Abstract
BACKGROUND
Vagal nerve stimulation (VNS) is an approved treatment for epilepsy and depression. Wrapping the helical electrodes around the nerve can prove technically challenging. However, a quick and efficient method to slightly elevate the nerve can highly facilitate this part of the procedure.
OBJECTIVE
To provide useful surgical tips to facilitate the procedure.
METHODS
Based on experience of more than 150 adult cases for mainly epilepsy (primary lead implant), the authors share their surgical technique to provide the experienced surgeons or newcomers to the field of VNS with some useful tips. All patients signed informed consent according to the local ethics committee guidelines.
RESULTS
The article consists of a detailed step-by-step description of the whole procedure illustrated through high-resolution colored photographs of the surgical field. Special reference is made to the usefulness of polyvinyl alcohol (PVA) sponge cubes to elevate the nerve instead of the commonly used silicon vessel loops.
CONCLUSION
The use of surgical microscope and PVA sponge cubes to elevate the nerve constitute key points to make VNS an easy surgery.
Keywords: Vagal nerve stimulation, Step-by-step, Technique, Wrapping, Operative
ABBREVIATIONS
- PVA
polyvinyl alcohol
- SCM
sternocleidomastoid muscle
- VNS
Vagal nerve stimulation
Vagal nerve stimulation (VNS) is well established for pharmacoresistant epilepsy.1 Significant reduction in seizure frequency has been reported in randomized controlled trials, multiple open-label studies, and systematic reviews.2-4 Quality of life has been also shown to be dramatically improved5-7 with associated positive impacts on mood, vigilance, and cognitive function.8-10 In July 2005, VNS was approved for chronic and recurrent depression.19,20 Recent attempts to use VNS for headaches,21,22 Crohn disease or disorders of consciousness18 have also provided promising insights.23
Mechanisms of action of VNS have yet to be fully understood. There is evidence that part of the antidepressant and antiepileptic effects are mediated by the locus coeruleus and noradrenergic system.16,17 With hypersynchrony representing a cardinal feature of epileptic seizures, the desynchronizing effect of VNS has been postulated as a possible upstream mechanism of action of VNS.11-13 It has been demonstrated that epileptic patients that respond to VNS tend to display reduced interictal and ictal cortical synchronicity both on scalp EEG14 and on direct intracerebral recordings.15
A comprehensive review of the literature with respect to the surgical technique yields relatively few articles, and published high-resolution photographs of the surgical field are lacking (Table 1).22,25,27,28,30,31,34-40,42-47 Most existing literature focuses on replacement, revision, and removal of the lead or Implantable Pulse Generators, but few articles or book chapters thoroughly describe the surgical procedure.27,28
TABLE 1.
Main Articles and Book Chapters Referring to VNS Surgical Techniques
| Author | Journal | Title | Summary of article content | Method used to elevate the nerve | High Quality Photographs |
|---|---|---|---|---|---|
| Lozano27 | Text Book | Textbook of Stereotactic and Functional Neurosurgery | Step-by-Step description | Plastic sheet | No |
| Patil28 | Surgical Neurology | Single incision for implanting a vagal nerve stimulator system (VNSS): technical note. | A single incision is an alternate to the double incision procedure. | Not specified | X |
| Bauman30 | Neurosurgery | Subpectoral implantation of the vagus nerve stimulator. | Subpectoral implantation increased coverage, cosmesis, durability and resistance to infection. | Not specified | X |
| Espinosa34 | Surgical Neurology | Revision and removal of stimulating electrodes following long-term therapy with the vagus nerve stimulator. | Safe removal of electrode even after several years | Not specified | No |
| DeGiorgio35 | Vagus Nerve Stimul | Surgical anatomy, implantation technique, and operative complications. | Technical complications | Not specified | X |
| McGregor36 | Epilepsia | Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. | Feasibility of right sided VNS | Not specified | X |
| Tronnier31 | Prog Neurol Surg | Vagus Nerve Stimulation: Surgical Technique and Complications | Step by step illustration | Elevation by loop | Yes |
| Le37 | Pediatric Neurosurgery | Interscapular placement of a vagal nerve stimulator for prevention of wound tampering. Technical note. | Interscapular placement of the stimulator with lower risk of infection | Not specified | X |
| Bruce38 | Epilepsia | Neuro-Cybernetic prosthesis (NCP) system for the treatment of refractory partial seizures: surgical technique and outcomes. | Notes | Not specified | X |
| Dionigi37 | Epilepsia | Vagus nerve stimulation for standardized monitoring: technical notes for conventional and endoscopic thyroidectomy. | Critical view of safety of the VN stimulation with or without dissection. | Not specified | X |
| Aalbers40 | Acta Neurochirurgica | Vagus nerve stimulation lead removal or replacement: surgical technique, institutional experience, and literature overview. | Complete removal or replacement of the VNS system including lead and coils is feasible and safe | Not specified | No |
