Abstract
Vascular mimicry is induced by a wide array of genes with functions related to cancer stemness, hypoxia, angiogenesis and autophagy. Vascular mimicry competent (VM-competent) cells that form de novo blood vessels are common in solid tumors facilitating tumor cell survival and metastasis. VM-competent cells display increased levels of vascular mimicry selecting for stem-like cells in an O2-gradient-dependent manner in deeply hypoxic tumor regions, while also aiding in maintaining tumor cell metabolism and stemness. Three of the principal drivers of vascular mimicry are EphA2, Nodal and HIF-1α, however, directly or indirectly many of these molecules affect VE-Cadherin (VE-Cad), which forms gap-junctions to bind angiogenic blood vessels together. During vascular mimicry, the endothelial-like functions of VM-competent cancer stem cells co-opt VE-Cad to bind cancer cells together to create cancer cell-derived blood conducting vessels. This process potentially compensates for the lack of access to blood and nutrient in avascular tumors, simultaneously providing nutrients and enhancing cancer invasion and metastasis. Current evidence also supports that vascular mimicry promotes cancer malignancy and metastasis due to the cooperation of oncogenic signaling molecules driving cancer stemness and autophagy. While a number of currently used cancer therapeutics are effective inhibitors of vascular mimicry, developing a new class of vascular mimicry specific inhibitors could allow for the treatment of angiogenesis-resistant tumors, inhibit cancer metastasis and improve patient survival. In this review, we describe the principal vascular mimicry pathways in addition to emphasizing the roles of hypoxia, autophagy and select proangiogenic oncogenes in this process.
1. Introduction
Scientists were first aware of neovascularization in 1787 as it related to developmental biology (Fernandez-Cortes, Delgado-Bellido, & Oliver, 2019). Following careful investigation, some tumors possess angiogenic and non-angiogenic regions, while others grow independently of angiogenesis even when hypoxic (Bridgeman et al., 2017). In 1999, Maniotis et al. described a completely neoteric blood supply in malignant melanoma and observed how stem-like cancer cells transdifferentiate into an endothelial-like cell phenotype (Maniotis et al., 1999). Such angiogenesis-independent tumor growth is thought to be conferred by a process known as vascular mimicry. Vascular mimicry requires the formation of de novo vasculature with a dense laminin and collagen-containing basement membrane, lined with cancer cells on the posterior surface (El Hallani et al., 2010). Cancerous tissues often re-purpose embryological processes (such as vasculogenesis) to promote their survival and to adapt to cell stress. Vasculogenic mimicry enables the tumors to form matrix-embedded vascular structures, containing plasma and blood cells to meet the metabolic demands of rapidly growing tumors (Angara, Borin, & Arbab, 2017). Histologically, vasculogenic mimicry is distinguished by extracellular matrixes rich in laminin, proteoglycans, heparin sulfate and collagens, which are stained by Periodic Acid Schiff (PAS) staining for histological identification, often in combination with endothelial marker staining, to differentiate angiogenic vessels from vascular mimetic vessels (Chen, Maniotis, Majumdar, Pe’er, & Folberg, 2002; Clarijs, Otte-Holler, Ruiter, & de Waal, 2002; Clemente, Perez-Alenza, Illera, & Pena, 2010; Seftor et al., 2001; Vartanian, 2012).
Vascular mimicry occurs in aggressive melanomas (Zhang et al., 2019), cancers of breast (Shirakawa et al., 2001), ovarian (Sood et al., 2001), prostate (Sharma et al., 2002), lung (Passalidou et al., 2002), liver (Sun et al., 2006) and also glioblastoma (GBM; (Yue & Chen, 2005). Patient tumors with greatly overall vascular mimicry display poorer prognosis (Sun, Zhang, Zhao, Zhang, & Hao, 2004; Yang et al., 2016); vascular mimicry also positively correlates with tumor staging (Lin et al., 2012). It is likely that vascular mimicry plays a causal role in tumor progression, stimulating the invasive growth and metastasis of tumor cells clinically. Tumor cells involved in vascular mimicry also display increased cancer-stemness and endothelial-like gene expression. During the formation of vascular mimetic vessels, tumor cells are immediately adjacent to blood flow, making the detachment and intrastation of these cells more likely for metastasis to distant sites. Furthermore, increased levels of vascular mimicry are generally associated with metastasis as revealed in breast cancer where primary breast tumors are chiefly angiogenic, however, metastatic lung lesions originating from matched tumors of the breast are found to be non-angiogenic (Pezzella et al., 2000).
Tumors that are resistant to angiogenesis inhibitors display greater levels of vascular mimicry, and more importantly, angiogenesis inhibitors like endostatin that have broad activities to inhibit many angiogenic signaling molecules (VEGF, FGF-2, MMPs, HIF-1α), do not effectively suppress vascular mimicry (Hendrix, Seftor, Hess, & Seftor, 2003), indicating that vascular mimicry is a potential mechanism of angiogenesis-resistance clinically. In a study by Liu et al., vascular mimicry was shown to correlate with distant metastases, poor overall survival, and local cancer recurrence; they also observed that vascular mimicry was highly associated with MMP-2 levels (Liu, Yang, Meng, Zhang, & Xu, 2012). Nevertheless, the molecular mechanisms that give rise to vascular mimicry remain largely unknown. In this review, the triggers of vascular mimicry, it’s signaling pathways and key molecular interactions, as well as, in vivo models of vascular mimicry are discussed.
2. Vascular mimicry vessel structure
The vascular network is comprised of a variety of fluid and blood-conducting vessels with the overall goal of perfusing all the metabolic tissues of the body with essential nutrients and blood. Crucial differences exist between these vessels, which will be discussed in detail here. These vessels can take many forms, with the primary blood conducting vessels having endothelial cells lining their luminal blood-conducting space, i.e., angiogenic vessels (blood vessels) and lymphatic vessels. Only blood vessels, however, have supporting pericytic cells posterior to the vessel lumen and the basal lamina (basement membrane). Vascular mimetic vessels, however, are hollow tubes lined posteriorly by cancer cells resting on top of a non-continuous basement membrane like structure, created by dense deposits of extracellular matrix glycoproteins surrounding the central lumen (Valdivia et al., 2019) (Fig. 1). Represented among these glycoproteins include type I and IV collagens as well as Laminin5γ2 and its cleaved products 5γ2x and 5γ2’ (Liliensiek, Nealey, & Murphy, 2009; Yousif, Di Russo, & Sorokin, 2013). Using the simple illustration of these vessels in Fig. 1, clinically vascular mimicry is identified by positive and negative staining approaches; using periodic acid-Schiff (PAS) to stain glycoproteins pink and using antibody-based labeling to identify endothelial cell containing vessels using CD31 more commonly, CD34 is also used on occasion. The clinical standard to characterize the involvement of vascular mimicry in tumors remains the identification of PAS+/CD31− blood conducting vessels. Such analyses are unfortunately insufficient to image vascular mimicry in patients and animals in real-time for individual tumors without invasive tissue biopsies and histopathological analyses.
Fig. 1.
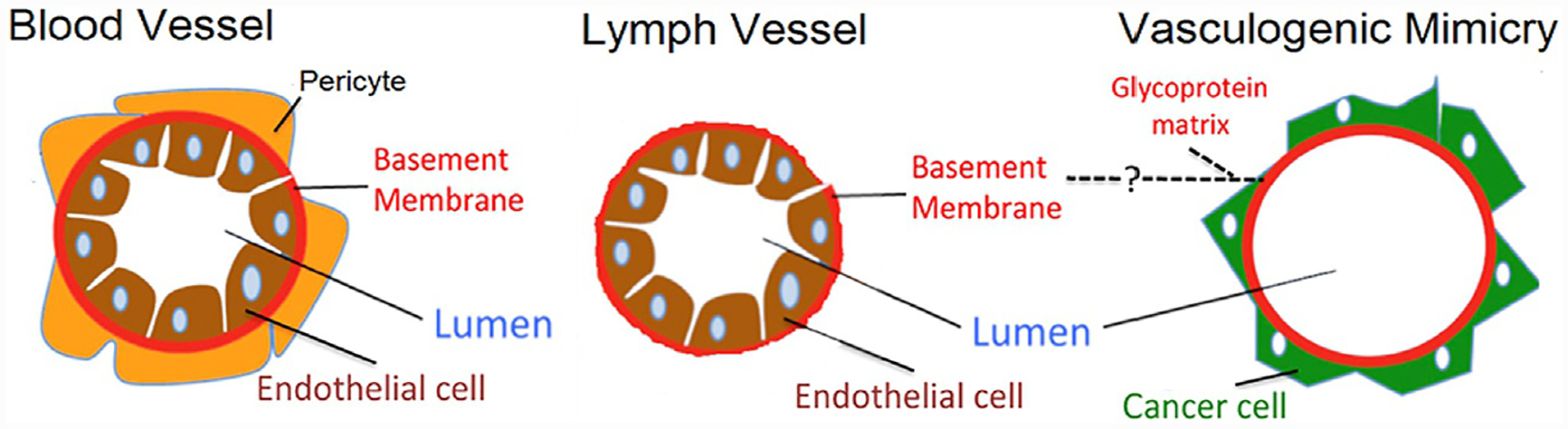
The structure of vascular mimetic vessels (right) compared to angiogenic blood vessels (left) and lymphatic vessels (center). During the course of vascular mimicry, cancer cells are endothelial cell-like, however, these cancerous cells line the basal surfaces relative to the lumen space in these vessels compared to the endothelial cell which align to the apical surface in lymphoid and blood vessels. The presence of supporting pericytes can also be used to differentiate between lymphatic and blood vessels, which both stain for endothelial markers relative to the endothelial cell void vascular mimetic vessels. From Valdivia, A., Mingo, G., Aldana, V., Pinto, M. P., Ramirez, M., Retamal, C., et al. (2019). Fact or fiction, it is time for a verdict on vasculogenic mimicry? Frontiers in Oncology, 9, 680. doi: 10.3389/fonc.2019.00680.
3. Primary vascular mimicry signaling pathways
While it is true that a myriad of extracellular factors, tumor hypoxia, and autophagy are implicated in vascular mimicry, the intracellular regulators of vascular mimicry follow three primary signaling pathways: VE-Cadherin, Notch and the HIF family of transcription factors. Additionally, oncogenes and tumors suppressor genes regulate important intracellular signaling processes and are prominent contributors to vascular mimicry to be discussed later in this review. In this section, the principal vascular mimicry induction modalities are discussed, from which other oncogenic drivers can be biochemically integrated forming a comprehensive understanding of these processes again discussed later in this review. For additional reviews on the fundamental aspects of these signaling pathways, please refer to the leaders in this field, without which the science of vascular mimicry would not be at its current state of understanding (Delgado-Bellido, Serrano-Saenz, Fernandez-Cortes, & Oliver, 2017; Hendrix et al., 2003; Kirschmann, Seftor, Hardy, Seftor, & Hendrix, 2012; Qiao et al., 2015; Valdivia et al., 2019).
Vascular mimicry involves the activation of signaling pathways associated with angiogenesis, cancer stemness and hypoxia to promote the cleavage of Laminin5γ2 into 5γ2x and 5γ2’ for the dense extracellular matrix protein deposition, forming de novo blood vessels in solid tumors. Frequently, vascular mimicry occurs in avascular, hypoxic tumor microenvironments, but in many cancer cells vascular mimicry can also occur during normoxia as is the case with many in vitro studies. The three primary signaling pathways inducing vascular mimicry include the VE-Cadherin/EphA2, HIF-1α/Stat3 and the Nodal/Notch signaling pathways. VE-Cadherin (VE-Cad) has the most extensive crosstalk with these other pathways, integrating signals from Nodal and serving as a transcriptional target of both HIF-1α (Tang et al., 2014) and HIF-2α (Le Bras et al., 2007). Vascular mimicry competent (VM-competent) cell sub-populations within heterogeneous tumors display marked cellular plasticity through at least one of the following pathways, culminating in the cleavage of laminin by MMP-2 (Fig. 2). These numerous biochemical pathways and their molecular regulation are discussed in detail below to put vascular mimicry in its proper biochemical context, ultimately for the design of pharmacological inhibitors to disrupt this driver of tumor growth, metastasis and cancer mortality.
Fig. 2.
