Abstract
Survivorship is an area of paramount importance to be addressed as early as possible after cancer diagnosis by all health care providers. On this regard, cancer care in young patients often poses several age-related considerations among which fertility and pregnancy-related issues have a crucial role. According to the available guidelines on the topic, all patients with cancer diagnosed during their reproductive years should be provided a proper oncofertility counselling before starting anticancer treatments. This is an important step in order to inform patients about the potential treatment-induced gonadotoxicity and the available strategies for fertility preservation so that they can be referred as early as possible to fertility specialists if potentially interested in these options.
In this manuscript, we aim to provide an up to date overview on the available efficacy and safety data with the main strategies for fertility preservation in male and female cancer patients in order to help optimising the oncofertility counselling performed by healthcare providers involved in cancer care and dealing with young patients. In male patients with cancer, sperm cryopreservation is the standard technique for fertility preservation. Oocyte/embryo cryopreservation, ovarian tissue cryopreservation and temporary ovarian suppression with luteinising hormone-releasing hormone agonists during chemotherapy are the main options in female patients with cancer.
A multidisciplinary management building a strong network between fertility and oncology/haematology units is crucial to properly address fertility care in all young patients with cancer, at both diagnosis and during oncologic follow-up. Discussing fertility and pregnancy-related issues with young patients with cancer has to be considered mandatory nowadays keeping in mind that returning to a normal life (including the possibility to have a family and to live with as few side effects as possible) should be considered an important ambition in cancer care in the 21st century.
Keywords: fertility, pregnancy, young adult cancer, cryopreservation, oncofertility
Introduction
In 2012, approximately one million new cancer cases have been diagnosed in young adults between the ages of 20 and 39 years.1 Global cancer burden in these patients varies substantially by sex, age, development level and geographical region; liver and testicular cancers are the two most commonly diagnosed malignancies among young male patients while breast and cervical cancer are the most frequent diagnosis in young female patients.1 Nowadays, thanks to improved survival rates, many cancer survivors face the consequences of short-term and long-term treatment-induced side effects; hence, survivorship is an area of paramount importance to be addressed as early as possible after cancer diagnosis by all healthcare providers.2
Cancer care in young patients often poses several age-related considerations among which fertility and pregnancy-related issues are of major importance. Anticancer therapies may have potential detrimental effects on the gonadal function and fertility potential of young patients.3–7 Treatment-induced gonadotoxicity is of concern to many young patients with newly diagnosed cancer.8–12 According to the available guidelines on the topic, all patients with cancer diagnosed during their reproductive years should be provided a proper oncofertility counselling before starting anticancer treatments.13 14 Nevertheless, the knowledge and attitudes of healthcare providers towards this important issue remain suboptimal.15–17
In the last years, the available evidence on fertility preservation in patients with cancer has markedly increased. In this manuscript, we aim to provide an up to date overview on fertility preservation in male and female cancer patients in order to help optimising the oncofertility counselling performed by healthcare providers involved in cancer care and dealing with young patients. The available efficacy and safety data with the main strategies for fertility preservation in male (sperm cryopreservation) and female (oocyte/embryo cryopreservation, ovarian tissue cryopreservation and temporary ovarian suppression with luteinising hormone-releasing hormone agonists (LHRHa)) patients with cancer are reviewed.
Conceiving after cancer
The majority of young patients with cancer report a strong interest in having biological children.18 However, despite the increasing availability of fertility preservation techniques, cancer survivors have reduced chances of conceiving following completion of anticancer treatments as compared with the general healthy population of a similar age,19 20 with higher rates for male (hazard ratio [HR] 0.74, 95% confidence intervals [CI] 0.71 to 0.78) than female (HR 0.61, 95% CI 0.58 to 0.64) patients.19 Nevertheless, the rates of post-treatment pregnancies appears to be similar to those of the general population for male and female survivors of melanoma and thyroid cancer.19 20 Among women, the chances of post-treatment pregnancies are particularly low for breast cancer survivors with prior history of hormone receptor-positive disease.21 22
Young cancer survivors and their treating oncologists/haematologists may express different concerns when considering to conceive following completion of anticancer treatments.16 23 However, evidence has become available to dispel most of them.
Globally, many studies have shown no apparent increased risk of congenital abnormalities for pregnancies of male and female cancer survivors with prior exposure to anticancer treatments.24–28 However, some studies have reported potential increased risks. Specifically, a slightly higher risk of congenital abnormalities was described in men using cryopreserved sperm or fresh post-treatment sperm; however, no strong conclusions can be derived on this regard considering the limited evidence coming mostly from register-based studies.29 Similarly, a recent metanalysis has shown a slightly higher risk of congenital abnormalities in babies born from women with prior cancer history; however, this result was considered being likely an artefact of the analysis.30
In terms of risk of developing pregnancy complications (including abortion, caesarean delivery, postpartum haemorrhage, preterm birth, small for gestational age, low birth weight), a higher risk for post-treatment pregnancies of adult women with prior cancer history has been described,25 26 30–35 while this has not been shown for male patients and their healthy partners.25 Notably, off-target effects of chemotherapy and radiotherapy in female patients may also be associated with structural and/or vascular uterine damages that can potentially lead to pregnancy complications.36 Therefore, post-treatment pregnancies in female cancer survivors should be monitored more closely than those in healthy women.
