Abstract
Background
Adipose and adipose derived regenerative cells (ADRCs) play an increasing role in androgenetic alopecia.
Objectives
The authors sought to evaluate the safety and feasibility of fat grafts enriched with ADRCs in early androgenetic alopecia.
Methods
Seventy-one patients were treated: 16 with Puregraft fat and 1.0 × 106 ADRCs/cm2 scalp; 22 with Puregraft fat and 0.5 × 106 ADRCs/cm2 scalp, 24 with Puregraft fat alone, and 9 with saline control. Treatments were delivered into the skin and subcutaneous layer of the scalp. A total of 40 cm2 of scalp was treated and macrophotography and global photography were obtained at baseline and at 6, 24, and 52 weeks.
Results
A total of 71 patients tolerated the procedures well. No unanticipated associated adverse events were reported. When evaluating all patients at 24 weeks, there were no statistical differences between any of the treatment groups with respect to nonvellus (terminal) hair counts or width. There were increases (mean change from baseline) in terminal hair count for the low-dose ADRC group in the Norwood Hamilton 3 subgroup at week 6 (13.90 ± 16.68), week 12 (11.75 ± 19.42), week 24 (16.56 ± 14.68), and week 52 (2.78 ± 16.15). For this subgroup, the difference in hair count between the low-dose ADRC group and no-fat saline control was statistically significant (P = 0.0318) at week 24.
Conclusions
Puregraft fat and ADRCs are safe and well tolerated. In early male hair loss, this therapy demonstrated a statistically significant increase in terminal hair counts relative to the control population at 24 weeks and represents a promising approach for early androgenetic alopecia.
Level of Evidence: 2
Androgenetic alopecia (AGA) is the most common cause of hair loss in men and women and is characterized by progressive hair loss of the scalp due to a combination of genetics and androgens. AGA is reported to affect up to 70% of Caucasian men and 42% of women during the course of a lifetime.1 The pathogenesis of this progressive condition is based on miniaturization of the hair follicles and gradual conversion of terminal hairs into vellus hairs while the anagen phase shortens.2
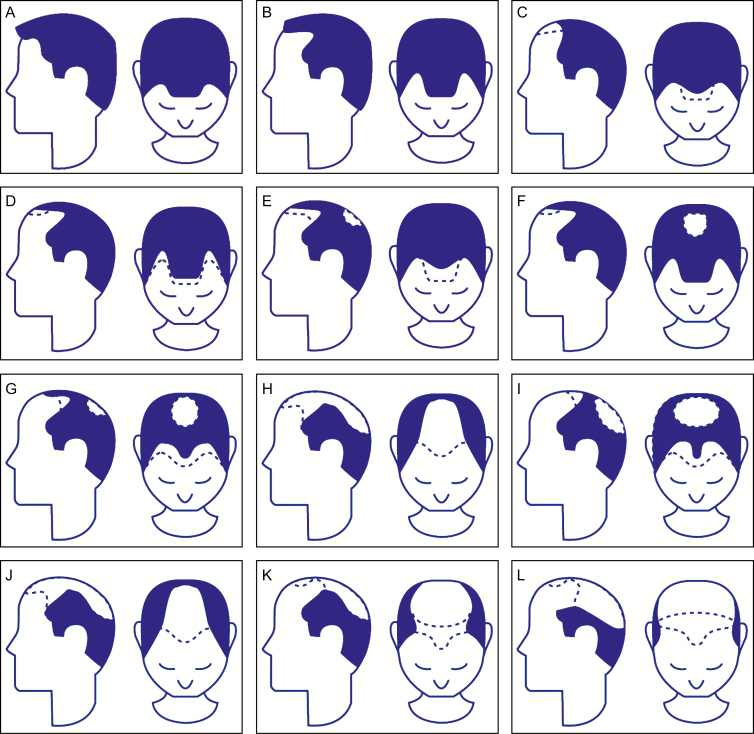
The degree of hair loss in the male patient is classified by the Norwood Hamilton Classification (Figure 1) in which hair loss is staged from Class 1 (absence of thinning) to Class 7 (very advanced hair loss), and for female hair loss the Savin scale is used for staging hair loss (Figure 2).
Figure 1.
(A-L) Norwood Hamilton classification for male hair loss. Men with Grades III, IIIA, III-Vertex, IV, and IV-A were included in this study.
Figure 2.
(A-F) Savin scale (partial) for female hair loss. Women with Grades I-3, I-4, II-1, and II-2 were included in this study.
