Abstract
Introduction:
The illicit opioid supply in the U.S. is increasingly adulterated with fentanyl. As such, persons with opioid use disorder (OUD) may be regularly exposed to fentanyl, however, the pharmacokinetics of repeated fentanyl exposure are not well understood. The current study aimed to quantify renal clearance of fentanyl in OUD patients presenting to residential treatment.
Methods:
Participants (N=12) who presented to a 28-day residential treatment program were enrolled if they tested positive for fentanyl at intake. Urine samples were collected every 2–3 days and were quantitatively tested for fentanyl, norfentanyl, and creatinine via liquid chromatography mass spectrometry (LC-MS). Fentanyl clearance was defined as the time since last illicit opioid use and the median time between last positive and first negative fentanyl urine screen.
Results:
Participants had a mean and standard deviation (SD) age of 28.9 (11.0), were 67% male, and 83% white. The mean (SD) time for fentanyl and norfentanyl clearance was 7.3 (4.9) and 13.3 (6.9) days, respectively. One participant continued to test positive for fentanyl for 19 days and norfentanyl for 26 days following their last use, and left treatment without testing negative for norfentanyl.
Conclusion:
Fentanyl clearance in persons with OUD is considerably longer than the typical 2–4 day clearance of other short-acting opioids. The findings of this study might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.
Keywords: Fentanyl, Opioid use disorder, Pharmacokinetic, Heroin, Opioid, Treatment
1. Introduction
Much of the illicit opioid supply in the U.S. is adulterated with fentanyl or fentanyl analogues, leading to a recent surge in fentanyl-related overdose deaths (Green and Gilbert, 2016; Macmadu et al., 2017; Jones et al., 2018). Fentanyl is now sold on the black market both as a stand-alone drug and mixed with heroin or other drugs, often unbeknownst to the buyer (Green and Gilbert, 2016; Cicero et al., 2017). There is considerable variability in the incidence of fentanyl adulteration in the illicit drug supply and the concentration of fentanyl between batches of fentanyl-cut heroin, which increases overdose risks for persons who use illicit opioids (Carroll et al., 2017). Likewise, many persons presenting to treatment with opioid use disorder (OUD) test positive for fentanyl at intake (Hayashi et al., 2018; Ochalek et al., 2019). Although illicit fentanyl use is not new, the number of individuals currently being exposed to fentanyl is unprecedented, and there is little research on the treatment implications of this phenomenon.
Fentanyl is highly lipophilic, allowing for fast diffusion between blood plasma and the central nervous system (Henthorn et al., 1999; Lötsch et al., 2013). In persons who use fentanyl regularly, this high lipophilicity can allow fentanyl to be sequestered in adipocytes or other tissue (Comer and Cahill, 2019), similar to persons who use marijuana regularly and experience protracted renal clearance of tetrahydrocannabinol (THC). Previous pharmacokinetic studies of fentanyl have relied on single dose administration or a few doses over a relatively short period of time (usually same day), and have reported half-lives ranging from 1.5–7 hours and 1.5–6 hours for intranasal (IN) and intravenous (IV) fentanyl, respectively (Mather, 1983; Lötsch et al., 2013). The pharmacokinetics of repeated IV and IN fentanyl administration have not been examined, likely because IV fentanyl is generally used to provide acute analgesia and fentanyl was not dominant in the illicit opioid drug supply until the last few years. However, persons who use illicit opioids have reported that daily fentanyl use has unintended consequences, such as unanticipated buprenorphine precipitated withdrawal (Silverstein et al., 2019). The current study quantified the potential long-term clearance of fentanyl in persons presenting to residential treatment for OUD. We hypothesized that fentanyl clearance would take longer than the standard 2–4 day clearance of other short-acting opioids.
2. Methods
2.1. Study Design
Participants from a 28-day closed residential addiction treatment program were enrolled if they: (1) were 18 or older, (2) were physically dependent on opioids, and (3) tested positive for fentanyl at intake based on a semi-quantitative urine fentanyl screen. Patients underwent an initial screen for eligibility that included questions about demographics and drug use history. Participants then provided urine samples every 2–3 days that were quantitatively tested for fentanyl, the metabolite norfentanyl, and creatinine via liquid chromatography mass spectrometry (LC-MS) (Huynh et al., 2005). The range for fentanyl and norfentanyl quantification was 0.5–500ng/mL and 0.5–200ng/mL, respectively. Qualitative tests with a 2ng/mL cutoff were also performed for common fentanyl analogues, namely acetyl fentanyl, acetyl norfentanyl, acrylfentanyl, carfentanil, furanyl fentanyl, and fluoroisobutyryrl/fluorobutyrl fentanyl. Previous studies have found that fentanyl is primarily excreted as metabolites such as norfentanyl, and only ~10% of the drug is unchanged in urinary excretion (Mather, 1983). All participants provided informed consent, and the Johns Hopkins University Institutional Review Board approved this study.