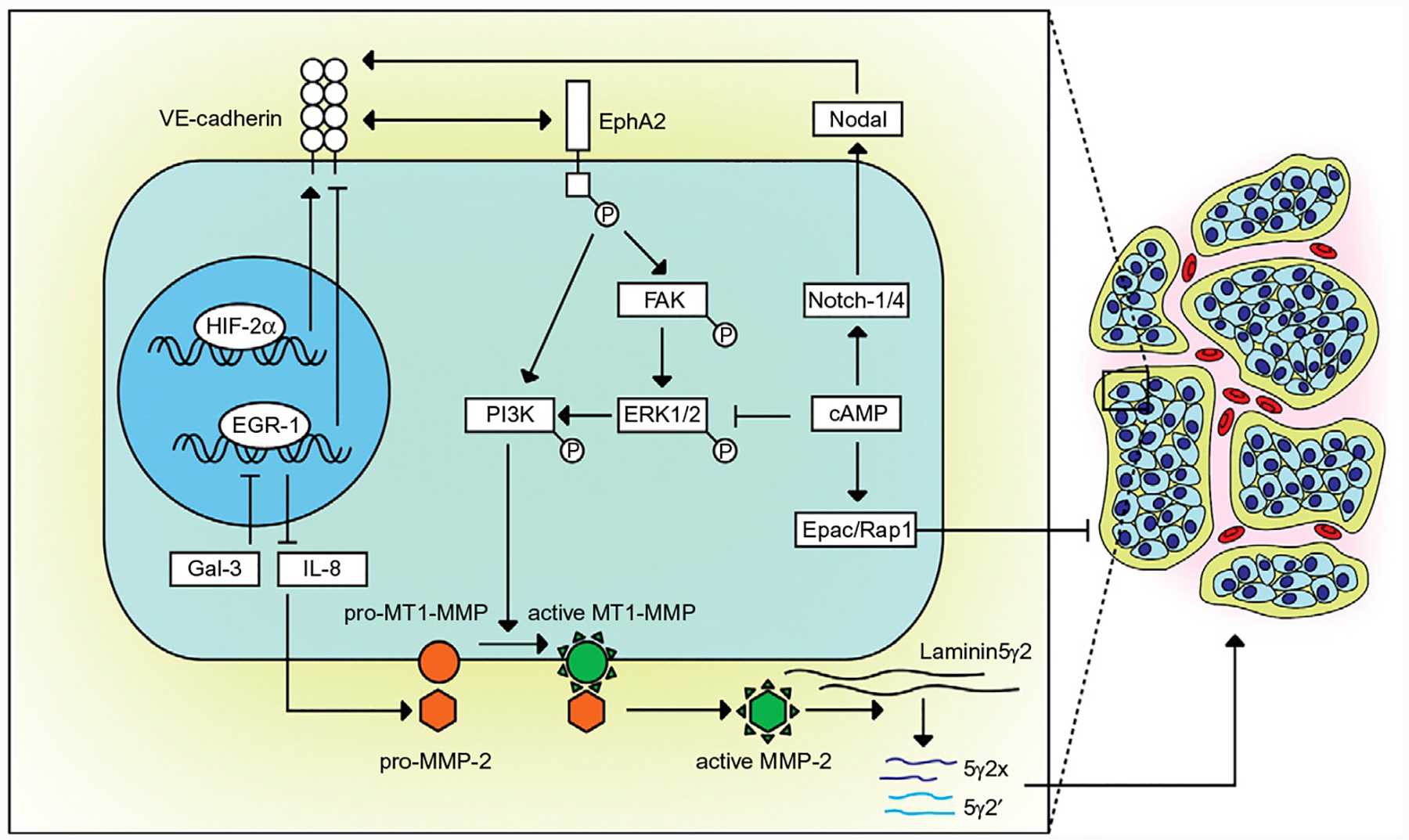
Summary of the principal signaling pathways driving vascular mimicry. Based on the work of Hendrix et al. and her model of the signaling cascades implicated in vascular mimicry (Hendrix et al., 2003). VE-Cad regulates the translocation of EphA2 to the membrane. Once activated by ligand binding, EphA2 induces focal adhesion kinase (FAK) activation to activate phosphoinositide 3-kinase (PI3K) via ERK1/2 as well as another mechanism for direct activation of PI3K independent of FAK signaling, both signaling cascades acting to induce MMP-2-mediated laminin cleavage. The Laminin5γ2 fragments into γ2′ and γ2x to exert pro-cancer cell migratory and invasiveness which may stimulate vascular mimicry. Galectin-3 can also stimulate vascular mimicry via the repression of EGR-1, the negative repressor of IL-8 to block the enhancement of the pro vascular mimicry protein MMP-2. cAMP inhibits vascular mimicry via the stimulation of Epac/Rap1 as well as via the direct repression of ERK1/2. Surprisingly cAMP can also promote vascular mimicry via its ability to increase Nodal expression and its subsequent up-regulation of VE-Cad. From Vartanian, A. A. (2012). Signaling pathways in tumor vasculogenic mimicry. Biochemistry (Mosc), 77(9), 1044–1055. doi: 10.1134/S000629791209012X.
3.1. VE-cadherin, EphA2, FAK
The relationship between VE-Cadherin (VE-Cad; also known as CD144) was first shown to drive vascular mimicry by Dr. Hendrix and colleagues where they linked highly vascular structures to VE-Cad expression (Hendrix et al., 2003). Functionally, VE-Cad is a transmembrane protein common to endothelial cells regulating their adhesion to other endothelial cells (Breier, Grosser, & Rezaei, 2014). Elevated VE-Cad is linked to poor prognosis in melanoma patients (Bartolome et al., 2017) and it is possible vascular mimicry contributes to these clinical observations. While the initial studies of vascular mimicry focused on melanomas, the vascular mimicry phenotype has now been observed in many other tumor types (Passalidou et al., 2002; Sharma et al., 2002; Shirakawa et al., 2001; Sood et al., 2001; Sun et al., 2006; Yue & Chen, 2005; Zhang et al., 2019). Additionally, increased VE-Cad expression is also evident in breast (Bartolome et al., 2017) and liver cancers (Sun et al., 2010), supporting the hypothesis that vascular mimicry driven by VE-Cad is likely generalizable to many different primary tumor types. The generalized effects of VE-Cad upon vascular mimicry may be based in its diverse effects upon Nodal-signaling, HIF-2α (Yang et al., 2017) and EphA2 (Yeo, Lee, & Lee, 2019). For a more detailed look at the structure and phosphorylation sites of VE-Cad, please see the following manuscripts (Delgado-Bellido et al., 2017; Taveau et al., 2008).
In 2006, Hess et al. documented that in vascular mimicry-competent melanoma cells VE-cadherin co-localized with EphA2 at cell–cell junctions and that these two molecules are able to interact during the process of vascular mimicry (Hess et al., 2006; Paulis, Soetekouw, Verheul, Tjan-Heijnen, & Griffioen, 2010). In highly aggressive melanoma cells, knock-down of VE-Cad forced EphA2 to re-localize to the cytoplasm, blocking its signaling effects on the cell surface to promote vascular mimicry (Hendrix et al., 2001). Considering how the disruption of EphA2 did not affect VE-Cad localization or signaling, this observation confirms that EphA2 is down-stream of VE-Cad. Furthermore, the suppression of EphA2 was sufficient to inhibit vascular mimicry in vitro (Hess et al., 2001; Margaryan et al., 2009), as well as metastasis in vivo (Duxbury, Ito, Zinner, Ashley, & Whang, 2004a). The final step in vascular mimicry entails the cleavage of laminin5γ2 into γ2′ and γ2x. EphA2 drives laminin cleavage via the activation of FAK (Duxbury, Ito, Zinner, Ashley, & Whang, 2004b), which is frequently overexpressed and hyper-phosphorylated in aggressive cancers (Tandon, Vemula, & Mittal, 2011; Walker-Daniels, Hess, Hendrix, & Kinch, 2003). Moreover, when FAK signaling is blocked, vascular mimicry is also inhibited. FAK activation also leads to the phosphorylation of extracellular signal-regulated kinase 1 and 2 (ERK1/2), driving MMP-2 and MT1-MMP induced laminin5γ2 cleavage, stimulating the formation of vascular mimetic vessels (Hess et al., 2005) as illustrated in Fig. 2.
While it is likely that there are many drivers of VE-Cad expression in cancer cells, Sun et al. found that Twist1 appears to be involved in hepatocellular carcinoma (HCC) (Sun et al., 2010). Twist1 binds the VE-Cad promoter to enhance its expression, leading to Nodal/notch induction (McAllister, Zhan, Weishaupt, Hsu, & Murphy, 2010; Topczewska et al., 2006) and the activation of the HIF-family of transcription factors (Tang et al., 2014; Zhao et al., 2012). Therefore, active VE-Cad signaling is sufficient for the formation of vascular mimetic vessels as observed by Dr. Hendrix, however, there are a number of other factors which are also involved and discussed later in this review.
3.2. Notch, Smad2/3
Nodal/Notch, referred to as Notch for the purpose of this review, has several intriguing characteristics as an inducer of vascular mimicry and its over-expression in tumors (Brzozowa-Zasada et al., 2017). Notch also plays a major role during development by maintaining the stemness of embryological stem cells in addition to its position as a member of the transforming growth factor β (TGF-β) superfamily (Sakaki-Yumoto, Katsuno, & Derynck, 2013). Cells undergoing vascular mimicry frequently undergo morphological changes via epithelial to mesenchymal transition (EMT) (Fan, Zheng, Tang, & Liang, 2013; Malek et al., 2017). Notch expression is also essential to promote cancer stem cell self-renewal in aggressive melanoma cells (Postovit, Seftor, Seftor, & Hendrix, 2007; Topczewska et al., 2006). Furthermore, the reexpression of notch allows cancerous cells to return to a stem-like state as well as facilitating vascular mimicry (Delgado-Bellido et al., 2017).
Notch is a transmembrane receptor with four different isoforms to which Nodal, its ligand, binds with assistance of the co-receptors Cripto and the activin-like kinase (ALK) receptors type I (ALK4/7) and type II (ActRIIB; Schier, 2003). Once activated, Notch phosphorylates SMAD2 and SMAD3, eventually binding SMAD4 and translocating to the nucleus to drive gene expression (Shen, 2007). Smad2/3 drives vascular mimicry in breast cancer cells in addition to inducing EMT in vitro and in vivo (Gong et al., 2016). Such activation also stimulates a negative feedback loop to inhibit notch signaling via SMAD4-mediated transactivation of the Nodal inhibitors LEFTY1 and 2, preventing excessive Nodal activation in the nucleus. In cancers it is therefore logical to investigate how the SMAD4 feedback loop would likely become inactivated to promote tumor progression, vascular mimicry and distant metastasis of these tumors. SMAD4 is a known tumor suppressor which is either inactivated by direct mutation or deleted in head and neck (Lin, Chang, Cheng, & Liu, 2019) and colorectal cancers (Ma, Yan, Li, Liu, & Sun, 2014; Zhou et al., 1998). These clinical observations confirm the basic biology of the involvement of nodal/notch signaling in cancer progression (Zhao, Mishra, & Deng, 2018) as cancers are driven not only to induce β-catenin/Wnt signaling, but also to remove repression via SMAD4 and LEFTY1 and 2.
Cancer cells with decreased levels of Notch signaling display lower levels of VE-Cad mRNA and protein while promoting greater pigment production. This is interesting because VE-Cad (CD144) expression is known to be associated with endothelial-like stem cells and the embryonic stem cell lineage (Nikolova-Krstevski, Bhasin, Otu, Libermann, & Oettgen, 2008), therefore one would anticipate less differentiated stem-like cancer cells to display increased levels of VE-Cad, not decreased levels as shown in this study. These observations indicate that in melanomas with less nodal signaling, tumor cells become less aggressive and display greater melanotic cell characteristics accompanied by their decreased overall stemness (Topczewska et al., 2006).
In tumors with increased stemness and Notch signaling, however, nodal targets Notch-4 directly at the transcriptional level (Hardy et al., 2010). Enhanced Notch-1 and Notch-4 levels, along with its effects upon VE-Cad (Hardy et al., 2010), support the aggressiveness of melanoma cells and there in vivo metastasis via β-catenin (Balint et al., 2005). The critical role of notch to vascularity in transgenic knockout mice (Ruchoux et al., 2003) and the observation of notch involvement in many tumor types (Aster, Pear, & Blacklow, 2017), support the generalizable role of Notch upon vascular mimicry (Gong et al., 2016). Finally, blocking notch signaling via antibody treatment, inhibits vascular mimicry functionally validating these assertions (Hardy et al., 2010). Notch is highly expressed during development and these levels decrease later in life once the vasculature is developed. The upregulation of Nodal and its signaling effects upon notch, drive cancer cell stemness to repurpose embryological functions, establishing tumor vascularity independent of apoptosis.
3.3. HIF-1α, STAT3
Among the primary pathways involved in vascular mimicry, the Hypoxia inducible transcription factors (HIF-1α and HIF-2α), in particular HIF-1α, as well as the signal transducer and activator of transcription 3 (STAT3) are primarily mediators of this process. This section discusses the intracellular signaling roles of HIF and STAT3 and describes how they promote vascular mimicry. Undoubtedly, these molecules are hypoxia effectors, however, in this section the contribution of these molecules to the signaling cascades of vascular mimicry beyond the hypoxic condition and cross-talk with relevant signaling pathways of interest are discussed, leaving a more detailed review of hypoxia in Section 4.1 of this review. The phosphorylation of STAT3 correlates with cancer stemness, EMT and VE-Cad expression in colorectal cancer cells (Han et al., 2017). While the overexpression and hyper-phosphorylation of STAT3 is frequently observed in tumors, inducing cancer invasion and EMT via IL-6 activation (Huang et al., 2011), STAT3 was confirmed to induce vascular mimicry using RNAi approaches. EMT suppression is also observed during STAT3 knockdown (Han et al., 2017), which contributes to the suppression of vascular mimicry.
The phosphorylation of STAT3 also enhances HIF-1α protein stabilization, binding its C-terminus to block HIF-ubiquitination by von Hippel-Lindau (pVHL) in fibroblast COS7 cells (Jung et al., 2008, 2005). During hypoxia, these levels of HIF-1α would be much greater, however, signals from other oncogenes inducing p-STAT3 drives these pro-vascular mimetic signals even during periods of normoxia to promote tumor aggressiveness. In an interesting renal cancer study, saracatinib (Src inhibitor) significantly decreased p-STAT3 levels with no effect upon HIF-1α protein levels (Pawlus, Wang, & Hu, 2014), indicating that careful consideration of the overall signaling contexts and cancer subtypes are necessary to understand such conflicting results and the integration of these stimuli, which are complex and require further clarification.