Safety concerns on the potential detrimental prognostic effect of pregnancy have been expressed for patients with hormone sensitive cancers.16 Recent data have dispelled these concerns.28 37–40 A meta-analysis of 19 retrospective (10 case–control and 9 cohort) studies showed that women with a pregnancy after prior history of breast cancer had a non-significant reduced risk of recurrence (HR 0.84, 95% CI 0.69 to 1.02) and a significantly improved overall survival (HR 0.63, 95% CI 0.51 to 0.79) as compared with those without a subsequent pregnancy.37 When including only the studies that controlled for the ‘healthy mother effect’ similar results were obtained with a reduced risk of death for women who had a subsequent pregnancy (HR 0.65, 95% CI 0.52 to 0.81).37 More recent findings from a large population-based retrospective cohort study,38 and a retrospective analysis within a phase III randomised trial,28 confirmed the lack of detrimental prognostic effect for post-treatment pregnancies in young breast cancer survivors. One large retrospective case–control study had specifically addressed the safety of conceiving in women with prior history of hormone receptor-positive breast cancer.39 41 Updated results after a median follow-up of approximately 10 years from initial breast cancer diagnosis showed no difference in disease-free survival (HR 0.94, 95% CI 0.70 to 1.26) nor in overall survival (HR 0.84; 95% CI, 0.60 to 1.18) between patients with or without a subsequent pregnancy after prior history of oestrogen receptor-positive disease.39 Among women with oestrogen receptor-negative breast cancer, those with a post-treatment pregnancy had similar disease-free survival (HR 0.75; 95% CI 0.53 to 1.06) but better overall survival (HR 0.57; 95% CI 0.36 to 0.90) than patients without a subsequent pregnancy. No impact on patients’ outcomes was shown for abortion, time to pregnancy and breastfeeding.39 Recent data have also supported the safety of pregnancy after breast cancer in young patients carrying germline BRCA pathogenic variants.40 This is highly relevant new information that can be shared during the oncofertility counselling of these patients considering the limited evidence available on this regard,42 43 and physicians’ safety concerns.44
Although there is no contraindication to pregnancy after treatment completion in breast cancer survivors irrespective of their tumour subtype,45 there is no proper evidence to counsel women with history of hormone receptor-positive breast cancer who are receiving 5–10 years of adjuvant endocrine therapy on the safety of a temporary treatment interruption for trying to conceive. An international multicentre trial (POSITIVE study: NCT02308085) investigating this issue has recently completed accrual and is expected to provide an important answer on this regard.46
Fertility preservation in male patients with cancer
Sperm cryopreservation
Sperm cryopreservation is the standard strategy for fertility preservation in male cancer patients (preferably aged ≤45–50 years) about to undergo gonadotoxic therapies or cancer surgery at risk of infertility and who may desire children in the future.47 It is a widely available method but a multicollaborative care pathway should be implemented in order to provide the patients with rapid and easy access to reproductive specialists and lab facilities for sperm cryopreservation.48 The most effective and widely adopted fertilisation method is represented by intracytoplasmic sperm injection (ICSI).49
In patients undergoing sperm cryopreservation, semen can be collected by masturbation, which is the most used whenever feasible, or by assisted ejaculation techniques such as penile vibratory stimulation, electroejaculation or testicular biopsy, when the patient cannot ejaculate by masturbation.50
The actual usage rate of sperm cryopreserved before starting anticancer therapies accounts for approximately 10%.51 In terms of efficacy, although large series are scarce, the rates of success using cryopreserved sperm from cancer patients for assisted reproduction are similar to or higher than outcomes with standard procedures used to treat infertile couples. A large study examined the rate of success of semen cryopreservation in 118 patients affected by different tumours: a total of 169 in vitro fertilisation (IVF)—ICSI were performed with a clinical pregnancy rate of 56.8%.52 Consistently with the literature, a significant higher pregnancy rate was registered using ICSI (50.3%, 85/169 effective cycles) compared with IVF (24.1%, 13/54 effective cycles).52 More recently, a systematic review included 30 studies and a total of 11 798 male patients with cancer who underwent sperm cryopreservation.53 The rate of success of assisted reproductive techniques (ART) was 23% in terms of clinical pregnancy per cycle (95% CI 21% to 26%) irrespectively of the fertilisation method used. When considering IVF comparing to intrauterine insemination (IUI), significantly better outcomes were observed: the clinical pregnancy rate per cycle was 30% (95% CI 27% to 34%) for IVF and 13% (95% CI 10% to 17%) for IUI. As expected, IVF provides higher chances of pregnancy per cycle instead of IUI of thawed semen.53 The three main techniques (IUI, IVF and ICSI) were compared by van Casteren et al in a cohort of cancer patients with cancer.54 A total of 629 men who cryopreserved their semen were analysed. Out of the 37 couples who used the cryopreserved samples, 7 cycles of IUI, 32 of IVF and 53 of ICSI cycles were performed. The clinical pregnancy rate per cycle reported was 14.3% for IUI, 25% for IVF and 30.1% for ICSI.54
Notably, the ICSI procedure is the technique that revolutionised the effectiveness of sperm cryopreservation, allowing fertilisation even when the quality of the semen is poor (oligoasthenozoospermia, low mobility). For this reason, this approach should be considered the preferred method when available.54
As previously highlighted, despite the controversial and limited available literature on this regard, patients should be informed about a slightly increased risk of congenital abnormalities in offspring of cancer survivors obtained with cryopreserved sperm or fresh post-treatment sperm.29
Fertility preservation in female patients with cancer
Oocyte/embryo cryopreservation
Oocyte/embryo cryopreservation before starting anticancer therapies is a standard strategy and the first option to be proposed to all female cancer patients (preferably aged ≤40 years) who wish to preserve their fertility.13 14 This strategy requires approximately 2 weeks of controlled ovarian stimulation before oocyte pick up; this amount of time is mandatory for the procedure. Despite the possibility to initiate controlled ovarian stimulation also during the luteal phase with the so-called ‘random-start stimulation’ protocols,55–58 this strategy cannot be proposed to patients who have urgent need to start anticancer treatment. On the contrary, in women who can delay the start of anticancer therapies of more than 4 weeks, a double controlled ovarian stimulation, beginning after picking up the former oocytes stimulated, can be considered to increase the potential chances of success.59
