ABSTRACT
Introduction
Main clinical manifestations of SARS-CoV-2 infection are characterized by fever, dyspnea, and interstitial pneumonia, frequently evolving in acute respiratory distress syndrome (ARDS).
Areas covered
Features of coronavirus disease 2019 (COVID-19) presents some common points with interstitial lung disease (ILD) both idiopathic and related to rheumatoid arthritis (RA), typically characterized by a chronic progression over time and possibly complicated by acute exacerbation (AE). The study of common pathogenetic mechanisms, such as the involvement of toll-like receptor 4, could contribute to the knowledge and treatment of idiopathic and RA-ILD. Moreover, hyperinflammation, mainly characterized by increase of effector T-cells and inflammatory cytokines, and activation of coagulation cascade, observed in COVID-19 related ARDS have been already shown in patients with AE of idiopathic and RA-ILD. A literature search was performed in PubMed, Embase, Scopus, and Web of Science, together with a manual search in COVID-resource centers of the main journals.
Expert opinion
Despite the uncertainty about pathogenetic aspects about COVID-19- pneumonia, it could be a possible model for other forms of ILD and AE. The great amount of data from studies on COVID-19 could be helpful in proposing safe therapeutic approaches for RA-ILD, in understanding pathogenesis of usual interstitial pneumonia and to develop new therapeutic strategies for AE.
KEYWORDS: COVID-19, rheumatoid arthritis, interstitial lung disease, toll-like receptor, idiopathic pulmonary fibrosis, acute exacerbation
1. Introduction
In December 2019 a novel infectious disease by a coronavirus named SARS-CoV-2 has been detected in the city of Wuhan in China and rapidly widespread worldwide. World Health Organization declared the stage of pandemic on 11 March 2020 [1].
Main clinical manifestations are fever, cough, dyspnea and interstitial pneumonia, frequently evolving in an acute respiratory distress syndrome (ARDS). Increasing data are reporting other systemic clinical manifestations, including anosmia, vomit, diarrhea, but also fatal thrombotic events and septic shock [1,2].
The main cause of death of COVID-19 patients is characterized by respiratory failure due to interstitial pneumonia [3,4]. a state of hyperinflammation induced by the viral infection could be responsible for the severe pulmonary involvement, frequently leading to a respiratory failure [5].
Features of COVID-19 pneumonia present some common characteristics with interstitial lung disease both idiopathic, i.e. the idiopathic interstitial pneumonias, particularly idiopathic pulmonary fibrosis (IPF), and usual interstitial pneumonia (UIP) related to rheumatoid arthritis (RA) and connective tissue diseases (CTDs), typically characterized by a chronic progression over some years [6,7].
Aim of this review is to describe the clinical characteristics of these conditions, possible common pathogenetic aspects, to suggest possible therapeutic options for COVID-19 patients and to generate new hypotheses for the treatment of idiopathic or RA-ILD.
2. Literature search
a literature search was performed in some electronic databases, including PubMed, PubMed, Embase, Scopus, and Web of Science including the terms coronavirus 2019, COVID-19 pneumonia, SARS-CoV2, and pathogenesis of interstitial pneumonia, interstitial lung disease, usual interstitial pneumonia. Moreover, a manual search in COVID-resource centres of the main medical journals, including the categories “Internal Medicine,” “Infectious Diseases,” “Immunology,” “Respiratory System,” and “Rheumatology,” was also performed searching for recently online published articles.
3. COVID-19 interstitial pneumonia
Fever, cough, sore throat dyspnea, fatigue and myalgia represent the most common clinical manifestations at the onset of the disease. The majority of patients develop flu-like symptoms [1,2]. Pneumonia may frequently occur, characterized by nonspecific features at chest high resolution computed tomography (HRCT), including ground-glass and/or consolidative opacities. About 10–15% of the patients develop a severe respiratory disease, that in 5% of the cases, result in a critical disease, characterized by severe respiratory failure, septic shock, and/or multiple organ dysfunction or failure [1,4].
In this latter group, the acute worsening of respiratory function occurs about a week later the onset of the systemic symptoms, causing a clinical condition that require mechanical ventilation and support in intensive care unit, with possible progression to severe acute respiratory distress syndrome (ARDS) [4,8–10].
At HRCT, ground glass opacities (GGO), in some cases associated to consolidations, are the most common findings [11,12]. In 81 Chinese patients the HRCT alterations changed according to the stage of the disease. At the clinical onset, the main CT abnormalities were unilateral, multifocal GGO, but also interlobular septal thickening, thickening of the adjacent pleura, nodules, round cystic changes, bronchiolectasis, pleural effusion. In a more advanced stage (one week after the onset of the disease) lesions became bilateral and diffuse, while two weeks later the predominant CT features were GGO, whereas appearance of consolidations was also observed in some cases. Finally, GGO and reticular pattern were the predominant findings in the last stage (2–3 weeks after symptoms onset) [13] (Figure 1).
Figure 1.
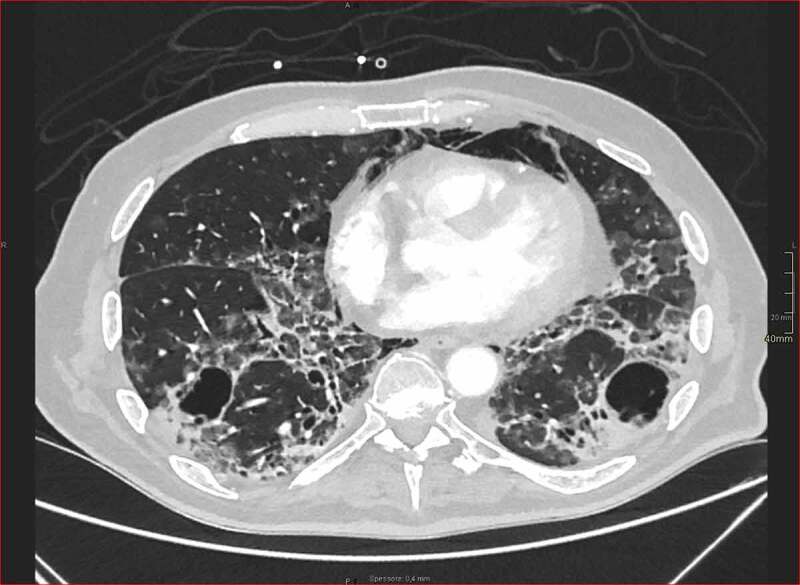
High-resolution lung base image from contrast-enhancement arterial scan for pulmonary embolism detection in patient with long-standing COVID-19 pneumonia and pneumomediastinum. Ground-glass opacities are detected in subpleural areas mixed with focal consolidations. Moreover, computed tomography shows initial fibrotic changes with architectural distortion and bronchiolectasis. Multiple thin-walled cysts are also recognized, in keeping with smoking-related changes (courtesy by Gabriele D’Andrea, Radiology Unit, San Gerardo Hospital, ASST Monza, Monza, Italy).
4. Pathogenesis of COVID-19
Pathogenesis of SARS-Cov2 infection is not fully understood, and both viral and host factors appear to be involved. The virus is transmitted mainly via respiratory droplet and contact [8,14]. Primary viral replication is presumed to occur in mucosal epithelium of upper respiratory tract, with further multiplication in lower respiratory tract [14], giving rise to a mild viremia. Many infections are controlled at this point and remain asymptomatic [1,14]. In contrast, clinical findings showed exuberant inflammatory responses during SARS-CoV-2 infection, further resulting in uncontrolled pulmonary inflammation, likely a leading cause of mortality. The antigen presentation cells (APC) play a central role in antiviral immunity. Antigenic peptides are presented by human leukocyte antigen (HLA) and then recognized by virus-specific cytotoxic T lymphocytes. Unfortunately, there is still lack of report about antigen presentation of SARS-CoV-2, and we can only get some information from previous researches on SARS-CoV and MERS-CoV, showing numerous HLA polymorphisms correlate to the susceptibility to these viruses [15]. Differences in the susceptibility of individuals to infection, regarding the spectrum of COVID-19 symptoms and in particular the development of ARDS remain to be fully understood. Genetic variability in histocompatibility complex (MHC) class I genes plays a role, specifically individuals presenting HLA-B*46:01 gene product may be more vulnerable to COVID-19 – due to reduced capacity for viral antigen presentation to immune cells while patients with HLA-B*15:03 may be more likely to develop immunity [16]. Nevertheless, considerable variation in disease behavior and severity among patients with pulmonary infection secondary SARS-CoV-2 have been also observed.
Moreover, advanced age, male sex and preexisting conditions such as cardiovascular, pulmonary, and renal diseases make a subject more vulnerable to the more severe health consequences of COVID-19 [17,18].
The initial onset of rapid viral replication may cause massive epithelial and endothelial cell death and vascular leakage, triggering the production of exuberant proinflammatory cytokines and chemokines [14].
4.1. Role of toll-like receptor 4 in the pathogenesis of COVID-19 pneumonia
As mentioned above, an aberrant immune response against the virus plays a key role in the immunopathogenesis of the disease, resulting in an hyperinflammatory state that causes pulmonary tissue damage, functional impairment, and reduced lung capacity [5,9,10].
Some possible mechanisms have been investigated in pathogenesis of COVID-19 pneumonia, mainly involving both innate and adaptive response and angiotensin-converting enzyme 2 (ACE2)-mediated lung fibrosis. ACE2 has been reported to have a protective role in lung fibrosis, while SARS-CoV-2 and its spike protein can reduce ACE2 expression during viral infection. The decreased ACE2 expression could lead to lung fibrosis by upregulating angiotensin 2 and activating TGF- signaling [19].
