Abstract
Inflammatory cytokines are highly inducible small glycoproteins or regulatory proteins of low molecular weight secreted by different cell types. They regulate intercellular communication and mediate a number of physiological functions in the human immune system. Numerous prospective studies report that inflammatory cytokines strongly predict coronary artery disease, myocardial infarction, heart failure and other adverse cardiac events. Inflammatory cascade is believed to be a causative factor in the development of atherosclerotic process. Several aspects of atherogenesis are accelerated by cytokines. This article provides an overall overview of current understanding of cytokines in various cardiovascular events. Besides, inflammatory cytokines trigger cellular events that can induce malignancy and carcinogenesis. Elevated expression of several cytokines such as interleukin-1, interleukin-6, interleukin-10, tumor necrosis factor-α, macrophage migration inhibitory factor and transforming growth factor-β are involved in tumor initiation and progression. Thus, they exert a pivotal role in cancer pathogenesis. This review highlights the role of several cytokines in various events of tumorigenesis. Actually, this article summarizes the contributions of cytokines in the pathogenesis of cardiovascular disease and cancer.
Keywords: Cytokines, inflammation, cardiovascular diseases, cancers
Introduction
Inflammation is a normal response of body’s tissue to injury.1 It is a basic process whereby the body reacts to injury or infection.2 It is a tissue-destroying localized biological reaction initiated by various factors ranging from microbial infection and chemical injury that result in cell death or cell injury.3,4 Actually, it is a nonspecific immune response. The main features of inflammation are redness, pain, warmth and swelling.2 There are mainly two types of inflammation, that is, acute and chronic.3 Acute inflammation is of a short duration and this inflammatory response is characterized by rapid onset. It is caused by the migration of neutrophils and exudation of plasma proteins and fluids into the injured part of the body.3 Chronic inflammation manifests by the presence of macrophages and lymphocytes. It is of a prolonged duration.3 This inflammatory response may be a causative factor in the development of a variety of degenerative diseases like cancer, Alzheimer, rheumatoid arthritis, multiple sclerosis, asthma, congestive heart failure (CHF), diabetes, inflammatory bowel disease, atherosclerosis, gout, aging, acquired immunodeficiency syndrome and central nervous system (CNS) depression.5,6 Besides, it is mentioned that prolonged action or exaggeration of inflammation may directly harm the body in many ways.2
It has been described that inflammatory mediators such as cytokines contribute a significant role in inflammation.7,8 Inflammatory responses are coordinated by cytokines.4 Cytokines are glycoproteins or regulatory soluble proteins of low molecular weight secreted by different cell types including WBC (white blood cell) in the body. Various cytokines modulate the proliferation and differentiation of immune cells.4,9 Cytokines are also known as lymphokines, monokines and interleukins as they are secreted by lymphocytes, monocytes and leukocytes accordingly. Moreover, one particular type of cytokines such as monocyte inflammatory protein (MIP-1α and MIP-1β), monocyte chemoattractant protein (MCP-1) and growth-related oncogene (GRO/KC) is known as chemokines as they induce chemotaxis.10 Pober and Cotran11 in 1990 reported that lymphocytes and activated tissue macrophages primarily secrete inflammatory cytokines in response to various inflammatory stimuli, such as endotoxin, chemical and physical injury. There are two major groups of inflammatory cytokines: those responsible for acute inflammation and those involved in chronic inflammation. Acute inflammation is caused by cytokines such as interleukin (IL)-1, IL-6, IL-8, IL-11, TNF-α (tumor necrosis factor-α), IL-16, IL-17, G-CSF (granulocyte colony stimulating factor) and GM-CSF (granulocyte-macrophage colony-stimulating factor).12 Besides, involvement of cytokines in chronic inflammation can be subdivided into two classes: cytokines coordinating humoral responses are IL-4, IL-5, IL-6, IL-7 and IL-13, and those for cellular responses are IL-1, IL-2, IL-3, IL-4, IL-7, IL-9, IL-10, IL-12, interferons, and TNFα and β. Few cytokines significantly play a role in both acute and chronic inflammation, such as IL-1, IL-6, IL-11, IL-17 and TNF-α.12 Besides, the IL-1 family of cytokines also comprises two ligands with anti-inflammatory activity (IL-37, IL-38).13
This review highlights the role of inflammatory cytokines in the pathogenesis of cardiovascular diseases (CVDs) and cancer.
Inflammatory cytokines and CVDs
CVD is a chronic inflammatory state of the blood vessels. It is a major socioeconomic problem and also a threat of public health throughout the world, since they significantly contribute to the global morbidity. Some common CVDs, including atherosclerosis, vascular disease and heart disease, occupy a strong position in the structure of disability and mortality worldwide.14,15
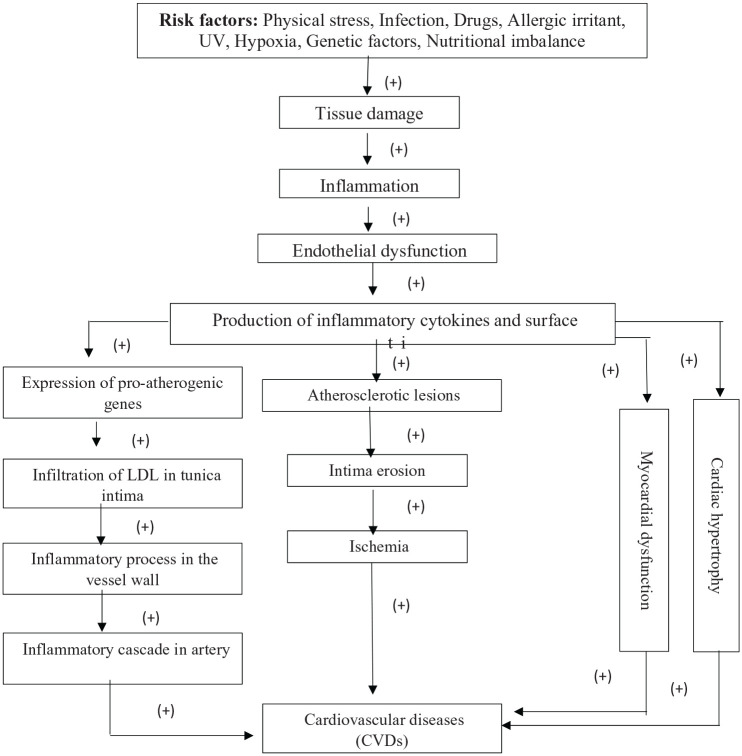
Different heart diseases, including coronary heart disease (CHD), atherosclerotic heart disease and CHF, are associated with increased levels of proinflammatory cytokines such as interferon-γ (IFN-γ), IL-1β, IL-6 and TNF-α.16 These cytokines contribute a significant role in the formation of atherosclerotic plaque. The atheroma, an accumulation of macrophages, lipid-laden cells, mast cells, T cells and other degenerative material, occurs in the inner layer (also known as “tunica intima”) of artery walls (endothelium) by a fatty streak.16–19 Then various inflammatory molecules, cytokines and chemokines are released by the action of activated macrophages which lead to tissue damage and, ultimately, to inflammation. Thus, the formation of atherosclerotic plaque is promoted.19–21 Actually, inflammatory cytokines contribute a great role in the development of atherosclerotic plaques. Importantly, such plaques or lesions induce endothelial dysfunction. Thus, normal function of endothelium is disrupted and primary stage of atherosclerotic process stimulated.14 Besides, inflammatory cytokines are also well known to induce numerous chronic inflammatory disorders. Recent study has reported that increased release of cytokines, including TNF-α, IL-6 and IL-1, promote the expression of pro-atherogenic gene.22 The retention and infiltration of the tunica intima of endothelium stimulate an inflammatory process in the vessel wall.19 Modification of lipoprotein LDL (low-density lipoprotein), through enzymatic attack or oxidation in the inner layer, stimulates the release of phospholipids. Thus, endothelial cells can be activated which may accelerate the expression of inflammatory genes. Hence, the accumulation of lipid-laden cells or lipids may start an inflammatory cascade in the artery.23,24 Within the tunica intima, chemokines recruit monocytes which differentiate into macrophages that trigger the development of foam cells. The macrophages also multiply and secrete various inflammatory cytokines and growth factors, thus amplifying proinflammatory signals.25 This step is very much essential for the proper development of atherosclerotic lesions.26 Besides, this step upregulates toll-like receptors.19 Such type of receptors through stimulating a signal cascade leads to cell activation. The activated macrophage releases cytotoxic oxygen, proteases, nitrogen radical molecules and inflammatory cytokines.27 Mast cells, dendritic cells and endothelial cells also show similar effects. Numerous toll-like receptors recognize bacterial toxins, DNA motifs and stress proteins.27 Furthermore, oxidized LDL particles and heat-shock protein 60 of human being may stimulate toll-like receptors.28,29 Cells in atherosclerotic plaques show a spectrum of these receptors and inflammation of atherosclerosis may be dependent on this pathway.30,31
