Abstract
The effect of standard therapeutic strategies on Helicobacter pylori infection is diminished over time owing to the emergence of drug resistant strains. In this study, we would like to confirm the enhanced effect of L. paracasei HP7, which has been reported to exert antibacterial and gastric mucosal protective effects, in combination with Perilla frutescens var. acuta (P. frutescens)and Glycyrrhiza glabra (G. glabra) extracts.
P. frutescens extract and G. glabra extract were found to inhibit the growth of H. pylori in a concentration-dependent manner, and the combination of L. paracasei HP7 and P. frutescens extract and G. glabra extract effectively inhibited H. pylori from attaching to AGS a gastric epithelial cells. Moreover, L. paracasei HP7 complex mixture containing P. frutescens and G. glabra extracts has been shown to inhibit H. pylori virulence genes such as AlpA, CagA, FlaA and UreA. When H. pylori-infected mice were administered a complex mixture of L. paracasei HP7 containing P. frutescens and G. glabra extract, the infection rate of H. pylori was significantly reduced. In addition, the L. paracasei HP7 complex mixture significantly reduced serum IL-8 levels and stomach inflammation in H. pylori infected mice.
These results suggest that a complex mixture of L. paracasei HP7 containing P. frutescens and G. glabra extracts may be an alternative to treating diseases caused by H. pylori infection.
Keywords: Lactobacillus paracasei, HP7, Helicobacter pylori, Perilla frutescens var. acuta, Glycyrrhiza glabr
Introduction
Helicobacter pylori, a major causative pathogen of chronic gastritis [1] and gastric ulcers [2], is a spiral of gram-negative bacteria associated with an increased risk of gastric cancer [3, 4]. Vaccination with antibiotics to remove gastric H. pylori can reduce H. pylori-associated gastrointestinal diseases [5, 6] and reduce the risk of gastric cancer [7]. The standard recommended therapy for H. pylori uses two antibiotics, usually a triple combination therapy, including clarithromycin and a proton pump inhibitor with amoxicillin or metronidazole [8, 9]. However, the efficacy of the triple therapy has currently reduced over time. Recent cure rates of less than 80% are mainly due to the increased prevalence of resistant H. pylori strains in metronidazole and clarithromycin [10–12]. In addition, some patients showed allergic side effects to antibiotics and can sometimes cause side effects if H. pylori is not treated [13]. Long-term vaccination with antibiotics is not recommended for the prevention of H. pylori infection. Therefore, it is important to develop new non-antibacterial agents for the treatment of H. pylori [14].
Lactobacillus spp. is recommended as an additive to the standard recommended treatment for H. pylori treatment, and it is possible to improve the patient’s adaptability by reducing the side effects of antibacterial agents [15, 16]. In our previous study, we reported that the lactic acid bacterium Lactobacillus paracasei HP7 (L. paracasei HP7) isolated from kimchi, a fermented vegetable dish widely consumed in Korea, had inhibitory effects against H. pylori in-vitro and in-vivo [17].
Recently, there has been a clear increase in demand for natural compounds from plant extracts that are effective antibacterial agents against a wide range of bacteria to control human infection and for the preservation of food [18]. Recently, the inhibitory effect of Glycyrrhiza glabra (G. glabra) on H. pylori and the therapeutic effect on infected patients have been reported [19–21]. In addition, antibacterial activities [22–24] and anti-inflammatory [25–27] effects of Perilla frutescens var. acuta (P. frutescens) have been reported.
In this study, we aimed to determine whether the combination of L. paracasei HP7 and P. frutescens and G. glabra extracts had a synergistic effect on the inhibition of H. pylori infection.
Methods/experimental
Bacterial strains
L. paracasei HP7 was incubated at Man-Rogosa-Sharpe broth (Difco Laboratories, Detroit, Mich.) at 35 °C for 24 h. H. pylori strain SS1 (B0890; Korean Jeongeup Korean Collection) was cultured overnight at 37 °C. under microaerobic conditions in brain-heart infusion medium containing 10% fetal bovine serum (FBS) and grown to density ~ 2.0 × 109 CFU/mL. The cultured bacteria were then transferred to phosphate buffered saline (PBS) before the test.
Herbal extract
Each of the herbal extracts of G. glabra and P. frutescens were obtained from Korea Yakult Co., Ltd.
Cell culture
Human gastric cell line AGS cells (human gastric adenocarcinoma) were obtained from the Korean Cell Line Bank (cellbank.snu.ac.kr) and used. For maintenance and proliferation of cells, passage was performed every 2 days at 37 °C and 5% CO2 using Ham’s F-12 medium containing 10% FBS and 1% antibiotic. For analysis of H. pylori infection to gastric cells, antibiotics were not added to the culture medium.
H. pylori growth inhibition
To confirm the anti-H.pylori activity of P. frutescens and G. glabra extracts, Alamar blue assay was performed by referring to the study of Tsukasa M et al. [28]. H. pylori was suspended in DMEM / F-12 containing 5 mM L-lactic acid with a turbidity of 0.005 (1 × 105 CFU/mL). One hundred microliter suspension was added to 96 well culture plate and then incubated for 4 h at 37 °C with the test material (P. frutescens and G. glabra extracts) under micro-aerophilic conditions. After incubation, inhibition of H. pylori growth was measured by Alamar blue according to manufacturer’s criteria (Alamar Bio-Sciences, Sacramento, CA, U.S.A.). H. pylori inhibitory activity of the tested material was calculated by the following formula:
A: cultured without test sample. B: cultured with test sample. C: medium alone.
