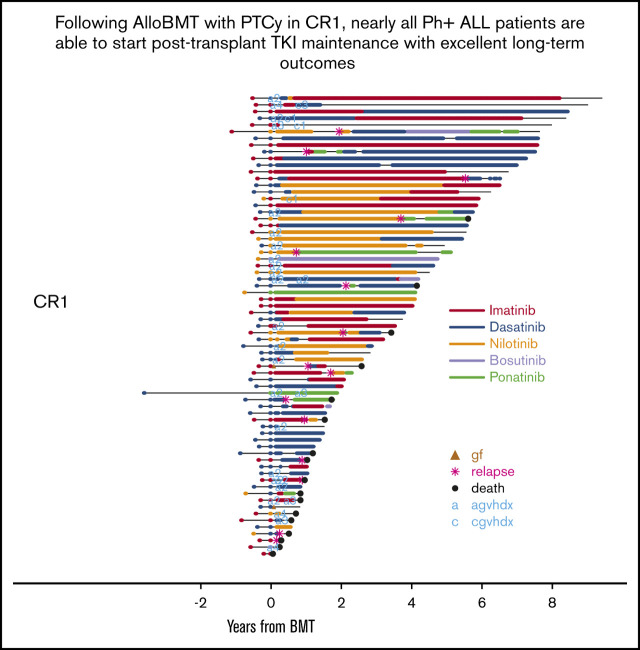
Key Points
In Ph+ ALL in CR1, alloBMT with PTCy yielded favorable outcomes with nonmyeloablative conditioning after initial induction with dasatinib.
Patients with MRD by multicolor flow cytometry but not BCR-ABL polymerase chain reaction on pretransplant evaluation had poorer RFS and OS.
Abstract
Allogeneic blood or marrow transplantation (alloBMT) is standard of care for adults with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in first complete remission (CR1). The routine pretransplant and posttransplant use of tyrosine kinase inhibitors (TKIs) has dramatically improved outcomes, but the optimal conditioning regimen, donor type, and TKI remain undefined. The bone marrow transplant database at Johns Hopkins was queried for adult patients with de novo Ph+ ALL who received alloBMT using posttransplantation cyclophosphamide (PTCy) as a component of graft-versus-host disease (GVHD) prophylaxis from 2008 to 2018. Among transplants for Ph+ ALL, 69 (85%) were performed in CR1, and 12 (15%) were performed in second or greater remission (CR2+). The majority of transplants (58%) were HLA haploidentical. Nearly all patients (91.4%) initiated TKI posttransplant. For patients in CR1, the 5-year relapse-free survival (RFS) was 66%. The use of nonmyeloablative conditioning, absence of measurable residual disease (MRD) according to flow cytometry at transplant, and the use of dasatinib vs imatinib at diagnosis were associated with improved overall survival (OS) and RFS. Neither donor type nor recipient age ≥60 years affected RFS. When analyzing all transplants, alloBMT in CR1 (vs CR2+) and the absence of pretransplant MRD were associated with improved RFS. Most relapses were associated with the emergence of kinase domain mutations. The cumulative incidence of grade 3 to 4 acute GVHD at 1 year was 9%, and moderate to severe chronic GVHD at 2 years was 8%. Nonmyeloablative alloBMT with PTCy for Ph+ ALL in an MRD-negative CR1 after initial treatment with dasatinib yields favorable outcomes.
Visual Abstract
Introduction
Before the introduction of imatinib, 5-year overall survival (OS) with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) was <20% with chemotherapy alone but improved to ∼40% with allogeneic blood or marrow transplantation (alloBMT) in first complete remission (CR1).1,2 These outcomes improved dramatically with tyrosine kinase inhibitors (TKIs), and studies incorporating TKIs with chemotherapy followed by alloBMT in CR1 continue to suggest this approach yields the best outcomes for transplant-eligible patients.3-5 These reports have largely relied on myeloablative conditioning (MAC) with nonrelapse mortality (NRM) of at least 25% and required an HLA-matched donor.3,6 Unfortunately, most patients do not have an HLA-matched sibling, and <30% of US ethnic minorities have a matched unrelated donor (MUD) option.7
Early transplants were restricted to matched sibling donors (MSDs). However, alternative donor options are expanding with excellent outcomes. For example, in Ph+ ALL, the European Group for Blood and Marrow Transplantation (EBMT) reported improved leukemia-free survival due to decreased relapse among MUD transplants compared with MSD transplants.8 AlloBMT using haploidentical related (haplo) donors is available to most patients with outcomes similar to matched donor alloBMT when high-dose posttransplantation cyclophosphamide (PTCy) is used as a component of graft-versus-host disease (GVHD) prophylaxis.9-13 Previous analyses of haplo alloBMT have pooled Ph+ ALL with other hematologic malignancies, and thus more data are needed on its efficacy in Ph+ ALL.
MAC leads to high rates of NRM,6 and it is unsuitable for older patients, in whom Ph+ ALL is common.14 Nonmyeloablative conditioning (NMAC) reduces NRM and can be used in older and less fit patients. Center for International Bone Marrow Transplant Research data show reduced NRM but increased relapse incidence, yielding comparable OS for patients receiving reduced-intensity conditioning transplants compared with MAC for Ph+ ALL.6 The transplants in this dataset were performed before second-generation TKIs were routinely used in Ph+ ALL, and most patients did not receive posttransplant TKI maintenance. Although the prophylactic use of TKIs posttransplant remains controversial, an EBMT registry study suggested it was associated with improved leukemia-free survival and OS, as have prospective studies.8,15,16 Thus, when combined with NMAC, posttransplant TKI maintenance may overcome the advantage of leukemia control associated with MAC.
In addition to the need for data on outcomes using alternative donors and NMAC for Ph+ ALL, a better understanding of the role of TKIs in Ph+ ALL is needed, including the optimal agent, dosing, and duration of posttransplant maintenance. Some studies have limited posttransplant maintenance to 1 year, whereas others have extended maintenance up to 5 years.4,8 One prospective study randomized patients to receive prophylactic posttransplant imatinib vs initiating imatinib upon detection of measurable residual disease (MRD) and found similar survival outcomes; however, the patients were enrolled after transplantation, leading to the potential for selection bias.17
The current article presents results for Ph+ ALL patients who underwent transplant with MAC or NMAC and universally received PTCy. By including all Ph+ ALL patients undergoing transplant at our institution over a decade, we describe a comprehensive experience with the initiation, dosing, and duration of posttransplant TKI prophylaxis, including patterns of relapse.
Patients and methods
Patients
The BMT database at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins was screened for adults (age ≥18 years) with de novo Ph+ ALL who received alloBMT using PTCy between January 2008 and August 2018. Patient demographic characteristics, disease characteristics, and pretransplantation treatment were obtained. This study was conducted with a waiver of informed consent following approval by the Johns Hopkins Institutional Review Board.