2.2. Data Analyses
Renal fentanyl clearance was examined by quantifying the time elapsed since last use (usually the day before or the day of treatment entry) and: (1) time until the last fentanyl and norfentanyl positive urine drug screen, (2) time until the first fentanyl and norfentanyl negative urine screen, and (3) the median between last positive and first negative urine screen, which might be most indicative of renal fentanyl clearance given the 2–3 day gap between sample collection. Data were creatinine-adjusted to control for varying levels of urine concentration, and presented as nanograms of fentanyl/norfentanyl per milligram of creatinine. For data presented in Figure 1, days with no urine screens were imputed within each individual based on a decay function for initial clearance and linear function for protracted clearance, consistent with trends observed in the data. Descriptive analyses on participant characteristics and renal fentanyl and norfentatnyl clearance were performed using SPSS (IBM, Armonk, NY).
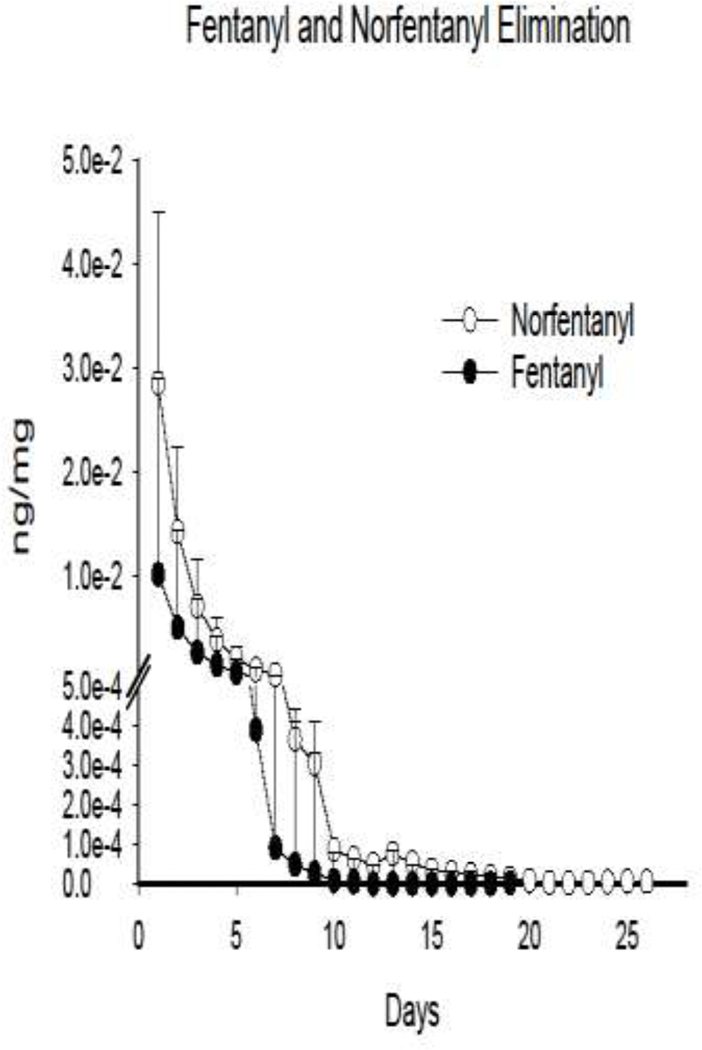
Figure 1.
Mean time course for fentanyl and norfentanyl positive urine drug screens since day of last opioid use. Data is creatinine-adjusted and presented as nanogram of fentanyl/norfentanyl per milligram of creatinine. Data for participants who left against medical advice were censored following their last fentanyl positive test. Days with no urine screens were imputed for each individual based on a decay function for initial clearance and linear function for protracted clearance, consistent with trends observed in the data. The length of fentanyl and norfentanyl lines represents the longest time observed for positive urine screens since day of last use, which was 19 and 26 days, respectively. Error bars represent 95% confidence intervals.