Although the success in cryopreserving oocytes has improved also thanks to the development of vitrification,60 more limited efficacy data than with embryo cryopreservation are available in patients with cancer.61 The limits of cryopreserving unfertilised oocytes might be referred to their biological characteristics: they are retrieved in metaphase II of cellular cycle and are large cells with a low ratio between surface and volume, highly capable of retaining water and thus to be potentially damaged during the freezing procedure.61 They also are particularly vulnerable to osmotic stress caused by cryoprotective agents applied in order to preserve the cell from the intracellular ice-formation during the process. Cryopreservation has shown to also impact the genomic material in the nucleus of the oocyte, by deregulating genes involved in protection from oxidative stress, cell cycle and structural cell maintenance.62 Nevertheless, oocyte cryopreservation is largely used and preferred also considering that it provides a patient reproductive autonomy. This technique is suitable to women who do not have a partner, do not wish to use donor sperm, or have religious or ethical objections to embryo freezing as well as in countries like Italy where embryo cryopreservation in cancer patients is not allowed by law. Notably, it was demonstrated that storage time does not affect the transcriptome of cryopreserved mature oocytes,63 which is particularly relevant for cancer survivors that may use their material several after years after cryostorage. Despite the limited data on the efficacy of oocyte cryopreservation specifically in the oncological population, the response to controlled ovarian stimulation could be considered the same as the non-cancer population.64
The success of oocyte/embryo cryopreservation is strongly dependent on the number of mature oocytes collected following controlled ovarian stimulation which is strongly influenced by the age and ovarian reserve of the patient at the time of the procedure.65 The live birth rates using all cryopreserved oocytes after one stimulation cycle are around 35% in women <35 years.66 67 In women around 40 years of age, the success rates are substantially lower.66 68 Specifically, a study showed that the age-associated live birth rate per warmed oocyte ranged from 8.7% in women aged <30 years to 1.1% in women aged 43–44 years, with an overall oocyte to child efficiency of 6.7%.69 Recent data indicate that oocytes from women with cancer show reduced fertilisation rates and embryos lower implantation rates than in women freezing oocytes for non-medical reasons, resulting in a lower live birth rate.68 In women under 36 years of age, oocyte survival was 81.2% vs 91.4%, implantation rates 32.5% vs 42.6%, and cumulative live birth rates 41.1% vs 68.8% in patients with or without cancer diagnosis, respectively.68 A reduction in the number of retrieved oocytes in cancer patients has not been shown by other studies.65 Even when analysing the efficacy profile of embryo cryopreservation, live birth rate is strongly related to the age of the patients at the time of the procedure: the chances of pregnancy per embryo implanted ranged from 13.2% at the age of 25–29 years to 9.8% at the age of 35–39 years.66
In terms of safety, controlled ovarian stimulation can lead in rare cases to high-grade hyperstimulation70; pelvic infection and ovarian bleeding are potential but uncommon complications during the pick-up. The main safety concern with controlled ovarian stimulation is its use in patients with breast cancer particularly in those with estrogen-sensitive tumours.16 The limited evidence on this regard suggests the lack of potential detrimental effect of the ovarian stimulation on breast cancer outcomes.71–73 However, more prospective efforts are needed on this regard.74 75 For trying to counteract the possible negative effect of the increased oestrogen serum concentration during controlled ovarian stimulation, alternative protocols including the use of letrozole76 77 or tamoxifen78 have been developed. Considering the larger and prospective available data with the use of letrozole, this agent should be preferred to be added to controlled ovarian stimulation protocols in breast cancer patients.79
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation is an alternative strategy to preserve fertility before starting gonadotoxic treatments.13 14 The experimental designation has been recently revised,80 81 and some countries already consider it to be a standard strategy,82 which can be offered preferably to women aged ≤36 years. Importantly, this is the only option available for prepubertal girls.83
The main advantages over oocyte/embryo cryopreservation are represented by the short timeframe to perform the procedure (controlled ovarian stimulation is not required), and the possibility to preserve not only fertility but also gonadal function. For this reason, the best candidates to ovarian tissue cryopreservation are patients who do not have enough time before starting anticancer therapies to perform ovarian stimulation for oocyte/embryo cryopreservation. However, it should be highlighted that this is a more complex surgical procedure consisting in biopsies of the ovarian cortex or unilateral ovariectomy usually done by laparoscopy, and then subsequent transplantation following the end of anticancer treatments. To cryopreserve ovarian cortex, despite encouraging results have been reported with vitrification, slow freezing is still the standard method.84 Although this technique should be performed only in centres with the adequate expertise, ovarian surgery can be done locally and tissue transported to a central laboratory (with the so called ‘hub and spoke’ model).85
Ovarian function restoration has been reported in more than 90% of transplanted patients within 4–9 months, and the duration of ovarian functions ranges from less than 1 up to 10 years (mean 4–5 years).85 86 To date, almost 200 babies have been born with the use of this procedure, with a live birth rate per patient estimated to be approximately 40%.87 Age (together with the expertise of the centre) is the strongest determinant for the success of this strategy: ovarian tissue cryopreservation should not be proposed to patients older than 36 years.88 Half of the pregnancies reported so far were natural conceptions, while around one third where conceived by ART.87 Notably, there is not uniformity on whether ART treatment should be initiated right after transplantation or women should be allowed a period to attempt natural conception before.89 On one hand, since ovarian function is restored in the majority of patients, a natural and less invasive approach could be preferred by women.90 91 However, other groups claim that immediate ART treatment after regaining ovarian function maximises the chances of success, because of a greater pool of follicles available.82
In terms of safety, while surgical complications with ovarian tissue cryopreservation are rare, the main concern with its use in oncology is represented by the potential risk of reintroducing cancer cells at the time of transplantation (eg, for patients with leukaemia).92 Hence, special attention should be paid in analysing the tissue before any transplantation procedure. The possibility to perform ovarian tissue cryopreservation after a first course of gonadotoxic treatment in order to decrease the risk of transplanting cancer cells has been successfully reported in a patient with leukaemia.93 The possibility to grow follicles from ovarian tissue fragments in vitro, in a matrix of fibrin (‘the artificial ovary’), is being studied, but without clinical application in humans yet.94 However, since patients will probably use the cryopreserved tissue many years after the diagnosis, the state of art may change.
Another important concern is represented by the use of this strategy in patients with hereditary cancer syndromes associated with an increased risk of ovarian cancer like in women with germline BRCA pathogenic variants.43 Despite the success of the strategy has been reported also in BRCA-mutated patients,95 96 this is not the preferred option in this setting.