Initial treatment for AGA is usually medical, and the 2 US Food and Drug Administration-approved medications are minoxidil and finasteride. Apart from these, in the past several years there has been an increased interest in low-level laser therapy (photobiomodulation) and prostaglandins as well as in regenerative therapies such as microneedling, platelet-rich plasma (PRP), and stem cell treatments. Hair transplantation remains a surgical option to restore hair in which hair is harvested from a “permanent” donor zone of the scalp (occipital and parietal areas), and then placed into areas affected with hair loss.
The potential of regenerative medicine, especially the use of adipose tissue and adipose derived regenerative cells (ADRCs) is growing in all fields of medicine. Scientific focus on the role of this tissue in the hair growth cycle has shown correlation between these 2 forms of cell therapy and hair growth.3 It has been established that the adipose tissue is an integral part of the normal hair cycle, and it is reported that the hair loss and decreased volume of subcutaneous tissue in the scalp occur together.3 ADRCs maintain the ability to differentiate into mesenchymal lineage cells but also secrete various growth factors that seem to play a role in neovascularization, which is important in treating various hair loss conditions.4 The addition of adipose tissue in the scalp thickens the subcutaneous layer that is typically associated with thinning in AGA.5
Cell enrichment of adipose tissue with ADRCs has been shown to prolong graft retention as described in many preclinical and clinical studies to date.6 Zhu et al have used an animal model to show that coating 1 volume of adipose tissue with cells isolated from another volume of adipose tissue resulted in an approximate doubling of graft retention at 6 and 9 months.6
Most of the studies published on hair regeneration utilizing ADRCs are in either cultured hair follicles, animal models, or human patients employing ADRC-conditioned medium.7-9 Apart from ADRC-conditioned medium application, there was 1 study where stromal vascular fraction, with autologous adipose tissue, was utilized on 9 patients with a follow-up of 6 months.10 Despite some limitations of the study (small sample size, poor follow-up data, nonblinded analysis), this study showed that stromal vascular fraction with fat injection is a safe and promising alternative approach to treating hair loss in men and women and served as the precursor to the phase II investigation described in this report.
METHODS
The STYLE Trial was a prospective, randomized, multi-center device trial intended to evaluate the safety and efficacy of the Kerastem Technologies (Solana Beach, CA, manufactured under exclusive license by Cytori Therapeutics) and Puregraft (Solana Beach, CA) Systems in the processing and preparation of an autologous fat graft enriched with ADRCs in the treatment of early AGA.
This study was performed at 4 different sites in the United States (Los Angeles, CA; Miami, FL; Highland Park, NJ; and New York City, NY) in accordance with the guidelines set forth by the International Conference on Harmonisation on Good Clinical Practice. In addition, this study was conducted under United States Food and Drug Administration Investigational Device Exemption #16488 and approved by Quorum (Seattle, WA; now Advarra; www.advarra.com) Investigational Review Board. The study’s date range was December 2015 to September 2017.
Following informed consent and screening evaluations, eligible patients underwent preoperative testing. Patients randomized into the study were assigned the treatment corresponding to the next available number in a computer-generated randomization schedule. The randomization process was managed by an independent contract research organization (Peachtree BioResearch Solutions, Marietta, GA) and occurred on the procedure day prior to the start of liposuction. Patients underwent a fat harvest utilizing local anesthesia with or without conscious sedation. Patients were randomly assigned to receive a fat graft cell enriched with ADRCs (available in 2 different doses), a fat graft without cell enrichment (fat alone control), or a saline injection (no-fat control) in a 2:2:2:1 ratio.
The key primary inclusion and exclusion criteria can be found at www.clinicaltrials.gov (NCT02503852) and are listed below.
The inclusion criteria included males with a diagnosis of Alopecia Androgenetica; females with a diagnosis of Alopecia Androgenetica; males with hair loss consistent with Grades III, IIIA, III-Vertex, IV, and IV-A based on Norwood-Hamilton Scale (Figure 1); females with hair loss consistent with Grades I-3, I-4, II-1, and II-2 based on the Savin Scale (Figure 2); patients who provided written informed consent and complied with the study requirements; women of childbearing potential with a negative pregnancy test at the screening visit who agreed to maintain 2 forms of contraception for the duration of the study; patients who were willing to maintain a consistent hair length and natural hair color, without the utilization of any coloring agents, during the study period; patients with the ability to complete study procedures and patient surveys and who agreed to photographs; patients who were 18 years of age and older and who had a body mass index of less than 40kg/m2.