Preparative regimen, donor typing, and GVHD prophylaxis
Before 2015, the choice of preparative regimen (MAC vs NMAC) was at the discretion of the treating physician based on patient characteristics and available clinical trials. Beginning in 2015, patients received NMAC exclusively. Additional information regarding the definition of donor types, preparative regimens, and GVHD prophylaxis have been reported previously and can be found in the supplemental Methods.10,18-20
MRD assessment
Bone marrow biopsies were performed within 30 days before alloBMT and again 50 to 75 days’ posttransplant according to institutional standards. Multicolor flow cytometry (MFC) was performed on bone marrow aspirate samples, and MRD was defined with a level of sensitivity of 1/10 000 (0.01%) cells. Most patients also had BCR-ABL polymerase chain reaction (PCR) testing from the bone marrow or peripheral blood that was normalized to a control gene (ABL1). A quantitative value was reported for patients with a major transcript (p210) with a limit of detection of 0.002% BCR-ABL1 to ABL1 ratio after March 29, 2018, and 0.01% before that date.21-23 A qualitative value (ie, negative or positive) was reported for patients with a minor transcript (p190) with a limit of detection similar to that for the p210 transcript. This assay was performed at the time of standard bone marrow biopsies and intermittently after transplantation.
TKI usage
TKI use was determined from patient charts, including dates of initiation, discontinuation, and dosing. The goal for all patients was to initiate a TKI after transplantation, although the timing of initiation and the choice of TKI were at the discretion of the treating physicians. We evaluated the percentage of evaluable, posttransplant days over 12 months starting from day 31 that patients used TKIs. TKI use on ≥85% of days in the first posttransplant year has previously been defined as successful maintenance.16 For patients who relapsed, died, or were lost to follow-up before day 395, the number of evaluable days was calculated from day 31 until the first event. For relapsed patients, the results of kinase domain (KD) mutation testing were recorded when available.
Statistical analysis
The primary outcomes were OS, defined as the time from alloBMT to death or last follow-up, and relapse free survival (RFS), defined as the time from alloBMT to death, relapse, or last follow-up, whichever occurred first. Patients undergoing a second transplant were censored for OS at the time of their second transplant. Relapse was defined as the reappearance of blasts in the blood or bone marrow (>5%) or in any extramedullary site after CR.24 Characteristics of patients were summarized and compared according to conditioning regimen intensity by using the Student t test for continuous variables and Fisher’s exact test for categorical variables. Estimators of OS and RFS were reported by using the Kaplan-Meier method. Differences in time-to-event outcomes were estimated by using Cox proportional hazards models for RFS and OS, or Fine and Gray’s model for cumulative incidence of relapse/NRM considering competing events.25 Additional details of the statistical analysis are reported in the supplemental Methods.
Results
Patient characteristics
Between January 2008 and August 2018, a total of 81 alloBMTs involving 76 unique patients were performed for Ph+ ALL at Johns Hopkins using PTCy. All patients were in morphologic remission at transplant. Sixty-nine transplants (85%) were performed in CR1, and 12 transplants (15%) were performed in second or greater remission (CR2+). Demographic characteristics are presented in Table 1, including the hematopoietic cell transplantation–specific comorbidity index and Karnofsky performance status.26,27 Based on the reversed Kaplan-Meier method, the median follow-up was 4.7 years (range, 0.04-9.4 years). The median age at transplant was 49.2 years (range, 21-71.9 years) for transplants in CR1. The majority of patients undergoing transplant in CR1 had received the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) regimen (76.8%), whereas the remainder received therapy based on the E2993 protocol (11.6%) or other chemotherapy regimens (11.6%).1,28 Among patients undergoing transplant in CR1, baseline characteristics were equalized between patients who received MAC vs NMAC, except for a significantly higher burden of comorbidities as assessed by using the hematopoietic cell transplantation–specific comorbidity index among those undergoing transplant with NMAC. Among 12 patients transplanted in CR2+, the median duration of CR1 was 16 months, and 6 had undergone previous alloBMT.
Table 1.
Demographic characteristics according to remission status (CR1 vs CR2+) and conditioning regimen for patients in CR1
| Characteristic | All (N = 81) | CR1 MAC (n = 26) | CR1 NMAC (n = 43) | P | CR2+ (n = 12) |
|---|---|---|---|---|---|
| Female sex, n (%) | 46 (56.8) | 19 (73.1) | 21 (48.8) | .07 | 6 (50) |
| Median age (range), y | 49.2 (21.0-71.9) | 46.1 (21-63.1) | 50.4 (26.7-71.9) | .1 | 51.3 (29.5-65.5) |
| Age >60 y, n (%) | 17 (20.1) | 2 (7.7) | 12 (27.9) | .06 | 3 (25) |
| Median WBC at diagnosis (range), ×103/μL | 18 (1-386) | 19 (2-300) | 18 (1-386) | .79 | |
| Prior CNS involvement, n (%) | 7 (8.6) | 0 (0) | 2 (4.7) | 5 (41.7) | |
| Transcript at diagnosis, n (%) | |||||
| p190 alone | 49 (60.4) | 16 (61.5) | 25 (58.1) | .47 | 8 (66.7) |
| p210 alone | 12 (14.8) | 2 (7.7) | 8 (18.6) | 2 (16.7) | |
| Other | 3 (3.7) | 2 (7.7) | 1 (2.3) | 0 | |
| Unknown | 17 (21.0) | 6 (23.0) | 9 (20.9) | 2 (16.7) | |
| TKI at diagnosis, n (%) | .07 | ||||
| Imatinib | 15 (57.7) | 13 (30.2) | |||
| Dasatinib | 8 (30.7) | 25 (58.1) | |||
| Nilotinib | 3 (11.5) | 5 (11.6) | |||
| HCT-CI score, n (%)* | .001 | ||||
| 0 (low) | 25 (31.6) | 15 (57.5) | 7 (17.1) | 3 (25) | |
| 1-2 (intermediate) | 30 (38.0) | 8 (30.8) | 16 (39.0) | 6 (50) | |
| 3+ (high) | 24 (30.4) | 3 (11.5) | 18 (43.9) | 3 (25) | |
| Median KPS (range) | 90 (80-100) | 90 (90-100) | 90 (80-100) | .1 | 90 (80-100) |
| Prior allogeneic transplant, n (%) | 6 (50) | ||||
| Median duration of CR1 (range), mo | 16 (5.9-50.5) | ||||
| CR1 duration <1 y, n (%) | 5 (41.7) |
CNS, central nervous system; HCT-CI, hematopoietic cell transplantation–specific comorbidity index; KPS, Karnofsky performance status.
Pulmonary function test results were unavailable for 2 patients who were omitted from analysis for HCT-CI.
AlloBMT characteristics are summarized in supplemental Table 1. The majority (62.3%) of patients undergoing transplant in CR1 received NMAC, and most grafts were from haploidentical donors (55.1%); other donor types were MSDs (23.2%), MUDs (18.8%), and mismatched unrelated donors (mMUDs; 2.9%). Patients undergoing transplant with NMAC in CR1 were more likely to receive haplo grafts than those transplanted with MAC (76.7% vs 19.2%; P < .001). The majority of CR1 patients receiving MAC underwent transplant from 2008 to 2013 (92.3% vs 30.2% of NMAC transplants; P = .0001), with most receiving a chemotherapy-based preparative regimen. All patients continued their pretransplant TKI until the start of conditioning, with patients transplanted in CR1 taking dasatinib (46.4%), imatinib (34.8%), nilotinib (14.5%), ponatinib (2.9%), or bosutinib (1.4%). Among the transplants in CR2+, patients were taking ponatinib (n = 6), dasatinib (n = 3), or imatinib (n = 3) at transplant.