LHRHa during chemotherapy
Temporary ovarian suppression with the use of LHRHa during chemotherapy has been developed as an option to reduce the gonadotoxicity of cytotoxic systemic therapies in order to avoid endocrine-related side effects associated with the development of premature ovarian insufficiency (POI).97 This option has not been studied as a fertility preservation strategy.97 Therefore, it should not be considered an alternative to cryopreservation strategies and, on the other hand, it can be offered also to all premenopausal women (preferably aged ≤45 years) without pregnancy desire. Notably, the biological rationale behind its protective effect remains to be defined.98–101 Nevertheless, after many years of debate on the efficacy and safety of this approach,102–106 recent clinical data have led current guidelines to recommend its use as a strategy to preserve ovarian function during chemotherapy, mainly in the case of young women with breast cancer.13 14 107–110
Most of the evidence available on the efficacy and safety of this strategy exists for premenopausal patients with breast cancer. In this setting, most of the randomised trials have shown a statistically significant reduction in the risk of developing chemotherapy-induced POI with concurrent use of LHRHa.98 The highest level of evidence derives from an individual patient-level meta-analysis that included 873 patients randomised in five breast cancer trials.111 The rate of chemotherapy-induced POI was significantly reduced from 30.9% to 14.1% with the use of LHRHa (adjusted odds ratio [OR] 0.38, 95% CI 0.26 to 0.57). Moreover, a higher number of patients treated with LHRHa during chemotherapy had a post-treatment pregnancy (37 vs 20; incidence rate ratio 1.83, 95% CI 1.06 to 3.15) suggesting a potential fertility preservation role of this option. All patients irrespective of hormone receptor status, age, type and duration of chemotherapy derived benefit from the administration of LHRHa during chemotherapy.111 Similar results were observed in a large metanalysis based on abstracted data from 12 randomised trials conducted in breast cancer patients.112
In premenopausal women with haematological malignancies, no protective effect of LHRHa use during chemotherapy was observed.113–115 In a recent meta-analyses including 3 trials and 109 patients, similar POI rates (18.9% vs 32.1%; risk ratio [RR] 0.70, 95% CI 0.20 to 2.47) and post-treatment pregnancies (17 vs 18; RR 1.13, 95% CI 0.66 to 1.93) were observed between patients treated with LHRHa during chemotherapy or cytotoxic therapy alone.116
One randomised trial including 30 patients with ovarian cancer showed that LHRHa use during chemotherapy significantly reduced the rates of POI (33.3% vs 0.0%; p=0.02) but did not report post-treatment pregnancies.117
Regarding the safety of this strategy, administering LHRHa during chemotherapy increases the risk of developing menopausal symptoms (hot flashes and sweating); in most of the cases, they are of low severity and reversible.111 Prior concerns for breast cancer patients with hormone receptor-positive disease on a potential detrimental antagonism between chemotherapy and an endocrine agent have been recently dispelled with the observation of similar survival outcomes between patients receiving systemic cytotoxic therapy with or without concurrent LHRHa.111 118 In these patients, considering the known prognostic value of chemotherapy-induced amenorrhea and the role of ovarian function suppression,119–121 prolonging treatment with LHRHa up to 5 years should be considered as part of adjuvant endocrine therapy.109 110 122
Conclusions
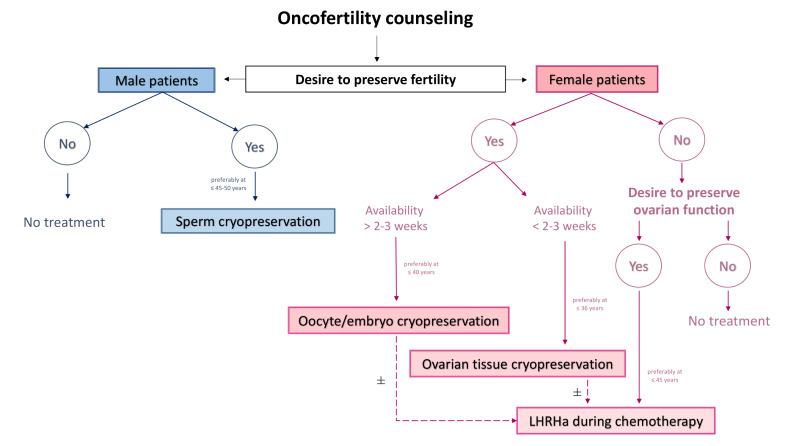
Having a family after prior cancer diagnosis and treatment completion is feasible but timing is crucial. A proper oncofertility counselling should be scheduled with all patients with cancer diagnosed during their reproductive years before treatment initiation.13 14 This is a crucial step to inform patients on the potential gonadotoxicity of the proposed anticancer therapies and to offer them the available strategies for fertility preservation (table 1)(figure 1).
Table 1.
Characteristics of the main available strategies for fertility preservation in male and female patients with cancer
| Type of strategy | Definition | Experimental or standard | Access to fertility unit needed | Hormonal stimulation needed | Potential delay in anticancer therapy initiation | Surgery needed | Fertility preservation outcomes | Gonadal preservation outcomes | |
| Male patients | Sperm cryopreservation | Freezing a sample of sperm to be used for IUI/IVF/ICSI | Standard | Yes | No | No | No | Clinical pregnancy rate per cycle:
|
NA |
| Female patients | Embryo cryopreservation | Harvesting eggs, IVF, and freezing of embryos | Standard | Yes | Yes | Yes | Yes | Live birth rate:
|
NA |
| Oocyte cryopreservation | Harvesting and freezing of unfertilised eggs | Standard | Yes | Yes | Yes | Yes | Live birth rate:
|
NA | |
| Ovarian tissue cryopreservation | Freezing of ovarian tissue and reimplantation after cancer treatment | Experimental/ standard |
Yes | No | No | Yes | Live birth rate approximately 40% (≤36 years) |
Ovarian function restoration in 90% of patients in 4–9 months | |
| Ovarian suppression with LHRHa | Use of hormonal therapies to protect ovarian tissue during chemotherapy | Standard* | No | No | No | No | Pregnancies:
IRR 1.83 95% CI 1.06 to 3.15 (breast cancer)
RR 1.13 95% CI 0.66 to 1.93 (lymphoma) |
POI rates:
OR 0.38 95% CI 0.26 to 0.57 (breast cancer)
RR 0.70 95% CI 0.20 to 2.47 (lymphoma) 33.3% vs 0.0% without LHRHa (ovarian cancer) |
*For ovarian function preservation, mostly in patients with breast cancer.
ICSI, intracytoplasmic sperm injection; IRR, incidence rate ratio; IUI, intrauterine insemination; IVF, in vitro fertilisation; LHRHa, luteinising hormone-releasing hormone agonist; NA, not applicable; POI, premature ovarian insufficiency; RR, relative risk.;
Figure 1.