The exclusion criteria included patients who have utilized minoxidil or any oral or topical medication including over-the-counter and herbal medications for the treatment of hair loss within 6 months of study screening, or finasteride or dutasteride within 12 months of study screening; treatment with an investigational product or procedure within 30 days or plans to participate in another clinical study; patients who had previously failed or were deemed nonresponsive to a previous experimental hair loss treatment; patients must have had no previous hair transplants, cell treatment, microneedling, or any other treatment in the last 6 months in the scalp; patients who were currently suffering from an active autoimmune disease such as serum lupus erythematosus or alopecia areata; patient is currently suffering from dermatological condition in the treatment area or had a significant scar in the hair treatment area that, in the opinion of the investigator, would make hair growth difficult (such as systemic burns, etc.); history of autoimmune disease or organ transplantation or a patient on immunosuppressive medication(s); diagnosis of cancer, receiving active treatment; active systemic infection; required chronic antibiotics or systemic corticosteroids; utilization of systemic agents that increase bleeding or clotting or disorders associated with these effects, including patients receiving GIIB/IIIa inhibitors within 2 weeks prior to the study procedure through to 1 week after the study procedure; clinically significant medical or psychiatric illness currently or within 30 days of study screening as determined by the investigator; prior surgery in the treatment area; any disease or condition (medical or surgical) that, in the opinion of the investigator, might compromise dermatological, hematological, cardiovascular, pulmonary, renal, gastrointestinal, hepatic, or central nervous system function; or any condition that would place the patient at increased risk; pregnant or lactating women or women trying to become pregnant; known allergic reaction to components of study treatment and/or study injection procedure; patient had any disorder that may prevent compliance to study procedures and visits; patient who was part of the study staff, a family member, or friend; diabetes or thyroid disorder; patient who had a sensitive, irritated, or abraded scalp area; women who had an alternate diagnosis associated with hair loss; body mass index <18 kg/m2; clinically significant abnormal findings on laboratory screening panels, including hemoglobin ≤10 g/dL; hepatic dysfunction, as defined as aspartate aminotransferase, alanine aminotransferase, or bilirubin levels greater than 1.5 times the upper limit of normal range prior to randomization; chronic renal insufficiency as defined by a serum creatinine greater than 1.5 mg/dL in men or greater than 1.2 mg/dL in women; and an elevated prothrombin time/partial thromboplastin time, international normalized ratio, or platelet count less than 100 × 109/L.
While patients underwent liposuction, lipoaspirate was processed in the Puregraft System (Video 1, available online www.aestheticsurgeryjournal.com) to remove the lipoaspirate of impurities and in the Kerastem Celution System to isolate and concentrate ADRCs.
Injection
After liposuction was completed, patients had, under a ring block local anesthesia, a subcutaneous (hypodermis) scalp injection of either 0.1 mL/cm2 of Puregraft purified autologous fat or saline (no-fat control) employing a blunt-tip cannula. Utilizing a standard fat-grafting technique, cannula were advanced at strategically placed insertion points and tunneled to the area of treatment. This was followed by separate second intradermal injections (multiple, covering the area of treatment) of either 0.1 mL/cm2 of ADRCs (available in 2 different doses), a visually matched blood saline solution (fat alone control), or saline (no-fat control) using a 1-inch-long standard 25G needle directly through the scalp. These injections were performed at a 45-degree angle to the scalp, and injection of cells was performed on withdrawal of the needle. A schematic of the procedure is shown in Figure 3.
Figure 3.
Schematic of the procedure.
All patients underwent clinical evaluations and laboratory testing prior to and after the procedure. For each patient, the study duration included a screening period of up to 28 days and a 52-week follow-up period.
Safety endpoints included the frequency of adverse events (AEs), serious AEs, unexpected adverse device events, changes in clinical laboratory test results (chemistry, hematology, and urinalysis), vital signs, and physical examination findings. In addition, an independent data monitoring safety board was commissioned to review safety data at various timepoints for the first 40 patients.
