75 (54.0) | 17 (65.4) | 30 (51.7) | 29 (52.7) |
| Non-Hispanic, Minority | 17 (12.3) | 3 (11.5) | 4 (6.9) | 9 (16.4) |
| Hispanic | 49 (33.9) | 6 (23.1) | 24 (41.4) | 17 (30.9) |
| Household composition | ||||
| One-caregiver household | 45 (32.4) | 6 (23.1) | 20 (34.5) | 19 (34.5) |
| Two-caregiver household | 94 (67.6) | 20 (76.9) | 38 (65.5) | 36 (65.5) |
| TPMT absolute value | 12.71 ± 3.61 | 10.06 ± 3.47 | 13.98 ± 3.51 | 12.66 ± 3.13 |
| TPMT genotype | ||||
| Heterozygote | 18 (14.4) | 12 (60) | 2 (3.8) | 4 (7.7) |
| Wild type | 107 (85.6) | 8 (40) | 51 (96.2) | 48 (92.3) |
Note. No significant differences between metabolite profiles on relevant demographic and medical characteristics. Heterozygous: present with intermediate levels of TPMT activity. Wild type present with normal or high TPMT activity.
Eligibility Criteria and Attrition Rates
To be eligible for study participation, participants needed to be prescribed a daily dosage of oral 6MP, diagnosed with ALL or LBL in remission, and in at least their second cycle of the maintenance phase of therapy. Participants were excluded from study participation (n = 7) if they were involved in foster care or did not have a primary caregiver available to participate (n = 2), or had known plans to relocate (n = 5). In accord with HIPAA guidelines, families were first contacted by their medical provider who assessed their willingness for study participation. If families approved, they were approached by study coordinators at each site to obtain parental permission and consent and written assent for patients aged ≥11 years. Verbal assent was obtained for patients <11 years. Of the 171 patients and families approached to participate, 18.7% (n = 32) refused participation: too busy (n = 12), not interested (n = 19), or no transportation (n = 1). Comparisons of families who participated in the study with those who did not indicated no differences (p’s > 0.05) with respect to patients’ age and gender. However, non-Hispanic, Caucasian families (n = 16, 9.4%) refused participation more frequently compared with Hispanic (n = 6, 3.5%) and non-Hispanic, minority (n = 10, 5.8%) families (V = 0.23; p = 0.01).
Twelve patients (8.6%) dropped out during the 15-month period for the following reasons: disease relapse (n = 9, 75%), maintenance therapy finished before study completion (n = 1, 8.3%), and relocation and transfer of care to another hospital (n = 2, 16.7%). Of these families, 8.3% (n = 1) completed baseline, 33.3% (n = 4) completed 3 months, 25% (n = 3) completed 6 months, 25% (n = 3) completed 9 months, and 8.3% (n = 1) completed 12 months. Comparisons of families who completed the study with those who did not indicated no differences (p’s > 0.05) with respect to patients’ cumulative behavioral adherence rates, age, and gender. On the other hand, Hispanic patients (n = 8) were dropped more frequently than non-Hispanic, Caucasian (n = 3) and non-Hispanic, minority patients (n = 1) (V = 0.22; p = 0.04).
Family-Centered Problem-Solving Intervention
Following the baseline study visit, patients and their primary caregivers were randomized in equal numbers to one of two groups using a stratified random permuted blocks scheme design: Family Problem-Solving Training Intervention (FPST) (n = 69) or Current Psychosocial Care (n = 70). The FPST intervention tested the efficacy of a family-centered intervention to address specific barriers to medication adherence that were commonly experienced by children and adolescents with cancer and their families, including enhancing adolescent–parent problem-solving strategies; facilitating parent–adolescent communication and collaboration; and using behavioral reinforcement to enhance problem-solving skills (Kato et al., 2008; Pai & Drotar, 2009). The intervention included five in-person visits and two phone visits.
Measures
Medical Characteristics and Prescribed Medical Treatment
Medical charts were reviewed at quarterly intervals from baseline to 15 months using standardized forms to obtain information regarding 6MP medication dosing and timing of administration. Prescribed medical treatment was standardized across all sites based on treatment protocols for ALL and LBL implemented by the Children’s Oncology Group.
Behavioral Medication Adherence Measures: Electronic Monitoring of 6MP
An electronic monitoring device (i.e., Medication Event Monitoring System [MEMS®] from the AARDEX Corporation) was used to monitor behavioral adherence to 6MP oral medication across 15 months. The electronic monitor is similar to a prescription bottle, but contains a micro-electronic chip in the cap that registers dates and times when the bottle is opened and closed. Patients and families were aware of adherence monitoring, but were not given feedback regarding their medication adherence. A standardized form was completed at quarterly intervals to capture information regarding extra openings, refills, and periods of nonuse during the previous 3-month period. Adherence was defined as the number of times that oral medication was taken as prescribed (Lau et al., 1998). Electronic monitoring of oral medication adherence has been used by a number of investigators in a range of pediatric chronic illnesses (Ingerski et al., 2011; Rapoff, 2010), including pediatric cancer (Bhatia et al., 2012; Lau et al., 1998; Rohan et al., 2013).
Pharmacological Measures of Medication Adherence: 6MP Metabolite Concentrations
Blood samples were obtained at six time points collected at quarterly intervals from baseline to 15 months. A validated high-performance liquid chromatography assay with ultraviolet detection was used to measure 6MP and concentrations of its two metabolites (TGN and MMP) in red blood cells (RBC) (Davies & Lilleyman, 1995; Dervieux & Boulieu, 1998; Traore et al., 2006). The use of RBC, TGN, and MMP concentrations was based on an extensive review of the extant literature on 6MP pharmacology and utility to detect 6MP (Davies & Lilleyman, 1995; Dervieux & Boulieu, 1998; Traore et al., 2006). Pharmacological measures of medication adherence have been used in previous research investigating adherence in pediatric ALL and LBL (Davies et al., 1993; Davies & Lilleyman, 1995; Hawwa et al., 2009; Lilleyman & Lennard, 1994; Traore et al., 2006). Metabolite levels at each time point, the last dosage amount, and the date and time of the last dose were mapped on and verified with electronic monitoring data.
