Abstract
Calcific tendinopathy of the rotator cuff is a common cause of shoulder pain and debility. Minimally invasive treatment options have been employed for management; however, ultrasonic tenotomy has not been previously described for management of calcific tendinopathy of the shoulder. The purpose of the current case series was to provide preliminary evidence in support of a novel treatment modality for calcific tendinopathy of the rotator cuff. This descriptive pilot case series included a total of 8 patients with calcific tendinopathy of the supraspinatus that underwent ultrasound-guided ultrasonic debridement in the sports medicine clinic. All procedures were performed by the same physician (JLE). All patients had confirmation of the diagnosis with MRI and ultrasound imaging. Pain was measured pre-procedure and followed until 3-months post-procedure. Very large, statistically significant, reductions (P < .01) in pain scores were observed at 1 (ES = 1.93), 2 (ES = 1.84) and 3 (ES = 2.20) months post-procedure, respectively. All patients experienced a significant reduction in pain scores, regardless of hardness of the calcium deposit, at 1 month post-procedure with pain scores remaining lower than at baseline at 2 and 3 months post-procedure. No adverse events were noted in any patients. Ultrasonic tenotomy and debridement appears to be a safe and effective treatment option for patients with calcific tendinopathy of the supraspinatus.
Keywords: calcific tendinitis, tendinopathy, percutaneous, ultrasound-guided, rotator cuff
Background
Calcific tendinopathy of the rotator cuff is a common cause of shoulder pain that predominantly affects adults between the ages of 40 to 60.1,2 Most often, the etiology of calcific tendinopathy is unknown. However, prior hypotheses point towards an accumulation of hydroxyapatite crystal deposition over time3 and it is currently accepted that the condition is not necessarily related to trauma or overuse. Rather, it is characterized by deposition of calcium deposits in 1 or more rotator cuff tendons with the supraspinatus being the most commonly affected tendon.4
Pain is often characterized as a deep ache in the shoulder that radiates down the side of the arm towards the elbow. It is common for patients to present with classic impingement symptoms, such as pain with overhead movement. Diagnosis can be confirmed with x-ray, ultrasound or magnetic resonance imaging (MRI).5 Currently, no consensus exists regarding the specific pathoeitology of the calcium deposition. It is generally accepted that the calcium deposits go through several phases as outlined in the work of Uhthoff and Loehr.6 The authors6 suggest the calcium deposit progresses through 3 main phases: pre-calcification, calcification and post-calcification phases, of which any given calcium deposit may show signs of being in more than 1 phase.6
Conservative treatment options typically include rest, physical therapy, non-steroidal anti-inflammatories (NSAIDS) and shock wave therapy.7 However, approximately ten percent of patients will be refractory to conservative treatment options.8 Historically, for those who fail conservative treatment options, corticosteroid injections, a minimally invasive lavage procedure or arthroscopic versus open surgery can be performed. The needle lavage procedure and its effectiveness have been previously reviewed.9,10 Castillo-Gonzalez et al demonstrated significantly reduced calcium deposit size as well as pain reductions in ultrasound-guided lavage patients compared to extracorporeal shockwave treatment and this effect was sustained at 12 months post-procedure.10 Recent meta-analysis found that ultrasound-guided lavage was more effective than extracorporeal shockwave treatment or a corticosteroid injection with clinically important effect in the short term, but questionable long term results.9 Complications from calcific tendinopathy can include pain, adhesive capsulitis, rotator cuff tears, greater tuberosity osteolysis and ossifying tendinitis as outlined in the review by Merolla et al.11
Ultrasonic tenotomy and debridement of tendinopathy across multiple tendons in the body is a novel treatment option that has been previously shown to be an effective treatment option for the common extensor tendon of the elbow,12-15 Achilles tendon,16,17 patellar tendon,18 gluteal tendon19 and biceps tendon20 among other areas.21,22 A common device used, manufactured by Tenex (Tenex Health, Lake Forest, CA) utilizes ultrasonic energy to debride and aspirate pathologic tendon tissue. The manufacturers have developed 3 different tips (TX1, TX2 and TXBone). The development of the longer TX2 tip and more powerful TXBone tip has provided opportunities for tendon pathology previously unreachable with a percutaneous approach.
