ABSTRACT
Introduction
Several months into the COVID-19 pandemic, safe and effective treatments against this global health disaster have yet to be identified. Clinical research trials around the world are underway testing a wide array of possible medications. In particular, the off-label use of hydroxychloroquine for COVID-19 prophylaxis and treatment has created many unprecedented challenges for the scientific community and the public.
Areas covered
We critically assessed major events from February – May 2020 that contributed to widespread use of hydroxychloroquine for the treatment and prophylaxis of COVID-19. We aimed to explore how opinions toward hydroxychloroquine may shift from early enthusiasm (based on in vitro and preliminary clinical data) to the hope for a miracle cure (through communication and promotion of questionable results) and, finally, to a rise of skepticism as more in-depth analyses are emerging.
Expert opinion
Mindful and rigorous acquisition of data, as well as its interpretation, are essential to an effective pandemic response. The rapid and premature promotion of results has had major implications for global crisis management, even creating distrust among the public. It is crucial for the medical and scientific community to incorporate the lessons learned from this situation.
KEYWORDS: Hydroxychloroquine, chloroquine, antimalarials, coronavirus disease 2019, SARS-CoV-2
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has disrupted all aspects of society including the economy, health care, and scientific research. In the midst of a global health disaster, there has been a collective race to find safe and effective treatments. The use of preprints as a medium of rapid scientific communication has surged in recent months in order to expedite the availability of study results, though not without peril. Preprints have not been peer-reviewed at the time of online distribution, vary in quality, and some may never be published in a peer-reviewed scientific journal. The use of chloroquine (CQ) and hydroxychloroquine (HCQ) for COVID-19 exemplifies the risks of both overinterpreting and amplifying preliminary data by those outside of the scientific community and was followed by swift corrective measures by researchers. This may represent the most rapid medical reversal in recent history, a full ‘pendulum swing’ from early enthusiasm to wide skepticism. In this report, we analyze the evolving waves of discourse regarding CQ/HCQ in relation to COVID-19, lessons learned, and implications for the future (Figure 1).
Figure 1.
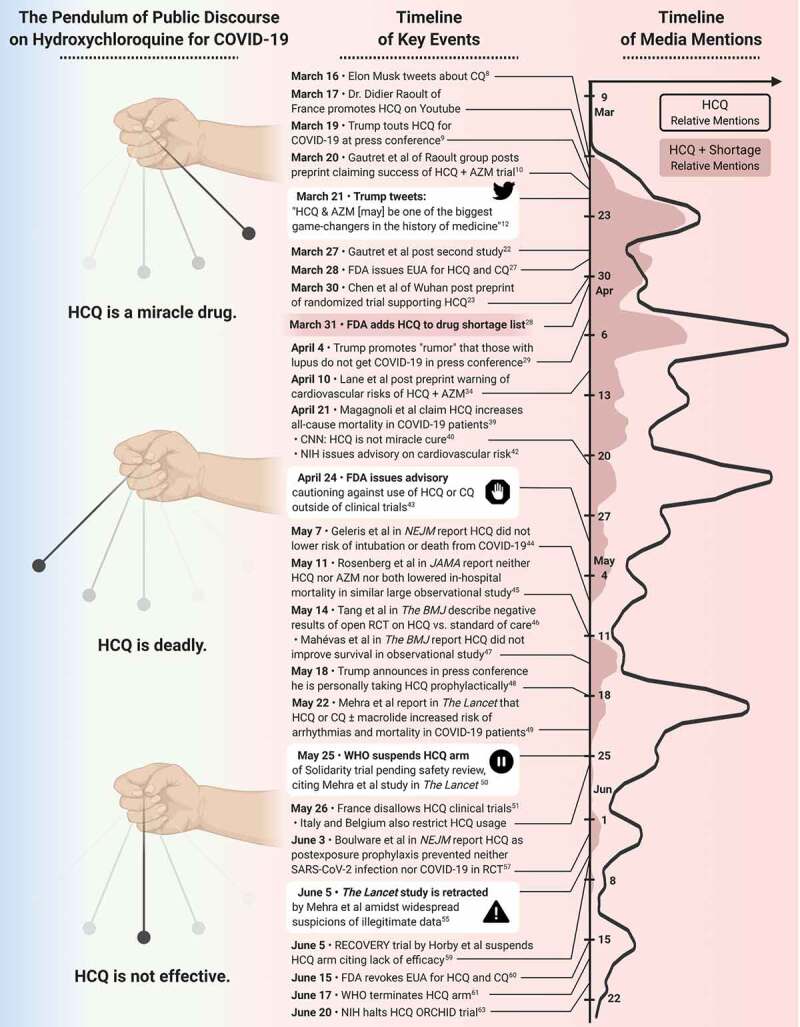
The swinging pendulum. Each swing of the pendulum (left column) illustrating changes in the discourse on HCQ use in COVID-19 as a result of key events (middle column). Timeline on the amount of media mentions of HCQ and HCQ + shortage (data extracted from www.mediacloud.org, source U.S. Top Sources 2018* collection) are presented graphically in right column. Curves show a 4-day mean of leading.
