Abstract
This study set out to examine the combined effects of the supplementation of a dietary palm oil (PO) and sunflower oil (SO) blend, 0. 25% L-Arginine (L-Arg), and different levels of vitamin E (Vit E) on growth performance, fat deposition, cytokine expression, and immune response in broilers. A total of 216 1-day-old male broiler chicks (Cobb500) were randomly distributed into six dietary groups as follows: Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg) + 20 mg/kg Vit E; Diet 4: (PO and SO blend + 0.25% L-Arg) + 50 mg/kg Vit E; Diet 5: (PO and SO blend + 0.25% L-Arg) + 100 mg/kg Vit E; and Diet 6: (PO and SO blend + 0.25% L-Arg) + 150 mg/kg Vit E. Weight gain and serum IgG and IgM increased while feed conversion ratio, fat deposition, and plasma cholesterol decreased in broilers fed Vit E with the oil blend and L-Arg, compared to those fed the negative control (Diet 1). Expression of IFN and TNF-α were reduced, whereas TGF-ß1 was up-regulated as the level of Vit E increased in the broiler diets. In summary, the combination of oil blend, L-Arg, and Vit E at a level of 50 mg/kg increased the performance and altered the expression of cytokines that may positively influence immune function in broiler chickens.
Keywords: cytokine expression, immune function, dietary oils, vitamin E, serum immunoglobulin, broiler chickens
Introduction
Dietary unsaturated fat supplementation in broiler diets leads to higher levels of available metabolizable energy owing to its greater solubility and digestibility when compared to saturated fat; this produces more energy at a lower expense and therefore increases productivity (1, 2). Studies show that a combination of plant oils is more beneficial for performance and has a more positive effect on serum parameters and fatty acid deposition in broilers than a single oil (3). Moreover, supplementation of unsaturated fats (a blend of palm oil and sunflower oil) in the diet beneficially alters the fatty acid profile of broiler muscle. As a result, cholesterol content is decreased in the meat (4) with subsequent health benefits to consumers. A high intake of meat containing highly saturated fat and cholesterol has been linked to a higher incidence of disease, particularly cardiovascular disease (5). Besides the health benefits on meat composition, dietary unsaturated fats and/or combination oils can reduce abdominal fat and total body fat deposition in chickens and rats when compared to saturated fats (1, 6). Excessive deposition of abdominal fat in chickens leads to a loss of dietary energy, which can decrease carcass yield and negatively affect its acceptability to the health-conscious consumer (7). Thus, fat or oil containing both unsaturated fatty acids and saturated fatty acids would be a valuable option for broiler production.
Arginine (Arg) is one of the most versatile and essential amino acids in chickens and it must be provided in their diet due to a lack of renal argininosuccinate synthetase and argininosuccinate lyase. Arg is required for the regulation of protein synthesis (8), tissue repair, and growth, and it also plays a role in decreasing body fat deposition in ducks (9) and chickens (10). Therefore, chickens, particularly broilers, have a requirement for Arg and are extremely dependent on the dietary intake of this amino acid (11). Arg also plays an important role as a precursor of nitric oxide (NO), which controls lipogenesis and energy partitioning. We previously reported that a diet containing 0.25% L-Arg and an oil blend (4% palm oil and 2% sunflower oil) had a complementary effect on the suppression of lipogenic genes such as acetyl–CoA carboxylase (ACC) and fatty acid synthetase (FAS), and reduced fat deposition in broilers (12). Azimi Youvalari et al. (13) suggest that adding 0.2% Arg beyond NRC (1994) requirements in broiler diets results in energy splitting toward protein deposition and decreased abdominal fat pads. Moreover, dietary Arg improves immune response by enhancing nitric oxide (NO) release from macrophages (14, 15). Hence, nutritional modifications are recommended to decrease fat and cholesterol accumulation and improve muscle yield to meet the prevailing health requirements of consumers.
Despite the benefits, the inclusion of dietary unsaturated fats, mostly polyunsaturated fatty acids (PUFAs), can increase lipid oxidation and unfavorably influence the color and flavor of meat (16), decrease the performance of chickens (17), and may encourage oxidative challenges that can affect health (18). The harmful effects of feeding unsaturated fat can be overcome by including antioxidants in the diet. Vit E (α-tocopherol) acts as an important antioxidant and can be used in the diet as an effective means of inhibiting lipid oxidation. It acts as an immunomodulator, shields membranes from free radical attack via the production of NO, and enhances immune function (19, 20). Furthermore, Vit E supplementation has been reported to improve liver function and increase the number of white blood cells and red blood cells, thus increasing immune status in broilers (21). Furthermore, a significant interactive effect between oil blends, L-Arg, and Vit E on meat quality has recently been reported (22). In our previous study (12), convincing results were obtained in terms of performance, fat deposition, fatty acid profile, and blood lipid profile in broilers fed a combination of palm oil (PO) and sunflower oil (SO; 4:2) with L-Arg (0.25%). However, a combined effect of different levels of Vit E in the latter diet on performance, cytokine expression, and immune response has not been previously explored. Thus, a diet including the above mentioned oil combination with L-Arg (0.25%) was chosen and supplemented with different levels of Vit E to test the hypothesis that concurrent supplementation of the PO and SO blend, L-Arg, and Vit E will improve broiler performance, cytokine expression, and immune function. Therefore, the study was conducted to assess this diet regarding growth performance, carcass characteristics, blood lipid profile, serum immunoglobulin, and splenic cytokine expression in broiler chickens.
Materials and Methods
Birds, Experimental Diets, and Management
All procedures followed the protocols endorsed by the Institutional Animal Care and Use Committee of Research Policy at Universiti Putra Malaysia (UPM/IACUC/AUP-R081/2016). A total of 216 1-day-old male broiler (Cobb 500) chicks were purchased from a commercial hatchery in Malaysia. The birds were immediately weighed, marked, and randomly divided into six dietary groups (treatments). Each dietary treatment contained six replicates with six birds in each replication. The experimental diets were: Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg) + 20 mg/kg Vit E; Diet 4: (PO and SO blend + 0.25% L-Arg) + 50 mg/kg Vit E; Diet 5: (PO and SO blend + 0.25% L-Arg) + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg) + 150 mg/kg Vit E. Arg was added as L-Arg monohydrochloride (99.6% L-Arg; Herbstore Ltd., USA), and Vit E was added in the form of DL-α-tocopherol acetate. Diets were formulated to meet the nutrient requirements for broiler chickens (23) by using FeedLIVE software (FeedLIVE 1.52., Thailand). The broilers were reared for six weeks; they were vaccinated at 7 and 14 days against infectious bronchitis (IB) and Newcastle disease (ND), respectively, and at 21 days against infectious bursal diseases (IBD). All broilers were fed a starter diet from 1 to 21 days of age and a finisher diet from 22 to 42 days of age; these diets are presented in Tables 1, 2, respectively.
Table 1.
Feed composition and calculated nutrient contents of the experimental diets fed to broiler chickens during the starter period.
