Origins
Two decades ago, if you were to ask a scientist what it would mean to regenerate the heart, you would regularly hear about the power of developmental biology in decoding the intricacy of organogenesis and the plasticity of stem cells recognized as nature’s ultimate ‘building blocks’. The conversation would then delve into the merits and drawbacks of embryonic stem cell technology, presented as the quintessential regenerative phenotype.
With an ever-broader scholarly engagement and a growing public awareness, the academic intrigue of stem cell-based therapy was exponentially fuelled by the practicality of mining adult stem cell reservoirs out of bone marrow and adipose tissue. Universally, this multinational transdisciplinary endeavour captured the imagination of patients and physicians/scientists alike, while recognizing the ensuing medical, ethical and societal opportunities, and potential risks.
The prospect of pioneering a change in disease management propelled this maturing field from science fiction to the rigor of the scientific bench and onwards to randomized clinical trials. Here, the tantalizing concept of rebuilding the body to reverse underlying pathology, as opposed to a battle to palliate disease, emerged as a paradigm shift. In parallel, the notion of a curative intervention held the promise of altering the economics of chronic disease management, relieving the health care system of the cost burden associated with an ageing population vulnerable to degenerative disease.
Reflection
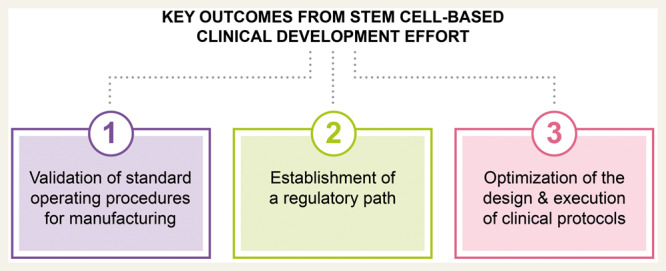
To date, the key outcomes from the collective cardiovascular clinical development effort have been:
validation of standard operating procedures needed for stem cell deri vation and manufacturing;
establishment of a regulatory path including the FDA ‘Expedited Programs for Regenerative Medicine Therapies for Serious Conditions’; and
optimization of the design and execution of clinical protocols for cell- based therapy.
Accordingly, the safety and feasibility of applying adult stem cell therapy to patients with cardiovascular disease have been repeatedly attained and independently documented. Mechanistic insights into how stem cells behave and influence the heart are also increasingly elucidated. With enrolment of patients into larger clinical trials, stratification criteria for selection of patient populations most likely to benefit has become progressively clearer. However, used as an investigational new drug stem cells in practice departed from traditional pharmacodynamics, introducing an increased complexity in the therapeutic paradigm. Moreover, even with efforts to ensure potency, purity, and homogeneity, dose-to-dose variance in outcomes precluded standardized regimens amenable for early adoption.
As we approach 2020, and despite significant clinical experience with thousands of patients treated globally, there is still no specific cell type registered definitively for use in cardiovascular practice.
Journey
The odyssey to achieve reproducible cardiac regeneration in a clinically meaningful manner is reminiscent of the efforts to develop vaccines for cancer. The notion that the immune system can be sensitized to eliminate cancer from the body was heralded for decades as the Holy Grail in oncology, elevating the armamentarium of care with introduction of a ‘fourth modality’, beyond the traditional triad of chemotherapy, surgery and radiation. Yet, in initial clinical applications, promising pre-clinical data did not materialize, yielding little reduction in tumour load and failure to prevent metastatic progression. Destined to remain on the side-lines of care, cancer immunotherapy did eventually attain dramatic breakthroughs. Catalysed by robust public/private partnerships, immunotherapies today are transforming daily oncological practice.
