Abstract
Background:
VRC01 is a human IgG1 broadly neutralizing antibody (bnAb) that binds to the HIV-1 envelope glycoprotein. It is being evaluated in two ongoing efficacy trials, the first assessment of a passively-administered bnAb for HIV-1 prevention. HVTN 104 was a Phase 1 trial of VRC01.
Setting:
We measured serum concentrations and serum neutralization of VRC01 in 1079 longitudinal samples collected after passive administration of VRC01 in 84 HVTN 104 participants. As assays for measuring VRC01 serum neutralization titers are resource-intensive, we investigated approaches to predicting such titers.
Methods:
Serum concentration was measured using an anti-idiotypic ELISA assay. Serum neutralization ID50 titers and in vitro neutralization potency IC50 of the VRC01 clinical lot were measured against Env-pseudoviruses. Three approaches were used to predict serum neutralization ID50 titers based on 1) observed serum concentration divided by IC50, 2) pharmacokinetics model-predicted serum concentration divided by IC50, and, 3) joint modeling of the longitudinal serum concentrations and ID50 titers.
Results:
All three approaches yielded satisfactory prediction of neutralization titers against viruses of varied sensitivities; the median fold-differences (FDs) of observed-over-predicted ID50 titers were between 0.95 and 1.37. Approach 3 generally performed the best with FDs between 0.95 and 0.99, and <70% mean squared prediction error relative to Approach 1. Similar results were obtained for ID80 titers.
Conclusion:
VRC01 serum neutralization could be accurately predicted, especially when using pharmacokinetics models. The proposed prediction approaches could potentially save significant resources for the characterization of serum neutralization of VRC01, including for other bnAbs and bnAb combinations.
Keywords: broadly neutralizing antibody, HIV, passive administration, pharmacokinetics modeling
1. Introduction
VRC01 is an IgG1 broadly neutralizing monoclonal antibody (bnAb) targeting the CD4 binding site of the HIV-1 envelope (Env) glycoprotein (e.g., 1,2). It is currently being evaluated in the two harmonized Phase 2b Antibody Mediated Prevention (AMP) efficacy trials (HVTN 704/HPTN 085 and HVTN 703/HPTN 081; NCT02716675 and NCT02568215), the first assessment of a passively-administered bnAb for HIV-1 prevention.3 Prior to AMP, the safety, pharmacokinetics (PK) and functional activity of VRC01 were evaluated in healthy adults in two Phase 1 trials, VRC6024 and HVTN 104.5,6
Virus neutralization activity is a key function to consider when evaluating the efficacy of bnAbs in preventing HIV infection (e.g., 7). However, assays for measuring serum neutralizing activity require more resources than those for measuring serum concentrations of post-administration bnAbs. Therefore, the identification of an approach for the prediction of serum neutralization, given known serum concentrations of bnAbs, could lead to more efficient use of serum samples from study participants and significant resource savings.
Using serum samples collected post administration of VRC01 in HVTN 104, we measured concentrations and neutralization titers of VRC01 against Env-pseudotyped viruses of diverse sensitivities to VRC01-mediated neutralization. We then compared three different approaches for predicting VRC01 serum neutralization titers. Our findings have implications for the clinical development of future bnAbs, and are also timely in the planning of assays for the AMP trials.
2. Methods
2.1. Study procedure
In HVTN 104, 84 healthy men (n=42) and women (n=42) aged 18 – 50 years received a loading dose of 40 mg/kg of VRC01 administered intravenously (IV), followed by 20 mg/kg IV every 4 weeks (Group 1); 10, 30 or 40 mg/kg IV of VRC01 every 8 weeks (Groups 2, 4, or 5); or a 40 mg/kg IV loading dose of VRC01, followed by 5 mg/kg of VRC01 subcutaneously, every 2 weeks for 5.5 months (Group 3).5,6 VRC01 serum concentrations and neutralization were measured at 3 days to 8 weeks after each administration, and at one hour post last infusion. All volunteers provided informed written consent prior to study participation. The institutional review boards at the Fred Hutchinson Cancer Research Center approved the study.
