Abstract
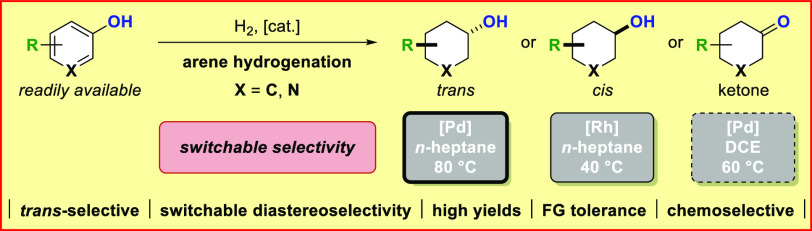
A trans-selective arene hydrogenation of abundant phenol derivatives catalyzed by a commercially available heterogeneous palladium catalyst is reported. The described method tolerates a variety of functional groups and provides access to a broad scope of trans-configurated cyclohexanols as potential building blocks for life sciences and beyond in a one-step procedure. The transformation is strategically important because arene hydrogenation preferentially delivers the opposite cis-isomers. The diastereoselectivity of the phenol hydrogenation can be switched to the cis-isomers by employing rhodium-based catalysts. Moreover, a protocol for the chemoselective hydrogenation of phenols to cyclohexanones was developed.
Keywords: arene hydrogenation, trans-selective, switchability, cyclohexanols, palladium catalysis
Arene hydrogenation is a powerful tool to transform simple, two-dimensional precursors into more complex, three-dimensional scaffolds.1 A plethora of readily available arenes and heteroarenes provided by established transformations (e.g., cross-coupling, aromatic substitution), offer potential access to a wide array of cyclic saturated motifs. The strategic importance of arene hydrogenation is exemplified by the industrial synthesis of cyclohexane2 and cyclohexene3 from benzene on multi-ton scale.
The field of arene hydrogenation has constantly evolved over the last several decades, and progress toward the stereo- and chemoselective hydrogenation of (hetero)aromatic compounds has been achieved. Chemoselective hydrogenation of arenes provides direct access to saturated carbo- and heterocycles containing reductively labile functional groups directly attached to the reactive center.1d,4 The competing hydrodefunctionalization pathway observed for fluorinated,4a−4c borylated4d,4e or silylated4f arenes can be limited by the choice of suitable reaction conditions. Additionally, the enantioselective hydrogenation of heteroarenes provides rapid access to chiral, saturated heterocycles through the controlled formation of stereocenters.5
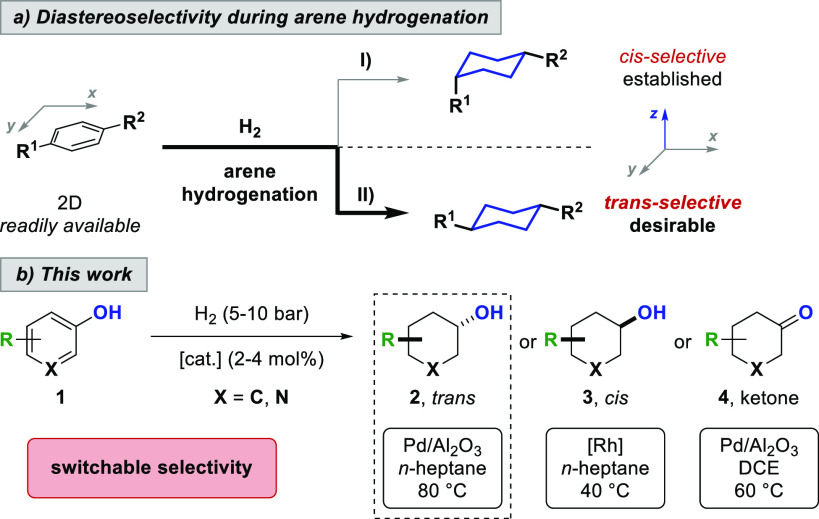
During the (chemoselective) hydrogenation of multisubstituted aromatic compounds, multiple stereocenters are formed in one step allowing for the formation of several product diastereomers. In general, the transition-metal catalyzed hydrogenation of arenes favors the corresponding all-cis configuration of the saturated analogues (Scheme 1a, path I).1,4 The cis-selectivity results from a fast, continuous hydrogenation of the substrate through a non-interrupted coordination to the catalyst. The corresponding trans-isomers require a π-facial exchange of the dearomatized diene or olefin intermediate via substrate desorption and readsorption to the catalyst, which is associated with binding to the sterically more hindered π-face. For this reason, the corresponding trans-isomers remain minor side products during arene hydrogenation.1,4 However, trans-isomers of multisubstituted saturated carbo- and heterocycles are desirable product motifs, and their synthesis starting from the corresponding cis-isomers is often limited, rendering a direct trans-selective hydrogenation of arenes desirable (Scheme 1a, path II).
Scheme 1. Diastereoselectivity in the Process of Arene Hydrogenation and Switchable (Stereo)selectivity in the Hydrogenation of Phenols.
Encouraged by a few rare examples with diminished cis-selectivity during our previous studies on the rhodium cyclic (alkyl)(amino)carbene (Rh–CAAC)-catalyzed chemoselective hydrogenation of arenes,4 we sought to investigate the underexplored and limited trans-selective hydrogenation of arenes.6 Especially unprotected phenols gave a relatively high ratio of the “undesired” trans-diastereomer in our previous rhodium-catalyzed studies. The hydrogenation of phenol is an important industrial process for the synthesis of cyclohexanone and cyclohexanols as the intermediates for Nylon-6 and 66.2,7 Substituted cyclohexanols are also extensively used as valuable chemical feedstock and synthetic intermediates in the pharmaceutical, petrochemical, and fine chemical industry. Moreover, the resulting hydroxyl group provides a diverse synthetic handle for further functionalization of the products.
Herein, we report the first comprehensive study toward a trans-selective hydrogenation of arenes. trans-Cyclohexanols are synthesized from abundant phenols in a one-step reaction that tolerates a variety of functional groups (Scheme 1b).