| Ingredient (g/kg) | Dietary treatments* | |||||
|---|---|---|---|---|---|---|
| Diet 1 (control) | Diet 2 (positive control) | Diet 3 | Diet 4 | Diet 5 | Diet 6 | |
| Corn | 441.8 | 435.1 | 435.1 | 435.1 | 435.1 | 435.1 |
| Soybean meal | 312.4 | 329.7 | 329.7 | 329.7 | 329.7 | 329.7 |
| Palm oil (PO) | 60.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Sunflower oil (SO) | 0.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Wheat bran | 38.6 | 39.5 | 39.5 | 39.5 | 39.5 | 39.5 |
| Wheat pollard | 75.3 | 70.5 | 70.5 | 70.5 | 70.5 | 70.5 |
| Fish meal | 39.6 | 27.9 | 27.9 | 27.9 | 27.9 | 27.9 |
| L-Lysine | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| DL-Methionine | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| DCP1 | 15.0 | 17.9 | 17.9 | 17.9 | 17.9 | 17.9 |
| Calcium carbonate | 5.0 | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 |
| Choline chloride | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Mineral premix2 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin premix3 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Antioxidant4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Toxin binder5 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Arginine | 0.0 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Vit E (mg/kg) | – | – | 20.0 | 50.0 | 100.0 | 150.0 |
| Chemical composition(Calculated)6 | ||||||
| ME (MJ/kg) | 12.9 | 12.9 | 12.9 | 12.9 | 12.9 | 12.9 |
| CP (g/kg) | 210 | 210 | 210 | 210 | 210 | 210 |
| Fat (g/kg) | 82.5 | 82.0 | 82.0 | 82.0 | 82.0 | 82.0 |
| Fiber (g/kg) | 42.9 | 43.6 | 43.6 | 43.6 | 43.6 | 43.6 |
| Calcium (g/kg) | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 |
| Available P (g/kg) | 4.60 | 4.60 | 4.60 | 4.60 | 4.60 | 4.60 |
| Lysine (g/kg) | 13.3 | 13.3 | 13.3 | 13.3 | 13.3 | 13.3 |
| Methionine (g/kg) | 5.50 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 |
| Arginine (g/kg) | 13.8 | 16.3 | 16.3 | 16.3 | 16.3 | 16.3 |
| Meth + cyst (g/kg) | 8.80 | 8.70 | 8.70 | 8.70 | 8.70 | 8.70 |
| Tryptophan (g/kg) | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 |
| α-tocopherol (analyzed, mg/kg) | 40.7 | 37.8 | 50.7 | 78.4 | 124 | 165 |
Di-calcium phosphate.
Vitamin premix contained: retinol 2 mg, α-tocopherol 0.02 mg, cholecalciferol 0.03 mg, menadione 1.33 mg, thiamin 0.83 mg, cobalamin 0.03 mg, riboflavin 2.0 mg, biotin 0.03 mg, folic acid 0.33 mg, niacin 23.30 mg, pantothenic acid 3.75 mg, and pyridoxine 1.33 mg.
Mineral premix contained: Fe 120 mg, Zn 100 mg, Mn 150 mg, Cu 20 mg, Mg 12 mg, Co 0.6 mg, Se 0.20 mg.
Antioxidant contained: butylatedhydroxyanisole (BHA).
Toxin binder contained: natural hydrated sodium calcium aluminum silicates (HSCAS).
The rations were formulated using FeedLIVE software (Thailand).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
Table 2.
Feed composition and calculated nutrient contents of the experimental diets fed to broiler chickens during the finisher period.
| Ingredient (g/kg) | Dietary treatments* | |||||
|---|---|---|---|---|---|---|
| Diet 1 (control) | Diet 2 (positive control) | Diet 3 | Diet 4 | Diet 5 | Diet 6 | |
| Corn | 495.8 | 490.9 | 490.9 | 490.9 | 490.9 | 490.9 |
| Soybean meal | 291.6 | 287.4 | 287.4 | 287.4 | 287.4 | 287.4 |
| Palm oil (PO) | 60.00 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Sunflower oil (SO) | 0.00 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Wheat bran | 34.0 | 45.5 | 45.5 | 45.5 | 45.5 | 45.5 |
| Wheat pollard | 50.5 | 43.4 | 43.4 | 43.4 | 43.4 | 43.4 |
| Fish meal | 27.7 | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 |
| L-Lysine | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| DL-Methionine | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| DCP1 | 18.2 | 18.3 | 18.3 | 18.3 | 18.3 | 18.3 |
| Calcium carbonate | 9.80 | 9.30 | 9.30 | 9.30 | 9.30 | 9.30 |
| Choline chloride | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 |
| Salt | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Mineral premix2 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Vitamin premix3 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Antioxidant4 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 |
| Toxin binder5 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| Arginine | 0.000 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Vit E (mg/kg) | – | – | 20.0 | 50.0 | 100 | 150 |
| Chemical composition (Calculated)6 | ||||||
| ME (MJ/kg) | 13.0 | 13.0 | 13.0 | 13.0 | 13.0 | 13.0 |
| CP (g/kg) | 195 | 195 | 195 | 195 | 195 | 195 |
| Fat (g/kg) | 83.1 | 84.0 | 84.0 | 84.0 | 84.0 | 84.0 |
| Fiber (g/kg) | 40.4 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 |
| Calcium (g/kg) | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| Available (g/kg) | 4.60 | 4.60 | 4.60 | 4.60 | 4.60 | 4.6 |
| Lysine (g/kg) | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 |
| Methionine (g/kg) | 5.30 | 5.30 | 5.30 | 5.30 | 5.30 | 5.30 |
| Arginine (g/kg) | 12.8 | 15.3 | 15.3 | 15.3 | 15.3 | 15.3 |
| Meth + cyst (g/kg) | 8.30 | 8.30 | 8.30 | 8.30 | 8.30 | 8.30 |
| Tryptophan (g/kg) | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 |
| α-tocopherol (analyzed, mg/kg) | 39.4 | 36.6 | 47.5 | 77.4 | 122 | 164 |
Di-calcium phosphate.
Vitamin premix contained: retinol 2 mg, α-tocopherol 0.02 mg, cholecalciferol 0.03 mg, menadione 1.33 mg, thiamin 0.83 mg, cobalamin 0.03 mg, riboflavin 2.0 mg, biotin 0.03 mg, folic acid 0.33 mg, niacin 23.30 mg, pantothenic acid 3.75 mg, and pyridoxine 1.33 mg.
Mineral premix contained: Fe 120 mg, Zn 100 mg, Mn 150 mg, Cu 20 mg, Mg 12 mg, Co 0.6 mg, and Se 0.20 mg.
Antioxidant contained: butylatedhydroxyanisole (BHA).
Toxin binder contained: natural hydrated sodium calcium aluminum silicates (HSCAS).
The rations were formulated using FeedLIVE software (Thailand).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
Sample and Data Collection
The body weight and feed intake of the broilers were measured weekly to calculate body weight gain (BWG) and feed conversion ratio (FCR). At 21 days, blood samples were collected for measurement of IgG and IgM. At 42 days, 12 broilers per treatment were slaughtered for sampling of blood, breast muscle, abdominal fat, and internal organs. Breast meat samples were kept in a −20°C freezer for analysis of chemical composition and α-tocopherol content of the meat. Spleen samples were snapped in liquid nitrogen and stored in a −80°C freezer for the measurement of cytokine expression. Internal organs and abdominal fat were collected to estimate their percentage, while serum and plasma samples were collected for the measurement of immunoglobulin (IgG and IgM) and lipid profile, respectively.
Determination of Vit E
The Vit E (α-tocopherol) content of meat samples was determined according to the method of Rutkowski and Grzegorczyk (24). 0.5 g of pulverized sample was placed into a screw cap centrifuged tube to which was added 0.5 mL of anhydrous ethanol followed by a vigorous mixing for 1 min. Approximately 3 mL of xylene was added into the tube and mixed thoroughly for 1 min. The tube was subsequently centrifuged at 1,500 × g for 10 min. After centrifugation, 1.5 mL of the upper layer (extract) was transferred to a separate test tube and 0.25 mL of a 6.02 mM solution of batophenanthroline was added and mixed in. Subsequently, test and standard samples were prepared, and absorbance was measured against a blank at 539 nm using a spectrophotometer (Secomam, Domont, France). The α-tocopherol content of meat samples was measured using the following formula as the amount of α-tocopherol: (μM) = (Ax/As) × Cs, where, Cs = concentration of standard; Ax = Absorbance of test sample; and As = Absorbance of standard.
Proximate Analysis of Meat
The breast meat samples were analyzed for moisture, ash, crude fat (EE), and crude protein content following the methods of AOAC (25).
Plasma Lipid Profile, Blood Protein, Albumin, and Liver Enzyme Estimation
At 42 days of age, blood samples were collected from six chickens per treatment into separate vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). The blood samples were mixed by inverting the tubes gently and were then stored in ice before centrifugation. Plasma was separated by centrifuge at 3,000 × g for 10 min, then was transferred into vials and stored at −80°C for further analysis. The plasma lipid profiles, aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), total protein, and albumin were measured using an Automatic Analyzer 902 (Hitachi, Germany). Serum very low density lipoprotein-cholesterol (VLDL-C) and LDL-C were determined according to the Friedewald Equation as described by Loh et al. (26).