Regarding innate immunity, the involvement of toll-like receptor 4 (TLR4) could have a specific potential role in pathogenesis of both idiopathic and interstitial pneumonias related to RA and CTDs, mainly usual interstitial pneumonia (UIP) [20–23].
Toll-like receptors (TLRs) and related pattern-recognition receptors represent the first line of host defense against infectious agents [20,24]. Cell surface receptors such as TLR4 and endosomal receptors such as TLR3 recognize both extrinsic and intrinsic particles, namely pathogen-associated molecule patterns (PAMPs) such as lipopolysaccharide (LPS), but also damage-associated molecule patterns (DAMPs), produced during different forms of noninfectious tissue injury [4,20,25,26].
TLR4 has been demonstrated to be involved in the induction of inflammatory damage during acute viral infections [27,28] and TLR4 activation has been clearly identified as responsible of the severity of some viral diseases in animal models [20,29]; in this regard, lethal infection of mice was prevented by treatment with TLR4 antagonists, highlighting the therapeutic potential role of these molecules [29–31].
Interestingly, TLR4 knockout mice had similar survival rates or disease severity to infected wild-type mice, suggesting that some degree of TLR4 activation might be required for a protective immune response against viruses, such as SARS-Cov2 [29,30].
Activation of TLR4 results in the recruitment of the intracellular adaptor protein, myeloid differentiation primary response 88 (MyD88), and/or toll/interleukin-1 receptor-domain-containing adapter-inducing interferon-β, ultimately resulting in the expression and secretion of proinflammatory mediators [31].
4.2. Role of TLR4 in idiopathic and RA-related usual interstitial pneumonia
As described, COVID-19-related interstitial pneumonia can be characterized by a various degree of pulmonary fibrosis, even if the long-term evolution of these alterations is unknown [11,32]. In particular, while in the early stages of COVID-19 pneumonia ground glass opacities represent the main finding of the disease, in more advanced stages the main manifestation is interstitial change of both lungs, such as fibrous cords and reticular opacities [33,34].
The spectrum of pulmonary fibrotic disease observed in COVID-19 is wide, ranging from fibrosis associated with organizing pneumonia to severe acute lung injury, in which there is evolution to widespread fibrotic change. In a follow-up study of 36 patients surviving MERS coronavirus infection, 12 (33%) had radiographic evidence of pulmonary fibrosis; these patients were older and had longer intensive care unit admissions. Given approximately 30% of survivors of SARS and MERS experienced persistent radiological and physiological abnormalities consistent with fibrotic lung disease, the repercussions of COVID-19 could include a large cohort of individuals with pulmonary fibrosis and persistent and potentially progressive physiological impairment [33,34].
The TLR4 pathway has been deeply investigated as possible pathogenetic mechanism of UIP both idiopathic and RA and CTDs related [22,23,35,36].
In fact, despite the presence of some conflicting data [35,37–41], many findings support a profibrotic role subsequent to TLR4 activation. For example, LPS induces lung fibroblast proliferation in mice by mean of a TLR4-dependent pathway [42] and some findings suggest that TLR4 inhibition might protect from LPS-induced pulmonary fibrosis [35].
It is well-known that the expression of both TLR2 and TLR4 is significantly increased in the lungs of patients with different ILD, suggesting either a protective compensatory response or aberrant activation leading to unresolved inflammation and tissue injury [37].
The suppression of TLR4 and TLR2 produces opposite results; in fact, the suppression of TLR4 induces inhibition of bleomycin-induced lung fibrosis, while the suppression of TLR2 exacerbates it [38–40].
Furthermore, IPF susceptibility and progression have been associated with functional genetic variants of TOLLIP, an inhibitory adaptor protein modulating innate immune responses through modulation of the canonical MyD88-dependent TLR2 and TLR4 signaling [43,44].
Interestingly, a novel antifibrotic compound, named Mogroside IIIE, markedly decreased fibrosis through regulation of the TLR4 signaling in a murine model of bleomycin-induced pulmonary fibrosis [45].
a possible role for TLR4 has been proposed also in the pathogenesis of fibrosis in CTDs. In particular, specific DAMPs are significantly upregulated in fibrotic skin and lungs from systemic sclerosis (SSc) patients and largely colocalized with TLR4-expressing myofibroblasts [46,47]. In mice, the genetic ablation of 2 DAMPs associated with SSc, namely fibronectin-EDA and tenascin-C, resulted in a significant attenuation of skin and lung fibrosis, suggesting a pathogenetic role for DAMP-TLR4 signaling in the development of chronic organ fibrosis [46,47]. Moreover, knock-out mice for functional TLR4, as well as with a fibroblast-specific deletion of TLR4, were protected from fibrosis in experimental models [22,46,48,49].
Rheumatoid arthritis (RA) is complicated by ILD in about 10–20% of cases, mainly showing UIP pattern, followed by a NSIP pattern [50–52]. The involvement of TLR4 in RA has been demonstrated in the pathogenesis of musculoskeletal and pulmonary involvement [21,23,53]. Innate immunity exhibits significant differences between idiopathic UIP and NSIP, justifying the hypothesis of possible distinctive pathogenetic features of UIP and NSIP in RA patients, also; these peculiarities could be responsible for the different clinical evolution and outcomes in RA patients showing an UIP or a NSIP pattern [23].
Differently by UIP, pathogenesis of NSIP is currently understood as an autoimmune and inflammatory-driven process and early histological studies examining lung biopsy samples show a mononuclear lymphocytic infiltrate. In NSIP a largely lymphocytic infiltrate expressing the B cell-specific antigen can be found but also a high CD4/CD8 ratio, exhibiting a Th1-type response, when compared with UIP tissue [54]. Moreover, a significantly higher expression of the chemokine receptor CXCR3 rather than CXCR4 have been found in lung tissue of patients with NSIP compared with those with IPF [55].
All these findings suggest the potential role of the host’s immune response in the pathogenesis of NSIP [54].
Because of the relevant role of TLR4 in pathogenesis of RA [21,23,51–53,56], inhibition of TLR4 has been explored as a possible therapeutic target; in experimental models, the TLR4 inhibitor TAK-242, inhibited the production of interleukin (IL)-6, IL-8, matrix metalloproteinase-1, and vascular endothelial growth factor in a LPS stimulated human synovial cell line [53].
However, an anti-TLR4 monoclonal antibody (NI-0101) has failed in demonstrating its efficacy in the treatment of the disease [57]. On the other hand, considering the significant role of TLR4 in lung complications of COVID-19, we believe that there is a rationale for considering this as a possible therapeutic approach for SARS-Cov2 pulmonary infection [20,31].
In RA, both conventional and biologic disease modifying antirheumatic drugs (DMARDs) have been implicated in the development of lung complications, both acute drug-related pulmonary toxicity and chronic lung fibrosis, with conflicting data [27,58].
Nevertheless, some biologic DMARDS have been recently associated to a more favorable evolution of RA-ILD, such as rituximab, abatacept, tocilizumab [51,59–61].
Specifically, in a murine model of ILD, abatacept showed an ability to significantly reduce the fibrogenic marker levels, T-cell proliferation, and M1/M2 macrophage lung infiltration [62,63], concurrently improving the fibrosis score at histology and the lung density at HRCT [62,63]. Moreover, in a retrospective cohorts of RA patients, some Authors observed a good safety and effectiveness profile of abatacept in patients complicated by ILD [64,65]. Finally, in a pooled analysis on more than 4000 RA patients treated with abatacept by the ‘Clinical Abatacept Trial Program’, the incidence rate of ILD in the long-extension analysis was very low [66] if compared with other similar study in RA [51].
Of interest, abatacept has been demonstrated to modulate the proinflammatory response upon cytokine-activated T cell and TLR ligand stimulation, including TLR4 [67].
Although speculative, this mechanism could explain the safety and possibly the effectiveness of abatacept in RA-ILD patients and the low rate incidence of ILD in RA patients treated with this drug. On the other hand, in consideration of its interaction with TLR4, abatacept could be evaluated as a possible therapeutic option against SARS-Cov2, before the appearance of severe ARDS.
Considering the rapid evolution of systemic and lung inflammatory life-threatening involvement, it is crucial to early identify clinical and humoral markers, consequence of the reactive hyperimmune response. In this regard, the correct timing of the treatment could deeply impact on the efficacy of the therapy and minimize the possible effects on the viral replication [5,68].
5. Similarities between COVID-19 pneumonia and acute exacerbation of interstitial lung disease
Acute exacerbation (AE) is a well-known complication of interstitial pneumonia, both idiopathic and secondary to other conditions, such as CTDs and RA [69]. Exceptionally, AE can appear also in patients without previous knowledge of ILD or represent the first clinical manifestation of ILD [70].
Diagnosis of AE of ILD in the context of rheumatic disease is quite difficult in clinical practice. Many confusing factors have to be considered, such as opportunistic infections. In this context, multidisciplinary discussion, involving pulmonologists, rheumatologists and thoracic radiologist is crucial to correctly classify the patients [70].
Currently, it is debated whether AE is an externally induced, incidental event or the result of underlying cellular mechanisms [71]. Lung pathology of IPF patients with AE is very similar to that of ARDS showing a diffuse alveolar damage (DAD) and hyaline membranes [69,70,72,73].
Pathogenesis and risk factors for AE in IPF and other ILD are poorly understood [73], but many aspects of DAD observed during SARS-Cov2 infection resemble AE observed in primary or secondary lung fibrosis [69,70,74] and DAD could probably be considered as the common end-stage of different lung pathologies [72].