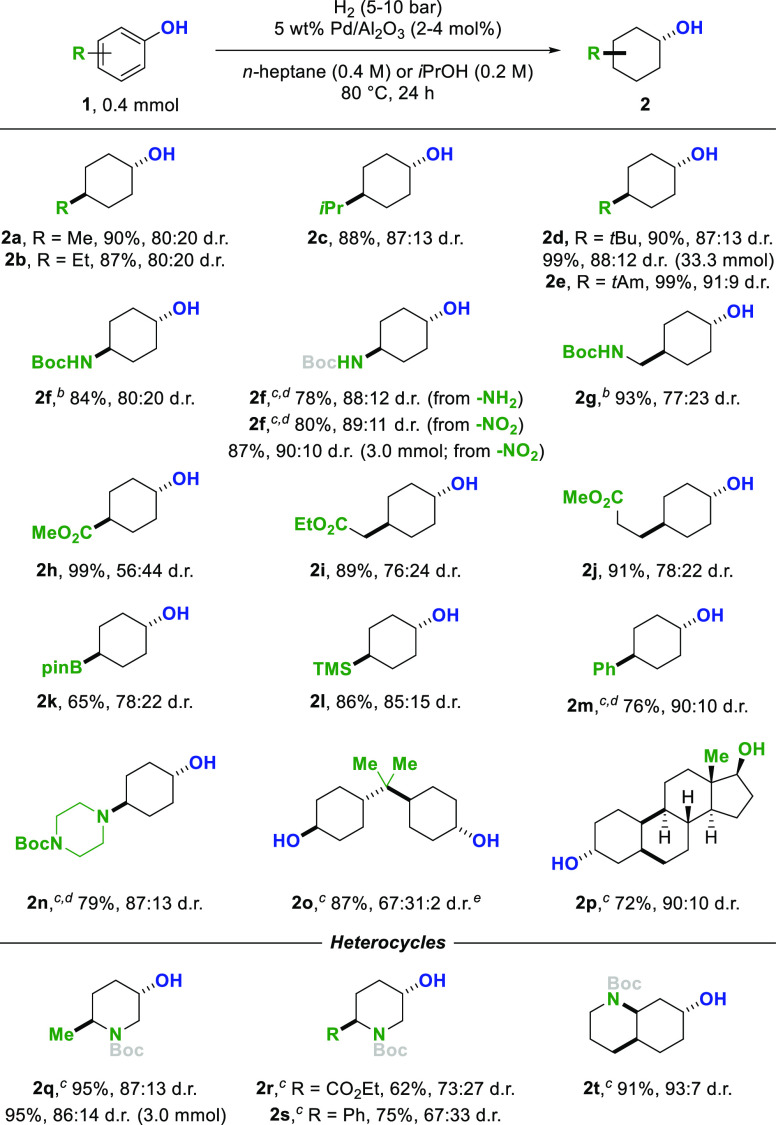
We commenced our studies by investigating different transition-metal based catalysts in the hydrogenation of p-cresol. When switching from rhodium-based catalysts to heterogeneous palladium catalysts, we noticed an inversion in the diastereoselectivity of the reaction. Investigating the efficacy of different palladium catalysts in the reaction revealed that a 5 wt % Pd/Al2O3 system had the best selectivity toward the desired trans-cyclohexanol product, accompanied by only trace amounts of the unwanted cyclohexanone intermediate. After optimization, employing commercially available 5 wt % Pd/Al2O3 in n-heptane as the solvent, under a low hydrogen pressure of 5 bar at 80 °C provided the best trans-diastereoselectivity, yield, and chemoselectivity of the reaction. Hydrogenation of p-cresol furnished 4-methylcyclohexanol 2a in 90% yield and 80:20 diastereomeric ratio (d.r., trans:cis).8 To evaluate the sensitivity of our developed protocol, we conducted a reaction-condition-based sensitivity screen.9 The influence on yield and diastereoselectivity was investigated by systematic variation of key reaction parameters (see Supporting Information).
With the optimized conditions in hand, we began investigating the scope of the reaction (Scheme 2). The hydrogenation of phenols bearing aliphatic substituents (2a–e) provided the corresponding trans-cyclohexanols in high yields. With increasing steric bulk in para-position, the diastereomeric ratio increased from 80:20 d.r. to 91:9 d.r. In addition, 4-tert-butylcyclohexanol 2d was obtained in quantitative yield and 88:12 d.r. after scale-up to 5.0 g scale (33.3 mmol). Boc-protected p-aminophenol was hydrogenated to protected 4-aminocyclohexanol (2f) in 84% yield and 80:20 d.r. By switching the solvent from n-heptane to polar isopropanol and adding K2CO3 as base, the unprotected p-aminophenol could be hydrogenated to 2f in high yield and with an increased diastereoselectivity of 88:12. Boc-protected trans-4-aminocyclohexanol could also be obtained after hydrogenation and protection of p-nitrophenol while reducing the nitro group and arene in a one-step procedure. Moreover, different ester substituents were tolerated furnishing trans-cyclohexanols with protected acids (2h−j). Aryl boronic acid derivative 1k was hydrogenated giving access to the synthetically valuable trans-configurated organoboron compound 2k. Additionally the organosilicon analogue 2l could be obtained in 86% yield and 85:15 d.r. Chemoselective hydrogenation of the more substituted phenol ring compared to the phenyl substituent in p-phenylphenol delivered 2m in 76% yield and 90:10 d.r. Additionally, a Boc-protected piperazine substituent was tolerated, giving the trans-configurated building block 2n with two orthogonal sites available for further functionalization. The hydrogenation of bisphenol A, a common precursor for polycarbonates, provided compound 2o bearing two trans-configurated cyclohexanols as the major isomer. Showcased by products 2a–2o, a wide array of achiral building blocks with potential applications in pharmaceutical sciences could be synthesized. Moreover, the female sex hormone estradiol could be transformed into the corresponding saturated analogue 2p in 72% combined yield and 90:10 d.r.