2. Waves of discourse
2.1. Initial movement: Early adopters of HCQ (February to mid-March 2020)
Based on previous evidence of the in vitro antiviral effects of CQ and HCQ against Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-1), both medications were used for the treatment of COVID-19 in China [1]. On February 4 in vitro data on the antiviral effect of CQ on SARS-CoV-2 was published, showing biological plausibility [2]. This data was announced by the State Council of China on February 17 regarding possible efficacy of CQ in the treatment of COVID-19 pneumonia [3]. As reported by Gao et al, this resulted in the development of multiple trials in CQ [4]. The authors also alluded to successful treatment of over 100 patients using CQ, however, no data was published at that time. Similar in vitro efficacy against SARS-CoV-2 was demonstrated with HCQ shortly thereafter [5].
Raoult and colleagues in France reported these findings as ‘hot topics’ in a showcase of their group’s research interests [6]. This was also promoted on social media by Raoult in late February [7]. By early March, interest in HCQ abruptly transitioned from mechanistic plausibility that would support its study in a clinical trial setting to rapid off-label use in patients with COVID-19, primarily fueled by promotion on social media, lay press, and celebrity influence [8].
2.2. Swift upswing: HCQ as a miracle cure (March 19 to mid-April 2020)
On March 17, results of the first study from the IHU-Méditerranée Infection on the use of HCQ and azithromycin (AZM) were revealed on Raoult’s YouTube channel (video no longer available). On March 19, President Trump endorsed HCQ for the treatment of COVID-19 in a press conference and commented on the drug’s presumed safety in this setting as ‘if things don’t go as planned, it’s not going to kill anybody’ [9]. The study by Raoult and colleagues was published as a preprint on March 20 and received immediate and at times uncritical media attention, as well as criticism from the scientific medical community regarding the study design and outcomes [10,11]. Following this, Trump posted on Twitter on March 21 describing the combination of HCQ and AZM potentially being ‘one of the biggest game-changers in the history of medicine’, also mentioning the Federal Drug Administration (FDA) efforts to approve combination and quoting data presented in the International Journal of Antimicrobial Agents [12]. Concurrently, new HCQ prescriptions for COVID-19 surged for both therapeutic and prophylactic use [13]. Without prior stockpiling, drug shortages quickly ensued [11,14]. Patients with rheumatic diseases appeared on news outlets and social media to shed light on the implications of HCQ and CQ drug shortages. In the same week, multiple state public health agencies and regulatory boards attempted to protect the HCQ supply chain by restricting prescriptions, for both COVID-19 and rheumatic diseases [15]. Multiple rheumatology organizations and governmental institutions also released guiding statements regarding scarce resource allocation during the pandemic [16–19].
With the enthusiasm around HCQ came reports of toxic ingestions, including at least one fatality, from chloroquine phosphate containing aquarium products as well as inappropriate prescriptions by healthcare providers possibly for hoarding purposes [20,21]. By late March, two new studies became publicly available: a second study from the group of IHU-Méditerranée Infection using HCQ and AZM in 80 patients with mild COVID-19 infection released on their webpage, and a preprint of the first randomized controlled trial of 62 patients from Wuhan reporting a difference in clinical time to recovery and radiologic findings with HCQ treatment [22,23]. With the increasing interest and examples of the irrational use of HCQ, physicians and rheumatologists raised concerns about the increasing use of HCQ and the inevitable impact on patients who rely on this medication [24–26]. However, the FDA issued an emergency use authorization (EUA) for the use of HCQ and CQ in hospitalized patients with COVID-19 on March 28 [27]. On March 31, HCQ was added to the FDA drug shortage list with several companies reporting limited supplies [28].
2.3. Momentum begins to shift: The rise of skepticism of HCQ (mid to late April 2020)
During a White House Press Conference on April 4, Trump suggested that a study had shown lupus patients were not contracting COVID-19; this hypothesis was unfortunately echoed by some scientists without supporting data [29,30]. These assertions that lupus patients were protected against COVID-19 were swiftly countered with emerging data from the Global Rheumatology Alliance and emerging data from Italy [31–33].
There were concerns that the use of HCQ, particularly in combination with AZM, might induce arrhythmias, as new onset cardiomyopathy had been reported with severe COVID-19. Lane et al released a preprint in MedRvix on April 10, in which they used claims data from multiple international sources to study HCQ with or without AZM versus active comparators [34]. Although their study did not find a difference in short-term outcomes with 30-day follow-up, they did detect a concerning safety signal: HCQ combined with AZM was associated with an increased risk of cardiovascular death, angina, and heart failure. Further studies assessing HCQ with or without AZM on QT prolongation in COVID-19 patients emerged [35]. Several observational studies were published on the potentially arrhythmogenic impact of HCQ and azithromycin [36–38].