Blueprint
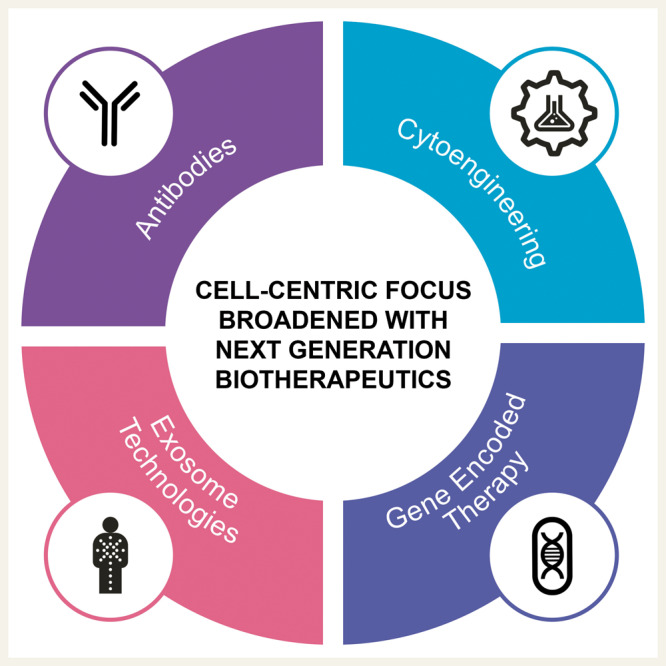
Highlighted by targeted antibodies and engineered chimeric antigen receptor (CAR) T-cell therapy, the era of biologics-based treatment is realized as a revolutionary advance in managing select refractory cancers. For the cardiac regeneration space, the playbook gathered from decades of trial and error in immunotherapy can in turn serve as a progressive blueprint towards realizing novel cardiovascular solutions. The culture of establishing the molecular basis underpinning clinical outcomes provides a remarkable inroad to identify the determinants of prospective benefit, and thus creates an informed framework to drive the success of novel therapies. If this iterative tactic holds true for improved cardiovascular disease management, the current cell-centred focus will likely be broadened to encompass next generation advances in the science of biotherapeutics, including antibodies, cytoengineering, gene encoded therapy, and exosome technologies.
Fitness
Although naïve T-cells are not primed to treat cancer, CAR engineering allows for a curative result. Likewise, stem cells utilized as a reparative measure in the heart, show among others, anti-inflammatory and provasculogenic properties, which are serendipitously relied upon to achieve regenerative outcomes. Use of emergent molecular platforms, exemplified by gene encoded technology and exosome science, creates a therapeutic lens enabling to zoom-in on the actionable target of therapy.
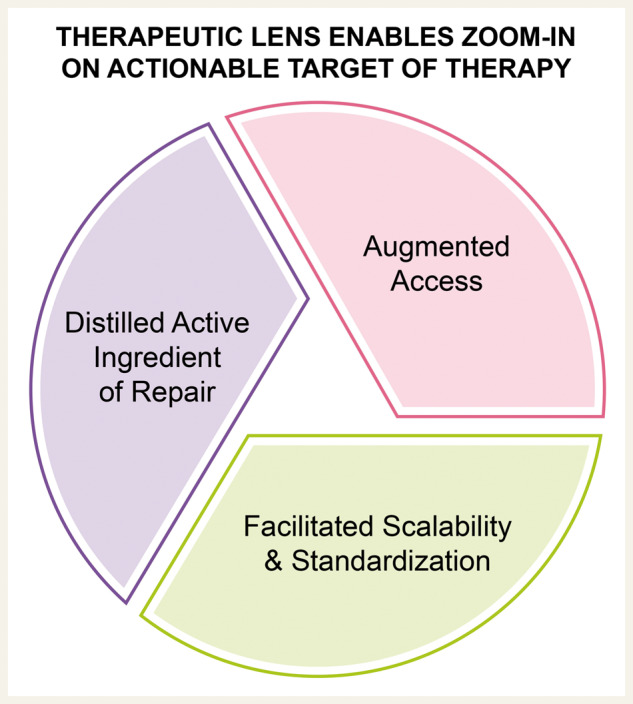
Mounting clinical experience in cardiovascular regeneration streamlines the mechanistic understanding of benefit to distil the active ingredient of repair. Scientific, scalability and standardization barriers, which at present have marred progress and exposed scepticism, are poised to be breached, thanks to an expanding knowledge base and adoption of the increasingly precise, target-costumed toolkit that provides a new level of regenerative fitness.