Pretransplant MRD assessment
Pretransplant MRD was assessed by MFC and/or BCR-ABL PCR (supplemental Table 2). Most patients transplanted in CR1 had no detectable MRD according to MFC. Patients transplanted in CR1 using MAC were more likely to be MRD+ than those undergoing transplant with NMAC (23.1% vs 0%; P = .002). The presence of MRD according to MFC before transplantation in CR1 based on the TKI used at treatment initiation was 7.8% for imatinib, 6.1% for dasatinib, and 28.6% for nilotinib (P = .22). According to donor type, there was a trend toward more MRD according to MFC with MSD (25%) and MUD (14.3%) than haploidentical (6.4%) or mMUD (0%) (P = .18) (supplemental Table 3). When assessed by using PCR, 10.1% of patients transplanted in CR1 were MRD+, 62.3% were MRD–, and 27.5% were unknown. Among those who had pretransplant PCR testing, the frequency of MRD based on TKI used at treatment initiation was 13.6% for imatinib, 10% for dasatinib, and 33% for nilotinib (P = .31). Among patients with pretransplant MRD assessment according to MFC and PCR, the observed agreement was 78%, with a skewness distribution that most patients (74%) were negative by both (supplemental Table 4).
Incidence of GVHD and graft failure
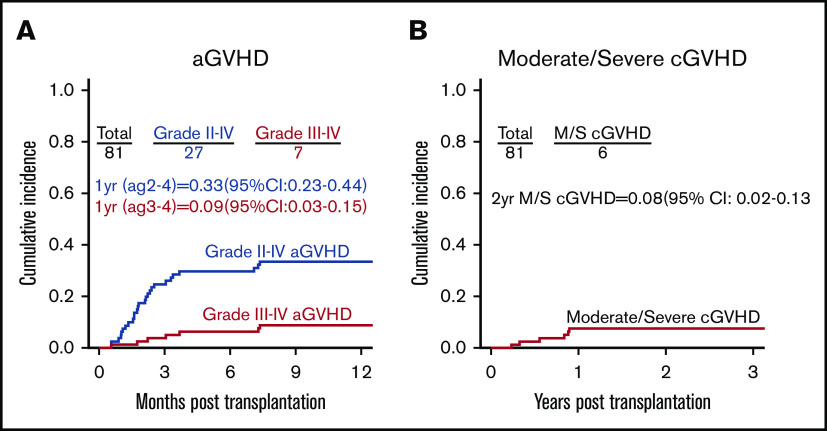
The incidence of graft failure for all 81 transplants was 2.5%. Among transplants in CR1, the incidence of graft failure was 0% with MAC vs 4.7% with NMAC (P = .52). For all patients, the cumulative incidence of grade 2 to 4 acute GVHD (aGVHD) at 1 year was 33% (95% CI, 23-44), and cumulative incidence of grade 3 to 4 aGVHD at 1 year was 9% (95% CI, 3-15). The incidence of moderate or severe cGVHD at 2 years was 8% (95% CI, 2-13) (Figure 1).
Figure 1.
Posttransplant GVHD incidence. (A) Cumulative incidence of grade 2 to 4 aGVHD and grade 3 to 4 aGVHD. (B) Cumulative incidence of moderate to severe (M/S) chronic GVHD (cGVHD).
TKI use after alloBMT
Posttransplant TKI maintenance was initiated after 91.4% of transplants. Seven patients failed to start TKI maintenance due to death within 100 days (n = 2), cytopenias (n = 4), and aGVHD (n = 1). Posttransplant TKI maintenance use is summarized in Table 2 and supplemental Figure 1. The median time to prophylactic posttransplant TKI initiation was 56 days (range, 31-389 days) and was similar regardless of conditioning regimen (MAC 59 days vs NMAC 55 days; P = .7). The median duration of continuous TKI use from posttransplant initiation until discontinuation or TKI change due to side effects, relapse, or death exhibited a significantly longer duration of continuous use for imatinib (median, 183 days) and nilotinib (median, 368 days) than dasatinib (median, 75 days; P = .02 for imatinib vs dasatinib and P = .007 for dasatinib vs nilotinib). The distribution of posttransplant TKI maintenance over 12 months starting on day 31 is shown in supplemental Figure 2. Overall, 44% of patients were on TKI for ≥85% of nonrelapse evaluable days during that time. Patients who developed grade 3 to 4 aGVHD were less likely to achieve this milestone than those who did not (0% vs 48.6%; P = .02).
Table 2.
Posttransplant TKI initiation, dosing, continuous use, and 85% use in year 1
| Variable | All (N = 81), n (%) | CR1 MAC (n = 26), n (%) | CR1 NMAC (n = 43), n (%) | P | CR2+ (n = 12), n (%) | Median time to posttransplant TKI start (range), d | Median duration of continuous treatment with first posttransplant TKI (range), d | Mean TKI dosing in year 1 posttransplant |
|---|---|---|---|---|---|---|---|---|
| First posttransplant TKI | ||||||||
| Imatinib | 19 (23.5) | 9 (34.6) | 9 (20.9) | 1 (8.3) | 48.5 (36-143) | 183 (1-2698) | 393.2 mg | |
| Dasatinib | 35 (43.2) | 11 (42.3) | 20 (46.5) | 4 (33.3) | 53.5 (32-389) | 75 (1-1664) | 79.6 mg | |
| Nilotinib | 13 (16) | 4 (15.34) | 8 (18.6) | 1 (8.3) | 66 (36-117) | 368 (23-1607) | 300.8 mg | |
| Bosutinib | 1 (1.2) | 0 | 1 (2.3) | 75 | 1654 | 500 mg | ||
| Ponatinib | 6 (7.4) | 0 | 2 (4.7) | 4 (33.3) | 61 (31-119) | 153 (9-916) | 26.5 mg | |
| None | 7 (8.6) | 2 (7.7) | 3 (7.0) | 2 (16.7) | ||||
| Received TKI on 85% of nonrelapse evaluable days 31-395 | 36 (44.4) | 9 (34.6) | 22 (51.1) | .22 | 5 (41.7) | Dosing is daily, except nilotinib, which is twice daily |
Overall outcomes
At 5 years, RFS for all 81 transplants was 56% (95% CI, 43.5-66.7). In univariate analysis, transplant in CR1 vs CR2+ and the presence of MRD according to MFC before transplant affected 5-year RFS, as shown in Table 3 and Figure 2. There was no difference in 5-year RFS when MRD was assessed according to PCR. Factors that did not lead to a statistically significant difference in RFS included whether the transplant was performed from 2008 to 2013 vs 2014 to 2018, conditioning intensity (NMAC vs MAC), age >60 years, and donor type.
Table 3.