On April 21, Magagnoli et al. released a preprint of their study from the Veterans Health Administration that showed an increased risk of death from any cause in those who had been treated with HCQ for COVID-19 versus those who did not [39]. Although the observational design had limitations, it’s release was followed by articles in the press highlighting concerns of harm with HCQ use [40]. Additionally, Silva-Borba et al. published a study demonstrating that high dose CQ was associated with a higher risk of mortality versus low dose [41]. With mounting evidence, the National Institute of Health (NIH) made an advisory on April 21, followed by an FDA advisory on April 24 [42,43].
2.4. What goes up must come down: The pendulum swings back (May-June 2020)
By May, the pendulum’s return swing gained force as multiple studies were published in high impact journals. On May 7 and 11, two large observational studies of hospitalized COVID-19 patients in New York found nonsignificant associations between the use of HCQ and major outcomes such as intubation or death, or in-hospital mortality [44,45]. Two studies were published on May 14: a negative open-label RCT of HCQ versus standard of care on the outcome of seroconversion at 28 days, and an observational study of patients requiring supplemental oxygen for COVID-19 that did not demonstrate a significant association between the use of HCQ versus no HCQ on the outcome of survival without ICU transfer within 21 days [46,47]. Counter to this new evidence, on May 18 Trump announced at a press conference that he was personally taking HCQ for the prevention of COVID-19. He cited anecdotes that frontline healthcare workers were doing the same [48].
On May 22, an observational study using purported de-identified international registry data on nearly 100,000 patients with COVID-19 found an increased risk of mortality and new arrhythmias for HCQ or CQ alone, and either antimalarial in combination with a macrolide, versus comparators, receiving neither an antimalarial nor a macrolide [49].
These findings prompted near immediate safety reviews of multiple international trials involving HCQ use. Less than 24 hours after publication, enrollment in the HCQ arm of the Solidarity trial was suspended pending an interim Data Safety Monitoring Board (DSMB) review [50]. Within days, France’s public health agency recommended against HCQ’s use as treatment for COVID-19 treatment and revoked authorization for its use in clinical trials [51,52]. Similar measures were taken simultaneously by drug safety agencies in Italy and Belgium [53].
However, the plausibility of Surgisphere’s data was soon questioned by the scientific community. For example, the study contained electronic data derived from the digital hospital records of almost a third of all COVID-19 cases and 70% of COVID-19 related deaths in Africa at the time [54]. Clinicians and researchers working in Africa found it implausible that these extensive digital platforms existed and that this proportion of COVID-19 cases across the continent were admitted to hospitals with these digital facilities. Multiple other questions were raised, including the lack of a data availability statement and the lack of ethical approval. After third party reviewers were not given access to verify the data, this study and a related NEJM study by the same group were retracted [55,56].
2.5. Returning to center: The resting pendulum (June 2020 and beyond)
In addition to friction from scientific dissent, results from multiple large RCTs on HCQ for prevention or treatment of COVID-19 provided a restoring force to the pendulum’s swing. On June 3, a double blind placebo controlled RCT for HCQ as post-exposure prophylaxis against COVID-19 infection involving 821 subjects showed no significant differences in subsequent infection rates between the treatment and control groups [57]. Most subjects were healthcare workers and infections were self-reported by participants as either PCR-confirmed or symptomatically compatible due to limited testing availability in the US. No arrhythmias or deaths were reported. The authors also included data on concurrent zinc use which has been a topic of considerable interest amongst the general public [58].
On June 5, the lead investigators of the RECOVERY trial announced the suspension of the HCQ arm in a statement citing lack of efficacy [59]. An interim DSMB review showed no significant difference comparing those treated with HCQ alone to those who received supportive care regarding the primary endpoint of 28-day mortality or secondary outcomes such as length of hospitalization or mechanical ventilation. These data were also cited by the FDA in a detailed memorandum which revoked the EUA for CQ/HCQ on June 15 [60]. Shortly thereafter, the WHO announced that the HCQ arm of the Solidarity trial, which included the French Discovery trial data, had been stopped due to inefficacy on June 17 [61,62]. Following an interim DSMB review on June 19 involving 470 enrolled subjects, the NIH suspended the ORCHID study due to lack of efficacy between the HCQ arm and placebo in hospitalized patients [63].
3. The patient perspective
The discourse surrounding COVID-19 in the US has forced patients to disseminate accurate information to the public and combat the politicization of the narrative. It has been extremely frustrating for patients to ensure that the general public, and especially patients with rheumatic diseases, have been receiving correct information. Patients, especially those with chronic illness, tend to latch on to any hope they find. Public support for the use of HCQ as a cure for COVID-19 has put patients on these medications in danger as a massive influx of people tried to access HCQ who did not have prior prescriptions. Patients already on HCQ were facing shortages and some were questioning if they should alter their doses. Some wanted to decrease the risks that they would contract COVID-19 while others were seeking to lower their doses in the face of obstacles to receiving their prescriptions. These actions have important consequences, as this increased their risk of worsening disease activity.