Immune Response
The IgG and IgM concentrations were estimated using Chicken IgM ELISA Kits (Immunology Consultants Laboratories, Inc., USA) and Chicken IgG ELISA Kits (CEA544Ga, Cloud-Clone Corp., USA), respectively. Serum samples were removed from the freezer and allowed to thaw and were then mixed gently by inverting. The samples were diluted based on an expected concentration of the analyte to fall within the ranges of the standard curve. The IgM standard was prepared by adding 1 mL de-ionized water into the supplied IgM calibrator in order to obtain a concentration of 54.20 μg/mL. The tubes were labeled for the preparation of IgM standard at 100, 50, 25, 12.5, 6.25, and 3.125 ng/mL using the supplied diluent. A duplicate 100 μL of sample, blank, and standard were placed into pre-assigned wells and incubated for 30 min and then washed four times with wash solutions. After that, 100 μL of diluent enzyme antibody conjugate was added to each well, covered, and incubated at 22°C for 30 min, and washed four times. TMB substrate (3, 3′, 5, 5′-tetramethyl benzidine) solution was dispensed into each well and kept in the dark at room temperature for 10 min. Finally, 100 μL of 0.3 M sulfuric acid (stop solution) was added into each well, and absorbance of the solution was measured at 450 nm using a microplate reader (Model 3550-UV, Bio-Rad). Serum IgM concentrations in each sample were estimated using standard curves which were obtained by plotting the OD450 against standard concentrations.
The IgG concentration of serum samples was determined by using Chicken IgG ELISA Kits. The serum samples were thawed and diluted 2,000-fold by using 0.01 mol/L PBS (pH = 7.0–7.2). The standard was prepared by diluting stock to obtain a concentration of stock solution 100 μL/mL and then diluted at 100, 33.33, 11.11, 3.70, and 1.23 ng/mL. Aliquots of 50 μL of respective standard, sample, and blank in duplicate were dispensed into the previously selected wells; subsequently, the same amount (μL) of diluent A was added and the solution was incubated at 37°C for 1 h. After 1 h, the solution was washed four times and diluent B (100 μL) was added, followed by an incubation period of 30 min at 37°C. The solution was then washed and 90 μL of substrate solution (TMB) was added; it was then covered and incubated in the dark at 37°C for 15 min. Thereafter, 50 μL of 0.3 M sulfuric acid (stop solution) was added into each well, and absorbance was measured at 450 nm using a microplate reader (Model 3550-UV, Bio-Rad). Serum IgG concentrations were calculated using the equation from the standard curves. The concentrations of IgM and IgG were expressed as ng/mL of blood serum.
RNA Isolation and Real Time PCR
Spleen samples were collected immediately after slaughter, snap frozen in liquid nitrogen, and stored in a −80°C freezer until used for RNA isolation. About 30 mg of splenic frozen tissue from each bird was homogenized and total RNA was extracted and purified using an RNeasy plus Mini kit (QIAGEN, Germany) according to the manufacturer's protocol. The purity and concentration of RNA was assessed using a NanoDrop ND-1000 UV-Vis Spectrophotometer at absorbance 260/280 nm, and absorbance ranged from 1.9 to 2.1 for all samples. Total RNA was then reverse transcribed to cDNA using a QuantiNova™ Reverse transcription kit (Qiagen, Hilden, Germany) as described in the manufacturer's protocol. Then, 1 μg of purified RNA was reacted with 4 μL of RT buffer, 1 μL transcriptase, 1 μL RT primer mix, 2 μL gDNA wipeout buffer, and RNase free water to produce a final volume of 20 μL.
Cytokine expression was performed with real time PCR (Bio-Rad, Hercules, CA. USA) using a QuantinovaTM SYBRR Green PCR kit (Qiagen) according to the manufacturer's instructions. The mRNA of β-actin (QT00600614, Qiagen) was used as the internal control gene to normalize the target genes. Real time qPCR analysis was carried out using a QuantiTech Primer Assay (200) [IFN (Cat. QT00598059), TGF-ß1 (Cat.QT00644049), TNF-α (Cat.QT00596106), and IL8 (Cat. QT00600446), Qiagen]. Each reaction (20 μL) comprised of 10 μL SYBR Green PCR Master mix, 1 μL cDNA, 1 μL of each (forward and reverse) primer, and 7 μL of RNase free water. Thermal cycling was initiated at 95°C for 2 min. Subsequently, 40 PCR cycles of denaturation for 5 s at 94°C were run, followed by annealing and extension for 10 s at 59°C. Each sample was run in duplicate and the mean of the duplicates was used to allocate cycle threshold (Ct) values. The concentration of test genes in relation to those of the reference gene (β-actin) mRNA expression were estimated based on comparative Ct value methods. The 2−ΔΔC(t) method was used to determine the relative changes in the mRNA expression of genes estimated from real time PCR (27).
Statistical Analysis
The experiments followed a completely randomized design (CRD). All data for broiler performance, carcass characteristics, IgG and IgM concentration, and gene expression were statistically analyzed using PROC GLM of SAS (28) using the following model:
Where, Yij is the dependent variable; μ is overall mean; Fi is the effect of dietary treatment (i = 1 to 6); and eij random error associated with each record, distributed as N (0, σ2).
The replicate was the experimental unit. The means were separated using Tukey's Honestly Significant Difference test and the level of significance was set at P < 0.05.
Results
Broiler Performance
The performance of broilers fed a dietary oil blend, supplemented with 0.25% L-Arg, and different levels of Vit E is shown in Table 3. Body weight and body weight gain were higher for broilers fed the PO and SO blend and supplemented with L-Arg and different levels of Vit E than for those fed the control diet (Diet 1) during the finisher period whereas in the starter period Diet 4 and 6 showed significantly higher values of these parameters. Moreover, FCR was greater (P < 0.05) in broilers fed the control diet than those fed other dietary treatments. However, no significant differences (P > 0.05) were found in body weight gain and FCR between the broilers fed different levels of Vit E compared to those fed the positive control diet (Diet 2).
Table 3.
Growth performance of broilers fed PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E.
| Parameter | Treatments* | SEM | |||||
|---|---|---|---|---|---|---|---|
| Diet 1 (control) | Diet 2 (positive control) | Diet 3 | Diet 4 | Diet 5 | Diet 6 | ||
| 1–21 days | |||||||
| Initial body weight (g) | 41.00 | 41.36 | 41.61 | 40.14 | 40.39 | 40.33 | 0.034 |
| Body weight (g) | 669b | 745a | 723a | 744a | 715ab | 738a | 1.14 |
| Body weight gain (g) | 628b | 704a | 681ab | 704a | 675ab | 698a | 6.85 |
| Feed intake (g) | 1072 | 1082 | 1061 | 1081 | 1070 | 1099 | 8.46 |
| FCR | 1.71a | 1.54b | 1.56b | 1.54b | 1.59b | 1.58b | 0.015 |
| 22–42 days | |||||||
| Body weight (g) | 2316b | 2577a | 2542a | 2580a | 2551a | 2530a | 3.53 |
| Body weight gain (g) | 1647b | 1832a | 1819a | 1836a | 1835 a | 1792a | 14.8 |
| Feed intake (g) | 2834 | 2913 | 2894 | 2851 | 2933 | 2905 | 13.6 |
| FCR | 1.72a | 1.59b | 1.59b | 1.55b | 1.60b | 1.62b | 0.011 |
Means with different superscripts in the same row differ significantly (P < 0.05).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
SEM, standard error of the means.
Carcass Characteristics and Internal and Lymphoid Organs
The effects of a dietary PO and SO blend + 0.25% L-Arg + different levels of Vit E on carcass characteristics and internal and lymphoid organs of broiler chickens are presented in Table 4. The broilers fed Diet 1 had significantly (P < 0.05) lower carcass and breast meat yields compared to those fed other diets. However, no significant differences were observed between chickens supplemented with different levels of Vit E (Diets 3–6) and Diet 2. The leg yield and the internal organs (liver, gizzard, and heart) percentage did not differ (P > 0.05) among the dietary treatments. However, the abdominal fat percentage was higher (P < 0.05) in chickens fed the control diet compared to other dietary treatments. The lymphoid organs (spleen and bursa of Fabricius weight) were not influenced (P > 0.05) by the dietary treatments.