Independent of the p-STAT3/HIF-1α hypoxia pathway, HIF-2α can also induce vascular mimicry by upregulating VE-Cadherin (Le Bras et al., 2007), assisting EphA2 translocation for signaling through the VE-Cad/EphA2/FAK signaling pathway. HIF-2α-induced VE-Cad is hypoxia-independent and involves the cooperative binding of HIF-2α and the Ets-1 transcription factor to the 140-base pair VE-Cad promoter (Le Bras et al., 2007). Data suggests HIF-1α may also upregulate VE-Cad expression in esophageal cancer cells driving vascular mimicry, however, the mechanism of these effects have not been fully characterized and warrant further investigation (Tang et al., 2014). Most importantly, the HIF-2α/VE-Cad/EphA2 pathway is thought to drive vascular mimicry, in a manner analogous to endothelial cell promoted angiogenesis (Song, Zhao, Song, & Shang, 2013), lending additional support for this signaling pathway in endothelial-like cancer stem cells.
4. Hypoxia and autophagy signaling
The absence of oxygen is lethal to organisms and cells that are dependent upon aerobic respiration. In response to these evolutionary selective pressures, human cells have developed numerous biochemical processes to promote cell survival during periods of sub-physiological oxygen levels. Rapidly growing cancers and micrometastases are frequently hypoxic as these new tissues which have grown beyond the support of the existing vasculature. Cancers adapt to the challenges of hypoxia by the activation of hypoxia inducible factors (HIFs) to alter gene expression (Heddleston et al., 2010), the upregulation of cancer stem cell markers (Zhang et al., 2017) and the activation of protective autophagy (Bellot et al., 2009). Below, we address how the hypoxic tumor microenvironment triggers these adaptations, their molecular pathways and how these effects drive cancer cell dedifferentiation into cancer stem cells leading to vascular mimicry.
4.1. Hypoxia
When aerobic lifeforms are deprived of oxygen and gas exchange, respiring tissues rapidly acidy as carbon dioxide accumulates forming carboxylic acid, and when cancer cells become dependent on glycolysis and the Warburg effect they produce lactic acid (Jiang, 2017). Acidic intracellular pH (pHi) is lethal so tumor cells maintain their neutral alkaline pHi values ranging from 7.2 to 7.5, while driving extracellular pH to 5.6–6.8 via proton pumps (known as pHi regulators) such as monocarboxylate transporters, transporters and the carbonic anhydrases to promote cell survival in these cytotoxic conditions (Chiche, Brahimi-Horn, & Pouyssegur, 2010; Wojtkowiak, Verduzco, Schramm, & Gillies, 2011). Highly acidic extracellular microenvironments are associated with malignant cells as these metabolic shifts occur a pH gradient forms along an oxygen gradient (pH decreases as oxygen levels decrease). Concomitant with this pH gradient, oncogenes activate, tumor suppressor activity is lost along with the activation of HIF-1α and HIF-2α as tumor cells shift toward anaerobic cellular metabolism, leading to augmented extracellular acidosis, further compounding these effects (Chiche et al., 2010). These effects are driven primarily via HIF-1α which amplifies the gene expression of many of the glucose transporters such as GLUT1, GLUT3 and many of the glycolytic enzymes to keep pace with the demands of anaerobic metabolism which requires the breakdown of ~18–19 times more glucose molecules relative to aerobic respiration (reviewed in Pouyssegur, Dayan, & Mazure, 2006; Semenza, 2003; Xiang & Semenza, 2019).
On a conceptual level, tumor hypoxia may seem to only pertain to extremely large, rapidly growing tumors. However, this is not the case as Li et al. observed microscopic tumors with sub-millimeter dimensions can be extremely hypoxic, while 1–4mm tumors are not significantly hypoxic when assessed using this approach (Li et al., 2007). From these data and their subsequent studies, it was implied that tumors first become hypoxic when they are avascular and reach several hundred micrometers (Li & O’Donoghue, 2008). Using a dorsal skin-fold window chamber system, the initial phases of tumor angiogenesis are evident prior to tumor hypoxia, with an intensification of these tumor angiogenic effects occurring once these tumors became hypoxic (as imaged in real time using 4T1 cells with a HIF:GFP imaging reporter) (Cao et al., 2005). Three of the most important hypoxic signaling factors involved in hypoxia are HIF-1α, HIF-2α and signal transducers and activations of transcription factor 3 (STAT3). STAT3 is upregulated in cancers during hypoxia (Yakata et al., 2007), driving the aggressiveness of tumors. Under normoxia, HIF-1α is rapidly degraded, however, during hypoxia, STAT3 blocks HIF-1α degradation while promoting HIF-1α protein synthesis, leading to an overall increase in HIF-1α signaling (Song et al., 2014). Due to the interaction of hypoxia or cancers over-expressing p-STAT3, HIF-1α transactivates genes containing hypoxia response elements (HRE) such as MMP-2, driving vascular mimicry (Xu et al., 2005). MMP-2 also enhances tumor invasion and metastasis by the p-STAT3/HIF-1α/MMP-2 vascular mimicry pathway (Li, Meng, Guan, Guo, & Han, 2016).
While the sequence of events and triggers of vascular mimicry are still unknown, we would like to put-forth a general framework for this understanding. During hypoxia, HIF-1α and HIF-2α are up-regulated altering gene expression leading hypoxic tumor cells toward cancer stemness in these hypoxic tumor microenvironments. These hypotheses are supported by the observations that HIF-1α is known to induce stemness and vascular mimicry via Oct3/4, Nanog and c-MYC clinically (Song et al., 2014). HIF-2α regulates the transactivation of Oct4, a crucial inducer and marker of cancer stem cells (Covello et al., 2006; Kitajima et al., 2018). While, HIF-1α and HIF-2α may compete with one another biochemically (Menrad et al., 2010), serving differing roles in acute versus chronic hypoxia (Hu et al., 2006; Sahin et al., 2012), they both play a crucial role in this process. Following the enrichment of cancer stem cells that occurs in hypoxic tumor microenvironments, the release of pro-angiogenic cytokines not only act systemically to drive angiogenesis, but they also act locally to transdifferentiate these stem-like cancer cells into vascular mimetic channels. This is particularly evident in glioma stem-like cells, VEGFR-2 positivity increases (Yao et al., 2013), indicating that VEGF ligand binding plays a role in vascular mimicry and in the hypoxic tumor microenvironment in vivo. Intriguingly, hypoxia can also induce vascular mimicry independently of the HIF-family via reactive oxygen species (ROS), which directly stimulate the ERK/Akt pathway, driving MMP-2 laminin cleavage (Liu et al., 2018). Further detailed biochemical investigations are necessary to elucidate novel molecular targets to disrupt hypoxia-induced vascular mimicry clinically.
4.2. Autophagy
Autophagy is a double-edged sword that can in specific contexts be protective and under other situations can be toxic (Bhutia et al., 2013). Cancerous cells can become addicted to protective autophagy-based signals due to the role of this process as a regulator of cancer cell survival and tumor growth (Liu & Debnath, 2016). More importantly, the effects of autophagy are dependent upon their cellular signaling context, their redox status among other factors. Autophagy process is described as a progression by which long-lived proteins and organelles are broken down to produce catabolic energy and biochemical monomers by sequestering these proteins and organelles into autophagosomes, which later fuse with lysosomes, generating the autophagolysosome (Bhutia et al., 2013; Liu & Debnath, 2016; Xu & Ren, 2012).
Autophagy is induced by hypoxia, however, autophagy signaling can also occur during cell starvation or cell stress which are common in tumors to modulate these effects. For example treatment with the autophagy inducer Rapamycin and the autophagy inhibitor Chloroquine have shown that autophagy plays a crucial role during vascular mimicry (Huang et al., 2014; Wu et al., 2017). The effects of hypoxia upon tumor cells alone is insufficient to recapitulate the effects of the hypoxic tumor microenvironment in vivo (Kim, Lin, Glazer, & Yun, 2018). Considering these factors, this section will carefully examine several questions: (1) Does Beclin-1, a key autophagy inducer, support the vascular mimicry phenotype? (2) Is autophagy-induced vascular mimicry in cells induced by the cAMP/AMPK/mTOR cell starvation pathway? (3) Is hypoxia required for autophagy-mediated vascular mimicry?
4.3. Beclin-1
Beclin-1 was initially cloned in 1998 (Liang et al., 1998), its principal function is to facilitate the formation of autophagosomes and was the first known autophagy-inducing gene (Liang et al., 1999). Vascular mimicry was first linked with Beclin-1 by Ding et al. in 2014, as Beclin-1 levels were significantly elevated during vascular mimicry while the inhibition of Beclin-1 blocked vascular mimicry and the expression of vascular mimicry-related genes (Ding, Zhao, Wu, & Xing, 2014). Furthermore, Beclin-1 mRNA and protein were elevated in vascular mimicry positive glioma (Duan, 2018), in VM-positive melanomas (Han et al., 2011), and in Oral squamous cell carcinoma clinical samples (Wang et al., 2018). Further correlative studies indicated that Beclin-1 also correlated with expression of HIF-1α and VEGF (Li et al., 2015). Furthermore, by knocking down Beclin-1 expression the levels of HIF-1α, VEGF, VE-Cad, EphA2 and MMP-2 decreased, thereby inhibiting hypoxia-induced vascular mimicry (Duan, 2018). This compelling evidence and additional studies indicated that Beclin-1-induced vascular mimicry was likely due to the generation of reactive oxygen species (ROS) and Akt activation, inducing angiogenesis (Goyal, Neill, Owens, Schaefer, & Iozzo, 2014; Nishikawa et al., 2010). Therefore, Beclin-1 affects vascular mimicry by mediating various molecules such as ERK (Liu et al., 2018). Relating these concepts to the clinic, Beclin-1 expression is correlated with VM formation in patient glioma samples along with increased VEGF and MMP-2 (Duan, 2018).
Beclin-1 induced vascular mimicry appears to occur via the ROS/ERK/MMP-2 pathway (Aggarwal et al., 2019), following the principal vascular mimicry pathways discussed in Section 3. However, when Beclin-1 was initially cloned, it was identified as a myosin-like Bcl-2 interacting protein (reviewed in Wechman et al., 2018). The genetic overexpression of Bcl-2 and the role of Bcl-2 signaling upon vascular mimicry was studied by real-time PCR analysis using hepatocellular carcinoma samples (Zhao et al., 2015). This analysis indicated that miR-27a and miR-17 levels decreased in Bcl-2 overexpressing cells, indicating that when Bcl-2 is over-expressed, the levels of VE-Cad, MMP-2, HIF-1α and VEGF-A are significantly increased, all of which induce vascular mimicry (Zhao et al., 2015). Bcl-2 inhibits Beclin-1 by binding the Beclin-1 BH3 domain (Marquez & Xu, 2012). It is unclear at this time how BH3 mimetics affect vascular mimicry or how understanding this effect will relate to B-cell lymphomas, which overexpress Bcl-2. It also remains to be determined if these effects are mediated by Bcl-2 globally and which specific BH3-family members are involved in facilitating these processes.
4.4. cAMP/AMPK/mTOR
Cyclic AMP (cAMP) is a second messenger, controlling many cellular processes related to cellular metabolism and vascularity. Once extracellular signals interact with their appropriate receptors, cAMP is produced by adenylyl cyclase to phosphorylate many targets including protein kinase A (PKA), Akt (Fan & Sun, 2010), MAPK, MEK1/2 and ERK1/2 (Dumaz & Marais, 2005), which are closely associated with vascular mimicry. Intriguingly, cAMP may also mediate the binding between Epac, Rap1 and p-Akt (Misra, Kaczowka, & Pizzo, 2008; Misra & Pizzo, 2005), which also significantly cross-talk with vascular mimicry signaling. When intracellular cAMP levels are high, cells are depleted of ATP which signals cellular starvation via PKA. Surprisingly, increased cAMP levels inhibit vascular mimicry through several modes of action. (1) Activation of Epac/Rap1 increases the levels of the Ras inhibitor Rap-GFP. (2) cAMP increases forskolin to inhibit PI3K and Akt signals (Hayashi & Sudo, 2009). (3) cAMP also inhibits ERK1/2 phosphorylation, via forskolin, independent of its action upon Epac and PKA, inhibiting Ras/MAPK-induced vascular mimicry (Lissitzky et al., 2009).