Two things stand out to patients: 1) the need to combat misinformation and 2) coordination of physician-researchers, patient advocacy groups, and patient care partners. It is expected that some people will take the media, scientists, and politicians at their word with no further search for the truth. Others will seek to read the published data, but when the data is uninterpretable due to study design or methodological issues, patients are left in a difficult position. Most rheumatic disease patients go to advocates and patient-oriented organizations as their source for information related to their health, sources that are outside of their care team. For these people, it is imperative that the groups have a wealth of accurate information. This is not only where precise lay summaries are helpful, but also references to the sources of this information. If nothing else, we are taught that going forward we need to have better systems in place and infrastructure to work together to disseminate factual and transparent scientific information to the public in a comprehensible way. This best helps us to avoid instances of people taking matters into their own hands.
4. Lessons learned
4.1. The perils of accessibility
Much of the early enthusiasm for HCQ originated from preprints listed on MedRxiv, a database launched in 2019 with the aims of enhancing scientific collaboration and increasing accessibility of scientific findings. However, unfettered access to preliminary reports has proven to be a double-edged sword with widespread dissemination via social media and the press serving as dangerous substitutes for peer review. Communicating accurate and accessible scientific information, including the limitations of current understanding, is crucial in preserving the public’s trust during the uncertainties inherent in public health emergencies.
4.2. Impact on research
Several authors have drawn comparisons between COVID-19 and the 2014–2015 Ebola virus disease outbreak in West Africa where widespread investigational drug use outside of well-designed clinical trials confounded the search for safe effective treatments [64]. Despite these reminders, close to 200 clinical trials involving HCQ for COVID-19 had been registered on clinicaltrials.gov including 93 which were actively enrolling participants as of late May. Most of the trials are too small to detect a meaningful effect size despite the immeasurable resources required to develop and conduct these trials.
The FDA’s EUA, issued prior to the availability of rigorous clinical data demonstrating efficacy or safety, allowed widespread access to HCQ/CQ outside of clinical trials. Consequently, enrollment in large RCTs rapidly decreased, delaying the critical safety and efficacy data necessary to evaluate these treatments in a timely fashion.
5. Conclusion
While the story of HCQ for COVID-19 is unique and has many unprecedented factors, it is also not the first time that humanity has pinned its hopes on this compound. Discovered in the bark of the cinchona tree, high in the mountains of the Andes in the 1600s after the Pope died of malaria, quinine and its derivatives have been an essential weapon in pandemics, wars, politics and empire building throughout history [65,66]. During the 1918 flu, it is reported that ‘Londoners refused to be fobbed off with advice to gargle with salt water, and besieged chemists and doctors’ surgeries demanding quinine’ [67,68].
Although data sharing and dissemination of data are important and enabled now by current forms of communication, the role of peer review is more crucial now than ever. As much as social media plays a role in the dissemination of information and scientific findings, scientists need to adequately direct and discuss these non-scientists, especially in challenging times such as a pandemic. While research is at times difficult to translate to the public, we can improve communication by providing patient-centered summaries at the time of publication.
6. Expert opinion
In many ways, the swift uptake and downfall for the recommended use of antimalarials, particularly HCQ, for COVID-19 serves as a cautionary tale for the conduct of research during a pandemic. The timeline of events has been particularly illustrative of the various types of pitfalls of research and scientific communication. That these events have occurred in a compressed timespan of several months is even more remarkable.
Scientific rigor needs to be upheld even in critical situations, such as pandemics, where there is an urgent need for new and efficacious treatments [69]. Transparency and accessibility in the publication and dissemination of results must remain key parts of research. Data availability, open peer review, and open access are emerging tools to improve scientific integrity. Maintaining these principles of the scientific method is even more vital during critical times, since results and findings will rapidly influence decision making. As observed with the use of antimalarials, rapid clinical implementation was based on data with clear limitations, without an adequate understanding of either benefits or potential harms. Furthermore, the impact of these decisions was not only restricted to its use in COVID-19 but had repercussions on patients who depended on these medications for other data-driven indications. It also led to inadequate allocation of resources that could have been employed for other vital interventions or equipment.
Effective, timely, and open peer review is critical to the validity of the data and the interpretation of the results. The widespread use of pre-print publications has highlighted the importance of adequate peer review, but also highlighted the value of the ‘community review’ of pre-prints when formal peer review has failed. With the contracted process from data collection to publication, the continued promotion of publication ethics is important to protect scientific integrity and trust in the system.
