Coronavirus disease 2019 (COVID-19)–associated coagulopathy, responsible for high rates of pulmonary thrombosis, is poorly characterized. Clinical studies identified several biomarkers, such as D-dimer. Different mechanisms have been proposed, including neutrophil and complement activation, vascular damage, and tissue factor expression. The intrinsic pathway of coagulation, which not only amplifies fibrin generation but also links to inflammation, including plasma kallikrein and bradykinin, both proposed to contribute to COVID-19, has not been studied. We performed a comprehensive analysis on the intrinsic pathway to characterize its role in COVID-19. By simultaneously studying potential triggers of the intrinsic pathway, we were able to identify neutrophils, neutrophil extracellular traps (NETs), and complement activation as potential drivers of this complex immunothrombotic disease.
We included 228 consecutive patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confirmed by real-time polymerase chain reaction (Table 1). Disease severity was classified as mild in patients not admitted to the hospital, moderate in admitted patients requiring oxygen via nasal cannula, and progressive/severe in patients requiring oxygen via a face mask, or admitted to the intensive care unit for invasive ventilation or those who died ≤7 days. Thrombotic events were uncommon in mild (n/N=2/54) and moderate COVID-19 (n/N=3/68) as compared with progressive/severe disease (n/N=23/106; P=0.002). At presentation and during follow-up, blood was obtained; sputum was collected in selected cases. This study was approved by the local ethics committee (no. 2020-1315), with a waiver of informed consent.
Table 1.
Baseline Characteristics of 228 Patients With Coronavirus Disease 2019
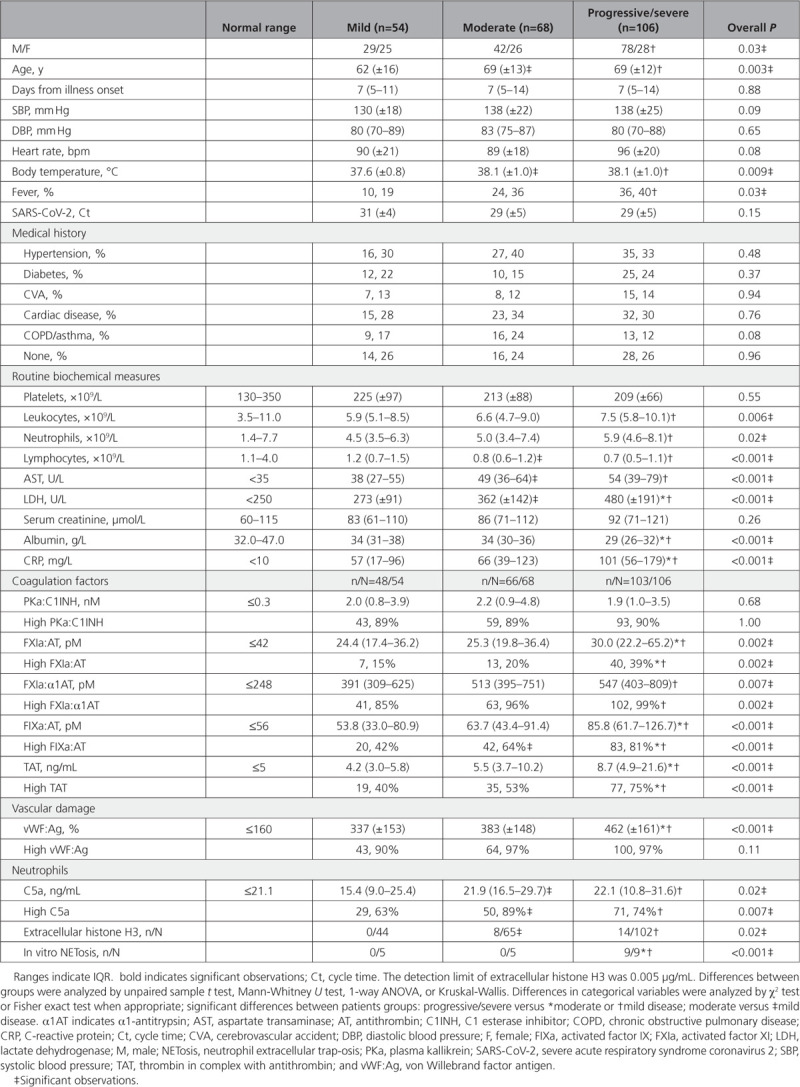
To delineate activation of coagulation, we used enzyme-linked immunosorbent assays to measure activated coagulation factors in complex with their natural inhibitors as described with modifications.1 Hypercoagulability was found in 131 (60%) out of 217 tested patients as indicated by increased levels of thrombin:antithrombin. We report for the first time that hypercoagulability was driven, at least partly, through contact activation as indicated by increased levels of plasma kallikrein:C1 esterase inhibitor (n/N=195/217), FXIa:α1 antitrypsin (n/N=206/217), FXIa:antithrombin (n/N=60/217), and FIXa:antithrombin (n/N=145/217). The finding that these circulating complexes remained stable at day 5 (±2; n=53) and day 10 (±2; n=40) in patients with progressive/severe COVID-19 treated with low molecular weight heparin corroborates this conclusion as heparins exert only mild effects on contact activation. It is remarkable that our data show that contact activation is already ongoing in most patients with mild disease, but the downstream markers FXIa:antithrombin, FIXa:antithrombin, and thrombin:antithrombin are clearly linked to COVID-19 severity. This might explain the high incidence of thrombotic events in patients with progressive/severe disease. Also, von Willebrand factor antigen was associated with disease severity, linking the pulmonary vasculature and platelets to hypercoagulability.