Inhibition of H. pylori adhesion to AGS cells
AGS cells were cultured in 6-well plates for 16 h. When the cells reached 90% confluence, the medium was replaced with serum and antibiotics-free F-12 medium. An overnight cultured H. pylori SS1 was suspended in Ham’s F-12 medium. For co-culture of bacteria and gastric epithelial cells, H. pylori SS1 (107 CFU) were added to wells containing 106 AGS cells and incubated for 4 h in the absence or presence of herbal extracts and L. paracasei HP7. The adhesion of H. pylori was measured using a Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) as in our previous paper [17]. Forward and reverse sequences of primers for amplifying the H. pylori 16S RNA gene were as follows: 5′-TCG GAA TCA CTG GGC GTA A-3′ and 5′-TTC TAT GGT TAA GCC ATA GGA TTT CAC-3′.
Detect of H. pylori virulence gene expression
H. pylori SS1 cells were cultured in brain-heart infusion broth at ~ 1.0 × 107 CFU/mL. Cultured H. pylori were treated with G. glabra extract (3μg / mL), P. frutescens extract (25μg/mL), and L paracasei HP7 (1.0 × 107 CFU/mL) and incubate at 37 °C for 2 h. cDNA was synthesized using murine leukemia virus reverse transcriptasptase with random hexamer. Primer sequence for H. pylori virulence genes are listed in Table 1. AlpA is genes that H. pylori attaches to the gastric mucosa, and CagA plays the role of H. pylori invading gastric cells. FlaA is related to the mobility of H. pylori, and UreA is genes that H. pylori uses to neutralize gastric acid [29].
Table 1.
PCR primer sequence for H. pylori virulence genes
| Gene name | Sequence | Tm (° C) | Reference |
|---|---|---|---|
| alpA | F: AAACCGCTCTGTGGATATGG | 55.0 | NZ_CP009259.1 |
| R: GAACTGGAAGTGCGTGTTATTG | 45.6 | ||
| cagA | F: TCACTCTTGGCGATATGGAAAT | 57.5 | |
| R: ACACAGAAGACAGAGCGTTATT | 57.7 | ||
| flaA | F: GCTAAGAGCATCAATGTGGTTTC | 58.3 | |
| R: CGGTAACATCGCGCAAATTC | 58.5 | ||
| ureA | F: AGTGGGTATTGAAGCGATGTT | 57.6 | |
| R: AAGAACAACTCACCAGGAACTAA | 57.6 |
Animals
Specific pathogen free (SPF) male C57BL/6 mice weighing 20–24 g were purchased from Samtako Co. (Osan, Korea) and were maintained at the inspection facility of Wonkwang University (Iksan, Korea) for 1 week before experiments. Thereafter, the mice were maintained in an SPF barrier room with regulated temperature (23 °C ± 1 °C) and humidity (50% ± 5%) and a 12:12-h light/dark cycle. The animals were fed a sterilized pellet diet (Purina, Seoul, Korea) and sterilized water ad libitum. All studies were performed in accordance with the Guide for Animal Experimentation of Wonkwang University and were approved by the Institutional Animal Care and Use Committee of Wonkwang University (approval no. WKU 2019-08-22).
H. pylori inoculation
Animals were intragastrically inoculated three times, with a 3-day interval between inoculations, with H. pylori at ~ 1.0 × 109 CFU in 0.5 mL broth. The challenged animals were confirmed as H. pylori-positive by stool antigen analysis using the Bioline H. pylori Ag kit (Standard Diagnostics, Suwon City, Korea) as previously described [30].
In vivo study protocol
The inhibition of H. pylori growth by L. paracasei HP7 was investi in a mouse model. The mice were divided into six groups: negative control (NC, n = 10); H. pylori-infected without treatment (C, n = 10); H. pylori-infected with positive control Deglycyrrhizinated Licorice (DGL) [20] treatment (D, n = 10); H. pylori-infected with P. frutescens extract (PFE) 5 mg/kg + G. glabra extract (GGE) 1.2 mg/kg (COM 1, n = 10); H. pylori-infected with PFE 10 mg/kg + GGE 1.2 mg/kg (COM 2, n = 10); and H. pylori-infected with L. paracasei HP7 2.0 × 107 CFU + PFE 10 mg/kg + GGE 1.2 mg/kg (COM 3, n = 10). All substances were administered orally once daily for 4 weeks. At the end of the experiment, the animals were euthanized with ether, and then dissected. The stomach was further incised along the taiwanese valley, and washed with saline. The remaining portion was formalin fixed and inserted into paraffin for histological analysis. H. pylori colonies were confirmed by the aforementioned quick urease test (CLO-test) [30].
Blood analysis
Blood samples were collected from the hearts of sacrificed animals,centrifuged at 1000×g for 15 min at 4 °C, and the isolated plasma was stored at − 80 °C. Serum titers of anti-H. pylori antibodies were measured using a mouse anti-H. pylori antibody (IgG-1) ELISA kit (Cusabio Biotech, Wuhan, China) in accordance with the manufacturer’s instructions. IL-8 levels in mice were measured using the Mouse Interleukin 8 ELISA Kit (R&D System, Minneapolis, USA) in accordance with the manufacturer’s instructions.
Statistical analysis
Experimental results were compared between groups using Minitab (State College, PA, USA) and one-way ANOVA, a parametric multiple comparison procedure. The results were expressed as mean ± standard error and statistically significant when P < 0.05.