In this regard, 2 aspects have been specifically addressed in COVID-19 pneumonia: the hyperinflammation, mainly characterized by increase of effector T cells and inflammatory cytokines, in particular IL-6, IL-1, tumor necrosis factor alpha, interferon gamma [5,75–77], and the activation of coagulation cascade [14,20,26]; both findings have been already shown, with some differences, in patients with AE of interstitial pneumonia [72,78–82].
Despite evidence is limited to small cohorts, the macrophage activation is crucial in patients with AE in IPF and it consists not only of an upregulation of proinflammatory (M1) cytokines, but also of cytokines associated with M2 [73].
Two major types of macrophage activation have been described. Classical activation (M1) is mediated by Th1 cytokines and is characterized by the secretion of proinflammatory cytokines, such as TNF-, IL-6, and IL-12 [83]. M1 macrophages are involved in promoting inflammation, extracellular matrix destruction, and apoptosis. Instead, Th2 cytokines has been shown to induce alternatively activated type 2 macrophages (M2), secreting proteins involved in repair and healing, cell proliferation, and angiogenesis. The activated macrophages secrete anti-inflammatory molecules, such as IL-10 and TGF-, inducing down-regulation of the inflammatory processes initiated by Th1 cytokines [84].
In mice, overexpression of IL-1β induces acute lung injury and leads to chronic fibrosis [85,86]. Furthermore, it was reported that injury to alveolar epithelial cells induces M2 macrophage activation [87] and macrophage dependent fibrosis. Schupp observed that, many of the M2 cytokines upregulated in AE, were induced in IPF patients by IL-1β signaling such as CCL2, CCL22 and IL-1ra [88,89].
Of interest, although increased IL-8 and CCL18 serum levels have already been demonstrated to be a worse prognostic factor for survival in IPF, a relationship between these cytokines and an increased risk for AE in IPF has not been shown [73].
Moreover, Schupp recorded a considerable heterogeneity in cytokine production at the moment of IPF diagnosis. M2 cytokines were high in early disease in some patients, while were low even in advanced disease in others. Therefore, the Authors concluded that some patients could be predisposed to AE and the risk for AE was, at least in part, mirrored by M2 cytokine production levels [73].
In COVID-19, the role of M1 macrophages seems to be proved for they play an important role in the pathogenesis of lung disease [90,91], while the activity of M2 macrophages subtype has been less investigated; however, an imbalance in M1/M2 differentiation could be present also in SARS-Cov2 patients, explaining, at least partially, why only some patients undergo to severe ARDS [20,24,31,38,73].
Similar to that observed in COVID-19, early in the development of an IPF-AE, IL-6 and IL-8 peripheral blood levels are significantly increased, and high value of IL-6 and IL-8 levels are related to a higher risk of death in all IPF patients. IL-6 is considered a cardinal stimulator of the production of most acute phase proteins in response to varied stimuli as well as a promoter of specific cellular and humoral immune responses [92].
In particular, Collard and colleagues investigated the plasma biomarker profile of IPF-AE patients in comparison to that of stable IPF and acute lung injury patients measuring not only inflammatory markers such as IL-6, but also markers of type I and II alveolar cell injury/proliferation such as receptor for advanced glycation, Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D), of endothelial injury such as von Willebrand factor (vWF) and of the coagulation cascade such as protein C and plasminogen activator inhibitor-1 (PAI-1) [93]. The authors found that KL-6, SP-D, vWF, IL-6, total protein C and PAI-1 levels were significantly higher in IPF-AE patients than stable IPF patients. The authors concluded that in IPF-AE alveolar epithelial type II injury, endothelial damage and coagulation abnormalities were predominant [93].
An increase of other nonspecific inflammatory markers is observed during COVID-19 pneumonia, representing significant poor prognostic marker of disease and similar observations have been conducted on patients with noninfectious ILD [5,26,78,92].
In this regard, in IPF patients, the 3‐month survival rate was inversely correlated to serum ferritin levels and survival was significantly lower in patients with high values of ferritin (≥500 ng/mL) [94]; moreover, patients developing AE‐IPF showed higher levels of serum ferritin at the diagnosis of IPF [94].
Furthermore, despite the importance of ferritin in the pathogenesis and prognosis of AE‐IPF has yet to be clarified, immunohistochemical staining in autopsy specimens showed that alveolar macrophages were positive for ferritin [94].
According to recent data from the Chinese cohorts, higher levels of IL-6 (>24.3 pg/mL) and D-dimer (>0.28 μg/L) would be predictive of the appearance of severe COVID-19 pneumonia, with high sensitivity and specificity when using a combination of the 2 tests (IL6 and D-dimer) [95].
Even D-dimer and coagulation cascade have been deeply investigated in interstitial pneumonia [78,79,81,93].
Ishikawa evaluated 263 patients with interstitial pneumonia of different etiologies and diagnosed both as IPF, and other idiopathic interstitial pneumonias (namely desquamative interstitial pneumonia, respiratory bronchiolitis interstitial lung disease, acute interstitial lung disease, nonspecific interstitial pneumonia, etc.), CTD-ILD, or chronic pulmonary fibrosis with emphysema. Patients with elevated D-dimer levels (more than 0.4 mcg/mL) had an increased risk of developing AE in ILD within three months from each measurement. Since the median time to AE was between the first and second month from D-dimer measurement the Authors raised a question about the efficacy of prophylactic anticoagulation for subjects with high D-dimer levels [78]. Previous studies on animal and human with pulmonary fibrosis supported anticoagulation as a therapeutic approach in IPF [96,97]. The mortality rate from AE in subjects with IPF receiving anticoagulation (i.e., warfarin for maintenance and switching to low-molecular-weight heparin after developing AE) was low at 18% compared with 71% in patients who did not receive anticoagulation [79,81].
On the other hand, despite an elevated D-dimer suggesting a hypercoagulable state, anticoagulation may not necessarily be a therapeutic target, given the elevated D-dimer may simply reflect upstream tissue damage from the inflammatory process.
Activation of coagulation and excessive inflammatory response are intrinsic findings of ARDS patho-physiology [98].
Cellular damage during ARDS and sepsis can determine the release of mitochondrial DAMPs into the circulation, that further activate polymorphonuclear neutrophils, spreading the inflammatory response [99]. The innate host response to endothelial damage is associated with the activation of coagulation, which in turn regulates and is regulated by the inflammatory process.
The pathological role of coagulation in the innate host response has been defined as immunothrombosis [98,100,101], a humoral regulated process that could contribute both to the protection of endothelial integrity and to inflammatory process.
Endothelial injury triggered the immune-thrombotic process by the formation of microthrombi in the microvessels [97,98,101–104]. Diffuse endothelial damage provides to the exposure of subendothelial collagen, and to the expression of tissue factor and von-Willebrand factor on endothelial cells. According to the stage of disease, widespread presence of various morphological types of thromboemboli have been observed in patients with ARDS [103,105,106].
Despite some Authors reported that microthrombosis could represent a worse prognostic factor for survival [107], the exact role of thrombosis in the propagation of lung damage is not known.
According to the above reported findings, many therapeutic approaches to patients with COVID-19-related ARDS have been proposed, such as inhibition of IL-6, the use of Janus kinases 1–3 inhibitor baricitinib, corticosteroids, antithrombotic therapies (Table 1; in supplementary Table 2 are reported ongoing trials on tocilizumab) [5,68,108–110].
Table 1.
Conventional and biologic DMARDs involved in therapeutic research for COVID19 (updated at June 18,2020).
| Drugs | Rational | Population/Condition | Trials Status/RCTs with results (number) |
|---|---|---|---|
| Chloroquine/Hydroxycloroquine | Anti-inflammatory and immunomodulatory effects, both on adaptive and innate immunity, with inhibition of cytokine, leukotrienes, prostaglandins, proteases and oxyradicals production. It also interfers with lysosomal activity and autophagy, membrane stability and signaling pathways and transcriptional activity, which can result in modulation of certain costimulatory molecules and may interfere with viral infection and replication. Inhibition pH-dependent steps of the replication of several viruses, including SARS-CoV infection. Modulation of glycosylation of cellular receptors of SARS-CoV | From preventative treatment to severe forms of COVID-19, including COVID19-pneumonia | Recruiting or Authorized/0 |
| Leflunomide | Tyrosine kinase inhibition, Inhibition of pyrimidine de novo synthesis | COVID19-pneumonia | Recruiting or Authorized/0 |
| Cyclosporin a | Calcineurin inhibitor. It can inhibit the replication of several coronaviruses, including SARS-COV | Hospitalized COVID19 patients, COVID19-pneumonia | Authorized/0 |
| Tocilizumab | Immunosuppressive effect with IL6-inhibition, efficacy in cytokine release syndrome | COVID19, severe COVID19, COVID19-pneumonia | Recruiting or Authorized/0 |
| Sarilumab | Immunosuppressive effect with IL6-inhibition | Moderate-severe COVID19, Hospitalized COVID19 patients, COVID19-pneumonia | Authorized/0 |
| Anakinra | Immunosuppressive effect with IL1-inhibition, inhibition of the inlammasome | COVID19, COVID19-pneumonia, Cytokine storm syndrome in COVID19 | Authorized/0 |
| Canakinumab | Immunosuppressive effect with IL1-inhibition, inhibition of the inlammasome | COVID19-pneumonia | Authorized/0 |
| Adalimumab | Immunosuppressive effect with TNFalpha-inhibition | Severe COVID19-pneumonia | Not Recruiting/0 |
| Ixekizumab | Immunosuppressive effect with IL17-inhibition. IL17 functions are crucial in different settings of viral infections. For MERS-CoV, SARS-CoV and SARS-CoV-2, the severity of disease was shown to positively correlate with levels of IL-17. The excessive production of IL-17 has been observed in patients with ARDS | COVID19-pneumonia | Recruiting/0 |
| Baricitinib | Inhibition of JAK/STAT pathway, reduction of proinflammatory cytokines, potential inhibition of AP2 associated proteinkinase1 (AAK1) that SARS-CoV-2 uses to infect lung cells through binding with ACE2 | COVID19-pneumonia | Authorized/0 |
| Ruxolitinib | Inhibition of JAK/STAT pathway, reduction of proinflammatory cytokines | COVID19, severe COVID19, severe COVID19-pneumonia, COVID19-ARDS, Cytokine storm syndrome in COVID19 | Recruiting or Authorized/0 |
| Jakotinib | Inhibition of JAK/STAT pathway, reduction of proinflammatory cytokines | Severe and acute exacerbation COVID19-pneumonia | Recruiting/0 |
Table 2.