TPMT Activity
TPMT is an enzyme that metabolizes 6MP into two active metabolites: MMP and TGN (Relling, 1999). TPMT activity is a genetic trait that is inherited from both biological parents (i.e., one allele from the mother and one allele from the father). TPMT activity can be described as the absolute values of TPMT activity; or as a genotype (e.g., homozygous deficient, heterozygous, or homozygous wild type). TPMT activity is an important measure in the present study because it can affect how a patient metabolizes 6MP, which could potentially influence TGN and MMP levels and hence the metabolite profile group membership for that patient. When patients with a homozygous deficient genotype are adherent to their 6MP medication, they will often present with extremely high concentrations of TGN metabolites and little to no MMP metabolites. Heterozygous patients taking medication as prescribed will often present with high concentrations of TGN metabolites and low levels of MMP metabolites. Homozygous/wild-type patients who are adherent to 6MP will express low levels of TGN and high levels of MMP if they are taking medication as prescribed. TPMT absolute values and TPMT genotypes were obtained for all patients who consented to genetic testing (n = 125) (Table I). Absolute values were calculated using the average TPMT value for the first and last blood draw (baseline and 15-month values for most patients). Differences in TPMT activity were examined across the metabolite clusters.
Data Analytic Plan
Baseline demographic and medical factors were examined to determine which variables, if any, should be included as covariates in analyses that examined group differences among adherence subgroups. Based on previous research (Bhatia et al., 2012; Davies & Lilleyman, 1995), the following covariates were examined: patient gender, patient age, patient ethnicity/race, single parent versus two-parent households, TPMT activity (genotype), Randomized Controlled Trial (RCT) assignment, and data collection site. Results of the RCT indicated that there were no significant differences in behavioral medication adherence and metabolite profiles between those patients who participated in the family-centered problem-solving intervention compared with those who received clinical care as usual (p = .12, d = 0.21). Thus, RCT assignment was not included as a covariate.
6-MP Metabolite Profiles From Baseline to 15 months
Longitudinal hierarchical two-step cluster analysis (Aldenderfer & Blashfield, 1984; Garson, 2010) was used to identify metabolite profiles for the two metabolites (TGN and MMP) of 6MP. The purpose of cluster analysis was to define mutually exclusive groups of individuals who had similar patterns of metabolite levels over time, which could reflect nonadherence or suboptimal therapy (e.g., low levels of both metabolites) or adherence to 6MP (i.e., high levels of one metabolite and low levels of another metabolite) (Aldenderfer & Blashfield, 1984; Davies & Lilleyman, 1995; Hawwa et al., 2009; Lennard et al., 1995; Traore et al., 2006). Metabolite profiles were based on each individual’s metabolite results from baseline to 15 months. Thus, it was possible for a patient to be in the same metabolite group or a different metabolite group over time depending on his/her TGN-MMP metabolite values at the time the blood draw was collected. Standardized z-scores for TGN and MMP were used as the unit of analysis rather than absolute scores because cluster analysis required commensurability (i.e., equal scale units) (Aldenderfer & Blashfield, 1984). Although Traore et al. (2006) and Hawwa et al. (2009) identified two additional groups (i.e., low TGN–very high MMP; very high TGN–low MMP), a three-group model was hypothesized in the current study given the small sample sizes and lack of diagnostic importance of these two groups to adherence: one group demonstrating low levels of both metabolites; and two groups demonstrating a negative correlation between the two metabolites: high TGN–low MMP and low TGN–high MMP (Lilleyman & Lennard, 1994).
The two-step cluster analysis first identified “pre-clusters” and then treated those “pre-clusters” as single cases in hierarchical cluster analysis (Garson, 2010). Cluster membership was determined by the cluster distances approach: between-groups differences were maximized and within-group differences were minimized to generate groups with similar metabolic profiles (Aldenderfer & Blashfield, 1984; Garson, 2010). The Bayesian information criterion (BIC) was used to determine the appropriate number of clusters, which was based on the lowest BIC (Aldenderfer & Blashfield, 1984; Garson, 2010). Patient age and ethnicity, household composition, randomized intervention status (intervention versus control), and site were examined across the three metabolite profiles to determine whether these variables should be included as covariates in the cluster analysis. There was a significant association between site and metabolite profile (p < 0.05), and site was used as a covariate in the cluster analysis. No other potential covariates were significant.
Relationship Between Behavioral and Pharmacological Measures of Adherence: Mean Differences in 5-Day Adherence Rates
The relationship between indirect behavioral measures and direct pharmacological measures of medication adherence was examined. It was hypothesized that there would be a moderate relationship between 6MP metabolites and behavioral adherence rates over time. The half-life of 6-TGN and 6-MMP is approximately five days following the medication dose (Fishman & Mrozek-Orlowsk, 1999). Thus, behavioral adherence rates were mapped on to the assay data and mean behavioral adherence rates were examined at 5 days before the date of the blood draw. It was hypothesized that those individuals who presented with low levels of both TGN and MMP metabolites would also have the lowest behavioral adherence rates across time compared with the other metabolite groups.