Scheme 2. Substrate Scope for the trans-Selective Hydrogenation of Phenols.
Combined yields of isolated product after column chromatography are given. The d.r. values were determined by GC-MS or 1H NMR analysis prior to purification. Piperidines and amines were trapped with Boc2O prior to isolation. For details, see Supporting Information.
48 h.
iPrOH as solvent.
K2CO3 as additive.
The ratio is trans/trans:trans/cis:cis/cis.
Since saturated heterocycles are an important structural motif, we extended the scope of the trans-selective hydrogenation to different heteroarenes (2q–t). By switching to isopropanol as polar solvent, poisoning of the palladium catalyst could be prevented.10,1h Hydroxypyridine 1q was hydrogenated to the corresponding piperidine in 95% yield and 87:13 d.r. The reactivity and selectivity were preserved after scale-up of the developed method to 3 mmol. Reductively labile groups like esters (2r) and phenyl substituents (2s) were tolerated, enabling a trans- and chemoselective hydrogenation of pyridines. Additionally, quinoline derivative 1t was trans-selectively hydrogenated, furnishing the perhydro product in 91% yield and 93:7 diastereomeric ratio. In almost all cases, the major diastereomer could be separated by column chromatography, providing access to the diastereomerically pure trans-configurated (heterocyclic) cyclohexanol derivatives with a broad variety of functional groups. The trans-selectivity of the hydrogenation was confirmed by X-ray diffraction analysis of products 2f, 2m, 2o, and 2q. Furthermore, the configuration of the majority of products were confirmed by NMR analysis.8
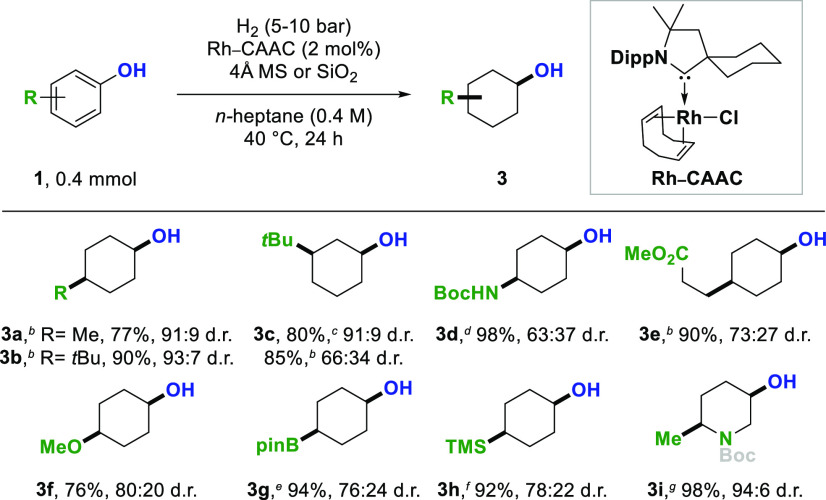
In many cases both product diastereomers of a newly developed method are of synthetic interest. However, switching the diastereoselectivity of established and newly developed protocols often remains non-trivial, and long synthetic routes are necessary. The cis-selective hydrogenation of phenols is frequently limited by poor functional group tolerance and cleavage of the carbon–oxygen bond by hydrogenolysis when employing standard heterogeneous catalysts (e.g., Rh/C, Rh/Al2O3) or [Rh(COD)Cl]2 in combination with various catalyst supports.11,6d When using Rh–CAAC as a precatalyst12 and 4 Å molecular sieves or silica gel as catalyst support, we could switch the diastereoselectivity to the corresponding cis-diastereomers without the undesired hydrogenolysis (Scheme 3).
Scheme 3. Substrate Scope for the cis-Selective Hydrogenation of Phenols.
Combined yields of isolated product after column chromatography are given. The d.r. values were determined by GC-MS or 1H NMR analysis prior to purification. Piperidines were trapped with Boc2O prior to isolation. For details, see Supporting Information. CAAC = cyclic (alkyl)(amino)carbene. Dipp = 2,6-diisopropylphenyl.
[Rh(COD)Cl]2 (2 mol %).
5 wt % Pd/Al2O3 (4 mol %).
48 h.
H2 (50 bar).
H2 (20 bar).
iPrOH as solvent.
Phenols with simple alkyl chains could be hydrogenated using [Rh(COD)Cl]2 as a catalyst. The hydrogenation of p-cresol and p-tert-butylphenol provided the corresponding cyclohexanols 3a and 3b in high yields and diastereoselectivities. m-tert-Butylphenol was hydrogenated and 3-tert-butylcyclohexanol 3c was obtained in 85% yield and 66:34 d.r. (cis:trans) when using [Rh(COD)Cl]2 as a catalyst. The diastereoselectivity of the reaction could be increased to 91:9 d.r. when using palladium on alumina instead. For phenols decorated with more labile functional groups, the Rh–CAAC system was used. Boc-protected cis-4-aminocyclohexanol 3d and cyclohexanol 3e with an ester substituent were obtained in excellent yields. Moreover, ether (3f), organoboron (3g), and organosilicon (3h) functionalities were well tolerated, rendering cis-configurated cyclohexanols with various functional groups accessible. The cis-selective hydrogenation protocol could be extended to pyridines, demonstrated by the hydrogenation of hydroxypyridine 2i. When using [Rh(COD)Cl]2 as the catalyst undesired hydrogenolysis of functional groups was observed. For example, cleavage of the carbon–oxygen bond (3f), carbon–silicon bond (3h), and hydrodeborylation (3g) could be detected (see Supporting Information for more details).