2.2. Lab assays
VRC01 concentrations in serum samples of study participants were quantified by the anti-idiotype enzyme-linked immunosorbent assay (ELISA)4; values below the lower limit of quantification (LLoQ = 1.1 mcg/mL) of the assay indicate non-detectable levels of post-administration VRC01 by the assay. Neutralization activity against HIV-1 Env-pseudotyped viruses by VRC01 (either the clinical lot of VRC01 in vitro or post-administration VRC01 in serum samples) was measured by the TZM-bl target cell neutralization assay.8,9 For the clinical lot of VRC01, 50% and 80% inhibitory concentration (IC50 and IC80) titers were assessed in vitro against 2 tier 1 Env-pseudotyped viruses (clade B: MN.3, Clade C: MW965.26) and a global panel of 11 tier 2 Env-pseudotyped viruses (246-F3_C, 25710–2., 398-F1_F, CH119.10, CNE55, CNE8, Ce703010, PVO.4, TRO.11, X1632-S2, and X2278_C2)10 (Table S1). Tier 1 viruses are highly susceptible to neutralization by easily-induced antibodies that target an open Env-conformation. Most circulating strains have evolved a closed Env-conformation that enables the virus to evade these antibodies while remaining sensitive to bnAbs − a phenotype that is classified as tier 2.11 For post-administration VRC01, 50% and 80% inhibitory dose (ID50 and ID80) titers were assessed against MN, MW965.26, and PVO.4 Env-pseudotyped viruses for all collected serum samples and against the global panel of viruses for serum samples at 5 time-points from 6 participants in group 5. Values below the LLoQ (10 for tier 2 and 20 for tier 1 viruses) of the TZM-bl target cell assay indicate a non-detectable level of the post-administration VRC01 by the assay.
2.3. Statistical analysis
Two approaches were used to predict serum ID50 titers against each tier 1 and global panel Env-pseudotyped virus: Approaches 1 and 2, respectively, consisted of dividing the measured or model-based VRC01 serum concentration by the IC50 of the VRC01 clinical lot. In Approach 2, the population pharmacokinetics (popPK) model described previously5 was used. Approach 3 consisted of a joint pharmacokinetics/pharmacodynamics (PK/PD) modeling of longitudinal serum concentration and neutralization titers. This approach was used to predict serum neutralization ID50 titers against MN, MW965.26, and PVO.4, but not the other viruses due to data sparsity. The same modeling exercises were repeated for predicting serum ID80 titers, except that IC80 values of the VRC01 clinical lot were used. To avoid overfitting, a 3-fold cross-validation procedure based on 500 iterations was used to evaluate the prediction performance of Approach 3. The following measurements were used to quantify the performance of the prediction methods: fold difference (FD) -- the ratio of the observed over predicted titers; relative fold difference (RFD) -- the difference of the log-transformed observed and predicted titers over the log-transformed observed titers; relative mean squared error (RMSE) -- the average of the squared difference between the observed and predicted log-transformed titers of each approach relative to Approach 1; and concordance correlation coefficients for repeated measurements (CCCrm)12,13 of the longitudinal observed vs. predicted log-transformed titers.
3. Results
A total of 1079 serum samples were collected from 84 participants post-VRC01 administration. We first assessed the relative sensitivity of the ELISA and TZM-bl target cell assays qualitatively. We found that when using more sensitive Env-pseudotyped viruses, such as MN.3 (IC50 = 0.04 mcg/mL, IC80= 0.12 mcg/mL) and MW965.26 (IC50 = 0.10, IC80= 0.26 mcg/mL) with IC50 or IC80 lower than the LLoQ of the ELISA assay, the TZM-bl assay was more sensitive than the ELISA assay in detecting the presence of post-administration VRC01 in serum (Table S2). As expected, the TZM-bl assay was less sensitive than the ELISA assay if less sensitive viruses [e.g. PVO.4 (IC50/80 = 1.04/3.17 mcg/mL) and other global panel viruses, Table S3] were used.