6MP Dosing Regimens and Relationship to Metabolite Clusters
Clinical guidelines suggest that patients who are treated with 6MP during the maintenance phase of treatment should be prescribed a target dose of 6MP to achieve maximum benefit (Relling et al., 2011; Relling, 1999). Patients may receive less than the target dose for a number of reasons including, but not limited to, reducing the frequency and type of adverse side effects. Thus, it is important to determine whether the low TGN–low MMP metabolite group reflected nonadherence to prescribed 6MP or reflected potentially suboptimal dosing. Patient’s prescribed 6MP dose was compared with his/her target 6MP dose. Based on clinical guidelines for 6MP dosing in pediatric cancer treatment, target dose was calculated by multiplying a standard dose of 65 mg by the patient’s body surface area (Relling et al., 2011; Relling, 1999). 6MP only comes in 50 mg tablets, thus 6MP target dose recommendations were compared with the prescribed dose to create three subgroups: 50 mg within target, 50 mg above target, and 50 mg below target.
Results
6-MP Metabolite Profiles From Baseline to 15 months
Consistent with hypotheses, a two-step longitudinal hierarchical cluster analysis indicated that a three-group model had the best fit based on the BIC criterion when controlling for site differences: (1) high TGN–low MMP metabolite profile (n = 113, 14.8%), (2) low TGN–high MMP metabolite profile (n = 340, 44.4%), and (3) low TGN–low MMP metabolite profile (n = 312, 40.8%). Table II provides the average TGN and MMP values for the metabolite profiles over time. As shown in Table II, TGN and MMP metabolite levels were relatively stable over time. Consistent with previous research (Davies & Lilleyman, 1995; Lennard et al., 1995), there is more variability in MMP metabolite values over time relative to TGN metabolite values.
Table II.
Descriptive Statistics for Metabolite Profiles: TGN and MMP Absolute Values and Dosing Recommendations (M ± SD), Range or n (%)
| Timepoint | High TGN - low MMP |
Low TGN - High MMP |
Low TGN - Low MMP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-TGN | 6-MMP | Within or above Recommendations | Below Recommendations | 6-TGN | 6-MMP | Within or above Recommendations | Below Recommendations | 6-TGN | 6-MMP | Within or above Recommendations | Below Recommendations | |
| Baseline | 1,386 ± 535 | 6,238 ± 5,143 | 23 (88.5) | 3 (11.5) | 585 ± 229 | 30,832 ± 10,756 | 58 (100) | 0 (0) | 381 ± 215 | 5,598 ± 4,939 | 44 (80) | 11 (20) |
| (860–3,233) | (0–18,609) | (232–1,482) | (15,027–74,494) | (0–811) | (0–17,755) | |||||||
| 3 months | 1,345 ± 536 | 5,100 ± 4,151 | 16 (94.1) | 1 (5.9) | 580 ± 218 | 30,100 ± 11,453 | 53 (98.1) | 1 (1.9) | 449 ± 218 | 6,206 ± 5,088 | 57 (91.9) | 5 (8.1) |
| (844–2,647) | (122–14,734) | (249–1,141) | (15,660–82,130) | (0–831) | (0–17,459) | |||||||
| 6 months | 1,292 ± 332 | 7,066 ± 6,098 | 18 (94.7) | 1 (5.3) | 535 ± 189 | 28,831 ± 10,248 | 59 (100) | 0 (0) | 437 ± 194 | 6,786 ± 5,212 | 49 (90.7) | 5 (9.3) |
| (881–2,248) | (116–23,707) | (253–1,102) | (15,356–65,568) | (0–794) | (0–17,380) | |||||||
| 9 months | 1,189 ± 367 | 6,578 ± 4,534 | 20 (100) | 0 (0) | 586 ± 236 | 32,821 ± 11,441 | 53 (98.1) | 1 (1.9) | 417 ± 203 | 7,709 ± 5,468 | 53 (93) | 4 (7.0) |
| (844–2,178) | (226–14,934) | (198–1,123) | (14,682–62,171) | (0–766) | (0–17,494) | |||||||
| 12 months | 1,175 ± 366 | 8,635 ± 8,854 | 17 (100) | 0 (0) | 562 ± 213 | 28,498 ± 9,868 | 62 (100) | 0 (0) | 414 ± 173 | 5,954 ± 4,655 | 35 (85.4) | 6 (14.6) |
| (846–1,957) | (230–30,263) | (209–1,008) | (16,199–60,967) | (115–775) | (123–17,187) | |||||||
| 15 months | 1,277 ± 484 | 4,388 ± 5,551 | 14 (100) | 0 (0) | 580 ± 175 | 30,301 ± 9,895 | 53 (100) | 0 (0) | 386 ± 198 | 5,234 ± 4,231 | 40 (93) | 3 (7) |
| (873–2,551) | (156–17,299) | (266–1,006) | (17,173–56,040) | (0–792) | (0–15,709) | |||||||
| Overall mean | 1284 ± 445 | 6,398 ± 5,850 | N/A | N/A | 571 ± 210 | 30,176 ± 10,628 | N/A | N/A | 416 ± 202 | 6,307 ± 5,020 | N/A | N/A |
| (844–3,233) | (0–30,263) | (198–1,482) | (14,682–82,130) | (0–831) | (0–17,755) | |||||||
Relationship Between Behavioral and Pharmacological Measures of Adherence
Differences in 5-day behavioral adherence rates were examined for the three metabolite profiles using repeated measures mixed models (SAS Proc Mixed). Overall group differences were examined, in addition to differences in 5-day adherence rates when controlling for dosing and/or TPMT. Significant differences were examined using appropriate post hoc comparisons (Tukey’s HSD or independent t-tests). Results are provided in Tables III and IV.