5’ AMP-activated protein kinase (AMPK) serves as an energy sensor to detect cellular starvation. cAMP activates PKA which then inhibits AMPK (MacPherson et al., 2016), blocking glucose metabolism and favoring β-oxidation (Huang et al., 2016). Once AMPK is activated, it stimulates Beclin-1 (Thr-388); (Zhang et al., 2016) inhibiting the mammalian target of rapamycin (mTOR), as well as inhibiting Akt, HIF-1α and glycolysis (Lyu et al., 2018; Salminen, Kaarniranta, & Kauppinen, 2016). AMPK negatively regulates glycolysis (The Warburg effect), suppressing tumor growth (Faubert et al., 2013). While the suppressive effects of AMPK upon vascular mimicry have not been rigorously confirmed at this time, it is clear that considering its signaling properties, which affect vascular pathology, that AMPK likely plays a critical role in these processes (Shirwany & Zou, 2014).
mTOR has a long-established role in autophagy regulation. mTOR actively inhibits the functions of autophagosome maturation inducer proteins (WIPI2, UVRAG), while upregulating autophagy inhibitors such as p300 and directly repressing Beclin-1 autophagosome complexes (Dossou & Basu, 2019). In terms of vascular mimicry, mTOR has been shown to be very important in glioma development directly correlating with mTOR expression, as confirmed in the analysis of 34 vascular mimicry positive (~27%, 34/127) glioma patient tumor samples (Huang et al., 2014). These authors then showed that the mTOR inhibitor rapamycin impeded vascular mimicry in U87 cells independent of hypoxia, supporting the substantial signaling consequences down-stream of mTOR (Chamcheu et al., 2019; Zarogoulidis et al., 2014). In terms of interactions with vascular mimicry-related molecules, rapamycin inhibits HIF-1α in a dose-dependent manner. Rapamycin is also an autophagy inducer, so Dr. Wu studied the effects of ATG5 genetic knock-down and chemically using the autophagosome maturation inhibitor chloroquine. In this study, chloroquine treatment and ATG5 knockdown inhibited vascular mimicry in glioma stem cells, while autophagy induction via rapamycin treatment enhanced vascular mimicry (Wu et al., 2017). This study revealed important mechanistic insights in terms of the effects of autophagy and bioenergetics upon vascular mimicry. Combining these concepts, when ATP levels are low and cAMP levels are high, PKA activation occurs to inhibit AMPK driving vascular mimicry. mTOR can also induce vascular mimicry via HIF-1α signaling, but these effects can be overcome by AMPK activation. AMPK is a universal energy sensor regulating aerobic and anaerobic metabolism in transformed and non-transformed cells and the integration of these autophagy/metabolic signals are critical to understand how cancer therapeutics targeting these pathways affect vascular mimicry.
5. Oncogenes associated with vascular mimicry
As vascular mimicry is a neovascularization process by which cancer cells become endothelial-like, promoting cancer invasion, metastasis and predicting poor survival it is therefore anticipated that vascular mimicry is correlated with the expression of several well-studied oncogenes. The following oncogenic signaling molecules were selected for their potential effects on vascular mimicry as these genes are known to drive tumors to become highly angiogenic (Casanova et al., 2002; Ciesielski et al., 2018; Minder, Zajac, Quigley, & Deryugina, 2015). As discussed in detail, many of these genes correlate with the VE-Cad/EphA2/FAK signaling pathway as discussed in Section 3.1 of this review. This section highlights select genes of interest to encourage further consideration of how specific biochemical pathways may cross-talk with other oncogenes and tumor types which highly express these signaling molecules in the context of vascular mimicry.
5.1. Ras
Simultaneously co-discovered by multiple scientists around the same time in 1982 (Cox & Der, 2010), Ras is one of the most well-studied oncogenes and implicated with transformation in many cancer types, promoting invasion and metastasis. The overexpression of this potent oncogene stimulates angiogenic branching, cancer cell stemness and EMT; all hallmarks of vascular mimicry (Liu et al., 2016). In terms of the relationship between Ras and vascular mimicry, K-Ras specifically induces PI3K via p38 (Choi et al., 2004), perhaps acting through the EphA2 pathway. Ras has also been shown to activate the RAF>MEK1/2>ERK1/2 pathways, which are down-stream of EphA2 (Macrae et al., 2005). Data analyzing the expression of the endogenous Ras inhibitor p120RasGAP indicated that endogenous Ras inhibition was limited to quiescent endothelial cells, which were VEGF-insensitive in many model systems (Westenskow et al., 2013). While it remains unclear, Ras seems to drive vascular mimicry and the endothelial-cell like adaptation of cancer stem-like cells. The true nature of Ras-induced vascular mimicry has not been clearly elucidated at this time, however, the Ras signaling axis very likely involves the VE-Cad/EphA2 pathway. Studies with next generation Ras inhibitors (Cruz-Migoni et al., 2019) will be essential to determine their therapeutic effects upon primary tumors, metastasis and vascular mimicry.
5.2. EGFR
The epidermal growth factor receptor drives tumorigenesis and is recognized as a biomarker for cancer therapeutic resistance, affecting a wide-array of cellular functions. EGFR is principally involved in stress-induced autophagy (Sigismund, Avanzato, & Lanzetti, 2018) and as an inducer of vascular mimicry (Delgado-Bellido et al., 2017). When the binding of EGFR to the extracellular matrix (ECM) and αvβ3 is blocked, vascular mimicry is inhibited using a CL4 aptamer suppressing in vivo tumor growth (Camorani et al., 2017). The CL4 oligonucleotide displays superior anti-EGFR efficacy as shown in vitro and in vivo and represses vascular mimicry more effectively than erlotinib and cetuximab. EGFR and EGFRvIII are potent inducers of vascular mimicry through αvβ3 in addition to their crosstalk with Ras, PI3K, acting primarily through the VE-Cad/EphA2 pathway.
5.3. HER2
Human epidermal growth factors receptor 2 (HER2) is overexpressed in around 25% of breast cancer tumors, enhancing breast tumorigenesis making it a compelling cancer therapeutic target (Mitri, Constantine, & O’Regan, 2012). Increased HER2 levels were histopathologically scored and correlated with increased levels of vascular mimicry clinically (Liu et al., 2013). Furthermore MCF-7 cells stably over-expressing HER2 display greater VE-Cad protein levels, supporting the relationship between HER2 and the VE-Cad/EphA2 vascular mimicry pathway (Liu et al., 2013). HER2-induced vascular mimicry may also include a number of different biochemical pathways including MAPK, PI3K, and Akt (Feng et al., 2018). HER2 is unique, however, because it has been revealed in terms of resistance to the HER2 inhibitor Lapatinib. This feedback loop was identified by treating breast cancer cells with another HER2 inhibitor trastuzumab, followed by antigen screening arrays. These screens identified 9 upregulated antigens, all of which were related to endothelial and stem cell phenotypes, i.e., vascular mimicry (Hori et al., 2019). The mechanisms of such resistance will require further study to identify new therapeutic modalities, however, it seems clear that Lapatinib-resistant breast cancer cells up-regulate RAF/MEK/ERK to drive vascular mimicry (Matkar, An, & Hua, 2017).
5.4. Twist1
Twist1 stimulates tumor progression by inhibiting cellular differentiation, inducing EMT and vascular mimicry (Chen & Wu, 2016). P53 suppresses Twist1 by inducing its ubiquitination to block its oncogenic effects (Yang-Hartwich et al., 2019), blocking Twist1-induced EMT and vascular mimicry. In a reciprocal mechanism, Twist1 can also repress p53 by binding its twist sequence on the C-terminus of p53, facilitating its degradation to prevent cell cycle arrest and the apoptosis effects of p53 (Maestro et al., 1999). Twist1 is frequently overexpressed in breast and cervical cancers (Ren et al., 2016; Zhao, Rahman, Chen, & Shin, 2017). Furthermore, Twist1 overexpression is sufficient to transform cancer cells into cancer stem cells with elevated levels of CD44, low levels of CD24 as well as increased levels of ALDH1 and rapid EMT transition (Strati, Nikolaou, Georgoulias, & Lianidou, 2019). Other vascular mimicry signaling molecules affected by Twist1 include the induction of Akt, β-Catenin, HIF-1α and NF-κB which also aid in the maintenance of EMT-associated cancer stem cells (Liu et al., 2016), likely inducing vascular mimicry via the STAT3/HIF-1α and Notch pathways leading to VE-Cad/EphA2 activation further down-stream.
5.5. NF-κB
NF-κB contributes to the alterations of oncogenic transcription factors and is a mediator of many cellular factors driving tumor progression and its activation is considered a hallmark of cancer (Ji, He, Regev, & Struhl, 2019). These roles include the control of cellular apoptosis, resistance to cancer therapeutic treatments, cancer stemness, hypoxia, EMT and more recently, vascular mimicry (Zakaria, Mohd Yusoff, Zakaria, Widera, & Yahaya, 2018). During treatment with the angiogenesis and vascular mimicry inhibitor thalidomide, tumor necrosis was induced, suppressing the levels of NF-κB along with alterations in other vascular mimicry related molecules such as VEGF, MMP-2 and MMP-9 (Zhang et al., 2008).
Studies in hepatocellular carcinoma reveal a role of miR-3928v in inducing EMT and vascular mimicry via the Hepatitis B virus X protein (HBx) in an NF-κb-dependent manner (Zhang et al., 2018). KVEPQDPSEW (AATP) binds to NF-κB and inhibits the MMP activation via blocking MAPK and NF-κB, experimentally inhibiting vascular mimicry and cancer metastasis in vivo (Gong et al., 2019), indicating the significant potential of NF-κB inhibitors as vascular mimicry inhibitors.
6. In vivo models of vascular mimicry
Most investigators in the vascular mimicry field are still validating the molecular markers associated with this process. The most common approach histologically is the use of immunological staining for the endothelial markers CD31 or CD34 followed by counterstaining with PAS (Chen et al., 2002; Clarijs et al., 2002; Clemente et al., 2010; Seftor et al., 2001; Vartanian, 2012). Most in vivo model systems of vascular mimicry have relied upon murine xenografts. At this time, no transgenic models have been reported to consistently form vascular mimicry positive tumors. Also, lending to the heavy dependence upon murine tumor models, vascular mimicry can only be assessed post-mortem, introducing limitations in follow-up studies as not all animals or tumors will develop vascular mimicry with uniformity. Real-time imaging is paramount to accelerate the pre-clinical development of vascular mimicry inhibitors.
To date, most publications studying vascular mimicry have used subcutaneous xenografts in immuno-compromised mice. To study vascular mimicry in this context, tumors must be dissected, sectioned and examined histologically to observe if cancer therapeutics of interest block vascular mimicry. Xenograft models are valuable to demonstrate the efficacy of therapeutics against human-derived cancers, however, access to transgenic animal models that form vascular mimicry-positive tumors spontaneously or in an inducible manner will be essential for the pre-clinical evaluation of vascular mimicry inhibitors. Such models would allow standardization of these approaches and accelerate vascular mimicry drug discovery. An ideal model would spontaneously form these tumors quickly within 3 months with a significant proportion of these displaying the presence of vascular mimetic channels (~75% of tumors or greater). Considering oncogenes and pathways that have been discussed throughout this review, this section will now discuss prospective murine transgenic models which are most likely to meet these criteria.
RASGRP1 mice form spontaneous tumors of the skin (Oki-Idouchi & Lorenzo, 2007). However, the role of or frequency of vascular mimicry in these tumors are unknown (Oki-Idouchi & Lorenzo, 2007). The VE-Cad-Cre-ER inducible model appears to be very interesting in regard to its utility for studying neo-angiogenesis and tumor growth (Monvoisin et al., 2006). Lastly there is an intriguing Smad4 haploinsufficiency intestinal cancer model with 100% tumor penetrance in the cells for the upper gastrointestinal (GI) tract (Alberici et al., 2006). Smad4+/E6sad carries a single nucleotide deletion in the exon 6 splice acceptor site, destabilizing Smad4 mRNA, knocking out Smad4 protein expression (Hohenstein et al., 2003). In the heterozygote state, this mutation induces gastric and duodenal tumors with characteristics of juvenile intestinal polyps. As discussed in greater detail in Section 3.2, Smad4 facilitates the translocation of Smad2/3 into the nucleus but Smad4 also upregulates the nodal inhibitors Lefty1 and 2, blocking prolonged nodal signaling. This Smad4 feedback loop is frequently mutated in many GI and pancreatic cancers (Hahn et al., 1996; Salovaara et al., 2002). Intriguingly, the tumor suppressor Smad4 also induces a cell lethal form of EMT, while not bypassing nodal/TGF-β-induced cancer progression (David et al., 2016), in addition to the roles of K-Ras, β-catenin and Wnt signaling to these processes (Zhao et al., 2018). Smad4+/E6sad animals cannot be breed as homozygotes as homozygosity is lethal at E9.5 due to gastrulation and mesodermal defects during development. Perhaps more importantly, vascular mimicry has been observed in colorectal cancers (Han et al., 2017) making it very likely that these tumors will form vascular mimicry networks in vivo. At present, no murine model has been developed which universally leads to formation of rapid vascular mimicry positive tumors with high penetrance. It is likely such models, could prove invaluable for the vascular mimicry field, permitting standardization of in vivo studies and once established, would be of benefit to cancer patients clinically through accelerated small molecule discovery targeting vascular mimicry.
7. Markers of vascular mimicry
For histological identification of vascular mimicry, there is a need for a wider array of positive and negative markers of vascular mimicry (Table 1). Negative markers differentiate angiogenic vessels, as is the case with most common in vivo approaches involving staining endothelial cells using CD31 and CD34 counterstained with PAS (Chen et al., 2002; Clarijs et al., 2002; Clemente et al., 2010; Seftor et al., 2001; Vartanian, 2012). Positive markers for vascular mimicry would stain vascular mimetic vessels, but not angiogenic ones. VE-Cad stains for vascular mimicry positive cells in vitro. In vivo however, VE-Cad is also highly expressed in normal endothelial cells making it a poor marker for histological analysis. Down-stream of VE-Cad, EphA2 would be nearly ideal as a vascular mimetic marker. EphA2 is highly expressed during vascular mimicry, but has low expression in endothelial cells (Delgado-Bellido et al., 2017). Other “positive” markers include MMP-2 and laminin, which are associated with the ECM maturation of vascular mimetic vessels (Angara et al., 2017), as well as other ECM factors such as type I/IV Collagen among other molecules of significant interest to better understand vascular mimicry. In this section, an array of vascular mimicry markers are discussed for the development of enhanced in vivo imaging.
Table 1.