We next assessed the ELISA and TZM-bl assays quantitatively. For serum samples with both detectable VRC01 concentration and neutralizing activity, we used three approaches to predict serum neutralization titers based on serum concentration and in vitro neutralization potency of the clinical lot of VRC01 against the MN.3, MW965.26, and PVO.4 Env-pseudotyped viruses. All three approaches yielded satisfactory prediction of neutralization titers against all three Env-pseudotyped viruses (Figures 1 and 2), supporting that post-administration serum VRC01 neutralizing titers can be accurately predicted by post-administration serum VRC01 concentration and neutralization titer of the VRC01 clinical lot. Of the three approaches, Approach 3 generally showed the best prediction performance across the three viruses, with FDs closest to 1 (means 1.00–1.04), RFDs closest to 0 (means −0.02 to 0.00), and the smallest RMSEs relative to Approach 1 (<70%) across ID50 and ID80 titer predictions (Figure 1). In terms of concordance between observed and predicted ID50 titers, Approach 3 also showed the best performance [CCCrm = 0.90 (95% CI: 0.87, 0.92), 0.90 (0.88, 0.92), and 0.86 (0.81, 0.90) for MN.3, MW965.26, and PVO.4, respectively]. For ID80 titers, there was also high concordance between observed and predicted ID80 titers based on Approach 3 [CCCrm= 0.92 (0.90, 0.94), 0.85 (0.82, 0.88), and 0.76, (0.70, 0.81) for MN.3, MW965.26, and PVO.4, respectively] (Figure 2).
Figure 1: Fold difference, relative fold difference, and relative mean squared error of predicted vs. observed ID50 (Panel A) and ID80 (Panel B) titers against MN.3, MW965.26, and PVO.4 Env-pseudotyped viruses based on prediction Approaches 1–3.

The fold difference is calculated as the ratio of observed over predicted titers; the relative fold difference is calculated as the difference of the log-transformed observed and predicted titers over the log-transformed observed titers; the relative mean squared error is calculated as the average of the squared difference between the predicted and observed log-transformed titers of each approach relative to Approach 1. Data were generated for HVTN 104 participants enrolled in one of five groups receiving a loading dose of 40 mg/kg of VRC01 administered IV, followed by 20 mg/kg IV every 4 weeks (Group 1); 10, 30, or 40 mg/kg IV of VRC01 every 8 weeks (Groups 2, 4, or 5); or a 40 mg/kg IV loading dose of VRC01, followed by 5 mg/kg of VRC01 subcutaneously, every 2 weeks for 5.5 months (Group 3).5,6
Figure 2: Observed vs. predicted serum neutralization ID50 (Panel A) and ID80 (Panel B) titers against MN.3, MW965.26, and PVO.4 Env-pseudotyped viruses using Approaches 1, 2, and 3.
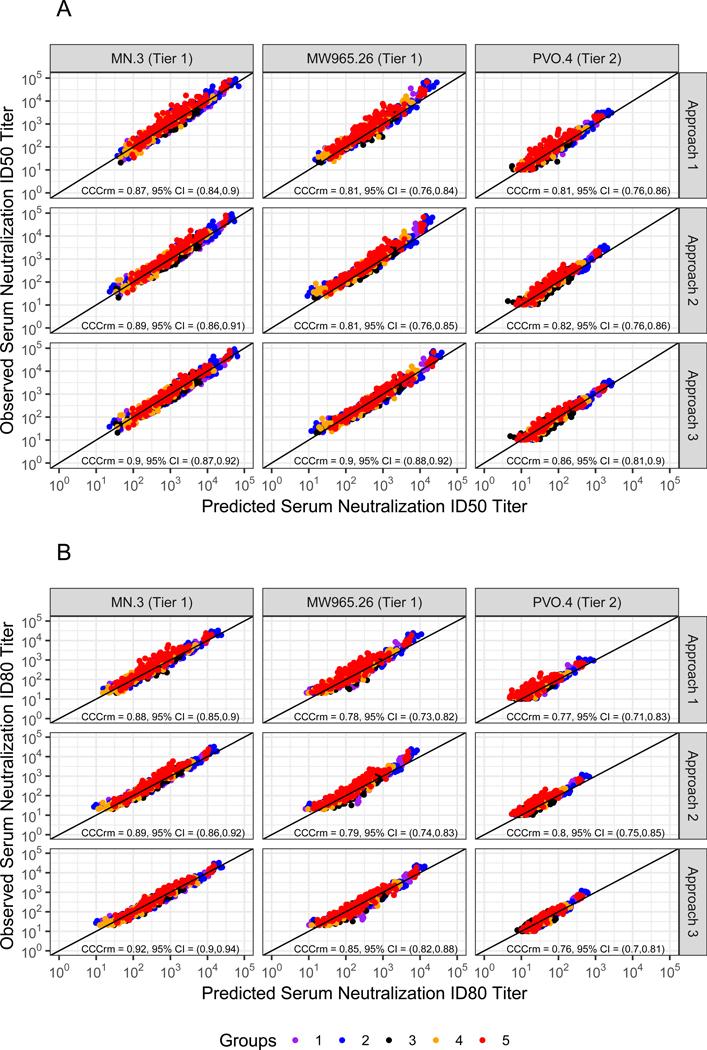
The concordance correlation coefficients for repeated measurements, CCCrm12,13 of the predicted vs. observed log-transformed titers, and 95% confidence intervals (CIs) are displayed. Data were generated for HVTN 104 participants enrolled in one of five groups receiving a loading dose of 40 mg/kg of VRC01 administered IV, followed by 20 mg/kg IV every 4 weeks (Group 1); 10, 30, or 40 mg/kg IV of VRC01 every 8 weeks (Groups 2, 4, or 5); or a 40 mg/kg IV loading dose of VRC01, followed by 5 mg/kg of VRC01 subcutaneously, every 2 weeks for 5.5 months (Group 3).5,6
