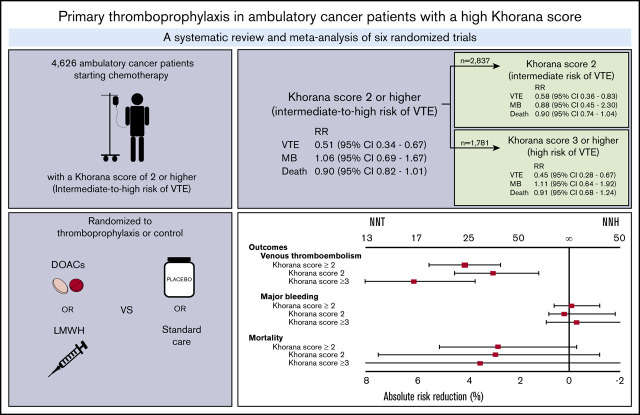
Abstract
Guidelines suggest thromboprophylaxis for ambulatory cancer patients starting chemotherapy with an intermediate to high risk of venous thromboembolism (VTE) according to Khorana score. Data on thromboprophylaxis efficacy in different Khorana score risk groups remain ambiguous. We sought to evaluate thromboprophylaxis in patients with an intermediate- to high-risk (≥2 points) Khorana score and an intermediate-risk score (2 points) or high-risk score (≥3 points) separately. MEDLINE, Embase, and CENTRAL were searched for randomized controlled trials (RCTs) comparing thromboprophylaxis with placebo or standard care in ambulatory cancer patients. Outcomes were VTE, major bleeding, and all-cause mortality. Relative risks (RRs) were calculated in a profile-likelihood random-effects model. Six RCTs were identified, involving 4626 cancer patients. Thromboprophylaxis with direct oral anticoagulants (DOACs) or low molecular weight heparin (LMWH) significantly reduced VTE risk in intermediate- to high-risk (RR, 0.51; 95% confidence interval [CI], 0.34-0.67), intermediate-risk (RR, 0.58; 95% CI, 0.36-0.83), and high-risk patients (RR, 0.45; 95% CI, 0.28-0.67); the numbers needed to treat (NNTs) were 25 (intermediate to high risk), 34 (intermediate risk), and 17 (high risk), respectively. There was no significant difference in major bleeding (RR, 1.06; 95% CI, 0.69-1.67) or all-cause mortality (RR, 0.90; 95% CI, 0.82-1.01). The numbers needed to harm (NNHs) for major bleeding in intermediate- to high-risk, intermediate-risk, and high-risk patients were 1000, −500, and 334, respectively. The overall NNH was lower in DOAC studies (100) versus LMWH studies (−500). These findings indicate thromboprophylaxis effectively reduces the risk of VTE in patients with an intermediate- to high-risk Khorana score, although the NNT is twice as high for intermediate-risk patients compared with high-risk patients.
Visual Abstract
Introduction
Venous thromboembolism (VTE) is a frequent complication in cancer patients, occurring in ∼7% during the first 6 months after cancer diagnosis.1,2 The VTE risk is up to sevenfold higher compared with patients without cancer.3 VTE can lead to interruption or postponement of cancer treatment, decreased quality of life,4 morbidity, and death.5,6
The goal of primary thromboprophylaxis is to reduce the risk of first-time VTE and, consequently, its short- and long-term sequelae. Several randomized trials have evaluated the efficacy and safety of prophylactic-dose low molecular weight heparin (LMWH) for VTE prevention in ambulatory cancer patients. A systematic review and metaanalysis that aggregated evidence of 9 of these studies enrolling 3284 patients with advanced cancer in total estimated that LMWH prophylaxis significantly reduced the risk of symptomatic VTE by ∼46% during a median follow-up of 10 months, resulting in a number needed to treat (NNT) of 30.7
The NNT can be lowered by selecting only those cancer patients at high risk of VTE. A risk score developed for this purpose, the Khorana score, has been incorporated into guidelines on thromboprophylaxis in ambulatory cancer patients starting chemotherapy.8,9 This score uses 5 clinical variables to classify ambulatory cancer patients who initiate chemotherapy as low (0 points), intermediate (1-2 points), or high risk (≥3 points; Table 1).10
Table 1.
Khorana score for prediction of VTE in cancer patients
| Variable | Points |
|---|---|
| Site of cancer* | |
| Very high-risk cancer (stomach, pancreas) | 2 |
| High-risk cancer (lung, lymphoma, gynecological, bladder, or testicular) | 1 |
| Prechemotherapy platelet count ≥350 × 109/L | 1 |
| Prechemotherapy hemoglobin level <100 g/L or use of red cell growth factors | 1 |
| Prechemotherapy leukocyte count >11 × 109/L | 1 |
| Body mass index ≥35 kg/m2 | 1 |
| Traditional risk categories | |
| High | ≥3 |
| Intermediate | 1-2 |
| Low | 0 |
| Currently proposed risk categories | |
| Intermediate to high | ≥2 |
| Low | <2 |
The AVERT trial included brain tumors as very high-risk cancer and myeloma as high-risk cancer.
Based on limited post hoc analysis data of 2 randomized controlled trials (RCTs), several guidelines had suggested that thromboprophylaxis may be considered in high-risk patients (≥3 points) only (supplemental Table 1).11-13 Recently, 2 placebo-controlled RCTs (AVERT and CASSINI) evaluated the safety and efficacy of prophylactic direct oral anticoagulants (DOACs) in ambulatory cancer patients starting chemotherapy during a 6-month period.14,15 These trials included patients based on Khorana score; however, conventional high-risk patients (≥3 points) as well as intermediate-risk patients (2 points) were included, with both risk groups combined into a single group called intermediate to high risk (≥2 points).14,15 After the publication of these trials, new guidelines adopted the threshold of ≥2 points for intermediate- to high-risk patients in whom thromboprophylaxis may be prescribed.8,9,16
Lowering the Khorana threshold from 3 to 2 points will have important consequences, because the group of patients eligible for thromboprophylaxis will increase from 17% to up to 47%.17 Because the incidence in patients at intermediate risk is lower than in patients at high VTE risk, the NNT will increase accordingly.6,17 Although the 2 trials demonstrated the efficacy of prophylactic DOACs in all patients with an intermediate to high risk (≥2 points), it remains unclear whether the effect of thromboprophylaxis is consistent in patients at intermediate risk compared with those at high risk, because these trials did not have enough statistical power for this subgroup analysis.
The aim of the systematic review and metaanalysis reported here is to provide a summary estimate of the safety and efficacy of primary thromboprophylaxis in ambulatory cancer patients at intermediate to high risk (Khorana score ≥2) of VTE, as is adopted by current guidelines, and in the groups with intermediate risk (Khorana score 2 points) and high risk (Khorana score ≥3) separately.