| Robbins24 | Interdisciplinary Neurosurgery | Novel implantation of vagus nerve stimulator AspireSR pulse generator: Technical note, | Heartbeat detection procedure. | Not specified | X |
| Nakano41 | Interdisciplinary Neurosurgery | Cosmetic procedure for vagus nerve stimulation, | Cosmotic step for the inside of sternocleidmastoid and back side of omohyoid muscle anchoring of cable tie-down | Not specified | No |
| Maniker42 | Surgical Neurology | Positioning of vagal nerve stimulators: technical note, | Changing the implant position help to prevent deactivation in the MRI | Not specified | No |
| Ralston43 | Journal of neurosurgery Pediatrics | In Situ repair of vagus nerve stimulator lead damage: technical note. | Lead in Situ repair | Not specified | Yes |
| O’Neill44 | Neurosurgery | Revision of vagal nerve stimulator electrodes through a posterior cervical triangle approach: technical note. | Posterior cervical triangle approach | Not specified | X |
| MacDonald45 | Acta Neurochirurgica | Revision of vagal nerve stimulator electrodes: technical approach. | Electrode can be removed from the vagus nerve and repositioned without significant consequence. | Not specified | X |
| Ortler46 | Journal of neurosurgery Pediatrics | Complete removal of vagus nerve stimulator generator and electrodes: technical note. | Safe removal | Not specified | X |
| Ng47 | J Int Soc Pediatr Neurosurg | Revision of Vagal Nerve Stimulation (VNS) Electrodes: Review and Report on Use of Ultra-Sharp Monopolar Tip | Ultra-sharp monopolar tip for safe dissection and removal of the electrode from the vagus nerve. | Not specified | X |
| Schneider25 | Surgical Neurology | Implantation of a new Vagus Nerve Stimulation (VNS) Therapy(R) generator, AspireSR(R): considerations and recommendations during implantation and replacement surgery–comparison to a traditional system. | Considerations and recommendations | Not specified | X |
| Giordano48 | Epilepsia | Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity | Indications, technique, outcome and complications | Not specified | No |
“X” mark refers to unavailability.
Herein, the authors based on their cohort of more than 150 cases for epilepsy describe the whole procedure step-by-step (Table 2 provides the demographic data of the cohort along with main postoperative complications). Particular attention is paid to some key points such as the use of polyvinyl alcohol (PVA) sponge cubes, inserted below the nerve at both ends, as a new alternative method to facilitate the wrapping step. The optional use of the operative microscope will also be discussed. For illustration, photographs of the surgical field and a video of the key steps are provided. Local ethics committee gave approval for the study.
TABLE 2.
Demographic Data and Main Postoperative Complications of the Cohort
| Mean age in years (min-max) | 37.4 (18.6-72.7) |
|---|---|
| Number of patients | 155 |
| Sex ratio M (%)/F (%) | 76 (49%)/79 (51%) |
| VNS indication (Epilepsy/Depression) | 150/5 |
| Postoperative dysphonia (number/percentage) | 3 (1.9%) |
| Wound infection | 1 (0.6%) |
| Deep infection | 0 (0%) |
| Cervical hematoma | 2 (1.2%) one immediate, one delayed (6 d postop) |
METHODS
Installation
The patient is positioned, as for a classical cervical anterior discectomy, in dorsal decubitus with slight head hyperextension.
Incisions
Two lines are marked with a sterile skin marker after rigorous antiseptic preparation. One is located at the middle cervical region starting from the midline to the anterior border of the sternocleidomastoid muscle (SCM) along Langer skin lines29 for the approach to the left vagus nerve and the other incision is made in the subclavicular area. Some surgeons resort to a single transverse incision.28 As several authors, we favor making 2 separate incisions. In our opinion, a single incision is not advisable on several grounds (large incision and subsequent scar, limited latitude as to where the generator is inserted, potential technical difficulties in anchoring the stimulator).
Subclavicular Pocket
The first step consists of preparing the pocket in which to lodge the stimulator. The model (model 102 or 106) requires a tailored dissected pocket size. A quick dissection readily leads to the aponeurosis of the great pectoral muscle. In very thin patients, we recommend going below the aponeurosis or even below the muscle as reported by Baumam et al.30 Then, we dissect cranially and medially towards the middle cervical region and above the clavicle to create a track to facilitate the tunneling step of the electrode later in the procedure.
Vagus Nerve Dissection
After skin incision, the platysma muscle is longitudinally divided, then the superficial cervical fascia is opened. By passing anterior and deep to SCM, the carotid sheath can be identified and opened. One of the key notions is that the vagus nerve in most cases is located posteriorly within the carotid sheath, which then must be widely opened and dissected. The vagus nerve is then located in a deep and posterior position between the common carotid artery medially and internal jugular laterally (Figure 1).
FIGURE 1.