Five-Day Behavioral Adherence Rates (Not Controlling for TPMT or Dosing)
As expected, there was a significant difference in 5-day behavioral adherence rates between the three metabolite clusters (p = .008). Post hoc comparisons indicated mean differences in 5-day adherence rates at 6, 9, and 12 months. Those in the low TGN–high MMP metabolite profile consistently demonstrated higher behavioral adherence rates compared with the low TGN–low MMP group. There were no other significant differences (Table III). On the other hand, behavioral adherence rates for the three metabolite profiles did not significantly change over time and remained relatively stable (p = .85) with adherence rates ranging between 72% and 78% for the low TGN–low MMP metabolite profile compared with rates of 85% to 90% for the low TGN–high MMP profile. The high TGN–low MMP metabolite group had adherence rates of 86% to 89% from 3 to 12 months, dropping to 72% adherence at 15 months. There was not a significant interaction between metabolite cluster and time (p = .68).
Table III.
Results of Mixed Effects Modeling (Not Controlling for TPMT): 5-Day Behavioral Adherence Rates by Metabolite Cluster (M ± SD, n)
| Cluster 1 | Cluster 2 | Cluster 3 | Overall |
1 versus 2 |
1 versus 3 |
2 versus 3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | ES | p | ES | p | ES | p | ES | ||||
| Overall sample | |||||||||||
| Metabolite profiles (between groups) | 4.96** | 0.07 | |||||||||
| 3 months | 88 ± 31 (16) | 85 ± 31 (47) | 78 ± 34 (53) | 0.70 | 0.01 | 0.95 | 0.09 | 0.60 | 0.27 | 0.61 | 0.19 |
| 6 months | 86 ± 31 (17) | 89 ± 26 (54) | 74 ± 34 (47) | 3.09* | 0.05 | 0.95 | 0.10 | 0.34 | 0.36 | 0.04* | 0.49 |
| 9 months | 88 ± 27 (17) | 90 ± 19 (45) | 73 ± 35 (49) | 4.72** | 0.08 | 0.97 | 0.09 | 0.14 | 0.46 | 0.01** | 0.60 |
| 12 months | 89 ± 30 (14) | 88 ± 24 (51) | 72 ± 36 (35) | 3.52* | 0.07 | 0.99 | 0.01 | 0.18 | 0.49 | 0.04* | 0.56 |
| 15 months | 72 ± 40 (12) | 90 ± 24 (45) | 78 ± 34 (33) | 2.54 | 0.05 | 0.15 | 0.66 | 0.80 | 0.19 | 0.20 | 0.42 |
| Time (within groups) | 0.35 | 0.01 | |||||||||
| Metabolite profile*time | 0.72 | 0.02 | |||||||||
| Controlling for 6MP dose | |||||||||||
| Metabolite profiles (between groups) | 4.35** | 0.06 | |||||||||
| 3 months | 87 ± 32 (15) | 84 ± 31 (46) | 78 ± 34 (50) | 0.57 | 0.01 | 0.97 | 0.01 | 0.67 | 0.24 | 0.65 | 0.18 |
| 6 months | 85 ± 31 (16) | 89 ± 26 (54) | 71 ± 34 (43) | 4.18* | 0.07 | 0.91 | 0.13 | 0.26 | 0.41 | 0.02* | 0.59 |
| 9 months | 88 ± 27 (17) | 91 ± 19 (44) | 73 ± 35 (47) | 4.82** | 0.08 | 0.94 | 0.13 | 0.15 | 0.45 | 0.01** | 0.62 |
| 12 months | 89 ± 30 (14) | 88 ± 24 (51) | 71 ± 35 (29) | 3.64* | 0.07 | 0.99 | 0.01 | 0.15 | 0.52 | 0.03* | 0.60 |
| 15 months | 72 ± 40 (12) | 90 ± 24 (45) | 77 ± 35 (30) | 2.69 | 0.06 | 0.15 | 0.66 | 0.87 | 0.14 | 0.16 | 0.45 |
| Time (within groups) | 0.62 | 0.02 | |||||||||
| Metabolite profile*time | 0.88 | 0.05 | |||||||||
Note. **p < .01; *p < .05; Effect sizes (ES) for F tests are partial eta squared (η2p) and those for t-tests are Cohen’s d.
Behavioral Adherence Rates (Controlling for TPMT Activity)
Previous research suggests that patients with a high TGN–low MMP metabolite profile may have deficient or intermediate levels of TPMT activity and could have trouble metabolizing 6MP (Relling et al., 2011). In the current sample, 60% of patients in the high TGN–low MMP profile were identified as having intermediate levels of TPMT activity (Table I, heterozygotes). Given potential difficulties with metabolizing 6MP, patients in the high TGN–low MMP metabolite profile (n = 26) and heterozygote TPMT patients in the other profiles (n = 6) were omitted from analysis. As expected, there was a significant difference in 5-day adherence rates between the low TGN–low MMP metabolite profile and the low TGN–high MMP profile (p = 0.003). Independent sample t-tests indicated that patients in the low TGN–low MMP profile demonstrated lower behavioral adherence rates across time relative to the low TGN–high MMP profile at 6, 9, 12, and 15 months. There were no other significant differences (Table IV). On the other hand, behavioral adherence rates did not significantly change over time and remained relatively stable even when controlling for TPMT activity (p = .80) with adherence rates ranging from 70% to 76% for the low TGN–low MMP profile compared with rates of 84% to 90% for the low TGN–high MMP profile. There was no significant interaction between metabolite cluster and time (p = .47).
Table IV.