Methods
This report was prepared with the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses; supplemental Table 2).18 The review was registered at Open Science Framework.19
Search strategy
Embase, MEDLINE, and the Cochrane Central Register of Trials were searched for RCTs that compared LMWH or DOACs for primary VTE prophylaxis with placebo or observation in ambulatory cancer patients with an intermediate to high risk of VTE (Khorana score ≥2). Because guidelines recommend calculating the Khorana score before starting chemotherapy, only studies in which patients had not yet started chemotherapy at baseline were included.8,10 Studies in which the Khorana score was evaluated post hoc were considered eligible if the risk score was calculated with data collected at baseline and before the start of chemotherapy. To be able to compare different risk groups (intermediate vs high risk), studies were only included if data on the Khorana score were available and when all patients had at least an intermediate-risk score based on tumor type only. No restrictions on language or year of publication were applied. In addition, we did not restrict on study design to be able to identify RCTs in which the Khorana score was calculated in post hoc analyses. The full search strategy is shown in supplemental Table 3.
Data collection and risk of bias assessment
Two authors (F.I.M. and F.T.M.B.) independently screened potentially eligible studies based on title, abstract, and full text using Rayyan Qatar Computing Research Institute software.20 Both authors independently collected data in standardized data collection forms and assessed risk of bias using version 2.0 of the Cochrane risk of bias tool for randomized studies.21 Disagreements were resolved by discussion.
Study outcomes
The primary efficacy outcome was objectively confirmed VTE, defined as symptomatic or asymptomatic deep vein thrombosis (DVT) of the upper or lower extremities, symptomatic or incidental pulmonary embolism (PE), or PE-related death, defined as fatal PE or unexplained death for which PE could not be ruled out.
The primary safety outcome was major bleeding, as per the criteria of the International Society on Thrombosis and Haemostasis, defined as fatal bleeding, bleeding in a critical organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or overt bleeding causing a drop in hemoglobin level of ≥2 g/dL (1.24 mmol/L) or leading to transfusion of ≥2 units of whole blood or red cells.22 The secondary safety outcome was all-cause mortality.
Because the Khorana score was validated in studies with 6-month follow-up, just as in the CASSINI and AVERT trials, all outcomes were assessed at 6 months after randomization.2,14,15,23 The efficacy outcome was evaluated in the intention-to-treat (ITT) population (ie, all randomized patients) or in the modified ITT population (ie, randomized patients who received at least 1 dose of the assigned study drug). The primary safety outcome was assessed during the on-treatment period (defined as treatment up to a maximum of 3 days after the last study drug was used) in the per-protocol population as defined by the individual studies. If major bleeding was not assessed in the per-protocol population and/or during the on-treatment period, data from the ITT population and/or complete study period were used.
Statistical analysis
The efficacy and safety of thromboprophylaxis were assessed in ambulatory cancer patients with an intermediate to high risk of VTE (Khorana score ≥2) and subsequently in patients at intermediate risk of VTE (Khorana score 2) and at high risk of VTE (Khorana score ≥3) separately. Subgroup analyses were performed of studies evaluating LMWH and those evaluating DOACs. A sensitivity analysis was performed, which was restricted to double-blind placebo-controlled studies without high risk of bias.
Summary relative risks (RRs) were calculated in a profile-likelihood random-effects model with inverse variance weighting.24,25 This random-effects model provides a better accounting of uncertainty than the more often used DerSimonian-Laird random-effects model and gives a more accurate summary estimation in metaanalyses with few studies.26 RRs were logit transformed before entry in the model. Forest plots were constructed to visualize summary estimates with 95% confidence intervals (CIs). Heterogeneity between studies was evaluated by estimating τ2 with maximum likelihood estimation and by I2, where <50% was regarded as low heterogeneity, 50% to 75% as moderate heterogeneity, and >75% as considerable heterogeneity. Subgroup differences were assessed with a χ2 test.
Overall absolute risk reductions (ARRs) were calculated by applying the summary RR to the 6-month risk of VTE reported in observational studies.17 This method is preferred over pooling of absolute risks, because RRs are usually more consistent across studies.27 The baseline VTE incidence in the different risk groups used in this metaanalysis was derived from a systematic review and metaanalysis, which included data from >27 000 ambulatory cancer patients.17 The 6-month incidence was 8.3% for intermediate- to high-risk (Khorana score ≥2), 7.1% for intermediate-risk (Khorana score 2), and 11.1% for high-risk patients (Khorana score ≥3). For intermediate-risk patients, the 6-month incidence of VTE in the aforementioned metaanalysis was only reported for patients with a Khorana score of 1 or 2 combined. The incidence in patients with a Khorana score of 2 only was calculated post hoc for this metaanalysis. Because no large observational data on the risk of major bleeding or all-cause mortality for different Khorana scores could be identified, major bleeding and all-cause mortality in the control groups of the included studies were used as baseline risk.
The NNT and the number needed to harm (NNH) were calculated for all outcomes with 95% CIs. As per the Cochrane Handbook for Systematic Reviews of Interventions, in the case of nonstatistically significant ARR, 1 of the confidence limits will indicate benefit and the other harm, passing through infinity; for example, an ARR of 5% (95% CI, −2% to 10%) will give an NNT of 20 (95% CI, NNT 10 to ∞ to NNH 50).28,29
Publication bias was visually assessed with a funnel plot.30 Certainty of evidence was graded according to the GRADE guidelines and presented in a summary of findings table with GRADEpro GDT software.18 R computing software was used for all analyses, in particular the metaLik package (version 0.43.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
The electronic database search identified 358 records, of which 69 were duplicates. Based on the screening of titles and abstracts, 8 were considered ineligible. Based on full-text assessment, 2 additional records were excluded. One record, consisting of 2 RCTs, was excluded because randomization had been performed after start of chemotherapy.31 One study was excluded because it was unclear whether chemotherapy had already been started at randomization (supplemental Figure 1).32
Two RCTs that met the inclusion criteria were identified directly in the search.14,15 The outcomes in these RCTs for patients with Khorana score of 2 or 3 or higher separately were derived from a post hoc analysis.33 Four included studies were identified through 1 individual patient data metaanalysis in which the Khorana score was calculated post hoc for participants in 7 RCTs evaluating thromboprophylaxis.34 Four of these RCTs fulfilled the eligibility criteria and were included in the analysis.35-38
The 6 included RCTs had enrolled a total of 7180 ambulatory cancer patients, of whom 4626 (64%) had an intermediate to high risk of VTE based on a Khorana score of ≥2 points. Study characteristics are listed in Table 2. One study used the modifications proposed by Ay et al23 to calculate the Khorona score, in which primary brain cancer is considered a very high-risk tumor and myeloma a high-risk tumor type.14 The included studies compared placebo (n = 3)14,15,35 or observation (n = 3)36-38 with either 2.5 mg of apixaban twice daily,14 10 mg of rivaroxaban once daily,15 5000 IU of dalteparin once daily,38 20 mg of semuloparin once daily,35 3500 IU of bemiparin once daily,37 or weight-adjusted enoxaparin (1 mg/kg) for 3 months followed by 40 mg once daily.36
Table 2.