To assess efficacy, global and macrophotography were captured at baseline, 6 weeks, 24 weeks, and 52 weeks employing the Global Hair Device and Macro Canon VEOS-SLR (Canfield Scientific, Inc., Fairfield, NJ). This platform system utilizes a chin cup and head support attached to a camera (with accompanying flash) to provide consistent, fixed distance global photography. For macrophotography, a fixed size glass contact plate (defines the measurement site) is attached to the camera and is applied directly to the patient’s skin to obtain the macrophotograph. To ensure the same location is measured each time, all patients first underwent a semi-permanent micro tattooing process prior to any data acquisition. Images captured at sites were securely uploaded, underwent a third-party (Canfield) quality assurance review, and evaluated employing validated software in a blinded fashion by the vendor. Macrophotography included hair counts and hair widths for vellus (<30 microns in diameter), nonvellus (>30 microns in diameter), and total (vellus + nonvellus). In addition, investigator and patient satisfaction surveys were performed at 24 and 52 weeks. The analysis sets employed were the intent-to-treat (ITT) population (all patients randomized prior to liposuction), the per-treatment-evaluable population (all randomized patients who received treatment and for whom follow-up information was available), the modified ITT (mITT) population (all ITT patients excluding 6 patients based on the reassessment of baseline hair loss category by the principal investigator), and the safety population (all treated patients).
As a phase II, exploratory study, the primary endpoint was safety and tolerability of the procedure, and as such, the investigators believed that a study design of 20 patients per arm and a 10 patient no-fat saline control was adequate. Therefore, the sample size for the trial was set at 70 based on clinical considerations and was not based on formal power calculations. The authors believe this is consistent with exploratory phase II studies.
Safety and efficacy endpoints were summarized by treatment group utilizing descriptive statistics (n, mean, SD, median, minimum, and maximum) for quantitative variables and frequencies and percentages for categorical variables. Laboratory data were also summarized employing shift tables between before procedure and at day 1, postprocedure, with values categorized as less than the lower limit of normal range, within normal range, and above upper limit of normal range. All statistical tests were 2-sided, and statistical significance was assessed with respect to a nominal P value of 0.05. Related AEs were analyzed separately for those related to ADRCs and the delivery of ADRCs (ie, injection site telogen effluvium). Consistent with a phase II investigation, efficacy analyses were conducted on the ITT population, the per-treatment-evaluable population, and the mITT population (including ad hoc populations such as males with Norwood Hamilton III, females). Global photographic data were not included this quantitative analysis. The change from baseline at weeks 24 and 52 in hair counts and hair width was analyzed employing analysis of covariance models with adjustment for the baseline value.
RESULTS
A total of 71 patients were randomized and treated in this study. This included 17 females and 54 males. The mean age of participants was 40.7 years (range, 24-73 years). Of the 71 patients treated, a total of 60 (84.5%) completed the 12-month study. Loss to follow-up was the primary reason for discontinuation, and mean time of follow-up was 46 weeks (range, 4-52 weeks). None of the patients were withdrawn due to AEs.
Hair Count
Nonvellus hairs, also known as terminal hairs, are the mature form of hair and are defined in this study as having a diameter greater than 30 microns. There were increases in nonvellus hair count for the low-dose ADRC group in the baseline NW3 ad hoc subgroup at week 6 (mean change from baseline, 13.90 ± 16.68), week 12 (mean change from baseline, 11.75 ± 19.42), week 24 (mean change from baseline, 16.56 ± 14.68), and week 52 (mean change from baseline, 2.78 ± 16.15). The difference in nonvellus hair count between the low-dose ADRC group and the no-fat saline control was statistically significant (P = 0.0318) at week 24. This is represented in Figure 4.
Figure 4.
Absolute mean change in terminal hairs from baseline to 24 weeks for the Norwood-Hamilton III population (per protocol). This figure shows that in this group of men with early hair loss, those treated with fat and a low dose of regenerative cells responded best when evaluating terminal (mature) hair counts. Compared with the control group within the same group at 24 weeks, this difference was found to be statistically significant. Of note, the group treated with fat and a high dose did not respond. The authors hypothesize this may be due to the presence of excess white blood cells involved with inflammation.
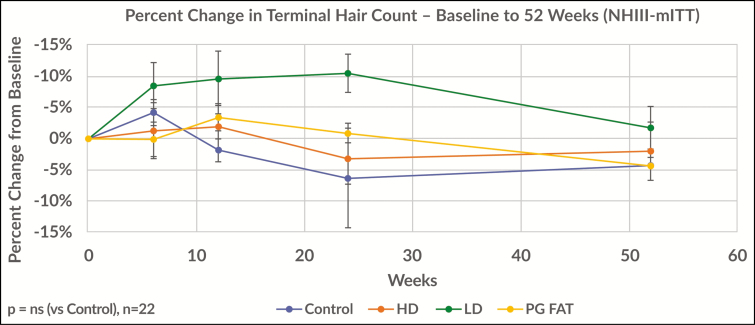
Figure 5 demonstrates the percent change from baseline at 52 weeks for the Norwood-Hamilton III population. For this group of men with early hair loss, the effect observed at 24 weeks noted above was not maintained at 1 year (2% increase). For the corresponding control group, they maintained a loss of −4% at 52 weeks. This difference between the fat + low dose regenerative cell group and control group was not statistically significant. This result suggests that this therapeutic approach may drive short-term hair cycle activity but may need to be repeated or utilized in combination with other therapeutic approaches for sustained effect.