Approaches 1 and 2 showed slightly lower, and generally more variable prediction performance. When Approach 1 was used, the FDs tended to be greater than 1.0 (means 1.15–1.49) and the RFDs tended to be positive (means 0.01–0.07). Similar results were obtained when Approach 2 was used (means 1.14–1.48 and 0.01–0.06, respectively) (Figure 1). These findings imply that neutralization titers predicted by either of these two approaches are generally lower than the observed titers, regardless of whether the measured or model-based serum concentration is used. Approach 2 generally performed marginally better than Approach 1 (i.e. FDs closer to 1, RFDs closer to 0, and smaller MSEs), likely because use of the PK model attenuates the impact of assay measurement error. In contrast to Approach 3, some variation in prediction performance by the two approaches was seen across the three viruses, but this did not appear to correlate with tier [i.e. slightly worse prediction for neutralization titers against MW965.26 (tier 1) than against MN.3 (tier 1) or PVO.4 (tier 2)]. Consistent with this finding, similar results were obtained for predictions of neutralization titers against the global panel of viruses (Figure S1). In terms of concordance between observed and predicted titers, Approaches 1 and 2 also showed satisfactory performance (Figure 2, Figure S2).
4. Discussion
To our knowledge, this is the first study that systematically assesses the predictability of post-administration serum neutralization activity based on serum concentrations and in vitro neutralization titers of a bnAb. Using data from HVTN 104, we found that all three proposed approaches could accurately predict post-administration serum VRC01 neutralization titers based on samples with detectable VRC01 by both the concentration and neutralization assays. We found that while Approach 1 is highly resource-efficient (i.e. because longitudinal sample storage and PK modeling is not needed), incorporating popPK modeling of longitudinal serum concentrations considerably increased prediction accuracy. This finding suggests that it is important to collect PK samples at post-administration time-points in the AMP study and in future passive immunization studies of similar bnAbs. Such data will not only allow an accurate characterization of the pharmacokinetics of the bnAb and the inter- and inter-individual variabilities of PK in the study population, but could also aid in the prediction of serum neutralization against viruses of diverse sensitivities to neutralization by bnAbs.
We also found that prediction accuracy improves when paired serum concentration and neutralization longitudinal data are used in Approach 3. In our analyses, this finding held true even when such paired data were available for a relatively small subset of 56 participants, while longitudinal serum concentration data were available from all 84 participants. Prediction of serum neutralization titers for the remaining 28 VRC01-recipients based on Approach 3 was improved compared to Approaches 1 and 2, which do not rely on paired serum concentration and neutralization data. Based on this finding, for AMP and future clinical trials of similar bnAbs, we recommend that marker studies be designed to generate serum neutralization data at the same time-points as serum concentration data – but on only a subset of VRC01-recipients, with data for other participants being predicted using Approach 3. Given a linear relationship was observed between the longitudinal serum concentration and serum neutralization titers within each given participant, a minimum of 3–5 longitudinal time-points spanning the dynamic ranges are recommended for reasonable performance of Approach 3. In addition, serum concentration data are expected to be available from a larger subset of participants for more stable building of the PK model.14 In this regard, for future research a two-phase modeling approach (e.g., 15) may be appealing to gain additional efficiency. Furthermore, once the bnAbs are better characterized against more diverse panels of pseudotyped viruses and clinical viruses, it is possible that Approach 2 could be used to save resources from assaying longitudinal neutralization titers in the future.