Results
H. pylori growth inhibition
We measured the H. pylori growth inhibitory activity of 140 plant extracts including G. glabra and P. frutescens. Excluding non-edible plants, G. glabra and P. frutescens extracts showed the best inhibitory effect on the growth of H. pylori. In particular, G. glabra 90% ethanol extract and P. frutescens 50% ethanol extract showed high activity (data not shown).
There have been several reports of antibacterial and Helicobacter pylori inhibitory activity of P. frutescens and G. glabra [19–24]. However, there have been few reports of synergistic effects of H. pylori inhibitory activity of P. frutescens and G. glabra. Therefore, the H. pylori growth inhibitory activity of each of the P. frutescens extract (PFE) and G. glabra extracts (GGE) was investigated, and whether the two extracts had a synergistic effect on H. pylori inhibition was examined. PFE and GGE inhibited the growth of H. pylori in a concentration-dependent manner. PFE and GGE almost completely inhibited the growth of H. pylori at concentrations of 12.5 μg/mL and 50 μg/mL, respectively, and the IC50 of each extract was 23.84 μg/mL and 2.88 μg/mL. (Fig. 1a). When the extract corresponding to IC50 was co-treated, the growth of H. pylori was inhibited by about 90% (Fig. 1b). This suggests that P. frutescens and G. glabra are synergistic in inhibiting the growth of H. pylori.
Fig. 1.
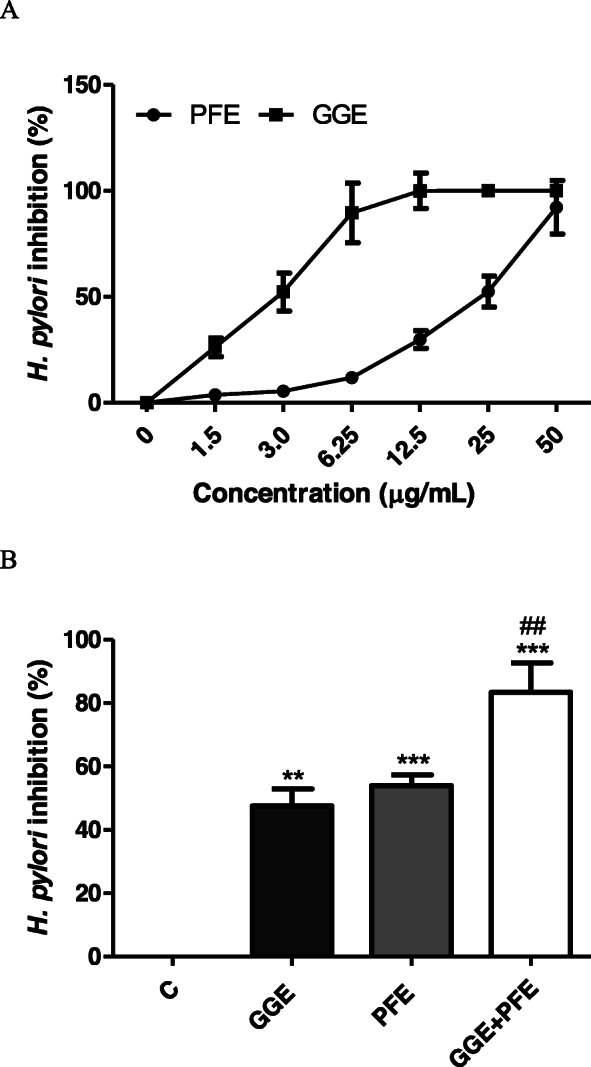
Inhibitory effects of P. frutescens and G. glabra extract against growth of H. pylori. a H. pylori growth inhibitory activity of P. frutescens and G. glabra extracts at various concentrations. b H. pylori inhibitory activity following co-treatment of P. frutescens and G. glabra extract. PFE, P. frutescens extract; GGE, G. glabra extract. **Significantly different from the non treated control C (P < 0.01). ***Significantly different from the non treated control C (P < 0.001). ##Significantly different from the PFE and GGE treated group (P < 0.01)
Suppression of H. pylori adhesion to gastric epithelial cells
In a previous study, we confirmed that hp7 inhibits Helicobacter pylori adhesion to gastric epithelial cells [17]. The complex mixture of L. paracasei HP7 containing PFE and GGE significantly inhibited H. pylori adhesion to gastric cells than L. paracasei HP7 or PFE or GGE alone (Fig. 2a). These results demonstrate that L. paracasei HP7 and P. frutescens and G. glabra extracts are synergistic in inhibiting bacterial adhesion to gastric epithelial cells.
Fig. 2.