Hazard ratios with 95% CIs for RFS, OS, CIR, and NRM according to pretransplant factors for all transplants and those in CR1
| Variable | RFS (95% CI) | OS (95% CI) | CIR (95% CI) | NRM (95% CI) |
|---|---|---|---|---|
| All transplants | ||||
| 2008-2013 (N = 45) vs 2014-2018 (N = 36) | 2.14 (0.99-4.65), P = .052 | 2.36 (0.93-5.98), P = .07 | 2.07 (0.82-5.24), P = .13 | 1.91 (0.5-7.25), P = .34 |
| NMAC (n = 54) vs MAC (n = 27) | 0.61 (0.30-1.22), P = .16 | 0.43 (0.19-0.98), P = .045 | 0.65 (0.28-1.51), P = .32 | 0.72 (0.20-2.54), P = .61 |
| Age ≥60 y (n = 17) vs age <60 y (n = 64) | 0.97 (0.42-2.22), P = .93 | 0.73 (0.25-2.15), P = .57 | 0.70 (0.24-2.03), P = .51 | 1.66 (0.42-6.46), P = .47 |
| MRD+ Pre by MFC (n = 9) vs MRD– (n = 70) | 2.57 (1.05-6.28), P = .039 | 2.49 (1.28-4.86), P = .007 | 2.28 (0.71-7.34), P = .17 | 1.99 (0.46-8.61), P = .36 |
| MRD+ Pre by PCR (n = 9) vs MRD– (n = 50) | 1.12 (0.38-3.27), P = .84 | 1.01 (0.29-3.51), P = .99 | 1.19 (0.31-4.55), P = .80 | 0.78 (0.08-6.88), P = .82 |
| MRD+ Pre by PCR (n = 9) vs MRD–/untested (n = 72) (sensitivity analysis) | 1.18 (0.41-3.35), P = .76 | 1.13 (0.33-3.81), P = .19 | 1.19 (0.32-4.43), P = .80 | 0.89 (0.10-7.61), P = .92 |
| Donor (vs MSD (n = 16)) | ||||
| MUD (n = 16) | 0.66 (0.22-1.97), P = .46 | 0.75 (0.22-2.6), P = .65 | 1.04 (0.32-3.37), P = .95 | NA |
| Haplo (n = 47) | 0.87 (0.37-2.06), P = .75 | 0.77 (0.27-2.16), P = .62 | 0.74 (0.27-2.02), P = .55 | 1.23 (0.27-5.71), P = .79 |
| CR1 (n = 69) vs CR2+ (n = 12) | 0.25 (0.12-0.52), P = .0002 | 0.23 (0.1-0.53), P = .0006 | 0.34 (0.14-0.81), P = .015 | 0.27 (0.08-0.87), P = .03 |
| CR1 only | ||||
| TKI at Dx (vs imatinib, n = 28) | ||||
| Dasatinib (n = 33) | 0.21 (0.07-0.66), P = .007 | 0.16 (0.06-0.75), P = .02 | 0.29 (0.08-1.03), P = .06 | 0.16 (0.02-1.35), P = .09 |
| Nilotinib (n = 8) | 0.75 (0.22-2.6), P = .66 | 0.66 (0.14-2.99), P = .59 | 1.34 (0.33-5.49), P = .69 | NA |
| NMAC (n = 43) vs MAC (n = 26) | 0.37 (0.16-0.88), P = .02 | 0.15 (0.04-0.53), P = .0035 | 0.48 (0.18-1.3), P = .15 | 0.29 (0.06-1.56), P = .15 |
| Age ≥60 y (n = 14) vs age <60 y (n = 55) | 1.14 (0.42-3.11), P = .79 | 0.63 (0.14-2.83), P = .55 | 0.82 (0.24-2.77), P = .75 | 2.06 (0.38-11.32), P = .41 |
| MRD+ Pre by MFC (n = 6) vs MRD– (n = 62) | 3.65 (1.22-10.9), P = .02 | 5.63 (1.74-18.2), P = .004 | 3.55 (0.94-13.3), P = .06 | 2.09 (0.28-15.74), P = .48 |
| MRD+ Pre by PCR (n = 7) vs MRD– (n = 43) | 0.75 (0.17-3.32), P = .71 | 0.61 (0.08-4.86), P = .64 | 0.43 (0.05-3.62), P = .44 | 2.13 (0.21-21.11), P = .52 |
| MRD+ Pre by PCR (n = 7) vs MRD–/untested (n = 62) (sensitivity analysis) | 0.82 (0.19-3.5), P = .79 | 0.62 (0.08-4.77), P = .65 | 0.49 (0.06-4.15), P = .52 | 1.85 (0.21-16.5), P = .58 |
| Donor (vs MSD, n = 16) | ||||
| MUD (n = 13) | 0.37 (0.1-1.43), P = .15 | 0.36 (0.07-1.88), P = .23 | 0.57 (0.14-2.3), P = .43 | NA |
| Haplo (n = 38) | 0.64 (0.25-1.63), P = .35 | 0.51 (0.16-1.62), P = .26 | 0.61 (0.21-1.78), P = .37 | 0.81 (0.16-4.2), P = .8 |
| 2008-2013 (N = 37) vs 2014-2018 (N = 32) | 2.75 (1.00-7.52), P = .05 | 2.14 (0.67-6.84), P = .20 | 3.23 (0.92-11.3), P = .07 | 1.74 (0.33-9.28), P = .52 |
CI, confidence interval; CIR, cumulative incidence of relapse; Dx, diagnosis; NA, not applicable; Pre, pretransplant.
Figure 2.