Imagine
Imagine a potassium channel selective antibody that can restore heart rhythm, while avoiding the long-term hazards of amiodarone. Or, a cell engineered to specifically secrete endothelial nitric oxide synthase, hence enhancing microvascular efficiency. How about tissue engineering for scar revision leveraging induced pluripotent stem cell-derived cardiac scaffolds? Or, gene encoded therapy for discriminate reversal of genetic lesions causing cardiomyopathies in no option patients or in order to postpone organ transplantation in others.
Furthermore, a refined understanding of cell-to-cell communication has emerged with the exploration of extracellular vesicles, often referred to as exosomes. Use of derived exosomes has the potential to harness salient features driving benefit from cell therapy, while concentrating the resolved active ingredient to endow flexibility of dosing biopotency in a ready-to-use product. If successfully derived and formulated, exosome-based therapies would eliminate the need for ensuring cell survival, freeze–thaw issues and could overall simplify the skillset and infrastructure needed in the application of biologics-based therapies.
If non-cellular biologics are realized as an off-the-shelf platform, suddenly therapy at the time of acute syndromes, such as a heart attack or stroke, would become a feasible indication for point-of-care regenerative medicine not necessarily limited to tertiary care centres.
Thus, milestones achieved over the 20-year odyssey of cardiac regeneration have brought us ever closer to the prospect of reaching a ‘therapeutic Ithaca’ with adoption of advances in biologic modalities championing a wave of synergistic innovation to complete this journey aimed at the wellbeing of patients.
Access
As cardiology gradually adopts therapeutic capabilities stemming from biologics, there is an added opportunity not only to achieve the full potential of disruptive therapies, but also to evade major costs inherent to latest generation technologies. With introduction of biologics in the oncology space, a reset in the norms of how much can be charged for a dose of a transformative or curative therapy has arisen. One may ask, if organ transplant to save a life costs in excess of $500 000, why cannot a cure for cancer or heart failure be as costly?
The approach to scientific advance is often achieved in the laboratory without a conscious decision or the know-how necessary to design cost-effective processes that would limit manufacturing and delivery costs. The objective of the discovery process is to prove a paradigm, not necessarily figure out how to achieve scale-up or proper dosing. Yet, both components are critical in advancing practice utilization. And if successful and exciting, the momentum of getting the technology ready for the clinic typically impedes post hoc introduction of cost efficiency and seamless delivery.
Although a technology can save lives in its current state, it is important to also ask how many lives can it save. A very large global population suffers from cardiovascular disease accounting for one-third, or nearly 20 million of all deaths worldwide. A large portion responsible for this staggering epidemiology occurs in emerging economies, where individuals in the most productive years of their lives suffer from debilitating heart attacks or strokes. Thus, in order to be impactful, not only does the next decade of efforts in cardiac regeneration need to overcome efficacy hurdles of the past; but also, there is a pressing need to build and apply technologies that fiscally and logistically are realistic on a global scale ensuring both validity and utility.
Horizon
To safeguard from healthcare disparities, it behoves the whole community of practice to utilize discovery not only as an engine to achieve translational and ultimately therapeutic success—but importantly, to embed innovation stringency as a means to break or prevent socioeconomic divides, augment access and level the playing field of care. In principle, biotherapies manufactured and delivered at a low cost of goods, as a room temperature stable product, would provide the unique ability to offer a generation of therapies across diverse environments and even for emerging nations.
If we could advance far enough where the need for a living therapeutic entity is no longer a pre-requisite to achieve regeneration, then suddenly new horizons of innovation and implementation would open-up. Achieving cell-free biologics to feature the ease of use and uniform benefit is an exciting evolution towards regeneration for all.
Conflict of interest: Mayo Clinic, A.B. and A.T. have interests in Rion LLC.