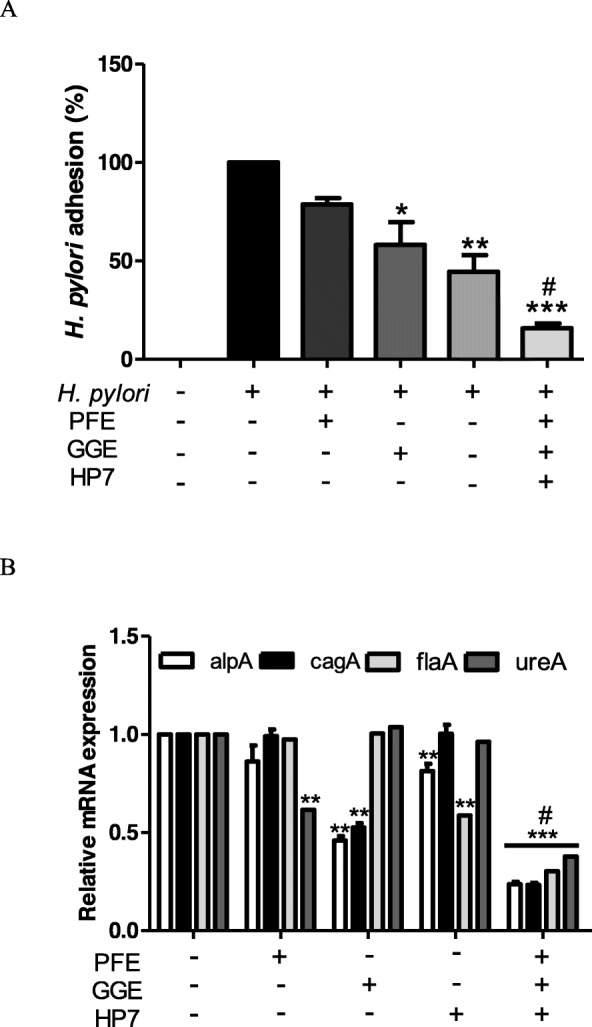
Effect of complex mixture of L. paracasei HP7 containing P. frutescens and G. glabra extract on H. pylori adhesion and H. pylori virulence genes expression (a) Degree of H. pylori attached to AGS cells (b) alpA, cagA, flaA and ureA mRNA expression in L. paracasei HP7, P. frutescens extract, G. glabra extract and complex mixture treated H. pylori SS1. PFE, P. frutescens extract 25 μg/mL; GGE, G. glabra extract 3 μg/mL; HP7, L. paracasei HP7 1.0 × 107 CFU/Ml. *Significantly different from non-treated control C (P < 0.05). **Significantly different from the non-treated control C (P < 0.01). ***Significantly different from the non-treated control C (P < 0.001). #Significantly different from the PFE, GGE and HP7 treated group (P < 0.05)
Inhibition of H. pylori virulence factor
H. pylori produces urease to decompose the urea in the stomach, reduce the acidity around it, move using flagella, and attach to epithelial cells through adhesion factors such as AlpA. In addition, the CagA protein secreted by H. pylori inflames gastric epithelial cells and causes gastric cell changes known as the “hummingbird phenomenon” [1, 2, 29]. Therefore, we investigated the effect of a complex mixture of L. paracasei HP7 containing PFE and GGE on the mRNA expression of genes encoding AlpA, Cag, FlaA, and UreA of H. pylori.
PFE significantly reduced ureA and GGE decreased alpA and cagA. HP7 significantly reduced flaA associated with H. pylori motility. Meanwhile, the HP7 complex mixture significantly reduced H. pylori virulence genes compared to PPE or GGE or HP7 alone (Fig. 2b).
Anti-H. pylori antibody titer in serum
To confirm the colonization of H. pylori in mice, the absorbance of IgG serum against H. pylori was also related to H. pylori colonization, so anti-Helicobacter IgG-1 serum levels were measured [31]. The serum antibody titers were elevated 4 weeks after H. pylori inoculation, to values of 1.48 ± 0.06, 0.94 ± 0.07, and 0.95 ± 0.04 in the H. pylori infection (Group C), positive control DGL (Group D), and H. pylori infection/L. paracasei.
HP7 + PPE + GGE (Group COM3) treatment groups, respectively, as compared with 0.25 ± 0.01 in control animals (Group NC) (Fig. 3).
Fig. 3.
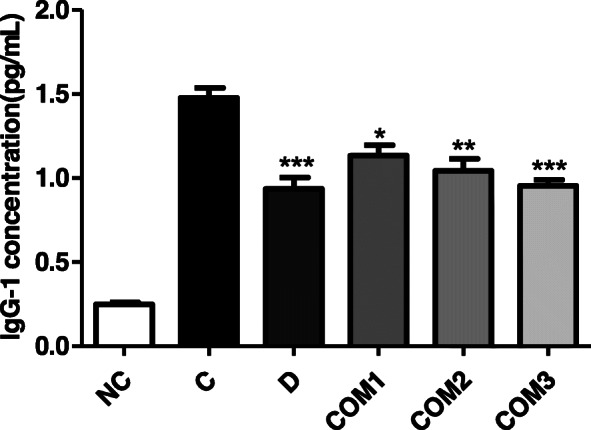
Results of Helicobacter pylori antibody (IgG) test with mice serum. *Significantly different from the infection control Group C (P < 0.05). **Significantly different from the infection control Group C (P < 0.01). ***Significantly different from the infection control Group C (P < 0.001)
These results indicated that H. pylori infection was significantly reduced by treatment with a complex mixture of L. paracasei HP7 containing extracts of PPE and GGE.
Decrease of H. pylori colonization
Repeated intragastric inoculation of C57BL/6 mice treated with H. pylori (1.0 × 109 CFU/mouse, three times) led to a positive reaction in the gastric mucosal campylobacter-like organism (CLO) test (Table 2). Positive percentages were increased 4 weeks after H. pylori inoculation, with values of 100% (CI 72.2–100), 30% (CI 10.8–60.3), 10% (CI 1.8–40.4) in the H. pylori infection (Group C), positive control DGL (Group D) and H. pylori infection/L. paracasei HP7 + PPE + GGE (Group COM3) treatment groups, respectively, compared with 0% (CI 0–27.6) in control animals (Group NC) (Table 2).
Table 2.