Results of Mixed Effects Modeling (Controlling for TPMT): 5-Day Behavioral Adherence Rates by Metabolite Cluster (M ± SD, n)
| Cluster 2 | Cluster 3 | 2 versus 3 |
||
|---|---|---|---|---|
| F, t | ES | |||
| Overall sample | ||||
| Metabolite profiles (between groups) | 9.25** | 0.09 | ||
| 3 months | 84 ± 31 (40) | 76 ± 36 (45) | 1.02 | 0.22 |
| 6 months | 88 ± 26 (50) | 70 ± 34 (39) | 2.74** | 0.59 |
| 9 months | 89 ± 20 (39) | 74 ± 34 (45) | 2.30* | 0.50 |
| 12 months | 88 ± 25 (48) | 70 ± 35 (29) | 2.65** | 0.62 |
| 15 months | 90 ± 24 (43) | 74 ± 36 (29) | 2.24* | 0.55 |
| Time (within groups) | 0.41 | 0.02 | ||
| Metabolite profile*time | 0.86 | 0.03 | ||
| Controlling for 6MP dose | ||||
| Metabolite profiles (between groups) | 6.99** | 0.07 | ||
| 3 months | 84 ± 31 (40) | 76 ± 37 (42) | 1.03 | 0.23 |
| 6 months | 88 ± 26 (50) | 69 ± 34 (38) | 2.85** | 0.61 |
| 9 months | 89 ± 20 (38) | 75 ± 34 (43) | 2.35* | 0.52 |
| 12 months | 88 ± 25 (48) | 70 ± 34 (24) | 2.63** | 0.66 |
| 15 months | 90 ± 24 (43) | 72 ± 37 (24) | 2.43* | 0.62 |
| Time (within groups) | 0.23 | 0.01 | ||
| Metabolite profile*time | 0.88 | 0.04 | ||
Note. **p < .01; *p < .05; Effect sizes (ES) for F tests are partial eta squared (η2p) and those for t-tests are Cohen’s d.
Behavioral Adherence Rates (Controlling for Dosing)
To determine whether the low TGN–low MMP metabolite group reflected nonadherence to 6MP or reflected suboptimal dosing, a patient’s prescribed 6MP dose was compared with his/her target 6MP dose based on pediatric guidelines for 6MP dosing (Relling et al., 2011; Relling, 1999). Table II provides the percentage of patients who received 6MP dosing within 50 mg of target, 50 mg above target, or 50 mg below target dose recommendations. The majority of patients across the three metabolite profiles were prescribed a dosage of 6MP that was within the recommended dose from baseline to 15 months. To control for patients with suboptimal dosing, those patients who received 6MP doses below standardized dose recommendations were removed from analysis. Patients who were prescribed 6MP within or above target recommendations were included.
As expected, there was a significant difference in 5-day adherence rates between the three metabolite profiles even when controlling for dose (p = .01). Post hoc comparisons indicated differences in 5-day adherence rates at 6, 9, and 12 months. Those in the low TGN–high MMP metabolite profile consistently demonstrated higher behavioral adherence rates compared with the low TGN–low MMP group. There were no other significant differences (Table III). On the other hand, behavioral adherence rates for the three metabolite profiles did not significantly change over time and remained relatively stable even when controlling for dose (p = .65). Adherence rates for the low TGN–low MMP metabolite profile ranged from 71% to 78% compared with rates of 84% to 91% for the low TGN–high MMP profile across 15 months, and rates of 85% to 89% from 3 to 12 months for the high TGN–low MMP profile dropping to 72% at 15 months. There was no significant interaction between metabolite cluster and time (p = .53).
Behavioral Adherence Rates (Controlling for Dosing and TPMT Activity)
To determine whether similar findings were observed when controlling for both dosing and TPMT activity, differences in behavioral adherence rates from 3 to 15 months were also examined for the low TGN–low MMP and the low TGN–high MMP profiles (excluding heterozygote patients given differences in how 6MP is metabolized). As expected, there was a significant difference in 5-day adherence rates between the three metabolite profiles even when controlling for dose and TPMT (p = .01). Independent sample t-tests indicated patients in the low TGN–low MMP metabolite profile had significantly lower behavioral adherence rates relative to the low TGN–high MMP profile at 6, 9, 12, and 15 months. There were no other significant group differences. See Table IV. On the other hand, behavioral adherence rates for the three metabolite profiles did not significantly change over time and remained relatively stable even when controlling for dose and TPMT (p = .92) with adherence rates ranging from 69 to 76% for the low TGN–low MMP profile compared with rates of 84–90% for the low TGN–high MMP profile across the 15-month period. There was no significant interaction between metabolite cluster and time (p = .47).