Study characteristics
| VTE, n/N (%) | Major bleeding, n/N (%) | All-cause mortality, n/N (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Intervention | Cancer type | Randomized patients, n | Khorana score ≥2, n (%) | Prophylaxis | Control | Prophylaxis | Control | Prophylaxis | Control |
| CASSINI (2019)15 | Double blind | DOAC: rivaroxaban (10 mg) OD for 6 mo | Solid tumor or lymphoma | 841 | 841 (100) | 25/420 (6) | 37/421 (9) | 8/405 (2) | 4/404 (1) | 84/420 (20) | 100/421 (24) |
| AVERT (2019)14 | Double blind | DOAC: apixaban (2.5 mg) BID for 6 mo | Various types of new diagnoses or progressive solid cancers | 574 | 574 (100) | 12/291 (4) | 28/283 (10) | 6/288 (2) | 3/275 (1) | 35/291 (12) | 27/283 (10) |
| FRAGMATIC (2016)38 | Open label | LMWH: dalteparin (5000 IU) OD for 24 wk | Primary bronchial small-cell or non–small-cell carcinoma | 2202 | 1,343 (61) | 26/654 (4) | 45/689 (7) | 9/654 (2) | 13/689 (2) | 219/654 (33) | 243/689 (35) |
| CONKO-004 (2015)36 | Open label | LMWH: enoxaparin (1 mg/kg) for 3 mo, followed by 40 mg OD until disease progression | Nonresectable advanced pancreatic cancer | 312 | 298 (96)* | 5/154 (3) | 21/144 (15) | 13/154 (8) | 10/144 (7) | 48/154 (31) | 48/144 (33) |
| ABEL (2013)37 | Open label | Ultra LMWH: bemiparin (3500 IU) OD for 26 wk or until disease progression | Limited disease small-cell lung cancer | 39 | 19 (49) | 0/11 (0) | 0/8 (0) | 0/11 (0) | 1/8 (13) | 1/11 (9) | 2/8 (25) |
| SAVE-ONCO (2012)35 | Double blind | Ultra LMWH: semuloparin (20 mg) OD during chemotherapy | Locally advanced or metastatic cancer of lung, pancreas, stomach, colon, bladder, or ovary | 3212 | 1,551 (48) | 12/764 (2) | 30/787 (4) | 9/764 (2) | 11/787 (1) | 198/764 (26) | 242/787 (31) |
BID, twice daily; OD, once daily.
Khorana score could not be calculated in 14 patients.
In all 4 studies that evaluated LMWH,35-38 the Khorana score was calculated post hoc.34 In 5 studies, chemotherapy was initiated after randomization in all study patients,14,15,35-37 whereas in 1 study, 83% of patients were treated with chemotherapy after randomization.38 Maximum follow-up duration varied between studies: 6 months,14,15 12 months,35 18 months,36 or until death.37,38
Risk of bias assessment
Two studies were considered to be at high risk of bias in the domain of measurement of the outcome, because they were open-label studies without adjudication of study outcomes in a blinded fashion (supplemental Figure 2).36,38 One study was judged to have some concerns in this domain because the primary outcome included asymptomatic screening-detected DVT, which may be an imperfect surrogate marker for symptomatic VTE in thromboprophylaxis trials.15,39 Visual inspection of the funnel plot did not indicate evidence of publication bias (supplemental Figure 3).
Patients at intermediate to high risk of VTE
Data on a total of 4626 ambulatory cancer patients with an intermediate to high risk of VTE (Khorana score ≥2) were included.14,15,36-38,40 Thromboprophylaxis was associated with a 49% reduction in the risk of VTE compared with the comparison groups (RR, 0.51; 95% CI, 0.34-0.67; I2 = 28%; high-quality evidence; Figure 1). Based on a baseline 6-month risk of VTE of 8.3%,17 the anticipated ARR with thromboprophylaxis was 4.1% (95% CI, 2.7% to 5.5%), corresponding to an NNT of 25 (95% CI, 19-38; Figure 2).
Figure 1.