Table 4.
Carcass characteristics and internal and lymphoid organs of broilers fed PO and SO blend end, supplemented with 0.25% L-Arg, and different levels of Vit E.
| Parameter | Treatments* | SEM | |||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | ||
| Carcass yield (%) | 74.1b | 77.6a | 78.7a | 79.0a | 78.5a | 78.6a | 0.337 |
| Breast yield (%) | 32.2b | 34.8ab | 35.2ab | 36.8a | 35.3a | 36.7a | 0.398 |
| Leg yield (%) | 25.3 | 26.1 | 26.5 | 26.1 | 27.0 | 26.2 | 0.230 |
| Abdominal fat (%) | 1.76b | 1.57a | 1.53a | 1.52a | 1.52a | 1.56 a | 0.022 |
| Gizzard (%) | 2.25 | 2.45 | 2.23 | 2.24 | 2.44 | 2.39 | 0.049 |
| Heart (%) | 0.390 | 0.390 | 0.430 | 0.420 | 0.410 | 0.420 | 0.087 |
| Bursa of Fabricius (%) | 0.090 | 0.100 | 0.110 | 0.120 | 0.120 | 0.110 | 0.003 |
| Spleen (%) | 0.100 | 0.110 | 0.130 | 0.130 | 0.140 | 0.120 | 0.003 |
Means with different superscripts in the same row differ significantly (P < 0.05).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
SEM, standard error of the means.
Vit E (α-Tocopherol) Concentration of Breast Meat
The Vit E concentration of breast meat taken from broilers fed a dietary PO and SO blend, supplemented with 0.25% L-Arg, and different levels of Vit E, is shown in Table 5. The α-tocopherol concentration of meat was significantly increased along with increased levels of α-tocopherol in the diet. The concentration of α-tocopherol in meat increased noticeably when the broiler diet was supplemented with at least 50 mg/kg or more of Vit E.
Table 5.
Vitamin E (α-tocopherol; mg/kg) concentration of the meat of broilers fed PO and SO blend, supplemented with 0.25% L-Arg, and different levels of Vit E.
| Dietary Treatments* | Vitamin E (mg/kg) |
|---|---|
| Diet 1 | 5.67e ± 0.087 |
| Diet 2 | 5.14f ± 0.075 |
| Diet 3 | 6.37d ± 0.101 |
| Diet 4 | 10.1c ± 0.110 |
| Diet 5 | 13.1b ± 0.091 |
| Diet 6 | 13.7a ± 0.106 |
Means with different superscripts in the same column differ significantly (P < 0.05).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
The values are all presented as mean ± standard error.
Chemical Composition of Breast Meat
The chemical composition of breast muscle in broilers fed a dietary PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E, is presented in Table 6. The percentage of moisture, ash, and fat content of breast meat were unaffected across the dietary treatments. The crude protein content of the breast meat of broilers fed Diet 1 was lower (P < 0.05) than those of broilers fed other dietary treatments.
Table 6.
Chemical composition of the breast meat of broilers fed PO and SO blend, supplemented with 0.25% L-Arg, and different levels of Vit E.
| Parameter (%) | Treatments* | SEM | |||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | ||
| Moisture | 70.6 | 69.0 | 69.2 | 68.9 | 68.7 | 68.8 | 0.252 |
| Ash | 1.59 | 1.63 | 1.62 | 1.63 | 1.62 | 1.63 | 0.005 |
| Crude protein | 23.7b | 25.9a | 25.9a | 26.1a | 26.4a | 26.2a | 0.276 |
| Crude fat | 3.60 | 3.24 | 3.14 | 3.11 | 3.05 | 3.13 | 0.090 |
Means with different superscripts in the same row differ significantly (P < 0.05).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
SEM, standard error of the means.
Total Protein and Albumin Contents in Blood
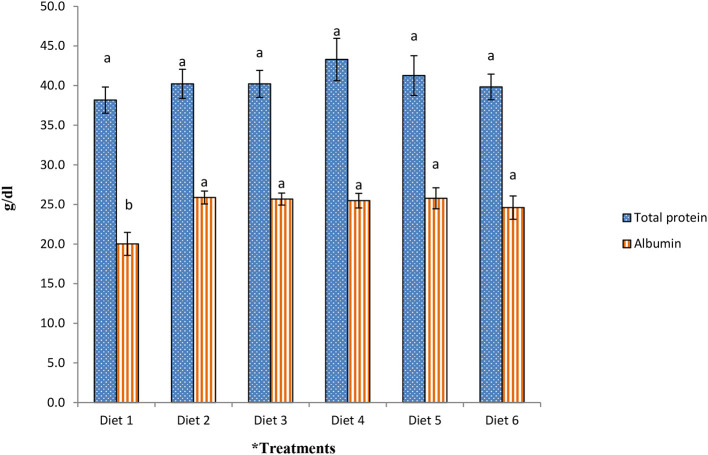
The total protein and albumin contents in blood plasma in broilers fed a dietary PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E, are illustrated in Figure 1. No significant difference (P > 0.05) was observed in total plasma protein among the dietary treatments, although numerically higher total protein levels were found in the plasma of broilers fed a diet supplemented with either 0.25% L-Arg alone or in combination with Vit E. The chickens fed Diet 1 had a lower (P < 0.05) plasma albumin level than those fed other diets.
Figure 1.
Effect of the supplementation of PO and SO blend, 0.25% L-Arg, and different levels of Vit E on plasma total protein and albumin content of broilers. a,b,cBars with the same color and no common letter differ significantly (P < 0.05). *Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
IgG and IgM Concentrations in Blood Serum
The effects of supplementation of a dietary PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E, on serum IgG and IgM concentrations for 21 days old broilers are presented in Table 7. Serum IgG and IgM concentrations increased (P < 0.05) for broilers fed the above mentioned supplements compared to the control. Moreover, chickens fed an oil-blend diet with 0.25% L-Arg combination and Vit E amounts ranging from 50mg to 150 mg/kg (Diet 3–6) had higher (P < 0.05) IgG and IgM concentrations than other dietary treatments; however, IgG and IgM concentrations were statistically similar (P > 0.05) among broilers fed Diets 3–6.
Table 7.
Effect of the supplementation of PO and SO blend, L-Arg, and different levels of Vit E on serum IgM and IgG (log10) concentrations in broilers at 21 days of age.
| Parameter | Treatments* | SEM | |||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | ||
| IgG | 2.98b | 3.01ab | 3.01ab | 3.03a | 3.04a | 3.04a | 0.006 |
| IgM | 2.71b | 2.82ab | 2.89a | 2.90a | 2.90a | 2.89a | 0.017 |
Means with different superscripts in the same row differ significantly (P < 0.05).
Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
SEM, standard error of the means.
Blood Lipid Profile and Liver Enzyme Function
The effects of a dietary PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E, on blood lipid profiles and liver enzymes in broilers are shown in Table 8. The concentrations of total cholesterol, TG, and VLDL-C (mmol/L) were greater (P < 0.05) in the blood plasma of broilers fed Diet 1 compared with those fed other dietary treatments, whereas the LDL-C (mmol/L) was significantly lower in Diet 6 than other dietary treatments. The level of HDL-C was not affected (P > 0.05) by dietary treatment, although the highest level (2.28 mmol/L) was found in broilers fed Diet 2 and Diet 5. In contrast, the lowest level (2.13 mmol/L) was observed in Diet 1. Dietary treatments did not affect (P > 0.05) alanine amino transferase (ALT) and aspartate amino transferase (AST) in broilers.
Table 8.