Clinical trials on COVID-19 involving Tocilizumab.
| Trial number and Title | Status | Conditions | Interventions | Study Type | Phase | Population | Primary Purpose | Allocation | Masking | Intervention Model | Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04317092 Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | Recruiting | COVID-19 Pneumonia | Tocilizumab | Interventional | II | 400 | Treatment | N.a. | Open Label | Single Group | One-month mortality rate; Interleukin-6 level; Lymphocyte count; CRP level; PaO2/FiO2 ratio; Change of the SOFA; Number of participants with treatment-related side effects as assessed by CTCAE version 5.0; Radiological response; Duration of hospitalization; Remission of respiratory symptoms |
| NCT04345445 Study to Evaluate the Efficacy and Safety of Tocilizumab Versus Corticosteroids in Hospitalized COVID-19 Patients With High Risk of Progression | Not yet recruiting | COVID-19 | Tocilizumab; Methylprednisolone | Interventional | III | 310 | Treatment | Randomized | Open Label | Crossover | The proportion of patients requiring mechanical ventilation; Mean days of ventilation; The proportion of patients requiring ICU admission; Overall 28-day survival; Change in symptom severity assessed by the WHO COVID19 ordinal scale measured daily up to 7 days from baseline; Duration of hospital and ICU stay |
| NCT04331795 Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Noncritically Ill Patients With COVID-19 Pneumonitis | Completed | COVID-19 | Tocilizumab | Interventional | II | 32 | Treatment | Nonrandomized | Open Label | Crossover | Clinical response; Biochemical response; Overall survival; Survival to hospital discharge; Progression of COVID-19 pneumonitis; Rate of nonelective mechanical ventilation; Duration of mechanical ventilation; Time to mechanical ventilation; Rate of vasopressor/inotrope utilization; Duration of vasopressor/inotrope utilization; and 3 more |
| NCT04412772 a RCT – Safety & Efficacy of Tocilizumab – Tx of Severe COVID-19: ARCHITECTS | Recruiting | COVID-19 | Tocilizumab; Placebo | Interventional | III | 300 | Treatment | Randomized | Double | Parallel | Clinical status at day 28; Clinical improvement; Mechanical Ventilation; Oxygenation |
| NCT04377750 The Use of Tocilizumab in the Management of Patients Who Have Severe COVID-19 With Suspected Pulmonary Hyperinflammation | Recruiting | Covid19 Pneumonia | Tocilizumab | Interventional | IV | 500 | Treatment | Randomized | Open Label | Parallel | Survival |
| NCT04361032 Assessment of Efficacy and Safety of Tocilizumab Compared to DefeROxamine, Associated With Standards Treatments in COVID-19 (+) Patients Hospitalized In Intensive Care in Tunisia | Not yet recruiting | COVID19 Intensive Care Unit | Tocilizumab Injection; Deferoxamine | Interventional | III | 260 | Treatment | Randomized | Open Label | Parallel | mortality rate |
| NCT04359667 Serum IL-6 and Soluble IL-6 Receptor in Severe COVID-19 Pneumonia Treated With Tocilizumab | Not yet recruiting | COVID-19 Severe Pneumonia | Tocilizumab | Observational, Prospective | N.a. | 30 | N.a. | N.a. | N.a. | N.a. | Serum IL-6 and soluble IL-6 receptor as biomarkers of clinical outcomes in patients with severe COVID-19 pneumonia treated with tocilizumab |
| NCT04332094 Clinical Trial of Combined Use of Hydroxychloroquine, Azithromycin, and Tocilizumab for the Treatment of COVID-19 | Recruiting | COVID-19 | Tocilizumab; Hydroxychloroquine; Azithromycin | Interventional | II | 276 | Treatment | Randomized | Open Label | Parallel | In-hospital mortality; Need for mechanical ventilation in the ICU |
| NCT04377659 Tocilizumab for Prevention of Respiratory Failure in Patients With Severe COVID-19 Infection | Recruiting | COVID-19 | Tocilizumab | Interventional | II | 40 | Treatment | Randomized | Open Label | Parallel | Progression of respiratory failure or death |
| NCT04424056 An Open Randomized Therapeutic Trial Using ANAKINRA, TOCILIZUMAB Alone or in Association With RUXOLITINIB in Severe Stage 2b and 3 of COVID19- associated Disease | Not yet recruiting | COVID-19 | Anakinra ± Ruxolitinib; Anakinra and Ruxolitinib; Tocilizumab ± ruxolitinib; Tocilizumab and Ruxolitinib; Standard of care | Interventional | III | 216 | Treatment | Randomized | Open Label | Parallel | Ventilation free days at Day 28 |
| NCT04346355 Efficacy of Early Administration of Tocilizumab in COVID-19 Patients | Recruiting | COVID-19 Pneumonia | Tocilizumab | Interventional | II | 398 | Treatment | Randomized | Open Label | Parallel | Entry into ICU with invasive mechanical ventilation or death from any cause or clinical aggravation; Death from any cause; Tocilizumab toxicity; Levels of IL-6 and CRP and their correlation with the effectiveness of the treatment; Evaluate the progress of the PaO2/FiO2 ratio; Evaluate the trend over time of the lymphocyte count |
| NCT04335071 Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19) | Recruiting | COVID-19 | Tocilizumab; Placebo | Interventional | II | 100 | Treatment | Randomized | Quadruple | Parallel | Number of patients with ICU admission; Number of patients with intubation; Number of patients with death; Illness severity; Number of patients with clinical improvement; Time to clinical improvement; Duration of hospitalization; Time to ICU admission; Duration of ICU stay; Time to intubation; Duration of mechanical ventilation |
| NCT04412291 a Study in Patients With COVID-19 and Respiratory Distress Not Requiring Mechanical Ventilation, to Compare Standard-of care With Anakinra and Tocilizumab Treatment The Immunomodulation-CoV Assessment (ImmCoVA) Study | Not yet recruiting | COVID-19 | Anakinra; Tocilizumab; Standard-of care treatment | Interventional | II | 120 | Treatment | Randomized | Open Label | Parallel | Time to recovery; Mortality; Number of Days on mechanical ventilation; Number of days of supplemental oxygen use; Number of patients requiring initiation of mechanical ventilation; Time to improvement in oxygenation for at least 48 hours; Mean change in the 8-point ordinal scale; Proportion of patients on level e-h on the 8-point ordinal scale at day 15; Time to improvement in one category from admission using the 8- point ordinal scale; Mean change in SOFA; and 19 more |
| NCT04403685 Safety and Efficacy of Tocilizumab in Moderate to Severe COVID-19 With Inflammatory Markers | Recruiting | COVID SARS Pneumonia; Cytokine Release Syndrome | Tocilizumab | Interventional | III | 150 | Treatment | Randomized | Open Label | Parallel | Evaluation of clinical status; All-cause mortality; Inpatient Mortality; Improvement of SOFA scale; Ventilator free days; Time until oxygen support independence; Need of mechanical ventilation support; Days to mechanical ventilation support.; Duration of hospitalization; Other infections; Incidence of thromboembolic events; Incidence of adverse events |
| NCT04356937 Efficacy of Tocilizumab on Patients With COVID-19 | Not yet recruiting | COVID-19 | Tocilizumab; Placebo | Interventional | III | 300 | Treatment | Randomized | Double | Parallel | Proportion of patients that require mechanical ventilation; Requirement for inotropes and/or vasopressors; 8-level Clinical improvement Scale; Duration of mechanical ventilation; Hospital discharge; Mortality; Duration of ICU stay; Duration of time on supplemental oxygen; The proportion of patients who require renal replacement therapy or have doubling of creatinine |
| NCT04372186 a Study to Evaluate the Efficacy and Safety of Tocilizumab in Hospitalized Participants With COVID-19 Pneumonia | Recruiting | COVID-19 Pneumonia | Placebo; Tocilizumab | Interventional | III | 379 | Treatment | Randomized | Double | Parallel | Cumulative Proportion of Participants Requiring Mechanical Ventilation by Day 28; Time to Improvement of at Least 2 Categories Relative to Baseline on a 7-Category Ordinal Scale of Clinical Status; Time to Clinical Failure,; Mortality Rate by Day 28; Time to Hospital Discharge or ‘Ready for Discharge’; Percentage of Participants with Adverse Events; Percentage of Participants with any Post-Treatment Bacterial and/or Fungal Infection; Incidence of Posttreatment Acute Kidney injury (defined by 50% increase of creatinine from baseline) |