Clinical Relevance of Metabolite Clusters and Behavioral Adherence
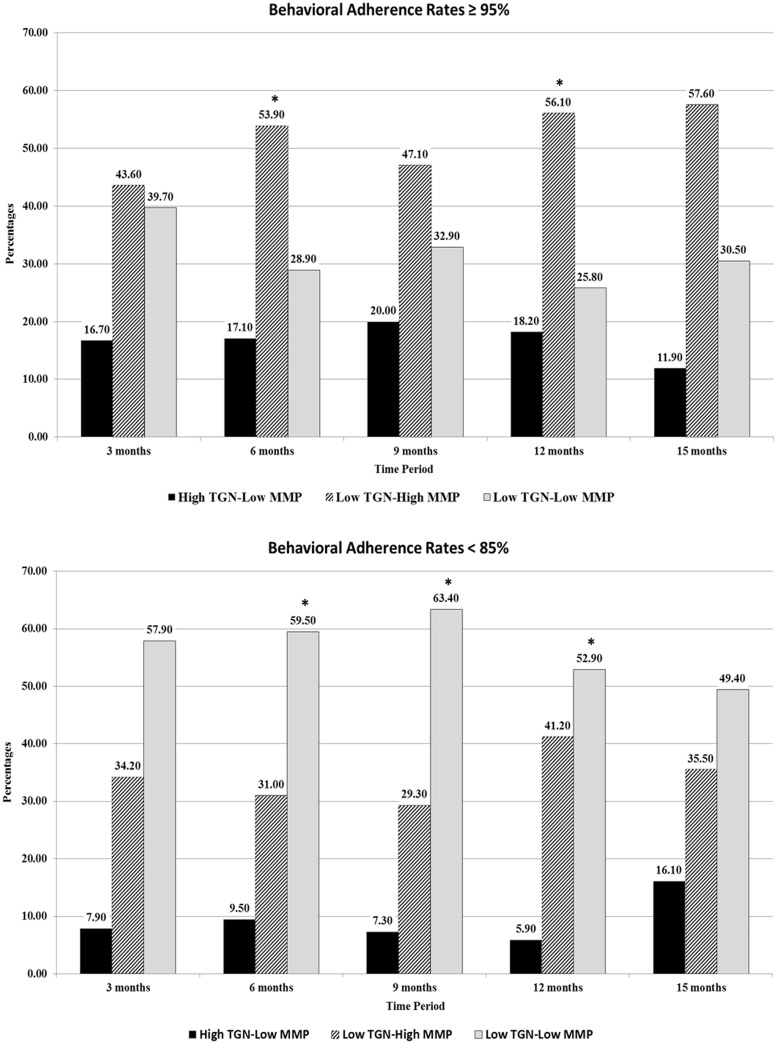
Bhatia et al. (2012) found that behavioral adherence rates<95% were associated with an increased risk for disease relapse. Across the 15-month period, patients in the three metabolite profiles had average 5-day adherence rates either >95% or <85% (see Figure 1 ). As shown, the majority of patients within the low TGN–high MMP group and the high TGN–low MMP group demonstrated adherence rates >95% across the 15-month period with the exception of the high TGN–low MMP group at 15 months. The majority of patients with low levels of both metabolites had adherence levels below 85%.
Figure 1.
Percentages of patients within each metabolite cluster with 5-day adherence rates ≥95% versus those with adherence rates <85%. Note. *Denotes significant differences (p < .05) between the three metabolite group profiles.
Discussion
To our knowledge, the current study is the first to validate the prospective relationship between an indirect measure of 6MP behavioral adherence (i.e., daily 6MP adherence using electronic monitors) and a direct measure of 6MP pharmacological adherence (i.e., metabolites of 6-mercaptopruine) during the maintenance phase of cancer treatment for pediatric ALL and LBL. Examination of pharmacological measures of medication adherence using hierarchical cluster analysis indicated three distinct metabolite profiles across 15 months. The percentage of patients within each of the metabolite profiles and the absolute levels of the TGN and MMP metabolites were relatively consistent with previous research (Hawwa et al., 2009; Traore et al., 2006). A major strength of the present study was the use of longitudinal hierarchical two-step cluster analysis to generate profiles of mutually exclusive groups of individuals who have similar patterns of 6MP metabolite levels across a 15-month period. Longitudinal hierarchical two-step cluster analysis was used in the present study rather than cross-sectional or iterative profile classifications given a patient could have similar TGN and MMP results over time, and hence, profiles should be classified based on TGN and MMP levels across the entire study duration rather than at each time point.
The primary contribution of the current study was the validation of metabolite profiles with behavioral measures of adherence (electronic monitoring). Those in the low TGN–low MMP group consistently had lower behavioral adherence rates relative to the other metabolite profiles, which suggests that the low TGN–low MMP group is indicative of nonadherence to 6MP. To determine whether 6MP dosing or TPMT genetic traits impacted the relationship between metabolite levels and behavioral adherence, we controlled for TPMT activity and dosing. Even when controlling for TPMT activity and 6MP dosing, the low TGN–low MMP group continued to have lower behavioral adherence rates over time. On the other hand, there were no significant differences in behavioral adherence between the high TGN–low MMP and the low TGN–high MMP group. These findings suggest that these two groups likely represent better adherence to 6MP with mean 5-day behavioral adherence rates >85%, which is consistent with previous research (Davies, Lennard & Lilleyman, 1993; Davies & Lilleyman, 1995). It is notable that those in the low TGN–high MMP profile demonstrated adherence rates between 84% and 91% across the entire 15-month period even when controlling for dosing and TPMT, whereas those in the high TGN–low MMP profile had behavioral adherence rates of 84–89% from 3 to 12 months, which dropped to 72% at 15 months. Although previous research (Davies & Lilleyman, 1995; Hawwa et al., 2009; Lennard et al., 1995; Traore et al., 2006) identified both the high TGN–low MMP and the low TGN–high MMP profiles as being indicative of adherence to 6MP, future research should further investigate these profiles, given the decreased behavioral adherence rates at 15 months for the high TGN–low MMP profile. Although the present study did not identify significant differences in behavioral adherence between the three groups at 3 months even after controlling for TPMT and dosing, or at 15 months before controlling for TPMT, it is notable that the low TGN–low MMP profile had adherence rates 10% lower than the other two groups at both periods. The overall differences in the behavioral adherence rates between the high TGN–low MMP and low TGN–high MMP groups versus the low TGN–low MMP group suggest that adherence behaviors do influence metabolite levels and hence exposure to medication (Kenna et al., 2005).
Bhatia et al. (2012) noted that adherence rates of ≥95% are a protective factor for relapse. In the present study, close to 50% or more of patients in the low TGN–high MMP group demonstrated 5-day behavioral adherence rates of ≥95% across the 15-month period, while over half of the patients in the low TGN–low MMP group demonstrated adherence rates <85% across the 15-month period, putting these patients at an increased risk for relapse (Bhatia et al., 2012). Our findings support the importance of monitoring adherence to 6MP using objective measures of medication adherence during treatment given the known relationship of adherence to disease remission. Future research should investigate the relationship between pharmacological and behavioral measures of 6MP and their relationship to clinical outcomes, including disease relapse, health-care use, and clinical biomarkers.