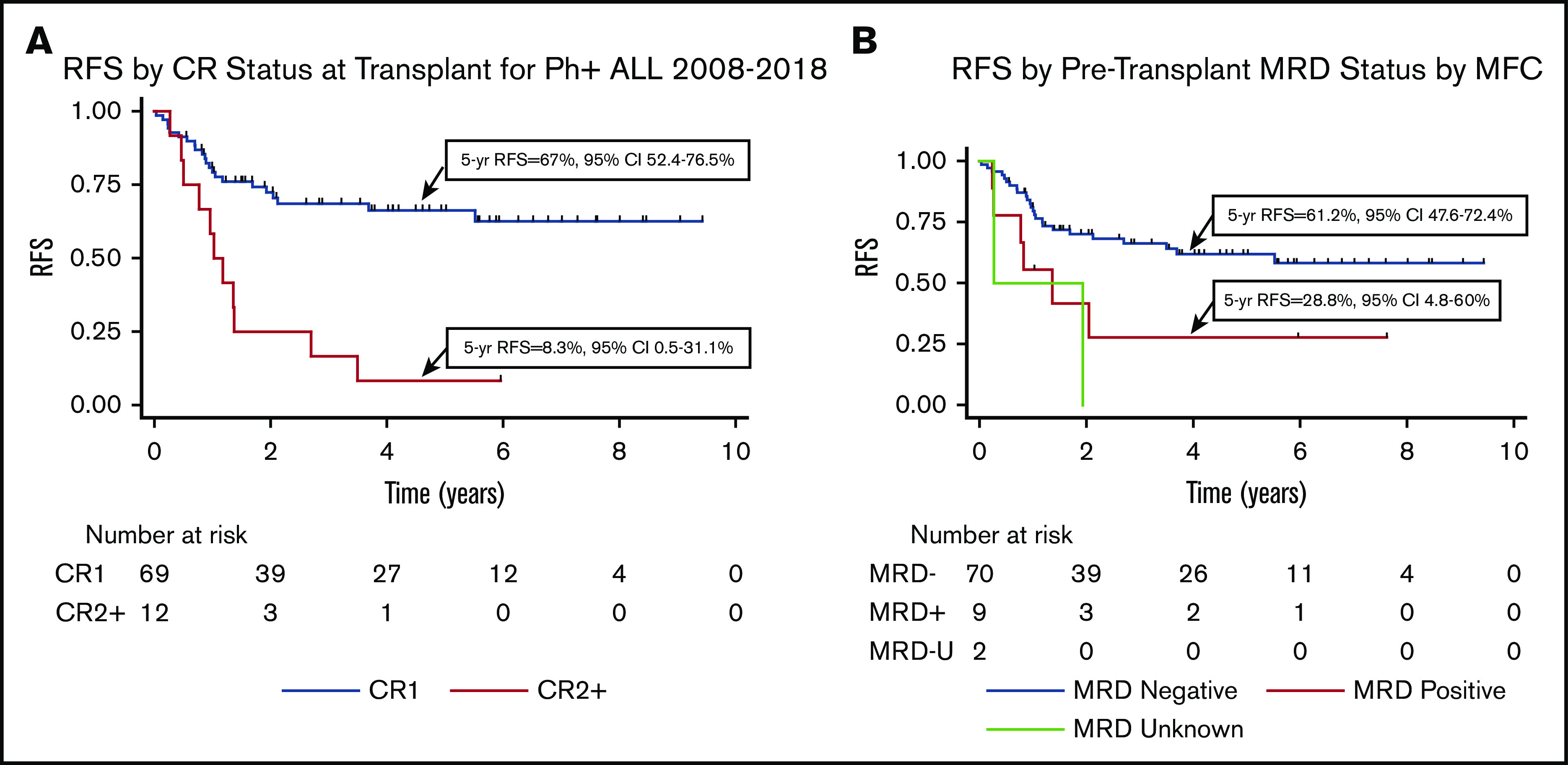
Transplant outcomes for all transplants. (A) RFS for transplants in CR1 vs CR2 or beyond (CR2+). (B) RFS based on MRD status at transplant as assessed by using MFC. MRD-U, MRD unknown.
Among patients transplanted in CR1, the 5-year OS was 77.6% (95% CI, 64.8-86.2), and RFS was 66% (95% CI, 52.4-76.5). The 5-year NRM was 9.9% (95% CI, 4.6-20.9), and cumulative incidence of relapse was 26.7% (95% CI, 16.8-40.8). As shown in Table 3 and Figure 3, in univariate analysis, the absence of pretransplant MRD according to MFC, NMAC, more recent transplant (2014-2018 vs 2008-2013), and the use of dasatinib at leukemia diagnosis were all associated with improved RFS. When considering the TKI used at diagnosis, 5-year RFS was 83.0% for those treated with dasatinib, 49.9% with imatinib, and 62.5% with nilotinib. Donor type and age >60 years did not lead to a difference in RFS. As shown in supplemental Figure 3, patients transplanted in CR1 with a haploidentical donor had a significantly better RFS following NMAC than MAC (hazard ratio, 0.13; P = .002), whereas this difference was not significant for those transplanted with a matched donor (hazard ratio, 0.32; P = .27). The small patient numbers preclude a multivariable analysis.
Figure 3.
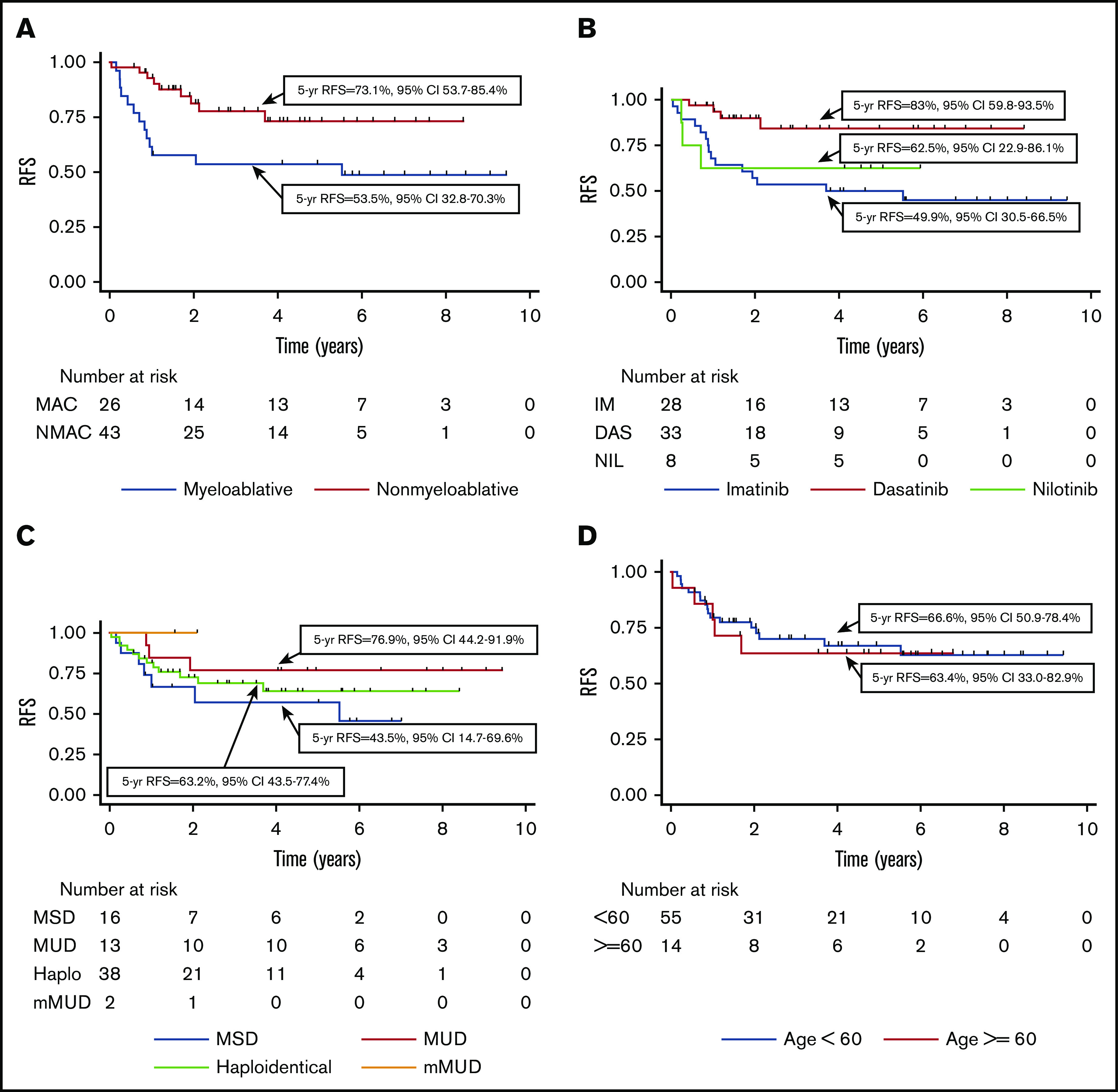
Transplant outcomes for CR1 transplants only. (A) RFS depending on conditioning intensity. (B) RFS depending on TKI used at diagnosis. (C) RFS according to donor type. (D) RFS according to recipient age (<60 years vs ≥60 years).