| NCT04320615 a Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | Active, not recruiting | COVID-19 Pneumonia | Tocilizumab; Placebo | Interventional | III | 450 | Treatment | Randomized | Double | Parallel | Clinical Status Assessed Using a 7-Category Ordinal Scale; Time to Clinical Improvement; |
| NCT04363736 a Study to Investigate Intravenous Tocilizumab in Participants With Moderate to Severe COVID-19 Pneumonia | Recruiting | COVID-19 Pneumonia | Tociliuzumab | Interventional | II | 100 | Treatment | Randomized | Open Label | Parallel | Serum Concentration of IL-6 Following Administration of Tocilizumab; Serum Concentration of Soluble Interleukin-6 Receptor Following Administration of Tocilizumab; Serum Concentration of Ferritin Following Administration of Tocilizumab; Serum Concentration of C-reactive Protein Following Administration of Tocilizumab; Pecentage of Participants with Adverse Events; SARS-CoV-2 Viral Load Over Time; Time to Real-Time Polymerase Chain Reaction Virus Negativity; |
| NCT04377503 Tocilizumab Versus Methylprednisolone in the Cytokine Release Syndrome of Patients With COVID-19 | Not yet recruiting | Cytokine Release Syndrome by Covid-19 | Tocilizumab; Methylprednisolone | Interventional | II | 40 | Treatment | Randomized | Open Label | Crossover | Patient clinical status 15 days after randomization; Improving oxygenation; Thorax CT improvement; ICU length of stay; Duration of mechanical ventilation; Incidence of acute kidney with necessity of renal replacement therapy |
| NCT04332913 Efficacy and Safety of Tocilizumab in the Treatment of SARS-Cov-2 Related Pneumonia | Recruiting | COVID-19 Pneumonia | Observational, Prospective | N.a. | 30 | N.a. | N.a. | N.a. | N.a. | Percentage of patients with complete recovery defined as fever disappearance and return to normal peripheral oxygen saturation values (SpO2) after 14 days from the end of treatment with tocilizumab.; | |
| NCT04363853 Tocilizumab Treatment in Patients With COVID-19 | Recruiting | COVID-19 | Tocilizumab | Interventional | II | 200 | Treatment | N.a: | Open Label | Single group | Hematic biometry; Blood chemistry; Blood gas; blood gas; thorax radiography |
| NCT04409262 a Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia | Not yet recruiting | COVID-19 Pneumonia | Remdesivir; Tocilizumab; Placebo | Interventional | III | 450 | Treatment | Randomized | Double | Parallel | Clinical Status as Assessed by the Investigator Using a 7- Category Ordinal Scale of Clinical Status on Day 28; Time to Clinical Improvement; |
| NCT04335305 Checkpoint Blockade in COVID-19 Pandemic | Recruiting | COVID-19 Pneumonia | Tocilizumab; Pembrolizumab (MK-3475) | Interventional | II | 24 | Treatment | Randomized | Open Label | Parallel | Percentage of patients with normalization of SpO2 > 96% on room air; Proportion of patients discharged from the emergency department; Number of days of patient hospitalization; Change from baseline in organ failure parameters; Proportion of mortality rate; Analysis of the remission of respiratory symptoms; Evaluation of the radiological response; Time to first negative in SARS-CoV-2 RT-PCR test; Change from baseline of absolute lymphocyte count, white blood cell count and white blood cell differential count; Change from baseline of hemoglobin |
| NCT04306705 Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID-19 | Recruiting | COVID-19 SARS; Cytokine Storm; Cytokine Release Syndrome | Tocilizumab; Standard of care; | Observational, Retrospective | N.a. | 120 | N.a. | N.a. | N.a. | N.a. | Proportion of Participants With Normalization of Fever and Oxygen Saturation Through Day 14; Duration of hospitalization; Proportion of Participants With Normalization of Fever Through Day 14; Change from baseline in white blood cell and differential count; Time to first negative in 2019 novel Corona virus RT-PCR test; All-cause mortality; Change from baseline in hsCRP; Change from baseline in cytokines IL-1 R, IL-10, sIL-2 R, IL-6, IL-8 and TNF- alfa; Change from baseline in proportion of CD4+ CD3/CD8+ CD3 T cells |
| NCT04310228 Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | Recruiting | COVID-19 | Favipiravir Combined With Tocilizumab; Favipiravir; Tocilizumab | Interventional | N.a. | 150 | Treatment | Randomized | Open Label | Parallel | Clinical cure rate; Viral nucleic acid test negative conversion rate and days from positive to negative; Duration of fever; Lung imaging improvement time; Mortality rate because of COVID-19; Rate of noninvasive or invasive mechanical ventilation when respiratory failure occurs; Mean in-hospital time |
| NCT04370834 Tocilizumab for Patients With Cancer and COVID-19 Disease | Recruiting | Pneumonitis; Severe Acute Respiratory Distress Syndrome; Symptomatic COVID-19 Infection | Tocilizumab | Interventional | II | 217 | Treatment | N.a. | Open Label | Single group | Clinical outcome as evaluated by the 7-category Clinical Status Ordinal Scale |
| NCT04339712 Personalized Immunotherapy for SARS-CoV-2 (COVID-19) Associated With Organ Dysfunction | Recruiting | COVID-19 Disease; Macrophage Activation Syndrome; | Anakinra; Tocilizumab | Interventional | II | 40 | Treatment | Nonrandomized | Open Label | Factorial | Change of baseline total SOFA score; Improvement of lung involvement measurements; Increase of pO2/FiO2 ratio; Comparison of change of baseline total SOFA score in enrolled subjects toward historical comparators; Change of SOFA score; Rate of Mortality; Cytokine stimulation; Gene expression; Serum/plasma proteins; Classification of the immune function; |
| NCT04315480 Tocilizumab for SARS-CoV2 (COVID-19) Severe Pneumonitis | Active, not recruiting | COVID-19 | Tocilizumab | Interventional | II | 38 | Treatment | N.a. | Open Label | Single group | Arrest in deterioration of pulmonary function; improving in pulmonary function; need of oro-tracheal intubation; death |
| NCT04333914 Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection | Suspended | COVID-19 in Advanced or Metastatic Hematological or Solid Tumor | Chloroquine analog (GNS651); Nivolumab; Tocilizumab; Standard of care; Avdoralimab; Monalizumab | Interventional | II | 384 | Treatment | Randomized | Open Label | Parallel | 28-day survival rate; Time to clinical improvement; Clinical status; Mean change in clinical status from baseline to days; Overall survival; Length of stay in ICU; Duration of mechanical ventilation or high flow oxygen devices; Duration of hospitalization; Rate of throat swab negativation; Quantitative SARS-CoV-2 virus in throat swab and blood samples; and 4 more |
| NCT04423042 Tocilizumab in Coronavirus-19 Positive Patients | Not yet recruiting | COVID-19 Severe Acute Respiratory Syndrome; | Tocilizumab | Interventional | III | 30 | Treatment | Nonrandomized | Open Label | Single group | All-cause mortality; Ordinal Scale for evaluating subject clinical status at days 3, 8, 15, 30, 60 post treatment. |
| NCT04361552 Tocilizumab for the Treatment of Cytokine Release Syndrome in Patients With COVID-19 (SARSCoV-2 Infection) | Recruiting | Cerebrovascular Accident; Chronic Obstructive Pulmonary Disease; Chronic Renal Failure; Coronary Artery Disease; Diabetes Mellitus; Malignant Neoplasm; COVID-19 | Best Practice; Tocilizumab | Interventional | III | 180 | Treatment | Randomized | Open Label | Parallel | 7-day length of invasive mechanical ventilation (MV); 30-day mortality rate; Rate of ICU transfer; Rate of invasive mechanical ventilation; Rate of tracheostomy; Length of ICU stay; Length of hospital stay |
| NCT04330638 Treatment of COVID-19 Patients With Anti-interleukin Drugs | Recruiting | COVID-19 | Usual Care; Anakinra; Siltuximab; Tocilizumab | Interventional | III | 342 | Treatment | Randomized | Open Label | Factorial | Time to Clinical Improvement; Time to improvement in oxygenation; Mean change in oxygenation; Number of days with hypoxia; Number of days of supplemental oxygen use; Time to absence fever for more than 48 h without antipyretics; Number of days with fever; Time to halving of CRP levels compared to peak value during trial; Time to halving of ferritin levels compared to peak value during trial; Incidence of AEs; and 29 more |