Figure 5.
This figure demonstrates the percent change from baseline at 52 weeks for the Norwood-Hamilton III population. For this group of men with early hair loss, the effect observed at 24 weeks noted above was not maintained at 1 year (2% increase). For the corresponding control group, they maintained a loss of −4% at 52 weeks. This difference between the fat and a low-dose regenerative cell group and the control group was not statistically significant.
In the same NW3 ad hoc subgroup, statistically significant increases in total hair count (nonvellus + vellus) were observed for the low-dose ADRC group at week 6 (P = 0.0219) and week 12 (P = 0. 0434). This was also observed for the high-dose ADRC group at week 6 (P = 0.0465).
In the ITT group, there was a statistically significant change from baseline in hair count for the low-dose ADRC group at week 6 (mean change from baseline, 13.65 ± 18.01; 95% confidence interval = 5.22, 22.08). In this population, there were no apparent changes from baseline in hair count for any of the other treatment groups at week 6 or for any of the treatment groups at week 12, week 24, or week 52. There were no statistically significant changes in total hair count between the low-dose ADRC, high-dose ADRC, or fat-alone control groups and the no-fat saline control group at any timepoint for the ITT population.
Hair Width
There were no statistically significant changes in total hair width between the low-dose ADRC, high-dose ADRC, or fat-alone control groups and the no-fat saline control group at any timepoint for the ITT population or for any of the sensitivity analyses. In the ITT population, other than an increase in hair width at week 12 for the no-fat saline control group, the fat-alone control group and the no-fat saline groups had decreased mean terminal hair widths at all timepoints. Evaluations of the ad hoc populations (mITT, baseline NW3, male not baseline NW3, and female) revealed a similar pattern of response to the ITT population for hair width.
In the Norwood-Hamilton III population, early increases in mean terminal hair width were observed at week 6, week 12, and week 24 for the low-dose group. These changes were not sustained at week 52. Figure 6 below illustrates the percent change from baseline in terminal hair width for the Norwood-Hamilton III population over the duration of the study.
Figure 6.
This figure illustrates the percent change from baseline in terminal hair width for the Norwood-Hamilton III population over the duration of the study.
Global Photography
Figures 7 and 8 are global photographs obtained in STYLE from various patients. Please note that for all patients, the area treated was limited to 40 cm2 of scalp. Additional global photographs from the study can be viewed as Supplemental Figures 1-3, available online at www.aestheticsurgeryjournal.com.
Figure 7.
The photo shows this 34-year-old male (low-dose adipose and adipose derived regenerative cells in a Norwood Hamilton III patient) patient. The patient’s vertex was treated. (A) Baseline global photography, (B) 24-week global photography, and (C) 52-week global photography.
Figure 8.
The photo shows this 63-year-old female (low-dose adipose derived regenerative cells—Savin-I) patient. The patient’s midline (and surrounding area) were treated. (A) Baseline global photography, (B) 24-week global photography, and (C) 52-week global photography. Although the patient did not have an increase in nonvellus hair count, her terminal hair width increased 23% over the 52-week period.
Hair Satisfaction Questionnaires
Questionnaires were provided on paper for both the investigator and patient. The survey was not anonymous because it was distributed and administered by the study coordinator(s) (site & initials of coordinators: Los Angeles: R.L., Z.B.; Miami: J.S., G.K.; Highland Park: J.G.R.; New York: J.M., B.S.) at each clinical site at baseline and each follow-up visit.
The investigator hair satisfaction questionnaire evaluated the patient’s hair loss area getting smaller and/or filling in (Question 1), appearance of the patient’s hair (Question 2), growth of the patient’s hair in the treated area (Question 3), effectiveness of the procedure in slowing the patient’s hair loss (Question 4), and satisfaction with the appearance of the treated area (Question 5).