Reactivity in the CLO test of gastric mucosa from mice infected with H. pylori followed by treatment with L. HP7 and herbal extracts
| Group | Treatment | n | Positive %a | Therapeutic % |
|---|---|---|---|---|
| NC | Normal control | 10 | 0%, CIb0–27.6 | 100%,CI72.2–100 |
| C | H. pylori | 10 | 100%, CI 72.2–100 | 0%, CI 0–27.6 |
| D | H. pylori + DGL | 10 | 30%, CI 10.8–60.3 | 70%, CI 39.7–89.2 |
| COM1 | H. pylori + PPE5 + GGE1.2 | 10 | 50%, CI 23.7–76.3 | 50%, CI 23.7–76.3 |
| COM2 | H. pylori + PPE10 + GGE1.2 | 10 | 30%, CI 10.8–60.3 | 70%, CI 39.7–89.2 |
| COM3 | H. pylori + HP7+ PPE10+ GGE1.2 | 10 | 10%, CI 1.8–40.4 | 90%, CI 60.0–98.2 |
DGL Deglycyrrhizinated Licorice, HP7 L. paracasei HP7, PPE P. frutescens var. acuta extract, GGE Glycyrrhiza glabra extract
aA positive percentage reflects H. pylori colonization, which was observed as medium color change from yellow to red
bIncidence (95% confidential interval [CI]) was calculated using MiniTab statistical software
CLO scores were decreased by H. pylori infection/L. paracasei HP7 + PPE + GGE (Group COM3) relative to H. pylori-infected animals without treatment (Group C) (P < 0.01; Fig. 4). Therefore, L. paracasei HP7 + PPE + GGE may reduce the colonization rate of H. pylori.
Fig. 4.
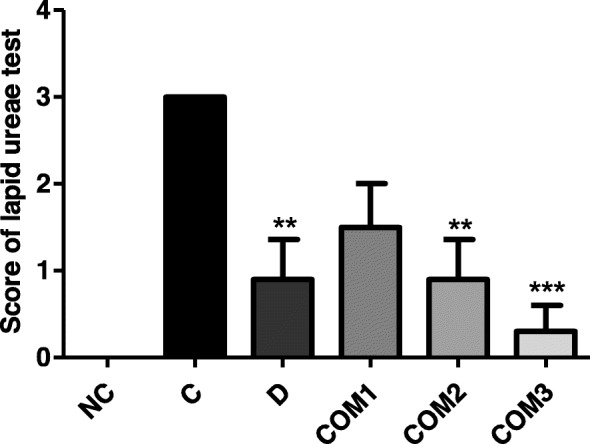
Reactivity in the CLO test of gastric mucosa from mice infected with H. pylori followed by treatment with complex mixture of L. paracasei HP7 and herbal extracts. **Significantly different from the infection control Group C (P < 0.01). ***Significantly different from the infection control Group C (P < 0.001)
Alleviation of gastric mucosal lesions caused by H. pylori
Pathological changes in the gastric mucosa were minimal in animals not infected with H. pylori (Group NC). In contrast, Group C (H. pylori inoculated) mice exhibited gastric atrophy and severely shortened villi. However, mice in Group COM3 (H. pylori infected/L. paracasei HP7 + PPE + GGE) showed a significant improvement in gastric mucosa. These results were confirmed by an increase in villus length in Group COM3 compared with Group C (Fig. 5).
Fig. 5.
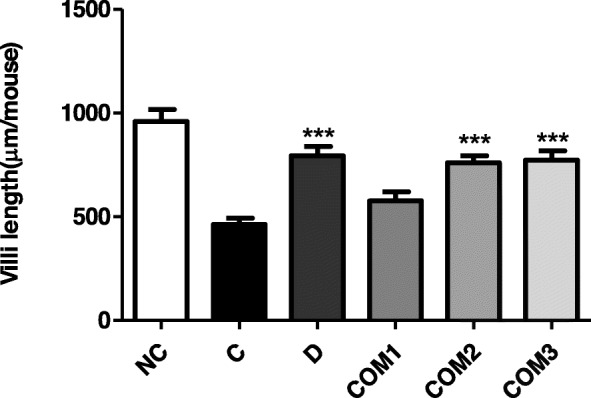
Gastric mucosal viili lengths of mice infected with H. pylori followed by treatment with complex mixture of L. paracasei HP7 and herbal extracts. ***Significantly different from the infection control Group C (P < 0.001)
Suppression of H. pylori-induced IL-8 production
Blood IL-8 levels were elevated 4 weeks after H. pylori inoculation, with values of 7.39 ± 0.70, 5.73 ± 0.63, 5.16 ± 0.49 in the H. pylori infection (Group C), positive control DGL (Group D) and H. pylori infection/L. paracasei HP7 + PPE + GGE (Group COM3) treatment groups, respectively, as compared to 5.36 ± 0.59 in control animals (Group NC) (Fig. 6).
Fig. 6.
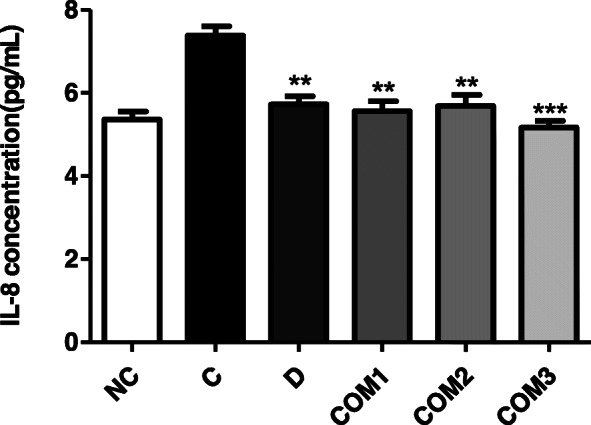
Serum IL-8 levels of mice infected with H. pylori followed by treatment with complex mixture of L. paracasei HP7 and herbal extracts. **Significantly different from the infection control Group C (P < 0.01). ***Significantly different from the infection control Group C (P < 0.001)
Discussion
Lactic acid bacteria suppress the growth of human bacterial pathogens by secreting compounds, such as antibiotics, organic acids, and bacteriocins, to lower the pH of the environment and control gastrointestinal infections [31, 32]. The inhibitory activity of H. pylori has been reported in several Lactobacillus spp., including L. acidophilus [32], L. casei [33], L. johnsonii [34], L. reuteri [35], and L. salivarius [36].