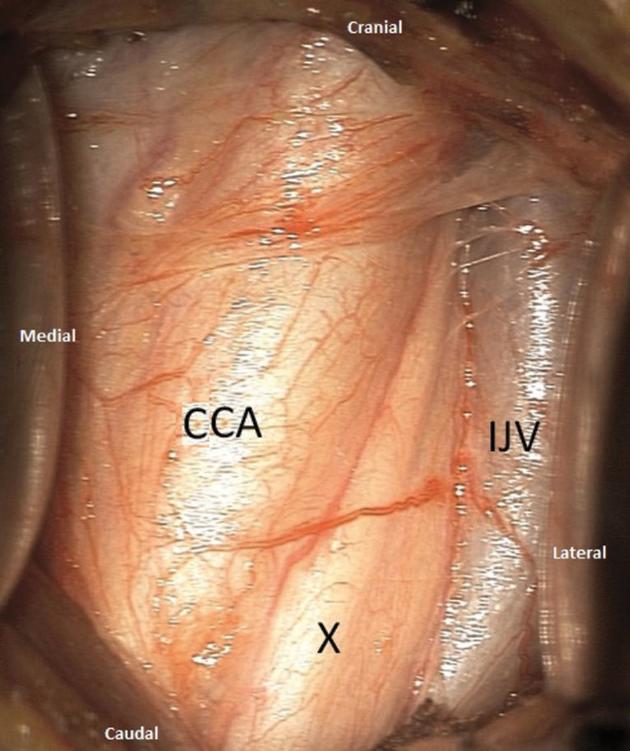
Intraoperative photograph at the beginning of the dissection showing the posterior position of the nerve (X) between the common carotid artery, medially, and the internal jugular vein, laterally.
During the approach to the vagus nerve, some technical difficulties may be encountered even by the experienced neurosurgeon. Care must be taken not to take an unusually anteriorly situated vagal nerve in the retractor before having seen it. In some patients, the approach to the carotid sheath can be complicated by the presence of large venous branches of the jugular vein. In most cases, it is possible to pass below or above those veins or dissect them so that they can be more easily retracted. In some instances, they need to be ligated or coagulated and divided. The surgeon should not be prevented from having enough space to work around the nerve. Big lymph nodes or omohyoid muscle may sometimes need to be resected or divided in order to provide enough room. The wrapping step may be rendered more difficult by insufficient dissection of the structures surrounding the nerve.
The ansa cervicalis of the 12th cranial nerve may be mistaken for the vagus nerve because of its course along the axis of both the carotid artery and internal jugular, but it is always superficial to the carotid sheath.
Once correctly identified, the surgeon must endeavor to completely free the nerve from any surrounding connective tissue over a minimal length of 4 cm (Figure 2). Failure to do so may result in remaining bridges of connective tissue and adhesions, with the risk of rendering the wrapping step more difficult. However, an overly extensive dissection may lead to compromise of the vascular supply of the nerve and could cause postoperative dysphonia.
FIGURE 2.
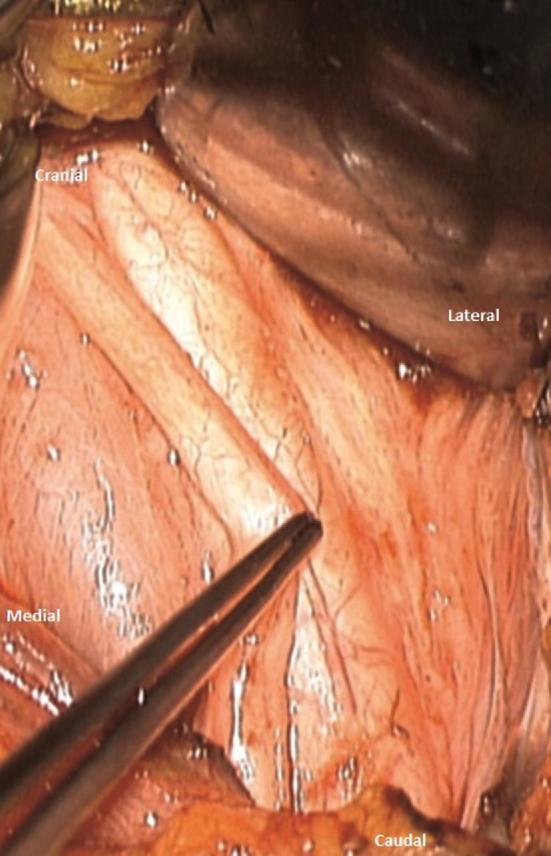
Intraoperative photographs showing the careful dissection of the vagal nerve with gentle microsurgical handling.
Microsurgical tools and a gentle microsurgical technique should be employed. Manipulation of the nerve carries the risk of significant damage. It is possible to pinch the epineurium (Figure 2). Bipolar coagulation or suction should be avoided in the close vicinity of the nerve. In our experience, the use of the optical magnification for this step proves helpful.
Tunneling of the Electrode
We suggest that this step be done before wrapping the electrode around the nerve. Performing this step after wrapping of the electrode may involve unwanted traction upon the nerve during the process of tunneling. Indeed, the tunneling step implies the use of some force to penetrate subcutaneous soft tissue and fascia, which may be transmitted to the nerve and even pull out a previously properly wrapped electrode.