T cells, the immune cells, also contribute in atherogenesis.25 Natural killer T cells are available in early atherosclerotic plaques. Lipid antigens are recognized by these cells. The activation of these killer cells induces atherosclerotic lesions in ApoE knockout mice.32 This type of cells also recognizes various viral antigens, which may be available in the plaques. Moreover, activation of these cells in knockout mice may promote the arterial cell death and initiate or stimulate atherosclerosis.33 T-cell activation also triggers the secretion of a set of inflammatory cytokines. Two stereotypical responses in inbred mice can be obtained: response of the type 1 helper T cells (Th1) activates inflammatory macrophages and type 2 helper T cells (Th2) accelerates allergic inflammatory processes.19,34 Cytokines which induce a Th1 response are available in the atherosclerotic plaques.19,35 Therefore, activated T cells mature into Th1 effector cells and start releasing a macrophage-stimulating cytokine, IFN-γ, which in turn promotes the synthesis of inflammatory TNF and IL-1.34 These cytokines synergistically contribute a role in the production of different cytotoxic and inflammatory molecules in vascular cells.36 All these effects trigger the formation of atherosclerotic lesions (Figure 1). Smoking, hypercholesterolemia, hypertension, diabetes and obesity are well-recognized atherogenic risk factors.36,37
Figure 1.
Mechanism of CVDs induced by inflammation (“+” = increased).
Cytokines in the development of myocardial infarction and coronary artery disease
Inflammation contributes a significant role in the etiology of myocardial infarction (MI), hypertension, angina pectoris (AP), and ischemic heart disease (IHD) or coronary artery disease (CAD).19,38 MI is caused by atheromatous process, since this process blocks coronary artery inhibiting proper blood flow through it.19 Atherosclerosis is the major cause of IHD. Intima erosion is caused by atherosclerosis, which leads to subsequent ischemia.38 Chemokines, including MCP-1, MIP-α, IFN-γ-inducible protein (IP-10) and eotaxin, play a significant role in the pathogenesis of CAD.39 MCP-1, also known as CCL2 (MCP-1/CCL2), is chemotactic to monocytes and contributes a role in the development of disorders associated with infiltration of monocytes.40,41 Besides, several studies have reported that elevated levels of C-reactive protein (CRP) and IL-6 are strongly associated with the risk of CAD.38 CRP is a plasma protein which comes from the liver. The synthesis of CRP is generally regulated by IL-6, which in turn is upregulated by IL-1 and TNF-α.42 Moreover, smooth muscle cells (SMCs) and monocytic cells also produce CRP in atherosclerotic plaques, more specifically in the tunica intima of endothelium, where it co-localizes with lipoproteins, inflammatory monocytes and monocyte-derived macrophages. This localization ensures a significant contribution to the atherosclerosis.
Furthermore, CRP directly facilitates and amplifies innate immunity, which in turn initiates CHD.41 Therefore, there is a strong relationship between increased level of high-sensitivity CRP (hsCRP) and inflammatory coronary events.38 CRP is a causative factor and also an independent predictor of MI, peripheral arterial disease, stroke and other cardiac events.14 Cytokines contribute a cytoprotective role in the primary stage of MI, by decreasing cell apoptosis. CRP facilitates further inflammatory incidence of myocardial cells.43 Besides, different types of pathogens, including herpes family viruses, have a contribution to CAD. Cytomegalovirus induces atherosclerosis through modulating vascular cell activity. It contributes to atherosclerotic lesions causing graft rejection.19
Cytokines in the development of heart failure
Heart failure (HF) is a multistep disease. Gullestad et al.44 in 2012 stated that “Heart failure (HF) is a highly complex multistep disorder in which a number of physiological systems participate.” Inflammation participates in the development of HF.44 Numerous studies have described increased expression and secretion of inflammatory cytokines including IL-1, IL-6, IL-18, TNF-α and cardiotrophin-1 (CT-1), as well as various chemokines such as monocyte chemoattractant peptide (MCP)-1/CCL2, IL-8/CXCL8, CXCL16 and CCL21 in HF patients. Levels of these inflammatory molecules in plasma appear to be increased in direct proportion to deterioration of cardiac performance.44 Increased expression of these inflammatory mediators or molecules has also been shown within the failing myocardium.45,46 Numerous researches have explored that the biological effects of inflammatory mediators, including cytokines, may explain different aspects of the syndrome of chronic HF. Furthermore, the pathogenic role of these cytokines in HF is supported by many transgenic animal models.44 Recently, many researches in gene-modified mice have revealed a link between IL-6, as well as several chemokines (e.g. MCP-1), and the development or progression of HF.47 Various inflammatory mediators and cytokines are upregulated in HF.44 By directly acting on cardiomyocytes and fibroblasts, cytokines stimulate hypertrophy and fibrosis. Cytokines impair myocardial contractile function through acting on intracellular calcium transport. They may also stimulate genes involved in myocardial remodeling. Thus, cytokines or inflammatory mediators may modulate myocardial functions.48 Besides, numerous studies have observed increased CRP levels in HF. Hence, CRP is believed to contribute to chronic HF.44
Inflammatory cytokines and cancers
Cancer is a genetic disorder. It is mainly characterized by uncontrolled proliferation of abnormal cells.49,50 Chronic inflammatory responses are directly associated with different types of cancers. The inflammatory response is a cascade of physiological and immunological processes. Various cytokines and chemokines are involved in the inflammatory mechanism.51 Besides, proinflammatory mediators recruit inflammatory response–related immune cells such as natural killer (NK) cells, macrophages, activated T-cytotoxic cells and neutrophils to the inflammatory region. These mediators also induce the production of several acute phase reactive proteins including CRP and serum amyloid A (CAA). All of these accelerate the inflammatory processes.16 Furthermore, epidemiological evidence suggests that chronic inflammation is responsible for up to 25% of all cancers.52
How cytokines contribute to cancer development?