Another relevant contribution of this study was the measurement and description of TPMT activity in analyses of metabolite levels of 6MP. It is well known that pediatric cancer patients with TPMT deficiencies (intermediate or low/absent TPMT) will have difficulty metabolizing 6MP, which could influence metabolite levels and health outcomes (Davies & Lilleyman, 1995; Relling et al., 2011). In the present study, the majority of patients with the heterozygous TPMT genotype were in the high TGN–low MMP metabolite group (67%). Furthermore, even after controlling for 6MP dosing, the metabolite group with low levels of TGN and MMP continued to demonstrate lower behavioral adherence rates. These findings provide further support that low levels of both TGN and MMP metabolites cannot be explained by metabolic differences and are likely indicative of nonadherence or poor bioavailability of the medication (Davies & Lilleyman, 1995). This is particularly important for evaluating results obtained in clinical trials and ensuring therapeutic efficacy of chemotherapy medications in pediatric cancer (Kenna et al., 2005).
Several limitations should be considered when interpreting these findings and developing future research. It is notable that seven patients of Hispanic ethnicity were dropped from the study owing to disease relapse, which is consistent with previous research conducted by Bhatia et al. (2012) who noted higher rates of disease relapse for pediatric patients of Hispanic ethnicity. Future studies need to be cross-validated in a heterogeneous age range (infancy to young adulthood) in a larger sample of pediatric cancer patients with greater ethnic diversity.
Furthermore, it is unknown whether 6MP adherence generalizes to other chemotherapy medications. It will be important to assess adherence across multiple medications during the entire duration of maintenance to determine treatment burden and its relationship to adherence. Finally, future studies should describe extensive PK modeling over time for a pediatric sample of patients diagnosed with ALL or LBL to inform clinical care and optimize dosing regimens.
Previous research has discussed the benefits and drawbacks from using objective measures of medication adherence, including the potentially high costs associated with using these measures (Drotar & Rohan, 2013; Ingerski et al., 2011; Riekert & Rand, 2002; Rohan et al., 2013). The advantages of using these measures in an effort to reduce morbidity, mortality, and health-care use owing to nonadherence far exceeds the costs of these objective measures. The findings presented in the current study suggested that using direct and indirect measures of medication adherence can provide an effective method for identifying patients who are in need of intensive adherence promotion intervention. Using behavioral and pharmacological measures of medication adherence at the onset of treatment could assist with identification of patients who have nonadherence patterns that negatively impact their clinical outcomes. Intensive adherence promotion interventions delivered early in treatment could significantly improve the health outcomes of these patients, and hence decrease their risk for disease relapse. Interventions such as those described in Kato et al. (2008) in which patients played a videogame to promote adherence to 6MP and other medications have been shown to be effective. Moreover, our experience suggests that a multidisciplinary team of providers can use principles of anticipatory guidance during medical follow up of ALL and LBL in an effort to prevent adherence problems from occurring during treatment (e.g., education concerning importance of adherence, to identify potential barriers to adherence, and to follow up on adherence promotion efforts) (Pai & Drotar, 2009).
Another potential use of the ongoing measurement of behavioral and pharmacological adherence measures of 6MP over the course of pediatric cancer treatment includes preventing the medical toxicity associated with undetected nonadherence to ALL and LBL treatment. In some instances, nonadherence to prescribed medications could be misinterpreted by a treating physician as poor absorption of the medication. In turn, the physician may prescribe a higher medication dose, which could result in significant drug toxicity if patients resume taking the medication at this higher dose (Davies & Lilleyman, 1995; Hawwa et al., 2009; Lau et al., 1998; Traore et al., 2006). Assessment of behavioral and pharmacological adherence measures could assist clinicians with 6MP dose recommendations, significantly reducing the risk for adverse events associated with drug toxicity. Finally, using both behavioral and pharmacological measures of medication adherence in clinical care not only identifies patients with chronic or moderate nonadherence, but also serves as a mechanism for identifying patients who are not receiving therapeutic doses of 6MP.
The findings in the present study provide strong evidence for the importance of using both indirect and direct objective measures of medication adherence, not only in the management of pediatric cancer but also in other pediatric chronic illness populations. Self-reported adherence rates are often inaccurate and provide overinflated representations of individual behavior. On the other hand, objective measures, such as electronic monitoring, provide real-time information about adherence patterns over time (e.g., daily, weekly, monthly, and overall adherence rates; information about dose timing to inform therapeutic efficacy; and information about prolonged periods where medication was not used by the patient). Metabolite levels are equally important because although electronic monitoring provides information regarding daily medication use, it does not provide us with information about whether medication was ingested by the patient or whether the dosing is therapeutically effective. The development and implementation of objective measures of adherence in routine care for pediatric chronic illness, specifically pediatric cancer, provide an important area to develop innovative adherence promotion interventions in an effort to prevent disease relapse and reduce health-care costs associated with disease morbidity and mortality (Bhatia et al., 2012; Rohan et al., 2013).
Acknowledgments
The efforts of children, adolescents, mothers, fathers, and health-care providers who gave their time and energy to this work are gratefully acknowledged. Data collection and data management of this study were facilitated by a talented group of research assistants and fellows: Andrea Perry, Leanue Bolo, Megan DeRosier, Matthew Maley, Megan Miller, Claire Peterson, Katharina March, Octavio Zavala, Brenda Quinonez, Karla Castillo, Joanna Cohen, Leela Jackson, Dailyn Martinez, Gabriela Reed, Jaime Crowley, Lauren Smith, Kate Sargeant, Chelsea Howe, Alina Vaisleib, Jennifer Ing, and Daphne Papadopoulos. The authors greatly appreciate the assistance of several undergraduate student research assistants who assisted with the processing and cleaning of data. Finally, special acknowledgement is given to Eric D. Kodish, MD, Department of Bioethics, Cleveland Clinic Foundation, Lenner College of Medicine for his support of the early development of this line of work.