Finally, it is important for clinicians and scientists to realize that their role as communicators is now more vital than ever. Media, including social media, are powerful tools of dissemination. As experienced during this crisis, the handling of data dissemination is critical and can have an either positive or negative impact in the public’s image of the scientific community. As the COVID-19 pandemic persists in the coming months, the scientific community must reemerge as a reliable source for guidance in interpreting the evolving knowledge base. This should be accomplished in tandem with stakeholders, such as patient advocates and public health officials, to carefully and effectively communicate findings to a wide audience.
Acknowledgments
Drs. Sattui, Liew, and Graef and contributed equally.
Drs. Kim and Sparks contributed equally.
Figure was created with BioRender.com.
Funding Statement
This paper was not funded.
Article highlights
The COVID-19 pandemic has resulted in the rapid dissemination of research, both through scientific and non-scientific channels.
We highlight the narrative of antimalarial therapy for the treatment of COVID-19 over the course of the pandemic from February through June 2020.
Researchers and clinicians should be mindful of the public’s uptake of both pre-print and published data and provide meaningful interpretations.
Premature use of unstudied therapies can delay clinical trial enrollment and potentially harm recipients.
Well-designed rigorous clinical trials are possible and necessary during a public health emergency.
Data availability
Data was extracted from the U.S. Top Sources 2018 collection, a collection of the top U.S. newspapers and digital native sources of 2018, based on research from the Pew Research Center published in Aug 2019.
Declaration of interest
S Sattui is supported by the Vasculitis Clinical Research Consortium (VCRC)/Vasculitis Foundation Fellowship. The VCRC is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Science (NCATS). The VCRC is funded through collaboration between NCATS and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR057319). No conflicts of interest or competing interests, and no funding relevant to this manuscript. J Liew is supported by a grant from the NIH/NIAMS outside of the submitted work. No conflicts of interest or competing interests relevant to this manuscript. F Berenbaum reports personal fees from Boehringer, Bone Therapeutics, Expanscience, Galapagos, Gilead, GSK, Merck Sereno, MSD, Nordic, Novartis, Pfizer, Regulaxis, Roche, Sandoz, Sanofi, Servier, UCB, Peptinov, TRB Chemedica, and 4P Pharma outside of the submitted work. No funding relevant to this manuscript. M Ugarte-Gil is supported by grants from Pfizer and Janssen outside of the submitted work. No conflicts of interest or competing interests, and no funding relevant to this manuscript. M Konig is supported by NIH/NIAMS T32AR048522, and received personal fees from Bristol-Myers Squibb and Celltrion, unrelated to the submitted work. No conflicts of interest or competing interests. P Korsten reports personal fees from GlaxoSmithKline, Sanofi-Aventis, Pfizer, AbbVie, Novartis Pharma, Lilly, and Bristol-Myers Squibb outside of the submitted work. No funding relevant to this manuscript. M Putman is supported by the Rheumatology Research Foundation outside of the submitted work. No conflicts of interest or competing interests. P Robinson reports personal fees from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche and UCB and research grant funding from Janssen, Novartis and UCB all outside of this work. A Kim is supported by grants from NIH/NIAMS, Rheumatology Research Foundation, and GlaxoSmithKline as well as personal fees from Exagen Diagnostics, Inc. and GlaxoSmithKline outside of the submitted work. J Sparks is supported by grants from NIH/NIAID/Autoimmune Centers of Excellence, Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund as well as personal fees from Bristol-Myers Squibb, Gilead, Inova, Janssen, and Optum outside of the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Dyall J, Gross R, Kindrachuk J, et al. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017. December;77(18):1935–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020. March;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antimalarial drug confirmed effective on COVID-19 [Internet]. China: the state council of the people’s Republic of China; 2020. March 17. [cited 2020 May 28]. Available from: http://english.www.gov.cn/statecouncil/ministries/202002/17/content_WS5e4a944dc6d0595e03c20f35.html
- 4.Gao J, Tian Z, Breakthrough: YX.. Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020. March 16;14(1):72–73. [DOI] [PubMed] [Google Scholar]
- 5.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;ciaa237. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colson P, Rolain JM, Raoult D.. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020. March;55(3):105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raoult D Coronavirus:versune sortie de crise? [Video]. IHU Méditerranée-Infection: youTube [Internet]; 2020. February 25 cited 2020 May27. Available from: https://www.youtube.com/watch?v=8L6ehRif-v8&feature=youtu.be
- 8.Musk E “Maybe worth considering chloroquine for C19“. [Internet]. @elonmusk: Twitter; 2020. March 16. cited 2020 May27. Available from: https://twitter.com/elonmusk/status/1239650597906898947?s=20
- 9.Edney A Trump Touts Drug That FDA Says Isn’t Yet Approved for Virus [News Article]. Prognosis: Bloomberg.com [Internet]; 2020. March 19 updated March19, 2020, 4:12 PM EDT 2020 March19. Available from: https://www.bloomberg.com/news/articles/2020-03-19/trump-touts-malaria-drug-as-potential-coronavirus-treatment
- 10.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020. Mar 20;105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Kim AHJ, Sparks JA, Liew JW, et al. A Rush to Judgment? Rapid Reporting and Dissemination of Results and Its Consequences Regarding the Use of Hydroxychloroquine for COVID-19. Ann Intern Med. 2020. June 16;172(12):819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Analysis of the initial studies reporting on HCQ in COVID-19, as well as the impact and issues of the interpretation and dissemination of these.