Effect of dietary supplementation of PO and SO blend, L-Arg, and different levels of Vit E on plasma lipid profiles and liver function of broilers at 42 days of age.
| Parameter | Treatments* | SEM | |||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | ||
| Lipid profile (mmol/L) | |||||||
| CHOL | 3.79a | 3.31b | 3.31b | 3.27b | 3.26b | 3.02b | 0.065 |
| TG | 0.780a | 0.540ab | 0.550ab | 0.520b | 0.500b | 0.500b | 0.029 |
| HDL-C | 2.13 | 2.28 | 2.24 | 2.20 | 2.28 | 2.25 | 0.051 |
| VLDL-C | 0.150a | 0.100b | 0.110b | 0.110b | 0.100b | 0.100b | 0.006 |
| LDL-C | 1.51a | 0.930ab | 0.960ab | 0.960ab | 0.880ab | 0.670b | 0.076 |
| Liver function | |||||||
| Alt (U/L) | 5.05 | 4.72 | 4.83 | 4.80 | 4.45 | 4.08 | 0.219 |
| Ast (U/L) | 329 | 284 | 287 | 320 | 290 | 310 | 8.76 |
Means with different superscripts in the same row differ significantly (P < 0.05).
Diet 1: 6% palm oil (control), Diet 2: (6% PO and SO blend + 0.25% L-Arg; positive control), Diet 3: (6% PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E), Diet 4: (6% PO and SO blend + 0.25% L-Arg + 50 mg/kg Vit E), Diet 5: (6% PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E), Diet 6: (6% PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E).
SEM, standard error of the means.
Splenic mRNA Expression
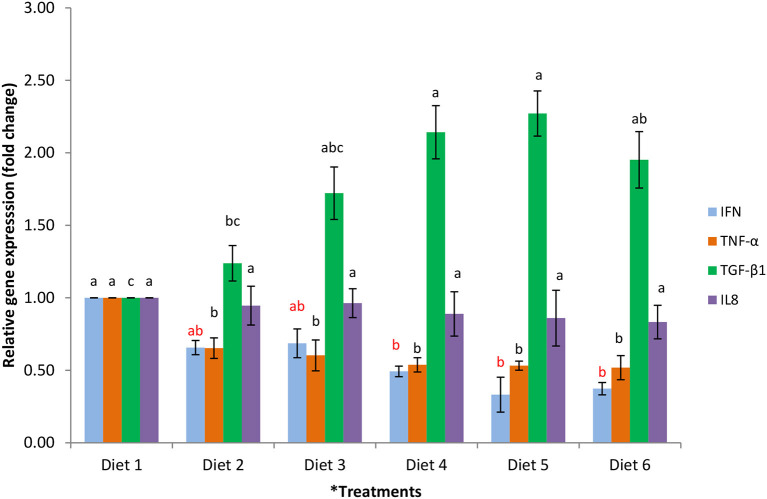
Splenic mRNA expression in broilers fed a dietary PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E, is shown in Figure 2. Dietary treatments did not affect (P > 0.05) the expression of IL8 in broilers. The expression of IFN in those broilers fed Diet 1 was greater (P < 0.05) when compared with those fed other dietary treatments. The expression of IFN decreased (P < 0.05) as the level of Vit E increased in the diet. Diet 1 upregulated the expression of TNF-α in broilers compared with other dietary treatments. Diet 1 down-regulated the expression of TGF-ß1 in broilers compared to those fed Diets 4, 5, and 6. The expression of TGF-ß1 in the broilers fed Diets 2 and 3 did not differ from those fed other dietary treatments. Moreover, no differences were observed in TGF-ß1, IFN, and TNF-α mRNA expression among broilers fed the PO and SO blend, supplemented with 0.25% L-Arg and Vit E ranging from 50 to 150 mg/kg feed (Diets 4–6).
Figure 2.
Comparison of IFN, TNF-α, TGF-β1, and IL8 expression in the spleen tissue of broiler chickens fed PO and SO blend, supplemented with 0.25% L-Arg and different levels of Vit E. Values were normalized with a housekeeping gene, β-actin. Subsequently, treated samples were expressed relative to gene expression of the control group (Diet 1). *Diet 1: 6% palm oil (negative control); Diet 2: PO and SO blend (4% palm oil and 2% sunflower oil) + 0.25% L-Arg (positive control); Diet 3: (PO and SO blend + 0.25% L-Arg + 20 mg/kg Vit E); Diet 4: (PO and SO blend + 0.25% L-Arg+ 50 mg/kg Vit E); Diet 5: (PO and SO blend + 0.25% L-Arg + 100 mg/kg Vit E); and Diet 6: (PO and SO blend + 0.25% L-Arg + 150 mg/kg Vit E). a,b,cBars with the same color and no common letter differ significantly (P < 0.05).
Discussion
Performance of Chickens
Broilers fed a diet containing an oil blend of SO and PO, 0.25% L-Arg, and different levels of Vit E had a higher body weight, greater body weight gain, and a lower FCR compared to those fed the control diet (Diet 1). These results might be due to the higher levels of unsaturated fats in the oil blend compared to only PO present in Diet 1. Improved growth rates and FCR have been reported for broilers fed diets containing higher proportions of long-chain fatty acids, due to their high energy-yielding capacity and digestibility compared to diets rich in saturated fats (4). In addition, supplementation of 0.25% L-Arg in the diet also plays an important role in the growth of broilers as L-Arg is involved in the biosynthesis of protein, glutamine, polyamines, ornithine, and proline. Wu et al. (9) state that L-Arg augments body weight gain in ducks. Furthermore, dietary inclusion of an oil blend (castor oil) is reported as a good option for increasing growth performance, even in coccidiosis-challenged broilers (29). In this aspect, the current findings suggest a synergistic effect among oil blend, L-Arg, and Vit E in increasing body weight gain and decreasing FCR during days 1–21 and 22–42 of age. Likewise, growth performance and FCR improved linearly when broilers were fed a diet containing a combination of canola oil (rich in PUFA) and 2–4 g/kg L-Arg (11).
Carcass Characteristics, Internal, and Lymphoid Organs
The carcass and breast muscle yield of broilers was significantly increased and abdominal fat was decreased in diets supplemented with PO and SO blend and 0.25% L-Arg, compared to the control diet. The carcass yield stimulation effect could be credited to L-Arg as it is involved in the synthesis of protein, proline, and hydroxyproline, which are essential for the formation of connective tissue, particularly through the gain of breast muscle and reduction of abdominal fat via alterations in carbohydrate and fat metabolism. Jiao et al. (30) report that supplementation of dietary Arg could enhance muscle growth and increase carcass and breast yield in broilers. Due to its faster accumulation, abdominal fat content is used as an indicator to assess total body fat content in broiler chickens (31). The dietary inclusion of oils containing long chain fatty acid, such as SO, soybean oil, and combinations of PO and SO (oil blend), enhanced oxidation and reduced the synthesis of fatty acids, resulting in less accumulation of abdominal fat in broilers (1, 4). The significant reduction of abdominal fat and higher carcass yield reported in the present study could also be due to the combined effects of L-Arg and the PO and SO blend inhibiting the expression of hepatic FASmRNA and ACCmRNA, which suppresses the synthesis of fatty acids and leads to less abdominal fat deposition (12).
The size of the bursa of Fabricius was not affected by Vit E supplementation, and no significant differences were found between the dietary treatments. Similar results were observed by Rostami et al. (32) and El-Gogary (33) who report that the dietary supplementation of Vit E or rosemary does not affect the size of the bursa of Fabricius or the weight of other lymphoid tissues. However, Biswas et al. (34) report that the supplementation of combined L-Arg and Vit E in broiler diets changed the size of the bursa of Fabricius. The relative spleen weight in the current study was not influenced by dietary treatments. This observation corroborates with the findings of Abdulkalykova and Ruiz-Feria (35), who observed that the supplementation of L-Arg and Vit E had no effect on the weight of spleens in broilers.
Tocopherol Content of Breast Muscle
The inclusion of antioxidants, particularly Vit E, in the diet is an important approach to increase the antioxidant capacity of meat to prevent lipid oxidation. In the present study, the α-tocopherol content of meat increased as the level of Vit E increased in the diet. Similarly, Skrivan et al. (36) report that dietary supplementation of higher levels of α-tocopherol increased the α- tocopherol content in both the thigh and breast muscle of broilers. In addition, Zhang et al. (37) reveal that increased levels of dietary α-tocopherol concentration significantly increased the α-tocopherol content of liver tissue and blood serum in chickens. The higher concentration of α-tocopherol in meat is helpful in preservation, as it protects sensitive compounds, such as UFA and PUFA, from oxidation. The increased Vit E concentration in the broiler diet increased Vit E content and decreased lipid peroxidation in meat, thus highlighting the benefits of additional Vit E for maintaining fat quality in broiler meat (38). Our recent findings report an effect of Vit E on lipid oxidation in broiler meat during the postmortem storage, and a significant reduction of MDA production in meat observed by adding 50–150 kg/mg Vit E with the oil blend of PO and SO and L-Arg (22).