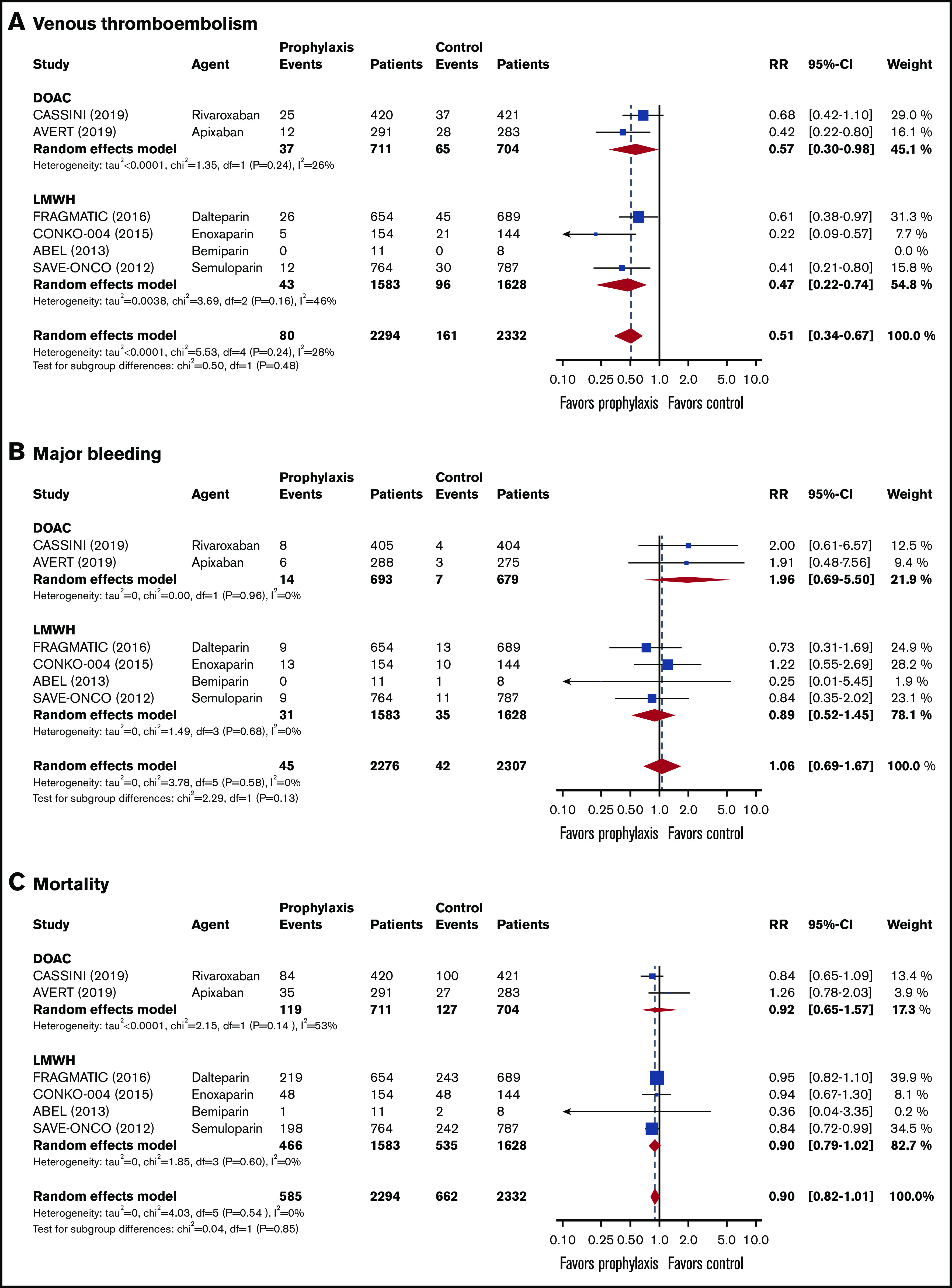
Forest plots for study outcomes in cancer patients with an intermediate to high risk of VTE (Khorana score ≥2). VTE (A), major bleeding (B), and all-cause mortality (C). df, degree of freedom.
Figure 2.
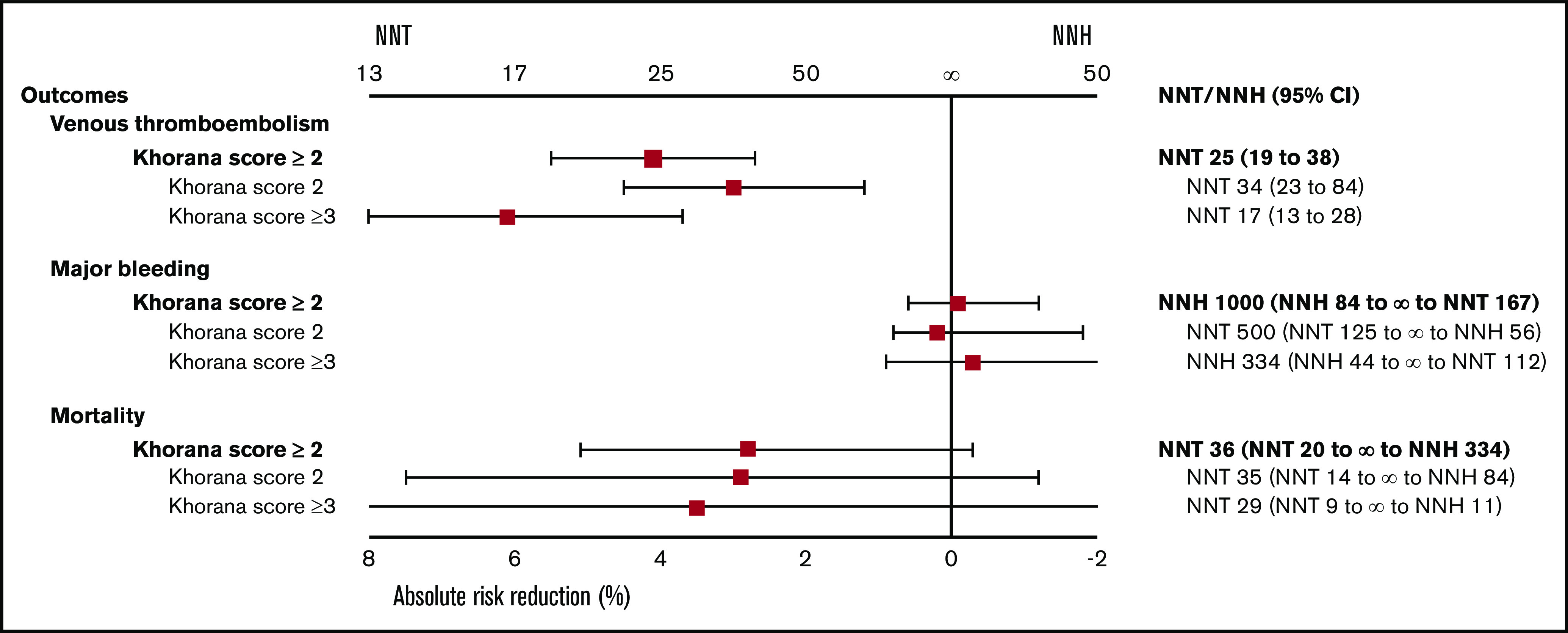
ARR and NNT or NNH of thromboprophylaxis in cancer patients for the outcomes of VTE, major bleeding, and all-cause mortality with different Khorana risk scores.
The risk of major bleeding was comparable in the thromboprophylaxis and comparison groups (RR, 1.06; 95% CI, 0.69-1.67; I2 = 0%; moderate-quality evidence; Figure 1). Based on the baseline risk of major bleeding of 1.8% in the comparison groups, the absolute risk increase was 0.1% (95% CI, −0.6% to 1.2%), corresponding to an NNH of 1000 (95% CI, NNH 84 to ∞ to NNT 167).
All-cause mortality was not different between the thromboprophylaxis and comparison groups (RR, 0.90; 95% CI, 0.82 to 1.01; I2 = 0%; moderate-quality evidence). Based on a baseline all-cause mortality risk of 28.4% in the comparison groups, the ARR was 2.8% (95% CI, −0.3% to 5.1%), with an NNT of 36 (95% CI, NNT 20 to ∞ to NNH 334; Figure 1; Table 3).
Table 3.