3. Results
Fourteen participants provided at least one urine sample and two left treatment against medical advice, leaving N=12. Participants had a mean and standard deviation (SD) age of 28.9 (11.0), body mass index of 25.3 (3.8), were 67% male and 83% white (Table 1). All participants tested positive for fentanyl and norfentanyl at initial testing. Two participants also tested positive for acetyl norfentanyl and one participant tested positive for fluoroisobutyrl/fluorobutyrl fentanyl. At 2–3 days following last self-reported opioid use, 50% of participants continued to test at the maximum level of norfentanyl assessed by LC-MS (>200ng/mL). The mean (SD) days between last positive and first negative fentanyl and norfentanyl screen was 7.3 (4.9) and 13.3 (6.9), respectively. Men and women did not differ in mean (SD) fentanyl elimination [7.3 (5.8) vs 7.5 (3.0), respectively] or norfentanyl elimination [13.8 (7.7) vs 12.1 (5.9), respectively]. One participant continued to test positive for fentanyl for 19 days and norfentanyl for 26 days following their last use, and left treatment without testing negative for norfentanyl. Mean creatinine adjusted fentanyl and norfentanyl clearance is depicted in Figure 1.
Table 1.
Participant Characteristics
| Age M(SD) | 28.9(11.0) |
| Sex (% Male) | 67% |
| Race (% White) | 83% |
| Body Mass Index M(SD) | 25.3(3.8) |
| Fentanyl Clearance | |
| Days until Last Positive Urine Screen M(SD) | 5.9 (4.7) |
| Days until First Negative Urine Screen M(SD) | 8.8 (4.8) |
| Median Split between Last Positive and First Negative Urine Screen M(SD) | 7.3 (4.9) |
| Norfentanyl Clearance | |
| Days until Last Positive Urine Screen M(SD) | 11.8 (6.9) |
| Days until First Negative Urine Screen M(SD) | 14.7 (7.1) |
| Median Split between Last Positive and First Negative Urine Screen M(SD) | 13.3 (6.9) |
Demographics and fentanyl-clearance characteristics for study participants. Data analyses included the number of days from last opioid use until the last fentanyl and norfentanyl positive urine screen, number of days from last opioid use until the first fentanyl and norfentanyl negative urine screen, and the median between last positive and first negative urine screens, as this was likely to be the most representative of renal fentanyl clearance. M = Mean, SD = Standard deviation.
4. Discussion
4.1. Physiological Considerations
The mean renal clearance time for fentanyl in OUD patients presenting to residential treatment was highly variable, yet consistently longer than the 2–4 day clearance of other short-acting opioids. Given that the majority of fentanyl clearance happens via fentanyl metabolites (Mather, 1983), the results of this study suggest that mean clearance is approximately two weeks, but might take four weeks or longer in some persons who use fentanyl regularly. Fentanyl is highly lipophilic and can be stored in adipocytes (Roy and Flynn, 1988; Pöyhiä and Seppälä, 1994), which likely accounts for the protracted clearance observed in this study. There is very little information available on the pharmacokinetic and pharmacodynamic properties of norfentanyl, but it is likely less lipophilic than fentanyl (Smith et al., 2018) given that norfentanyl loses a long carbon chain during fentanyl metabolization (National Center for Biotechnology Information, 2020). It is also unclear whether norfetnanyl acts on the mu-opioid receptor and what effect that might have on analgesic and euphorigenic outcomes.
Fentanyl was initially developed as an analgesic for acute pain treatment (Comer and Cahill, 2019), and not as an opioid that would be used repeatedly over longer periods of time. The transdermal fentanyl patch is an exception (Nelson and Schwaner, 2009), and while there are published data demonstrating that long-term fentanyl exposure results in high levels of fentanyl and norfentayl in urine (often higher levels than reported in overdose cases), and that urine concentrations of fentanyl and norfentanyl are highly variable between individuals on the same transdermal dose (Poklis and Backer, 2004), studies examining transdermal fentanyl clearance have focused on relatively short administration times (1–15 days) and a narrow window to collect clearance data (e.g. 72 hours) (Varvel et al., 1989; Portenoy et al., 1993). There are no published data, to our knowledge, regarding long-term fentanyl clearance in chronic pain patients who use long-term transdermal fentanyl therapy. Likewise, there are no published data, to our knowledge, on the degree to which low levels of fentanyl and norfentanyl might continue to exert meaningful effects for days or weeks since last use in persons with OUD who use fentanyl regularly. Nevertheless, the protracted renal clearance of high levels of fentanyl over the first 3–4 days since last use (see Figure 1), and low levels of fentanyl over the following days and weeks, is fundamentally different than the clearance patterns observed with other short-acting opioids, such as morphine and oxycodone (Pöyhiä and Seppälä, 1994).