Wrapping of the Electrode
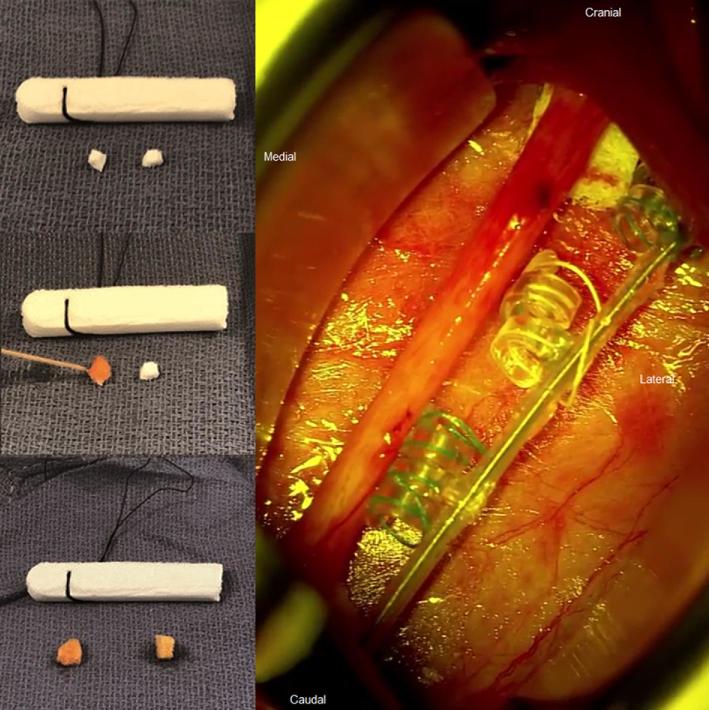
The wrapping step is often regarded as the most challenging. The nerve must have been sufficiently freed from its environment. We advise starting with the anchor tether and then continuing with the 2 helical electrodes. For this step, several advantages can be achieved by optical magnification. One of the key notions is first to properly position the retractors in order to obtain sufficient room to work around the nerve. Another crucial point is the need for the nerve to be elevated in order to easily work below and above it as needed. To do so, some surgeons use silicone vessel loops.31 Instead, we prefer to insert small cubes of PVA hemostatic sponge, such as Merocel nasal dressing (Medtronic Inc, Minneapolis, Minnesota) below both ends of the nerve (Figure 3).
FIGURE 3.
Photograph showing the preparation of small PVA sponges cubes from Merocel nasal dressing (upper left), inflation of cubes after irrigation with a few drops of Rifadin saline (middle and lower left), and the intraoperative positioning of the cubes below both ends of the nerve to elevate it (right).
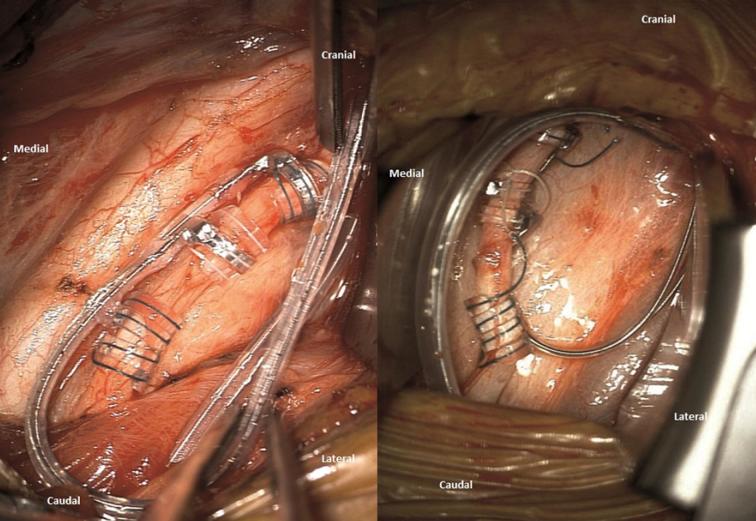
Once the nerve has been dissected over a length of at least 4 cm, the retractors are positioned and the operative microscope is focused on the surgical field. The electrode should not be stuck below a retractor and not have been tunneled too high up in relation to the plane of the nerve. This might result in upward traction on the nerve given the intrinsic rigidity of the lead (PerenniaFLEX® Model 304, Livanova Inc). A small piece of PVA sponge cube (4 by 4 mm) is inserted below both ends of the nerve with microsurgical forceps (Video). Then the sponges are humidified with a few drops of Rifadin-tinted sterile isotonic fluid (rifampicin, Sanofi Aventis France). After humidification the sponge pieces will swell up and gently lift up the nerve. The purpose of using 2 sponges located below both ends of the nerve is to elevate the nerve in the middle and by doing so provide room to manipulate the lead coils easily around the nerve. The surgeon must pass alternatively above and below the nerve with a little traction to be exerted on the small green and white threads. In our experience, it is best to take the threads quite proximally from the plastic part to unfold and stretch them efficiently. Once the anchor tether has been placed, it should be able to slide easily along the nerve. In order to be at ease, a gentle traction upon the lead exerted at the level of the subclavicular pocket will allow the anchor tether to slide distally so that the first helical electrode appears in the middle of the field where the nerve is best elevated. Nothing in the field will be an obstacle to the manipulation of the coils. If required, the same maneuvercan be repeated for the second helical electrode. The wrapping procedure takes about 3 to 4 min. The cubes must be removed as soon as all coils are properly positioned around the nerve. At the end, a picture showing the proper positioning is taken and kept in the patient's medical records (Figure 4).