Chemical Composition of Breast Muscle
The proximate composition of meat can be altered through dietary manipulation. The present findings indicated that different levels of Vit E with PO and SO blend and 0.25% L-Arg did not influence the moisture, ash, and fat content of meat. However, supplementation of L-Arg with the PO and SO blend increased the crude protein content of breast meat. This observation could be because Arg promotes muscle development as opposed to fat deposition in carcasses. This observation concurs with that of Tan et al. (39), who report that Arg supplementation increases protein content in the skeletal muscle of pigs.
In the current study, supplementation of different levels of Vit E did not affect the protein content nor other proximate components in boiler breast meat. This finding aligns with those of Souza et al. (40), who observed that dietary fat and Vit E had no effect on the proximate composition of broiler breast meat.
Blood Lipid Profile and Liver Enzyme Function
The results of the current study showed that broilers fed PO and SO blend and 0.25% L-Arg, alone or in combination with different levels of Vit E, had significantly decreased total cholesterol, TG, VLDL-C, and LDL-C as compared to the control. This observation might be due to increased levels of PUFAs in the oil blend, or because L-Arg inhibits the activity of stearoyl-CoA desaturase expression in the liver (12), which leads to a reduced level of triglycerides and VLDL secretion from the liver into the blood in chickens. These results are in agreement with those of Fouad et al. (10) and Velasco et al. (41) who report that serum total cholesterol, triglycerides, and LDL-C decreased in chickens following dietary supplementation of L-Arg and increased levels of SO (rich in PUFAs) in the diet, respectively. L-Arg could reduce blood cholesterol concentration through regulation of the metabolism and biosynthesis of cholesterol through the NO production pathway. On the other hand, in the current study the lipid profile did not differ among birds fed different levels of Vit E. Similarly, Ahmed et al. (42) report that dietary supplementation of antioxidants did not influence blood plasma cholesterol, triglycerides, or VLDL. In contrast, Gumus and Imik (43) observe that administration of Vit E reduces total cholesterol, triglycerides, HDL, and VLDL in heat stressed birds.
Concentrations of the liver enzymes ALT and AST are important indicators in the assessment of liver function and damage to hepatic cells. These enzymes also indicate abnormalities of the liver, which could develop due to various changes in physiological and medical conditions. In the present results, plasma ALT and AST concentration showed a trend toward decreased values in broilers fed diets containing the PO and SO blend, L-Arg, and Vit E compared to the control diet; however, no significant differences were observed among the treatments. This observation is supported by the findings of Ahmed et al. (42) who note that ALT and AST did not differ significantly among broilers supplemented with Vit E or synthetic antioxidants compared with a control diet. However, Attia et al. (44) report a favorable effect of PUFA on hepatic cell integrity and observed a decreased level of plasma AST when broilers were fed increased levels of PUFA (fish oil and a mixture of three oils). These differences among the studies might be due to differences in oil sources, Vit E levels, and environment and management practices of the birds.
IgG and IgM Concentrations in Blood Serum
IgG and IgM concentrations were examined to study the immune status of broilers fed the PO and SO blend + 0.25% L-Arg + Vit E. Nutritional supplementation is an effective strategy to increase immune response and improve avian health (45). In the present study, it was observed that serum IgG and IgM concentrations increased in broilers fed the PO and SO blend + 0.25% L-Arg + Vit E. The higher level of immunoglobulins could be attributed to increased levels of PUFAs, 0.25% L-Arg, and Vit E in the broiler diets, as both L-Arg and Vit E influence the immune response in chickens. Vit E is an antioxidant which decreases the formation of free-radicals and shields free radical attacks on double bonds of UFA in lipids. The ability of Vit E to reduce oxidative stress might be through the prevention of oxidation of UFAs and also through the alteration of eicosanoid metabolism and modulation of transcription factors which enhance avian immunity. Muir et al. (46) report that Vit E increases IgA concentration in intestinal scraps against anti-T toxoid. Vit E is particularly essential for the inhibition of fatty acid oxidation. Fatty acids can act as immunoregulatory particles that facilitate membrane fluidity, cellular communication, and second messenger elaboration; furthermore, the inhibitory action of Vit E on the stability of fatty acids may itself be immunoregulatory (38).
In addition, L-Arg modulates immune responses as Arg is involved in the synthesis of polyamine and proline, which are responsible for the proliferation of lymphocytes and the repair of damaged tissues, respectively. Moreover, via the NO production pathway, Arg repairs tissues through the stimulation of vasodilatation and the modulation of immune response (47). Hence, in the present study, increased IgG and IgM concentrations might be due to the combined effect of L-Arg and Vit E on broiler immune response.
Plasma Total Protein and Albumin Concentrations
Blood protein acts as a carrier to transport different substances, such as lipids, proteins, hormones, minerals, and vitamins, and also plays an important role in maintaining the osmotic pressure of blood. In the present study, supplementation of 0.25% L-Arg with the PO and SO blend increased plasma albumin levels compared to the control diet. This observation agrees with the findings of Emadi et al. (48) who report that the supplementation of L-Arg had no effect on total protein but significantly increased the albumin concentration in blood plasma. Attia et al. (49) report that heat stress negatively affected chicken immune status by decreasing the blood plasma protein, γ-globulin (innate immunity), and blood plasma albumin (non-specific immune protein), which was recovered by supplementation with Vit E alone or with a combination of Vit C. In the present study, greater levels of plasma albumin in broilers fed the combination of PO and SO blend + 0.25% L-Arg + Vit E might be due to the greater body weight of broiler chickens in these groups, which had a higher concentration of plasma protein compared to lighter broilers in the control group; this suggests an increased demand for maintaining lean tissue growth. A synergistic effect of PO and SO blend, L-Arg, and Vit E was observed in blood plasma albumin (non-specific immune-protein), meat crude protein content, serum IgG and IgM level, and Vit E concentrations of meat, which could explain the superior performance of broilers in these treatment groups.
Cytokine Expression
A decrease in the incidence of disease reflects changes in immune regulation via cytokine secretion. Nutrients such as amino acids, vitamins, and minerals act as immunomodulators and can be used to alter the immune response to pathogens by various pathways. Among amino acids, Arg acts as an immunologic modulator in a non-inflammatory manner because of its function as a substrate in the immune system. Moreover, vitamins, particularly Vit E, are antioxidants and have been reported to influence immune function in birds, including humoral and cellular response (19, 50).
In the current study, feeding broilers a dietary combination of 0.25% L-Arg and 50–150 mg/kg Vit E decreased pro-inflammatory cytokines IFN and TNF-α. This observation could be due to the modulatory effect of L-Arg and Vit E, which enhance non-specific immunity in the body by non-specifically killing fungi, bacteria, tumor cells, and parasites, thereby decreasing the pathogenic load. Arg produces NO as a metabolic product, playing a key role in modulating the immune response and inflammation via various pathways (51). The reduction in the expression of IFN and TNF-α in L-Arg- and Vit E-fed broilers corroborates the findings of Wu et al. (52), who observed that dietary supplementation of Arg and glutamine decreased TNF and IL2 in growing pigs.
Moreover, cytokine TGF-ß plays a key role in T-lymphocyte development and has anti-inflammatory action. In the present study, the expression of TGF-ß1 increased in broilers fed the PO and SO blend supplemented with 0.25% L-Arg and 50–150 mg/kg Vit E. This result concurs with that of Kaiser et al. (20) who observe that supplementation of higher levels of natural Vit E improves anti-inflammatory TGF-ß expression in broiler chickens. Liu et al. (53) observe that chickens fed a combination of Arg and Vit E had higher heterophil, oxidative burst, monocyte, and lymphocyte proliferation as compared with birds fed control diets. It is reported that dietary supplementation of both natural and synthetic Vit E increases one of the most important immune and inflammatory mediators, cytokine IL-6, compared to cyclophosphamide immunosuppressed broilers; this indicates that Vit E could enhance the secretion of some cytokines and thus improve immune status (50). Hence, in the current study the combined effects of 0.25% L-Arg and Vit E might alter the expression of cytokines and influence immune response in broilers. However, more studies are required to fully understand the combined effects of PO and SO blend, Arg, and Vit E and their interactions under stress conditions and specific disease challenges (parasitic and virus) in broiler chickens.