| NCT04322773 Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure | Recruiting | COVID-19 | Tocilizumab iv/sc; sarilumab sc; Standard medical care | Interventional | II | 200 | Treatment | Randomized | Open Label | Sequential | Time to independence from supplementary oxygen therapy; Number of deaths; Days out of hospital and alive; Ventilator free days alive and out of hospital; CRP level; Number of participants with serious adverse events |
| NCT04386239 Study on the Use of Sarilumab in Patients With COVID-19 Infection | Not yet recruiting | COVID-19 | Sarilumab | Interventional | I | 40 | Treatment | N.a. | Open Label | Single Group | Proportion of patients who show an improvement of the respiratory function; Evaluation of the time to resolution of fever; Evaluation of the viral load on blood and sputum for COVID-19; Evaluation of the plasma concentration of GM-CSF; Evaluation of the plasma concentration of Il-6; Evaluation of the plasma concentration of TNF-alfa; Evaluation of the rate of progression of White Blood Cell fraction |
| NCT04381936 Randomized Evaluation of COVID-19 Therapy | Recruiting | COVID-19 | Lopinavir/Ritonavir; Corticosteroid; Hydroxychloroquine; Azithromycin; Convalescent plasma; Tocilizumab | Interventional | II/III | 12,000 | Treatment | Randomized | Open Label | Factorial | All-cause mortality; Duration of hospital stay; Need for (and duration of) ventilation; Composite endpoint of death or need for mechanical ventilation or ECMO |
| NCT04331808 CORIMUNO-19 – Tocilizumab Trial – TOCI (CORIMUNO-TOCI) | Active, not recruiting | COVID-19 | Tocilizumab | Interventional | II | 228 | Treatment | Randomized | Open Label | Parallel | Survival without needs of ventilator utilization at day 14. Group 1; WHO progression scale ≤5 at day 4; Cumulative incidence of successful tracheal extubation at day 14. Group 2.; WHO progression scale at day 4. Group 2.; WHO progression scale; Survival; 28-day ventilator free-days; respiratory acidosis at day 4; PaO2/FiO2 ratio; time to oxygen supply independency; and 4 more |
| NCT04380818 Low Dose Anti-inflammatory Radiotherapy for the Treatment of Pneumonia by COVID-19 | Recruiting | Pneumonia, Viral | Low dose radiotherapy; Hydroxychloroquine Sulfate; Ritonavir/lopinavir; Tocilizumab; Azithromycin; Corticosteroid; Low molecular weight heparin; | Interventional | N.a. | 106 | Treatment | Randomized | Open Label | Parallel | Efficacy of low-dose pulmonary irradiation assessed by change in PAFI O2 by 20%; Number of participants with treatment-related adverse events as assessed by CTCAE v5.0; Change of the radiological image; Overall mortality; Measure of proinflammatory interleukins; Measure of transforming growth factor; Measure of tumor necrosis factor alpha; Determining overexpression of proinflammatory selectin; Determining cell adhesion molecules; Measure of marker of oxidative stress PON-1 |
| NCT04349410 The Fleming [FMTVDM] Directed CoVid-19 Treatment Protocol | Enrolling by invitation | COVID-19 | Hydroxychloroquine/Azithromycin; Hydroxychloroquine/Doxycycline; Hydroxychloroquine/Clindamycin; Hydroxychloroquine/Clindamycin/Primaquine/Remdesivir; Tocilizumab; Methylprednisolone; Interferon Alpha 2B; Losartan; Convalescent Serum | Interventional | II/III | 500 | Treatment | Randomized | Single | Factorial | Improvement in FMTVDM Measurement with nuclear imaging.; Ventilator status; Survival status |
| NCT04394182 Ultra Low Doses of Therapy With Radiation Applicated to COVID-19 | Recruiting | COVID-19 Pneumonia, Viral Cytokine Storm | Radiation: Ultra-Low-dose radiotherapy; Device: ventilatory support with oxygen therapy; Lopinavir/ritonavir; Hydroxychloroquine; Azithromycin; Piperacillin/tazobactam; Low molecular weight heparin; Corticosteroid injection; Tocilizumab | Interventional | N.a. | 15 | Supportive Care | N.a. | Open label | Single group | Oxygen Therapy Status at Day 2; Oxygen Saturation at Day 2; Blood Gas Analysis at Day 2; Blood Test at Day 2; Oxygen Therapy Status at Day 5; Oxygen Saturation at Day 5; Blood Test at Day 5; Oxygen Therapy Status at Day 7; Oxygen Saturation at Day 7; Blood Test at Day 7; and 5 more |
Legend: CRP: C-reactive protein; PaO2: partial pressure of oxygen; FiO2: fraction of inspired oxygen; SOFA: Sequential Organ Failure Assessment; CTCAE: Common Terminology Criteria for Adverse Event; COVID-19: Coronavirus Disease 2019; ICU: Intensive Care Unit; WHO: World Health Organization; IL-6: interleukin-6; ECMO: ExtraCorporeal Membrane Oxygenation; RT-PCR: reverse transcription-polymerase chain reaction; ARDS: Acute Respiratory Distress Syndrome; PK: Pharmacokinetic modeling; N.a.: Not available
Many trials are ongoing on these drugs, all targeting the immune response of the host and not the virus itself [68]. Other possible targets, as previously reported, could be TLR4, considering the involvement of innate immunity also in virus-induced ARDS [38–41,45,62,63,67].
For all these reasons, the IL-6 inhibitors, baricitinib and also TLR4 antagonists could be proposed in patients with AE related to other forms of ILD, such as IPF or RA-ILD. In these latter, where the involvement of the immune system is more evident, the use of these drugs could be more appropriate.
6. Conclusions
COVID-19 pneumonia is a main medical emergency involving many thousands of people around the World. Many efforts are ongoing to understand the pathogenesis of the disease and the possible therapeutic approach [1,111]. Many drugs, normally used in immune-mediated disorders have been proposed with different aims [5,68,112–114] and some trials are aiming to confirm their efficacy, mainly on the lung manifestations of COVID-19 [68].
As discussed by other authors, a relevant question concerns the possibility of identifying the right ‘window of opportunity’ for the therapeutic intervention [68]. In fact, different immune-mediated pathways are probably activated at various stages of the disease and in different patients, and our ability to identify them could be essential for the therapeutic response [2,5,68].
If on the one hand the similarities with other forms of acute and chronic lung diseases could allow us to successfully propose anti-inflammatory and immune-modulator drugs already used in such diseases, on the other the investigations on COVID-19 pathogenesis could help us to have a better understanding of other rare conditions and their treatment, also [5,68].
The ongoing research on COVID-19 could highlight many other similarities with UIP, possibly improving the knowledge in this field. For example, very recently, another common point with UIP has been reported, after the observation of a higher prevalence of smokers in COVID-19 related ARDS when compared with patients with mild disease [7,52,115].
In conclusion, despite the uncertainty about many pathogenetic aspects about COVID-19-related pneumonia, we are probably observing a possible model for other forms of ILD and AE. The great amount of data deriving from studies on COVID-19 could be helpful in proposing new therapeutic approach for AE of ILD and in understanding of pathogenesis of UIP related to autoimmune systemic diseases.
7. Expert opinion
Since 2009 our multidisciplinary team is cooperating in the diagnosis, clinical assessment and management of patients affected by systemic autoimmune disorders complicated by lung involvement. a multidisciplinary evaluation, including expert pulmonologists and rheumatologists, together with radiologists, cardiologists, thoracic surgeons, and pathologists is performed in selected cases.
Differential diagnosis of an AE-ILD in the context of a rheumatic disease includes mainly opportunistic infections, pulmonary embolism and heart failure. In this context, our multidisciplinary group actively collaborate in evaluating and treating these patients [51,60,61,70,116] (see Figure 2).
Figure 2.
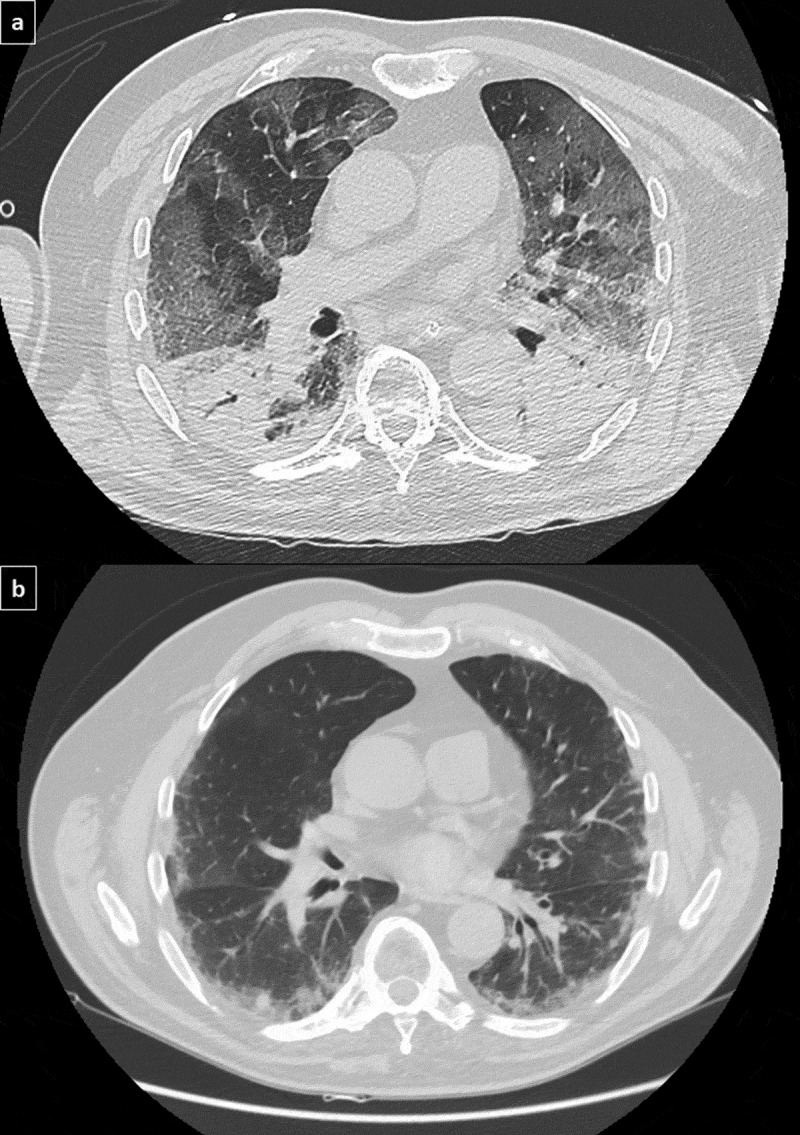
Ground-glass areas in COVID-19 pneumonia (Figure 2a) in a patient with ankylosing spondylitis and preexisting fibrosing nonspecific interstitial pneumonia (Figure 2b).
Management of both idiopathic and RA-related ILD is challenging. While in IPF the use of antifibrotic agents, nintedanib and pirfenidone, has been largely validated in various clinical trials, treatment of RA- or CTD-ILD is debated.