Summary of findings of thromboprophylaxis in cancer patients with intermediate, intermediate to high, or high risk of VTE according to Khorana score
| Study outcome at 6 mo | No. of patients (RCTs) | Certainty of evidence (GRADE) | RR (95% CI) | Anticipated absolute effects | ||
|---|---|---|---|---|---|---|
| Risk without thromboprophylaxis, %* | Risk difference with thromboprophylaxis (95% CI), % | NNT/NNH | ||||
| Intermediate to high risk of VTE (Khorana score ≥2) | ||||||
| VTE | 4626 (6) | ⨁⨁⨁⨁ (high) | 0.51 (0.34-0.67) | 8.3 | −4.1 (−5.5 to −2.7) | NNT, 25 (19-38) |
| Major bleeding | 4583 (6) | ⨁⨁⨁◯ (moderate)† | 1.06 (0.69-1.67) | 1.8 | 0.1 (−0.6 to 1.2) | NNH, 1000 (NNH 84 to ∞ to NNT 167) |
| All-cause mortality | 4626 (6) | ⨁⨁⨁◯ (moderate)† | 0.90 (0.82-1.01) | 28.4 | −2.8 (−5.1 to 0.3) | NNT, 36 (NNT 20 to ∞ to NNH 334) |
| Intermediate risk of VTE (Khorana score 2) | ||||||
| VTE | 2837 (6) | ⨁⨁⨁⨁ (high) | 0.58 (0.36-0.83) | 7.1 | −3.0 (−4.5 to −1.2) | NNT, 34 (23-84) |
| Major bleeding | 2806 (6) | ⨁⨁⨁◯ (moderate)† | 0.88 (0.45-2.30) | 1.4 | −0.2 (−0.8 to 1.8) | NNT, 500 (NNT 125 to ∞ to NNH 56) |
| All-cause mortality | 1894 (4) | ⨁⨁⨁◯ (moderate)† | 0.90 (0.74-1.04) | 28.8 | −2.9 (−7.5 to 1.2) | NNT, 35 (NNT 14 to ∞ to NNH 84) |
| High risk of VTE (Khorana score ≥3) | ||||||
| VTE | 1781 (6) | ⨁⨁⨁⨁ (high) | 0.45 (0.28-0.67) | 11.1 | −6.1 (−8.0 to −3.7) | NNT, 17 (13-28) |
| Major bleeding | 1770 (6) | ⨁⨁⨁◯ (moderate)† | 1.11 (0.64-1.92) | 2.5 | 0.3 (−0.9 to 2.3) | NNH, 334 (NNH 44 to ∞ to NNT 112) |
| All-cause mortality | 1326 (4) | ⨁⨁⨁◯ (moderate)† | 0.91 (0.68-1.24) | 38.6 | −3.5 (−12.3 to 9.3) | NNT, 29 (NNT 9 to ∞ to NNH 11) |
Risk of VTE in intervention group (and its 95% CI) is based on assumed risk in comparison group and relative effect of intervention (and its 95% CI). Assumed risk is derived from systematic review on VTE incidences with different Khorana scores by Mulder et al.17
Downgraded for imprecision because of possible positive and negative effects of thromboprophylaxis vs control.
Patients at intermediate risk of VTE
In the 2837 patients with an intermediate risk of VTE (Khorana score 2),14,15,35-38 the 6-month risk of VTE in the thromboprophylaxis groups was 42% lower than in the comparison groups (RR, 0.58; 95% CI, 0.36-0.83; I2 = 0%; high-quality evidence; Figure 3). Based on a baseline 6-month risk of VTE in patients with a Khorana score of 2 of 7.1%,17 the anticipated ARR was 3.0% (95% CI, 0.7% to 4.5%), corresponding to an NNT of 34 (95% CI, 23-84).
Figure 3.
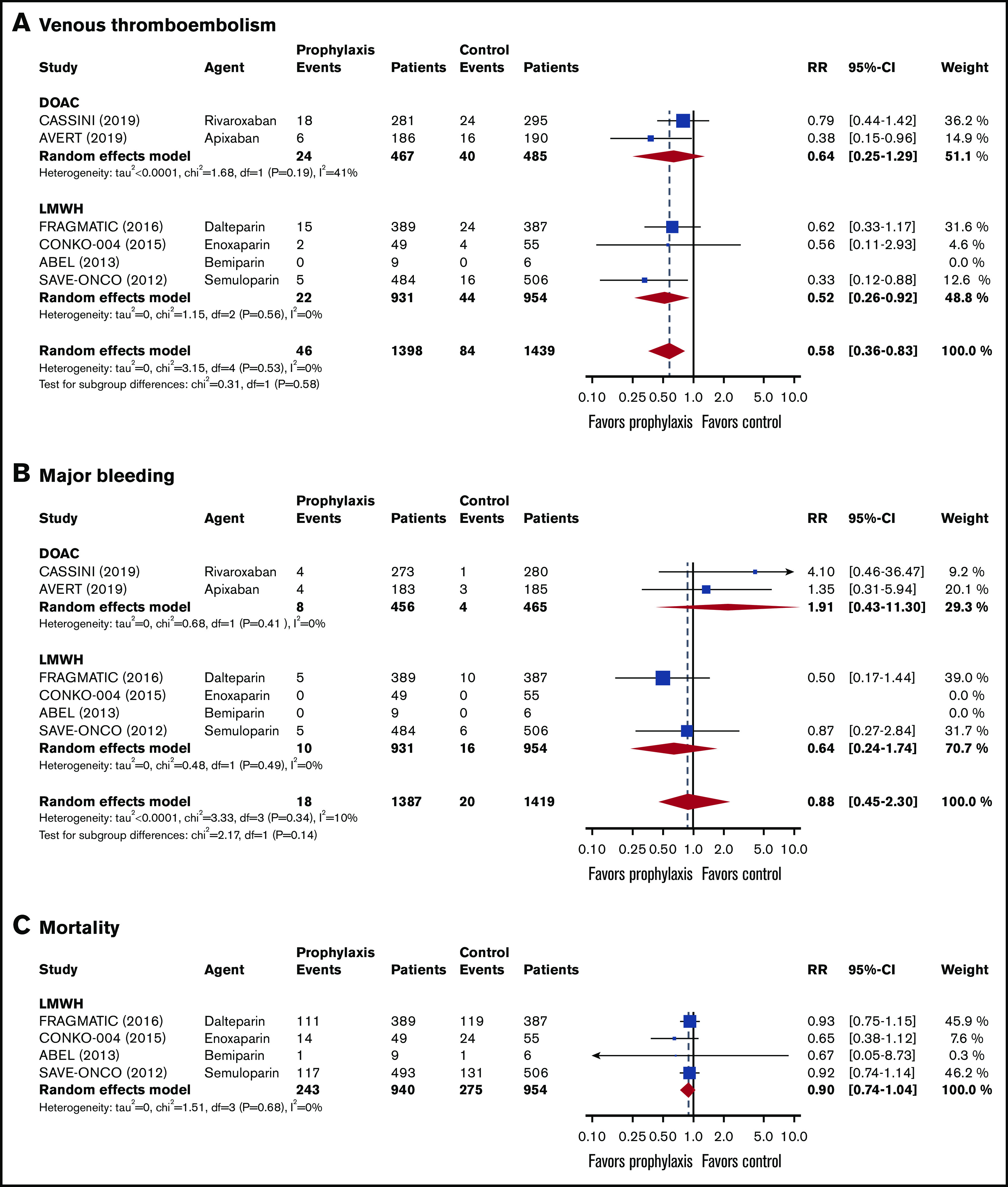
Forest plots for study outcomes in cancer patients with an intermediate risk of VTE (Khorana score 2). VTE (A), major bleeding (B), and all-cause mortality (C).