Compared with the no-fat control group, evaluation of the investigator hair satisfaction questionnaire revealed postprocedure improvements in the proportion of patients with positive responses for all questions for the low-dose ADRC, high-dose ADRC, and fat-alone groups. The low-dose ADRC group demonstrated the best overall response to treatment through 52 weeks postprocedure. Below is a summary of the responses of the investigator hair satisfaction questionnaire:
The low-fat ADRC group had the highest proportion of patients with positive responses at week 24 for Questions 1 through 4 and sustained that response level through week 52. For all of the questions, there was a shift to a larger percentage of patients with the most positive response category at week 52.
The high-fat ADRC group had the highest proportion of patients with positive responses at week 12 for all of the questions. Although the proportion of patients with positive responses decreased at week 24 compared with week 12, that response level was sustained through week 52 and was higher than the week 6 response level for all but Question 4 (effectiveness of the procedure has been in slowing down the patient’s hair loss).
The fat-alone control group had the highest proportion of patients with positive responses at week 24 for all of the questions but was lower at week 52 for all of the questions.
The patient hair satisfaction questionnaire (Appendix A) posed outcome-related questions and categorical responses to study participants at various timepoints. Supplemental Tables 1 and 2, available online at www.aestheticsurgeryjournal.com, report the patient satisfaction data reported for each of the 5 questions and comparison among study groups at 24 and 52 weeks, respectively. Compared with the no-fat control group, evaluation of the patient hair satisfaction questionnaire revealed postprocedure improvements in the proportion of patients with positive responses for all questions for the low-dose ADRC, high-dose ADRC, and fat-alone groups. The low-dose ADRC group demonstrated the best overall response to treatment.
The low-fat ADRC group had the highest proportions of patients with positive responses at week 24 for Questions 1 and 4 (and sustained that response level through week 52), and at week 52 for Questions 2, 3, and 5.
The high-fat ADRC group had the highest proportions of patients with positive responses at week 24 for Questions 1, 2, 3, and 5, and sustained that response level through week 52 for Question 2. There was a decrease in the proportions of patients with positive responses at week 52 for Questions 1, 3, and 5. For Question 4, the proportion of patients with positive response was highest at week 6 and 12 and lower at weeks 24 and 52.
The fat-alone control group had the highest proportions of patients with positive responses at week 24 for Questions 1-4 but was lower at week 52 for Questions 1-3.
Safety Analyses
Because the primary endpoint of the study was safety and tolerability, it was found that injections of fat grafts enriched with ADRCs were safe and well tolerated in patients with AGA. There were no serious AEs, deaths, or unexpected adverse device events in this study. There was no evidence of any clinically relevant changes in hematology or chemistry parameters, and although some patients had occasional hematology or clinical chemistry results that were abnormal, none were judged by the investigators to be clinically significant and none were reported as AEs. There were no apparent changes in vital signs over the course of the study, and no AEs were associated with vital sign abnormalities.
DISCUSSION
AGA is the most common form of both male and female pattern hair loss. For both sexes, the negative physical and psychological effects of AGA are well documented. In Stough’s report, the authors indicate that although the condition may not appear to cause direct physical harm in men, hair can protect against sunburn, cold temperatures, mechanical injury, and ultraviolet light.11 Beyond the physical, hair loss is known to psychologically affect the balding individual’s self-perception. This is particularly true when looking at female pattern hair loss, where Cash and co-workers reported that although AGA was a stressful experience for both sexes, it was substantially more distressing for women.12 In sum, the impact of the condition is substantially functional as well as aesthetic in nature. Therefore, serious and thoughtful investigation to develop satisfactory treatment regimens is proper and necessary.
The current treatment regimen for AGA recommended by medical professionals is a spectrum ranging from noninvasive pharmacologic approaches to surgical transplantation of thousands of follicular units. Initial treatment of early alopecia is typically noninvasive, and the 2 medications approved by the US Food and Drug Administration today are minoxidil and finasteride.13,14 For minoxidil, it is reported that approximately one-third of patients with AGA respond with new hair growth.15 Both of these medicines carry untoward side effects. In contrast, invasive transplantation options are generally not offered to patients with early AGA and if so, require significant financial resources. As a result, there remains a significantly large gap between these medical and surgical options. Hence there is a distinct medical need for treatments specific to patients with early alopecia.