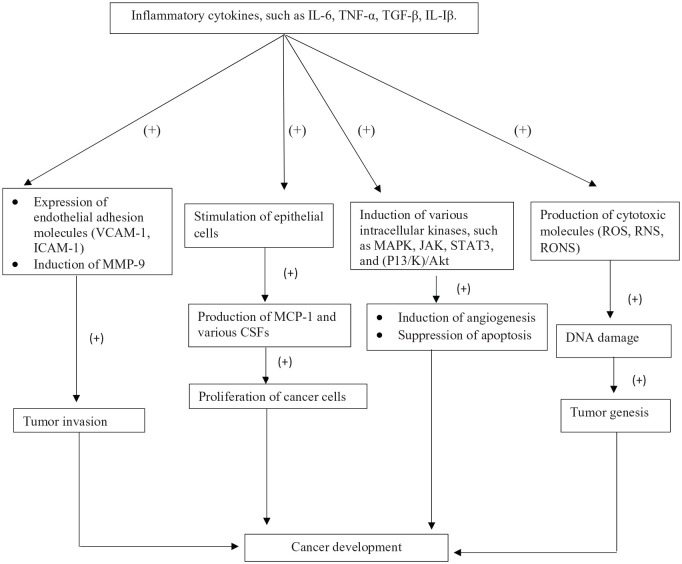
Cytokines regulate differentiation, proliferation, cell migration, cell death and immune cell activation.53 TNF-α is a carcinogenic cytokine and it has two receptors, known as TNF-α receptor-1 (TNF-α R-1) and TNF-α R-2.54 This cytokine induces tumor cell survival by promoting antiapoptotic mechanisms through the stimulation of certain genes. TNF-α also triggers the production of various genotoxic molecules such as reactive oxygen species (ROS) and nitric oxide, which can lead to DNA damage and ultimately to tumorigenesis.54,55 IL-6 is another cytokine that participates in carcinogenesis.56 It is a potent antiapoptotic and growth-promoting factor.55 By attaching to its receptor (IL-6Rα), IL-6 phosphorylates the STAT (signal transducers and activators of transcription) proteins: STAT1 and STAT3. STAT proteins contribute a significant role in the tumorigenic processes. STAT3 protein is an oncoprotein that is available in different types of cancers. It proliferates malignant cells. Actually, it exerts its activity by directly binding to target genes.54–58 Furthermore, IL-17 and IL-23 can initiate tumorigenesis by stimulating IL-6/STAT3 pathway in an experimental animal model.58 Besides, transforming growth factor-β (TGF-β) is a well-documented pleiotropic cytokine. It has three isoforms such as TGF-β1, TGF-β2 and TGF-β3. TGF-β contributes an important role in cell proliferation, invasion, embryogenesis, apoptosis and differentiation.59,60 TGF-β, IL-6 and TNF-α induce the generation of reactive oxygen and nitrogen species (RONS) in nonphagocytic cells. RONS promote DNA damage which accelerate or initiate tumorigenesis.54 Also, cytokine polymorphism on gene expression has a great impact on various cancers.60,61 Moreover, the epithelial mesenchymal transition (EMT) is a cellular reprogramming process during embryogenesis.62,63 Epithelial cells acquire fibroblast characteristics and show morphological changes during EMT.64,65 TGF-β promotes EMT progression by contributing a significant role in tumor development and embryogenesis in different EMT models. IL-6 and TNF-α synergistically mediate EMT progression via TGF-β signaling.54 Both inflammatory cytokines induce nuclear factor-κB (NF-κB) activation, which controls the expression of a variety of transcription factors such as ZEB, Snail and Twist involved in EMT.66,67
Low TNF-α levels accelerate tumor growth and promote angiogenesis that generates new blood vessels. Angiogenesis is important in cancer development since the new blood vessel network provides oxygen and nutrients to cancer cells.68,69 Tumor cells secrete various angiogenic factors such as vascular endothelial growth factor (VEGF). VEGF is mainly expressed in response to growth factor and cytokines.69 IL-6 is another angiogenic factor that promotes VEGF expression and induces angiogenesis by signaling the STAT3 pathway in cancer.54,70 Besides, several inflammatory cytokines modulate metastatic cascade. For example, TGF-β might contribute a significant role in inducing metastasis. During metastasis, neoplastic cell spread from one organ to another.54,71 Moreover, metastasis is closely associated with EMT that accelerates the migration of tumor cells.72
Cytokine-induced breast cancer
Breast cancer (BC) is an inflammation-linked disease affecting millions of people worldwide.73 Cytokines are well known to regulate tumor microenvironment. Several cytokines including IL-1, IL-6, IL-11 and TGF-β contribute a significant role in the development of BC.74 Actually, the IL-1 family of cytokines (IL-1α, IL-1β), the IL-1 receptors (IL-1RI and IL-1RII) and the receptor antagonist (IL-1Ra) have been observed to be expressed in human breast cancer cell lines.74 IL-1 is involved in the development of tumor through the stimulation of angiogenesis and metastasis. It also controls the expression of other tumorigenic cytokines.73 IL-1 is also known to stimulate expression of various endothelial adhesion molecules such as vascular adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1). ICAM-1 has a significant contribution in invasion of metastatic breast carcinoma cell lines.75 IL-1β, a member of IL-1 family, has been observed to be expressed in breast cancer. At the cellular membrane, IL-1β through binding of IL-1 receptor I (IL-1RI) induces cellular changes.75 IL-1β may be closely associated with induction of matrix metalloproteinase-9 (MMP-9), as reported by Wang et al.76 in 2005. Increased expression of MMP-9 contributes a role in tumor invasion. IL-1β may induce angiogenesis by controlling expression of various angiogenic factors. It also promotes expression of hypoxia-inducible factor-1α, a transcription factor for VEGF in breast cancer cells.75,76 Cytokine signaling is stimulated upon binding of certain cytokines to specific cognate receptors followed by initiation and induction of several intracellular kinases, including mitogen-activated protein kinase (MAPK), phosphatidyl ionositol-3-kinase (PI3/K)/Akt and Janus-activated kinase (JAK), with subsequent stimulation of transcription factors such as activator protein-1 (AP-1), NF-κB and STAT.77 IL-6 activates the JAK/STAT signaling pathway by binding to its receptor and coreceptor glycoprotein 130 (gp 130), thus regulating the expression of certain genes involved in suppression of apoptosis and acceleration of proliferation.78 IL-6 produces MCP-1 and various colony-stimulating factors (CSFs) by stimulating epithelial cells. Thus, serum circulating IL-6 induces breast cancer cell proliferation.75 Besides, TGF-β is also a regulator of differentiation, proliferation, migration and apoptosis. It promotes tumor vascularity by controlling the expression of VEGF and MCP-1. VEGF enhances the invasion of breast cancer cells through the activation of PI3K/Akt and MAPK signaling.79 Moreover, the expression of circulating TNF-α level has been observed in human breast carcinomas.79 TNF-α proliferates breast cancer cells and accelerates estrogen-induced cell