The risk of major bleeding was comparable in the thromboprophylaxis and comparison groups (RR, 0.88; 95% CI, 0.45-2.30; I2 = 0%; moderate-quality evidence; Figure 3). Based on the 1.4% risk of major bleeding in the comparison groups, the ARR was 0.2% (95% CI, −1.8% to 0.8%), with an NNT of 500 (95% CI, NNT 56 to ∞ to NNH 125).
All-cause mortality in patients with an intermediate risk of VTE was reported in 4 studies (n = 1894).35-38 There was no difference in all-cause mortality between patients in the thromboprophylaxis and comparison groups (RR, 0.90; 95% CI, 0.74-1.04; I2 = 51%; moderate-quality evidence). Based on a baseline all-cause mortality risk of 28.8% in the control arm, the ARR was 2.9% (95% CI, −1.2 to 7.5), with an NNT of 35 (95% CI, NNT 14 to ∞ to NNH 84; Figure 3; Table 3).
Patients at high risk of VTE
In the 1781 patients with a high VTE risk (Khorana score ≥3),14,15,35-38 the risk of VTE in the intervention groups was significantly lower than in the control groups (RR, 0.45; 95% CI, 0.28-0.67; I2 = 0%; high-quality evidence; Figure 4). This RR was not significantly different from that in patients with an intermediate risk of VTE (test for subgroup differences, P = .36). Based on a baseline 6-month risk of VTE in patients with a Khorana score of ≥3 of 11.1%,17 the anticipated ARR was 6.1% (95% CI, 3.7% to 8.0%), corresponding to an NNT of 17 (95% CI, 13-28).
Figure 4.

Forest plots for different outcomes in cancer patients with a high risk of VTE (Khorana score ≥3). VTE (A), major bleeding (B), and all-cause mortality (C).
The risk of major bleeding was not different between the thromboprophylaxis and comparison groups (RR, 1.11; 95% CI, 0.64-1.92; I2 = 0%; moderate-quality evidence; Figure 4). Based on a baseline major bleeding risk of 2.5% in the comparison groups, the absolute risk increase was 0.3% (95% CI, −0.9% to 2.3%), corresponding to an NNH of 334 (95% CI, NNH 44 to ∞ to NNT 112).
All-cause mortality in patients with a high risk of VTE was only presented in 4 studies (n = 1326).35-38 All-cause mortality was not different between patients treated with thromboprophylaxis and those receiving placebo or standard care (RR, 0.91; 95% CI, 0.68-1.24; I2 = 51%; moderate-quality evidence). Based on a baseline all-cause mortality risk of 38.6% in the comparison groups, the ARR was 3.5% (95% CI, −9.3% to 12.3%), with an NNT of 29 (95% CI, NNH 11 to ∞ to NNT 9; Figure 4; Table 3).
Sensitivity analysis
Results in the 3 separate risk groups were consistent in the sensitivity analysis restricted to the 3 double-blind placebo-controlled studies without high risk of bias (supplemental Table 4).
DOACs and LMWH
In the overall group of patients with an intermediate to high risk (Khorana score ≥2), the RR reduction of VTE seemed slightly lower in the DOAC studies (RR, 0.57; 95% CI, 0.30-0.98) than in LMWH studies (RR, 0.47; 95% CI, 0.22-0.74), although this difference was not statistically significant (test for subgroup difference, P = .48). In the same group, DOACs were associated with a nonsignificant increased risk of major bleeding (RR, 1.96; 95% CI, 0.69-5.50), with an NNH of 100 (95% CI, NNH 22 to ∞ to NNT 333), whereas the risk of major bleeding was not higher in patients receiving LMWH compared with those in the control groups (RR, 0.89; 95% CI, 0.52-1.45), with an NNT of 500 (95% CI, NNT 100 to ∞ to NNH 100). There was no statistically significant difference in the risk of major bleeding in patients treated with DOACs or LMWH (test for subgroup difference, P = .13). There was no difference in all-cause mortality between patients treated with DOACs or LMWH (test for subgroup differences, P = .85; Figures 1-3).
Discussion
This systematic review and metaanalysis included 6 randomized trials that evaluated primary VTE thromboprophylaxis in 4626 ambulatory cancer patients. Based on high-certainty evidence, thromboprophylaxis significantly reduced the risk of VTE in patients with an intermediate- to high-risk Khorana score of ≥2 (overall ARR, 4.1%; NNT, 25). Thromboprophylaxis is associated with a lower ARR in patients with an intermediate-risk Khorana score of 2 (overall ARR, 3.0%; NNT, 34) than in those with a high-risk Khorana score of ≥3 (overall ARR, 6.1%; NNT, 17). The overall risks of major bleeding (absolute risk increase, 0.1%; NNH, 1000) and all-cause mortality were comparable between the thromboprophylaxis and comparison groups, although the observed bleeding risk was higher in the DOAC group (absolute risk increase, 1.0%; NNH, 100). These results support the use of thromboprophylaxis in patients at intermediate to high risk of VTE (Khorana score ≥2), although the NNT is twice as high for intermediate-risk patients compared with high-risk patients (Figure 2).