Conclusions
A broiler diet including an oil blend of PO and SO (4:2) supplemented with 0.25% L-Arg and 50 to 150 mg/kg Vit E improved performance, immunoglobulin level, and immune-related cytokine expression, while resulting in decreased fat deposition and VLDL-C content in the blood. This diet could be used to decrease fat deposition, enhance broiler performance, and improve immune function in broilers.
Data Availability Statement
All datasets generated for this study are included in the article.
Ethics Statement
All the procedure in this animal study was reviewed, followed and approved by the Institutional Animal Care and Use Committee, Universiti Putra Malaysia (UPM/IACUC/AUP-R081/2016).
Author Contributions
JK and TL: conceptualization and investigation. JK, TL, HF, and HA: methodology. JK: data curation and drafting. JK, TL, and KK: formal analysis. TL and HA: supervision. JK, TL, HF, HA, and KK: review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to the Organization for Women in Science and Technology for the Developing World (OWSD), Trieste, Italy for the awarded PhD Fellowship to the first author.
Footnotes
Funding. This research was funded by the Long-Term Research Grant Scheme (LRGS NO. 5526000) from the Ministry of Higher Education, Malaysia.
References
- 1.Abdulla NR, Loh TC, Akit H, Sazili AQ, Foo HL. Effect of dietary oil sources and calcium: phosphorus levels on growth performance, gut morphology and apparent digestibility of broiler chickens. S Afr J Anim Sci. (2016) 46:42–53. 10.4314/sajas.v46i1.6 [DOI] [Google Scholar]
- 2.Thacker PA, Petri D. Nutritional evaluation of canola protein concentrate for broiler chickens. Asian-Australas J Anim Sci. (2011) 24:1607–14. 10.5713/ajas.2011.11161 [DOI] [Google Scholar]
- 3.Long S, Xu Y, Wang C, Li C, Liu D, Piao X. Effects of dietary supplementation with a combination of plant oils on performance, meat quality and fatty acid deposition of broilers. Asian-Australas J Anim Sci. (2018) 31:1773–80. 10.5713/ajas.18.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatun J, Loh TC, Akit H, Foo HL, Mohamad R. Fatty acid composition, fat deposition, lipogenic gene expression and performance of broiler fed diet supplemented with different sources of oil. Anim Sci J. (2017) 88:1406–13. 10.1111/asj.12775 [DOI] [PubMed] [Google Scholar]
- 5.Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, et al. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the health ABC study. Nutr Metab Cardiovasc Dis. (2011) 21:430–7. 10.1016/j.numecd.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongsuthavas S, Terapuntuwat S, Wongsrikeaw W, Katawatin S, Yuangklang C, Beynen C. Influence of amount and type of fat on deposition, adipocyte count and iodine number of abdominal fat in broiler chickens. J Anim Physiol Anim Nutr. (2018) 92:92–8. 10.1111/j.1439-0396.2007.00714.x [DOI] [PubMed] [Google Scholar]
- 7.Fouad AM, El-Senousey HK. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas J Anim Sci. (2014) 27:1057–68. 10.5713/ajas.2013.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. (2008) 138:867–72. 10.1093/jn/138.5.867 [DOI] [PubMed] [Google Scholar]
- 9.Wu LY, Fang YJ, Guo XY. Dietary L-arginine supplementation beneficially regulates body fat deposition of meat-type ducks. Br Poult Sci. (2011) 52:221–6. 10.1080/00071668.2011.559452 [DOI] [PubMed] [Google Scholar]
- 10.Fouad AM, El-Senousey HK, Yang XJ, Yao JH. Dietary L-Arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal. (2013) 7:1239–45. 10.1017/S1751731113000347 [DOI] [PubMed] [Google Scholar]
- 11.Khajali F, Tahmasebi M, Hassanpour H, Akbari MR, Qujeq D, Wideman RF. Effects of supplementation of canola meal-based diets with arginine on performance, plasma nitric oxide, and carcass characteristics of broiler chickens grown at high altitude. Poult Sci. (2011) 90:2287–94. 10.3382/ps.2011-01618 [DOI] [PubMed] [Google Scholar]
- 12.Khatun J, Loh TC, Akit H, Foo HL, Mohamad R, Kareem KY. Dietary supplementation with L-arginine and combinations of different oil sources beneficially regulates body fat deposition, lipogenic gene expression, growth performance and carcass yield in broiler chickens. Anim Prod Sci. (2020) 60:1409–17. 10.1071/AN19205 [DOI] [Google Scholar]
- 13.Azimi Youvalari S, Farhoomand P, Baghban Kanani P, Hosseintabar Ghasemabad B. Effects of copper sulfate and arginine supplements on performance and carcass traits in broiler chickens fed with canola meal based diet. Iranian J Appl Anim Sci. (2017) 7:647–54. [Google Scholar]
- 14.Emadi M, Jahanshiri F, Azizi Jalalian F, Kaveh K, Bejo MH, Ideris A, et al. Immunostimulatory effects of arginine in broiler chickens challenged with vaccine strain of infectious bursal disease virus. J Anim Vet Adv. (2010) 9:594–600. 10.3923/javaa.2010.594.600 [DOI] [Google Scholar]
- 15.Guo YW, Shi BL, Yan SM, Xu YQ, Li JL, Li TY. Effects of arginine on cytokines and nitric oxide synthesis in broilers. J Anim Plant Sci. (2015) 25:366–71. [Google Scholar]
- 16.Saleh H, Rahimi S, Torshizi MK, Golian A. Effect of dietary fish oil on oxidative stability and lipid composition of broiler chickens breast and thigh meat. J Anim Vet Adv. (2010) 9:2877–82. 10.3923/javaa.2010.2877.2882 [DOI] [Google Scholar]
- 17.Chekani-Azar S, Shahriar HA, Maheri-Sis N, Ahmadzadeh AR, Vahdatpoor T. Omega-3 fatty acids enrichment and organoleptic characteristics of broiler meat. Asian J Anim Vet Adv. (2008) 3:62–9. 10.3923/ajava.2008.62.69 [DOI] [Google Scholar]
- 18.Gaal T, Wagner L, Husveth F, Manilla HA, Vaidovich P, Balogh N, et al. Effects of saturated and unsaturated fats with vitamin E supplementation on the anti-oxidant status of broiler chicken tissues. Acta Vet Hung. (2000) 48:69–79. 10.1556/avet.48.2000.1.8 [DOI] [PubMed] [Google Scholar]
- 19.Zhao GP, Han MJ, Zheng MQ, Zhao JP, Chen JL, Wen J. Effect of dietary vitamin E on immunological stress of layers and their offspring. Anim Physiol Anim Nutr. (2000) 95:343–50. 10.1111/j.1439-0396.2010.01060.x [DOI] [PubMed] [Google Scholar]
- 20.Kaiser MG, Block SS, Ciraci C, Fang W, Sifri M, Lamont SJ. Effects of dietary vitamin E type and level on lipopolysaccharide-induced cytokine mRNA expression in broiler chicks. Poult Sci. (2012) 91:1893–8 10.3382/ps.2011-02116 [DOI] [PubMed] [Google Scholar]
- 21.Attia YA, Al-Harthi MA, El-Shafey AS, Rehab YA, Woo Kyun K. Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics, Ann Anim Sci. (2017) 17:1155–69. 10.1515/aoas-2017-0012 [DOI] [Google Scholar]
- 22.Khatun J, Loh TC, Akit H, Foo HL, Mohamad R, Shazali N. Effect of vitamin E, oil blend and L-Arginine on fatty acid profile, lipid and protein oxidation, and meat quality of broiler. S Afr J Anim Sci. (2020). [Google Scholar]
- 23.NRC Nutrient Requirements of Poultry. National Research Council. 9th rev. edn. Washington, DC: The National Academies Press; (1994). [Google Scholar]