A new Lactobacillus spp. isolated from kimchi by Korea Yakult Co. Ltd. was identified as L. paracasei and was named strain HP7. Kimchi is considered a healthy food as it is enriched in vitamins A, B, and C, and is high in fiber, but also contains a number of lactic acid bacteria [37]. The two herbs selected in this study were P. frutescens var. acuta and G. glabra, which showed the strong antibacterial activity of H. pylori by measuring the Helicobacter antibacterial activity (growth suppression) in the extraction of natural product candidates through the inhibitory clear zone test of H. pylori (data not shown).
G. glabra (licorice) was reported to exhibit antimicrobial activity against several gram-negative and gram-positive bacterial strains including H. pylori [38]. In addition, licorice also exerted beneficial effects against H. pylori through its antiadhesive properties [39]. Activity against ulcer and cancer, and clinical outcomes of H. pylori infection were also exhibited by licorice. The curative effect of deglycyrrhizinated licorice (DGL) on ulcers has been reported in vivo and in clinical studies [40–42], and the anticancer effect of licorice extract was shown in an in vitro study [43]. G. glabra was shown to possess anti-ulcerogenic properties that may be conferred by the cytoprotective mechanism of its antioxidant properties. These results supported the ethnomedical uses of licorice in the treatment of gastric ulcer [44].
Traditionally, P. frutescens var. acuta has been prescribed to treat depression- related disease, anxiety, asthma, chest stuffiness, vomiting, cough, cold, flu, phlegm, tumors, allergies, intoxication, fever, headache, stuffy nose, constipation, abdominal pain, and indigestion, and acts as an analgesic, anti-abortive agent, and a sedative [23]. The antibacterial activity of P. frutescens var. acuta has also been reported [24].
In this study, we confirmed in vitro and in vivo experiments of H. pylori inhibitory activity of a L. paracasei HP7 complex mixture containing P. frutescens var. acuta and G. glabra extracts. P. frutescens extract and G. glabra extract inhibited the growth of H. pylori in a dose-dependent manner, and the H. pylori growth inhibitory effect was increased when the two extracts were mixed at IC50 concentration. In addition, the inhibitory effect of adhesion of gastric epithelial AGS cells of H. pylori by the L. paracasei HP7 or P. frutescens extract and G. glabra extract, when applied in a complex mixture, rather than each individually, was confirmed to be larger. Also, we confirmed the inhibitory activity of a complex mixture of L. paracasei HP7 including the extracts of P. frutescens and G. glabra against H. pylori in a mouse model; a rapid urease test of mouse stomachs showed decreased H. pylori colonization. Thus, the eradication of H. pylori reduced inflammation and epithelial damage in the stomach, although it is also possible that a complex mixture of L. paracasei HP7 including the extract of P. frutescens and G. glabra had direct anti-inflammatory effects on the gastric mucosa.
Although triple therapy consisting of two antibiotics and a proton pump inhibitor is effective over a short term and helps to maintain patient compliance, many patients experience undesirable side effects such as diarrhea, epigastric pain, nausea, and bloating [45].
In comparison, a complex mixture of L. paracasei HP7, including the extracts of P. frutescens and G. glabra, is safe and therefore appropriate for the prevention and treatment of H. pylori infection. In this study, the therapeutic effect of a complex mixture of L. paracasei HP7 including the extract of P. frutescens and G. glabra, was partial, at 90%. However, H. pylori adhesion and a reduced inflammatory response was shown. Other researchers reported also that probiotics alone could not completely eliminate H. pylori, but could reduce the load of H. pylori in the stomach, and alleviate gastric mucosal inflammation [46, 47]. Accumulating evidence suggests an important role of IL-8 in H. pylori infection-associated chronic atrophic gastritis, peptic ulcer and gastric cancer [48]. The suppression of IL-8 by a complex mixture of L. paracasei HP7, including the extract of P. frutescens and G. glabra, can potentially prevent H. pylori-induced gastritis and carcinogenesis in the stomach.
Previously, the results of our study reported that L. paracasei HP7 alone was able, to some extent, suppress H. pylori infection [17]. This study was performed to confirm the elevation effect of compounds mixed with P. frutescens and G. glabra extract, which are known to have antibacterial and gastric mucosal protective effects other than L. paracasei HP7.
Conclusions
The administration of a complex mixture of L. paracasei HP7 containing an extract of P. frutescens and G. glabra was more effective than that of L. paracasei HP7 alone or P. frutescens extract or G. glabra extract, and the administration of a higher antibacterial effect of H. pylori and inflammation induced by H. pylori or it was confirmed to reduce the damage to the mucous membrane. The mechanism of this action resulted from the inhibitory effect of L. paracasei HP7 on the adhesion of H. pylori to the gastric mucosa, the antibacterial effect and antioxidative effect of G. glabra and P. frutescens extract, and the increased secretion of gastric mucosal mucin. It can be assumed that the anti-H.pylori effect and the protective effect on the gastric mucosa were induced. Thus, a complex mixture of L. paracasei HP7, including the extract of Perilla frutescens and Glycyrrhiza glabra can be used to treat patients with gastric symptoms, including ulcers caused by H. pylori.