With the publication of the CASSINI15 and AVERT14 trials, several guidelines now recommend or suggest that thromboprophylaxis with DOACs or LMWH may be administered to patients at intermediate to high risk of VTE (Khorana score ≥2).8,9,16,41 In contrast, previous versions of these guidelines suggested that thromboprophylaxis could be considered in selected high-risk patients,42,43 such as those with a high-risk Khorana score based on the positivity threshold of 3 points used in the derivation study (supplemental Table 1).10 This guideline change, which was driven by the results of the CASSINI and AVERT trials in which the Khorana positivity threshold was lowered to 2 points, is likely to affect many cancer patients, because the proportion of cancer patients in whom thromboprophylaxis is indicated may increase from 17% to 47%.17 With this possible profound increase in ambulatory cancer patients who are now eligible for thromboprophylaxis, it is important to be aware of the difference in the potential benefit of thromboprophylaxis in patients at intermediate risk compared with those at high risk. Although several studies have evaluated the cost effectiveness of primary thromboprophylaxis with DOACs in ambulatory cancer patients, no data are currently available for such an approach in the subgroups of intermediate- and high-risk patients specifically.44-46
The risk of major bleeding with thromboprophylaxis was not significantly increased in the overall population in this metaanalysis. However, in both DOAC trials, the RR of major bleeding in DOAC recipients was almost twofold higher than in the comparison groups (RR, 1.96; 95% CI, 0.69-5.50), although this difference was not statistically significant. The absolute risk difference in these trials was 1% (95% CI, −0.3% to 4.6%), translating to an NNH of 100 (95% CI, NNH 22 to ∞ to NNT 334). For LMWH, no increased risk of major bleeding was observed.35-38 A potential difference in bleeding risk could be the result of differences in pharmacodynamics, because DOACs are administered at a half-therapeutic dose in the setting of thromboprophylaxis, whereas LMWH is usually provided at a prophylactic dose. Other potential explanations include the higher risk of gastrointestinal and urogenital bleeding with DOACs in cancer patients, especially in those with gastrointestinal or urogenital cancer.47-49 Notably, the findings of 4 individual trials included in this metaanalysis previously reported a higher risk of clinically relevant nonmajor bleeding in patients receiving thromboprophylaxis than in those who received placebo or standard care.14,15,35,38 Therefore, bleeding risk should remain an important consideration in the decision to prescribe thromboprophylaxis.
The optimal way of identifying ambulatory cancer patients at high risk of VTE remains a matter of debate.2,6,17,40,50,51 In this metaanalysis, we focused on the Khorana score, because it is the most widely studied and used risk-stratification tool. However, a substantial variation in VTE risk has been observed across different tumor types within the same Khorana score risk group.17 This is characterized in this metaanalysis by the differences in VTE incidence in control groups from different studies. For example, in patients with a Khorana score of ≥3, the incidence in the control group of the FRAGMATIC trial was 7.0%, compared with 19.1% in the CONKO-004 trial.36,38 In addition, discrimination of the score seems to be poor in specific cancer types (eg, lung cancer), whereas it is much better in others.2,34,50 Finally, the sensitivity of the Khorana score is modest; 75% of VTE events do not occur in the group at high risk of VTE (Khorana score ≥3 or higher), and 50% do not occur in the group at intermediate to high risk of VTE (Khorana score ≥2). Consequently, half of all ambulatory cancer patients who develop VTE will not be identified as potential candidates for thromboprophylaxis with this score. It is important to note that the group of LMWH studies included various agents with various doses, hampering generalizability of the results, although it is reassuring that we did not find significant heterogeneity in any of the analyses. Another limitation is that no data on clinically relevant nonmajor bleeding or VTE-related death could be obtained, which are both important clinical outcomes when considering thromboprophylaxis. The CASSINI trial performed ultrasound screening to detect asymptomatic proximal DVT during follow-up, which may not correlate well with symptomatic VTE. Five of 6 trials included distal DVT as a primary outcome or did not specify whether proximal or distal DVT was included as a primary outcome. Only the AVERT trial limited the primary outcomes to proximal DVT and PE only, which precluded an analysis without distal DVT in the efficacy outcome. Strengths of this study include the use of a profile-likelihood random-effects model to provide a more conservative yet more reliable CI than the more widely used Dersimonian-Laird random-effects model.24,26 Certainty of evidence was rated using the standardized GRADE approach to aid in the interpretation of the findings. RRs were pooled, because it is well known that they are more consistent across studies than absolute risk differences.27 We then applied these RRs to baseline VTE risks obtained from a large systematic review in which the Khorana score was calculated for >27 000 ambulatory cancer patients with a wide variety of tumor types. This approach provides a better reflection of the estimated risk difference and NNT in clinical practice than when using data from a more selected group included in RCTs.52
In conclusion, among ambulatory cancer patients with an intermediate- to high-risk Khorana score of ≥2, thromboprophylaxis with DOACs or LMWH significantly reduces the risk of VTE (NNT, 25) without a significantly increased risk of major bleeding (NNH, 1000). Compared with LMWH studies, the major bleeding risk seemed to be higher in DOAC studies (NNH, 100). The NNT was twice as high in patients at intermediate risk (NNT, 34) compared with those at high risk (NNT, 17). Clinicians should consider these risks of VTE and bleeding when making decisions about thromboprophylaxis in ambulatory cancer patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
For original data, please contact Floris T. M. Bosch (f.t.bosch@amsterdamumc.nl).
Authorship
Contribution: All authors made a substantial contribution to the design of the study, interpreted the data, and reviewed the manuscript; F.T.M.B. and F.I.M. performed data extraction and analyses and wrote the first draft; and all authors critically revised the paper for important intellectual content, approved the final version, and agreed with the submission.
Conflict-of-interest disclosure: P.W.K. declares research funding from Daiichi Sankyo and Roche Diagnostics. S.M. reports grants and fees paid to her institution, outside the present work, from AbbVie, Bristol-Myers Squibb/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. N.v.E. reports receiving advisory board honoraria from Daiichi-Sankyo, LEO Pharma, and Bayer, which were transferred to his institution. The remaining authors declare no competing financial interests.
Correspondence: Floris T. M. Bosch, Department of Vascular Medicine, Amsterdam Cardiovascular Science, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: f.t.bosch@amsterdamumc.nl.