Proposed algorithm for managing fertility preservation in male and female patients with cancer1. LHRHa, luteinising hormone-releasing hormone agonist.
Implementing a strong network between fertility and oncology/haematology units is crucial to properly address fertility care in all young patients with cancer and for improving the access to fertility preservation strategies. A ‘hub and spoke’ model should be considered on this regard with different oncology/haematology units referring patients to centralised and more experienced fertility units.123 Nowadays, the oncofertility unit has to be considered integral part of the management of cancer patients not only at diagnosis but also during oncologic follow-up after the completion of anticancer therapies.12 In fact, it is important both to counsel patients on access to fertility preservation strategies before starting treatment in order to achieve future pregnancies and also to take care of their quality of life, sexuality, contraception and administration of specific therapies controlling the adverse effects of anticancer therapies.12 124 In this perspective, the oncofertility counselling assumes a wider significance and should be conducted in parallel with oncological follow-up, even when the patient does not desire (or desire yet) a pregnancy.
Discussing fertility and pregnancy-related issues with young cancer patients has to be considered mandatory nowadays keeping in mind that returning to a normal life (including the possibility to have a family and to live with as few side effects as possible) should be considered an important ambition in cancer care in the 21st century.
Twitter: @maclaudiaa, @lucarecco, @Cinzia Solinas, @matteolambe
Contributors: All authors contributed to this review article.
Funding: ML acknowledges the support of a grant from the Italian Ministry of Health '5×1000 funds 2017' for pursuing his research efforts in the field of oncofertility.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
Adapted from the recently published ESMO (European Society for Medical Oncology) guidelines on fertility preservation in patients with cancer.13
References
- 1.Fidler MM, Gupta S, Soerjomataram I, et al. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol 2017;18:1579–89. 10.1016/S1470-2045(17)30677-0 [DOI] [PubMed] [Google Scholar]
- 2.Jordan K, Aapro M, Kaasa S, et al. European Society for medical oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 2018;29:36–43. 10.1093/annonc/mdx757 [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Schover LR, Partridge AH, et al. American Society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–31. 10.1200/JCO.2006.06.5888 [DOI] [PubMed] [Google Scholar]
- 4.Lambertini M, Del Mastro L, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med 2016;14:1. 10.1186/s12916-015-0545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RA, Remedios R, Kirkwood AA, et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin's lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol 2018;19:1328–37. 10.1016/S1470-2045(18)30500-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-müllerian hormone levels in early breast cancer patients treated with Anthracycline- and Cyclophosphamide-based Chemotherapy. Front Oncol 2019;9:575. 10.3389/fonc.2019.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst 2019;111:86–94. 10.1093/jnci/djy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012;118:1710–7. 10.1002/cncr.26459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 2014;32:1151–6. 10.1200/JCO.2013.52.8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polland A, Berookhim BM. Fertility concerns in men with genitourinary malignancies: treatment dilemmas, fertility options, and medicolegal considerations. Urol Oncol 2016;34:399–406. 10.1016/j.urolonc.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 11.Lambertini M, Fontana V, Massarotti C, et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: results of the pilot phase of the pregnancy and fertility (prefer) study. Breast 2018;41:51–6. 10.1016/j.breast.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Massarotti C, Scaruffi P, Lambertini M, et al. Beyond fertility preservation: role of the oncofertility unit in the reproductive and gynecological follow-up of young cancer patients. Hum Reprod 2019;34:1462–9. 10.1093/humrep/dez108 [DOI] [PubMed] [Google Scholar]
- 13.Lambertini M, Peccatori FA, Demeestere I, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines. Ann Oncol 2020. 10.1016/j.annonc.2020.09.006. [Epub ahead of print: 16 Sep 2020]. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 15.Zapzalka DM, Redmon JB, Pryor JL. A survey of oncologists regarding sperm cryopreservation and assisted reproductive techniques for male cancer patients. Cancer 1999;86:1812–7. [DOI] [PubMed] [Google Scholar]
- 16.Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians' knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 2018;42:41–9. 10.1016/j.breast.2018.08.099 [DOI] [PubMed] [Google Scholar]
- 17.Vesali S, Navid B, Mohammadi M, et al. Little information about fertility preservation is provided for cancer patients: A survey of oncologists’ knowledge, attitude and current practice. Eur J Cancer Care 2019;28:e12947 10.1111/ecc.12947 [DOI] [PubMed] [Google Scholar]
- 18.Balthazar U, Fritz MA, Mersereau JE. Fertility preservation: a pilot study to assess previsit patient knowledge quantitatively. Fertil Steril 2011;95:1913–6. 10.1016/j.fertnstert.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 19.Stensheim H, Cvancarova M, Møller B, et al. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011;129:1225–36. 10.1002/ijc.26045 [DOI] [PubMed] [Google Scholar]
- 20.Anderson RA, Brewster DH, Wood R, et al. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod 2018;33:1281–90. 10.1093/humrep/dey216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambertini M, Boni L, Michelotti A, et al. Ovarian suppression with Triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA 2015;314:2632–40. 10.1001/jama.2015.17291 [DOI] [PubMed] [Google Scholar]
- 22.Shandley LM, Spencer JB, Fothergill A, et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril 2017;107:243–52. 10.1016/j.fertnstert.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senkus E, Gomez H, Dirix L, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 big 3-98. Psychooncology 2014;23:173–82. 10.1002/pon.3384 [DOI] [PubMed] [Google Scholar]
- 24.Azim HA, Metzger-Filho O, de Azambuja E, et al. Pregnancy occurring during or following adjuvant trastuzumab in patients enrolled in the HERA trial (big 01-01). Breast Cancer Res Treat 2012;133:387–91. 10.1007/s10549-012-1996-6 [DOI] [PubMed] [Google Scholar]
