Table 2.
The names, structures, targets, FBDD optimization strategy used, biophysical techniques used, and status in clinical trials of select drugs derived from the FBDD approach.
| Drug (Company) | Target | Original Fragment(s) * | Advanced Molecule and Progress in Clinical Trials | Techniques Used |
|---|---|---|---|---|
| Vemurafenib (Plexxikon) [224,226] | BRAF-V600E |
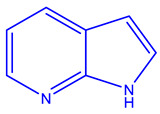
|
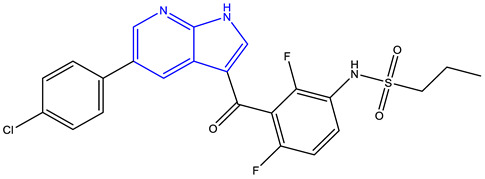 Approved |
high-concentration biochemical fragment screening, X-ray crystallography |
| Venetoclax (AbbVie, Genetech) [227,228,229,230] | BCL-2 |
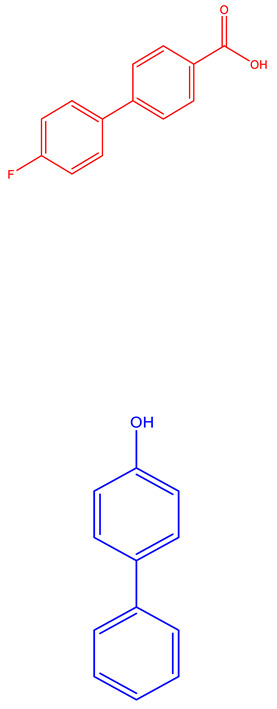
|
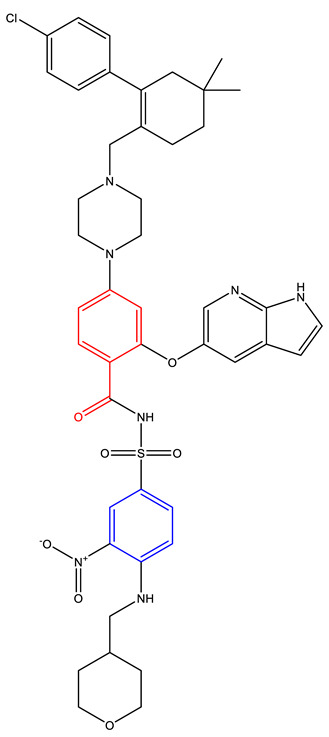 Approved |
NMR, X-ray crystallography |
| Ribociclib (Novartis Europharm Limited) [231] | CDK4 and 6 | Information Not Available |
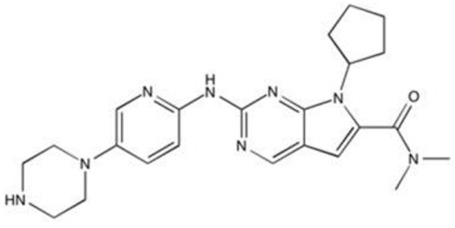 Approved |
Information not Available |
| PLX3397 (Plexxikon) [232,233,234] | FMS, KIT, and FLT3-ITD |
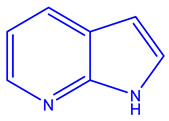
|
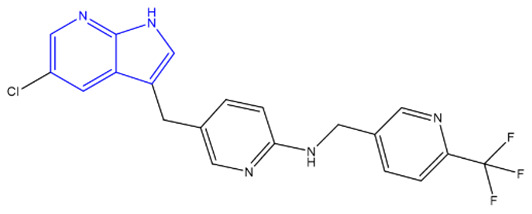 Phase 3 |
X-ray crystallography, Structure Confirmed by NMR, MS, and HPLC |
| Verubecestat (Merck) [235,236] | BACE1 |
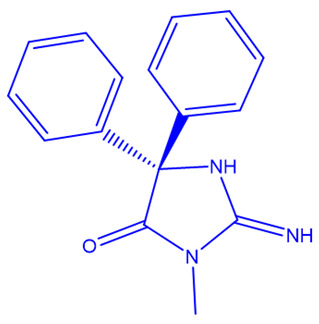
|
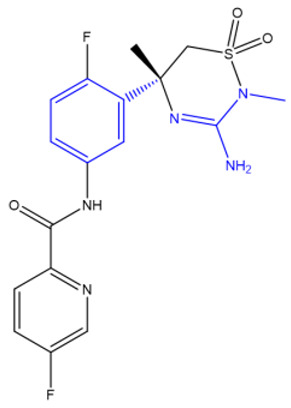 Phase 3 |
NMR, X-ray crystallography, inhibition of cathepsin D |
| Onalespib (Astex) [237,238] | HSP90 |
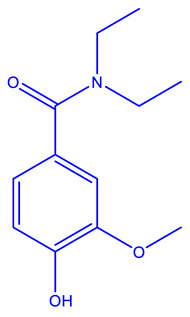
|
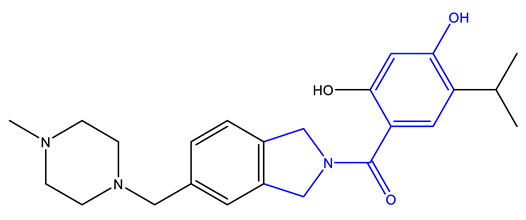 Phase 2 |
X-ray crystallography, isothermal titration calorimetry, NMR |
| AZD5099 [239,240] | Topoisomerase II |
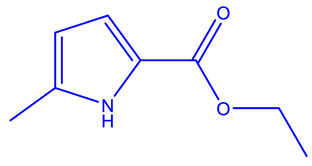
|
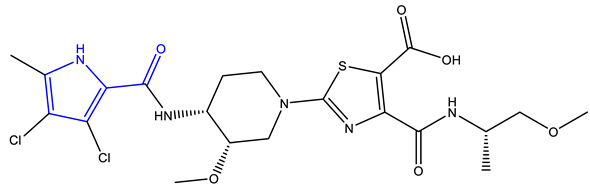 Phase 1 |
NMR, Surface Plasmon Resonance, isothermal calorimetry, X-ray cystallography |
| AT7519 [241,242,243,244] | CDK 1, 2, 4, and 5 |
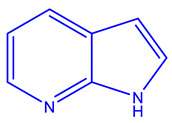
|
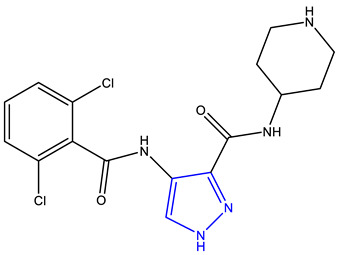 Phase 2 |
NMR, MS, X-ray crystallography |
* Structures for some of the fragments are taken directly from Dan Erlanson’s blog at [245].