Differences between vascular mimetic and endothelial cell markers.
| Normal endothelial cells | Vasculogenic mimicry cells |
|---|---|
| Similarities | |
| VE-cadherin positive | |
| E-selectin positive | |
| CD34 positive | |
| Differences | |
| TIE-2 positive | TIE-2 negative |
| VEGFR-1, 2 positive | VEGFR-1, 2 negative |
| P-selectin positive | P-selectin negative |
| VCAM-1/CD106 positive | VCAM-1/CD106 negative |
| CD31/PECAM-1 positive | CD31/PECAM-1 negative *13 (subpopulations PECAM-1 positive melanoma cells) |
| TIE-1 negative | TIE-1 positive |
| VEGF-C negative | VEGF-C positive |
| Neuropilin 1 negative | Neuropilin 1 positive |
| Endoglin negative | Endoglin positive |
| Tissue factor pathway inhibitor 1 (TFPI1) negative | Tissue factor pathway inhibitor 1 (TFPI1) positive |
| Laminin 5 gamma 2 chain (LAMC2) negative | Laminin 5 gamma 2 chain (LAMC2) positive |
| EphA-2 negative | EphA-2 positive |
From Delgado-Bellido, D., Serrano-Saenz, S., Fernandez-Cortes, M., & Oliver, F. J. (2017). Vasculogenic mimicry signaling revisited: Focus on non-vascular VE-cadherin. Molecular Cancer, 16(1), 65. doi: 10.1186/s12943-017-0631-x.
Laminin is an extracellular matrix factor which must be cleaved into the pro-migratory γ2′ and γ2x, which is present in vascular mimetic channels (Seftor et al., 2001). Importantly, laminin expression is higher in vascular mimetic vessels than in angiogenic vessels, allowing for direct identification of vascular mimicry vessels (Delgado-Bellido et al., 2017). Laminin is frequently stained via PAS, using angiogenic staining to differentiate angiogenic endothelial cell-lined vessels. However, direct laminin staining immunologically has been useful for studying vascular mimicry, but this approach has not been widely utilized (Angara et al., 2017). Lectin-based extracellular matrix protein staining may also be helpful for staining endothelial cell-lined vessels (Roussel & Dalion, 1988). While lectin could be of interest to study mosaic interactions between vascular mimicry and angiogenesis (Angara et al., 2017); head-to-head comparisons with CD31 and CD34 to determine if lectin provides more or less value for endothelial cell identification is necessary.
7.1. Contested markers of vascular mimicry
While the vascular mimicry markers EphA2, VE-Cad, MMP-2/9/14 are well established the role of other molecules in vascular mimicry are not well established. Aldehyde dehydrogenase 1 (ALDH1) expression correlates with cancer cell stemness and vascular mimicry in clinical breast tumor samples (Xing et al., 2018). More importantly, patients with tumors histologically scored with elevated vascular mimicry and ALDH1 display poorer disease-free and overall survival than patients bearing ALDH1 or vascular mimicry negative tumors. While ALDH1 is considered by some to be an inferior marker of cancer stem cells relative to the combination of CD24 or CD44 (discussed in; Horimoto et al., 2016; Li et al., 2017), ALDH1 may have promise as a biomarker for vascular mimicry-competent cancer cells. KiSS-1 is a tumor suppressor that when used therapeutically kills cancer cells systemically with high efficacy (Ly, Harihar, & Welch, 2020; McNally et al., 2010). Consistent with its tumor suppressor functions, KiSS-1 is frequently mono-allelically deleted in cancers, displaying lower expression in cancerous tissues than in matched normal controls (Yu et al., 2017). Therefore, KiSS-1 could serve as a negative marker for vascular mimicry. Conversely, MACC1 expression is significantly elevated in cancer cells relative to control tissue and correlates with vascular mimicry (Yu et al., 2017), making MACC1 a compelling positive marker for future study. The roles of ALDH1, KiSS-1, MACC1, CD133, CD44 and CD90 are known, but require further functional considerations and confirmation in vascular mimicry.
8. Vascular mimicry inhibitors
As our understanding of cancer malignancy increases, cancer therapeutics that can exploit these unique aspects of cancer cells at the molecular level are extremely appealing. The de novo formation of cancerous blood vessels by the process of vascular mimicry is induced by multiple mechanisms including tumor hypoxia, VE-Cad, Ras, HER2, VEGF, COX-2, autophagy and the Nodal/Smad pathways. To this end, the integration of these pathways and their available inhibitors are presented (Fig. 3) (Fan & Sun, 2010). Below we discuss known vascular mimicry inhibitors and the predicted challenges for the development of novel vascular mimicry inhibitors.
Fig. 3.

Vascular mimicry signaling pathways and points of cancer therapeutic intervention. Hypoxic activation of VEGF, Ras and MMP-2 pathways of vascular mimicry induction can be blocked in the following ways: (1) Genistein blocks channel formation directly via repressing VE-Cad cell signaling. (2) Thalidomide and bevacizumab inhibit vascular mimicry induced by VEGF and its interaction of the VEGFB receptor and the subsequent EphA2, FAK, ERK1/2 cascade in addition to its effects upon MMP-2 and laminin-5. (3) Forskolin inhibits vascular mimicry due to the repression of MAPK, but also PI3K which has considerable cross-talk with the Ras and EphA2/FAK signaling pathways. (4) COL-3 in addition to its effects as a VE-Cad inhibitor also actively represses active MMP-2 and MT1-MMP expression which are important for laminin-5 cleavage and maturation in to its pro-migratory fragments 2′ and 2x. (5) Along with COL-3 and Thalidomide, Doxycycline also maintains anti-vascular mimicry efficacy via the repression of active MMP-2 as well. Modified from Fan, Y. Z., & Sun, W. (2010). Molecular regulation of vasculogenic mimicry in tumors and potential tumor-target therapy. World Journal of Gastrointestinal Surgery, 2(4), 117–127. doi: 10.4240/wjgs.v2.i4.117.
In the continuous quest to develop innovative cancer therapeutics, there is a constant impetus to create new molecules, and to repurpose FDA approved drugs, to exploit these unique cancer inhibitory factors therapeutically. In the case of vascular mimicry and the dedifferentiation of these cells into endothelial-like stem cells, differentiation agents and all-trans retinoic acid and drugs targeting angiogenesis may be of use. The VEGF inhibitors thalidomide and bevacizumab efficiently inhibit vascular mimicry (Videira et al., 2011; Zhang & Luo, 2018). Thalidomide also inhibits MMP-2, blocking the cleavage of laminin5γ2 and vascular mimicry (Zhang et al., 2008). To the astute reader, vascular mimicry is thought to serve as a potential mechanism of angiogenesis-resistance. HER2 induced vascular mimicry is increased in cancer patient samples pre-treated with trastuzumab, which inhibits both HER2 and VEGF (Feng et al., 2018). All cancer cells resistant to trastuzumab efficiently form vascular mimicry vessels. Furthermore, trastuzumab-resistant cells are sensitive to vascular mimicry disruption via salinomycin treatment due to salinomycin’s ability to disrupt the actin cytoskeleton.
The VEGFA binding monoclonal antibody bevacizumab has been used clinically as a disrupter of angiogenesis since 2004 (Shih & Lindley, 2006). Following anti-angiogenic therapy, “vessel co-option” is common. Vessel co-option promotes poor responses to anti-angiogenic agents. Mechanistically such vessel co-option is due to the actin-related protein Arp2/3 (Frentzas et al., 2016), a protein that plays a role in vascular mimicry via notch and the maintenance of stem-like cancer cells (Zhang et al., 2017). Not surprisingly, vessel co-option is closely associated with vascular mimicry, however, these processes are distinct (reviewed in Donnem et al., 2013). Bevacizumab treatment directly leads to increased vascular mimicry in patient tumors (Xu et al., 2012). Specifically, bevacizumab treatment induces HIF-1α alone or in combination with cytotoxic therapeutics like cisplatin, increasing the density of vascular mimetic vessels. As stated in Section 4.1, hypoxia drives vascular mimicry by supporting cancer stemness, Nodal and VE-Cad. The effects of VEGF upon vascular mimicry is surprising (Xu et al., 2012) as parallel studies with bevacizumab at equivalent concentrations did not alter in vitro vascular mimicry while profound in vivo repression was observed, indicating that other microenvironmental factors may play a role in this phenomenon. In many cases, following prolonged angiogenic inhibitor treatment there is a release of alternative cytokines stimulating angiogenesis including, FGF-2 and SDF-1 as seen clinically in patients with glioblastoma multiforme (Batchelor et al., 2007).
It is likely that HER induced vascular mimicry includes a number of different biochemical pathways including MAPK, PI3K, and the Akt (Feng et al., 2018). Resistance to the HER2 inhibitor trastuzumab induces vascular mimicry via alteration to the actin cytoskeletal integrity (Hori et al., 2019). Prolonged VEGF inhibitor use also upregulates vascular mimicry (Angara et al., 2017), perhaps by creating selective pressure to maintain stem-like cancer cells leading to enhanced vascular mimicry (Gardner, Madu, & Lu, 2017). Chemotherapeutic targeting of vascular mimicry must address the stem-like nature of vascular mimetic cells and the incomplete and leaky tumor vasculature and the unique microenvironment of vascular mimetic channels. Cancer stem cells are well known for their abilities to over express drug-efflux pumps (Moitra, Lou, & Dean, 2011; Tripathi & Misra, 2016), limiting the effectiveness of drug-based vascular mimicry inhibitors. Studies with the differentiating agent retinoic acid effectively differentiates U87 stem-like cells to impair vasculogenic mimicry (Ling et al., 2015). Furthermore, by targeting vascular mimicry, these targeted cells will likely reside in deeply hypoxic avascular tumor regions of microscopic size (100-μM to 1-mm3), limiting the bioavailability of these drugs to these avascular niches when treated systemically. Dense extracellular matrix proteins can also block the spread of therapeutic viruses and drugs in vivo (Diop-Frimpong, Chauhan, Krane, Boucher, & Jain, 2011; Netti, Berk, Swartz, Grodzinsky, & Jain, 2000). Considering how collagen and laminin separate the vascular mimicry blood lumen from the cancer cells forming these vessels, this is also a factor which must be considered for therapeutic applications. These challenges will need to be addressed for the development of novel vascular mimicry inhibitors in addition to the need to identify which vascular mimicry biomarkers are of the greatest relevance clinically to monitor the efficacy of these drugs and cancer patient responses.
9. Discussion and future directions
Large multicellular organisms evolved complex vasculature networks to ensure that every cell is able to respire and remove metabolized sugars and fatty acids as carbon dioxide, while taking in oxygen to remove electrons from complex IV of the electron transport chain, thereby allowing further electron flow and optimal ATP synthesis. This fundamental aspect of biology underscores the evolutionary pressure of hypoxic adaptation and the existence of mechanisms to overcome these cellular stressors. In fully developed adult organisms, hypoxia rarely takes place. The exception to this rule, is when tumors and micrometastatic tumors grow quickly, producing avascular regions in excess of 1-mm3 in diameter (Li & O’Donoghue, 2008). Although these concepts are highlighted in this review, emphasis has been placed on the potential mechanism(s) by which cancer cells form functional blood vessels de novo and why this adaptation occurs.
In the hypoxic cores of tumors, where no endothelial cells are present, tumors must find ways to replace these endothelial cell functions to adapt to these metabolic stressors. Some of the primary examples include the secretion of vascular endothelial growth factor (VEGF) (Evans et al., 2017; Xu et al., 2019), platelet-derived growth factor (PDGF) (Shure, Senior, Griffin, & Deuel, 1992; Thijssen et al., 2018), Angiopoietin 2 and many other endothelial cell chemo-attractants to induce nearby angiogenic vessel branches to penetrate the rim of rapidly growing tumors during periods of hypoxia. While these cell-cell communications take time, and endothelial progenitor cells mobilize from the bone marrow, the hypoxic core of these tumors become dormant due to their suppressed overall metabolic rates, relying primarily on glycolysis and upregulated Glut1 expression to keep up with energy demands, since anaerobic metabolism requires ~18–19 times more glucose molecules to be equivalent to aerobic oxidative phosphorylation, which are traits of cancer stem cells. As recruited endothelial cell branches begin to penetrate the dividing rim cells of these tumors, cancer cells in these hypoxic cores do not have access to endothelial cells and thus must create their own blood conductive vessels. The hypoxic tumor microenvironment promotes the acquisition of stem-like traits leading to their transdifferentiation into endothelial-like vascular mimetic vessels. These vessels can then grow and can directly interface with the angiogenic vessels at the rim of these tumors to now convey blood from the tumor’s surroundings and perfuse the tumor interior with oxygen rich blood, further driving tumor growth. These de novo cancer-derived vessels would likely be “leaky,” as are tumor angiogenic vessels, forming the basis for vascular mimicry that contributes to the metastasis and invasion of cancer cells to regional and distant sites, since now these tumors have greater access to leaky vascular niches thereby permitting greater tumor cell intravasation. The molecular characteristics of leaky angiogenic vessels seem to be related to laminin α4 from reports using hemorrhagic KO mice (Thyboll et al., 2002), however, laminin5γ2 which actively participates in vascular mimicry is an α3 laminin (Schneider, Muhle, & Pacho, 2007).
To elucidate the fundamental nature of vascular mimicry and develop more effective inhibitors of this process, more authentic transgenic and xenograft model systems are necessary to answer some fundamental questions: (1) Are cancer initiating stem cells frequently vascular mimicry competent? (2) What features of cancer stem cells mark sub-populations for vascular mimicry? (3) Is it possible to target vascular mimicry via EphA2 antibody conjugates, VE-Cad or laminin? These crucial questions will likely be answered in the not too distant future as the reality of vascular mimicry-induced cancer malignancy is established in most if not all human cancers. The importance of vascular mimicry will also likely play a major role in resistance to cytotoxic chemotherapy. Resistance to chemotherapy is an important issue in the treatment of stem-like cancer cells. Combination treatments with vascular mimicry inhibitors are necessary to impact the avascular hypoxic cores of primary and metastatic tumors, as well as to prevent cancer recurrence, invasion and metastasis.