- 25.Stensheim H, Klungsøyr K, Skjaerven R, et al. Birth outcomes among offspring of adult cancer survivors: a population-based study. Int J Cancer 2013;133:n/a–705. 10.1002/ijc.28292 [DOI] [PubMed] [Google Scholar]
- 26.Haggar FA, Pereira G, Preen D, et al. Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: a population-based cohort study. PLoS One 2014;9:e113292. 10.1371/journal.pone.0113292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppänen VI, Artama MS, Malila NK, Pitkäniemi JM, et al. Risk for congenital anomalies in offspring of childhood, adolescent and young adult cancer survivors. Int J Cancer 2016;139:1721–30. 10.1002/ijc.30226 [DOI] [PubMed] [Google Scholar]
- 28.Lambertini M, Martel S, Campbell C, et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: analysis from the NeoALTTO (big 1-06) and ALTTO (big 2-06) trials. Cancer 2019;125:307–16. 10.1002/cncr.31784 [DOI] [PubMed] [Google Scholar]
- 29.Ståhl O, Boyd HA, Giwercman A, et al. Risk of birth abnormalities in the offspring of men with a history of cancer: a cohort study using Danish and Swedish national registries. J Natl Cancer Inst 2011;103:398–406. 10.1093/jnci/djq550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Kooi A-LLF, Kelsey TW, van den Heuvel-Eibrink MM, et al. Perinatal complications in female survivors of cancer: a systematic review and meta-analysis. Eur J Cancer 2019;111:126–37. 10.1016/j.ejca.2019.01.104 [DOI] [PubMed] [Google Scholar]
- 31.Mogos MF, Salihu HM, Aliyu MH, et al. Association between reproductive cancer and fetal outcomes: a population-based study. Int J Gynecol Cancer 2013;23:218–26. 10.1097/IGC.0b013e31827b877b [DOI] [PubMed] [Google Scholar]
- 32.Melin J, Heinävaara S, Malila N, et al. Adverse obstetric outcomes among early-onset cancer survivors in Finland. Obstet Gynecol 2015;126:803–10. 10.1097/AOG.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 33.Black KZ, Nichols HB, Eng E, et al. Prevalence of preterm, low birthweight, and small for gestational age delivery after breast cancer diagnosis: a population-based study. Breast Cancer Res 2017;19:11. 10.1186/s13058-017-0803-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartnett KP, Ward KC, Kramer MR, et al. The risk of preterm birth and growth restriction in pregnancy after cancer. Int J Cancer 2017;141:2187–96. 10.1002/ijc.30914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerstl B, Sullivan E, Ives A, et al. Pregnancy outcomes after a breast cancer diagnosis: a systematic review and meta-analysis. Clin Breast Cancer 2018;18:e79–88. 10.1016/j.clbc.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 36.Griffiths MJ, Winship AL, Hutt KJ. Do cancer therapies damage the uterus and compromise fertility? Hum Reprod Update 2020;26:161–73. 10.1093/humupd/dmz041 [DOI] [PubMed] [Google Scholar]
- 37.Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat 2016;160:347–60. 10.1007/s10549-016-3989-3 [DOI] [PubMed] [Google Scholar]
- 38.Iqbal J, Amir E, Rochon PA, et al. Association of the timing of pregnancy with survival in women with breast cancer. JAMA Oncol 2017;3:659–65. 10.1001/jamaoncol.2017.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst 2018;110:426–9. 10.1093/jnci/djx206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambertini M, Ameye L, Hamy A-S, et al. Pregnancy after breast cancer in patients With germline BRCA mutations. J Clin Oncol 2020;38:3012–23. 10.1200/JCO.19.02399 [DOI] [PubMed] [Google Scholar]
- 41.Lambertini M, Ameye L, Paesmans M, et al. Response. J Natl Cancer Inst 2018;110:919–20. 10.1093/jnci/djx292 [DOI] [PubMed] [Google Scholar]
- 42.Valentini A, Lubinski J, Byrski T, et al. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res Treat 2013;142:177–85. 10.1007/s10549-013-2729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev 2017;59:61–70. 10.1016/j.ctrv.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 44.Lambertini M, Di Maio M, Poggio F, et al. Knowledge, attitudes and practice of physicians towards fertility and pregnancy-related issues in youngBRCA-mutated breast cancer patients. Reprod Biomed Online 2019;38:835–44. 10.1016/j.rbmo.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 45.Lambertini M, Viglietti G. Pregnancies in young women with diagnosis and treatment of HER2-positive breast cancer. Oncotarget 2019;10:803–4. 10.18632/oncotarget.26611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast 2015;24:201–7. 10.1016/j.breast.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 47.Daudin M, Rives N, Walschaerts M, et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 2015;103:478–86. 10.1016/j.fertnstert.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 48.Wyns C, Collienne C, Shenfield F, et al. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod 2015;30:2022–30. 10.1093/humrep/dev161 [DOI] [PubMed] [Google Scholar]
- 49.Machen GL, Harris SE, Bird ET, et al. Utilization of cryopreserved sperm cells based on the indication for storage. Investig Clin Urol 2018;59:177–81. 10.4111/icu.2018.59.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyns C. Fertility preservation: current prospects and future challenges. Gynecol Endocrinol 2013;29:403–7. 10.3109/09513590.2012.754872 [DOI] [PubMed] [Google Scholar]
- 51.Muller I, Oude Ophuis RJA, Broekmans FJM, et al. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod Biomed Online 2016;32:147–53. 10.1016/j.rbmo.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 52.Hourvitz A, Goldschlag DE, Davis OK, et al. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril 2008;90:557–63. 10.1016/j.fertnstert.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 53.Ferrari S, Paffoni A, Filippi F, et al. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod Biomed Online 2016;33:29–38. 10.1016/j.rbmo.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 54.van Casteren NJ, van Santbrink EJP, van Inzen W, et al. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril 2008;90:2245–50. 10.1016/j.fertnstert.2007.10.055 [DOI] [PubMed] [Google Scholar]
- 55.Sönmezer M, Türkçüoğlu I, Coşkun U, et al. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril 2011;95:2125.e9–2125.e11. 10.1016/j.fertnstert.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 56.Cakmak H, Katz A, Cedars MI, et al. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril 2013;100:1673–80. 10.1016/j.fertnstert.2013.07.1992 [DOI] [PubMed] [Google Scholar]
- 57.Lambertini M, Pescio MC, Viglietti G, et al. Methods of controlled ovarian stimulation for embryo/oocyte cryopreservation in breast cancer patients. Expert Rev Qual Life Cancer Care 2017;2:47–59. 10.1080/23809000.2017.1270760 [DOI] [Google Scholar]