4.2. Treatment Considerations
These findings have several implications for OUD treatment. There have been recent reports of difficulties with buprenorphine inductions for patients who regularly use fentanyl (Bisaga, 2019), including buprenorphine-precipitated withdrawal during standard buprenorphine induction procedures (Silverstein et al., 2019). Protracted fentanyl clearance likely contributes to this phenomenon, and may require extended periods of opioid abstinence prior to buprenorphine induction, which could be challenging for new patients. It is also possible that fentanyl affects longer-term buprenorphine outcomes, as a recent study reported that those testing positive versus negative for fentanyl had lower opioid abstinence and marginally lower retention after six months of buprenorphine treatment (Wakeman et al., 2019). Protracted fentanyl clearance might also extend opioid withdrawal and post-withdrawal sequalae, changing the length and trajectory of acute care for persons undergoing supervised withdrawal. Likewise, induction onto oral or extended-release naltrexone might result in precipitated withdrawal or adverse events that would not be experienced by persons using other opioids. Finally, there are possible legal implications if a person tested positive for fentanyl for weeks after their last use, and was considered to have more recent use (and then be in violation of probation or could lose employment opportunities due to such a positive drug screen).
4.3. Limitations
This study is limited by the small sample size and gap in time between urine sample collection. We did not collect blood samples to compare plasma to urine fentanyl and norfentanyl concentrations; previous studies have demonstrated that the cytochrome P450 family of genes (specifically CYP3A5*3) affects plasma levels of fentanyl but not urinary excretion in persons exposed to fentanyl for surgery (Tanaka et al., 2014). The current study points to an urgent need to better understand the pharmacokinetics of fentanyl elimination in persons with OUD, as well as the treatment implications for OUD patients experiencing protracted fentanyl clearance.
5. Conclusion
Fentanyl clearance may take several weeks for persons with OUD who are exposed to fentanyl on a daily basis. Fentanyl will likely continue to be prevalent in the illicit opioid supply due to black market economics (Frank and Pollack, 2017), and treatment providers should be aware that clearance of fentanyl is fundamentally different than other opioids and can have serious negative implications during early OUD treatment. Improved treatment strategies for persons with OUD should account for the unique pharmacokinetics of fentanyl in persons who use fentanyl regularly.
Highlights.
Many persons with opioid use disorder (OUD) are exposed to fentanyl daily.
Fentanyl clearance was examined in OUD patients in residential treatment.
Mean fentanyl clearance was 2 weeks, with a range of 4–26 days.
Protracted fentanyl clearance might affect withdrawal and medications for OUD.
Acknowledgments
Author Disclosures: The work described in this manuscript was funded by the National Institute on Drug Abuse, Bethesda, MD: NIDA UG3DA048734 (Huhn).
ASH receives research funding from Ashley Addiction Treatment through his university. ECS has served on advisory boards, received grant funding from, and/or consulted for Alkermes, Analgesic Solutions, Caron, Indivior Pharmaceuticals, Innocoll Pharmaceuticals, The Oak Group/VitalHub, UpToDate, Otsuka Pharmaceutical Development and Commercialization, and Pinney Associates. He has received honoraria from the World Health Organization, and is currently collaborating with Innovative Health Solutions. All other authors report no financial disclosures.