proliferation. Also, it stimulates mammary tumorigenesis.75 This cytokine upregulates certain genes that are responsible for metastasis, proliferation and invasion. Besides, IL-19, IL-20 and IL-23 also induce breast tumorigenesis. IL-19 accelerates the proliferation of the Hs578T and MCF-7 breast cancer cell lines. IL-20 provides a tumor microenvironment.79,80 Numerous studies have demonstrated the role of chemokines in cell proliferation, invasion and migration. Chemokines are categorized into four groups: CC, XC, CXC and CX3C. Proinflammatory cytokines usually produce chemokines. Chemokines act by attaching with specific receptors in a paracrine or an autocrine manner.77 CC chemokine ligand-2 (CCL2) and CC chemokine ligand-5 (CCL5) have been studied broadly in breast carcinoma. Both chemokines have been detected in serum of patients with breast cancer. CCL2 contributes a crucial role in angiogenesis, while CCL5 promotes breast cancer cell migration and invasion.75 Furthermore, increased expression of CXC chemokine ligand-12 (CXCL12) and its receptor CXC chemokine receptor 4 (CXCR4) in breast carcinoma plays a significant role in tumor angiogenesis.75,81
Cytokine-induced prostate cancer
Prostate cancer (PCa) is a common malignant tumor in men.82 PCa has different mortality rate throughout the world. PCa mortality rate in Asia is lower than those in Europe or the United States.83 Cytokines facilitate the development of prostate tumor.84 IL-6 is extensively studied in PCa. It exerts its activity by attaching to its receptor and coreceptor. Circulating IL-6 activates the JAK, STAT3 and MAPK pathway, which stimulates the expression of androgen receptor-mediated gene.85 The IL-6/JAK/STAT signaling pathway has an effect on tumor initiation and progression, as highlighted by numerous studies.86 IL-6 accelerates cancer development by facilitating transformation of normal noncancer cells into cancer or tumor stem cells.87 IL-6 induces proliferation in prostate tumor cells such as in LNCaP and MDA PCa 2b.88,89 Besides, IL-1β is highly and IL-1α is rarely present in the tumor microenvironment. Both cytokines after binding to their receptors could potentiate IL-1 receptor-associated kinase (IRAK). IRAK is then attached to TNF receptor–associated factor (TRAF)-6. Finally, the IRAK/TRAF-6 complex induces the potentiation of NIK (NF-κB-inducing kinase).90 Thus, IL-1 family of cytokines contributes a significant role in cancer development via NF-κB pathway. Furthermore, macrophage migration inhibitory factor (MIF) accelerates tumorigenesis by preventing the tumor inhibitor gene p53. MIF promotes expression of a wide variety of inflammatory cytokines.91 It also interacts with chemokine receptor CXCR2 and CXCR4 and recruits both T cells and monocytes. MIF is overexpressed in various carcinomas including PCa.91,92 Moreover, PCa progression is also associated with TNF-α. This cytokine is mediated by two receptors, namely TNF-receptor I (TNFRI) and receptor II (TNFRII).85 TNF-α recruits monocytes and neutrophils to inflammatory sites. It also potentiates vascular endothelial cells to secrete several adhesion molecules for monocytes and neutrophils.91 Interestingly, TNF-α induces tumor necrosis and angiogenesis.93 Circulating TNF-α has been found in PCa.94 Besides, overexpression of TGF-β in tumors also promotes angiogenesis in prostate tumor.95 CXCL2 is such a chemokine that induces angiogenesis and regulates macrophage infiltration within the tumor, thus facilitating PCa development.96
Cytokine-induced colorectal cancer
Colorectal adenocarcinoma or colorectal cancer (CRC) is one of the most frequent types of cancer worldwide. Colorectal carcinogenesis is an aggressive type of carcinoma.97 Cytokines regulate cancer growth and also induce tumorigenesis, metastasis and invasion of tumors.98 As a proinflammatory cytokine, IL-6 participates in carcinogenesis.56 It acts as a growth factor for CRC cells.99 The circulating levels of IL-6 have been observed to be highly elevated and correlated to tumorigenesis in patients with colon cancer.99 IL-6/IL-6R complexes contribute in CRC development by inducing the activation of JAK/STAT3, PI3K/Akt and MAPK signaling pathways. In CRC cells, STAT3 is constitutively active.100–102 TNF-α accelerates the growth of certain cancer cells and causes carcinogenesis.103 This cytokine is an endogenous tumor promoter, as suggested by numerous preclinical studies. TNF-α has been observed to be expressed in CRC.77 Another powerful cytokine is TGF-β. Its role in cancer is complex and varying by stage of tumorigenesis. It acts as a tumor inhibitor in early stages of tumorigenesis, inducing apoptosis and suppressing cell proliferation. Later, TGF-β induces metastasis and invasion by promoting EMT.104 Moreover, MIF, an immune stimulatory cytokine, is overexpressed in CRC.91 IFN-γ and IL-2 have been rarely detected to be expressed in CRC. Recently, few studies have revealed gene expression of IL-10, IL-8, IL-6, IL-7 and TGF-γ in numerous colon cancer cell lines.97 An elevated expression of CXCL7 (a chemokine) and its receptor CXCR2 have been observed in patients with CRC, as reported by Uchiyama et al.105 Furthermore, TNF-α triggers the production of reactive oxygen species (ROS) and nitric oxide (NO). Interestingly, two prominent inflammatory cytokines are TGF-β and IL-6, which together with TNF-α induce the generation of RONS in nonphagocytic cells. RONS, ROS and NO accelerate tumorigenesis by inducing DNA damage.54,55 ROS and NO can initiate lipid peroxidation to produce other reactive molecules.106 NO is an important mediator linking inflammation and cancer. During arginine metabolism, NO is produced by various isoforms of nitric oxide synthase (NOS).107 During inflammation, inducible nitric oxide synthase (iNOS) in macrophages stimulate the production of NO (Figure 2). The levels of NO and iNOS have been detected to be increased in different carcinomas.108,109 The enhanced expression of iNOS has been observed in colorectal carcinomas.110
Figure 2.