These results demonstrated that treatment with a complex mixture of L. paracasei HP7, including the extract of P. frutescens and G. glabra could inhibit the growth of H. pylori and is thus a promising treatment for patients with gastric symptoms, such as gastritis, that are caused by H. pylori infection.
Acknowledgements
The studies were suported Korea Yakult Co. Ltd.
Authors’ contributions
We confirmed all authors’ contributions. The author(s) read and approved the final manuscript.
Funding
Funding information is not appicable.
Availability of data and materials
I declare that this manuscript has the availability of data and material.
Competing interests
I declare that this manuscript has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin N Am. 2000;29(3):559–578. doi: 10.1016/S0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58(10):2067–2069. [PubMed] [Google Scholar]
- 4.Maruta F, Ota H, Genta RM, Sugiyama A, Tatematsu M, Katsuyama T, Kawasaki S. Role of N-methyl-N-nitrosourea in the induction of intestinal metaplasia and gastric adenocarcinoma in Mongolian gerbils infected with Helicobacter pylori. Scand J Gastroenterol. 2001;36(3):83–90. doi: 10.1080/003655201750074591. [DOI] [PubMed] [Google Scholar]
- 5.Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T, Shimizu K, Kobayashi S. A multicenter, double- lind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6(3):254–261. doi: 10.1046/j.1523-5378.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 6.Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50(8):1517–1522. doi: 10.1007/s10620-005-2871-7. [DOI] [PubMed] [Google Scholar]
- 7.Maruta F, Sugiyama A, Ishizone S, Miyagawa S, Ota H, Katsuyama T. Eradication of Helicobacter pylori decreases mucosal alterations linked to gastric carcinogenesis in Mongolian gerbils. J Gastroenterol. 2005;40(1):104–105. doi: 10.1007/s00535-004-1501-z. [DOI] [PubMed] [Google Scholar]
- 8.Misiewicz JJ, Harris AW, Bardhan KD, Levi S, O'Morain C, Cooper BT, Kerr GD, Dixon MF, Langworthy H, Piper D. One week triple therapy for Helicobacter pylori: a multicentre comparative study. Lansoprazole Helicobacter Study Group Gut. 1997;41(6):735–739. doi: 10.1136/gut.41.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ. European helicobacter study group. Management of Helicobacter pylori infection-the Maastricht IV/ Florence consensus report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 10.Midolo PD, Lambert JR, Turnidge J. Metronidazole resistance: a predictor of failure of Helicobacter pylori eradication by triple therapy. J Gastroenterol Hepatol. 1996;11(3):290–292. doi: 10.1111/j.1440-1746.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 12.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Study group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 13.Buenz EJ, Bauer BA, Schnepple DJ, Wahner-Roedler DL, Vandell AG, Howe CL. A randomized phase I study of Atuna racemosa: a potential new anti-MRSA natural product extract. J Ethnopharmacol. 2007;114(3):371–376. doi: 10.1016/j.jep.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Liu CS, Cham TM, Yang CH, Chang HW, Chen CH, Chuang LY. Antibacterial properties of Chinese herbal medicines against nosocomial antibiotic resistant strains of Pseudomonas aeruginosa in Taiwan. Am J Chin Med. 2007;35(6):1047–1060. doi: 10.1142/S0192415X07005508. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi F, Cazzato A, Nista EC, Scarpellini E, Roccarina D, Gigante G, Gasbarrini G, Gasbarrini A. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter. 2007;12(Suppl 2):59–63. doi: 10.1111/j.1523-5378.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC, Song IS. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13(4):261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 17.Hong SS, Lee HA, Kim JY, Jeong JW, Shim JJ, Lee JL, Sim JH, Chung Y, Kim O. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacilllus paracasei HP7. Lab Anim Res. 2018;34(4):216–222. doi: 10.5625/lar.2018.34.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Kim YC, Choi UK. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary operation factorial design technique. Int J Mol Sci. 2011;12:2395–2407. doi: 10.3390/ijms12042395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asha MK, Debraj D, Prashanth D, Edwin JR, Srikanth HS, Muruganantham N, Dethe SM, Anirban B, Jaya B, Deepak M, Agarwal A. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J Ethnopharmacol. 2013;145(2):581–586. doi: 10.1016/j.jep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Puram S, Suh HC, Kim SU, Bethapudi B, Joseph JA, Agarwal A, Kudiganti V. Effect of GutGard in the management of Helicobacter pylori: a randomized double blind placebo controlled study. Evid Based Complementary Altern Med. 2013;2013:263805. doi: 10.1155/2013/263805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahnama M, Mehrabani D, Japoni S, Edjtehadi M, Firoozi MS. The healing effect of licorice (Glycyrrhiza glabra) on Helicobacter pylori infected peptic ulcers. J Res Med Sci. 2013;18(6):532–533. [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto H, Ogawa T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci Biotechnol Biochem. 2002;66:921–924. doi: 10.1271/bbb.66.921. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed HM. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt molecules. 2019;24(1):102. [DOI] [PMC free article] [PubMed]