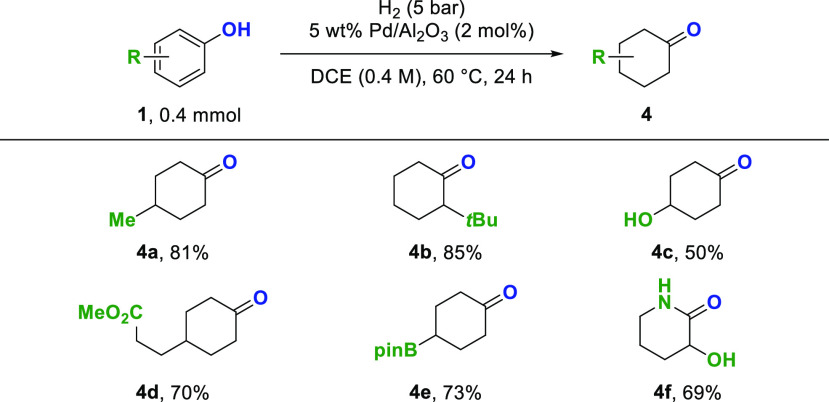
The chemoselective hydrogenation of phenols to cyclohexanones has been focus of different studies.13,4g The major challenge associated with chemoselective phenol hydrogenation is preventing the over-reduction of the reductively more labile carbonyl group. Herein, we present a simple variation of our protocol by using the same, commercially available 5 wt % Pd/Al2O3 as catalyst, 5 bar of hydrogen, and 1,2-dichloroethane (DCE) as solvent to target this challenge (Scheme 4). Hydrogenation of p-cresol and o-tert-butylphenol in DCE provided 4-methylcyclohexanone 4a in 81% yield and 2-tert-butylcyclohexanone 4b in 85% yield.
Scheme 4. Substrate Scope for the Ketone-Selective Hydrogenation of Phenols.
Yields of isolated product after column chromatography are given. DCE = 1,2-dichloroethane.
During the hydrogenation of hydroquinone, reduction of one carbonyl group of the diketo intermediate was observed, and 4-hydroxycyclohexanone 4c was obtained as the major product in 50% yield. Reductively labile groups like esters (4d) and pinacol boronic esters (4e) were tolerated, and the cyclohexanone derivatives were obtained in 70% and 73% yield, respectively. The major side products during the chemoselective hydrogenation of phenols to cyclohexanones were the corresponding cyclohexanols (see Supporting Information).
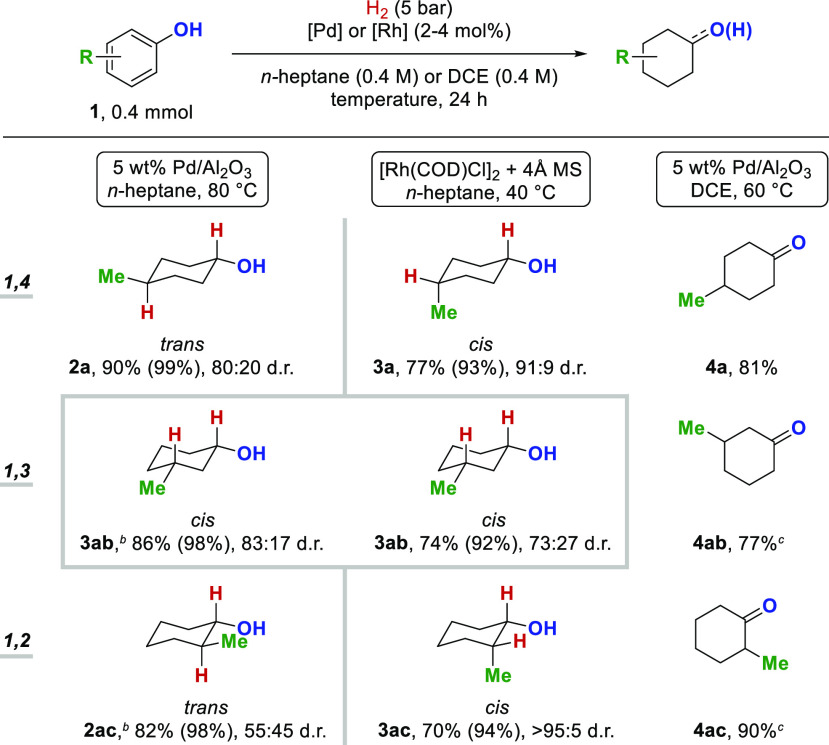
Having established three valuable protocols for the diastereo- and chemoselective hydrogenation of phenols, we sought to investigate the influence of the substitution pattern on reactivity and diastereoselectivity (Scheme 5). During the palladium-catalyzed hydrogenation of p-cresol, the thermodynamically more stable trans-4-methylcyclohexanol 2a was obtained as the major diastereomer. When employing a rhodium-based catalyst, the diastereoselectivity was inverted to the thermodynamically less stable cis-4-methylcyclohexanol 3a. When changing the substrate from p-cresol to m-cresol, cis-3-methylcyclohexanol 3ab was obtained as the major product for both catalytic systems (Pd and Rh). The same selectivity was previously observed in the hydrogenation of m-tert-butylphenol (Scheme 3). The use of palladium on alumina as the catalyst furnished a higher ratio of the thermodynamically more stable cis-diastereomer in the case of 1,3-disubsituted cycloalkanes. The palladium-catalyzed hydrogenation of o-cresol provided trans-2-cyclohexanol 2ac with low diastereoselectivity (55:45 d.r.), although trans-1,2-disubstituted cycloalkanes are thermodynamically favored. When employing the rhodium-based catalytic system, an excellent diastereoselectivity of >95:5 d.r. for cis-2-methylcyclohexanol 3ac was observed. The chemoselective hydrogenation of phenols to cyclohexanones showed no significant influence caused by the substitution pattern, giving access to the volatile methylcyclohexanones 4a–4ac in good yields. The catalytic system based on rhodium provides products generated by the all-cis addition1,4 of hydrogen atoms to the substrates, whereas the catalytic system based on palladium gives access to the thermodynamically more stable diastereomers.
Scheme 5. Substitution Pattern and Diastereoselectivity for the Hydrogenation of Phenols.
Combined yields of isolated product after column chromatography are given. Diastereoselectivity and yields in parentheses were determined by 1H NMR analysis. For details, see Supporting Information.