Cytokines in cancer development (“+” = increased).
Cytokine-induced epithelial ovarian cancer
Epithelial ovarian cancer (EOC) is the fifth leading cause of malignancy-related death in women. It is a fatal gynecological malignancy throughout the world.111,112 Different components stimulating inflammatory pathway such as cytokines, STAT3, NF-κB, iNOS, free radicals and VEGF have been observed to participate in the development of EOC.113 Cytokines induce ovarian tumor development in vivo.114 Circulating levels of IL-6, IL-7 and IL-10 have been detected to be enhanced in patients with ovarian cancer.115 Also, IL-1β and TNF-α have been observed to accelerate EOC progression.116,117 IL-6 triggers the development of EOC by inhibiting apoptosis, inducing cell proliferation and stimulating angiogenesis.114 It is an important immune regulatory cytokine. Besides, IL-6 has a significant role in autocrine growth of ovarian tumor cells.118 High serum levels of IL-6 have been observed in patients with advanced ovarian cancer.119 Interestingly, IL-6 activates STAT3, an oncoprotein that is overexpressed in EOC tissue. Activated STAT3 in EOC contributes a significant role in the prognosis and invasion.58,120 Like IL-6, IL-8 also modulates progression of ovarian cancer cells.121 Moreover, numerous studies have shown increased levels of TNF-α in patients with ovarian cancer. This cytokine is crucial in accelerating tumor growth. TNF-α exerts its activity by binding to its receptors (TNF-Rs).122 Besides, TGF-β is another cytokine that induces proliferation and differentiation of immune cells. It also prevents antitumor immune response. The expression of TGF-β is elevated in cancer cells.123 Furthermore, MIF is overexpressed in ovarian cancer. It also promotes expression of other inflammatory cytokines such as TNF-α, IFN-γ, IL-6, IL-8 and IL-1β in a feedback circuit.58
Cytokine-induced hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most frequent form of internal malignancy of the liver and the third most common cause of death from cancer throughout the world.124,125 Numerous studies report that chronic infection with different types of hepatitis viruses is a risk factor for HCC growth.126 Infectious agents induce carcinogenesis and are responsible for about 15% of human cancers. Inflammatory cytokines contribute a significant role in HCC development.127 TNF-α was shown to be elevated in patients with HCC.128,129 Moreover, the circulating levels of TNF-αRs were higher in patients with HCC when compared with normal healthy subjects.130 IFN-γ promotes TNF-α-mediated hepatic damage and also activates macrophages. In the liver, expression of IFN-γ causes chronic hepatitis. Recruitment of lymphomononuclear cells is responsible for this occurrence.131,132 Raised serum levels of TGF-β1 were detected in HCC patients.91 A member of the TGF-β family known as “Activin A” and its receptors are found in HCC patients and have been observed to accelerate VEGF transcription. Hence, TGF-β may enhance tumorigenesis.133 Elevated levels of MIF were shown in hepatocytes from liver cirrhosis patients.134 Increased levels of MIF associate with angiogenesis.135 Increased expression of IL-1β was detected in HCC patients versus healthy subjects.128 Numerous polymorphisms of IL-1β have been described. The IL-1β-511 genotype T/T and the IL-1β-511 genotype C/C were found to be highly expressed in HCC patients when compared with healthy individuals.136,137 Serum IL-2, IL-10, IL-12 and IL-15 were higher in HCC.127 In addition, elevated serum concentrations of IL-6 were found in HCC. IL-6 induces T-cell activation. Furthermore, it recruits neutrophils and enhances the proliferation and migration of T lymphocytes into the injured tissue.91
Cytokine-induced pancreatic cancer
Pancreatic cancer is another common cause of death from cancer in the Western world.138,139 Inflammation contributes an important role in the development of pancreatic cancer. Several biological processes including inflammation and immunity are regulated by cytokines. Elevated expression of various cytokines has been detected in pancreatic cancer.140 Increased levels of IL-6, IL-8, IL-10, IL-12, IL-18, TGF-β and MIF have been observed in patients with pancreatic cancer. In addition, IL-1β and TNF-α are also seen to be highly expressed in patients with pancreatic cancer versus normal healthy subjects.140 IL-6 participates in tumorigenesis by inducing the activation of MAPKs, P13Ks and STATs signaling pathways.141 It stimulates angiogenesis by regulating the secretion of VEGF in pancreatic tumor cells.142 By promoting the production of VEGF, VEGF receptors and neuropilin (NRP-2), IL-8 also induces angiogenesis in pancreatic cancer.143 Besides, TNF-α induces cancer cell proliferation by attaching to its receptor TNF-R2.144 Additionally, it has been observed that MIF also promotes pancreatic tumor cell proliferation.145 Furthermore, it has been shown that expression of IL-1β can induce tumor cell invasiveness in pancreatic cancer.145,146 It also contributes to metastasis.147 Schmid et al.148 showed that elevated levels of IL-1β enhance recruitment of macrophages in affected tissue.
Future directions of cytokines in cardiology and oncology
The possible role of inflammatory cytokines as diagnostic marker for cancer and CVD has been identified. Determining the serum levels of various cytokines can be associated with a tumorigenic process or poor prognosis.54 Although progress has been made in understanding these cytokines’ roles in the tumorigenic cycle, establishing a relationship between cytokine expression and disease progression, survival and therapy response remains a major challenge. A more detailed understanding of the body’s inflammatory activation pathways may lead to the development of unique therapies that do not compromise host defense against pathogenic agents.54,149 A variety of clinical trials in the field of therapy have been carried out to determine inhibitors of cytokine receptors or neutralizing antibodies that prevent the sustained exposure of the tumor progression and inflammation-promoting inflammatory mediators. Therapies explicitly directed against proinflammatory cytokines must be assessed in suitable prospective clinical trials in order to investigate their role in disease management.54,150 However, further studies are required to evaluate trusted cut-off values of circulating cytokines in order to have a direct causal association with cancer and CVD. Thus, effective approaches to tackle cardiovascular disorders and multiple cancers can be identified both within and outside the laboratory.
Limitations of this review
This review article is not a meta-analysis either. The key actors and their mechanism of action in the immunopathogenesis of various inflammatory disorders should be established in further studies. Such studies are a prerequisite for developing new CVD and cancer treatment approaches targeting inflammatory and immunopathogenic pathways in these disorders.