Given previous use of this technique to treat tendon pathology throughout the body, it was determined that treating calcific tendinopathy of the supraspinatus would be amenable to this approach using the TX2 or TXBone tips. We report our results in a limited case series where we successfully treated 8 cases in 8 patients (Males, n = 5; Females, n = 3) with ultrasonic tenotomy and debridement of the calcific deposit, who previously failed conservative therapies.
Methods
The study protocol was submitted through the Institutional Review Board Human Research Wizard and it was determined that IRB approval was not required. A letter documenting the exemption was received. All patients provided informed written consent for the procedure (ultrasound guided tenotomy and calcific deposit removal). All procedures were performed in the same clinical procedure suite by the same physician.
Physical Exam and Diagnostic Imaging
All patients presented to the sports medicine clinic in 2018 to 2020 for management of shoulder pain related to calcific tendinopathy. Patients reported a mean duration of 24 months of symptoms, which included shoulder pain, pain with overhead activity, pain at rest and failure to respond to a course of physical therapy. 100% of patients had at least 1 corticosteroid injection into the subacromial bursa.
In addition to the history and physical examination, all patients underwent an x-ray (AP external rotation, Internal Grashey, Axillary and scapular Y views) and MRI with or without intra-articular contrast dye to confirm the diagnosis of calcific tendinopathy of the rotator cuff and to exclude other pathology such as full thickness rotator cuff tearing. Figure 1 demonstrates an MRI showing a calcific deposit in the supraspinatus tendon from one the patients from the current case series. Finally, all patients underwent a diagnostic ultrasound examination to further characterize the calcific deposit and determine procedure planning. Diagnostic ultrasound is commonly used to add further value and even replace the MRI scan by noting factors such as subtle rotator cuff tears, focal tendinosis, suggested hardness of the calcific deposit based on echogenicity and shadowing.23-25 Hardness of the calcium deposit is presented in Table 1. Figure 2 presents an ultrasound image demonstrating calcific deposit in the supraspinatus tendon.
Figure 1.
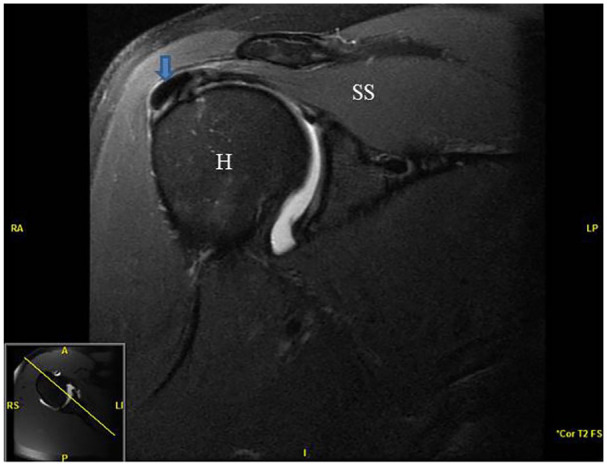
Pre-procedure MRI image of a patient with right shoulder calcific tendinopathy of the supraspinatus. Arrow signifies calcium deposit in the supraspinatus tendon.
Abbreviations: H, humeral head; SS, supraspinatus.
Table 1.
Patient Demographic Information. Data Presented as Mean, Standard Deviation (SD) and 95% Confidence Intervals (CI).