References
- 1.Ahlbrecht J, Dickmann B, Ay C, et al. . Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2012;30(31):3870-3875. [DOI] [PubMed] [Google Scholar]
- 2.van Es N, Di Nisio M, Cesarman G, et al. . Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica. 2017;102(9):1494-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Health. 2018;21(4):449-455. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. [DOI] [PubMed] [Google Scholar]
- 6.Mulder FI, Bosch FTM, van Es N. Primary thromboprophylaxis in ambulatory cancer patients: where do we stand? Cancers (Basel). 2020;12(2):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12:CD008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Key NS, Khorana AA, Kuderer NM, et al. . Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496-520. [DOI] [PubMed] [Google Scholar]
- 9.Wang TF, Zwicker JI, Ay C, et al. . The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17(10):1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streiff MB, Holmstrom B, Ashrani A, et al. . Cancer-associated venous thromboembolic disease, version 1.2015: Featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw. 2015;13(9):1079-1095. [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA. Targeted prophylaxis in cancer: the evidence accumulates. Intern Emerg Med. 2013;8(3):187-189. [DOI] [PubMed] [Google Scholar]
- 13.Khorana AA, Otten H-M, Zwicker JI, Connolly GC, Bancel DF, Pabinger I; Subcommittee on Haemostasis and Malignancy of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis . Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1928-1931. [DOI] [PubMed] [Google Scholar]
- 14.Carrier M, Abou-Nassar K, Mallick R, et al. ; AVERT Investigators . Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711-719. [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, Soff GA, Kakkar AK, et al. ; CASSINI Investigators . Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380(8):720-728. [DOI] [PubMed] [Google Scholar]
- 16.Farge D, Frere C, Connors JM, et al. ; International Initiative on Thrombosis and Cancer (ITAC) advisory panel . 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566-e581. [DOI] [PubMed] [Google Scholar]
- 17.Mulder FI, Candeloro M, Kamphuisen PW, et al. ; CAT-prediction collaborators . The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019;104(6):1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Framework Open Science. Primary thromboprophylaxis in cancer patients at high risk of venous thromboembolism: a systematic review and meta-analysis. https://osf.io/fgwer.
- 20.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Savović J, Page MJ, Sterne JA, eds. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). https://www.bristol.ac.uk/media-library/sites/social-community-medicine/images/centres/cresyda/RoB2-0_indiv_main_guidance.pdf. Accessed 15 June 2020.
- 22.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 23.Ay C, Dunkler D, Marosi C, et al. . Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377-5382. [DOI] [PubMed] [Google Scholar]
- 24.Cornell JE, Mulrow CD, Localio R, et al. . Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267-270. [DOI] [PubMed] [Google Scholar]
- 25.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. [DOI] [PubMed] [Google Scholar]
- 26.Guolo A, Varin C. Random-effects meta-analysis: the number of studies matters. Stat Methods Med Res. 2017;26(3):1500-1518. [DOI] [PubMed] [Google Scholar]
- 27.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575-1600. [DOI] [PubMed] [Google Scholar]
- 28.Schnemann HJ, Oxman AD, Vist GE, et al. . Interpreting results and drawing conclusions In: Higgins JPT, Thomas J, eds. Cochrane Handbook for Systematic Reviews of Interventions, Hoboken, NJ: Wiley; 2020:359-387. [Google Scholar]
- 29.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 31.Haas SK, Freund M, Heigener D, et al. ; TOPIC Investigators . Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18(2):159-165. [DOI] [PubMed] [Google Scholar]
- 32.Perry JR, Julian JA, Laperriere NJ, et al. . PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8(9):1959-1965. [DOI] [PubMed] [Google Scholar]
- 33.Li A, Kuderer NM, Garcia DA, et al. . Direct oral anticoagulant for the prevention of thrombosis in ambulatory patients with cancer: a systematic review and meta-analysis. J Thromb Haemost. 2019;17(12):2141-2151. [DOI] [PubMed] [Google Scholar]
- 34.van Es N, Ventresca M, Di Nisio M, et al. . The Khorana score for prediction of venous thromboembolism in cancer patients: an individual patient data meta‐analysis. J Thromb Haemost. 2020;18(8):1940-1951. [DOI] [PubMed] [Google Scholar]
- 35.Agnelli G, George DJ, Kakkar AK, et al. ; SAVE-ONCO Investigators . Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601-609. [DOI] [PubMed] [Google Scholar]
- 36.Pelzer U, Opitz B, Deutschinoff G, et al. . Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol. 2015;33(18):2028-2034. [DOI] [PubMed] [Google Scholar]
- 37.Lecumberri R, López Vivanco G, Font A, et al. . Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thromb Res. 2013;132(6):666-670. [DOI] [PubMed] [Google Scholar]
- 38.Macbeth F, Noble S, Evans J, et al. . Randomized phase III trial of standard therapy plus low molecular weight heparin in patients with lung cancer: FRAGMATIC trial. J Clin Oncol. 2016;34(5):488-494. [DOI] [PubMed] [Google Scholar]
- 39.Chan NC, Stehouwer AC, Hirsh J, et al. . Lack of consistency in the relationship between asymptomatic DVT detected by venography and symptomatic VTE in thromboprophylaxis trials. Thromb Haemost. 2015;114(5):1049-1057. [DOI] [PubMed] [Google Scholar]
- 40.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291-292. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network Cancer-associated venous thromboembolic disease (version 1. 2020). https://www.nccn.org/. Accessed 15 June 2020.
- 42.Lyman GH, Bohlke K, Khorana AA, et al. ; American Society of Clinical Oncology . Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network Cancer-Associated Venous Thromboembolic Disease (Version 1. 2014). https://www.nccn.org/. Accessed 15 June 2020. [DOI] [PubMed]
- 44.Kimpton M, Kumar S, Wells PS, Carrier M, Thavorn K Cost-utility analysis of apixaban compared to usual care for the primary thromboprophylaxis of ambulatory cancer patients initiating chemotherapy [abstract]. Blood. 2019;134(suppl 1). Abstract 329.
- 45.Ryan E, Salinaro J, Havrilesky LJ, Davidson BA. Venous thromboembolism prophylaxis in ambulatory cancer patients initiating chemotherapy: a cost-effectiveness analysis [abstract] J Clin Oncol. 2020;38.(suppl) Abstract 7074. [Google Scholar]
- 46.Du J, Wu B. New oral anticoagulants for thromboprophylaxis in patients with cancer receiving chemotherapy: an economic evaluation in a Chinese setting. Clin Drug Investig. 2020;40(7):653-663. [DOI] [PubMed] [Google Scholar]
- 47.Young AM, Marshall A, Thirlwall J, et al. . Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023. [DOI] [PubMed] [Google Scholar]
- 48.Raskob GE, van Es N, Verhamme P, et al. ; Hokusai VTE Cancer Investigators . Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. [DOI] [PubMed] [Google Scholar]
- 49.Prandoni P, Lensing AWA, Piccioli A, et al. . Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. 2002;100(10):3484-3488. [DOI] [PubMed] [Google Scholar]
- 50.Pabinger I, van Es N, Heinze G, et al. . A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts [published correction appears in Lancet Haematol. 2018;5(8):e332]. Lancet Haematol. 2018;5(7):e289-e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14(9):1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid CH, Lau J, McIntosh MW, Cappelleri JC. An empirical study of the effect of the control rate as a predictor of treatment efficacy in meta-analysis of clinical trials. Stat Med. 1998;17(17):1923-1942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.