Posttransplant TKI maintenance, relapse, KD mutations, and MRD
Among patients initiating TKI prophylaxis, the median duration of treatment was 1.7 years (range, 0.03-8.4 years). Maintenance therapy was discontinued in 20 cases (27.0%) before relapse. Among those discontinuing therapy, 12 (60%) remain in treatment-free remission with a median follow-up of 5.9 years (range, 1.5-9.4 years). Four patients (20%) died of nonrelapse causes, and 3 patients (15%) experienced a relapse at a median of 1.9 years (range, 1.7-2.1 years) posttransplant. One patient developed recurrent MRD according to BCR-ABL PCR, resumed dasatinib, and is in an ongoing MRD– remission. The median duration of posttransplant TKI prophylaxis before discontinuation was 46.5 months in those who remain in treatment-free remission vs 15.6 months in those who relapsed (P = .01). For those in whom maintenance therapy is ongoing, 34 patients remain in remission at a median of 3.9 years’ posttransplant (range, 0.6-8.4 years), and 2 patients died of nonrelapse causes. A total of 18 relapses occurred on maintenance at a median of 0.8 year posttransplant (range, 0.15-5.5 years); 67% of relapses occurred within 12 months of transplant. The median time to TKI initiation was 70 days among those who relapsed and 55 days among those who did not (P = .6). Results of KD mutation testing for relapsed patients are shown in Table 4.
Table 4.
KD mutations identified at relapse according to CR status at transplant and maintenance TKI at relapse
| Maintenance TKI at relapse | KD mutation | Total | |||||
|---|---|---|---|---|---|---|---|
| T315I | Y253H | F317I | T315I, Y253H | None | Testing unavailable | ||
| CR1 | |||||||
| Imatinib | 1 | 3* | 4 | ||||
| Dasatinib | 2 | 2 | 4 | ||||
| Nilotinib | 1 | 2 | 3 | ||||
| Bosutinib | 0 | ||||||
| Ponatinib | 0 | ||||||
| None | 1 | 1 | 2 | 1 | 5 | ||
| CR2+ | |||||||
| Imatinib | 0 | ||||||
| Dasatinib | 1 | 1 | 1* | 3 | |||
| Nilotinib | 1 | 1 | |||||
| Bosutinib | 1 | 1 | |||||
| Ponatinib | 2 | 2 | |||||
| None | 0 | ||||||
| Total | 5 | 4 | 1 | 1 | 3 | 9 | |
One patient on imatinib in CR1 and 1 patient on dasatinib in CR2 had isolated central nervous system relapses.
BCR-ABL PCR testing from the peripheral blood or bone marrow was performed in the first 75 days’ posttransplant after 66 transplants, showing 11 early molecular relapses (16.7%) without morphologic relapse and 55 molecular remissions (83.3%). The cumulative incidence of subsequent morphologic relapse was significantly increased in those with early molecular relapse compared with molecular remission (hazard ratio, 3.65; P = .02) as shown in supplemental Figure 4. All patients with early molecular relapse initiated a second- or third-generation TKI, and 5 patients achieved an MRD– remission that is ongoing at a median of 4.7 years’ posttransplant (range, 3.8-5.9 years). Among the 49 patients in continuous remission, 48 (98%) were MRD– according to PCR at their most recent assessment, and one was never assessed. Similarly, among the 10 cases of NRM, 9 (90%) were MRD– according to PCR at the last assessment before death, while one was not assessed.
Discussion
AlloBMT remains a standard of care for adults with Ph+ ALL who achieve remission.5,29 However, the optimal timing, donor type, conditioning regimen, and GVHD prophylaxis remain topics of debate. Although the use of posttransplant TKI maintenance is widely accepted,8 the choice of TKI as well as the optimal timing and duration of posttransplant treatment have not been established. This study shows the safety and efficacy of alloBMT for Ph+ ALL using PTCy as GVHD prophylaxis, including the near-universal initiation of posttransplant TKI maintenance. Using this approach, NMAC yielded better outcomes than MAC for transplants in CR1, especially among patients receiving haploidentical grafts, and treatment with dasatinib at diagnosis led to better outcomes than imatinib. Outcomes among patients transplanted in CR2+ and those with pretransplant MRD according to MFC were poor.
Patients transplanted in CR1 had improved RFS and OS after NMAC conditioning compared with MAC. Prior analyses have suggested comparable RFS and OS following reduced-intensity conditioning or MAC in Ph+ ALL due to increased NRM and decreased relapse incidence with MAC.6,8 In the current study, patients transplanted with NMAC had better outcomes despite an objectively higher burden of comorbidities. Furthermore, NMAC allows fit, older patients to undergo alloBMT, with a 5-year RFS of 63.4% in those aged >60 years, which was comparable to outcomes in younger patients. This replicates previous findings for NMAC transplants with PTCy that suggest age does not affect outcomes in selected patients.30 Although the Hyper-CVAD program with ponatinib has shown excellent outcomes in older patients without alloBMT,31 NRM with this regimen in older patients is as high as 38.5%.32 In addition, ponatinib is associated with vascular occlusive events, which are more frequent in older patients.32,33 Thus, NMAC transplant for older Ph+ ALL patients in CR1 represents a viable alternative to continuing chemotherapy. Although those patients undergoing NMAC alloBMT tended to be older with more comorbidities, patients transplanted with MAC were more likely to be MRD+, which also biases this comparison of outcomes between NMAC and MAC. In addition, chemotherapy-based conditioning regimens were used for the majority of MAC transplants, whereas recent evidence suggests better outcomes with total body irradiation–based regimens in ALL.34 The near-universal implementation of posttransplant TKI prophylaxis following both MAC and NMAC differentiates our study from previous studies, and it suggests that consistently excellent outcomes can be achieved with NMAC transplant for Ph+ ALL patients in an MRD-negative CR1 with posttransplant TKI maintenance, including older patients and those with comorbidities.
In addition to age, donor type did not affect outcomes in this study, similar to a previous study showing excellent survival with haploidentical transplant in Ph+ ALL,35 and the low incidence of moderate to severe cGVHD is consistent with previous reports after PTCy.9 Retrospective analyses have suggested similar outcomes after MAC and reduced-intensity conditioning haploidentical transplant,36 but the outcomes in this Ph+ ALL cohort were better after NMAC than after MAC. In addition to the role of PTCy in haploidentical transplant, randomized trials suggest the superiority of PTCy over conventional immunosuppression for matched transplants.37,38 RFS and OS for matched transplants with PTCy in CR1 in our cohort compare favorably to previous studies in Ph+ ALL using conventional immunosuppression.3,39 Thus, transplant with PTCy in Ph+ ALL facilitates matched and haploidentical transplant with limited toxicity, ensuring nearly all patients will have a donor.