| Case number | Sex | Age (years) | BMI (m/kg2) | Number of prior injections* | Length of prior symptoms (mo) | Energy time (sec) | Micro tip type | Calcification hardness |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 21.4 | 1 | 2 | 368 | TX2 | Medium |
| 2 | F | 39 | 25.5 | 2 | 5 | 248 | TX2 | Medium |
| 3 | M | 67 | 23.4 | 5 | 24 | 469 | TX2 | Hard |
| 4 | M | 67 | 23.4 | 5 | 24 | 360 | TXB | Hard |
| 5 | F | 50 | 36.3 | 2 | 12 | 265 | TX2 | Soft |
| 6 | M | 58 | 28.5 | 2 | 12 | 131 | TX2 | Soft |
| 7 | M | 36 | 28.0 | 2 | 15 | 282 | TXB | Hard |
| 8 | M | 46 | 38.4 | 2 | 72 | 362 | TXB | Hard |
| Mean | 51.69 | 33.57 | 1.89 | 30.89 | 247.97 | |||
| SD | 10.76 | 8.82 | 1.98 | 32.24 | 106.78 | |||
| 95% CI | 47.99, 55.38 | 33.57, 30.54 | 1.20, 2.57 | 19.81, 41.96 | 211.29, 284.65 |
Abbreviations: BMI, body mass index; Hard, thick hyperechoic rim with strong posterior acoustic shadowing; Medium, thin hyperechoic rim, inconstant, minimal posterior acoustic shadowing; mo, months; sec, seconds; Soft, amorphous appearance with no posterior acoustic shadowing; TX2, Tenex Health MicroTip 2; TXB, Tenex Health MicroTip bone.
Injections refer to prior corticosteroid injections.
Figure 2.

Ultrasound image demonstrating calcium deposit embedded in the supraspinatus tendon. Arrow—calcium deposit.
Abbreviations: RT, right; SS, supraspinatus.
All cases were also discussed with an orthopedic surgeon and the patients were presented with the option of an arthroscopic surgery versus an ultrasound-guided tenotomy and debridement with Tenex TX2 or TXBone devices given failed conservative treatments.
Procedure
Patients were placed supine with the head of the bed elevated 30 degrees. The procedure site was prepped using standard sterile technique. Under direct ultrasound visualization with a GE S7 (Logiq S7 R3 Expert; General Electric, Fairfield, CT, USA) a 2 inch, 25 gauge needle was used to deliver 1% plain lidocaine until adequate anesthesia was achieved. Again, under direct ultrasound visualization, an 11 blade scalpel was used to make a stab incision creating a tract down to the superficial fibers of the supraspinatus. Next, either a TX2 or TXBone device was inserted along the tract down to the desired location (calcific deposit). Figure 3 depicts the stepwise progression of the procedure. The tip of the device was activated via the attached foot pedal which allowed the tip of the device to move back and forth with low-amplitude, high frequency motion to gradually fragment and aspirate the deposit. The deposit was emulsified in a lateral to medial, superficial to deep fashion using real-time long axis imaging. Real-time visualization of debulked calcium deposit was noted. Loss of resistance with passing the tip of the device through the deposit was appreciated. See Figure 4 for ultrasound images demonstrating the progressive removal of a calcium deposit. Mean energy time (total treatment time) was noted to be 298.20 ± 104.20 seconds.
Figure 3.
Ultrasound images demonstrating progressive removal of the calcium deposit working lateral to medial (A-D).
Abbreviation: SS, supraspinatus.
Figure 4.
Ultrasound images demonstrating process of ultrasound-guided calcific tendon debridement. (A) Supraspinatus long axis. Arrows—calcium deposit. (B) Supraspinatus long axis. Needle insertion. (C) Supraspinatus long axis. 11-blade scalpel insertion. (D) Supraspinatus long axis. Needle is the Tenex Health TX2 tip mid-procedure.
Abbreviation: SS, supraspinatus.
In the immediate post-procedure period all patients were advised to avoid overhead activity and not lift greater than ten pounds with the affected extremity for 2 weeks. All patients were seen in follow-up 2 weeks post-procedure for incision check, range of motion, strength testing and pain scoring. Patients were then instructed to continue to avoid overhead activity for another 4 weeks while gradually increasing the weight restriction to 20 pounds. At the 6 week post-procedure visit range of motion, strength testing and pain scores were again checked. No formal physical therapy or home going post-procedure rehab protocol was implemented in any of the patients. All patients were allowed to gradually resume normal activities without restriction and instructed to return to clinic if any concerns arose in the future. See Figure 5 for a comparison of pre-procedure and post-procedure x-ray’s demonstrating removal of the calcium deposit.