An underutilized technology, which could help elucidate therapeutic effects in vivo in real-time, is Multispectral Optoacoustic Tomography (MSOT). At this time, there are a limited number of these machines currently utilized in the world (perhaps ~4–6). MSOT takes advantage of the bidirectional nature of the photoacoustic effect to produce light in response to ultrasound stimulation. Due to the endogenous differential resonance of oxygenated and deoxygenated hemoglobin molecules, MSOT can delineate between angiogenesis and vascular mimicry in vivo noninvasively, while also measuring O2 Saturation and tumor oxygenation with validation of CD31/PAS histology (Quiros-Gonzalez et al., 2018).
There is still much we do not understand about the process of vascular mimicry. Due to its very nature, it is implicit that vascular mimicry can occur in the hypoxic core of microscopic tumors, likely while these tumors remain undetectable as primary tumors, micrometastases, or once cancers become re-activated after remaining dormant for years promoting the invasive growth of these tumors. The history of how cancers utilize the process of vascular mimicry from early development informs us that this process is likely a manifestation of reversion to embryological vasculogenesis (reviewed in Paulis et al., 2010). As such, non-rodent models of vessel formation may reveal cancer therapeutic targets and processes that can be used to inhibit these extremely deleterious oncogenic phenomena. Overcoming the great challenge of targeting cancer stem cells, understanding the hypoxic niche and to treat and monitor the undetectable micrometastases where vascular mimicry is thought to occur, will be the next great hurdle in cancer therapy. To achieve future breakthroughs will require the collaboration of clinical oncologists, stem cell biologists, and imaging experts from around the world to detect what is now undetectable and to block cancer’s latest nefarious trick, i.e., vascular mimicry, thereby benefiting cancer patients worldwide.
Acknowledgments
Support for our laboratories was provided in part by National Institutes of Health Grants R01 CA097318 (P.B.F.), P50 CA058326 (Martin G. Pomper and P.B.F.), K12 GM093857 (Virginia Commonwealth University IRACDA) (S.L.W. and P.B.F.), and R01 CA244993 (D.S. and P.B.F.); the Samuel Waxman Cancer Research Foundation (P.B.F. and D.S.); National Foundation for Cancer Research (P.B.F.); NCI Cancer Center Support Grant to VCU Massey Cancer Center P30 CA016059 (P.B.F. and D.S.); and VCU Massey Cancer Center developmental funds (P.B.F.). P.B.F. and D.S. are SWCRF investigators. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
References
- Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, et al. (2019). Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules, 9(11). 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberici P, Jagmohan-Changur S, De Pater E, Van Der Valk M, Smits R, Hohenstein P, et al. (2006). Smad4 haploinsufficiency in mouse models for intestinal cancer. Oncogene, 25(13), 1841–1851. 10.1038/sj.onc.1209226. [DOI] [PubMed] [Google Scholar]
- Angara K, Borin TF, & Arbab AS (2017). Vascular mimicry: A novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Translational Oncology, 10(4), 650–660. 10.1016/j.tranon.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster JC, Pear WS, & Blacklow SC (2017). The varied roles of notch in cancer. Annual Review of Pathology, 12, 245–275. 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, et al. (2005). Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. The Journal of Clinical Investigation, 115(11), 3166–3176. 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome RA, Torres S, Isern de Val S, Escudero-Paniagua B, Calvino E, Teixido J, et al. (2017). VE-cadherin RGD motifs promote metastasis and constitute a potential therapeutic target in melanoma and breast cancers. Oncotarget, 8(1), 215–227. 10.18632/oncotarget.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. (2007). AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell, 11(1), 83–95. 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. (2009). Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Molecular and Cellular Biology, 29(10), 2570–2581. 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, et al. (2013). Autophagy: Cancer’s friend or foe? Advances in Cancer Research, 118, 61–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Grosser M, & Rezaei M (2014). Endothelial cadherins in cancer. Cell and Tissue Research, 355(3), 523–527. 10.1007/s00441-014-1851-7. [DOI] [PubMed] [Google Scholar]
- Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, Kostaras E, et al. (2017). Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. The Journal of Pathology, 241(3), 362–374. 10.1002/path.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowa-Zasada M, Piecuch A, Michalski M, Segiet O, Kurek J, Harabin-Slowinska M, et al. (2017). Notch and its oncogenic activity in human malignancies. European Surgery, 49(5), 199–209. 10.1007/s10353-017-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camorani S, Crescenzi E, Gramanzini M, Fedele M, Zannetti A, & Cerchia L (2017). Aptamer-mediated impairment of EGFR-integrin alphavbeta3 complex inhibits vasculogenic mimicry and growth of triple-negative breast cancers. Scientific Reports, 7, 46659 10.1038/srep46659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Li CY, Moeller BJ, Yu D, Zhao Y, Dreher MR, et al. (2005). Observation of incipient tumor angiogenesis that is independent of hypoxia and hypoxia inducible factor-1 activation. Cancer Research, 65(13), 5498–5505. 10.1158/0008-5472.CAN-04-4553. [DOI] [PubMed] [Google Scholar]
- Casanova ML, Larcher F, Casanova B, Murillas R, Fernandez-Acenero MJ, Villanueva C, et al. (2002). A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Research, 62(12), 3402–3407. [PubMed] [Google Scholar]
- Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu RN, Walker AL, et al. (2019). Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: A review of current status and future trends on natural and synthetic agents therapy. Cell, 8(8). 10.3390/cells8080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Maniotis AJ, Majumdar D, Pe’er J, & Folberg R (2002). Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Investigative Ophthalmology & Visual Science, 43(8), 2533–2539. [PubMed] [Google Scholar]
- Chen HF, & Wu KJ (2016). Endothelial transdifferentiation of tumor cells triggered by the Twist1-Jagged1-KLF4 Axis: Relationship between cancer stemness and angiogenesis. Stem Cells International, 2016, 6439864 10.1155/2016/6439864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche J, Brahimi-Horn MC, & Pouyssegur J (2010). Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. Journal of Cellular and Molecular Medicine, 14(4), 771–794. 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JA, Park MT, Kang CM, Um HD, Bae S, Lee KH, et al. (2004). Opposite effects of Ha-Ras and Ki-Ras on radiation-induced apoptosis via differential activation of PI3K/Akt and Rac/p38 mitogen-activated protein kinase signaling pathways. Oncogene, 23(1), 9–20. 10.1038/sj.onc.1206982. [DOI] [PubMed] [Google Scholar]
- Ciesielski M, Szajewski M, Peksa R, Lewandowska MA, Zielinski J, Walczak J, et al. (2018). The relationship between HER2 overexpression and angiogenesis in gastric cancer. Medicine (Baltimore), 97(42), e12854 10.1097/MD.0000000000012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarijs R, Otte-Holler I, Ruiter DJ, & de Waal RM (2002). Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Investigative Ophthalmology & Visual Science, 43(4), 912–918. [PubMed] [Google Scholar]
- Clemente M, Perez-Alenza MD, Illera JC, & Pena L (2010). Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Veterinary Pathology, 47(2), 265–274. 10.1177/0300985809353167. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. (2006). HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes & Development, 20(5), 557–570. 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, & Der CJ (2010). Ras history: The saga continues. Small GTPases, 1(1), 2–27. 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Migoni A, Canning P, Quevedo CE, Bataille CJR, Bery N, Miller A, et al. (2019). Structure-based development of new RAS-effector inhibitors from a combination of active and inactive RAS-binding compounds. Proceedings of the National Academy of Sciences of the United States of America, 116(7), 2545–2550. 10.1073/pnas.1811360116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. (2016). TGF-beta tumor suppression through a Lethal EMT. Cell, 164(5), 1015–1030. 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Bellido D, Serrano-Saenz S, Fernandez-Cortes M, & Oliver FJ (2017). Vasculogenic mimicry signaling revisited: Focus on non-vascular VE-cadherin. Molecular Cancer, 16(1), 65 10.1186/s12943-017-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhao K, Wu Y, & Xing C (2014). Expression and significance of Beclin-1 in vasculogenic mimicry formation of gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi, 17(7), 716–719. [PubMed] [Google Scholar]
- Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, & Jain RK (2011). Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2909–2914. 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, et al. (2013). Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Medicine, 2(4), 427–436. 10.1002/cam4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossou AS, & Basu A (2019). The emerging roles of mTORC1 in macromanaging autophagy. Cancers (Basel), 11(10). 10.3390/cancers11101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S (2018). Silencing the autophagy-specific gene Beclin-1 contributes to attenuated hypoxia-induced vasculogenic mimicry formation in glioma. Cancer Biomarkers, 21(3), 565–574. 10.3233/CBM-170444. [DOI] [PubMed] [Google Scholar]
- Dumaz N, & Marais R (2005). Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. The FEBS Journal, 272(14), 3491–3504. 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, & Whang EE (2004a). EphA2: A determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene, 23(7), 1448–1456. 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, & Whang EE (2004b). Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochemical and Biophysical Research Communications, 320(4), 1096–1102. 10.1016/j.bbrc.2004.06.054. [DOI] [PubMed] [Google Scholar]
- El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, et al. (2010). A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain, 133(Pt. 4), 973–982. 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IM, Kennedy SA, Paliashvili K, Santra T, Yamaji M, Lovering RC, et al. (2017). Vascular endothelial growth factor (VEGF) promotes assembly of the p130Cas interactome to drive endothelial chemotactic signaling and angiogenesis. Molecular & Cellular Proteomics, 16(2), 168–180. 10.1074/mcp.M116.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YZ, & Sun W (2010). Molecular regulation of vasculogenic mimicry in tumors and potential tumor-target therapy. World Journal of Gastrointestinal Surgery, 2(4), 117–127. 10.4240/wjgs.v2.i4.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YL, Zheng M, Tang YL, & Liang XH (2013). A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncology Letters, 6(5), 1174–1180. 10.3892/ol.2013.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. (2013). AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metabolism, 17(1), 113–124. 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. (2018). Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases, 5(2), 77–106. 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cortes M, Delgado-Bellido D, & Oliver FJ (2019). Vasculogenic mimicry: Become an endothelial cell “but not so much” Frontiers in Oncology, 9, 803 10.3389/fonc.2019.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. (2016). Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nature Medicine, 22(11), 1294–1302. 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner V, Madu CO, & Lu Y (2017). Anti-VEGF therapy in cancer: A double-edged sword. Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy, 385–410. 10.5772/66763. [DOI] [Google Scholar]
- Gong F, Chen MF, Zhang YY, Li CY, Zhou CX, Hong PZ, et al. (2019). A novel peptide from abalone (Haliotis discus hannai) to suppress metastasis and vasculogenic mimicry of tumor cells and enhance anti-tumor effect in vitro. Marine Drugs, 17(4), 244 10.3390/md17040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Sun B, Zhao X, Zhang D, Sun J, Liu T, et al. (2016). Nodal signaling promotes vasculogenic mimicry formation in breast cancer via the Smad2/3 pathway. Oncotarget, 7(43), 70152–70167. 10.18632/oncotarget.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Neill T, Owens RT, Schaefer L, & Iozzo RV (2014). Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biology, 34, 46–54. 10.1016/j.matbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. (1996). DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science, 271(5247), 350–353. 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Han C, Sun B, Wang W, Cai W, Lou D, Sun Y, et al. (2011). Overexpression of microtubule-associated protein-1 light chain 3 is associated with melanoma metastasis and vasculogenic mimicry. The Tohoku Journal of Experimental Medicine, 223(4), 243–251. 10.1620/tjem.223.243. [DOI] [PubMed] [Google Scholar]
- Han C, Sun B, Zhao X, Zhang Y, Gu Q, Liu F, et al. (2017). Phosphorylation of STAT3 promotes vasculogenic mimicry by inducing epithelial-to-mesenchymal transition in colorectal cancer. Technology in Cancer Research & Treatment, 16(6), 1209–1219. 10.1177/1533034617742312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, et al. (2010). Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Research, 70(24), 10340–10350. 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, & Sudo T (2009). Effects of the cAMP-elevating agents cilostamide, cilostazol and forskolin on the phosphorylation of Akt and GSK-3beta in platelets. Thrombosis and Haemostasis, 102(2), 327–335. 10.1160/TH08-12-0781. [DOI] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, & Rich JN (2010). Hypoxia inducible factors in cancer stem cells. British Journal of Cancer, 102(5), 789–795. 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, & Seftor RE (2003). Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nature Reviews Cancer, 3(6), 411–421. 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, et al. (2001). Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 8018–8023. 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Postovit LM, Margaryan NV, Seftor EA, Schneider GB, Seftor RE, et al. (2005). Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Research, 65(21), 9851–9860. 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, et al. (2001). Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: Role of epithelial cell kinase (Eck/EphA2). Cancer Research, 61(8), 3250–3255. [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, & Hendrix MJ (2006). VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: Implications for vasculogenic mimicry. Cancer Biology & Therapy, 5(2), 228–233. 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- Hohenstein P, Molenaar L, Elsinga J, Morreau H, van der Klift H, Struijk A, et al. (2003). Serrated adenomas and mixed polyposis caused by a splice acceptor deletion in the mouse Smad4 gene. Genes, Chromosomes & Cancer, 36(3), 273–282. 10.1002/gcc.10169. [DOI] [PubMed] [Google Scholar]