- 58.Wald K, Cakmak H, Mok-Lin E, et al. Back-to-back random-start ovarian stimulation prior to chemotherapy to maximize oocyte yield. J Assist Reprod Genet 2019;36:1161–8. 10.1007/s10815-019-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsampras N, Gould D, Fitzgerald CT, et al. Double ovarian stimulation (DuoStim) protocol for fertility preservation in female oncology patients. Hum Fertil 2017;20:248–53. 10.1080/14647273.2017.1287433 [DOI] [PubMed] [Google Scholar]
- 60.Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in art: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–55. 10.1093/humupd/dmw038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massarotti C, Scaruffi P, Lambertini M, et al. State of the art on oocyte cryopreservation in female cancer patients: a critical review of the literature. Cancer Treat Rev 2017;57:50–7. 10.1016/j.ctrv.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 62.Monzo C, Haouzi D, Roman K, et al. Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod 2012;27:2160–8. 10.1093/humrep/des153 [DOI] [PubMed] [Google Scholar]
- 63.Stigliani S, Moretti S, Anserini P, et al. Storage time does not modify the gene expression profile of cryopreserved human metaphase II oocytes. Hum Reprod 2015;30:2519–26. 10.1093/humrep/dev232 [DOI] [PubMed] [Google Scholar]
- 64.Quinn MM, Cakmak H, Letourneau JM, et al. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum Reprod 2017;32:568–74. 10.1093/humrep/dew355 [DOI] [PubMed] [Google Scholar]
- 65.von Wolff M, Bruckner T, Strowitzki T, et al. Fertility preservation: ovarian response to freeze oocytes is not affected by different malignant diseases-an analysis of 992 stimulations. J Assist Reprod Genet 2018;35:1713–9. 10.1007/s10815-018-1227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrenz B, Jauckus J, Kupka M, et al. Efficacy and safety of ovarian stimulation before chemotherapy in 205 cases. Fertil Steril 2010;94:2871–3. 10.1016/j.fertnstert.2010.06.054 [DOI] [PubMed] [Google Scholar]
- 67.Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod 2018;33:2051–9. 10.1093/humrep/dew158 [DOI] [PubMed] [Google Scholar]
- 68.Cobo A, García-Velasco J, Domingo J, et al. Elective and Onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018;33:2222–31. 10.1093/humrep/dey321 [DOI] [PubMed] [Google Scholar]
- 69.Doyle JO, Richter KS, Lim J, et al. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril 2016;105:459–66. 10.1016/j.fertnstert.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 70.Youssef MAF, Abdelmoty HI, Ahmed MAS, et al. Gnrh agonist for final oocyte maturation in GnRH antagonist co-treated IVF/ICSI treatment cycles: systematic review and meta-analysis. J Adv Res 2015;6:341–9. 10.1016/j.jare.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab 2016;101:1364–71. 10.1210/jc.2015-3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez-Wallberg KA, Eloranta S, Krawiec K, et al. Safety of fertility preservation in breast cancer patients in a register-based matched cohort study. Breast Cancer Res Treat 2018;167:761–9. 10.1007/s10549-017-4555-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moravek MB, Confino R, Smith KN, et al. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril 2018;109:349–55. 10.1016/j.fertnstert.2017.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lambertini M, Fontanella C. How reliable are the available safety data on hormonal stimulation for fertility preservation in young women with newly diagnosed early breast cancer? Breast Cancer Res Treat 2018;168:773–4. 10.1007/s10549-017-4654-1 [DOI] [PubMed] [Google Scholar]
- 75.Lambertini M, Anserini P, Del Mastro L. Reply to the letter "Safety of fertility preservation in women with breast cancer". Breast 2019;43:149–50. 10.1016/j.breast.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 76.Bonardi B, Massarotti C, Bruzzone M, et al. Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 2020;10:574669. 10.3389/fonc.2020.574669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oktay K, Turan V, Bedoschi G, et al. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol 2015;33:2424–9. 10.1200/JCO.2014.59.3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meirow D, Raanani H, Maman E, et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril 2014;102:488–95. 10.1016/j.fertnstert.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 79.Lambertini M, Goldrat O, Clatot F, et al. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol 2017;29:243–52. 10.1097/CCO.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 80.Martinez F, International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group . Update on fertility preservation from the Barcelona International Society for fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril 2017;108:407–15. 10.1016/j.fertnstert.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 81.Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a Committee opinion. Fertil Steril 2019;112:1022–33. 10.1016/j.fertnstert.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 82.Meirow D, Ra'anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril 2016;106:467–74. 10.1016/j.fertnstert.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 83.Wallace WHB, Smith AG, Kelsey TW, et al. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol 2014;15:1129–36. 10.1016/S1470-2045(14)70334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Q, Xie Y, Wang Y, et al. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep 2017;7:8538. 10.1038/s41598-017-09005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jensen AK, Kristensen SG, Macklon KT, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod 2015;30:2838–45. 10.1093/humrep/dev230 [DOI] [PubMed] [Google Scholar]
- 86.Andersen CY, Silber SJ, Bergholdt SH, et al. Long-term duration of function of ovarian tissue transplants: case reports. Reprod Biomed Online 2012;25:128–32. 10.1016/j.rbmo.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 87.Gellert SE, Pors SE, Kristensen SG, et al. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet 2018;35:561–70. 10.1007/s10815-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diaz-Garcia C, Domingo J, Garcia-Velasco JA, et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril 2018;109:478–85. 10.1016/j.fertnstert.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 89.Andersen ST, Pors SE, Poulsen LlaC, et al. Ovarian stimulation and assisted reproductive technology outcomes in women transplanted with cryopreserved ovarian tissue: a systematic review. Fertil Steril 2019;112:908–21. 10.1016/j.fertnstert.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 90.Schmidt KT, Rosendahl M, Ernst E, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril 2011;95:695–701. 10.1016/j.fertnstert.2010.07.1080 [DOI] [PubMed] [Google Scholar]
- 91.Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod 2016;31:2031–41. 10.1093/humrep/dew165 [DOI] [PubMed] [Google Scholar]