Figure 5.
An X-ray image demonstrating pre to post changes in calcium deposit. (A) Pre-procedure left shoulder. Arrow indicates calcific deposit. (B) Post-procedure left shoulder demonstrating complete resolution of the calcium deposit.
Abbreviation: L, left.
Pain Scores
Patients completed a baseline numeric pain scale with verbal anchors at 1 to 10. Follow-up phone calls were made monthly to assess pain scores at 3 months post-procedure. Patients were asked to rate their pain based on activities that had caused pain pre-procedure.
Statistical Analysis
Descriptive statistics were used to provide a summary of patient demographics and case history data. Data are presented as means ± standard deviation and 95% confidence intervals. A repeated measures analysis of variance was used to examine changes in pain scores over time. Alpha was set at P < .05 to determine statistical significance. Mean differences were calculated using LSD tests to determine where significance occurred. Cohen’s d effect size (ES) was calculated to determine the magnitude of change in pain scores and interpreted using the following criteria: <0.2 = trivial, 0.2-0.6 = small, 0.7-1.2 = moderate, 1.3-2.0 = large, and >2.0 = very large.
Results
A summary of patient demographics and case history is presented in Table 1. MicroTip selection and calcium hardness was also recorded and presented in Table 1. Large, statistically significant reductions (P < .01) in pain scores were observed at 1 (ES = 1.93), 2 (ES = 1.84) and very large at 3 (ES = 2.20) months post-procedure, respectively as seen in Figure 6. All patients experienced an immediate reduction in pain scores at 1 month post-procedure with pain scores remaining lower than at baseline at 2 and 3 months post-procedure as seen in Figure 7.
Figure 6.

Changes in post-procedure pain scores for all patients.
Abbreviation: ES, effect size.
Figure 7.
Changes in individual post-procedure pain scores.
Discussion
The primary aim of the current case series was to provide preliminary evidence of the efficacy and safety of ultrasonic tenotomy and debridement for calcific tendinopathy of the shoulder. The results of the current case series provides preliminary evidence that ultrasonic tenotomy and debridement can be used an effective alternative treatment option for patients with calcific tendinopathy of the supraspinatus and appears to be well tolerated. The ultrasonic tenotomy and debridement is purported to work by using an ultrasonic energy to emulsify and remove damaged tendon tissue or calcium deposits, while also stimulating a new healing response. Procedural options have historically included arthroscopic or open surgical debridement or a minimally invasive barbotage procedure. However, certain patients may not be a candidate for surgery based on medical co-morbidities such as suboptimal controlled diabetes mellitus, blood thinners or morbid obesity. Further, general anesthesia and prolonged recovery time from surgery is often a discouraging factor for patients, which provides strong rationale for the use of ultrasonic tenotomy and debridement as a less invasive alternative treatment option. Ultrasonic tenotomy and calcific deposit removal with Tenex Health Devices (TX1, TX2, TXBone) is a technique that has been available since 2011 and has previously shown to be safe and effective for treatment of tendon disorders throughout the body with minimal adverse events reported.12,16,19 For example, Baker and Mahoney presented a similar case series demonstrating comparable improvements in pain scores in 29 patients with gluteal tendinopathy. Barnes et al performed ultrasonic tenotomy for common flexor and common extensor tendinosis of the elbow in 19 patients with significant reductions in Visual Analogue Scale and QuickDASH scores at one year post-procedure and no complications.14
To the best of our knowledge the use of this procedure for calcific tendinopathy of the supraspinatus has not been published to date in the literature. We successfully treated 8 cases of calcific tendinopathy of the supraspinatus as evidenced by the reductions in pain scores for all patients, without any complications or adverse events. All patients tolerated the procedure well using local anesthetic only. No prescription pain medications were required post-procedure. Our patients experienced rapid and significant reductions in pain following completion of the procedure with pain scores continuing to remain significantly lower at 3 months post-procedure compared to pre-procedure. All patients returned their normal daily activities within 6 weeks of the procedure. Of note, 2 patients had the procedure performed without interruption of their blood thinner. One patient was on rivaroxaban and the other on apixaban. There were no bleeding complications during the procedure. Similarly, 4 patients in the current study had a hemoglobin A1C greater than 8.0 with no infection or healing complications observed. Several studies have identified imaging characteristics that correlate with failure of conservative treatment.23,25 Ultrasound characteristics of the calcium deposits were grouped as previously described into hard, medium or soft. Considering all patients had significant improvement in post-procedure pain scores compared to pre-procedure scores, these findings suggest that this treatment method may be beneficial regardless of previously described calcific deposit classification methods. Therefore, with further study, patients previously predicted to have a poor outcome with non-surgical treatment based on calcium deposit characteristics, may have an alternative treatment option. 100% of our patients’ answered “yes” when asked if they would recommend having this procedure done. While further investigation is required, this procedure may offer a safe alternative for patients who may not be able to stop anticoagulation or in whom suboptimal control of diabetes may pre-dispose them to surgical complications.