Concluding remarks
Inflammation is widely considered to be an important contributing factor of the pathophysiology of CVDs including CHD, and the inflammatory cascade is particularly important in the atherosclerotic process. Many large-scale prospective studies demonstrate that various inflammatory cytokines strongly and independently predict adverse cardiovascular events, including MI, ischemic stroke and sudden cardiac death. Besides, cytokines have been shown to stimulate cancer stem cells (CSCs) in different tumor models. They have also been shown to increase tumor cell mobility. The mixture of cytokines that is produced in the tumor microenvironment has an important role in cancer pathogenesis. Different clinical and epidemiological data suggest that cytokines are associated with an increased risk of cancer.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mohammad Nurul Amin  https://orcid.org/0000-0002-8296-3542
https://orcid.org/0000-0002-8296-3542
References
- 1. Henson PM. Dampening inflammation. Nat Immunol 2005; 6: 1179. [DOI] [PubMed] [Google Scholar]
- 2. Stankov SV. Definition of inflammation, causes of inflammation and possible anti-inflammatory strategies. Open Inflamm J 2012; 5: 1–9. [Google Scholar]
- 3. Iwalewa EO, McGaw LJ, Naidoo V, et al. Inflammation: the foundation of diseases and disorders: a review of phytomedicines of South African origin used to treat pain and inflammatory conditions. African J Biotechnol 2007; 6: 2868–2885. [Google Scholar]
- 4. Ashley NT, Weil ZM, Nelson RJ. Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst 2012; 43: 385–406. [Google Scholar]
- 5. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 2001; 85: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lho Y-M, Ha E, Cho C-H, et al. Inflammatory cytokines are overexpressed in the subacromial bursa of frozen shoulder. J Shoulder Elbow Surg 2013; 22(5): 666–672. [DOI] [PubMed] [Google Scholar]
- 8. Bunker TD, Reilly J, Baird KS, et al. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg Br 2000; 82(5): 768–773. [DOI] [PubMed] [Google Scholar]
- 9. Dinarello CA. Proinflammatory cytokines. Chest 2000; 118: 503–508. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J-M, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin 2007; 45: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation 1990; 50: 537–544. [DOI] [PubMed] [Google Scholar]
- 12. Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci 1997; 2: d12–d26. [DOI] [PubMed] [Google Scholar]
- 13. Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol 2019; 10: 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm 2010; 2010: 535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrukhin IS, Lunina EY. Cardiovascular disease risk factors and mortality in Russia: challenges and barriers. Public Health Rev 2011; 33: 436. [Google Scholar]
- 16. Tian R, Hou G, Li D, et al. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Scientific World J 2014; 2014: 780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med 2010; 16: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh J, Riek AE, Weng S, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem 2012; 287: 11629–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 20. Mehta JL, Saldeen TGP, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol 1998; 31(6): 1217–1225. [DOI] [PubMed] [Google Scholar]
- 21. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 22. Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link. Atherosclerosis 2000; 148(2): 209–214. [DOI] [PubMed] [Google Scholar]
- 23. Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol 2003; 14(5): 421–430. [DOI] [PubMed] [Google Scholar]
- 24. Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and-resistant regions of human vasculature. Proc Natl Acad Sci 2004; 101: 14871–14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006; 83: 456S–460S. [DOI] [PubMed] [Google Scholar]
- 26. Smith JD, Trogan E, Ginsberg M, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci 1995; 92: 8264–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medzhitov R, Janeway CA., Jr. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 28. Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol 2002; 22: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 29. Miller YI, Chang M-K, Binder CJ, et al. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol 2003; 14(5): 437–445. [DOI] [PubMed] [Google Scholar]
- 30. Edfeldt K, Swedenborg J, Hansson GK, et al. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 2002; 105: 1158–1161. [PubMed] [Google Scholar]
- 31. Björkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 2004; 10(4): 416–421. [DOI] [PubMed] [Google Scholar]
- 32. Tupin E, Nicoletti A, Elhage R, et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med 2004; 199: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludewig B, Freigang S, Jäggi M, et al. Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc Natl Acad Sci 2000; 97: 12752–12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szabo SJ, Sullivan BM, Peng SL, et al. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 2003; 21: 713–758. [DOI] [PubMed] [Google Scholar]
- 35. Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest 1996; 97: 2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001; 21: 1876–1890. [DOI] [PubMed] [Google Scholar]
- 37. Johnston TP, Li Y, Jamal AS, et al. Poloxamer 407-induced atherosclerosis in mice appears to be due to lipid derangements and not due to its direct effects on endothelial cells and macrophages. Mediators Inflamm 2003; 12: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bucova M, Bernadic M, Buckingham T. C-reactive protein, cytokines and inflammation in cardiovascular diseases. Bratisl Lek Listy 2008; 109(8): 333–340. [PubMed] [Google Scholar]
- 39. Rothenbacher D, Müller-Scholze S, Herder C, et al. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol 2006; 26(1): 194–199. [DOI] [PubMed] [Google Scholar]
- 40. Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell 1998; 2(2): 275–281. [DOI] [PubMed] [Google Scholar]
- 41. Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol 1999; 72: 7. [DOI] [PubMed] [Google Scholar]
- 42. Paffen E, demaat MPM. C-reactive protein in atherosclerosis: a causal factor? Cardiovasc Res 2006; 71: 30–39. [DOI] [PubMed] [Google Scholar]
- 43. Swiatkiewicz I, Kozinski M, Magielski P, et al. Value of C-reactive protein in predicting left ventricular remodelling in patients with a first ST-segment elevation myocardial infarction. Mediators Inflamm 2012; 2012: 250867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gullestad L, Ueland T, Vinge LE, et al. Inflammatory cytokines in heart failure: mediators and markers. Cardiology 2012; 122(1): 23–35. [DOI] [PubMed] [Google Scholar]
- 45. Devaux B, Scholz D, Hirche A, et al. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J 1997; 18(3): 470–479. [DOI] [PubMed] [Google Scholar]
- 46. Damas JK, Eiken HG, Oie E, et al. Myocardial expression of CC-and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res 2000; 47(4): 778–787. [DOI] [PubMed] [Google Scholar]
- 47. Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol 2008; 153 Suppl 1: S414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 2002; 91: 988–998. [DOI] [PubMed] [Google Scholar]
- 49. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10(8): 789–799. [DOI] [PubMed] [Google Scholar]