Next, we questioned whether the overwhelming immune response contributes to hypercoagulability. We hypothesized that neutrophils, and more specific NET formation with release of histones and DNA fragments, trigger contact activation.2 First, we assessed the presence of extracellular histone H3 (H3) in plasma and sputum by Western blot using an antihuman H3 polyclonal antibodies as described.3 Extracellular H3 was detected in patients with moderate and progressive/severe COVID-19 but not in those with mild disease. Extracellular H3 was citrullinated in 38 (73%) out of 52 cases, as detected by anticitrulline monoclonal antibodies, indicating NETs as its origin. The presence of citrullinated H3 in sputum from 9 patients requiring invasive ventilation linked neutrophil extracellular trap-osis (NETosis) to the lungs. Thereafter, we assessed whether patients with COVID-19 have circulating factors that induce NETosis by incubating neutrophils from healthy donors with patient serum diluted in medium for 4 hours at 37°C. Serum samples from all 9 patients with progressive/severe COVID-19 showed abundant formation of NETs that stain positive for DNA, citrullinated H3, neutrophil elastase, and myeloperoxidase on immunofluorescence microscopy, whereas serum from patients with mild and those with moderate COVID-19 did not; 2 (40%) out of 5 samples from patients with moderate disease only induced swelling of nuclei without NETosis. NETs have been shown to colocalize with thrombi in COVID-19.4 NETs with release of histones and DNA fragments, which trigger contact activation,2 may thereby contribute to COVID-19–associated coagulopathy.
It has been appreciated that neutrophils, especially NETs, engage with coagulation and complement in a triangular relationship to produce the immunothrombotic response to invading pathogens. The complement system plays a critical role in binding SARS-CoV-2’s N-protein, leading to the generation of the potent anaphylatoxin C5a. The subsequent attraction, priming, and activation of neutrophils causes an amplification loop of complement and neutrophil activation with more generation of C5a.5 C5a, quantified by enzyme immunoassay, was elevated in COVID-19 and increased significantly with disease severity.
In conclusion, our analysis of a large cohort of patients with COVID-19 presents strong indications that hypercoagulability and thrombotic events are driven by NETosis, contact activation, and complement. The triangular relationship with its multiple amplifying feedback loops urges therapeutic multiple-target strategies to dampen the immunothrombotic response effectively. Likely candidates are blockers of C5a, emerging inhibitors of plasma kallikrein and FXIa, and agents that neutralize extracellular histones. The challenge is to identify high-risk patients in the earliest possible stage of disease.