H2 (10 bar).
Yield determined by GC-FID analysis.
The proposed mechanism for the heterogeneous hydrogenation of phenols to cyclohexanols involves partially hydrogenated cyclohexenols (enol-forms) and the corresponding cyclohexanones (keto–enol tautomerism).14 The trans-isomers are believed to be formed through desorption and readsorption of intermediate cyclohexanone/cyclohexenol species (π-facial exchange) followed by the cis-addition of hydrogen to enol intermediates.1d,15
Mechanistic experiments showed a fast consumption of the starting material and formation of 4-tert-butylcyclohexanone as an intermediate (see Supporting Information for more details). However, direct hydrogenation of 4-tert-butylcyclohexanone resulted in reduced diastereoselectivity (67:33 d.r.), compared with the hydrogenation of p-tert-butylphenol (87:13 d.r.). Hydrogenation of “diene” intermediates (e.g., 4-tert-butylcyclohex-2-en-1-one and 4-tert-butylcyclohex-3-en-1-one) resulted in roughly the same diastereoselectivity as the direct hydrogenation of p-tert-butylphenol. Diene intermediates could never be observed because of the fast hydrogenation of alkene double bonds. Key to the increased trans-selectivity during phenol hydrogenation could be the low concentration of the cyclohexanone intermediate, which disfavors the slow, direct ketone hydrogenation, forming the minor cis-isomer. The trans-isomer could be formed through a desorption and readsorption process of diene and enol intermediates, which is facilitated through keto–enol tautomerism. Deuteration studies show deuterium scrambling at the 2-position, supporting the rapid interconversion of keto and enol intermediates on the catalyst surface. In addition, increasing steric bulk in 4-position as well as the high reaction temperature favor the process of desorption and readsorption (π-facial exchange) and therefore increase the ratio of trans-products. Moreover, diastereomerically pure trans- and cis-4-tert-butylcyclohexanols (>99:1 d.r.) were subjected to the standard conditions, and no isomerization was observed, excluding a thermodynamically driven isomerization process.
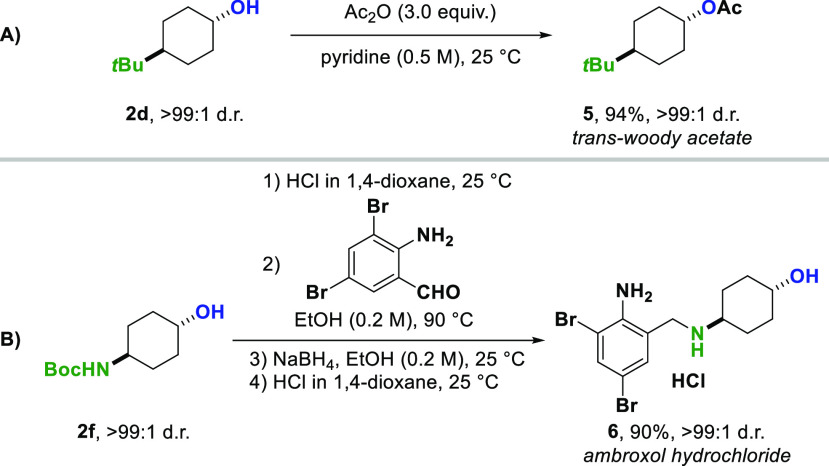
Having established an efficient and versatile method for the trans-selective hydrogenation of phenols, we sought to demonstrate the synthetic utility of the obtained products (Scheme 6). Acetylation of trans-4-tert-butylcyclohexanol 2d provided the trans-isomer of woody acetate 5, a fragrance ingredient used in cosmetics.16 Furthermore, ambroxol, a mucolytic agent used in the treatment of respiratory diseases, was synthesized.17 Starting from inexpensive p-nitrophenol, Boc-protected trans-4-aminocyclohexanol 2f was obtained in 87% yield and 90:10 d.r. on a 3 mmol scale in one step (Scheme 2). After separation of the diastereomers on silica gel, treatment of 2f with HCl in 1,4-dioxane provided diastereomerically pure 4-trans-aminocyclohexanol. Imine formation with 2-amino-3,5-dibromobenzaldehyde, followed by NaBH4 reduction and treatment with HCl gave access to ambroxol hydrochloride in 90% yield over four steps (Scheme 6B).
Scheme 6. Synthetic Applications of trans-Cyclohexanols.
For experimental details, see Supporting Information.
In conclusion, we have developed three sets of reaction conditions for the hydrogenation of abundant phenols providing access to either trans- or cis-configurated cyclohexanols, as well as cyclohexanones. The trans-selective hydrogenation of phenols is catalyzed by heterogeneous palladium on alumina and tolerates a variety of functional groups, giving access to building blocks with the opposite diastereoselectivity preferentially generated through the hydrogenation of arenes. The diastereoselectivity was inverted by employing rhodium-based catalysts and a simple and practical method for the chemoselective hydrogenation of phenols to cyclohexanones was established.
Acknowledgments
Generous financial support by the Deutsche Forschungsgemeinschaft (IRTG 2027 Münster-Toronto) and the European Research Council (ERC Advanced Grant Agreement No. 788558) are gratefully acknowledged. The authors thank Daniel Moock, Tobias Wagener (both WWU Münster) and Austin D. Marchese (University of Toronto) for helpful discussions. We also thank Dr. Constantin G. Daniliuc (WWU Münster) for X-ray crystallographic analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.0c03423.