There are several limitations of this study. First, the lack of sufficient serum concentration and neutralization data for bnAbs other than VRC01 precluded us from investigating whether our results are also applicable to other bnAbs. However, similar approaches could be adapted to analyze data from studies of other single bnAbs or combinations of bnAbs, should such data become available in the future. Possible synergistic or antagonistic pharmacokinetics and neutralization interactions among multiple bnAbs will need to be accounted for in studies of bnAb combinations. Second, due to the lack of replicates for the measurements of serum concentration and neutralization, the exact measurement errors of the assays could not be quantified and incorporated in the precision characterization of the prediction approaches. Third, our comparison of Approach 3 vs. Approaches 1 and 2 is based on a single tier 2 virus, such that future studies of additional viruses representative of exposing viruses are needed to better quantify the relative advantages of the three approaches. Lastly, our results do not inform on how well neutralization activity at mucosal sites can be predicted; this is arguably the most relevant parameter of interest in relation to mechanisms of prevention efficacy. Future studies with mucosal post-infusion sampling would be needed to address this question.
Overall, our results indicate that – for Env-pseudotyped viruses against which the neutralization titer of the VRC01 clinical lot is known – both measured and model-predicted serum concentrations of passively administered VRC01 can be used to obtain satisfactory predictions of serum neutralization titer against the same viruses. These results have practical implications for the AMP marker studies in assay planning. Future research is needed to validate the utility of the prediction approaches for additional viruses and for other bnAbs and bnAb combinations.
Supplementary Material
Acknowledgments
The authors thank the HVTN 104 study participants and study team for their dedication and contributions to the original study.
Conflicts of Interest and Source of Funding: At the time of the research, RB, JL and JM are employees of the National Institute of Allergy and Infectious Diseases (NIAID). This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) US. Public Health Service Grants UM1 AI068614 [LOC: HIV Vaccine Trials Network], UM1 AI068635 [HVTN SDMC FHCRC], UM1 AI068618 [HVTN Laboratory Center FHCRC]. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References:
- 1.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert PB, Juraska M, deCamp AC, et al. Basis and statistical design of the passive HIV-1 Antibody Mediated Prevention (AMP) test-of-concept efficacy trials. Stat Commun Infect Dis. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledgerwood JE, Coates EE, Yamshchikov G, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Zhang L, Ledgerwood J, et al. Population pharmacokinetics analysis of VRC01, an HIV-1 broadly neutralizing monoclonal antibody, in healthy adults. MAbs. 2017;9(5):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer KH, Seaton KE, Huang Y, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med. 2017;14(11):e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegu A, Borate B, Huang Y, et al. A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe. 2019;26(3):336–346 e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarzotti-Kelsoe M, Bailer RT, Turk E, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. [DOI] [PubMed] [Google Scholar]
- 10.deCamp A, Hraber P, Bailer RT, et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2014;88(5):2489–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montefiori DC, Roederer M, Morris L, et al. Neutralization tiers of HIV-1. Curr Opin HIV AIDS. 2018;13(2):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrasco JL, King TS, Chinchilli VM. The concordance correlation coefficient for repeated measures estimated by variance components. J Biopharm Stat. 2009;19(1):90–105. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco JL, Phillips BR, Puig-Martinez J, et al. Estimation of the concordance correlation coefficient for repeated measures using SAS and R. Comput Methods Programs Biomed. 2013;109(3):293–304. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Gilbert PB, Capparelli E, et al. Pharmacokinetics simulations for studying correlates of prevention efficacy of passive HIV-1 antibody prophylaxis in the Antibody Mediated Prevention (AMP) study. Arxiv:1801.08626 [q-bio.Qm] [preprint] available from: Jan 25, 2018 [cited Mar 14, 2019]. [Google Scholar]
- 15.Breslow NE, Lumley T, Ballantyne CM, et al. Improved Horvitz-Thompson estimation of model parameters from two-phase stratified samples: Applications in epidemiology. Stat Biosci. 2009;1(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