- Hori A, Shimoda M, Naoi Y, Kagara N, Tanei T, Miyake T, et al. (2019). Vasculogenic mimicry is associated with trastuzumab resistance of HER2-positive breast cancer. Breast Cancer Research, 21(1), 88 10.1186/s13058-019-1167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto Y, Arakawa A, Sasahara N, Tanabe M, Sai S, Himuro T, et al. (2016). Combination of cancer stem cell markers CD44 and CD24 is superior to ALDH1 as a prognostic indicator in breast cancer patients with distant metastases. PLoS One, 11(10), e0165253 10.1371/journal.pone.0165253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, & Simon MC (2006). Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Molecular and Cellular Biology, 26(9), 3514–3526. 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Chien YS, Chen YJ, Ajuwon KM, Mersmann HM, & Ding ST (2016). Role of n-3 polyunsaturated fatty acids in ameliorating the obesity-induced metabolic syndrome in animal models and humans. International Journal of Molecular Sciences, 17(10). 10.3390/ijms17101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Ke Y, Sun X, Yu L, Yang Z, Zhang Y, et al. (2014). Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1alpha. Oncology Reports, 32(5), 1973–1980. 10.3892/or.2014.3454. [DOI] [PubMed] [Google Scholar]
- Huang C, Yang G, Jiang T, Zhu G, Li H, & Qiu Z (2011). The effects and mechanisms of blockage of STAT3 signaling pathway on IL-6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma, 58(5), 396–405. 10.4149/neo_2011_05_396. [DOI] [PubMed] [Google Scholar]
- Ji Z, He L, Regev A, & Struhl K (2019). Inflammatory regulatory network mediated by the joint action of NF-kB, STAT3, and AP-1 factors is involved in many human cancers. Proceedings of the National Academy of Sciences of the United States of America, 116(19), 9453–9462. 10.1073/pnas.1821068116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B (2017). Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes and Diseases, 4(1), 25–27. 10.1016/j.gendis.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim HS, Lee CS, Shin YJ, Kim YN, Kang GH, et al. (2008). STAT3 inhibits the degradation of HIF-1alpha by pVHL-mediated ubiquitination. Experimental & Molecular Medicine, 40(5), 479–485. 10.3858/emm.2008.40.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, et al. (2005). STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. The FASEB Journal, 19(10), 1296–1298. 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- Kim H, Lin Q, Glazer PM, & Yun Z (2018). The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Research, 20(1), 16 10.1186/s13058-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, & Hendrix MJ (2012). Molecular pathways: Vasculogenic mimicry in tumor cells: Diagnostic and therapeutic implications. Clinical Cancer Research, 18(10), 2726–2732. 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Lee KL, Fujioka M, Sun W, You J, Chia GS, et al. (2018). Hypoxia-inducible factor-2 alpha up-regulates CD70 under hypoxia and enhances anchorage-independent growth and aggressiveness in cancer cells. Oncotarget, 9(27), 19123–19135. 10.18632/oncotarget.24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras A, Lionneton F, Mattot V, Lelievre E, Caetano B, Spruyt N, et al. (2007). HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene, 26(53), 7480–7489. 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- Li XF, Carlin S, Urano M, Russell J, Ling CC, & O’Donoghue JA (2007). Visualization of hypoxia in microscopic tumors by immunofluorescent microscopy. Cancer Research, 67(16), 7646–7653. 10.1158/0008-5472.CAN-06-4353. [DOI] [PubMed] [Google Scholar]
- Li H, Chen J, Zen W, Xu X, Xu Y, Chen Q, et al. (2015). Effect of hypoxia inducible factor-1 antisense oligonucleotide on liver cancer. International Journal of Clinical and Experimental Medicine, 8(8), 12650–12655. [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma H, Zhang J, Zhu L, Wang C, & Yang Y (2017). Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Scientific Reports, 7(1), 13856 10.1038/s41598-017-14364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Meng W, Guan Z, Guo Y, & Han X (2016). The hypoxia-related signaling pathways of vasculogenic mimicry in tumor treatment. Biomedicine & Pharmacotherapy, 80, 127–135. 10.1016/j.biopha.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Li XF, & O’Donoghue JA (2008). Hypoxia in microscopic tumors. Cancer Letters, 264(2), 172–180. 10.1016/j.canlet.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. (1999). Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature, 402(6762), 672–676. 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. (1998). Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. Journal of Virology, 72(11), 8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliensiek SJ, Nealey P, & Murphy CJ (2009). Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Engineering Part A, 15(9), 2643–2651. 10.1089/ten.TEA.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Chang KW, Cheng HW, & Liu CJ (2019). SMAD4 somatic mutations in head and neck carcinoma are associated with tumor progression. Frontiers in Oncology, 9, 1379 10.3389/fonc.2019.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Wang W, Sun BC, Cai WJ, Li L, Lu HH, et al. (2012). Vasculogenic mimicry is a key prognostic factor for laryngeal squamous cell carcinoma: A new pattern of blood supply. Chinese Medical Journal, 125(19), 3445–3449. [PubMed] [Google Scholar]
- Ling GQ, Liu YJ, Ke YQ, Chen L, Jiang XD, Jiang CL, et al. (2015). All-trans retinoic acid impairs the vasculogenic mimicry formation ability of U87 stem-like cells through promoting differentiation. Molecular Medicine Reports, 12(1), 165–172. 10.3892/mmr.2015.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissitzky JC, Parriaux D, Ristorcelli E, Verine A, Lombardo D, & Verrando P (2009). Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Research, 69(3), 802–809. 10.1158/0008-5472.CAN-08-2391. [DOI] [PubMed] [Google Scholar]
- Liu J, & Debnath J (2016). The evolving, multifaceted roles of autophagy in cancer. Advances in Cancer Research, 130, 1–53. [DOI] [PubMed] [Google Scholar]
- Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, et al. (2016). The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. Journal of Cellular and Molecular Medicine, 20(9), 1761–1769. 10.1111/jcmm.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Sun B, Zhao X, Gu Q, Dong X, Yao Z, et al. (2013). HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. Journal of Cellular and Molecular Medicine, 17(1), 116–122. 10.1111/j.1582-4934.2012.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang HL, Deng MJ, Wen XJ, Mo YY, Chen FM, et al. (2018). Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral cancer. Oxidative Medicine and Cellular Longevity, 2018, 3510970 10.1155/2018/3510970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang K, Meng C, Zhang Z, & Xu Y (2012). Vasculogenic mimicry is a marker of poor prognosis in prostate cancer. Cancer Biology & Therapy, 13(7), 527–533. 10.4161/cbt.19602. [DOI] [PubMed] [Google Scholar]
- Ly T, Harihar S, & Welch DR (2020). KISS1 in metastatic cancer research and treatment: Potential and paradoxes. Cancer and Metastasis Reviews. 10.1007/s10555-020-09868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu X, Wang J, Guo X, Wu G, Jiao Y, Faleti OD, et al. (2018). EBV-miR-BART1–5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathogens, 14(12), e1007484 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yan F, Li L, Liu L, & Sun J (2014). Deletion and down-regulation of SMAD4 gene in colorectal cancers in a Chinese population. Chinese Journal of Cancer Research, 26(5), 525–531. 10.3978/j.issn.1000-9604.2014.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson RE, Dragos SM, Ramos S, Sutton C, Frendo-Cumbo S, Castellani L, et al. (2016). Reduced ATGL-mediated lipolysis attenuates beta-adrenergic-induced AMPK signaling, but not the induction of PKA-targeted genes, in adipocytes and adipose tissue. American Journal of Physiology Cell Physiology, 311(2), C269–C276. 10.1152/ajpcell.00126.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, et al. (2005). A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell, 8(2), 111–118. 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, et al. (1999). Twist is a potential oncogene that inhibits apoptosis. Genes & Development, 13(17), 2207–2217. 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek R, Gajula RP, Williams RD, Nghiem B, Simons BW, Nugent K, et al. (2017). TWIST1-WDR5-Hottip regulates Hoxa9 chromatin to facilitate prostate cancer metastasis. Cancer Research, 77(12), 3181–3193. 10.1158/0008-5472.CAN-16-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. The American Journal of Pathology, 155(3), 739–752. 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaryan NV, Strizzi L, Abbott DE, Seftor EA, Rao MS, Hendrix MJ, et al. (2009). EphA2 as a promoter of melanoma tumorigenicity. Cancer Biology & Therapy, 8(3), 279–288. 10.4161/cbt.8.3.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez RT, & Xu L (2012). Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. American Journal of Cancer Research, 2(2), 214–221. [PMC free article] [PubMed] [Google Scholar]
- Matkar S, An C, & Hua X (2017). Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop. American Journal of Cancer Research, 7(7), 1476–1485. [PMC free article] [PubMed] [Google Scholar]
- McAllister JC, Zhan Q, Weishaupt C, Hsu MY, & Murphy GF (2010). The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. Journal of Cutaneous Pathology, 37(Suppl. 1), 19–25. 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally LR, Welch DR, Beck BH, Stafford LJ, Long JW, Sellers JC, et al. (2010). KISS1 over-expression suppresses metastasis of pancreatic adenocarcinoma in a xenograft mouse model. Clinical & Experimental Metastasis, 27(8), 591–600. 10.1007/s10585-010-9349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menrad H, Werno C, Schmid T, Copanaki E, Deller T, Dehne N, et al. (2010). Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology, 51(6), 2183–2192. 10.1002/hep.23597. [DOI] [PubMed] [Google Scholar]
- Minder P, Zajac E, Quigley JP, & Deryugina EI (2015). EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation. Neoplasia, 17(8), 634–649. 10.1016/j.neo.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Kaczowka SJ, & Pizzo SV (2008). Interaction between TCL1 and Epac1 in the activation of Akt kinases in plasma membranes and nuclei of 8-CPT-2-O-Me-cAMP-stimulated macrophages. Cellular Signalling, 20(1), 130–138. 10.1016/j.cellsig.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, & Pizzo SV (2005). Coordinate regulation of Forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: Effects of silencing CREB gene expression on Akt activation. The Journal of Biological Chemistry, 280(46), 38276–38289. 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- Mitri Z, Constantine T, & O’Regan R (2012). The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemotherapy Research and Practice, 2012, 743193 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K, Lou H, & Dean M (2011). Multidrug efflux pumps and cancer stem cells: Insights into multidrug resistance and therapeutic development. Clinical Pharmacology and Therapeutics, 89(4), 491–502. 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
- Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, & Iruela-Arispe ML (2006). VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Developmental Dynamics, 235(12), 3413–3422. 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, & Jain RK (2000). Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Research, 60(9), 2497–2503. [PubMed] [Google Scholar]
- Nikolova-Krstevski V, Bhasin M, Otu HH, Libermann T, & Oettgen P (2008). Gene expression analysis of embryonic stem cells expressing VE-cadherin (CD144) during endothelial differentiation. BMC Genomics, 9, 240 10.1186/1471-2164-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Tsuno NH, Okaji Y, Sunami E, Shuno Y, Sasaki K, et al. (2010). The inhibition of autophagy potentiates anti-angiogenic effects of sulforaphane by inducing apoptosis. Angiogenesis, 13(3), 227–238. 10.1007/s10456-010-9180-2. [DOI] [PubMed] [Google Scholar]
- Oki-Idouchi CE, & Lorenzo PS (2007). Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Research, 67(1), 276–280. 10.1158/0008-5472.CAN-06-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passalidou E, Trivella M, Singh N, Ferguson M, Hu J, Cesario A, et al. (2002). Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. British Journal of Cancer, 86(2), 244–249. 10.1038/sj.bjc.6600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, & Griffioen AW (2010). Signalling pathways in vasculogenic mimicry. Biochimica et Biophysica Acta, 1806(1), 18–28. 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Pawlus MR, Wang L, & Hu CJ (2014). STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene, 33(13), 1670–1679. 10.1038/onc.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F, Manzotti M, Di Bacco A, Viale G, Nicholson AG, Price R, et al. (2000). Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet, 355(9217), 1787–1788. [PubMed] [Google Scholar]
- Postovit LM, Seftor EA, Seftor RE, & Hendrix MJ (2007). Targeting Nodal in malignant melanoma cells. Expert Opinion on Therapeutic Targets, 11(4), 497–505. 10.1517/14728222.11.4.497. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, & Mazure NM (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441(7092), 437–443. 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, et al. (2015). Advanced research on vasculogenic mimicry in cancer. Journal of Cellular and Molecular Medicine, 19(2), 315–326. 10.1111/jcmm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros-Gonzalez I, Tomaszewski MR, Aitken SJ, Ansel-Bollepalli L, McDuffus LA, Gill M, et al. (2018). Optoacoustics delineates murine breast cancer models displaying angiogenesis and vascular mimicry. British Journal of Cancer, 118(8), 1098–1106. 10.1038/s41416-018-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Du P, Ge Z, Jin Y, Ding D, Liu X, et al. (2016). TWIST1 and BMI1 in cancer metastasis and chemoresistance. Journal of Cancer, 7(9), 1074–1080. 10.7150/jca.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel F, & Dalion J (1988). Lectins as markers of endothelial cells: Comparative study between human and animal cells. Laboratory Animals, 22(2), 135–140. 10.1258/002367788780864457. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, et al. (2003). Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. The American Journal of Pathology, 162(1), 329–342. 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin TT, Kasuya H, Nomura N, Shikano T, Yamamura K, Gewen T, et al. (2012). Impact of novel oncolytic virus HF10 on cellular components of the tumor microenvironment in patients with recurrent breast cancer. Cancer Gene Therapy, 19(4), 229–237. 10.1038/cgt.2011.80. [DOI] [PubMed] [Google Scholar]