- 12.Trump DJ “HYDROXYCHLOROQUINE & AZITHROMYCIN, taken together, have a real chance to be one of the biggest game changers in the history of medicine. The FDA has moved mountains - Thank You! Hopefully they will BOTH (H works better with A, International Journal of Antimicrobial Agents) …”. [Internet]. @realDonaldTrump: Twitter. 2020. March 21. cited 2020 May27 Available from: https://twitter.com/realDonaldTrump/status/1241367239900778501?s=20.
- 13.Horn A Online pharmacy saw a ‘panic-driven’ boom in drug touted by Trump. [Internet]: NPR.com; updated 2020 May14; cited 2020 May14; cited 2020 May14; cited 2020 May14; cited 2020 May26. Available from: https://www.npr.org/sections/coronavirus-live-updates/2020/05/14/855841849/online-pharmacy-saw-a-panic-driven-boom-in-drug-touted-by-trump
- 14.Mahase E. Covid-19: six million doses of hydroxychloroquine donated to US despite lack of evidence. BMJ. 2020. March;23(368):m1166. [DOI] [PubMed] [Google Scholar]
- 15.COVID-19: Hydroxychloroquine, Chloroquine, and Azithromycin. [Internet] . National Alliance of State Pharmacy Associations; updated 2020 April13; cited 2020 April13; cited 2020 April13; cited 2020 May13; cited 2020 May27. Available from: https://naspa.us/resource/hydroxychloroquine-chloroquine-and-azithromycin/
- 16.EULAR president: application of anti-malarials to tackle COVID-19 raises vital issues for rheumatic disease community in Europe 2020. [Internet]. Kilchberg, Switzerland: EULAR; 2020. April 3 cited 2020 May27. Available from: https://www.eular.org/sysModules/obxContent/files/www.eular.2015/1_42291DEB-50E5-49AE-5726D0FAAA83A7D4/eular_president_application_of_anti_malarials_to_tackle_covid_19_raises_vital_issues_for_rheumatic_disease_community_in_europe(1).pdf [Google Scholar]
- 17.American College of Rheumatology . Guiding principles from the American College of Rheumatology for scarce resource allocation during the COVID-19 pandemic: the case of hydroxychloroquine 2020. [Internet]. [updated 2020. April 22]. [cited 2020 May 28]. Available from: https://www.rheumatology.org/Portals/0/Files/Guiding-Principles-Scarce-Resource-Allocation-During-Covid-19.pdf
- 18.BFARM . Hydroxychloroquin - Sicherstellung der Versorgung von chronisch kranken Patientinnen und Patienten in den zugelassenen Indikationen. [Internet]. Germany: Bundesinstitut für Arzneimittel und Medizinprodukte; updated 2020 March4; cited 2020 May27]; Letter 2020 March4; cited 2020 March4; cited 2020 May4; cited 2020 May27. Available from: https://www.bfarm.de/DE/Service/Presse/Themendossiers/Coronavirus/Anlagen/Off_Label_Use_Hydroxychloroquin.pdf;jsessionid=0F3476A70525B464B743E623F671C154.2_cid507?__blob=publicationFile&v=1 [Google Scholar]
- 19.Statement: Lupus Foundation of America urges manufacturers of hydroxychloroquine and chloroquine to ensure supply to treat lupus . Lupus Foundation of America [Internet]. [Internet]; 2020. March 23 cited 2020 May27. Available from: https://www.lupus.org/news/lupus-foundation-statement-manufacturers-hydroxychloroquine-chloroquine
- 20.Waldrop T, Alsup D, McLaughlin E. Fearing coronavirus, Arizona man dies after taking a form of chloroquine used to treat aquariums [Internet]: CNN; updated 2020 March25; cited 2020 March25; cited 2020 March25; cited 2020 May25; cited 2020 May27. Available from: https://www.cnn.com/2020/03/23/health/arizona-coronavirus-chloroquine-death/index.html
- 21.Sanders T, Armstrong D, Kofman A. Doctors Are Hoarding Unproven Coronavirus Medicine by Writing Prescriptions for Themselves and Their Families [Internet]. ProPublica; [updated 2020, March 24]. [cited 2020 May 28]. Available from: https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families
- 22.Gautret P, Lagier J-C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020. Mar-Apr;34:101663. doi: 10.1016/j.tmaid.2020.101663. Epub 2020 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020. [cited 2020 May 28]. Available from: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3
- 24.Yazdany J, Kim AHJ. Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: what Every Clinician Should Know. Ann Intern Med. 2020. June 2;172(11):754–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romo V COVID-19 Patients Given Unproven Drug In Texas Nursing Home In ‘Disconcerting’ Move [Internet]: NPR.org; updated 2020 April10; cited 2020 April10; cited 2020 April10; cited 2020 May10; cited 2020 May27. Available from: https://www.npr.org/2020/04/10/830348837/covid-19-patients-given-unproven-drug-in-texas-nursing-home-garnering-criticism