For patients transplanted in CR1, the use of dasatinib at diagnosis (5-year RFS, 83.0%) led to improved OS and RFS compared with imatinib (5-year RFS, 49.9%). Previous studies in Ph+ ALL have suggested no significant difference in outcomes between imatinib- and dasatinib-treated patients in the absence of alloBMT.40 However, our results mirror those of a US intergroup study using dasatinib showing a 3-year RFS of 76% with alloBMT.4,40 The better outcomes may be explained by faster and deeper responses with dasatinib, as seen in chronic myeloid leukemia,41 and the intermittent dosing strategy that was initially used with imatinib in combination with chemotherapy for Ph+ ALL.42 Although we detected no differences in the frequency of pretransplant MRD between imatinib- and dasatinib-treated patients, it is conceivable that dasatinib yielded deeper responses below the limit of detection of our assays, explaining the trend toward decreased relapse after dasatinib treatment.
In our cohort, 5-year RFS was 66% after transplant in CR1 and just 8% after transplant in CR2+. The MD Anderson Cancer Center reported similarly poor transplant results for Ph+ ALL patients in CR2, with a 5-year OS of 9%.39 There is certainly debate about deferring alloBMT in select adult patients with Ph+ ALL,43,44 especially given the recent approvals of blinatumomab, inotuzumab, and chimeric-antigen receptor T cells, which can be effective in inducing an MRD-negative CR2 both alone and in combination with TKIs.45-49 Among patients transplanted in CR2+, many had at least one negative prognostic factor, including prior central nervous system involvement (41.7%), relapse after a previous alloBMT (50%), CR1 duration <1 year (41.7%), or persistent MRD before transplant (25%). Although there is reasonable evidence that successful salvage with transplant in an MRD– CR2 is achievable in Ph- ALL,50 similar evidence is currently lacking in Ph+ ALL and must factor into discussions about the wisdom of deferring alloBMT in CR1.
The optimal technique for evaluating MRD in Ph+ ALL and its impact on posttransplant prognosis is unclear. Three separate studies evaluating pretransplant MRD assessed by using BCR-ABL PCR have shown no difference in RFS between MRD+ and MRD– patients.6,8,39 Our data are consistent with this conclusion based on analyses of outcomes for MRD+ vs MRD– patients as well as a sensitivity analysis in which untested patients were assumed to be MRD–, as 91% were MRD– according to MFC. However, when MRD is assessed by using MFC, patients who were MRD+ at transplant had poorer RFS. This is consistent with prior studies.34 The fact that the Ph chromosome may be expressed in non-ALL cells such that its persistence may be an imperfect marker of residual leukemia partially explains this discrepancy.51 A more sensitive MRD assay that specifically tracks clonal rearrangements in B cells using immunoglobulin heavy-chain rearrangements may prove more predictive, as it has already shown a strong correlation with posttransplant outcomes in pediatric ALL.52
Posttransplant TKI prophylaxis was nearly universal in our cohort, and this provides insight about the use, initiation, and dosing of posttransplant TKIs. Previous prospective posttransplant studies have shown that imatinib and nilotinib can be initiated by days 21 and 81, respectively.15-17 In the current study, the median time to posttransplant TKI initiation was 56 days and did not differ according to TKI. This suggests that later-generation TKIs can be initiated as posttransplant prophylaxis earlier than previously reported. However, the timing of posttransplant TKI initiation is likely to remain an individualized decision with risk factors for relapse, such as MRD+ status and transplant in CR2+, being balanced by concerns about posttransplant toxicities, including GVHD, cytopenias, and infections. For example, patients who developed grade 3/4 aGVHD were unable to consistently take a posttransplant TKI. Although most patients on a posttransplant TKI took the dose recommended for chronic-phase chronic myeloid leukemia, the duration of continuous posttransplant use was significantly shorter with dasatinib than with nilotinib or imatinib. This approach suggests that dasatinib at conventional doses may not be as well tolerated posttransplant as alternative TKIs.
Posttransplant relapse was relatively rare after alloBMT in CR1, but the timing and mechanism of relapse may guide the use of posttransplant TKI prophylaxis. Among patients on posttransplant TKIs, 67% of relapses occurred within 1 year. All relapses among patients who stopped or did not start a posttransplant TKI occurred by day 776. Prior prospective studies have largely limited posttransplant TKI prophylaxis to 1 year,15,16 but the frequency of relapses among patients off of prophylaxis during the second year posttransplant suggests that TKI prophylaxis should be extended for at least 2 years when feasible. Although some patients relapsed or developed recurrent MRD >5 years after transplant, there is a risk of cardiovascular toxicity with second-generation TKIs such that their long-term use necessitates an individualized decision.53 Posttransplant monitoring of BCR-ABL PCR identified a subset of patients with early molecular relapse at increased risk of morphologic relapse, but 45% of patients with early molecular relapse became MRD– with TKI maintenance alone, leading to excellent long-term outcomes. This is similar to previous findings with posttransplant imatinib.17 Although there are no current standards for MRD monitoring in Ph+ ALL, the fact that patients with molecular relapse achieved long-term RFS suggests that routine monitoring can facilitate successful preemptive therapy.
Although immune escape mechanisms underlie many posttransplant relapses in AML,54-56 the majority of relapses in our patients were driven by KD mutations, conferring resistance to the TKI in use at relapse. As previously noted in Ph+ ALL patients treated with second-generation TKIs,57 T315I mutations were common, occurring in 50% of tested patients who relapsed in CR1. Although this mutation can be overcome by ponatinib, its cardiovascular toxicity limits its usefulness as posttransplant prophylaxis33; however, the allosteric ABL inhibitor asciminib may be effective in this setting and deserves further study.58
This study of PTCy-based alloBMT for Ph+ ALL shows the efficacy of NMAC for transplant in CR1 with a universal posttransplant maintenance approach especially following induction with dasatinib. These data also expand the evidence supporting the effectiveness of haploidentical alloBMT. Finally, these results strengthen previous evidence for the role of alloBMT in CR1 and show poorer outcomes among patients with persistent MRD according to MFC before transplantation.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by a grant from Swim Across America and grants from the National Institutes of Health, National Cancer Institute (P01 CA225618 and P30 CA06973).
Footnotes
Original data can be requested by contacting the corresponding author (Jonathan A. Webster; e-mail: jwebst17@jhmi.edu).
Authorship
Contribution: J.A.W. designed the study, completed data collection, and drafted the manuscript; J.A.W. and H.-L.T. conducted the statistical analysis; L.L., H.-L.T., P.H.I., R.J.J., and B.D.S. contributed to the study design and reviewed drafts of the manuscript; P.H.I. and J.B.-M. performed GVHD assessments; A.E.D. and K.W.P. assisted with data collection; and all authors provided clinical care of patients and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan A. Webster, Johns Hopkins, 1650 Orleans St, Room 245, Baltimore, MD 21287-0013; e-mail: jwebst17@jhmi.edu.