FIGURE 4.
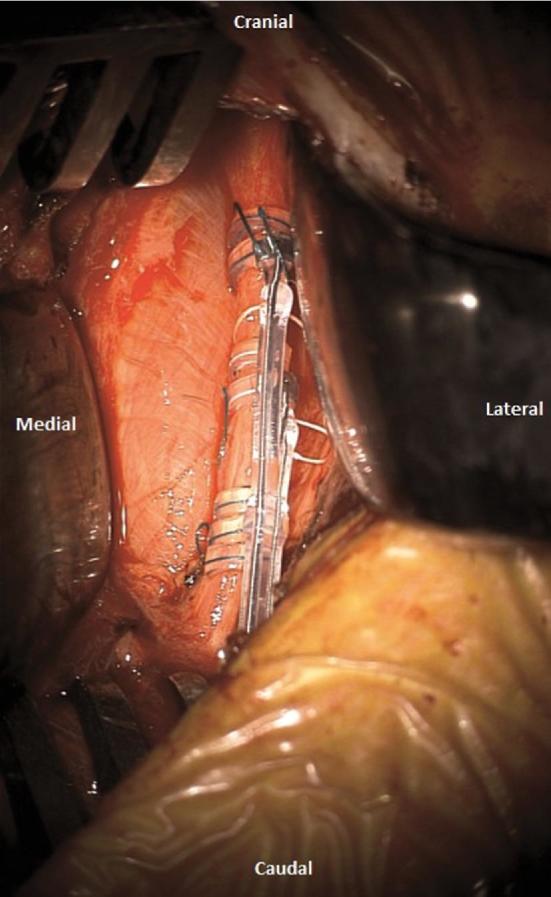
Intraoperative photographs after completion of the wrapping step, showing the proper positioning of the anchor tether and the 2 helical electrodes.
Security Loop
A so-called security loop, also known as strain-relief bend, should be made. This is critical to prevent the risk of pulling out the electrode when connecting the electrode at the level of the subclavicular pocket (Figure 5).
FIGURE 5.
Final intraoperative photograph showing the security loop before closure. All Intraoperative Photographs were taken by OPMI® PENTERO® 900 from Zeiss Operating Microscope (Carl Zeiss Surgicals Inc, Jena, Germany).
Securing the Electrode to the SCM
One additional step, recommended by Livanova (Cyberonics Co Ltd, Houston, Texas), is to secure the lead to the SCM with the help of 2 additional white silicone pieces.
Stimulator Insertion, Connection, and Impedance Testing
The lead pin must be completely inserted before screwing to secure the connection. Then we advise anchoring the stimulator to the musculoaponeurotic tissue with the help of a single nonresorbable suture. Impedance testing should then be performed. The anesthesiologist should be warned of the possible occurrence of bradycardia, reported incidence of which is between 0.1% and 2.7%.32,33 In the event of bradycardia, this does not preclude VNS implantation, but warrants caution when switching on the stimulator, which should be done with cardiac monitoring, using specific VNS parameters (small amplitude and pulse width).
Any contraction of cervical muscles during impedance testing is indicative of a problem. For responsive VNS devices, the check of heart rate detection is performed in order to verify that the device reliably detects heart rate (compare with OR monitor) before closure.26
Closure
The hemostasis should be meticulously checked. Uncontrolled bleeding may lead to a life-threatening obstructive cervical hematoma. For the prevention of hardware infection, we recommend washing the operative field several times with sterile isotonic fluid or Rifadin-diluted saline. The closure is classically made depending on operator's preference.
DISCUSSION
Significant progress has been made in widening the spectrum of VNS indications in the field of nonpharmacological modulation of brain activity. In experienced hands, VNS is a fairly quick and easy procedure to perform. However, wrapping the electrode around the nerve is often regarded as the most tedious or challenging step. The suggestions described here may be helpful not only for newcomers, but also for more advanced neurosurgeons. We emphasize 2 points of particular importance: the use of the operative microscope and our method of elevating the nerve with PVA sponge cubes.
Surgical Microscope
The use of the operating microscope is not mandatory to perform VNS surgery but, in our view, it is valuable for at least three main reasons. Firstly, it increases the comfort of the surgeon (anatomical structures are better seen and identified with magnification), and the wrapping step becomes significantly easier. Secondly, all personnel in the room can see what is occurring, and in a university hospital setting, teaching is facilitated. Thirdly, it is mandatory for us in case of removal or replacement of the electrode because of the fibrosis that surrounds the nerve in the carotid sheath. It may be argued that the microscope is time-consuming, but in our experience, the surgeon's comfort should be prioritized, and the time consumed by bringing the microscope is largely inferior to the amount of time gained by the consequent rapidity of the wrapping step.