- 24.Rutkowski M, Grzegorczyk K. Modifications of spectrophotometric methods for antioxidative vitamins determination convenient in analytic practice. Acta Sci Pol Technol Aliment. (2007) 6:17–28. [Google Scholar]
- 25.AOAC Official Methods of Analysis, 14th edn. Arlington, VI: Association of Official Analytical Chemists, Inc. (2007). [Google Scholar]
- 26.Loh TC, Choe DW, Foo HL, Sazili AQ, Bejo MH. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Vet Res. (2014) 10:149. 10.1186/1746-6148-10-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Method. (2001) 25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc SAS/STAT® User's Guide Version 9.4. Cary, NC: SAS Institute Inc; (2014). [Google Scholar]
- 29.Moraes PO, Cardinal KM, Gouvea FL, Schroeder B, Ceron MS, Lunedo R, et al. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poult Sci. (2019) 98:5456–64. 10.3382/ps/pez345 [DOI] [PubMed] [Google Scholar]
- 30.Jiao P, Guo Y, Yang X, Long F. Effects of dietary Arginine and Methionine levels on broiler carcass traits and meat quality. J Anim Vet Adv. (2010) 9:1546–51. 10.3923/javaa.2010.1546.1551 [DOI] [Google Scholar]
- 31.Chen JM, Wang M, Kong Y, Ma H, Zou S. Comparison of the novel compounds creatine and pyruvate on lipid and protein metabolism in broiler chickens. Animal. (2011) 5:1082–9. 10.1017/S1751731111000085 [DOI] [PubMed] [Google Scholar]
- 32.Rostami H, Seidavi AR, Dadashbeiki M, Asadpour Y, Simoes J, Shah AA, et al. Supplementing dietary rosemary (Rosmarinus officinalis L.) powder and vitamin E in broiler chickens: evaluation of humoral immune response, lymphoid organs, and blood proteins. Environ Sci Pollut Res. (2018) 25:8836–42. 10.1007/s11356-018-1209-x [DOI] [PubMed] [Google Scholar]
- 33.El-Gogary MR, Ismail FSA, El-Nadi MI. Effect of vitamin E supplementation and stocking density on broiler performance, Carcass traits and histological responses of lymphoid organs. Asian J Poult Sci. (2015) 9:70–84. 10.3923/ajpsaj.2015.70.84 [DOI] [Google Scholar]
- 34.Biswas A, Mohan J, Sastry KVH, Tyagi JS. Effect of higher levels of dietary vitamin E on performance and immune response in growing Japanese quail. J Appl Anim Res. (2008) 33:61–4. 10.1080/09712119.2008.9706897 [DOI] [Google Scholar]
- 35.Abdukalykova ST, Ruiz-Feria CA. Arginine and vitamin E improves the cellular and humoral immune response of broiler chickens. Int J Poult Sci. (2006) 5:121–7. 10.3923/ijps.2006.121.12719637028 [DOI] [Google Scholar]
- 36.Skrivan M, Dlouhá G, Englmaierová M, Cervinková K. Effects of different levels of dietary supplementalcaprylic acid and vitamin E on performance, breast muscle vitamin E and A, and oxidative stability in broilers. Czech J of Anim Sci. (2010) 55:167–73. 10.17221/221/2009-CJAS [DOI] [Google Scholar]
- 37.Zhang X, Wang G, Zhou Y, Wang T. Effect of RRR-α-tocopherol succinate on the meat quality and antioxidativestatus in broilers. S Afr J Anim Sci. (2012) 42 (4):340–53. 10.4314/sajas.v42i4.2 [DOI] [Google Scholar]
- 38.Pompeua MA, Cavalcantib LFL, Torala FLB. Effect of vitamin E supplementation on growth performance, meat quality, and immune response of male broiler chickens: a meta-analysis. Livest Sci. (2018) 208:5–13. 10.1016/j.livsci.2017.11.021 [DOI] [Google Scholar]
- 39.Tan B, Yin Y, Liu Z, Li X, Xu H, Kong X, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing finishing pigs. Amino Acid. (2009) 37:169–75. 10.1007/s00726-008-0148-0 [DOI] [PubMed] [Google Scholar]
- 40.Souza XR, Faria PB, Bressan MC. Proximate composition and meat quality of broilers reared under different production systems. Braz J Poult Sci. (2011) 13:15–20. 10.1590/S1516-635X2011000100003 [DOI] [Google Scholar]
- 41.Velasco S, Ortiz LT, Alzueta C, Rebole A, Trevino J, Rodriguez ML. Effect of inulin supplementation and dietary fat source on performance, blood serum metabolites, liver lipids, abdominal fat deposition, and tissue fatty acid composition in broiler chickens. Poult Sci. (2010) 89:1651–62. 10.3382/ps.2010-00687 [DOI] [PubMed] [Google Scholar]
- 42.Ahmed SK, Abdul-Abass MH, Al-Hammed SA. Effect of dietary supplementation of natural and synthetic antioxidants on broilers physiological and productive performance. Egypt Poult Sci J. (2015) 35:93–105. [Google Scholar]
- 43.Gumus R, Imik H. Effects of vitamin E (α-Tocopherol acetate) on serum lipid profile, Ca and P levels of broilers exposed to heat stress. Sch J Agric Vet Sci. (2016) 3:105–10. [Google Scholar]
- 44.Attia YA, Mohammed MA, Abo El-Maaty HM. The effects of different oil sources on performance, digestive enzymes, carcass traits, biochemical, immunological, antioxidant, and morphometric responses of broiler chicks. Front Vet Sci. (2020) 7:181. 10.3389/fvets.2020.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kidd MT. Nutritional modulation of immune function in broilers. Poult Sci. (2004) 83:650–7. 10.1093/ps/83.4.650 [DOI] [PubMed] [Google Scholar]
- 46.Muir WI, Husband AJ, Bryden WL. Dietary supplementation with vitamin E modulates avian intestinal immunity. Br J Nutr. (2002) 87:579–85. 10.1079/BJN2002562 [DOI] [PubMed] [Google Scholar]
- 47.Munir K, Muneer MA, Masaoud E, Tiwari A, Mahmud A, Chaudhry RM, et al. Dietary arginine stimulates humoral and cell-mediated immunity in chickens vaccinated and challenged against hydropericardium syndrome virus. Poult Sci. (2009) 88:1629–38. 10.3382/ps.2009-00152 [DOI] [PubMed] [Google Scholar]
- 48.Emadi M, Jahanshiri F, Kaveh K, Hair-Bejo M, Ideris A, Alimon AR. Nutrition and immunity: the effects of the combination of arginine and tryptophan on growth performance, serum parameters and immune response in broiler chickens challenged with infectious bursal disease vaccine. Avian Pathol. (2011) 40:63–72. 10.1080/03079457.2010.539590 [DOI] [PubMed] [Google Scholar]
- 49.Attia YA, Abou-Shehema BM, Abdellah AA, Aly OM, El-Naggar AS. Effect of ascorbic acid and/or alpha-tocopherol fortification on semen quality, metabolic profile, antioxidants status, and dna of roosters exposed to heat stress. J Anim Plant Sci. (2020) 30:325–35. 10.36899/JAPS.2020.2.0051 [DOI] [Google Scholar]
- 50.Cheng K, Song ZH, Zheng XC, Zhang H, Zhang JF, Zhang LL, et al. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult Sci. (2017) 96:1159–66. 10.3382/ps/pew336 [DOI] [PubMed] [Google Scholar]
- 51.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu GY. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. (2006) 17:571–88. 10.1016/j.jnutbio.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Wang W, Yao K, Zhou T, Yin J. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One. (2013) 8:e69502. 10.1371/journal.pone.0069502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Byrd JA, Farnell M, Ruiz-Feria CA. Arginine and vitamin E improve the immune response after a Salmonella challenge in broiler chicks. Poult Sci. (2014) 93:882–90. 10.3382/ps.2013-03723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article.