- 50. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 51. Fernandes JV, DE Medeiros Fernandes TA, de Azevedo JC, et al. Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncol Lett 2015; 9(3): 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perwez Hussain S, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 2007; 121: 2373–2380. [DOI] [PubMed] [Google Scholar]
- 53. Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 2011; 7(5): 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014: 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kabel AM. Relationship between cancer and cytokines. J Cancer Res Treat 2014; 2: 41–43. [Google Scholar]
- 56. Rose-John S, Schooltink H. Cytokines are a therapeutic target for the prevention of inflammation-induced cancers. Recent Results Cancer Res 2007; 174: 57–66. [DOI] [PubMed] [Google Scholar]
- 57. Fenton JI, McCaskey SJ, Woodworth HL. Molecular mechanisms of obesity, inflammation and cancer: the use of in vitro model approaches for targeted prevention strategies. Open Obes J 2010; 2: 23–37. [Google Scholar]
- 58. Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell 1999; 98: 295–303. [DOI] [PubMed] [Google Scholar]
- 59. Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 2011; 121(6): 233–251. [DOI] [PubMed] [Google Scholar]
- 60. Marson A, Levine SS, Cole MF, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 2008; 134: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jin P, Panelli MC, Marincola FM, et al. Cytokine polymorphism and its possible impact on cancer. Immunol Res 2004; 30(2): 181–190. [DOI] [PubMed] [Google Scholar]
- 62. Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 2008; 19(3): 294–308. [DOI] [PubMed] [Google Scholar]
- 63. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2010; 120: 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7(2): 131–142. [DOI] [PubMed] [Google Scholar]
- 65. Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res 2009; 19: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maier HJ, Schmidt-Straßburger U, Huber MA, et al. NF-κB promotes epithelial–mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 2010; 295: 214–228. [DOI] [PubMed] [Google Scholar]
- 67. Kumar M, Allison DF, Baranova NN, et al. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS ONE 2013; 8(7): e68597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li B, Vincent A, Cates J, et al. Low levels of tumor necrosis factor α increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res 2009; 69: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother 2005; 59: S340–S343. [DOI] [PubMed] [Google Scholar]
- 70. Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003; 22: 1517. [DOI] [PubMed] [Google Scholar]
- 71. Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442. [DOI] [PubMed] [Google Scholar]
- 72. Samatov TR, Tonevitsky AG, Schumacher U. Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer 2013; 12: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Al-Hassan AA, Al-Ghurabi BH, Al-Karkhi IH. Prognostic value of proinflammatory cytokines in breast cancer. J Biomol Res Ther 2012; 1: 2. [Google Scholar]
- 74. Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev 2006; 17: 325–337. [DOI] [PubMed] [Google Scholar]
- 75. Sirotković-Skerlev M, Kulić A, Bradić L, et al. Protumor effects of proinflammatory mediators in breast cancer. Period Biol 2012; 114: 489–496. [Google Scholar]
- 76. Wang F, Liu H, Liu S, et al. SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1 β in vivo and in vitro. Breast Cancer Res Treat 2005; 89: 5–14. [DOI] [PubMed] [Google Scholar]
- 77. Kundu JK, Surh Y-J. Inflammation: gearing the journey to cancer. Mutat Res 2008; 659(1-2): 15–30. [DOI] [PubMed] [Google Scholar]
- 78. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 2005; 41(16): 2502–2512. [DOI] [PubMed] [Google Scholar]
- 79. Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, et al. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res 2015; 35(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hsing C-H, Cheng H-C, Hsu Y-H, et al. Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome. Clin Cancer Res 2012; 18: 713–725. [DOI] [PubMed] [Google Scholar]
- 81. Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121: 335–348. [DOI] [PubMed] [Google Scholar]
- 82. Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin 1999; 49: 8–31. [DOI] [PubMed] [Google Scholar]
- 83. Rebbeck TR, Haas G. Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol 2014; 21(5): 7496–7506. [PMC free article] [PubMed] [Google Scholar]
- 84. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4(1): 71–78. [DOI] [PubMed] [Google Scholar]
- 85. Xu H, Hu M, Bai P, et al. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. Biomed Res Int 2015; 2015: 249741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gasche JA, Hoffmann J, Boland CR, et al. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer 2011; 129: 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim S-Y, Kang JW, Song X, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal 2013; 25(4): 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Colombatti M, Grasso S, Porzia A, et al. The prostate specific membrane antigen regulates the expression of IL-6 and CCL5 in prostate tumour cells by activating the MAPK Pathways. PLoS ONE 2009; 4: e4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Malinowska K, Neuwirt H, Cavarretta IT, et al. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer 2009; 16(1): 155–169. [DOI] [PubMed] [Google Scholar]
- 90. Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science 1996; 271: 1128–1131 [DOI] [PubMed] [Google Scholar]
- 91. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013; 14(6): e218–228. [DOI] [PubMed] [Google Scholar]
- 92. Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13(5): 587–596. [DOI] [PubMed] [Google Scholar]
- 93. Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011; 11: 886. [DOI] [PubMed] [Google Scholar]
- 94. De Miguel MP, Royuela M, Bethencourt FR, et al. Immunoexpression of tumour necrosis factor-α and its receptors 1 and 2 correlates with proliferation/apoptosis equilibrium in normal, hyperplasic and carcinomatous human prostate. Cytokine 2000; 12: 535–538. [DOI] [PubMed] [Google Scholar]
- 95. Wikström P, Stattin P, Franck Lissbrant I, et al. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998; 37: 19–29. [DOI] [PubMed] [Google Scholar]
- 96. Loberg RD, Ying C, Craig M, et al. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 2007; 9(7): 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Csiszar A, Szentes T, Haraszti B, et al. The pattern of cytokine gene expression in human colorectal carcinoma. Pathol Oncol Res 2004; 10(2): 109–116. [DOI] [PubMed] [Google Scholar]
- 98. Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 2004; 4(2): 97–105. [DOI] [PubMed] [Google Scholar]
- 99. Chung Y, Chang Y. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 2003; 83(4): 222–226. [DOI] [PubMed] [Google Scholar]
- 100. Su JL, Lai KP, Chen CA, et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res 2005; 65: 4827–4835. [DOI] [PubMed] [Google Scholar]
- 101. Chung YC, Chaen YL, Hsu CP. Clinical significance of tissue expression of interleukin-6 in colorectal carcinoma. Anticancer Res 2006; 26(5B): 3905–3911. [PubMed] [Google Scholar]
- 102. Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005; 7(6): 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gaiotti D, Chung J, Iglesias M, et al. Tumor necrosis factor-α promotes human papillomavirus (HPV) E6/E7 RNA expression and cyclin-dependent kinase activity in HPV-immortalized keratinocytes by a RAS-dependent pathway. Mol Carcinog 2000; 27: 97–109. [DOI] [PubMed] [Google Scholar]