During hair follicle growth phase (anagen), the hair follicle extends deep into the rich dermal macroenvironment as it grows to maturity where it is surrounded by large lipid-filled adipocytes. These intradermal adipocytes regenerate with faster kinetics than other adipose tissue depots, and such growth parallels with the hair cycle, suggesting that an interplay exists between hair follicle cells and adipocyctes.3 Thus, it has been established that adipose is an integral part of the normal hair cycle; it is hypothesized that telogen may be due to an absence of adipose tissue because it is reported that hair loss and a decrease in perifollicular adipocytes occur together.3,16
Therefore, the transplantation of adipose tissue, or autologous fat transfer, into the subcutaneous layer of fat in the scalp and into the dermis for purposes of stimulating hair growth is consistent with the reported literature that indicates hair loss and adipose loss occur in tandem.
In an autologous fat transfer procedure, a patient’s subcutaneous adipose tissue is readily aspirated from 1 location (most commonly the abdomen or thighs) and transferred into another depot of subcutaneous fat located throughout the body (eg, mid-face). These surgical techniques are well established throughout the aesthetic surgical literature. Currently, there are few, if any, minimally invasive, autologous cell therapeutic modalities being developed that address early alopecia. A previous pilot study employing similar, but not identical to, methods described in this study demonstrated stem cell-enriched fat grafting represents a promising alternative approach for treating baldness in men and women.10 The current study (the STYLE Trial) was a prospective, randomized, multicenter, device trial intended to evaluate the safety and efficacy of the Kerastem Therapy in the processing and preparation of an autologous fat graft enriched with ADRCs in the treatment of early AGA.
The authors’ key take-aways from the exploratory STYLE study include (1) dosing matters, and the lower dose of ADRC-enriched purified fat consistently performed better in both quantitative as well as qualitative methods; (2) this minimally invasive approach was superior only in men with early hair loss and likely requires either additional treatments and/or combination with complementary therapies, and (3) this procedure was safe and well tolerated by patients.
Dosing Matters
In evaluating the dose response of patients in STYLE, one of the dosing regimens was clearly superior. Namely, injection of purified fat with 0.5 × 106 ADRCs/cm2 of scalp demonstrated a clear and consistent superior result. The importance of dosing is often underappreciated in regenerative aesthetics. Furthermore, the data supported that more cells per area of scalp treated did not provide a better outcome. This seemingly paradoxical response has been reported previously in other stem cell therapy studies, notably those in cardiac therapies.17 When applied to AGA, the authors hypothesize this may be related to the degree of local inflammation, which was previously reported to play a role in patterned baldness.18,19 In looking at the make-up of the ADRCs, there is a relatively high percentage of tissue macrophages (approximately 25%). A relatively high dose of ADRCs injected into a local area of the scalp may promote micro-inflammation and may be responsible for the inferior response. Additional studies are likely needed to further investigate this hypothesis, including the role of potential complementary therapies such as PRP, microneedling, and cultured stem cell therapies.
Early Hair Loss
One of the goals of a phase II exploratory study, besides proving safety and tolerability, is to determine feasibility and directionality. In analysis of the population and subpopulations of this investigation, the optimum results were seen in men with early hair loss (Norwood-Hamilton 3). This does not appear to be a function of age, because the average age of men in this subpopulation was 41 years, which was also the average age of all participants. That said, it is understood in regenerative medicine approaches that the quality of autologous cells,20 and perhaps the extent of existing disease, plays a role in the regenerative capacity of therapies. Applied to STYLE, perhaps men in the early stages of the complex process of patterned hair loss are best suited to respond to regenerative approaches (perhaps in combination with other therapies), and future studies will no doubt target this population given the strong signal. This is of particular relevance given the clear benefits of treatments addressing this population.
Sustained Response, PRP, and the Role of Repeat Therapies
In the men with early hair loss who were observed to respond best to fat + low-dose regenerative cells at 24 weeks, this effect appeared to diminish over time as seen at 52 weeks. Although the relative trend between groups (eg, low dose vs others) generally held, the authors assert that this cellular therapy would likely require repeat approaches and/or combination with other adjunct therapies, including PRP, low-level laser therapy, microneedling, etc. Of note, polytherapy is historically a common approach to the treatment of patterned alopecia, likely reflecting the complex multi-factorial etiology underlying hair loss.