References
- 1.Rowe JM, Buck G, Burnett AK, et al. ; MRC/NCRI Adult Leukemia Working Party . Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760-3767. [DOI] [PubMed] [Google Scholar]
- 2.Fielding AK, Rowe JM, Richards SM, et al. . Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalandon Y, Thomas X, Hayette S, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) . Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia [published correction appears in Blood. 2015;126(10):1261]. Blood. 2015;125(24):3711-3719. [DOI] [PubMed] [Google Scholar]
- 4.Ravandi F, Othus M, O’Brien SM, et al. . US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv. 2016;1(3):250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Fakih R, Jabbour E, Ravandi F, et al. . Current paradigms in the management of Philadelphia chromosome positive acute lymphoblastic leukemia in adults. Am J Hematol. 2018;93(2):286-295. [DOI] [PubMed] [Google Scholar]
- 6.Bachanova V, Marks DI, Zhang MJ, et al. . Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anasetti C, Aversa F, Brunstein CG. Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant. 2012;18(suppl 1):S161-S165. [DOI] [PubMed] [Google Scholar]
- 8.Brissot E, Labopin M, Beckers MM, et al. . Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCurdy SR, Kasamon YL, Kanakry CG, et al. . Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 2017;102(2):391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luznik L, O’Donnell PV, Symons HJ, et al. . HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurdy SR, Kanakry JA, Showel MM, et al. . Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. . Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Malki MM, Yang D, Labopin M, et al. . Comparing transplant outcomes in ALL patients after haploidentical with PTCy or matched unrelated donor transplantation. Blood Adv. 2020;4(9):2073-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister T, Schwartz S, Bartram CR, Gökbuget N, Hoelzer D, Thiel E; GMALL study group . Patients’ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918-919. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter PA, Snyder DS, Flowers ME, et al. . Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter PA, Johnston L, Fernandez HF, et al. . Posttransplant feasibility study of nilotinib prophylaxis for high-risk Philadelphia chromosome positive leukemia. Blood. 2017;130(9):1170-1172. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer H, Wassmann B, Bethge W, et al. ; GMALL Study Group . Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-1262. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakry CG, Tsai HL, Bolaños-Meade J, et al. . Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25):3817-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasamon YL, Ambinder RF, Fuchs EJ, et al. . Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1(4):288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beillard E, Pallisgaard N, van der Velden VH, et al. . Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using “real-time” quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe Against Cancer program. Leukemia. 2003;17(12):2474-2486. [DOI] [PubMed] [Google Scholar]
- 22.Gabert J, Beillard E, van der Velden VH, et al. . Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17(12):2318-2357. [DOI] [PubMed] [Google Scholar]
- 23.Branford S, Cross NC, Hochhaus A, et al. . Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20(11):1925-1930. [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network Acute lymphoblastic leukemia version 2. Updated 2019. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#all. Accessed 5 January 2019.
- 25.Fine J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 26.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187-193. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantarjian H, Thomas D, O’Brien S, et al. . Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788-2801. [DOI] [PubMed] [Google Scholar]
- 29.Ravandi F. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2019;133(2):130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasamon YL, Bolaños-Meade J, Prince GT, et al. . Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabbour E, Short NJ, Ravandi F, et al. . Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618-e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorer DJ, Knickerbocker RK, Baccarani M, et al. . Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84-91. [DOI] [PubMed] [Google Scholar]
- 34.Pavlů J, Labopin M, Niittyvuopio R, et al. . Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Zhang C, Gao L, et al. . Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicenter study in southwest China. J Hematol Oncol. 2015;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huselton E, Slade M, Trinkaus KM, DiPersio JF, Westervelt P, Romee R. Propensity score analysis of conditioning intensity in peripheral blood haploidentical hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(10):2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolaños-Meade J, Reshef R, Fraser R, et al. . Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132-e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Jong CN, Meijer E, Bakunina K, et al. . Post-transplantation cyclophosphamide after allogeneic hematopoietic stem cell transplantation: results of the prospective randomized HOVON-96 trial in recipients of matched related and unrelated donors. Blood. 2019;134(suppl 1):1.31273001 [Google Scholar]
- 39.Kebriaei P, Saliba R, Rondon G, et al. . Long-term follow-up of allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact of tyrosine kinase inhibitors on treatment outcomes. Biol Blood Marrow Transplant. 2012;18(4):584-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravandi F, Jorgensen JL, Thomas DA, et al. . Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortes JE, Saglio G, Kantarjian HM, et al. . Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34(20):2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas DA, Faderl S, Cortes J, et al. . Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396-4407. [DOI] [PubMed] [Google Scholar]
- 43.Litzow MR. Allogeneic transplantation for patients with Philadelphia chromosome positive acute lymphoblastic leukemia: is it imperative in the tyrosine kinase inhibitor era? Best Pract Res Clin Haematol. 2018;31(4):357-360. [DOI] [PubMed] [Google Scholar]
- 44.Bachanova V. Deferring allogeneic transplantation for adult acute lymphoblastic leukemia: is there a second chance? Leuk Lymphoma. 2016;57(9):1988-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinelli G, Boissel N, Chevallier P, et al. . Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study [published corrections appear in J Clin Oncol. 2017;35(23):2722 and J Clin Oncol. 2017;35(24):2856]. J Clin Oncol. 2017;35(16):1795-1802. [DOI] [PubMed] [Google Scholar]
- 46.Assi R, Kantarjian H, Short NJ, et al. . Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897-901. [DOI] [PubMed] [Google Scholar]
- 47.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. . Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain N, Cortes JE, Ravandi F, et al. . Inotuzumab ozogamicin in combination with bosutinib for patients with relapsed or refractory Ph+ ALL or CML in lymphoid blast phase. Blood. 2017;130(suppl 1):143. [Google Scholar]
- 49.Maude SL, Laetsch TW, Buechner J, et al. . Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassaday RD, Alan Potts D Jr., Stevenson PA, et al. . Evaluation of allogeneic transplantation in first or later minimal residual disease—negative remission following adult-inspired therapy for acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57(9):2109-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schenk TM, Keyhani A, Bottcher S, et al. . Multilineage involvement of Philadelphia chromosome positive acute lymphoblastic leukemia. Leukemia. 1998;12(5):666-674. [DOI] [PubMed] [Google Scholar]
- 52.Pulsipher MA, Carlson C, Langholz B, et al. . IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016;2(5):625-632. [DOI] [PubMed] [Google Scholar]
- 54.Christopher MJ, Petti AA, Rettig MP, et al. . Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379(24):2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vago L, Perna SK, Zanussi M, et al. . Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478-488. [DOI] [PubMed] [Google Scholar]
- 56.Toffalori C, Zito L, Gambacorta V, et al. . Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603-611. [DOI] [PubMed] [Google Scholar]
- 57.Rousselot P, Coudé MM, Gokbuget N, et al. ; European Working Group on Adult ALL (EWALL) group . Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes TP, Mauro MJ, Cortes JE, et al. . Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.