- 104. Morrison CD, Parvani JG, Schiemann WP. The relevance of the TGF-β Paradox to EMTMET programs. Cancer Lett 2013; 341: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Uchiyama T, Takahashi H, Endo H, et al. IL-6 plays crucial roles in sporadic colorectal cancer through the cytokine networks including CXCL7. J Cancer Ther 2012; 3: 874. [Google Scholar]
- 106. Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat Res Mol Mech Mutagen 2005; 591: 34–44. [DOI] [PubMed] [Google Scholar]
- 107. Moncada S. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991; 43(2): 109–142. [PubMed] [Google Scholar]
- 108. Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethyl benzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog Publ Coop with Univ Texas MD Anderson Cancer Cent 2004; 40: 232–240. [DOI] [PubMed] [Google Scholar]
- 109. Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 2001; 281(3): G626–634. [DOI] [PubMed] [Google Scholar]
- 110. Wilson KT, Fu S, Ramanujam KS, et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res 1998; 58: 2929–2934. [PubMed] [Google Scholar]
- 111. Cannistra SA. Cancer of the ovary. N Engl J Med 2004; 351: 2519–2529. [DOI] [PubMed] [Google Scholar]
- 112. Yanaihara N, Anglesio MS, Ochiai K, et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol 2012; 41(3): 1094–1100. [DOI] [PubMed] [Google Scholar]
- 113. Ferrandina G, Ranelletti FO, Lauriola L, et al. Cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), and Her-2/neu expression in ovarian cancer. Gynecol Oncol 2002; 85(2): 305–310. [DOI] [PubMed] [Google Scholar]
- 114. Matte I, Lane D, Laplante C, et al. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2012; 2(5): 566–580. [PMC free article] [PubMed] [Google Scholar]
- 115. Lambeck AJA, Crijns APG, Leffers N, et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res 2007; 13: 2385–2391. [DOI] [PubMed] [Google Scholar]
- 116. Nowak M, Glowacka E, Szpakowski M, et al. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett 2010; 31(3): 375–383. [PubMed] [Google Scholar]
- 117. Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2011; 20(5): 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rabinovich A, Medina L, Piura B, et al. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res 2007; 27(1A): 267–272. [PubMed] [Google Scholar]
- 119. Scambia G, Testa U, Benedetti Panici P, et al. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer 1995; 71(2): 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Min H, Wei-hong Z. Constitutive activation of signal transducer and activator of transcription 3 in epithelial ovarian carcinoma. J Obstet Gynaecol Res 2009; 35(5): 918–925. [DOI] [PubMed] [Google Scholar]
- 121. Yang J, Wang Y, Gao Y, et al. Reciprocal regulation of 17β-estradiol, interleukin-6 and interleukin-8 during growth and progression of epithelial ovarian cancer. Cytokine 2009; 46: 382–391. [DOI] [PubMed] [Google Scholar]
- 122. Jammal MP, DA Silva AA, Filho AM, et al. Immunohistochemical staining of tumor necrosis factor-α and interleukin-10 in benign and malignant ovarian neoplasms. Oncol Lett 2015; 9(2): 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liao S, Liu J, Lin P, et al. TGF-β blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin Cancer Res 2011; 17: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002; 122: 1609–1619. [DOI] [PubMed] [Google Scholar]
- 125. Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol WJG 2014; 20: 4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat 2004; 11(5): 383–393. [DOI] [PubMed] [Google Scholar]
- 127. Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 2006; 80: 1197–1213. [DOI] [PubMed] [Google Scholar]
- 128. Huang YS, Hwang SJ, Chan CY, et al. Serum levels of cytokines in hepatitis C-related liver disease: a longitudinal study. Zhonghua Yi Xue Za Zhi (Taipei) 1999; 62(6): 327–333. [PubMed] [Google Scholar]
- 129. Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer 1992; 70: 709–713. [DOI] [PubMed] [Google Scholar]
- 130. Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-α) receptors in type C chronic liver disease. Clin Exp Immunol 1997; 109: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kakimi K, Lane TE, Wieland S, et al. Blocking chemokine responsive to γ–2/Interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of Hepatitis B Virus–specific cytotoxic T lymphocytes. J Exp Med 2001; 194: 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Toyonaga T, Hino O, Sugai S, et al. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc Natl Acad Sci 1994; 91: 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wagner K, Peters M, Scholz A, et al. Activin A stimulates vascular endothelial growth factor gene transcription in human hepatocellular carcinoma cells. Gastroenterology 2004; 126(7): 1828–1843. [DOI] [PubMed] [Google Scholar]
- 134. Akbar SMF, Abe M, Murakami H, et al. Macrophage migration inhibitory factor in hepatocellular carcinoma and liver cirrhosis; relevance to pathogenesis. Cancer Lett 2001; 171: 125–132. [DOI] [PubMed] [Google Scholar]
- 135. Hira E, Ono T, Dhar DK, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer 2005; 103: 588–598. [DOI] [PubMed] [Google Scholar]
- 136. Wang Y, Kato N, Hoshida Y, et al. Interleukin-1β gene polymorphisms associated with hepatocellular carcinoma in hepatitis C virus infection. Hepatology 2003; 37: 65–71. [DOI] [PubMed] [Google Scholar]
- 137. Hirankarn N, Kimkong I, Kummee P, et al. Interleukin-1β gene polymorphism associated with hepatocellular carcinoma in hepatitis B virus infection. World J Gastroenterol WJG 2006; 12: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 139. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010; 46(4): 765–781. [DOI] [PubMed] [Google Scholar]
- 140. Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett 2014; 345: 157–163. [DOI] [PubMed] [Google Scholar]
- 141. Yu Y, Wang W, Zhai S, et al. IL6 gene polymorphisms and susceptibility to colorectal cancer: a meta-analysis and review. Mol Biol Rep 2012; 39(8): 8457–8463. [DOI] [PubMed] [Google Scholar]
- 142. Tang RF, Wang SX, Zhang FR, et al. Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat Dis Int 2005; 4(3): 460–463. [PubMed] [Google Scholar]
- 143. Li M, Zhang Y, Feurino LW, et al. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci 2008; 99(4): 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Friess H, Guo X, Nan B, et al. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci 1999; 880: 110–121. [DOI] [PubMed] [Google Scholar]
- 145. Denz A, Pilarsky C, Muth D, et al. Inhibition of MIF leads to cell cycle arrest and apoptosis in pancreatic cancer cells. J Surg Res 2010; 160: 29–34. [DOI] [PubMed] [Google Scholar]
- 146. Greco E, Basso D, Fogar P, et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1β not by transforming growth factor-β1. Int J Biol Markers 2005; 20: 235–241. [DOI] [PubMed] [Google Scholar]
- 147. Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 2006; 25(3): 387–408. [DOI] [PubMed] [Google Scholar]
- 148. Schmid MC, Avraamides CJ, Foubert P, et al. Combined blockade of integrin-α4β1 plus cytokines SDF-1α or IL-1β potently inhibits tumor inflammation and growth. Cancer Res 2011; 71: 6965–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci 2005; 108(3): 205–213. [DOI] [PubMed] [Google Scholar]
- 150. Williams JW, Huang LH, Randolph GJ. Cytokine circuits in cardiovascular disease. Immunity 2019; 50: 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]