Elevating the Nerve with PVA Sponge Cubes
As previously mentioned, many neurosurgeons regard wrapping as challenging or tedious. We think that, in reality, it can become very easy to perform by simply adhering to some basic rules. First of all, dissection of the nerve should be performed completely and over a sufficient distance to be at ease. Even if the manufacturer states > 3 cm, it is not erroneous to dissect the nerve slightly more. We suggest freeing the nerve over a distance of around 4 cm (as shown in Figure 3E) for ease of surgical approach. A second crucial point to work readily around the nerve is to elevate it efficiently. Many surgeons use vessel loops to lift the nerve up from the tissue bed instead of PVA sponge cubes. Their use carries the risk of exerting excessive traction upon the nerve with potential subsequent dysphonia. It is not easy with this method to measure out the force of traction that will be tolerated. Our method has the advantage of gently elevating the nerve without any risk of excessive traction (the elevation of the nerve occurring passively during the swelling process of the PVA sponge cubes). Moreover, the recourse to vessel loops requires additional hands or forceps to hold them in the surgical field. With PVA sponge cubes, once in place, there is nothing other than the nerve and the lead with the coils and anchor tether as shown in video. This saves time, involves less manipulation, and takes up limited space in the operative field. Some surgeons consider that vessel loops also prove useful during the preparatory action of freeing the nerve from surrounding connective tissue and that it is, therefore, logical to use them for elevation for the next step. We tend to favor freeing the nerve with gentle microdissection technique following the approach of the nerve. Another advantage of PVA sponge cubes is that their swelling after humidification creates a smooth, progressive elevation of the nerve that proves sufficient to place the coils. It is atraumatic and will not exert too much traction upon both ends of the nerve. The cubes, serving as cushions, are quick to position and provide up to 10 to 15 mm of elevation. Additionally, they are not expensive. PVA sponges have been used for years mainly for hemostasis of nasal bleeding and, to our knowledge, without report of any biocompatibility issues. We have not encountered any complication specifically related to PVA sponge cubes. Because of theoretical risk of complications (increased infection risks or foreign body granulomatous reaction) that might occur if the PVA sponges are left in place, specific attention should be paid not to leaving them in situ. Taking a final picture at the end of the wrapping step after the removal of both PVA sponges is thus advisable.
Based on our experience, we are inclined to think that our technical contribution does translate into optimized clinical results (particularly less dysphonia). Our rate of postoperative dysphonia was found to be as low as 1.93% in the last 155 patients, which tends to compare favorably with the data reported in the literature (lower limit of range). Indeed, in series of more than 100 patients, the rate of dysphonia appears variable but was around 3.8% (1.4% and 5.6%).48-50 In our opinion, it is indeed very unlikely that using surgical microscope and PVA sponge cubes prove more harmful to the nerve than other technical alternatives.
CONCLUSION
VNS is a possible option in epilepsy surgery. The development of responsive VNS devices offering the automatic triggering of stimulation upon detection of ictal tachycardia will renew interest in the technique. The technical suggestions provided here will hopefully be helpful to newcomers and provide a detailed surgical guide. By adhering to the main principles and suggestions that are described in this article, VNS implantation can remain an easy and uncomplicated procedure.