In fact, the therapeutic approach to RA-ILD patients is complicated by some unresolved matters. First, both conventional and biologic DMARDs have been implicated in the development of drug-related pulmonary toxicity with conflicting data; secondly, there is no evidence that the lung involvement could benefit by the treatment of RA. Nevertheless, immunosuppressive drugs used in CTD or antifibrotic drugs are not effective on joint involvement of RA, suggesting that RA-ILD treatment is not the same as treating RA in patients with concurrent ILD.
For all these reasons, to enhance our knowledge on pathogenesis of RA-ILD, in particular UIP pattern, could contribute not only to the development of drugs effective on lung involvement, but also to increase the safety of DMARDs effective on arthritis.
TLR4 has been demonstrated to be involved in the induction of inflammatory damage during acute viral infections and TLR4 activation has been clearly identified as responsible of the severity of some viral diseases such as COVID-19 and might represent the link between viral infection, inflammation and fibrosis.
In this regard, abatacept has been demonstrated to modulate the proinflammatory response upon cytokine-activated T cell and TLR ligand stimulation, including TLR4 and this mechanism could be, at least partially, explains the good safety and effectiveness of such drugs on ILD the low incidence rate of ILD in RA patients treated with abatacept. Concurrently, the TLR4 modulation could reasonably justify the safety demonstrated by abatacept in the treatment of RA patients complicated by ILD. In particular, a very low rate of AE has been reported in RA-ILD patients treated with abatacept.
AE is one of the main causes of death in patients with interstitial pneumonia, both idiopathic and secondary to other conditions, such as CTDs and RA. COVID-19 pneumonia represents an impressive model of AE of ILD, resembling both the hyperinflammation, mainly characterized by increase of effector T cells and inflammatory cytokines, and the activation of coagulation cascade. Similar to that observed in COVID-19, early in the development of an IPF-AE, IL-6, and IL-8 peripheral blood levels are significantly increased, and high value of IL-6 and IL-8 levels are related to a higher risk of death in all IPF patients. Similarly, D-dimer increase, hypercoagulability and microembolism have been described in AE and anticoagulation has been evaluated as a possible therapeutic target in IPF.
Therefore, the formidable effort in research on COVID-19 could contribute to the development of novel therapeutic strategy for AE of both idiopathic and secondary-ILD. Many therapeutic approaches to patients with COVID-19-related ARDS have been proposed, such as inhibition of IL-6, the use of Janus kinases 1–3 inhibitor baricitinib, antithrombotic therapies (Table 1 and supplementary Table 2) and should be specifically evaluated in randomized clinical trials, rather than encourage the use of drugs without evidence. All these drugs could be properly investigated in AE of ILD, in particular in patients with RA or CTD-ILD, where the involvement of the immune system and the inflammatory state is more evident.
Although some experience with TLR4 inhibitors in the treatment of RA have been unsatisfactory, other molecules are currently under observation. However, since at least 15–20% of patients shows ILD, TLR4 might represent a possible target not only for the treatment of RA joint involvement, but also for a safe and additional therapy in patients with ILD.
Finally, specific randomized clinical trials should be conducted on some biologic DMARDs, such as abatacept, tocilizumab or baricitinib, both to evaluate the safety on RA-ILD and the ability in prevention and treatment of AE.
Supplementary Material
Acknowledgments
a special thanks to Gabriele D’Andrea from the Radiology Unit of San Gerardo Hospital of Monza, for the support in iconography
Funding Statement
This paper was not funded.
Article highlights
COVID-19 pneumonia resembles many pathogenetic and radiologic features of idiopathic and rheumatoid arthritis related usual interstitial pneumonia
Toll-like receptor 4 plays a crucial role in the pathogenesis of both COVID-19 and usual interstitial pneumonia
The modulation of toll-like receptor 4 could explain the safety of some disease modifying antirheumatic drugs, such as abatacept, in patients with rheumatoid arthritis related interstitial lung disease
Acute exacerbation of interstitial lung disease shares many features with acute respiratory distress syndrome in COVID-19 pneumonia
The use of tocilizumab, sarilumab and baricitinib, used in severe COVID-19 pneumonia, could also be suggested for the treatment of acute exacerbation of interstitial lung disease
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Supplementary material
Supplemental data for this article can be accessed here.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Lu X, Xu C, et al. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y, Deng H, Huang L, et al. Analysis of Characteristics in Death Patients with COVID-19 Pneumonia without Underlying Diseases. Acad Radiol. 2020;27:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 6.Solomon JJ, Fischer a. Connective tissue disease-associated interstitial lung disease. J Intensive Care Med. 2015;30:392–400. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Prim. 2017;3:17074. [DOI] [PubMed] [Google Scholar]; •• Cohomprensive review about idiopathic pulmonary fibrosis.
- 8.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Cohomprensive review on the radiological findings of COVID-19 pneumonia.
- 9.Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49:1–9. [PubMed] [Google Scholar]
- 10.Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020. Mar 13:nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai H, Zhang X, Xia J, et al. High-resolution chest CT features and clinical characteristics of patients infected with COVID-19 in Jiangsu, China. Int J Infect Dis. 2020;95:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salehi S, Abedi a, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020:1–7. DOI: 10.2214/AJR.20.23672 [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The article firstly analyzed the radiological features of COVID-19 pneumonia.
- 14.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020:NEJMoa2020283. DOI: 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godri Pollitt KJ, Peccia J, Ko AI, et al. COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum Genomics. 2020;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, et al. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallenave J-M, Guillot L.. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in Covid-19: key therapeutic targets? Front Immunol. 2020;11:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olejnik J, Hume AJ, Mühlberger E.. Toll-like receptor 4 in acute viral infection: too much of a good thing. PLoS Pathog. 2018;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The article fully explains the role of TLR4 in viral infection.
- 21.Pierer M, Wagner U, Rossol M, et al. Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen-induced arthritis in DBA1j mice. PLoS One. 2011;6:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharyya S, Wang W, Qin W, et al. TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. JCI Insight. 2018;3:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papanikolaou IC, Boki KA, Giamarellos-Bourboulis EJ, et al. Innate immunity alterations in idiopathic interstitial pneumonias and rheumatoid arthritis-associated interstitial lung diseases. Immunol Lett. 2015;163:179–186. [DOI] [PubMed] [Google Scholar]
- 24.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitfield SJC, Taylor C, Risdall JE, et al. Interference of the T cell and antigen-presenting cell costimulatory pathway using CTLA4-Ig (Abatacept) prevents staphylococcal enterotoxin B pathology. J Immunol. 2017;198:3989–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongartz T, Cantaert T, Atkins SR, et al. Summary for Policymakers. In: Intergovernmental Panel on Climate Change , editor. Clim. Chang. 2013 - Phys. Sci. Basis. 3rd ed. Cambridge: Cambridge University Press; 2017. p. 1–30. [Google Scholar]
- 28.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younan P, Ramanathan P, Graber J, et al. The toll-like receptor 4 antagonist eritoran protects mice from lethal filovirus challenge. Vogel SN, Patton JT, editors. MBio. 2017;8. DOI: 10.1128/mBio.00226-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marr N, Turvey SE. Role of human TLR4 in respiratory syncytial virus-induced NF-κB activation, viral entry and replication. Innate Immun. 2012;18:856–865. [DOI] [PubMed] [Google Scholar]
- 31.Kuzmich N, Sivak K, Chubarev V, et al. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel). 2017;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H, Han X, Cao Y, et al. CT screening for early diagnosis of SARS-CoV-2 infection – authors’ reply. Lancet Infect Dis. 2020;3099:20200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Q, Lu Y, Lure F, et al. Clinical and radiological features of novel coronavirus pneumonia. J Xray Sci Technol. 2020;28:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020. DOI: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The article reviews pathogenesis of fibrotic aspects of COVID-19 pneumonia and possible role of antifibrotic therapies.