Author Contributions
‡ (M.W., A.H.) These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews on arene hydrogenation, see:; a Wiesenfeldt M. P.; Nairoukh Z.; Dalton T.; Glorius F. Selective Arene Hydrogenation for Direct Access to Saturated Carbo- and Heterocycles. Angew. Chem., Int. Ed. 2019, 58, 10460. 10.1002/anie.201814471. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Giustra Z. X.; Ishibashi J. S. A.; Liu S.-Y. Homogeneous Metal Catalysis for Conversion between Aromatic and Saturated Compounds. Coord. Chem. Rev. 2016, 314, 134. 10.1016/j.ccr.2015.11.006. [DOI] [Google Scholar]; c Gualandi A.; Savoia D. Substrate Induced Diastereoselective Hydrogenation/Reduction of Arenes and Heteroarenes. RSC Adv. 2016, 6, 18419. 10.1039/C5RA23908G. [DOI] [Google Scholar]; d Qi S.-C.; Wei X.-Y.; Zong Z.-M.; Wang Y.-K. Application of Supported Metallic Catalysts in Catalytic Hydrogenation of Arenes. RSC Adv. 2013, 3, 14219. 10.1039/c3ra40848e. [DOI] [Google Scholar]; e Gual A.; Godard C.; Castillón S.; Claver C. Soluble Transition-Metal Nanoparticles-Catalysed Hydrogenation of Arenes. Dalton Trans. 2010, 39, 11499. 10.1039/c0dt00584c. [DOI] [PubMed] [Google Scholar]; Soluble Transition-Metal Nanoparticles-Catalysed Hydrogenation of Arenes. For books on arene hydrogenation, see:; f Foubelo F.; Yus M.. Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; Mortier J., Ed.; Wiley: Hoboken, 2016; p 337. [Google Scholar]; g Bianchini C.; Meli A.; Vizza F.. The Handbook of Homogeneous Hydrogenation; de Vries J. G.; Elsevier C. J., Eds.; Wiley-VCH: Weinheim, 2006; p 455. [Google Scholar]; h Nishimura S.Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; John Wiley & Sons: New York, 2001; p 414. [Google Scholar]; For selected reviews on the more general dearomatization of arenes, see:; i Wertjes W. C.; Southgate E. H.; Sarlah D. Recent Advances in Chemical Dearomatization of Nonactivated Arenes. Chem. Soc. Rev. 2018, 47, 7996. 10.1039/C8CS00389K. [DOI] [PubMed] [Google Scholar]; j Huck C. J.; Sarlah D. Shaping Molecular Landscapes: Recent Advances, Opportunities, and Challenges in Dearomatization. Chem. 2020, 6, 1589. 10.1016/j.chempr.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; k You S.-L., Ed., Asymmetric Dearomatization Reactions; Wiley-VCH: Weinheim, 2016. [Google Scholar]
- Weissermel K.; Arpe H.-J.. Industrial Organic Chemistry; Wiley-VCH: Weinheim, 2008; p 337. [Google Scholar]
- a Foppa L.; Dupont J. Benzene Partial Hydrogenation: Advances and Perspectives. Chem. Soc. Rev. 2015, 44, 1886. 10.1039/C4CS00324A. [DOI] [PubMed] [Google Scholar]; b Nagahara H.; Ono M.; Konishi M.; Fukuoka Y. Partial Hydrogenation of Benzene to Cyclohexene. Appl. Surf. Sci. 1997, 121–122, 448. 10.1016/S0169-4332(97)00325-5. [DOI] [Google Scholar]
- a Wiesenfeldt M. P.; Nairoukh Z.; Li W.; Glorius F. Hydrogenation of Fluoroarenes: Direct Access to all-cis-(Multi)fluorinated Cycloalkanes. Science 2017, 357, 908. 10.1126/science.aao0270. [DOI] [PubMed] [Google Scholar]; b Nairoukh Z.; Wollenburg M.; Schlepphorst C.; Bergander K.; Glorius F. The Formation of all-cis-(Multi)fluorinated Piperidines by a Dearomatization–Hydrogenation Process. Nat. Chem. 2019, 11, 264. 10.1038/s41557-018-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang X.; Ling L.; Luo M.; Zeng X. Accessing Difluoromethylated and Trifluoromethylated cis-Cycloalkanes and Saturated Heterocycles: Preferential Hydrogen Addition to the Substitution Sites for Dearomatization. Angew. Chem., Int. Ed. 2019, 58, 16785. 10.1002/anie.201907457. [DOI] [PubMed] [Google Scholar]; d Wollenburg M.; Moock D.; Glorius F. Hydrogenation of Borylated Arenes. Angew. Chem., Int. Ed. 2019, 58, 6549. 10.1002/anie.201810714. [DOI] [PubMed] [Google Scholar]; e Ling L.; He Y.; Zhang X.; Luo M.; Zeng X. Hydrogenation of (Hetero)aryl Boronate Esters with a Cyclic (Alkyl)(amino)carbene–Rhodium Complex: Direct Access to cis-Substituted Borylated Cycloalkanes and Saturated Heterocycles. Angew. Chem., Int. Ed. 2019, 58, 6554. 10.1002/anie.201811210. [DOI] [PubMed] [Google Scholar]; f Wiesenfeldt M. P.; Knecht T.; Schlepphorst C.; Glorius F. Silylarene Hydrogenation: A Strategic Approach that Enables Direct Access to Versatile Silylated Saturated Carbo- and Heterocycles. Angew. Chem., Int. Ed. 2018, 57, 8297. 10.1002/anie.201804124. [DOI] [PubMed] [Google Scholar]; g Wei Y.; Rao B.; Cong X.; Zeng X. Highly Selective Hydrogenation of Aromatic Ketones and Phenols Enabled by Cyclic (Amino)(alkyl)carbene Rhodium Complexes. J. Am. Chem. Soc. 2015, 137, 9250. 10.1021/jacs.5b05868. [DOI] [PubMed] [Google Scholar]