- 26.Graef ER, Liew JW, Putman MS, et al. Festina lente: hydroxychloroquine, COVID-19 and the role of the rheumatologist. Ann Rheum Dis. 2020. June;79(6):734–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration . Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease [Internet] 2020. March 28 [cited 2020 May 26]; Government Letter. Available from: https://www.fda.gov/media/136534/download
- 28.U.S. Food and Drug Administration . FDA Drug Shortages [Internet]; cited 2020 March31. Available from: https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm
- 29.Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing [Internet]. The White House April; 4, 2020. [cited 2020 May 27]. Available from: https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-coronavirus-task-force-press-briefing-19/
- 30.Joob B, Wiwanitkit V. SLE, hydroxychloroquine and no SLE patients with COVID-19: a comment. Ann Rheum Dis. 2020. June;79(6):e61. [DOI] [PubMed] [Google Scholar]
- 31.Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020. May; 2(5): e250–e253. Published online 2020 Apr 16. doi: 10.1016/S2665-9913(20)30095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First report of the incidence of COVID-19 infection in patients with rheumatic diseases from COVID-19 Global Rheumatology Alliance registry.
- 32.Konig MF, Kim AH, Scheetz MH, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020. May 7:annrheumdis-2020-217690. doi: 10.1136/annrheumdis-2020-217690. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Analysis from the COVID-19 Global Rheumatology Alliance Registry showing no difference in COVID-19 outcomes in lupus patients with or without baseline HCQ use.
- 33.Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020. June;79(6):837–839. [DOI] [PubMed] [Google Scholar]
- 34.Lane JCE, Weaver J, Kostka K, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. 2020. [cited 2020 May 28]. Available from: https://www.medrxiv.org/content/10.1101/2020.04.08.20054551v2
- 35.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. May 1;e201834. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleh M, Gabriels J, Chang D, et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020. June;13(6):e008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bessiere F, Roccia H, Deliniere A, et al. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020. May 1;e201787. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020. May 12;S1547-5271(20)30435-5. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med. 2020. Apr 21;2020.04.16.20065920. doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen E, Nigam M. Study finds no benefit, higher death rate in patients taking hydroxychloroquine for Covid-19 [Internet]. CNN.com; [updated 2020 April21; cited 2020 April21; cited 2020 April21; cited 2020 May21; cited 2020 May27]. Available from: https://www.cnn.com/2020/04/21/health/hydroxychloroquine-veterans-study/index.html
- 41.Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Network Open. 2020. April 24;3(4):e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. National Institutes of Health; 2020. updated 2020 May12 cited 2020 May12 cited 2020 May12 cited 2020 May12 cited 2020 May27. Available from: https://www.covid19treatmentguidelines.nih.gov/.
- 43.Hydroxychloroquine or Chloroquine for COVID-19: Drug Safety Communication - FDA Cautions Against Use Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems . [Internet]. [Internet]: U.S. Food and Drug Administration. Drug Safety Communication; 2020, April 24 cited 2020 May27]. Available from: https://www.fda.gov/safety/medical-product-safety-information/hydroxychloroquine-or-chloroquine-covid-19-drug-safety-communication-fda-cautions-against-use
- 44.Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020. June 18;382(25):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]; • US-based observational study showing no decreased or increased risk of intubation or death with HCQ in hospitalized COVID-19 patients.
- 45.Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020. May 11;323(24):2493-2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]; • US-based observational study showing no differences in in-hospital mortality for patients treated with HCQ, AZ or both, compared to neither in COVID-19 patients.
- 46.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020. May;14(369):m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahevas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;14(369):m1844. May. [DOI] [PMC free article] [PubMed] [Google Scholar]; • French multicenter observational study showing no differences in ICU transfers, survival, incidence of ARDS in hospitalized COVID-19 patients treated with HCQ or standard of care.