Sources of Funding
H.t.C. and H.M.H.S. receive grant support by the Netherlands Heart Foundation (CVON2014-09), Reappraisal of Atrial Fibrillation: Interaction between HyperCoagulability, Electrical Remodeling, and Vascular Destabilization in the Progression of Atrial Fibrillation (RACE V), and from REG-MED XB: Cardiovascular Moonshot. H.t.C. and H.M.H.S. are member of the Dutch Coalition on Thrombosis in COVID-19 (DCTC) supported by ZON-MW and the Netherlands Thrombosis Foundation. H.t.C. was supported by a fellowship of the Gutenberg University Mainz. M.N. is supported by grant funding from REG-MED XB: Cardiovascular Moonshot. M.V. is supported in part by funding from a Health-Holland project/partnership grant (LSHM 9111).
Disclosures
H.t.C. and H.M.H.S. received funding for research from Bayer and Pfizer; they are stakeholder in Coagulation Profile; H.t.C. is consultant for Alveron and has served on advisory boards for Bayer, Pfizer, Daiichi, Leo, and Gilead. C.P.R. and G.A.F.N. are coinventors of a patent describing use of low anticoagulant heparins in sepsis and owned by Maastricht University. C.P.R. is scientific consultant for Matisse Pharmaceuticals and Annexin Pharmaceuticals. The other authors report no conflicts.
Footnotes
Drs Busch and Timmermans contributed equally.
Drs Reutelingsperger and van Paassen contributed equally.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Contributor Information
Matthias H. Busch, Email: matthias.busch@mumc.nl.
Sjoerd A.M.E.G. Timmermans, Email: sjoerd.timmermans@mumc.nl.
Magdolna Nagy, Email: m.nagy@maastrichtuniversity.nl.
Mayken Visser, Email: m.visser@maastrichtuniversity.nl.
Joram Huckriede, Email: j.huckriede@maastrichtuniversity.nl.
Joop P. Aendekerk, Email: joop.aendekerk@mumc.nl.
Femke de Vries, Email: femke.devries@student.maastrichtuniversity.nl.
Judith Potjewijd, Email: judith.potjewijd@mumc.nl.
Borefore Jallah, Email: papay.jallah@mumc.nl.
Renée Ysermans, Email: renee.ysermans@mumc.nl.
Astrid M.L. Oude Lashof, Email: a.oudelashof@mumc.nl.
Paul H. Breedveld, Email: ph.breedveld@mumc.nl.
Marcel C.G. van de Poll, Email: marcel.vande.poll@mumc.nl.
Iwan C.C. van de Horst, Email: iwan.vander.horst@mumc.nl.
Bas C.T. van Bussel, Email: bas.van.bussel@mumc.nl.
Ruud O.M.F.I.H. Theunissen, Email: r.theunissen@maastrichtuniversity.nl.
Henri M.H. Spronk, Email: henri.spronk@maastrichtuniversity.nl.
Jan G.M.C. Damoiseaux, Email: jan.damoiseaux@mumc.nl.
Hugo ten Cate, Email: h.tencate@maastrichtuniversity.nl.
Gerry A.F. Nicolaes, Email: g.nicolaes@maastrichtuniversity.nl.
Chris P. Reutelingsperger, Email: c.reutelingsperger@maastrichtuniversity.nl.
References
- 1.Govers-Riemslag JW, Smid M, Cooper JA, Bauer KA, Rosenberg RD, Hack CE, Hamulyak K, Spronk HM, Miller GJ, ten Cate H. The plasma kallikrein-kinin system and risk of cardiovascular disease in men. J Thromb Haemost. 2007;5:1896–1903. doi: 10.1111/j.1538-7836.2007.02687.x [DOI] [PubMed] [Google Scholar]
- 2.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wildhagen KC, García de Frutos P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984 [DOI] [PubMed] [Google Scholar]
- 4.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camous L, Roumenina L, Bigot S, Brachemi S, Frémeaux-Bacchi V, Lesavre P, Halbwachs-Mecarelli L. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564 [DOI] [PubMed] [Google Scholar]