- a For reviews on enantioselective hydrogenation of (hetero)arenes, see:Zhao D.; Candish L.; Paul D.; Glorius F.. N-Heterocyclic Carbenes in Asymmetric Hydrogenation. ACS Catal. 2016, 6, 5978. [Google Scholar]; b He Y.-M.; Song F.-T.; Fan Q.-H. Advances in Transition Metal-Catalyzed Asymmetric Hydrogenation of Heteroaromatic Compounds. Top. Curr. Chem. 2013, 343, 145. 10.1007/128_2013_480. [DOI] [PubMed] [Google Scholar]; c Wang D.-S.; Chen Q.-A.; Lu S.-M.; Zhou Y.-G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557. 10.1021/cr200328h. [DOI] [PubMed] [Google Scholar]; d Glorius F. Asymmetric Hydrogenation of Aromatic Compounds. Org. Biomol. Chem. 2005, 3, 4171. 10.1039/b512139f. [DOI] [PubMed] [Google Scholar]
- Only few limited examples of trans-selective arene hydrogenation (trans/cis = >50/<50) with scarce scope entries have been reported in the literature:; a Murugesan K.; Senthamarai T.; Alshammari A. S.; Altamimi R. M.; Kreyenschulte C.; Pohl M.-M.; Lund H.; Jagadeesh R. V.; Beller M. Cobalt-Nanoparticles Catalyzed Efficient and Selective Hydrogenation of Aromatic Hydrocarbons. ACS Catal. 2019, 9, 8581. 10.1021/acscatal.9b02193. [DOI] [Google Scholar]; b Li H.; Wang Y.; Lai Z.; Huang K.-W. Selective Catalytic Hydrogenation of Arenols by a Well-Defined Complex of Ruthenium and Phosphorus–Nitrogen PN3–Pincer Ligand Containing a Phenanthroline Backbone. ACS Catal. 2017, 7, 4446. 10.1021/acscatal.7b01316. [DOI] [Google Scholar]; c Tungler A.; Szabados E. Overcoming Problems at Elaboration and Scale-up of Liquid-Phase Pd/C Mediated Catalytic Hydrogenations in Pharmaceutical Production. Org. Process Res. Dev. 2016, 20, 1246. 10.1021/acs.oprd.6b00073. [DOI] [Google Scholar]; d Maegawa T.; Akashi A.; Yaguchi K.; Iwasaki Y.; Shigetsura M.; Monguchi Y.; Sajiki H. Efficient and Practical Arene Hydrogenation by Heterogeneous Catalysts under Mild Conditions. Chem. - Eur. J. 2009, 15, 6953. 10.1002/chem.200900361. [DOI] [PubMed] [Google Scholar]; e Jansat S.; Picurelli D.; Pelzer K.; Philippot K.; Gómez M.; Muller G.; Lecante P.; Chaudret B. Synthesis, Characterization and Catalytic Reactivity of Ruthenium Nanoparticles Stabilized by Chiral N-Donor Ligands. New J. Chem. 2006, 30, 115. 10.1039/B509378C. [DOI] [Google Scholar]; f Yadav G. D.; Goel P. K. Stereoselective Hydrogenation of p-tert-Butylphenol over Supported Rhodium Catalyst. J. Mol. Catal. A: Chem. 2002, 184, 281. 10.1016/S1381-1169(02)00009-2. [DOI] [Google Scholar]
- a Van de Vyver S.; Román-Leshkov Y. Emerging Catalytic Processes for the Production of Adipic Acid. Catal. Sci. Technol. 2013, 3, 1465. 10.1039/C3CY20728E. [DOI] [Google Scholar]; b Musser M. T.Cyclohexanol and Cyclohexanone. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2011. [Google Scholar]
- See the Supporting Information for experimental details, full optimization data, and structural elucidation.
- Pitzer L.; Schäfers F.; Glorius F. Rapid Assessment of the Reaction-Condition-Based Sensitivity of Chemical Transformations. Angew. Chem., Int. Ed. 2019, 58, 8572. 10.1002/anie.201901935. [DOI] [PubMed] [Google Scholar]
- a Bartholomew C. H. Mechanisms of Catalyst Deactivation. Appl. Catal., A 2001, 212, 17. 10.1016/S0926-860X(00)00843-7. [DOI] [Google Scholar]; b Forzatti P.; Lietti L. Catalyst Deactivation. Catal. Today 1999, 52, 165. 10.1016/S0920-5861(99)00074-7. [DOI] [Google Scholar]
- a Shu R.; Li R.; Lin B.; Wang C.; Cheng Z.; Chen Y. A Review on the Catalytic Hydrodeoxygenation of Lignin-Derived Phenolic Compounds and the Conversion of Raw Lignin to Hydrocarbon Liquid Fuels. Biomass Bioenergy 2020, 132, 105432. 10.1016/j.biombioe.2019.105432. [DOI] [Google Scholar]; b Shafaghat H.; Rezaei P. S.; Ashri Wan Daud W. M. Effective Parameters on Selective Catalytic Hydrodeoxygenation of Phenolic Compounds of Pyrolysis Bio-Oil to High-Value Hydrocarbons. RSC Adv. 2015, 5, 103999. 10.1039/C5RA22137D. [DOI] [Google Scholar]; c Saidi M.; Samimi F.; Karimipourfard D.; Nimmanwudipong T.; Gates B. C.; Rahimpour M. R. Upgrading of Lignin-Derived Bio-Oils by Catalytic Hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103. 10.1039/C3EE43081B. [DOI] [Google Scholar]; d Wang D.-W.; Lu S.-M.; Zhou Y.-G. A Simple and Highly Effective Method for Hydrogenation of Arenes by [Rh(COD)Cl]2. Tetrahedron Lett. 2009, 50, 1282. 10.1016/j.tetlet.2008.12.108. [DOI] [Google Scholar]; e Hiyoshi N.; Bando K. K.; Sato O.; Yamaguchi A.; Rode C. V.; Shirai M. Stereoselective Hydrogenation of 4-Alkylphenols over Carbon-Supported Rhodium Catalyst in Supercritical Carbon Dioxide Solvent. Catal. Commun. 2009, 10, 1702. 10.1016/j.catcom.2009.05.011. [DOI] [Google Scholar]