Limitations
This was a limited case series with no control group. Formal measures of strength, range of motion and functional assessment of activities of daily living were also not completed during this series and may further contribute to our understanding of how this procedure can impact a patient’s quality of life. All patients did undergo formal physical therapy and had at least 1 corticosteroid injection previously, but it is possible if some patients were simply given more time, their condition would have improved regardless of treatment. It is important to note that all procedures were performed by the same physician and this may limit the generalizability of the procedure. Further research is needed to determine if results are sustained at longer term intervals as our case series only had a three-month follow-up period. Another limitation of the current case series was the lack of post-procedural imaging for all patients.
Conclusion
To the best of our knowledge, this case series is the first to report successful treatment of calcific tendinopathy of the supraspinatus and partial tears of the supraspinatus using percutaneous ultrasonic energy. This limited case series highlights the benefits of ultrasonic tenotomy and debridement for calcific tendinopathy of the rotator cuff as a viable and safe consideration for patients that have failed multiple conservative treatments. Standard conservative therapies should be employed first for the majority of patients with calcific tendinopathy of the supraspinatus tendon. However, when conservative measures fail, it is reasonable to consider ultrasound-guided tenotomy and debridement as an effective and potentially definitive treatment option for calcific tendinopathy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jacob L. Erickson  https://orcid.org/0000-0002-9975-8836
https://orcid.org/0000-0002-9975-8836
Andrew R. Jagim  https://orcid.org/0000-0002-6651-5096
https://orcid.org/0000-0002-6651-5096
References
- 1. Speed CA, Hazleman BL. Calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1582-1584. [DOI] [PubMed] [Google Scholar]
- 2. De Carli A, Pulcinelli F, Rose GD, Pitino D, Ferretti A. Calcific tendinitis of the shoulder. Joints. 2014;2:130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gartner J, Simons B. Analysis of calcific deposits in calcifying tendinitis. Clin Orthop Relat Res. 1990;254:111-120. [PubMed] [Google Scholar]
- 4. Loew M, Jurgowski W, Mau HC, Thomsen M. Treatment of calcifying tendinitis of rotator cuff by extracorporeal shock waves: a preliminary report. J Shoulder Elbow Surg. 1995;4:101-106. [DOI] [PubMed] [Google Scholar]
- 5. Chianca V, Albano D, Messina C, et al. Rotator cuff calcific tendinopathy: from diagnosis to treatment. Acta Biomed. 2018;89:186-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183-191. [DOI] [PubMed] [Google Scholar]
- 7. Hurt G, Baker CL., Jr. Calcific tendinitis of the shoulder. Orthop Clin N Am. 2003;34:567-575. [DOI] [PubMed] [Google Scholar]
- 8. Louwerens JK, Sierevelt IN, van Noort A, van den Bekerom MP. Evidence for minimally invasive therapies in the management of chronic calcific tendinopathy of the rotator cuff: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2014;23:1240-1249. [DOI] [PubMed] [Google Scholar]