- 35.Karampitsakos T, Woolard T, Bouros D, et al. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol. 2017;808:35–43. [DOI] [PubMed] [Google Scholar]
- 36.Ebener S, Barnowski S, Wotzkow C, et al. Toll-like receptor 4 activation attenuates profibrotic response in control lung fibroblasts but not in fibroblasts from patients with IPF. Am J Physiol - Lung Cell Mol Physiol. 2017;312:L42–L55. [DOI] [PubMed] [Google Scholar]
- 37.Go H, Koh J, Kim HS, et al. Expression of toll-like receptor 2 and 4 is increased in the respiratory epithelial cells of chronic idiopathic interstitial pneumonia patients. Respir Med. 2014;108:783–792. [DOI] [PubMed] [Google Scholar]
- 38.Yang H-Z, Wang J-P, Mi S, et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–292. [DOI] [PubMed] [Google Scholar]
- 39.Yang H-Z, Cui B, Liu H-Z, et al. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J Immunol. 2009;182:692–702. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H, Leu S-W, Shi L, et al. TLR4 is a negative regulator in noninfectious lung inflammation. J Immunol. 2010;184:5308–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Z, Zhu Y, Jiang H. Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir Res. 2009;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Z, Gao Y, Deng Y, et al. Lipopolysaccharide induces lung fibroblast proliferation through toll-like receptor 4 signaling and the phosphoinositide3-kinase-akt pathway. Sanchez-Margalet V, editor. PLoS One. 2012;7:e35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noth I, Zhang Y, Ma S-F, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doz E, Noulin N, Boichot E, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. [DOI] [PubMed] [Google Scholar]
- 45.Tao L, Yang J, Cao F, et al. Mogroside IIIE, a novel anti-fibrotic compound, reduces pulmonary fibrosis through toll-like receptor 4 pathways. J Pharmacol Exp Ther. 2017;361:268–279. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharyya S, Kelley K, Melichian DS, et al. Toll-like receptor 4 signaling augments transforming growth factor-β responses. Am J Pathol. 2013;182:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharyya S, Tamaki Z, Wang W, et al. FibronectinEDA promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med. 2014;6:232ra50–232ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T, Asano Y, Ichimura Y, et al. Amelioration of tissue fibrosis by toll-like receptor 4 knockout in murine models of systemic sclerosis. Arthritis Rheumatol. 2015;67:254–265. [DOI] [PubMed] [Google Scholar]
- 49.Saigusa R, Asano Y, Taniguchi T, et al. Multifaceted contribution of the TLR4-activated IRF5 transcription factor in systemic sclerosis. Proc Natl Acad Sci. 2015;112:15136–15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balbir-Gurman a, Guralnik L, Yigla M, et al. Imaging aspects of interstitial lung disease in patients with rheumatoid arthritis: literature review. Autoimmun Rev. 2018;17:87–93. [DOI] [PubMed] [Google Scholar]
- 51.Cassone G, Manfredi a, Vacchi C, et al. Treatment of rheumatoid arthritis-associated interstitial lung disease: lights and shadows. JCM. 2020;9:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brito Y, Glassberg MK, Ascherman DP. Rheumatoid arthritis-associated interstitial lung disease: current concepts. Curr Rheumatol Rep. 2017;19. DOI: 10.1007/s11926-017-0701-5 [DOI] [PubMed] [Google Scholar]
- 53.Samarpita S, Kim JY, Rasool MK, et al. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res Ther. 2020;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teoh AKY, Corte TJ. Nonspecific interstitial pneumonia. Semin Respir Crit Care Med. 2020;41:184–201. [DOI] [PubMed] [Google Scholar]
- 55.Yoshinouchi T, Naniwa T, Shimizu S, et al. Expression of chemokine receptors CXCR3 and CCR4 in lymphocytes of idiopathic nonspecific interstitial pneumonia. Respir Med. 2007;101:1258–1264. [DOI] [PubMed] [Google Scholar]
- 56.Sacre SM, Drexler SK, Andreakos E, et al. Could toll-like receptors provide a missing link in chronic inflammation in rheumatoid arthritis? Lessons from a study on human rheumatoid tissue. Ann Rheum Dis. 2007;66:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monnet E, Choy EH, McInnes I, et al. Efficacy and safety of NI-0101, an anti-toll-like receptor 4 monoclonal antibody, in patients with rheumatoid arthritis after inadequate response to methotrexate: a phase II study. Ann Rheum Dis. 2020;79:316–323. [DOI] [PubMed] [Google Scholar]
- 58.Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2014;43:613–626. [DOI] [PubMed] [Google Scholar]
- 59.Schwind LTF, Arrieira RL, Dias JD, et al. The structure of planktonic communities of testate amoebae (Arcellinida and uglyphida) in three environments of the Upper Paraná River basin, Brazil. J Limnol. 2016;75:78–89. [Google Scholar]
- 60.Manfredi a, Cassone G, Furini F, et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicenter retrospective study. Intern Med J. 2019:imj.14670. DOI: 10.1111/imj.14670 [DOI] [PubMed] [Google Scholar]
- 61.Guaraldi G, Meschiari M, Cozzi-Lepri a, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020. DOI: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The article firstly describes a large cohort of COVID-19 patients treated with tocilizumab.
- 62.Boleto G, Guignabert C, Pezet S, et al. T-cell costimulation blockade is effective in experimental digestive and lung tissue fibrosis. Arthritis Res Ther. 2018;20:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiménez-Alvarez L, Arreola JL, Ramírez-Martínez G, et al. The effect of CTLA-4Ig, a CD28/B7 antagonist, on the lung inflammation and T cell subset profile during murine hypersensitivity pneumonitis. Exp Mol Pathol. 2011;91:718–722. [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Díaz C, Loricera J, Castañeda S, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: a national multicenter study of 63 patients. Semin Arthritis Rheum. 2018;48:22–27. [DOI] [PubMed] [Google Scholar]
- 65.Mochizuki T, Ikari K, Yano K, et al. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod Rheumatol. 2019;29:413–417. [DOI] [PubMed] [Google Scholar]
- 66.Weinblatt ME, Moreland LW, Westhovens R, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol. 2013;40:787–797. [DOI] [PubMed] [Google Scholar]
- 67.Wenink MH, Santegoets KCM, Platt AM, et al. Abatacept modulates proinflammatory macrophage responses upon cytokine-activated T cell and Toll-like receptor ligand stimulation. Ann Rheum Dis. 2012;71:80–83. [DOI] [PubMed] [Google Scholar]
- 68.Ferro F, Elefante E, Baldini C, et al. COVID-19: the new challenge for rheumatologists. Clin Exp Rheumatol. 2020;38:175–180. [PubMed] [Google Scholar]; • The article explores the possible role of antirheumatic drugs in COVID-19 pneumonia.
- 69.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med. 2016;194:265–275. [DOI] [PubMed] [Google Scholar]
- 70.Manfredi a, Sebastiani M, Cerri S, et al. Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: a prospective study and review of the literature. J Thorac Dis. 2019;11:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The article exhaustively review the findings of acute exacerbation of interstitial lung disease in rheumatic patients and describes a prospective cohort.
- 71.Johannson KA, Vittinghoff E, Lee K, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung O-Y, Graziano P, Smith ML. Acute lung injury. In: Pract. Pulm. Pathol. a Diagnostic Approach. 3rd ed. Elsevier; 2018. p. 125–146.e3. [Google Scholar]
- 73.Schupp JC, Binder H, Jäger B, et al. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One. 2015;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–839. [DOI] [PubMed] [Google Scholar]
- 75.Yildizhan E, Kaynar L. Cytokine release syndrome. J Oncol Sci. 2018;4:134–141. [Google Scholar]
- 76.Rooney C, Sauer T. Modeling cytokine release syndrome. Nat Med. 2018;24:705–706. [DOI] [PubMed] [Google Scholar]
- 77.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. iagnosis-and-management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishikawa G, Acquah SO, Salvatore M, et al. Elevated serum D-dimer level is associated with an increased risk of acute exacerbation in interstitial lung disease. Respir Med. 2017;128:78–84. [DOI] [PubMed] [Google Scholar]
- 79.Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–1482. [DOI] [PubMed] [Google Scholar]
- 80.Kondoh Y, Azuma a, Inoue Y, et al. Thrombomodulin alfa for acute exacerbation of idiopathic pulmonary fibrosis: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2020;201:1110–1119. [DOI] [PubMed] [Google Scholar]
- 81.Kubo H, Yanai M, Azuma A. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis: a hidden subgroup? Am J Respir Crit Care Med. 2013;187(9):1029–1030. [DOI] [PubMed] [Google Scholar]
- 82.Noth I, Olman M. Reply: a placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis: a hidden subgroup? Am J Respir Crit Care Med. 2013;187:1030. [DOI] [PubMed] [Google Scholar]
- 83.Mora AL, Torres-González E, Rojas M, et al. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 85.Kolb M, Margetts PJ, Anthony DC, et al. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ganter MT, Roux J, Miyazawa B, et al. Interleukin-1β causes acute lung injury via αvβ5 and αvβ6 integrin–dependent mechanisms. Circ Res. 2008;102:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osterholzer JJ, Olszewski MA, Murdock BJ, et al. Implicating exudate macrophages and Ly-6C high monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brieland JK, Flory CM, Jones ML, et al. Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-alpha, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1995;12:104–109. [DOI] [PubMed] [Google Scholar]
- 89.Rodenburg RJT, Brinkhuis RFB, Peek R, et al. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1 β, tumor necrosis factor α, and lipopolysaccharide. J Leukoc Biol. 1998;63:606–611. [DOI] [PubMed] [Google Scholar]
- 90.Ren YH, Wang SY, Liu M, et al. When COVID-19 encounters interstitial lung disease: challenges and management. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E039. [DOI] [PubMed] [Google Scholar]
- 91.Sallenave JM, Guillot L. Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front Immunol. 2020;11:1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papiris SA, Tomos IP, Karakatsani a, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine. 2018;102:168–172. [DOI] [PubMed] [Google Scholar]
- 93.Collard HR, Calfee CS, Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Cell Mol Physiol. 2010;299:L3–L7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J. 2018;12:2378–2389. [DOI] [PubMed] [Google Scholar]
- 95.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Günther a, Mosavi P, Ruppert C, et al. Enhanced tissue factor pathway activity and fibrin turnover in the alveolar compartment of patients with interstitial lung disease. Thromb Haemost. 2000;83:853–860. [PubMed] [Google Scholar]
- 97.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–S220. [DOI] [PubMed] [Google Scholar]
- 98.Frantzeskaki F, Armaganidis a, Orfanos SE. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 101.van der Poll T, Herwald H. The coagulation system and its function in early immune defense. Thromb Haemost. 2014;112:640–648. [DOI] [PubMed] [Google Scholar]
- 102.Schulz C, Engelmann B, Massberg S. Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost. 2013;11(Suppl 1):233–241. [DOI] [PubMed] [Google Scholar]
- 103.Maniatis NA, Kotanidou a, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramirez GA, Rovere-Querini P, Sabbadini M, et al. Parietal and intravascular innate mechanisms of vascular inflammation. Arthritis Res Ther. 2015;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomashefski JF, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greene R, Zapol WM, Snider MT, et al. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124:593–601. [DOI] [PubMed] [Google Scholar]
- 108.Stebbing J, Phelan a, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fragkou PC, Belhadi D, Peiffer-Smadja N, et al. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGonagle D, Sharif K, O’Regan a, et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gendelman O, Amital H, Bragazzi NL, et al. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmun Rev. 2020;19:102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vardavas C, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luppi F, Cerri S, Taddei S, et al. Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern Emerg Med. 2015;10:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