- For mechanistic analysis on the Rh(CAAC)-catalyzed arene hydrogenation, see:; a Moock D.; Wiesenfeldt M. P.; Freitag M.; Muratsugu S.; Ikemoto S.; Knitsch R.; Schneidewind J.; Baumann W.; Schäfer A. H.; Timmer A.; Tada M.; Hansen M. R.; Glorius F. Mechanistic Understanding of the Heterogeneous, Rhodium-Cyclic (Alkyl)(Amino)Carbene-Catalyzed (Fluoro-)Arene Hydrogenation. ACS Catal. 2020, 10, 6309. 10.1021/acscatal.0c01074. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tran B. L.; Fulton J. L.; Linehan J. C.; Balasubramanian M.; Lercher J. A.; Bullock R. M. Operando XAFS Studies on Rh(CAAC)-Catalyzed Arene Hydrogenation. ACS Catal. 2019, 9, 4106. 10.1021/acscatal.8b04929. [DOI] [Google Scholar]; c Tran B. L.; Fulton J. L.; Linehan J. C.; Lercher J. A.; Bullock R. M. Rh(CAAC)-Catalyzed Arene Hydrogenation: Evidence for Nanocatalysis and Sterically Controlled Site-Selective Hydrogenation. ACS Catal. 2018, 8, 8441. 10.1021/acscatal.8b02589. [DOI] [Google Scholar]
- For selected references on the chemoselective hydrogenation of phenols to cyclohexanones, see:; a Kong X.; Gong Y.; Mao S.; Wang Y. Selective Hydrogenation of Phenol.. ChemNanoMat 2018, 4, 432. 10.1002/cnma.201800031. [DOI] [Google Scholar]; b Wei Y.; Zeng X. Catalytic Strategies toward Selective Hydrogenation of Aromatic Ketones and Phenols: Facile Synthesis of Cyclohexyl Ketones. Synlett 2016, 27, 650. 10.1055/s-0035-1560382. [DOI] [Google Scholar]; c Zhong J.; Chen J.; Chen L. Selective Hydrogenation of Phenol and Related Derivatives. Catal. Sci. Technol. 2014, 4, 3555. 10.1039/C4CY00583J. [DOI] [Google Scholar]; d Nelson N. C.; Manzano J. S.; Sadow A. D.; Overbury S. H.; Slowing I. I. Selective Hydrogenation of Phenol Catalyzed by Palladium on High-Surface-Area Ceria at Room Temperature and Ambient Pressure. ACS Catal. 2015, 5, 2051. 10.1021/cs502000j. [DOI] [Google Scholar]; e Snelders D. J. M.; Yan N.; Gan W.; Laurenczy G.; Dyson P. J. Tuning the Chemoselectivity of Rh Nanoparticle Catalysts by Site-Selective Poisoning with Phosphine Ligands: The Hydrogenation of Functionalized Aromatic Compounds. ACS Catal. 2012, 2, 201. 10.1021/cs200575r. [DOI] [Google Scholar]; f Wang Y.; Yao J.; Li H.; Su D.; Antonietti M. Highly Selective Hydrogenation of Phenol and Derivatives over a Pd@Carbon Nitride Catalyst in Aqueous Media. J. Am. Chem. Soc. 2011, 133, 2362. 10.1021/ja109856y. [DOI] [PubMed] [Google Scholar]; g Liu H.; Jiang T.; Han B.; Liang S.; Zhou Y. Selective Phenol Hydrogenation to Cyclohexanone Over a Dual Supported Pd–Lewis Acid Catalyst. Science 2009, 326, 1250. 10.1126/science.1179713. [DOI] [PubMed] [Google Scholar]
- a Hiyoshi N.; Rode C. V.; Sato O.; Tetsuka H.; Shirai M. Stereoselective Hydrogenation of tert-Butylphenols over Charcoal-Supported Rhodium Catalyst in Supercritical Carbon Dioxide Solvent. J. Catal. 2007, 252, 57. 10.1016/j.jcat.2007.08.011. [DOI] [Google Scholar]; b Konuspaev S. R.; Zhanbekov K. N.; Kul’kova N. V.; Murzin D. Y. Kinetics of 4-tert-Butylphenol Hydrogenation over Rhodium. Chem. Eng. Technol. 1997, 20, 144. 10.1002/ceat.270200212. [DOI] [Google Scholar]; c Smith H. A.; Stump B. L. A Study of the Catalytic Hydrogenation of Hydroxybenzenes over Platinum and Rhodium Catalysts. J. Am. Chem. Soc. 1961, 83, 2739. 10.1021/ja01473a032. [DOI] [Google Scholar]
- a Weitkamp A. W. Stereochemistry and Mechanism of Hydrogenation of Naphthalenes on Transition Metal Catalysts and Conformational Analysis of the Products. Adv. Catal. 1968, 18, 1. 10.1016/S0360-0564(08)60428-9. [DOI] [Google Scholar]; b Siegel S.; Smith G. V.; Dmuchovsky B.; Dubbell D.; Halpern W. The Stereochemistry of the Hydrogenation of the Isomeric Xylenes and p-tert-Butyltoluene over a Platinum Catalyst. J. Am. Chem. Soc. 1962, 84, 3136. 10.1021/ja00875a020. [DOI] [Google Scholar]
- Brown G.; Mangan D.; Miskelly I.; Moody T. S. A Facile Stereoselective Biocatalytic Route to the Precursor of Woody Acetate. Org. Process Res. Dev. 2011, 15, 1036. 10.1021/op200166a. [DOI] [Google Scholar]
- a Weiser T.; Wilson N. Inhibition of Tetrodotoxin (TTX)-Resistant and TTX-Sensitive Neuronal Na+ Channels by the Secretolytic Ambroxol. Mol. Pharmacol. 2002, 62, 433. 10.1124/mol.62.3.433. [DOI] [PubMed] [Google Scholar]; b Latli B.; Hrapchak M.; Switek H.-K.; Retz D. M.; Krishnamurthy D.; Senanayake C. H. Synthesis of Labeled Ambroxol and its Major Metabolites. J. Labelled Compd. Radiopharm. 2010, 53, 15. 10.1002/jlcr.1694. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.