- 9. Lafrance S, Doiron-Cadrin P, Saulnier M, et al. Is ultrasound-guided lavage an effective intervention for rotator cuff calcific tendinopathy? A systematic review with a meta-analysis of randomised controlled trials. BMJ Open Sport Exerc Med. 2019;5:e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castillo-Gonzalez FD, Ramos-Alvarez JJ, Rodriguez-Fabian G, González-Pérez J, Calderón-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2014;4: 220-225. [PMC free article] [PubMed] [Google Scholar]
- 11. Merolla G, Bhat MG, Paladini P, Porcellini G. Complications of calcific tendinitis of the shoulder: a concise review. J Orthop Traumatol. 2015;16:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altahawi F, Li X, Demarest B, Forney MC. Percutaneous ultrasonic tenotomy with the TX-1 device versus surgical tenotomy for the treatment of common extensor tendinosis [published online July 9, 2020]. Skeletal Radiol. doi: 10.1007/s00256-020-03540-7 [DOI] [PubMed] [Google Scholar]
- 13. Barayeva D, Edelstein Y, Stickevers SM. Poster 179 ultrasound guided percutaneous tenotomy for refractory lateral epicondylitis. PM R. 2016;8:S220. [DOI] [PubMed] [Google Scholar]
- 14. Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24:67-73. [DOI] [PubMed] [Google Scholar]
- 15. Battista CT, Dorweiler MA, Fisher ML, Morrey BF, Noyes MP. Ultrasonic percutaneous tenotomy of common extensor tendons for recalcitrant lateral epicondylitis. Tech Hand Up Extrem Surg. 2018;22:15-18. [DOI] [PubMed] [Google Scholar]
- 16. Stover D, Fick B, Chimenti RL, Hall MM. Ultrasound-guided tenotomy improves physical function and decreases pain for tendinopathies of the elbow: a retrospective review. J Shoulder Elbow Surg. 2019;28:2386-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanchez PJ, Grady JF, Saxena A. Percutaneous ultrasonic tenotomy for achilles tendinopathy is a surgical procedure with similar complications. J Foot Ankle Surg. 2017;56:982-984. [DOI] [PubMed] [Google Scholar]
- 18. Baria MR, Vasileff WK, Miller M, et al. Percutaneous ultrasonic tenotomy effectively debrides tendons of the extensor mechanism of the knee: a technical note. Knee. 2020;27:649-655. [DOI] [PubMed] [Google Scholar]
- 19. Baker CL, Jr, Mahoney JR. Ultrasound-guided percutaneous tenotomy for gluteal tendinopathy. Orthop J Sports Med. 2020;8:2325967120907868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greditzer HG, Kaplan LD, Lesniak BP, Jose J. Ultrasound-guided percutaneous long head of the biceps tenotomy: a novel technique with case report. HSS J. 2014;10:240-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall MM, Woodroffe L. Ultrasonic percutaneous tenotomy for recalcitrant calcific triceps tendinosis in a competitive strongman: a case report. Curr Sports Med Rep. 2017;16:150-152. [DOI] [PubMed] [Google Scholar]
- 22. Pourcho AM, Hall MM. Percutaneous ultrasonic fasciotomy for refractory plantar fasciopathy after failure of a partial endoscopic release procedure. PM R. 2015;7:1194-1197. [DOI] [PubMed] [Google Scholar]
- 23. Bazzocchi A, Pelotti P, Serraino S, et al. Ultrasound imaging-guided percutaneous treatment of rotator cuff calcific tendinitis: success in short-term outcome. Br J Radiol. 2016;89:20150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farin PU, Jaroma H. Sonographic findings of rotator cuff calcifications. J Ultrasound Med. 1995;14:7-14. [DOI] [PubMed] [Google Scholar]
- 25. Ogon P, Suedkamp NP, Jaeger M, Izadpanah K, Koestler W, Maier D. Prognostic factors in nonoperative therapy for chronic symptomatic calcific tendinitis of the shoulder. Arthritis Rheum. 2009;60:2978-2984. [DOI] [PubMed] [Google Scholar]