Funding
The work reported in this article was funded by the National Cancer Institute at the National Institutes of Health (grant numbers 1F31CA168307 to J.M.R., 1R01CA119162 to D.D.). This work was also supported by the National Center for Research Resources (grant number UL1RR024134 to M.A.) and the National Center for Advancing Translational Sciences (grant number UL1TR000003 to M.A.).
Conflicts of interest: None declared.
References
- Aldenderfer M. S. Blashfield R. K. (1984). Cluster analysis (Vol. 44). Beverly Hills, CA: Sage Publications, Inc. [Google Scholar]
- Bhatia S. Landier W. Shangguan M. Hageman L. Schaible A. N. Carter A. R. Hanby C. L. Leisenring W. Yasui Y. Kornegay N. M. Mascarenhas L. Ritchey A. K. Casillas J. N. Dickens D. S. Meza J. Carroll W. L. Relling M. V. Wong F. L. (2012). Nonadherence to oral mercaptopurine and risk of relapse in hispanic and non-hispanic white children with acute lymphoblastic leukemia: A report from the children’s oncology group. Journal of Clinical Oncology , 30, 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. A. Lennard L. Lilleyman J. S. (1993). Variable mercaptopurine metabolism in children with leukaemia: a problem of non-compliance?. British Medical Journal , 306, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. A. Lilleyman J. S. (1995). Compliance with oral chemotherapy in childhood lymphoblastic leukaemia. Cancer Treatment Reviews , 21, 93–103. [DOI] [PubMed] [Google Scholar]
- Dervieux T. Boulieu R. (1998). Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clinical Chemistry , 44, 551–555. [PubMed] [Google Scholar]
- Drotar D. Rohan J. M. (2013). Pediatric Adherence and Health Behavior Change. In L. R. Martin & M. R. DiMatteo (Eds.), The Oxford Handbook of Health Communication, Behavior Change, and Treatment Adherence (pp. 387-407). New York, NY: Oxford University Press.
- Fishman M. Mrozek-Orlowsk M. (1999). Cancer chemotherapy guidelines and recommendations for practice. Pittsburgh, PA: Oncology Nursing Press. [Google Scholar]
- Garson G. D. (2010). Cluster analysis statnotes: Topics in multivariate analysis. Retrieved January 29, 2010. [Google Scholar]
- Hawwa A. F. Millership J. S. Collier P. S. McCarthy A. Dempsey S. Cairns C. McElnay J. C. (2009). The devlopment of an objective methodology to measure medication adherence to oral thiopurines in paediatric patients with acute lymphoblastic leukaemia: An exploratory study. European Journal of Clinical Pharmacology , 65, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Ingerski L. M. Hente E. A. Modi A. C. Hommel K. A. (2011). Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. Journal of Pediatrics , 159, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato P. M. Cole S. W. Bradlyn A. S. Pollock B. H. (2008). A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics , 122, e305–e317. [DOI] [PubMed] [Google Scholar]
- Kenna L. A. Labbé L. Barrett J. S. Pfister M. (2005). Modeling and simulation of adherence: Approaches and applications in therapeutics. The AAPS Journal , 7, E390–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau R. C. W. Matsui D. Greensberg M. Koren G. (1998). Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Medical and Pediatric Oncology , 30, 85–90. [DOI] [PubMed] [Google Scholar]
- Lennard L. Welch J. Lilleyman J. (1995). Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: A possible indicator of non-compliance?. British Journal of Cancer , 72, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleyman J. S. Lennard L. (1994). Mercaptopurine matabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet , 343, 1188–1190. [DOI] [PubMed] [Google Scholar]
- Pai A. L. H. Drotar D. (2009). Medication adherence in pediatric oncology. In Kazak A. E., Kupst M. J., Pao M., Patenaude A. F., Wiener L. (Eds.), Quick reference for oncology clinicians: The psychiatric and psychological dimensions of cancer symptom management (pp. 90–96). Charlottesville, VA: IPOS Press. [Google Scholar]
- Rapoff M. A. (2010). Adherence to pediatric medical regimens (2nd ed.). Springer New York Dordrecht Heidelberg London: Springer Science + Business Media LLC. [Google Scholar]
- Relling M. Gardner E. Sandborn W. Schmiegelow K. Pui C. Yee S. Stein C. M. Carrillo M. Evans W. E. Klein T. ; Clinical Pharmacogenetics Implementation Consortium. (2011). Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clinical Pharmacology and Therapeutics , 89, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling M. V. Hancock M. L. Boyett J. M. Pui C. H. Evans W. E. (1999). Prognostic importnace of 6-Mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood , 93, 2817–2823. [PubMed] [Google Scholar]
- Riekert K. A. Rand C. (2002). Electronic monitoring of adherence: When is high-tech the best?. Journal Of Clinical Psychology in Medical Settings , 9, 25–34. [Google Scholar]
- Rohan J. M. Drotar D. Alderfer M. Donewar C. W. Ewing L. Katz E. R. Muriel A. (2015). Electronic monitoring of medication adherence in early maintenance phase treatment for pediatric leukemia and lymphoma: Identifying patterns of nonadherence. Journal of Pediatric Psychology, 40(1), 75–84. doi: 10.1093/jpepsy/jst093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore F. O’Riordan M. A. Myers C. Groth K. Hoff A. Angiolillo A. Rheingold S. Drotar D. Kodish E. (2006). How low is too low? Use of cluster analysis to define low levels of mercaptopurine metabolites. Pediatric Blood and Cancer , 46, 187–192. [DOI] [PubMed] [Google Scholar]