- 48.Gearan A, Laurie M, Lenny B, et al. Trump says he is taking hydroxychloroquine to protect against coronavirus, dismissing safety concerns [Internet]: The Washington Post; [updated 2020, May 18]. Available from: https://www.washingtonpost.com/politics/trump-says-he-is-taking-hydroxychloroquine-to-protect-against-coronavirus-dismissing-safety-concerns/2020/05/18/7b8c928a-9946-11ea-ac72-3841fcc9b35f_story.html
- 49.Mehra MR, Desai SS, Ruschitzka F, et al. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020. May 22;S0140-6736(20)31180-6. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.WHO Director-General’s opening remarks at the media briefing on COVID-19-25 May 2020 [Internet]. The World Health Organization; 2020. May 25. [cited 2020 May 28]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—25-may-2020
- 51.COVID-19: l’ANSM souhaite suspendre par précaution les essais cliniques évaluant l’hydroxychloroquine dans la prise en charge des patients - Point d’Information. [Internet]: ANSM; [updated 2020 May26]. Available from: https://www.ansm.sante.fr/S-informer/Actualite/COVID-19-l-ANSM-souhaite-suspendre-par-precaution-les-essais-cliniques-evaluant-l-hydroxychloroquine-dans-la-prise-en-charge-des-patients-Point-d-Information
- 52.Haut Conseil de la Santé Publique. Covid-19: utilisation de l’hydroxychloroquine [Internet]. Paris: Haut Conseil de la Santé Publique; [updated 2020 March26; cited 2020 March26; cited 2020 March26; cited 2020 June26; cited 2020 June25]. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=837
- 53.Blamont M, Smout A, Parodi E. EU governments ban malaria drug for COVID-19, trial paused as safety fears grow [Internet]. Reuters; [updated 2020 May27; cited 2020 May27; cited 2020 May27; cited 2020 June27; cited 2020 June25]. Available from: https://www.reuters.com/article/health-coronavirus-hydroxychloroquine-fr/eu-governments-ban-malaria-drug-for-covid-19-trial-paused-as-safety-fears-grow-idUSKBN2340A6
- 54.Outbreak Brief 13: COVID-19 Pandemic – 14 April 2020 [Internet]. [Africa CDC; Outbreak Briefs]. [cited 2020 May 28]. Available from: https://africacdc.org/download/outbreak-brief-number-13-covid-19-pandemic-14-april-2020/
- 55.Mehra MR, Ruschitzka F, Patel AN. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020. June 13;395(10240):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehra MR, Desai SS, Kuy S, et al. Retraction: cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020. June 25;382(26):2582. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boulware DR, Pullen MF, Bangdiwala AS, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020. Jun 3;NEJMoa2016638. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• US-based RCT showing no benefit of HCQ for post-exposure prophylaxis in prevention of illness or confirmed COVID-19 infection after high- or moderate-risk exposure.
- 58.Balasubramanian S Zinc: no Evidence Yet To Support Its Effects On Coronavirus. [Internet]. Forbes.com; [updated April10, 2020; cited 2020; cited 2020 April10, 2020; cited 2020 June10, 2020; cited 2020 June27]. Available from: https://www.forbes.com/sites/saibala/2020/04/10/zinc-no-evidence-yet-to-support-its-effects-on-coronavirus/#d1e173c41513
- 59.P H. Statement from the Chief Investigators of the Randomised Evaluation of COVID-19 Therapy (RECOVERY) Trial on hydroxychloroquine: university of Oxford; [updated 5June 2020; 2 2020 June5]. Available from: https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf
- 60.U.S. Food and Drug Administration . Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine [Internet]; [updated June15, 2020]; FDA News Release 2020 June15]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- 61.Update on hydroxychloroquine and the Solidarity Trial - 27 May 2020 . [Internet]. World Health Organization; [updated 2020. June 17]; Rolling updates on coronavirus disease (COVID-19)]. [cited 2020 May 28]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 62.Cochrane Response . Targeted Update: safety and efficacy of hydroxychloroquine or chloroquine for treatment of COVID-19. In: the COVID-NMA project, editor: Cochrane Response; 2020. p. 18.
- 63.NIH halts clinical trial of hydroxychloroquine . [Internet]. National Institutes of Health; 2020. June 20 [cited 2020 June25]. Available from: https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine
- 64.Kalil AC. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA. 2020. March 24. doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 65.Rocco F. The miraculous fever tree: malaria and the quest for a cure that changed the world. 1st ed. New York, NY: HarperCollins; 2003. [Google Scholar]
- 66.Gordon I The chloroquine chronicles: A history of the drug that conquered the world. [Internet]. The World from PRX; [updated April 15, 2020]. [cited 2020 May 28]. Available from: https://www.pri.org/stories/2020-04-15/chloroquine-chronicles-history-drug-conquered-world
- 67.Spinney L What the 1918 flu pandemic can teach us about coronavirus drug trials. [Internet]. The Guardian; [updated April 5, 2020]. Available from: https://www.theguardian.com/commentisfree/2020/apr/05/1918-flu-pandemic-coronavirus-drug-trials-scientists-treatments-evidence
- 68.Honigsbaum M. Living with enza: the forgotten story of Britain and the great flu pandemic of 1918. London; New York: Macmillan; 2009. [Google Scholar]
- 69.London AJ, Kimmelman J. Against pandemic research exceptionalism. Science. 2020. May 1;368(6490):476–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data was extracted from the U.S. Top Sources 2018 collection, a collection of the top U.S. newspapers and digital native sources of 2018, based on research from the Pew Research Center published in Aug 2019.