- 92.Bastings L, Beerendonk CCM, Westphal JR, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update 2013;19:483–506. 10.1093/humupd/dmt020 [DOI] [PubMed] [Google Scholar]
- 93.Shapira M, Raanani H, Barshack I, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril 2018;109:48–53. 10.1016/j.fertnstert.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 94.Chiti MC, Dolmans M-M, Mortiaux L, et al. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet 2018;35:41–8. 10.1007/s10815-017-1091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jensen AK, Macklon KT, Fedder J, et al. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet 2017;34:325–36. 10.1007/s10815-016-0843-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lambertini M, Goldrat O, Ferreira AR, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol 2018;29:237–43. 10.1093/annonc/mdx639 [DOI] [PubMed] [Google Scholar]
- 97.Lambertini M, Richard F, Nguyen B, et al. Ovarian function and fertility preservation in breast cancer: should gonadotropin-releasing hormone agonist be administered to all premenopausal patients receiving chemotherapy? Clin Med Insights Reprod Health 2019;13:117955811982839. 10.1177/1179558119828393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lambertini M, Horicks F, Del Mastro L, et al. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev 2019;72:65–77. 10.1016/j.ctrv.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 99.Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health 2019;13:117955811987016. 10.1177/1179558119870163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poggio F, Lambertini M, Bighin C, et al. Potential mechanisms of ovarian protection with gonadotropin-releasing hormone agonist in breast cancer patients: a review. Clin Med Insights Reprod Health 2019;13:117955811986458. 10.1177/1179558119864584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scaruffi P, Stigliani S, Cardinali B, et al. Gonadotropin releasing hormone agonists have an anti-apoptotic effect on cumulus cells. Int J Mol Sci 2019;20:6045. 10.3390/ijms20236045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Del Mastro L, Lambertini M. Temporary ovarian suppression with gonadotropin-releasing hormone agonist during chemotherapy for fertility preservation: toward the end of the debate? Oncologist 2015;20:1233–5. 10.1634/theoncologist.2015-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dolmans M-M, Taylor HS, Rodriguez-Wallberg KA, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in women receiving chemotherapy: pros and cons. Fertil Steril 2020;114:725–38. 10.1016/j.fertnstert.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 104.Lambertini M, Falcone T, Unger JM, et al. Debated role of ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in women with cancer. J Clin Oncol 2017;35:804–5. 10.1200/JCO.2016.69.2582 [DOI] [PubMed] [Google Scholar]
- 105.Blumenfeld Z. Fertility preservation by endocrine suppression of ovarian function using gonadotropin-releasing hormone agonists: the end of the controversy? J Clin Oncol 2018;36:1895–7. 10.1200/JCO.2018.78.9347 [DOI] [PubMed] [Google Scholar]
- 106.Poggio F, Conte B, Lambertini M. Treatment-Induced early menopause and the protective role of gonadotropin-releasing hormone agonists during chemotherapy. Breast Cancer Res Treat 2018;171:245–6. 10.1007/s10549-018-4806-y [DOI] [PubMed] [Google Scholar]
- 107.Paluch-Shimon S, Cardoso F, Partridge AH. ESO-ESMO 4th International consensus guidelines for breast cancer in young women (BCY4). Ann Oncol 2020;S0923-7534:36363–8. [DOI] [PubMed] [Google Scholar]
- 108.Lambertini M, Cinquini M, Moschetti I, et al. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: a grade approach for evidence evaluation and recommendations by the Italian association of medical oncology. Eur J Cancer 2017;71:25–33. 10.1016/j.ejca.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 109.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1194–220. 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 110.Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 2019;30:1541–57. 10.1093/annonc/mdz235 [DOI] [PubMed] [Google Scholar]
- 111.Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol 2018;36:1981–90. 10.1200/JCO.2018.78.0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lambertini M, Ceppi M, Poggio F, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol 2015;26:2408–19. 10.1093/annonc/mdv374 [DOI] [PubMed] [Google Scholar]
- 113.Behringer K, Wildt L, Mueller H, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol 2010;21:2052–60. 10.1093/annonc/mdq066 [DOI] [PubMed] [Google Scholar]
- 114.Demeestere I, Brice P, Peccatori FA, et al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J Clin Oncol 2013;31:903–9. 10.1200/JCO.2012.42.8185 [DOI] [PubMed] [Google Scholar]
- 115.Demeestere I, Brice P, Peccatori FA, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol 2016;34:2568–74. 10.1200/JCO.2015.65.8864 [DOI] [PubMed] [Google Scholar]
- 116.Senra JC, Roque M, Talim MCT, et al. Gonadotropin-Releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:77–86. 10.1002/uog.18934 [DOI] [PubMed] [Google Scholar]
- 117.Gilani MM, Hasanzadeh M, Ghaemmaghami F, et al. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia Pac J Clin Oncol 2007;3:79–83. 10.1111/j.1743-7563.2007.00089.x [DOI] [Google Scholar]
- 118.Regan MM, Walley BA, Francis PA, et al. Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: an exploratory analysis of text and soft. Ann Oncol 2017;28:2225–32. 10.1093/annonc/mdx285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lambertini M, Viglietti G, de Azambuja E. Controversies in oncology: which adjuvant endocrine therapy is to be given to premenopausal patients with hormone receptor-positive breast cancer? ESMO Open 2018;3:e000350. 10.1136/esmoopen-2018-000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lambertini M, Blondeaux E, Perrone F, et al. Improving adjuvant endocrine treatment tailoring in premenopausal women with hormone receptor-positive breast cancer. J Clin Oncol 2020;38:1258–67. 10.1200/JCO.19.02242 [DOI] [PubMed] [Google Scholar]
- 121.Parisi F, Razeti MG, Blondeaux E, et al. Current state of the art in the adjuvant systemic treatment of premenopausal patients with early breast cancer. Clin Med Insights Oncol 2020;14:117955492093181. 10.1177/1179554920931816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 2016;34:1689–701. 10.1200/JCO.2015.65.9573 [DOI] [PubMed] [Google Scholar]
- 123.Razeti MG, Spinaci S, Lambertini M. Implementing the hub and spoke model for the oncofertility units. Breast 2019;48:100. 10.1016/j.breast.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 124.Condorelli M, Lambertini M, Del Mastro L, et al. Fertility, sexuality and cancer in young adult women. Curr Opin Oncol 2019;31:259–67. 10.1097/CCO.0000000000000540 [DOI] [PubMed] [Google Scholar]