- 24.Choi UK, Lee OH, Lim SI, Kim YC. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Pseudomonas aeruginosa using the evolutionary operation-factorial design technique. Int J Mol Sci. 2010;11(10):3922–3932. doi: 10.3390/ijms11103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omer EA, Khattab ME, Ibrahim ME. First cultivation trial of Perilla frutescens L. in Egypt. Flavour Fragr J. 1998;13:221–225. doi: 10.1002/(SICI)1099-1026(1998070)13:4<221::AID-FFJ716>3.0.CO;2-Z. [DOI] [Google Scholar]
- 26.Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 27.Wang XF, Li H, Jiang K, Wang QQ, Zheng YH, Tang W, Tan CH. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. Fitoterapia. 2018;130:61–5. [DOI] [PubMed]
- 28.Tsukasa M, Tetsufumi T, Haruki Y. A novel approach for screening of new anti-Helicobacter pylori substances. Biol Pharm Bull. 2008;31(1):143–145. doi: 10.1248/bpb.31.143. [DOI] [PubMed] [Google Scholar]
- 29.Selbach M, Moese S, Meyer TF, Backert S. Functional analysis of the helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70(2):665–671. doi: 10.1128/IAI.70.2.665-671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon DI, Shin EH, Oh HG, Oh JS. Hong S, Chung Y, Kim O. usefulness of a Helicobacter pylori stool antigen test for diagnosing H. pylori infected C57BL/6 mice. Lab Anim Res. 2013;29(1):27–32. doi: 10.5625/lar.2013.29.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreuning J, Lindeman J, Biemond I, Lamers CB. Relation between IgG and IgA antibody titres against Helicobacter pylori in serum and severity of gastritis in asymptomatic subjects. J Clin Pathol. 1994;47(3):227–231. doi: 10.1136/jcp.47.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130(2S Suppl):396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- 33.Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004;70(1):518–526. doi: 10.1128/AEM.70.1.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol. 2005;12(12):1378–1386. doi: 10.1128/CDLI.12.12.1378-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lionetti E, Miniello VL, Castellaneta SP, Magistá AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24(10):1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 36.Ryan KA, Daly P, Li Y, Hooton C, O'Toole PW. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J Antimicrob Chemother. 2008;61(4):831–834. doi: 10.1093/jac/dkn040. [DOI] [PubMed] [Google Scholar]
- 37.Ki MR, Ghim SY, Hong IH, Park JK, Hong KS, Ji AR, Jeong KS. In vitro inhibition of Helicobacter pylori growth and of adherence of cagA-positive strains to gastric epithelial cells by Lactobacillus paraplantarum KNUC25 isolated from kimchi. J Med Food. 2010;13(3):629–634. doi: 10.1089/jmf.2009.1265. [DOI] [PubMed] [Google Scholar]
- 38.Gupta VK, Fatima A, Faridi U, Negi AS, Shanker K, Kumar JK, Rahuja N, Luqman S, Sisodia BS, Saikia D, Darokar MP, Khanuja SPS. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008;116(2):377–380. doi: 10.1016/j.jep.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Wittschier N, Faller G, Hensel A. Aqueous extracts and polysaccharides from Liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Ethnopharmacol. 2009;125(2):218–223. doi: 10.1016/j.jep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Larkworthy W, Holgate PF. Deglycyrrhizinized liquorice in the treatment of chronic duodenal ulcer. A retrospective endoscopic survey of 32 patients. Practitioner. 1975;215(1290):787–792. [PubMed] [Google Scholar]
- 41.Bennett A, Clark-Wibberley T, Stamford IF, Wright JE. Aspirin-induced gastric mucosal damage in rats: cimetidine and deglycyrrhizinated liquorice together give greater protection than low doses of either drug alone. J Pharm Pharmacol. 1980;32(2):151. doi: 10.1111/j.2042-7158.1980.tb12879.x. [DOI] [PubMed] [Google Scholar]
- 42.Jalilzadeh-Amin G, Najarnezhad V, Anassori E, Mostafavi M, Keshipour H. Antiulcer properties of Glycyrrhiza glabra L. extract on experimental models of gastric ulcer in mice. Iran J Pharm Res. 2015;14(4):1163–1170. [PMC free article] [PubMed] [Google Scholar]
- 43.Khazraei-Moradian S, Ganjalikhani-Hakemi M, Andalib A, Yazdani R, Arasteh J, Kardar GA. The effect of licorice protein fractions on proliferation and apoptosis of gastrointestinal Cancer cell lines. Nutr Cancer. 2017;69(2):330–339. doi: 10.1080/01635581.2017.1263347. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee M, Bhaskaran N, Srinath R, et al. Anti-ulcer and antioxidant activity of GutGard. Indian J Exp Biol. 2010;48(3):269–274. [PubMed] [Google Scholar]
- 45.Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47(5):709–710. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- 46.Salas-Jara MJ, Sanhueza EA, Retamal-Díaz A, González C, Urrutia H, García A. Probiotic Lactobacillus fermentum UCO-979C biofilm formation on AGS and Caco-2 cells and Helicobacter pylori inhibition. Biofouling. 2016;32(10):1245–1257. doi: 10.1080/08927014.2016.1249367. [DOI] [PubMed] [Google Scholar]
- 47.Song HY, Zhou L, Liu DY, Yao XJ, Li Y. What roles do probiotics play in the eradication of Helicobacter pylori? Current Knowledge and Ongoing Research Gastroenterol Res Pract. 2018;16:9379480. doi: 10.1155/2018/9379480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, Choi SY, Jung YD. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. 2013;19(45):8192–8202. doi: 10.3748/wjg.v19.i45.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I declare that this manuscript has the availability of data and material.