- Sakaki-Yumoto M, Katsuno Y, & Derynck R (2013). TGF-beta family signaling in stem cells. Biochimica et Biophysica Acta, 1830(2), 2280–2296. 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, & Kauppinen A (2016). AMPK and HIF signaling pathways regulate both longevity and cancer growth: The good news and the bad news about survival mechanisms. Biogerontology, 17(4), 655–680. 10.1007/s10522-016-9655-7. [DOI] [PubMed] [Google Scholar]
- Salovaara R, Roth S, Loukola A, Launonen V, Sistonen P, Avizienyte E, et al. (2002). Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut, 51(1), 56–59. 10.1136/gut.51.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF (2003). Nodal signaling in vertebrate development. Annual Review of Cell and Developmental Biology, 19, 589–621. 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schneider H, Muhle C, & Pacho F (2007). Biological function of laminin-5 and pathogenic impact of its deficiency. European Journal of Cell Biology, 86(11–12), 701–717. 10.1016/j.ejcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, et al. (2001). Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Research, 61(17), 6322–6327. [PubMed] [Google Scholar]
- Semenza GL (2003). Targeting HIF-1 for cancer therapy. Nature Reviews Cancer, 3(10), 721–732. 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sharma N, Seftor RE, Seftor EA, Gruman LM, Heidger PM Jr., Cohen MB, et al. (2002). Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: Role in vasculogenic mimicry. Prostate, 50(3), 189–201. 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- Shen MM (2007). Nodal signaling: Developmental roles and regulation. Development, 134(6), 1023–1034. 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Shih T, & Lindley C (2006). Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clinical Therapeutics, 28(11), 1779–1802. 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Tsuda H, Heike Y, Kato K, Asada R, Inomata M, et al. (2001). Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Cancer Research, 61(2), 445–451. [PubMed] [Google Scholar]
- Shirwany NA, & Zou MH (2014). AMPK: A cellular metabolic and redox sensor. A minireview. Frontiers in Bioscience (Landmark Edition), 19, 447–474. 10.2741/4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure D, Senior RM, Griffin GL, & Deuel TF (1992). PDGF AA homodimers are potent chemoattractants for fibroblasts and neutrophils, and for monocytes activated by lymphocytes or cytokines. Biochemical and Biophysical Research Communications, 186(3), 1510–1514. 10.1016/s0006-291x(05)81577-3. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Avanzato D, & Lanzetti L (2018). Emerging functions of the EGFR in cancer. Molecular Oncology, 12(1), 3–20. 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YY, Sun LD, Liu ML, Liu ZL, Chen F, Zhang YZ, et al. (2014). STAT3, p-STAT3 and HIF-1alpha are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma. Oncology Letters, 8(1), 431–437. 10.3892/ol.2014.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhao XP, Song K, & Shang ZJ (2013). Ephrin-A1 is up-regulated by hypoxia in cancer cells and promotes angiogenesis of HUVECs through a coordinated cross-talk with eNOS. PLoS One, 8(9), e74464 10.1371/journal.pone.0074464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, et al. (2001). Molecular determinants of ovarian cancer plasticity. The American Journal of Pathology, 158(4), 1279–1288. 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati A, Nikolaou M, Georgoulias V, & Lianidou ES (2019). Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in EpCAM-positive circulating tumor cells from early stage breast cancer patients. Cell, 8(7). 10.3390/cells8070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao X, et al. (2006). Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncology Reports, 16(4), 693–698. [PubMed] [Google Scholar]
- Sun B, Zhang S, Zhao X, Zhang W, & Hao X (2004). Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyo-sarcomas. International Journal of Oncology, 25(6), 1609–1614. [PubMed] [Google Scholar]
- Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, et al. (2010). Expression and functional significance of Twist1 in hepatocellular carcinoma: Its role in vasculogenic mimicry. Hepatology, 51(2), 545–556. 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- Tandon M, Vemula SV, & Mittal SK (2011). Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opinion on Therapeutic Targets, 15(1), 31–51. 10.1517/14728222.2011.538682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NN, Zhu H, Zhang HJ, Zhang WF, Jin HL, Wang L, et al. (2014). HIF-1alpha induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World Journal of Gastroenterology, 20(47), 17894–17904. 10.3748/wjg.v20.i47.17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveau JC, Dubois M, Le Bihan O, Trepout S, Almagro S, Hewat E, et al. (2008). Structure of artificial and natural VE-cadherin-based adherens junctions. Biochemical Society Transactions, 36(Pt. 2), 189–193. 10.1042/BST0360189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen VL, Paulis YW, Nowak-Sliwinska P, Deumelandt KL, Hosaka K, Soetekouw PM, et al. (2018). Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. The Journal of Pathology, 246(4), 447–458. 10.1002/path.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, et al. (2002). Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Molecular and Cellular Biology, 22(4), 1194–1202. 10.1128/mcb.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: Role in melanoma aggressiveness. Nature Medicine, 12(8), 925–932. 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Tripathi A, & Misra K (2016). Inhibition of P-glycoprotein mediated efflux of paclitaxel by coumarin derivatives in cancer stem cells: An in silico approach. Combinatorial Chemistry & High Throughput Screening, 19(6), 497–506. 10.2174/1386207319666160517115158. [DOI] [PubMed] [Google Scholar]
- Valdivia A, Mingo G, Aldana V, Pinto MP, Ramirez M, Retamal C, et al. (2019). Fact or fiction, it is time for a verdict on vasculogenic mimicry? Frontiers in Oncology, 9, 680 10.3389/fonc.2019.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian AA (2012). Signaling pathways in tumor vasculogenic mimicry. Biochemistry (Mosc), 77(9), 1044–1055. 10.1134/S000629791209012X. [DOI] [PubMed] [Google Scholar]
- Videira PA, Piteira AR, Cabral MG, Martins C, Correia M, Severino P, et al. (2011). Effects of bevacizumab on autocrine VEGF stimulation in bladder cancer cell lines. Urologia Internationalis, 86(1), 95–101. 10.1159/000321905. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Hess AR, Hendrix MJ, & Kinch MS (2003). Differential regulation of EphA2 in normal and malignant cells. The American Journal of Pathology, 162(4), 1037–1042. 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Zhang Y, Yu L, Zhu B, Wu S, et al. (2018). Vasculogenic mimicry and expression of ALDH1, Beclin1, and p16 correlate with metastasis and prognosis in oral squamous cell carcinoma. International Journal of Clinical and Experimental Pathology, 11(3), 1599–1609. [PMC free article] [PubMed] [Google Scholar]
- Wechman SL, Pradhan AK, DeSalle R, Das SK, Emdad L, Sarkar D, et al. (2018). New insights into beclin-1: Evolution and pan-malignancy inhibitor activity. Advances in Cancer Research, 137, 77–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenskow PD, Kurihara T, Aguilar E, Scheppke EL, Moreno SK, Wittgrove C, et al. (2013). Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. The Journal of Clinical Investigation, 123(11), 4900–4908. 10.1172/JCI70230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtkowiak JW, Verduzco D, Schramm KJ, & Gillies RJ (2011). Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Molecular Pharmaceutics, 8(6), 2032–2038. 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HB, Yang S, Weng HY, Chen Q, Zhao XL, Fu WJ, et al. (2017). Autophagy-induced KDR/VEGFR-2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy, 13(9), 1528–1542. 10.1080/15548627.2017.1336277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, & Semenza GL (2019). Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Advances in Cancer Research, 141, 175–212. [DOI] [PubMed] [Google Scholar]
- Xing P, Dong H, Liu Q, Zhao T, Yao F, Xu Y, et al. (2018). ALDH1 expression and vasculogenic mimicry are positively associated with poor prognosis in patients with breast cancer. Cellular Physiology and Biochemistry, 49(3), 961–970. 10.1159/000493227. [DOI] [PubMed] [Google Scholar]
- Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. (2005). Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene, 24(36), 5552–5560. 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Q, Li XY, Yang QY, Xu WW, & Liu GL (2012). Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. Journal of Experimental & Clinical Cancer Research, 31, 16 10.1186/1756-9966-31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, & Ren J (2012). Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clinical and Experimental Pharmacology & Physiology, 39(2), 200–208. 10.1111/j.1440-1681.2011.05638.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Zong Y, Gao Y, Sun X, Zhao H, Luo W, et al. (2019). VEGF induce vasculogenic mimicry of choroidal melanoma through the PI3k signal pathway. BioMed Research International, 2019, 3909102 10.1155/2019/3909102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, & Sekine I (2007). Expression of p-STAT3 in human gastric carcinoma: Significant correlation in tumour invasion and prognosis. International Journal of Oncology, 30(2), 437–442. [PubMed] [Google Scholar]
- Yang JP, Liao YD, Mai DM, Xie P, Qiang YY, Zheng LS, et al. (2016). Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis, 19(2), 191–200. 10.1007/s10456-016-9500-2. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhu DM, Zhou XG, Yin N, Zhang Y, Zhang ZX, et al. (2017). HIF-2alpha promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget, 8(29), 47801–47815. 10.18632/oncotarget.17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Hartwich Y, Tedja R, Roberts CM, Goodner-Bingham J, Cardenas C, Gurea M, et al. (2019). p53-Pirh2 complex promotes Twist1 degradation and inhibits EMT. Molecular Cancer Research, 17(1), 153–164. 10.1158/1541-7786.MCR-18-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M, et al. (2013). Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS One, 8(3), e57188 10.1371/journal.pone.0057188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C, Lee HJ, & Lee EO (2019). Serum promotes vasculogenic mimicry through the EphA2/VE-cadherin/AKT pathway in PC-3 human prostate cancer cells. Life Sciences, 221, 267–273. 10.1016/j.lfs.2019.02.043. [DOI] [PubMed] [Google Scholar]
- Yousif LF, Di Russo J, & Sorokin L (2013). Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhesion & Migration, 7(1), 101–110. 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X, et al. (2017). Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diagnostic Pathology, 12(1), 23 10.1186/s13000-017-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue WY, & Chen ZP (2005). Does vasculogenic mimicry exist in astrocytoma? The Journal of Histochemistry and Cytochemistry, 53(8), 997–1002. 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- Zakaria N, Mohd Yusoff N, Zakaria Z, Widera D, & Yahaya BH (2018). Inhibition of NF-kappaB signaling reduces the stemness characteristics of lung cancer stem cells. Frontiers in Oncology, 8, 166 10.3389/fonc.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarogoulidis P, Lampaki S, Turner JF, Huang H, Kakolyris S, Syrigos K, et al. (2014). mTOR pathway: A current, up-to-date mini-review (review). Oncology Letters, 8(6), 2367–2370. 10.3892/ol.2014.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hai L, Zhu M, Yu S, Li T, Lin Y, et al. (2017). Actin cytoskeleton regulator Arp2/3 complex is required for DLL1 activating Notch1 signaling to maintain the stem cell phenotype of glioma initiating cells. Oncotarget, 8(20), 33353–33364. 10.18632/oncotarget.16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Imani S, Shasaltaneh MD, Hosseinifard H, Zou L, Fan Y, et al. (2019). The role of vascular mimicry as a biomarker in malignant melanoma: A systematic review and meta-analysis. BMC Cancer, 19(1), 1134 10.1186/s12885-019-6350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y, et al. (2008). Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. Journal of Experimental & Clinical Cancer Research, 27, 60 10.1186/1756-9966-27-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, & Luo H (2018). Effects of thalidomide on growth and VEGF-A expression in SW480 colon cancer cells. Oncology Letters, 15(3), 3313–3320. 10.3892/ol.2017.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Song G, Yao L, Liu Y, Liu M, Li S, et al. (2018). miR-3928v is induced by HBx via NF-kappaB/EGR1 and contributes to hepatocellular carcinoma malignancy by down-regulating VDAC3. Journal of Experimental & Clinical Cancer Research, 37(1), 14 10.1186/s13046-018-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang D, Wang W, Sun X, Xu D, Wang C, Zhang Q, et al. (2016). AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy, 12(9), 1447–1459. 10.1080/15548627.2016.1185576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Zhang Y, Cheng S, Wu Z, Liu F, & Zhang J (2017). CD133 positive U87 glioblastoma cells-derived exosomal microRNAs in hypoxia-versus normoxia-microenviroment. Journal of Neuro-Oncology, 135(1), 37–46. 10.1007/s11060-017-2566-x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Mishra L, & Deng CX (2018). The role of TGF-beta/SMAD4 signaling in cancer. International Journal of Biological Sciences, 14(2), 111–123. 10.7150/ijbs.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Rahman MA, Chen ZG, & Shin DM (2017). Multiple biological functions of Twist1 in various cancers. Oncotarget, 8(12), 20380–20393. 10.18632/oncotarget.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu ZY, et al. (2012). Hypoxia-induced vasculogenic mimicry formation via VE-cadherin regulation by Bcl-2. Medical Oncology, 29(5), 3599–3607. 10.1007/s12032-012-0245-5. [DOI] [PubMed] [Google Scholar]
- Zhao N, Sun BC, Zhao XL, Wang Y, Meng J, Che N, et al. (2015). Role of Bcl-2 and its associated miRNAs in vasculogenic mimicry of hepatocellular carcinoma. International Journal of Clinical and Experimental Pathology, 8(12), 15759–15768. [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Buckhaults P, Zawel L, Bunz F, Riggins G, Dai JL, et al. (1998). Targeted deletion of Smad4 shows it is required for transforming growth factor beta and activin signaling in colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2412–2416. 10.1073/pnas.95.5.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]