It is also worth noting for the reader that PRP is increasingly being explored as an intervention for treating early hair loss. This current study did not involve the study of PRP, and therefore the authors are not in a position to directly compare and contrast the efficacy of the 2 techniques. A direct comparison employing published reports is also limited by considerably varying methods of measuring and reporting hair growth. However, in a recent meta-analysis on the utilization of PRP, Gupta and co-workers reported a standardized mean difference in hair density of 0.58, in favor of PRP.21 Although this reported benefit is encouraging, the authors also recommend initial repeated monthly sessions of PRP (once monthly × 3 treatments) followed by a 3- to 6-month maintenance period. Further, according to the International Society for Hair Restoration Surgery, no definitive regimen or conclusive studies on the role of PRP efficacy exist.22 Although both cell- and platelet-based therapies show evidence supporting hair growth, both approaches are likely to benefit from repeated treatments and therefore must be made available as cost-effective solutions to patients. Furthermore, the investigators hypothesize that although cell-based approaches may initiate a stimulation of the hair cycle, frequent interventions are necessary to counter the constant forces of follicular miniaturization at work with genetic alopecia.
Study Limitations
The authors acknowledge the potential limitations of this study include a relatively small sample size for the findings in men with early hair loss (Norwood-Hamilton III). However, the robustness of the data, even with this small sample, speaks to the potential merit of the approach. In addition, the authors note the emerging role of alternative approaches (eg, PRP, microneedling) to the treatment of early hair loss, and our work does not directly compare and contrast alternative approaches. Future work will address these limitations.
CONCLUSIONS
The straightforward technical aspects of this approach to hair loss are likely behind the positive safety data. In addition, patients screened and enrolled into the study were generally healthy, with minimal comorbidities. In short, this is an enriched fat-grafting procedure in which practitioners comfortable with these standard techniques may be suited to explore this approach. In conclusion, the authors believe this novel regenerative approach is promising and warrants continued development in the treatment of early patterned hair loss.
Supplementary Material
Disclosures
Dr Daniels is an officer of Kerastem Technologies and owns stock in Kerastem Technologies. Dr Washenik has been a consultant for Kerastem Technologies. Drs Kuka, Epstein, Aronowitz, Glasgold, Rogal, Brown, and Geronemus received funding as participating clinical investigators.
Funding
This study was funded by Kerastem Technologies, Solana Beach, CA. The sponsor participated in the design of the study and in the collection, analysis, and interpretation of the data and writing of the manuscript.
REFERENCES
- 1. Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32(1):11-22. [DOI] [PubMed] [Google Scholar]
- 2. Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28(5 Pt 1): 755-763. [DOI] [PubMed] [Google Scholar]
- 3. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678-685. [DOI] [PubMed] [Google Scholar]
- 5. Hori H, Moretti G, Rebora A, Crovato F. The thickness of human scalp: normal and balds. J Inv Derm. 1972;58(6):396-399. [DOI] [PubMed] [Google Scholar]
- 6. Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64(2):222-228. [DOI] [PubMed] [Google Scholar]
- 7. Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15:e10. [PMC free article] [PubMed] [Google Scholar]
- 8. Fukuoka H, Narita K, Suga H. Hair regeneration therapy using proteins secreted by adipose derived stem cells. Curr Stem Cell Res Ther. 2017;12(7):531-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol. 2015;54(6):730-735. [DOI] [PubMed] [Google Scholar]
- 10. Perez-Meza D, Ziering C, Sforza M, Krishnan G, Ball E, Daniels E. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stough D, Stenn K, Haber R, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005;80(10):1316-1322. [DOI] [PubMed] [Google Scholar]
- 12. Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568-575. [DOI] [PubMed] [Google Scholar]
- 13. Ross EK, Shapiro J. Management of hair loss. Dermatol Clin. 2005;23(2):227-243. [DOI] [PubMed] [Google Scholar]
- 14. Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). J Investig Dermatol Symp Proc. 2003;8(1):20-23. [DOI] [PubMed] [Google Scholar]
- 15. Magerl M, Paus R, Farjo N, et al. Limitations of human occipital scalp hair follicle organ culture for studying the effects of minoxidil as a hair growth enhancer. Exp Dermatol. 2004;13(10):635-642. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt B, Horsley V. Unravelling hair follicle-adipocyte communication. Exp Dermatol. 2012;21(11):827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 1992;127(3):239-246. [DOI] [PubMed] [Google Scholar]
- 19. Mahé YF, Michelet JF, Billoni N, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39(8):576-584. [DOI] [PubMed] [Google Scholar]
- 20. Renewed interest in regenerative medicine. EBioMedicine. 2018;27:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta AK, Cole J, Deutsch DP, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45(10):1262-1273. [DOI] [PubMed] [Google Scholar]
- 22. Platelet Rich Plasma. ISHRS.org https://ishrs.org/patients/treatments-for-hair-loss/medications/platelet-rich-plasma/. Accessed September 1, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.