Abbreviations:
- OUD
Opioid use disorder
- THC
tetrahydrocannabinol
- IN
intranasal
- IV
intravenous
- LC-MS
liquid chromatography mass spectrometry
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bisaga A, 2019. What should Clinicians do as Fentanyl Replaces Heroin? Addiction. 14(5), 782. [DOI] [PubMed] [Google Scholar]
- Carroll JJ, Marshall BD, Rich JD, Green TC, 2017. Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: A mixed methods study. International Journal of Drug Policy. 46, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA, 2017. Increases in Self-Reported Fentanyl use among a Population Entering Drug Treatment: The Need for Systematic Surveillance of Illicitly Manufactured Opioids. Drug Alcohol Depend. 177, 101–103. [DOI] [PubMed] [Google Scholar]
- Comer SD, Cahill CM, 2019. Fentanyl: Receptor Pharmacology, Abuse Potential, and Implications for Treatment. Neuroscience & Biobehavioral Review. 106, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RG, Pollack HA, 2017. Addressing the Fentanyl Threat to Public Health. New England Journal of Medicine. 376(7), 605–607. [DOI] [PubMed] [Google Scholar]
- Green TC, Gilbert M, 2016. Counterfeit medications and fentanyl. JAMA Internal Medicine. 176, 1555–1557. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Milloy M, Lysyshyn M, DeBeck K, Nosova E, Wood E, Kerr T, 2018. Substance use patterns associated with recent exposure to fentanyl among people who inject drugs in Vancouver, Canada: a cross-sectional urine toxicology screening study. Drug Alcohol Depend. 183, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn TK, Liu Y, Mahapatro M, Ng K, 1999. Active transport of fentanyl by the blood-brain barrier. J Pharmacol Exp Ther. 289, 1084–1089. [PubMed] [Google Scholar]
- Huynh N, Tyrefors N, Ekman L, Johansson M, 2005. Determination of Fentanyl in Human Plasma and Fentanyl and Norfentanyl in Human Urine using LC–MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 37(5), 1095–1100. [DOI] [PubMed] [Google Scholar]
- Jones CM, Einstein EB, Compton WM, 2018. Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016. JAMA. 319, 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G, 2013. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin Pharmacokinet. 52, 23–36. [DOI] [PubMed] [Google Scholar]
- Macmadu A, Carroll JJ, Hadland SE, Green TC, Marshall BD, 2017. Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non-medically. Addict Behav. 68, 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather LE, 1983. Clinical pharmacokinetics of fentanyl and its newer derivatives. Clin Pharmacokinet. 8, 422–446. [DOI] [PubMed] [Google Scholar]
- Nelson L, Schwaner R, 2009. Transdermal Fentanyl: Pharmacology and Toxicology. Journal of Medical Toxicology. 5(4), 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochalek TA, Parker MA, Higgins ST, Sigmon SC, 2019. Fentanyl exposure among patients seeking opioid treatment. J Subst Abuse Treat. 96, 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis A, Backer R, 2004. Urine Concentrations of Fentanyl and Norfentanyl during Application of Duragesic® Transdermal Patches. Journal of Analytical Toxicology, 28(6), 422–425. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Southam MA, Gupta SK, Lapin J, Layman M, Inturrisi CE, Foley KM, 1993. Transdermal Fentanyl for Cancer Pain Repeated Dose Pharmacokinetics. Anesthesiology. 78(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Pöyhiä R, Seppälä T, 1994. Liposolubility and Protein Binding of Oxycodone in Vitro. Pharmacology & Toxicology, 74(1), 23–27. [DOI] [PubMed] [Google Scholar]
- Roy SD, Flynn GL, 1988. Solubility and Related Physicochemical Properties of Narcotic Analgesics. Springer. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Daniulaityte R, Martins SS, Miller SC, Carlson RG, 2019. “Everything is not right anymore”: Buprenorphine experiences in an era of illicit fentanyl. International Journal of Drug Policy. 74, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Coetzee JF, Fisher IW, Borts DJ, Mochel JP, 2018. Pharmacokinetics of Fentanyl Citrate and Norfentanyl in Holstein Calves and Effect of Analytical Performances on Fentanyl Parameter Estimation. Journal of Veterinary Pharmacology and Therapeutics. 41(4), 555–561. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Naito T, Yagi T, Doi M, Sato S, Kawakami J, 2014. Impact of CYP3A5* 3 on Plasma Exposure and Urinary Excretion of Fentanyl and Norfentanyl in the Early Postsurgical Period. Therapeutic Drug Monitoring. 36(3), 345–352. [DOI] [PubMed] [Google Scholar]
- Varvel JR, Shafer SL, Hwang SS, Coen PA, Stanski DR, 1989. Absorption Characteristics of Transdermally Administered Fentanyl. Anesthesiology. 70(6), 928–934. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, Chang Y, Regan S, Yu L, Flood J, Metlay J, Rigotti N, 2019. Impact of Fentanyl use on Buprenorphine Treatment Retention and Opioid Abstinence. Journal of Addiction Medicine. 13(4), 253–257 [DOI] [PubMed] [Google Scholar]