Disclosures
This work has been carried out within the FHU EPINEXT, with the support of the A*MIDEX project (ANR-11-IDEX-0001-02), funded by the “Investissements d’Avenir” French Government program managed by the French National Research Agency (ANR). Cyberonics and Livanova provided some financial and material support to Dr Carron for participation in scientific meetings. Dr Carron has received honoraria from Livanova for sharing his expertise in VNS surgery. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Krahl SE. Vagus nerve stimulation for epilepsy: a review of the peripheral mechanisms. Surg Neurol Int. 2012;3(Suppl 1):S47-S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(s2):S40-S43. [DOI] [PubMed] [Google Scholar]
- 3. Privitera MD, Welty TE, Ficker DM, Welge J. Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev. 2002;(1):CD002896 https://www.ncbi.nlm.nih.gov/pubmed/11869641. [DOI] [PubMed] [Google Scholar]
- 4. Uthman BM, Wilder BJ, Penry JK et al.. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43(7):1338-1338. [DOI] [PubMed] [Google Scholar]
- 5. Englot DJ, Hassnain KH, Rolston JD, Harward SC, Sinha SR, Haglund MM. Quality-of-life metrics with vagus nerve stimulation for epilepsy from provider survey data. Epilepsy Behav. 2017 Jan;66:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mikati MA, Ataya NF, El-Ferezli JC et al.. Quality of life after vagal nerve stimulator insertion. Epileptic Disord. 2009;11(1):67-74. [DOI] [PubMed] [Google Scholar]
- 7. Ryvlin P, Gilliam FG, Nguyen DK et al.. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: The PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014;55(6):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schachter SC. Vagus nerve stimulation: mood and cognitive effects. Epilepsy Behav. 2004;5(Suppl 1):56-59. [DOI] [PubMed] [Google Scholar]
- 9. Klinkenberg S, Majoie HJM, van der Heijden MM, Rijkers K, Leenen L, Aldenkamp AP. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clin Neurol Neurosurg. 2012;114(4):336-340. [DOI] [PubMed] [Google Scholar]
- 10. Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord. 2017;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zanchetti A, Wang SC, Moruzzi G. The effect of vagal afferent stimulation on the EEG pattern of the cat. Electroencephalogr Clin Neurophysiol. 1952;4(3):357-361. [DOI] [PubMed] [Google Scholar]
- 12. Marrosu F, Santoni F, Puligheddu M et al.. Increase in 20–50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clin Neurophysiol. 2005;116(9):2026-2036. [DOI] [PubMed] [Google Scholar]
- 13. Jaseja H. EEG-desynchronization as the major mechanism of anti-epileptic action of vagal nerve stimulation in patients with intractable seizures: Clinical neurophysiological evidence. Med Hypotheses. 2010;74(5):855-856. [DOI] [PubMed] [Google Scholar]
- 14. Bodin C, Aubert S, Daquin G et al.. Responders to vagus nerve stimulation (VNS) in refractory epilepsy have reduced interictal cortical synchronicity on scalp EEG. Epilepsy Res. 2015 Jul;113:98-103. [DOI] [PubMed] [Google Scholar]
- 15. Bartolomei F, Bonini F, Vidal E et al.. How does vagal nerve stimulation (VNS) change EEG brain functional connectivity? Epilepsy Res. 2016 Oct;126:141-146. [DOI] [PubMed] [Google Scholar]
- 16. De Taeye L, Vonck K, van Bochove M et al.. P3 event-related potential is a biomarker for the efficacy of vagus nerve stimulation in patients with epilepsy. Neurotherapeutics. 2014;11(3):612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimonprez A, Raedt R, Portelli J et al.. The antidepressant-like effect of vagus nerve stimulation is mediated through the locus coeruleus. J Psychiatr Res. 2015 Sep;68:1-7. [DOI] [PubMed] [Google Scholar]
- 18. Corazzol M, Lio G, Lefevre A et al.. Restoring consciousness with vagus nerve stimulation. Curr Biol CB. 2017;27(18):R994-R996. [DOI] [PubMed] [Google Scholar]
- 19. Daban C, Martinez-Aran A, Cruz N, Vieta E. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15. [DOI] [PubMed] [Google Scholar]
- 20. Martin JLR, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155. [DOI] [PubMed] [Google Scholar]
- 21. Yuan H, Silberstein SD. Vagus nerve stimulation and headache. Headache. 2017;57(Suppl 1):29-33. [DOI] [PubMed] [Google Scholar]
- 22. Mosqueira AJ, López-Manzanares L, Canneti B et al.. [Vagus nerve stimulation in patients with migraine]. Rev Neurol. 2013;57(2):57-63. [PubMed] [Google Scholar]
- 23. Bonaz B, Sinniger V, Hoffmann D et al.. Chronic vagus nerve stimulation in Crohn's disease: A 6-month follow-up pilot study. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2016;28(6):948-953. [DOI] [PubMed] [Google Scholar]
- 24. Robbins JW, Lacy J, Puccioni M. Novel implantation of vagus nerve stimulator AspireSR pulse generator: technical note. Interdiscip Neurosurg. 2017;10:20-23. [Google Scholar]
- 25. Schneider UC, Bohlmann K, Vajkoczy P, Straub H-B. Implantation of a new Vagus Nerve Stimulation (VNS) Therapy® generator, AspireSR®: Considerations and recommendations during implantation and replacement surgery–comparison to a traditional system. Acta Neurochir. 2015;157(4):721-728. [DOI] [PubMed] [Google Scholar]
- 26. Fisher RS, Afra P, Macken M et al.. Automatic vagus nerve stimulation triggered by ictal tachycardia: Clinical outcomes and device performance-The U.S. E-37 trial. Neuromodulation. 2016;19(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amar AP, Elder B, Apuzzo M. Vagal nerve stimulation for seizures. In: Textbook of Stereotactic and Functional Neurosurgery. Lozano H, Andres M, Gildenberg, Philip L, Tasker, Ronald R; 2009:2802-22. [Google Scholar]
- 28. Patil A, Chand A, Andrews R. Single incision for implanting a vagal nerve stimulator system (VNSS): technical note. Surg Neurol. 2001;55(2):103-105. [DOI] [PubMed] [Google Scholar]
- 29. Langer K. On the anatomy and physiology of the skin: I. The cleavability of the cutis. Br J Plast Surg. 1978;31(1):3-8. [PubMed] [Google Scholar]
- 30. Bauman JA, Ridgway EB, Devinsky O, Doyle WK. Subpectoral implantation of the vagus nerve stimulator. Neurosurgery. 2006;58(4 Suppl 2):ONS-322-325; discussion ONS-325-326. [DOI] [PubMed] [Google Scholar]
- 31. Tronnier VM. Vagus nerve stimulation: Surgical technique and complications. Prog Neurol Surg. 2015;29:29-38. https://www.ncbi.nlm.nih.gov/pubmed/26393499. [DOI] [PubMed] [Google Scholar]
- 32. Ardesch JJ, Buschman HPJ, van der Burgh PH, Wagener-Schimmel LJJC, van der Aa HE, Hageman G. Cardiac responses of vagus nerve stimulation: Intraoperative bradycardia and subsequent chronic stimulation. Clin Neurol Neurosurg. 2007;109(10):849-852. [DOI] [PubMed] [Google Scholar]
- 33. Asconapé JJ, Moore DD, Zipes DP, Hartman LM, Duffell WH. Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: a rare complication of intraoperative device testing. Epilepsia. 1999;40(10):1452-1454. [DOI] [PubMed] [Google Scholar]
- 34. Espinosa J, Aiello MT, Naritoku DK. Revision and removal of stimulating electrodes following long-term therapy with the vagus nerve stimulator. Surg Neurol. 1999;51(6):659-664. [DOI] [PubMed] [Google Scholar]
- 35. DeGiorgio CM, Amar A, Apuzzo MLJ. Surgical anatomy, implantation technique, and operative complications. Vagus Nerve Stimul. 2001:31-50. [Google Scholar]
- 36. McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005;46(1):91-96. [DOI] [PubMed] [Google Scholar]
- 37. Le H, Chico M, Hecox K, Frim D. Interscapular placement of a vagal nerve stimulator pulse generator for prevention of wound tampering. Technical note. Pediatr Neurosurg. 2002;36(3):164-166. [DOI] [PubMed] [Google Scholar]
- 38. Bruce D, Li M, Fraser R, Alksne. The Neuro-Cybernetic prosthesis (NCP) system for treatment of refractory partial seizures: surgical technique and outcomes. Epilepsia. 1998:92-3. [Google Scholar]
- 39. Dionigi G, Kim HY, Wu C-W et al.. Vagus nerve stimulation for standardized monitoring: technical notes for conventional and endoscopic thyroidectomy. Surg Technol Int. 2013 Sep;23:95-103. https://www.ncbi.nlm.nih.gov/pubmed/23860931. [PubMed] [Google Scholar]
- 40. Aalbers MW, Rijkers K, Klinkenberg S, Majoie M, Cornips EMJ. Vagus nerve stimulation lead removal or replacement: surgical technique, institutional experience, and literature overview. Acta Neurochir. 2015;157(11):1917-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakano N, Miyauchi M, Murakami S et al.. Cosmetic procedure for vagus nerve stimulation. Interdiscip Neurosurg. 2016;6:1-3. https://www.researchgate.net/publication/302594847_Cosmetic_procedure_for_vagus_nerve_stimulation. [Google Scholar]
- 42. Maniker A, Liu WC, Marks D, Moser K, Kalnin A. Positioning of vagal nerve stimulators: technical note. Surg Neurol. 2000;53(2):178-181. [DOI] [PubMed] [Google Scholar]
- 43. Ralston A, Ogden P, Kohrman MH, Frim DM. In situ repair of vagus nerve stimulator lead damage: technical note. J Neurosurg Pediatr. 2016;25(6):679-682. [DOI] [PubMed] [Google Scholar]
- 44. O’Neill B, Wilberger E J. Revision of vagal nerve stimulator electrodes through a posterior cervical triangle approach: technical note. Neurosurgery. 2010 Dec;67:457-60. https://www.ncbi.nlm.nih.gov/pubmed/21099572. [DOI] [PubMed] [Google Scholar]
- 45. MacDonald J, Couldwell WT. Revision of vagal nerve stimulator electrodes: technical approach. Acta Neurochir (Wien). 2004;146(6):567-570; discussion 570. [DOI] [PubMed] [Google Scholar]
- 46. Ortler M, Unterhofer C, Dobesberger J, Haberlandt E, Trinka E. Complete removal of vagus nerve stimulator generator and electrodes. J Neurosurg Pediatr. 2010;5(2):191-194. [DOI] [PubMed] [Google Scholar]
- 47. Ng WH, Donner E, Go C, Abou-Hamden A, Rutka JT. Revision of vagal nerve stimulation (VNS) electrodes: review and report on use of ultra-sharp monopolar tip. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2010;26(8):1081-1084. [DOI] [PubMed] [Google Scholar]
- 48. Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(Suppl 1):85-90. [DOI] [PubMed] [Google Scholar]
- 49. Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18(1):97-104. [DOI] [PubMed] [Google Scholar]
- 50. Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: a single center longitudinal study of 143 patients. Seizure. 2013;22(10):827-833. [DOI] [PubMed] [Google Scholar]